Effect of Illite Treatment on Quality Characteristics and Antioxidant Activity of Broccoli (Brassica oleracea L. var. italica) Sprouts
Abstract
1. Introduction
2. Results and Discussion
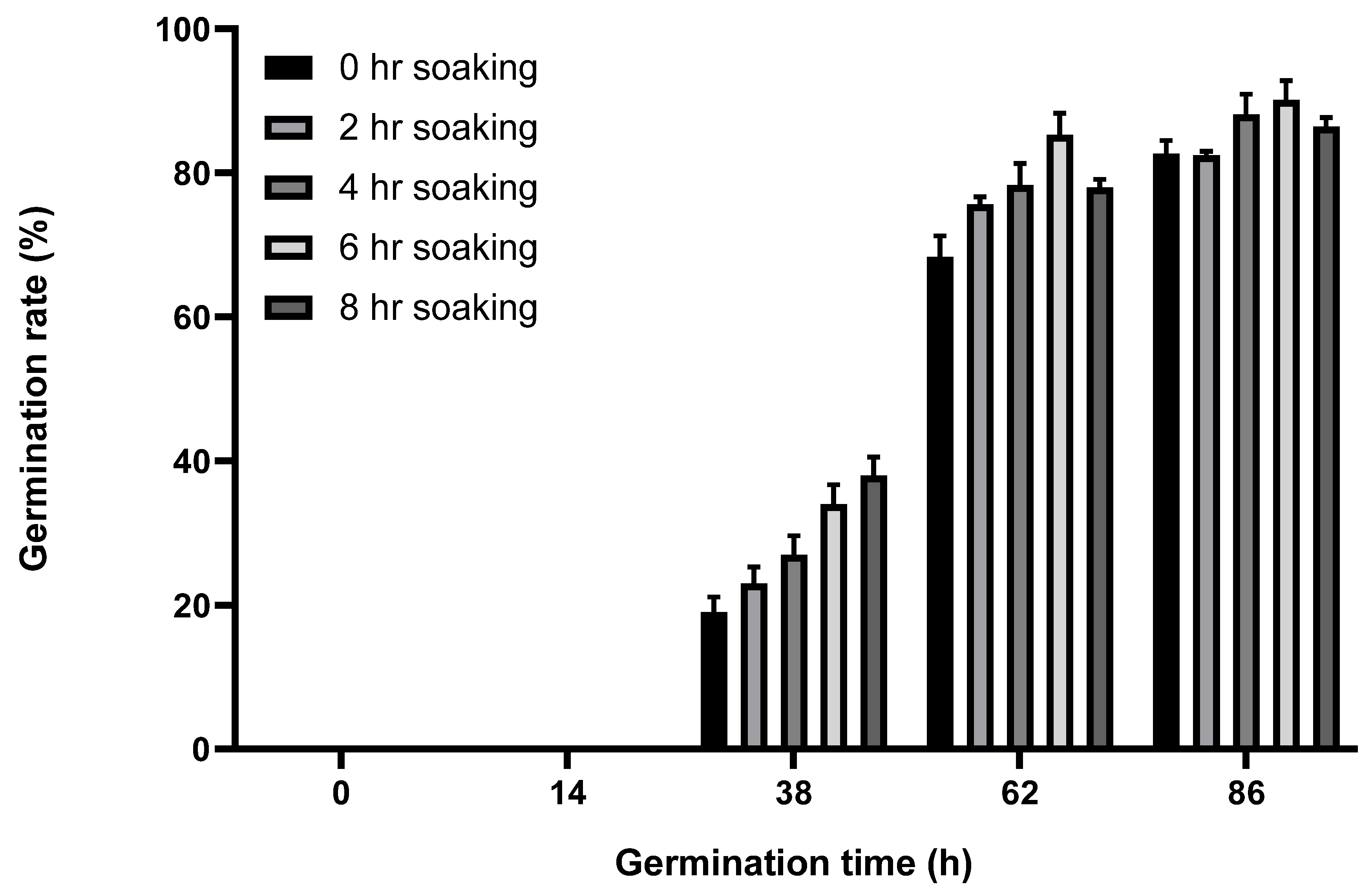
2.1. Effect of Soaking Time on Germination Rate of Broccoli Seeds
2.2. Total Weight and Vitamin C Content
2.3. Mineral Contents
| Element | Control | IS 1% | IS 3% | IS 5% | IS 7% |
|---|---|---|---|---|---|
| Ca | 16.55 ± 0.34 | 22.15 ± 1.34 | 27.84 ± 3.4 | 34.42 ± 4.42 | 38.91 ± 2.37 |
| Zn | 0.01 ± 0.001 | 0.01 ± 0.0006 | 0.03 ± 0.0004 | 0.09 ± 0.016 | 0.10 ± 0.012 |
| Fe | 0.55 ± 0.02 | 0.89 ± 0.04 | 1.73 ± 0.08 | 2.58 ± 0.12 | 3.36 ± 0.16 |
| K | 6.75 ± 0.45 | 19.29 ± 4.5 | 25.88 ± 4.77 | 41.66 ± 2.26 | 53.37 ± 1.84 |
| Na | 17.01 ± 2.18 | 27.22 ± 1.65 | 36.53 ± 1.92 | 43.25 ± 5.56 | 46.71 ± 5.45 |
| Mg | 0.44 ± 0.14 | 0.66 ± 0.15 | 0.833 ± 0.15 | 0.55 ± 0.02 | 0.86 ± 0.02 |
| Mn | ND | ND | ND | ND | ND |
| Cu | ND | ND | 0.01 | 0.01 | 0.02 |
| Cd | ND | ND | ND | ND | ND |
| As | ND | ND | ND | ND | ND |
| Hg | ND | ND | ND | ND | ND |
| Pb | ND | ND | ND | ND | ND |
| Total | 41.33505 | 70.243195 | 92.8627078 | 122.5665369 | 143.3464516 |
2.4. Hunter’s Color Value
2.5. Free Amino Acid Content
2.6. Sulforaphane Content
2.7. Volatile Compounds
2.8. DPPH Radical Scavenging Activity, Total Flavonoid and Polyphenol Contents
3. Materials and Methods
3.1. Materials and Samples
3.2. Broccoli Seed Germination Test
3.3. Sprouting Method
3.4. Determination of Vitamin C Content
3.5. Color Measurement
3.6. Determination of Mineral Composition
3.7. Quantification of Free Amino Acid Content
3.8. Extraction and Determination of Volatile Compounds (GC/MS)
3.9. Determination of Sulforaphane Content (LC/MS)
3.10. Determination of Antioxidant Activities
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajan, P.; Lada, R.R.; MacDonald, M.T. Advancement in indoor vertical farming for microgreen production. Am. J. Plant Sci. 2019, 10, 1397. [Google Scholar] [CrossRef]
- Michell, K.A.; Isweiri, H.; Newman, S.E.; Bunning, M.; Bellows, L.L.; Dinges, M.M.; Grabos, L.E.; Rao, S.; Foster, M.T.; Heuberger, A.L. Microgreens: Consumer sensory perception and acceptance of an emerging functional food crop. J. Food Sci. 2020, 85, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.k.; Rehal, J.; Kaur, A.; Jyot, G. Enhancement of Attributes of Cereals by Germination and Fermentation: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Kayahara, H.; Tsukahara, K.; Tatai, T. Flavor, health and nutritional quality of pre-germinated brown rice. In Proceedings of the 10th International Flavor Conference, Paros, Greece, 4–7 July 2000. [Google Scholar]
