Efficient Charge Carriers Separation and Transfer Driven by Interface Electric Field in FeS2@ZnIn2S4 Heterojunction Boost Hydrogen Evolution
Abstract
1. Introduction
2. Results and Discussion
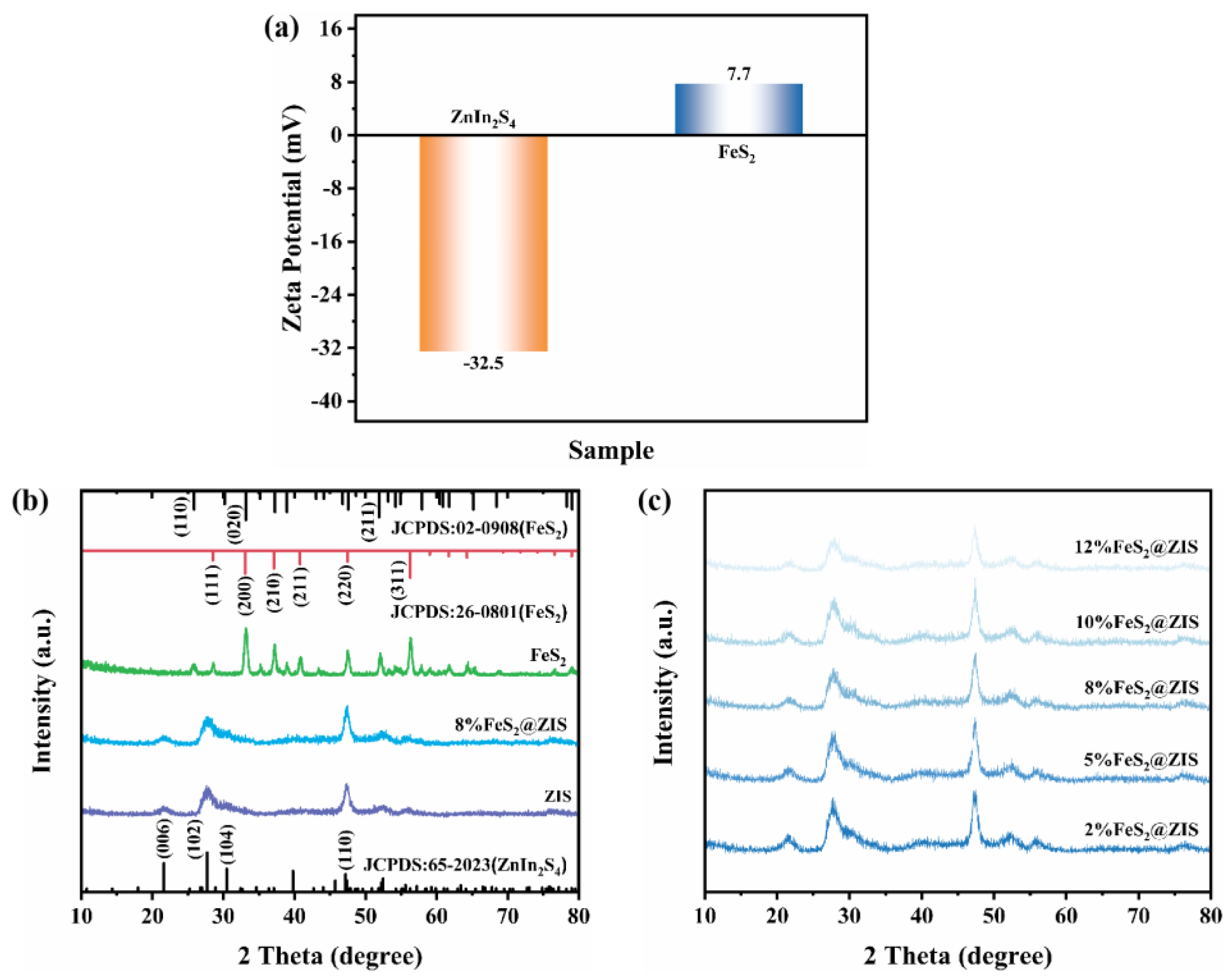