- Kang, P.; Kaur, S.; Singh, J.; Rasane, P. Broccoli and Cress Sprouts. In Advances in Plant Sprouts: Phytochemistry and Biofunctionalities; Majid, I., Kehinde, B.A., Dar, B., Nanda, V., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 331–362. [Google Scholar]
- Jaiswal, A.K. Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Villaño, D.; López-Chillón, M.T.; Zafrilla, P.; Moreno, D.A. Bioavailability of broccoli sprouts in different human overweight populations. J. Funct. Foods 2019, 59, 337–344. [Google Scholar] [CrossRef]
- Bokić, J.; Škrobot, D.; Tomić, J.; Šeregelj, V.; Abellán-Victorio, Á.; Moreno, D.A.; Ilić, N. Broccoli sprouts as a novel food ingredient: Nutritional, functional and sensory aspects of sprouts enriched pasta. LWT 2022, 172, 114203. [Google Scholar] [CrossRef]
- Nandini, D.B.; Rao, R.S.; Deepak, B.S.; Reddy, P.B. Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J. Oral Maxillofac. Pathol. 2020, 24, 405. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Suzuki, K. The integrative role of sulforaphane in preventing inflammation, oxidative stress and fatigue: A review of a potential protective phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef]
- Schepici, G.; Bramanti, P.; Mazzon, E. Efficacy of sulforaphane in neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 8637. [Google Scholar] [CrossRef]
- Lee, S.; Choi, B.R.; Kim, J.; LaFerla, F.M.; Park, J.H.Y.; Han, J.S.; Lee, K.W.; Kim, J. Sulforaphane Upregulates the Heat Shock Protein Co-Chaperone CHIP and Clears Amyloid-β and Tau in a Mouse Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2018, 62, 1800240. [Google Scholar] [CrossRef]
- Su, X.; Jiang, X.; Meng, L.; Dong, X.; Shen, Y.; Xin, Y. Anticancer activity of sulforaphane: The epigenetic mechanisms and the Nrf2 signaling pathway. Oxidative Med. Cell. Longev. 2018, 2018, 5438179. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Novel seeds pretreatment techniques: Effect on oil quality and antioxidant properties: A review. J. Food Sci. Technol. 2021, 58, 4451–4464. [Google Scholar] [CrossRef]
- Miyahira, R.F.; Antunes, A.E.C. Bacteriological safety of sprouts: A brief review. Int. J. Food Microbiol. 2021, 352, 109266. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.-C.; Im, D.-Y.; Park, H.-S.; Dhungana, S.K.; Kim, I.-D.; Shin, D.-H. Seed treatment with illite enhanced yield and nutritional value of soybean sprouts. Molecules 2022, 27, 1152. [Google Scholar] [CrossRef] [PubMed]
- Han, D.-H.; Kim, H.-J.; Kim, S.-H.; Kim, I.-D.; Adhikari, A.; Kim, J.-H. Effect of illite pretreatment on germinated Brown rice with Special Reference to amino acids, antioxidants, texture, and mineral elements. Heliyon 2024, 10, e28843. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Yoo, C.H.; Lee, I.-H.; Bae, J.-Y.; Hong, S.-H.; Cha, J.-M. Effect of broccoli sprouts germination by soaking water condition. Korean J. Biotechnol. Bioeng. 2008, 23, 551–552. [Google Scholar]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Natella, F.; Maldini, M.; Nardini, M.; Azzini, E.; Foddai, M.S.; Giusti, A.M.; Baima, S.; Morelli, G.; Scaccini, C. Improvement of the nutraceutical quality of broccoli sprouts by elicitation. Food Chem. 2016, 201, 101–109. [Google Scholar] [CrossRef]
- Nishikawa, F.; Kato, M.; Hyodo, H.; Ikoma, Y.; Sugiura, M.; Yano, M. Effect of sucrose on ascorbate level and expression of genes involved in the ascorbate biosynthesis and recycling pathway in harvested broccoli florets. J. Exp. Bot. 2005, 56, 65–72. [Google Scholar] [CrossRef]
- Salama, Z.A.; El Baz, F.K.; Gaafar, A.A.; Zaki, M.F. Antioxidant activities of phenolics, flavonoids and vitamin C in two cultivars of fennel (Foeniculum vulgare Mill.) in responses to organic and bio-organic fertilizers. J. Saudi Soc. Agric. Sci. 2015, 14, 91–99. [Google Scholar] [CrossRef]
- Uchendu, E.E.; Leonard, S.W.; Traber, M.G.; Reed, B.M. Vitamins C and E improve regrowth and reduce lipid peroxidation of blackberry shoot tips following cryopreservation. Plant Cell Rep. 2010, 29, 25–35. [Google Scholar] [CrossRef]
- Kathi, S.; Laza, H.; Singh, S.; Thompson, L.; Li, W.; Simpson, C. Simultaneous biofortification of vitamin C and mineral nutrients in arugula microgreens. Food Chem. 2024, 440, 138180. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi Goharrizi, K.; Riahi-Madvar, A.; Rezaee, F.; Pakzad, R.; Jadid Bonyad, F.; Ghazizadeh Ahsaei, M. Effect of Salinity Stress on Enzymes’ Activity, Ions Concentration, Oxidative Stress Parameters, Biochemical Traits, Content of Sulforaphane, and CYP79F1 Gene Expression Level in Lepidium draba Plant. J. Plant Growth Regul. 2020, 39, 1075–1094. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, H.; Zhao, Y.; Cheng, X.; Wang, L.; Guo, X. Effect of light quality on polyphenol biosynthesis in three varieties of mung bean sprouts with different color seed coats. Plant Cell Rep. 2023, 42, 253–268. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development and Metabolism; Springer Nature: Berlin, Germany, 2023. [Google Scholar]