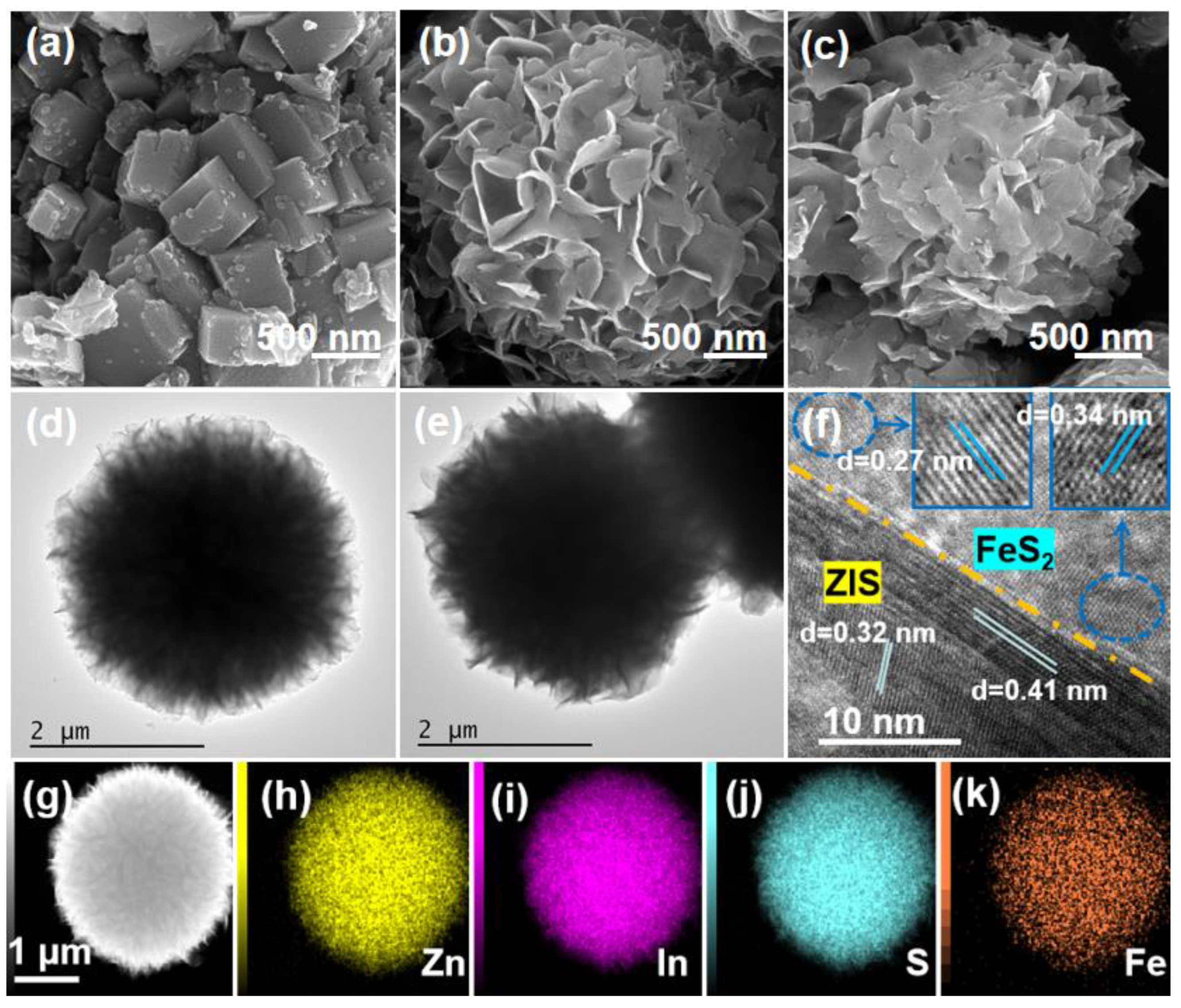
2.1. Structure and Morphology Analysis