- Yu, S.P. Regulation and critical role of potassium homeostasis in apoptosis. Prog. Neurobiol. 2003, 70, 363–386. [Google Scholar] [CrossRef]
- Assunção, A.G.L.; Cakmak, I.; Clemens, S.; González-Guerrero, M.; Nawrocki, A.; Thomine, S. Micronutrient homeostasis in plants for more sustainable agriculture and healthier human nutrition. J. Exp. Bot. 2022, 73, 1789–1799. [Google Scholar] [CrossRef]
- Dey, S.; Nagababu, B.H. Applications of food color and bio-preservatives in the food and its effect on the human health. Food Chem. Adv. 2022, 1, 100019. [Google Scholar] [CrossRef]
- Ashraf, U.; Kiran, M.; Shahid, M.N.; Anjum, S.A.; Khan, I. Biofortification Through Seed Priming in Food Crops: Potential Benefits and Future Scope. In Mineral Biofortification in Crop Plants for Ensuring Food Security; Hasanuzzaman, M., Tahir, M.S., Tanveer, M., Shah, A.N., Eds.; Springer Nature: Singapore, 2023; pp. 261–296. [Google Scholar]
- Oplatowska-Stachowiak, M.; Elliott, C.T. Food colors: Existing and emerging food safety concerns. Crit. Rev. Food Sci. Nutr. 2017, 57, 524–548. [Google Scholar] [CrossRef]
- Díaz-Gómez, J.; Twyman, R.M.; Zhu, C.; Farré, G.; Serrano, J.C.E.; Portero-Otin, M.; Muñoz, P.; Sandmann, G.; Capell, T.; Christou, P. Biofortification of crops with nutrients: Factors affecting utilization and storage. Curr. Opin. Biotechnol. 2017, 44, 115–123. [Google Scholar] [CrossRef]
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Amino acids in plants: Regulation and functions in development and stress defense. Front. Plant Sci. 2021, 12, 772810. [Google Scholar] [CrossRef]
- Heinemann, B.; Hildebrandt, T.M. The role of amino acid metabolism in signaling and metabolic adaptation to stress-induced energy deficiency in plants. J. Exp. Bot. 2021, 72, 4634–4645. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Signorelli, S.; Höfte, M. γ-Aminobutyric acid and related amino acids in plant immune responses: Emerging mechanisms of action. Plant Cell Environ. 2020, 43, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Textor, S.; Bartram, S.; Kroymann, J.; Falk, K.L.; Hick, A.; Pickett, J.A.; Gershenzon, J. Biosynthesis of methionine-derived glucosinolates in Arabidopsis thaliana: Recombinant expression and characterization of methylthioalkylmalate synthase, the condensing enzyme of the chain-elongation cycle. Planta 2004, 218, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, J.; Ullah, S.; Ahmad, I. Sulforaphane and its protective role in prostate cancer: A mechanistic approach. Int. J. Mol. Sci. 2023, 24, 6979. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Fimognari, C. Cytotoxic and antitumor activity of sulforaphane: The role of reactive oxygen species. BioMed Res. Int. 2015, 2015, 402386. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.F.; Yuan, G.F.; Wang, Q.M. Effect of NaCl treatments on glucosinolate metabolism in broccoli sprouts. J. Zhejiang Univ. Sci. B 2013, 14, 124–131. [Google Scholar] [CrossRef]
- Li, L.; Song, S.; Nirasawa, S.; Hung, Y.-C.; Jiang, Z.; Liu, H. Slightly acidic electrolyzed water treatment enhances the main bioactive phytochemicals content in broccoli sprouts via changing metabolism. J. Agric. Food Chem. 2018, 67, 606–614. [Google Scholar] [CrossRef]
- Melchini, A.; Traka, M.H. Biological profile of erucin: A new promising anticancer agent from cruciferous vegetables. Toxins 2010, 2, 593–612. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Zhao, Z.; Sheng, X.; Shen, Y.; Gu, H. Natural variation of glucosinolates and their breakdown products in broccoli (Brassica oleracea var. italica) seeds. J. Agric. Food Chem. 2019, 67, 12528–12537. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Zielińska-Dawidziak, M.; Siger, A. Effect of elevated accumulation of iron in ferritin on the antioxidants content in soybean sprouts. Eur. Food Res. Technol. 2012, 234, 1005–1012. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Sonne, C.; Kim, K.-H. Heavy metals and arsenic stress in food crops: Elucidating antioxidative defense mechanisms in hyperaccumulators for food security, agricultural sustainability, and human health. Sci. Total Environ. 2023, 874, 162327. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.N.; Gaur, J.P. Relationship between copper-and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta 2004, 219, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists; Association of Official Agricultural Chemists (US). Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Rockville, MD, USA, 1931; Volume 3. [Google Scholar]
- Skujins, S. Handbook for ICP-AES (Varian-Vista). A Short Guide to Vista Series ICP-AES Operation. Varian Int; Version 1.0; AG Zug: Zug, Switzerland, 1998. [Google Scholar]
- Je, J.-Y.; Park, P.-J.; Jung, W.-K.; Kim, S.-K. Amino acid changes in fermented oyster (Crassostrea gigas) sauce with different fermentation periods. Food Chem. 2005, 91, 15–18. [Google Scholar] [CrossRef]
- Howard, K.L.; Mike, J.H.; Riesen, R. Validation of a solid-phase microextraction method for headspace analysis of wine aroma components. Am. J. Enol. Vitic. 2005, 56, 37–45. [Google Scholar] [CrossRef]