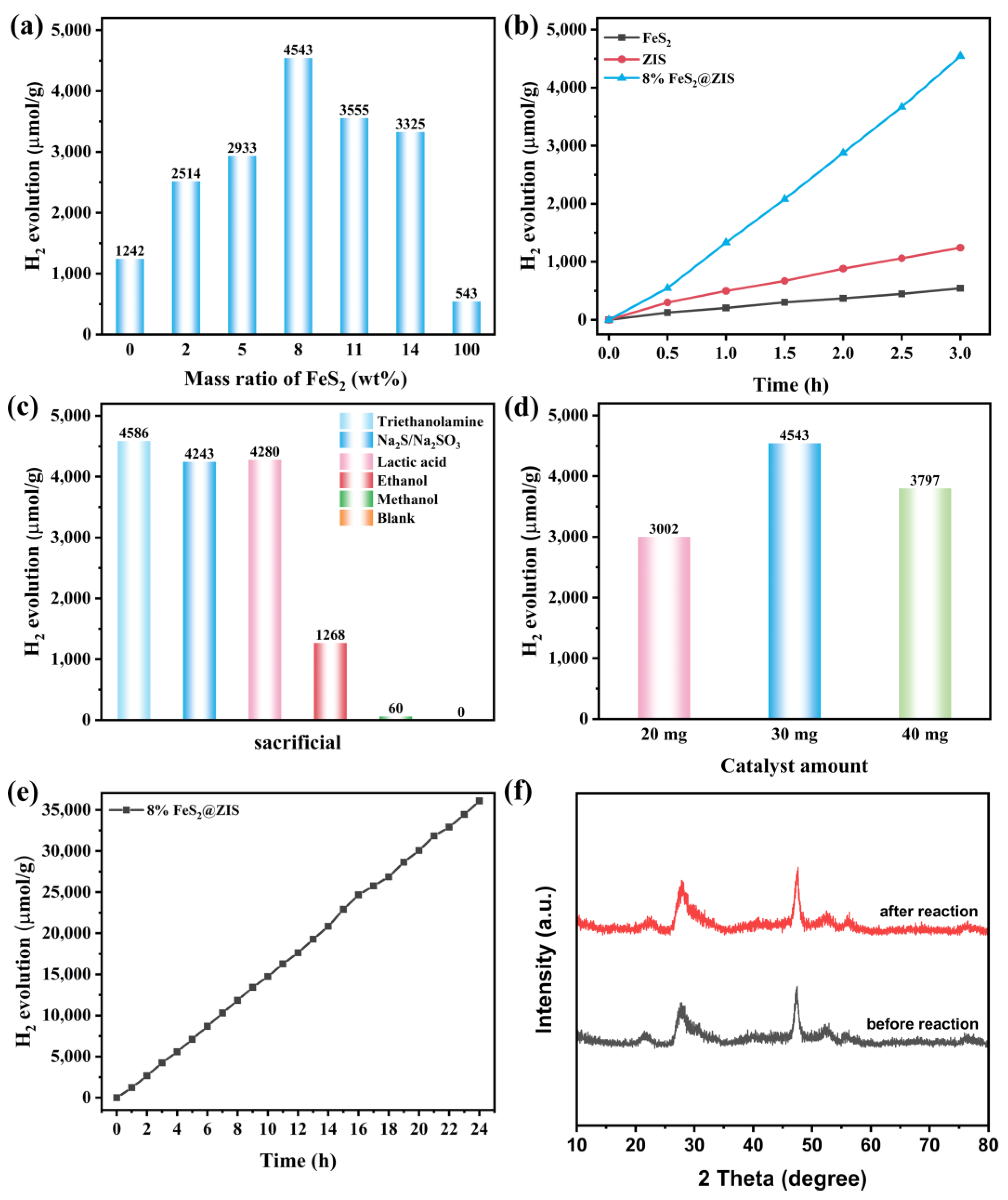
2.2. HER Performance
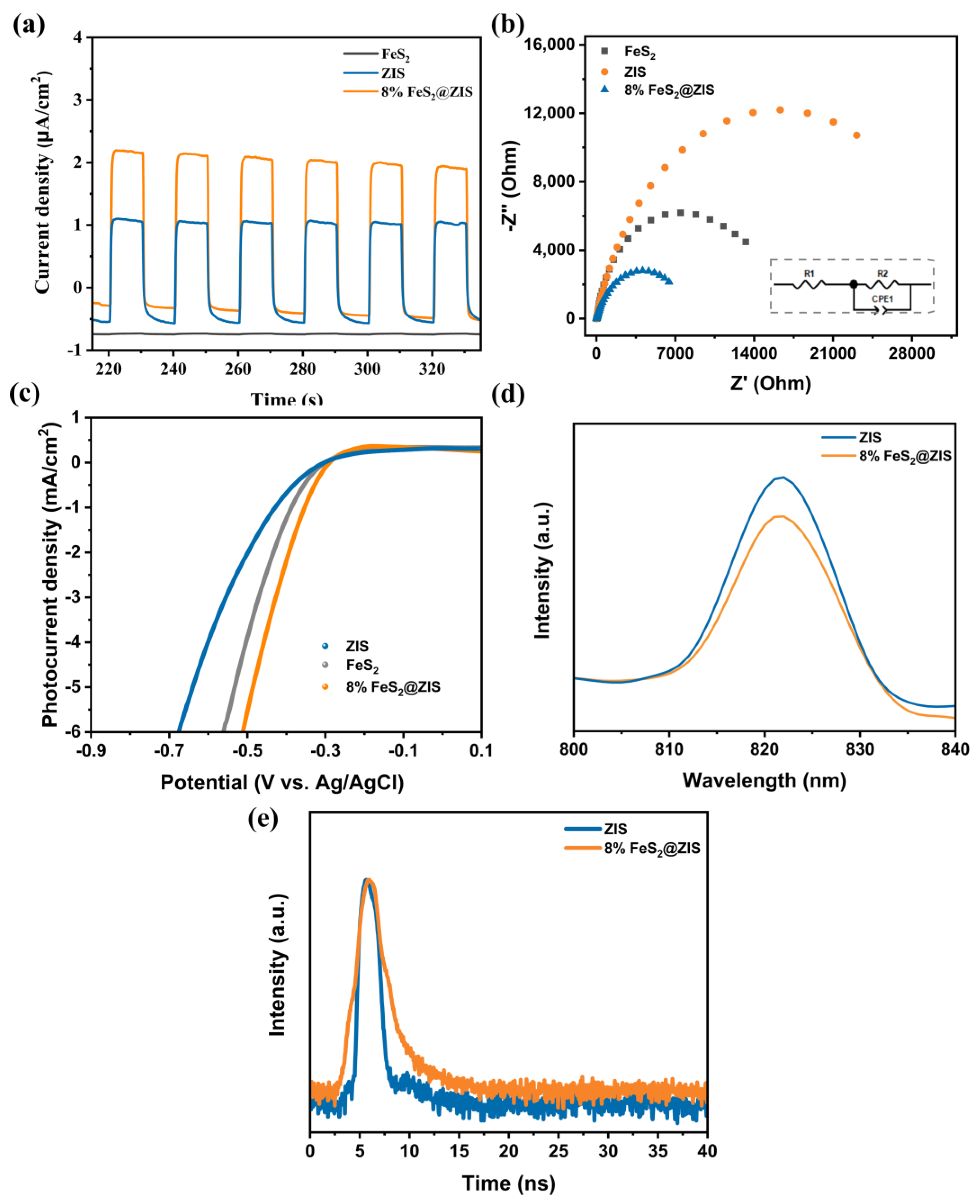