- Adhikari, B.; Dhungana, S.K.; Ali, M.W.; Adhikari, A.; Kim, I.-D.; Shin, D.-H. Resveratrol, total phenolic and flavonoid contents, and antioxidant potential of seeds and sprouts of Korean peanuts. Food Sci. Biotechnol. 2018, 27, 1275–1284. [Google Scholar] [CrossRef]
- Mohdaly, A.A.A.; Sarhan, M.A.; Smetanska, I.; Mahmoud, A. Antioxidant properties of various solvent extracts of potato peel, sugar beet pulp and sesame cake. J. Sci. Food Agric. 2010, 90, 218–226. [Google Scholar] [CrossRef]
| Sample | Total Weight (g) | Vitamin C Content (mg/100 g of dw) |
|---|---|---|
| Control | 101.44 ± 5.32 b | 45.45 ± 1.75 |
| IPB-1 | 116.79 ± 1.89 a | 53.8 ± 3.17 |
| IPB-3 | 107.51 ± 9.50 ab | 55.55 ± 3.74 |
| IPB-5 | 105.10 ± 7.16 b | 50.5 ± 3.37 |
| IPB-7 | 104.97 ± 4.81 b | 49.99 ± 1.32 |
| Sample (1) | |||||
|---|---|---|---|---|---|
| Element | Control | IPB-1 | IPB-3 | IPB-5 | IPB-7 |
| Ca | 4.67 ± 0.1 d (2) | 5.48 ± 0.15 c | 5.9 ± 0.21 b | 6.26 ± 0.27 a | 5.53 ± 0.34 bc |
| Cu | ND (3) | 0.01 ± 0.00 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b | ND |
| Fe | 0.07 ± 0.00 b | 0.08 ± 0.00 a | 0.07 ± 0.00 b | 0.07 ± 0.00 c | 0.04 ± 0.00 d |
| K | 3.92 ± 0.19 | 4.56 ± 0.22 | 4.68 ± 0.3 | 4.99 ± 0.1 | 3.61 ± 0.15 |
| Mg | 2.22 ± 0.06 b | 2.32 ± 0.02 a | 2.35 ± 0.02 a | 2.24 ± 0.01 b | 1.78 ± 0.06 c |
| Na | 0.94 ± 0.00 d | 1.14 ± 0.02 b | 1.08 ± 0.01 c | 1.29 ± 0.01 a | 0.79 ± 0.01 e |
| Zn | ND | ND | ND | ND | ND |
| P | 5.03 ± 0.11 | 5.88 ± 0.064 | 6.05 ± 0.06 | 6.16 ± 0.14 | 4.87 ± 0.22 |
| S | 7.14 ± 0.17 | 8.76 ± 0.42 | 9.04 ± 0.44 | 8.69 ± 0.34 | 8.32 ± 0.4 |
| Mn | 0.01 ± 0.00 e | 0.01 ± 0.00 c | 0.02 ± 0.00 b | 0.02 ± 0.00 a | 0.01 ± 0.00 d |
| Total | 24.04 | 28.245 | 29.155 | 29.73 | 24.98 |
| Sample (1) | Color Value (2) | ||
|---|---|---|---|
| L* (Lightness) | a* (Redness) | b* (Yellowness) | |
| Control | 65.85 ± 0.66 ab (3) | 0.96 ± 0.07 b | 18.83 ± 0.47 b |
| IPB-1 | 65.32 ± 0.41 b | 0.95 ± 0.09 ab | 18.19 ± 0.16 c |
| IPB-3 | 66.63 ± 0.81 a | 0.80 ± 0.10 b | 19.06 ± 0.37 b |
| IPB-5 | 66.29 ± 0.69 ab | 1.10 ± 0.06 a | 19.42 ± 0.23 b |
| IPB-7 | 64.80 ± 0.51 b | 1.12 ± 0.06 a | 20.29 ± 0.22 a |
| Sample (1) | |||||
|---|---|---|---|---|---|
| Amino Acid | Control | IPB-1 | IPB-3 | IPB-5 | IPB-7 |
| Essential amino acids Threonine | 0.96 ± 0.02 e (2) | 1.40 ± 0.02 a | 1.01 ± 0.02 d | 1.38 ± 0.02 b | 1.32 ± 0.02 c |
| Valine | 1.10 ± 0.02 d | 1.56 ± 0.02 a | 1.23 ± 0.03 c | 1.45 ± 0.02 b | 1.44 ± 0.02 b |
| Methionine | 0.64 ± 0.01 b | 0.51 ± 0.01 c | 0.67 ± 0.01 a | 0.50 ± 0.01 c | 0.53 ± 0.01 c |
| Isoleucine | 0.96 ± 0.01 d | 1.39 ± 0.02 a | 1.07 ± 0.02 c | 1.29 ± 0.02 b | 1.29 ± 0.02 b |
| Leucine | 1.63 ± 0.02 c | 2.04 ± 0.03 a | 1.65 ± 0.03 c | 1.98 ± 0.03 b | 1.95 ± 0.02 b |
| Phenylalanine | 1.04 ± 0.01 d | 1.35 ± 0.02 a | 1.05 ± 0.02 d | 1.32 ± 0.01 b | 1.28 ± 0.02 c |
| Lysine | 1.76 ± 0.03 d | 2.27 ± 0.03 a | 1.74 ± 0.03 d | 2.24 ± 0.03 b | 2.12 ± 0.02 c |
| Histidine | 0.64 ± 0.01 c | 0.83 ± 0.01 a | 0.64 ± 0.01 c | 0.82 ± 0.02 a | 0.78 ± 0.01 b |
| Subtotal | 8.73 | 11.35 | 9.06 | 10.98 | 10.72 |
| Nonessential amino acids Aspartic acid | 1.57 ± 0.02 c | 2.04 ± 0.02 a | 1.49 ± 0.03 d | 2.06 ± 0.02 a | 1.93 ± 0.01 b |
| Serine | 0.65 ± 0.01 c | 1.09 ± 0.02 a | 0.61 ± 0.01 d | 1.11 ± 0.01 a | 1.01 ± 0.02 b |
| Glutamic acid | 3.09 ± 0.03 d | 4.62 ± 0.04 b | 2.89 ± 0.03 e | 4.87 ± 0.03 a | 4.43 ± 0.03 c |
| Glycine | 0.41 ± 0.01 a | 0.38 ± 0.01 b | 0.39 ± 0.01 b | 0.39 ± 0.01 b | 0.38 ± 0.01 b |
| Alanine | 0.91 ± 0.01 c | 1.14 ± 0.02 a | 1.85 ± 0.01 d | 1.17 ± 0.02 a | 1.08 ± 0.01 b |
| Tyrosine | 0.62 ± 0.01 d | 0.91 ± 0.01 a | 0.67 ± 0.01 c | 0.84 ± 0.02 b | 0.82 ± 0.02 b |
| Arginine | 1.51 ± 0.03 c | 1.87 ± 0.02 a | 1.86 ± 0.02 c | 1.79 ± 0.02 b | 0.92 ± 0.01 d |
| Proline | ND (3) | ND | ND | ND | ND |
| Subtotal | 8.74 | 12.04 | 8.42 | 12.22 | 11.45 |
| Essential to nonessential amino acid ratio | 0.99 | 0.94 | 1.08 | 0.90 | 0.94 |
| Other amino acids Phosphoserine | 0.21 ± 0.01 a | 0.18 ± 0.01 b | 0.21 ± 0.02 a | 0.17 ± 0.01 b | 0.18 ± 0.01 b |
| Taurine | ND | ND | ND | ND | ND |
| Phosphoethanolamine | ND | ND | ND | ND | ND |
| Urea | 0.39 ± 0.01 b | 0.30 ± 0.01 d | 0.47 ± 0.01 a | 0.34 ± 0.02 c | 0.36 ± 0.02 c |
| Sarcosine | ND | ND | ND | ND | ND |
| α-Amino adipic acid | ND | 0.01 ± 0.01 a | ND | 0.01 ± 0.01 a | ND |
| Citrulline | 2.22 ± 0.02 c | 2.35 ± 0.03 a | 2.21 ± 0.03 c | 2.36 ± 0.03 a | 2.27 ± 0.03 b |
| α-Amino-n-butyric acid | 0.01 ± 0.01 a | ND | 0.01 ± 0.01 a | ND | ND |
| Cystine | 0.41 ± 0.02 b | 0.47 ± 0.03 a | 0.43 ± 0.01 b | 0.47 ± 0.01 a | 0.48 ± 0.02 a |
| Cystathionine | 0.03 ± 0.01 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a |
| β-alanine | 0.04 ± 0.01 b | 0.09 ± 0.01 a | 0.05 ± 0.01 b | 0.08 ± 0.01 a | 0.09 ± 0.01 a |
| β-amino isobutyric acid | 0.03 ± 0.01 a | 0.02 ± 0.01 a | 0.03 ± 0.01 a | 0.02 ± 0.01 a | ND |
| γ-amino-n-butyric acid | 0.06 ± 0.01 a | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 0.06 ± 0.01 a | 0.06 ± 0.01 a |
| Ethanolamine | 0.04 ± 0.01 b | 0.06 ± 0.01 a | 0.05 ± 0.01 b | 0.07 ± 0.01 a | 0.06 ± 0.01 a |
| Hydroxylysine | 0.02 ± 0.01 a | ND | ND | ND | ND |
| Ornithine | 0.02 ± 0.01 b | 0.05 ± 0.01 a | 0.05 ± 0.01 a | 0.05 ± 0.01 a | 0.05 ± 0.01 a |
| 1-methylhistidine | 0.02 ± 0.01 c | 0.32 ± 0.02 a | 0.02 ± 0.01 c | 0.32 ± 0.03 a | 0.26 ± 0.02 b |
| 3-methylhistidine | ND | ND | ND | ND | ND |
| Anserine | 0.25 ± 0.02 a | 0.15 ± 0.02 b | 0.22 ± 0.02 a | 0.11 ± 0.01 c | 0.15 ± 0.01 b |
| Carnosine | ND | ND | ND | ND | ND |
| Hydroxyproline | 0.9 ± 0.03 e | 1.01 ± 0.03 de | 1.47 ± 0.03 c | 2.04 ± 0.02 a | 1.90 ± 0.02 b |
| Sub-total | 5.28 | 6.12 | 5.30 | 6.13 | 5.90 |
| Total free amino acids | 21.70 | 28.52 | 24.13 | 29.33 | 27.21 |
| Sample (1) | Control | IPB-1 | IPB-3 | IPB-5 | IPB-7 |
|---|---|---|---|---|---|
| Sulforaphane (μg/g of DW) | 15.23 ± 1.12.07 b (2) | 22.36 ± 0.87 a | 21.36 ± 1.09 a | 22.49 ± 0.24 a | 22.14 ± 0.63 a |
| Sample (1) | DPPH (% Inhibition) | Total Flavonoids (mg QE (2)/100 g) | Total Polyphenols (mg GAE (3)/100 g) |
|---|---|---|---|
| Control | 81.51 ± 0.16 b (4) | 185.65 ± 5.25 a | 74.25 ± 0.09 b |
| IPB-1 | 82.09 ± 0.31 a | 186.00 ± 4.55 a | 74.41 ± 0.07 a |
| IPB-3 | 79.53 ± 0.55 c | 177.93 ± 1.31 b | 74.35 ± 0.02 a |
| IPB-5 | 78.18 ± 0.71 d | 166.00 ± 8.20 c | 74.37 ± 0.12 a |
| IPB-7 | 80.10 ± 0.65 c | 166.35 ± 3.47 c | 74.41 ± 0.12 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Dhungana, S.K.; Kim, I.-D.; Adhikari, A.; Kim, J.-H. Effect of Illite Treatment on Quality Characteristics and Antioxidant Activity of Broccoli (Brassica oleracea L. var. italica) Sprouts. Molecules 2024, 29, 4347. https://doi.org/10.3390/molecules29184347
Kim S-H, Dhungana SK, Kim I-D, Adhikari A, Kim J-H. Effect of Illite Treatment on Quality Characteristics and Antioxidant Activity of Broccoli (Brassica oleracea L. var. italica) Sprouts. Molecules. 2024; 29(18):4347. https://doi.org/10.3390/molecules29184347
Chicago/Turabian StyleKim, So-Hyun, Sanjeev Kumar Dhungana, Il-Doo Kim, Arjun Adhikari, and Jeong-Ho Kim. 2024. "Effect of Illite Treatment on Quality Characteristics and Antioxidant Activity of Broccoli (Brassica oleracea L. var. italica) Sprouts" Molecules 29, no. 18: 4347. https://doi.org/10.3390/molecules29184347
APA StyleKim, S.-H., Dhungana, S. K., Kim, I.-D., Adhikari, A., & Kim, J.-H. (2024). Effect of Illite Treatment on Quality Characteristics and Antioxidant Activity of Broccoli (Brassica oleracea L. var. italica) Sprouts. Molecules, 29(18), 4347. https://doi.org/10.3390/molecules29184347