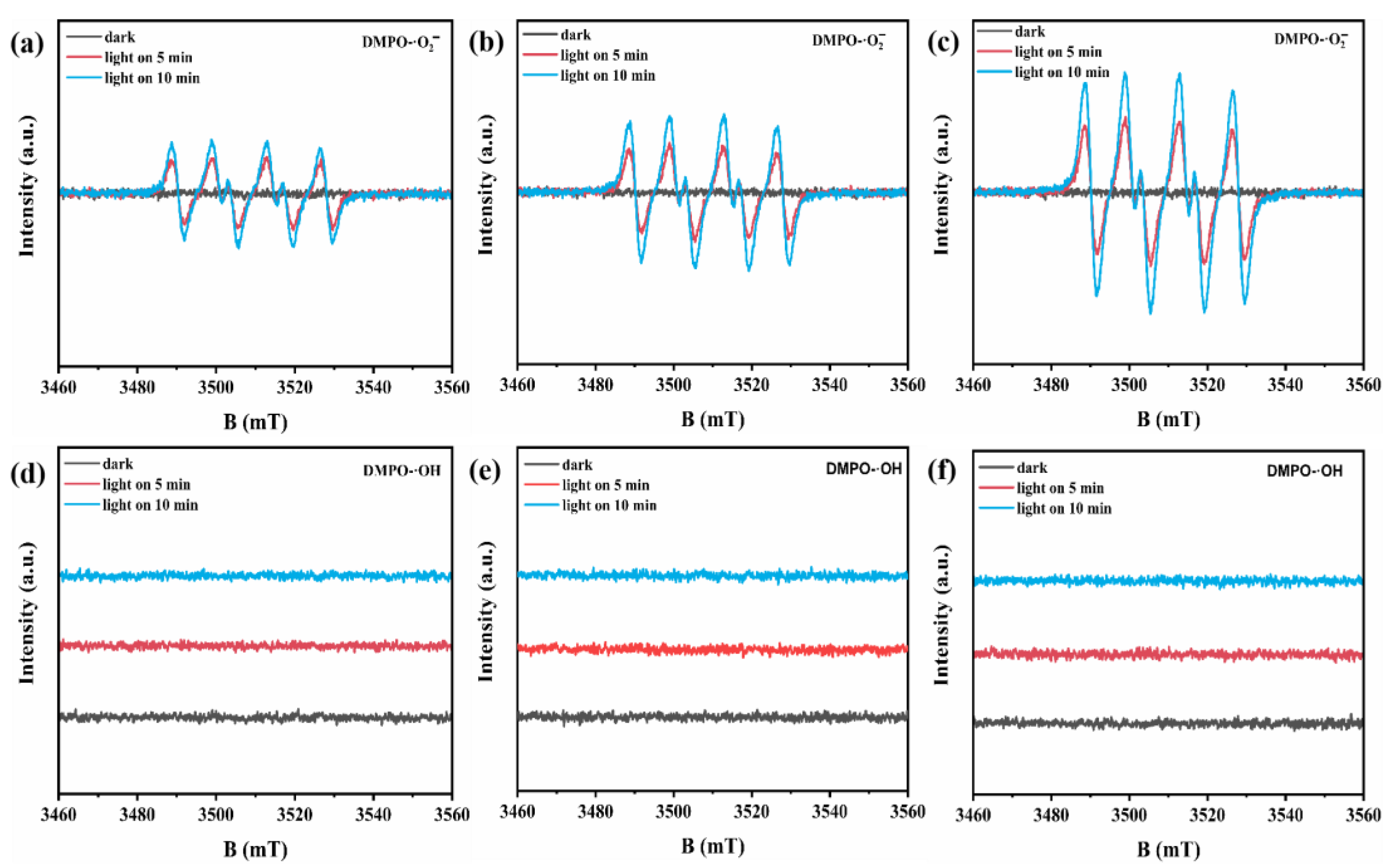
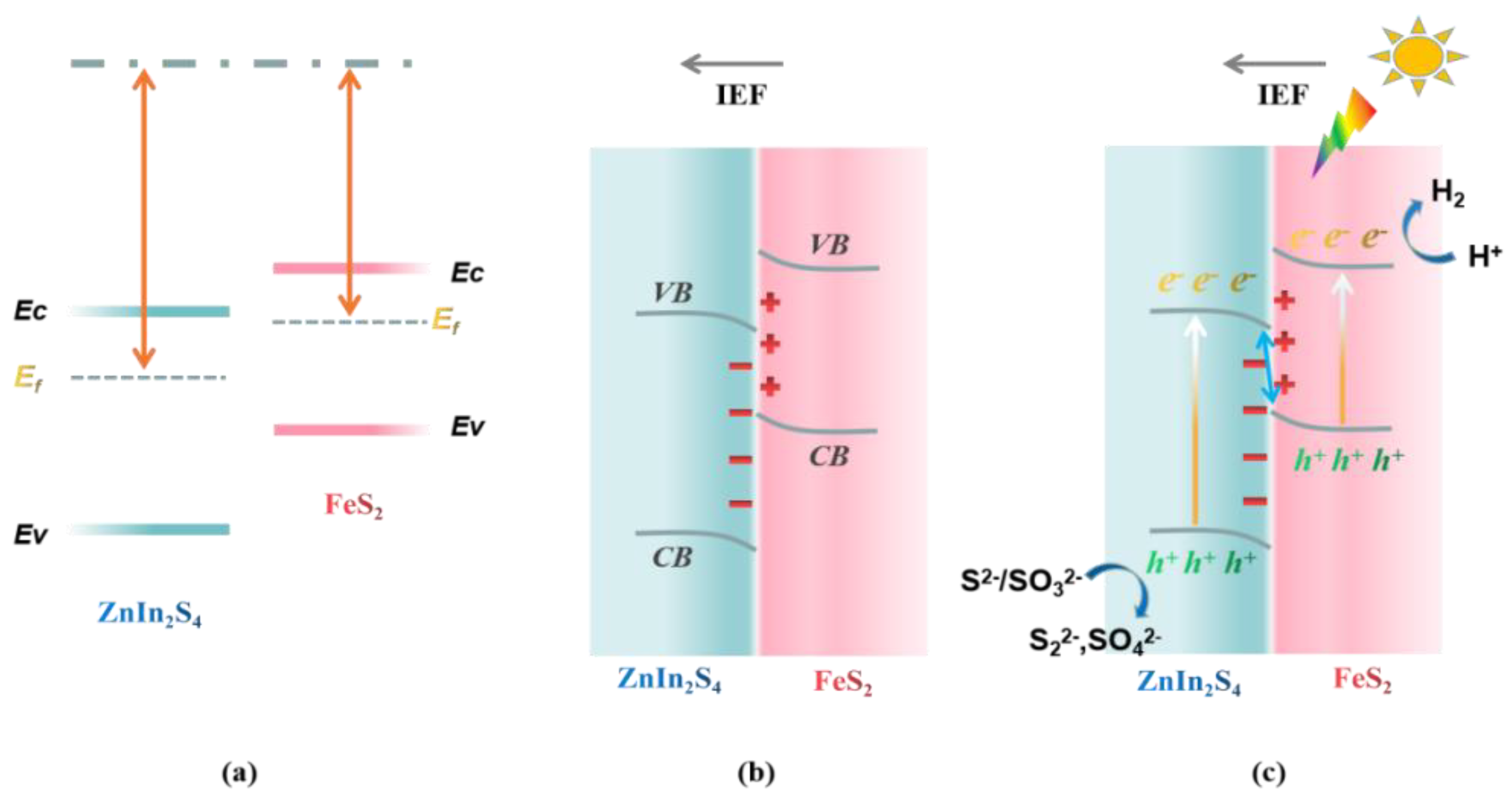
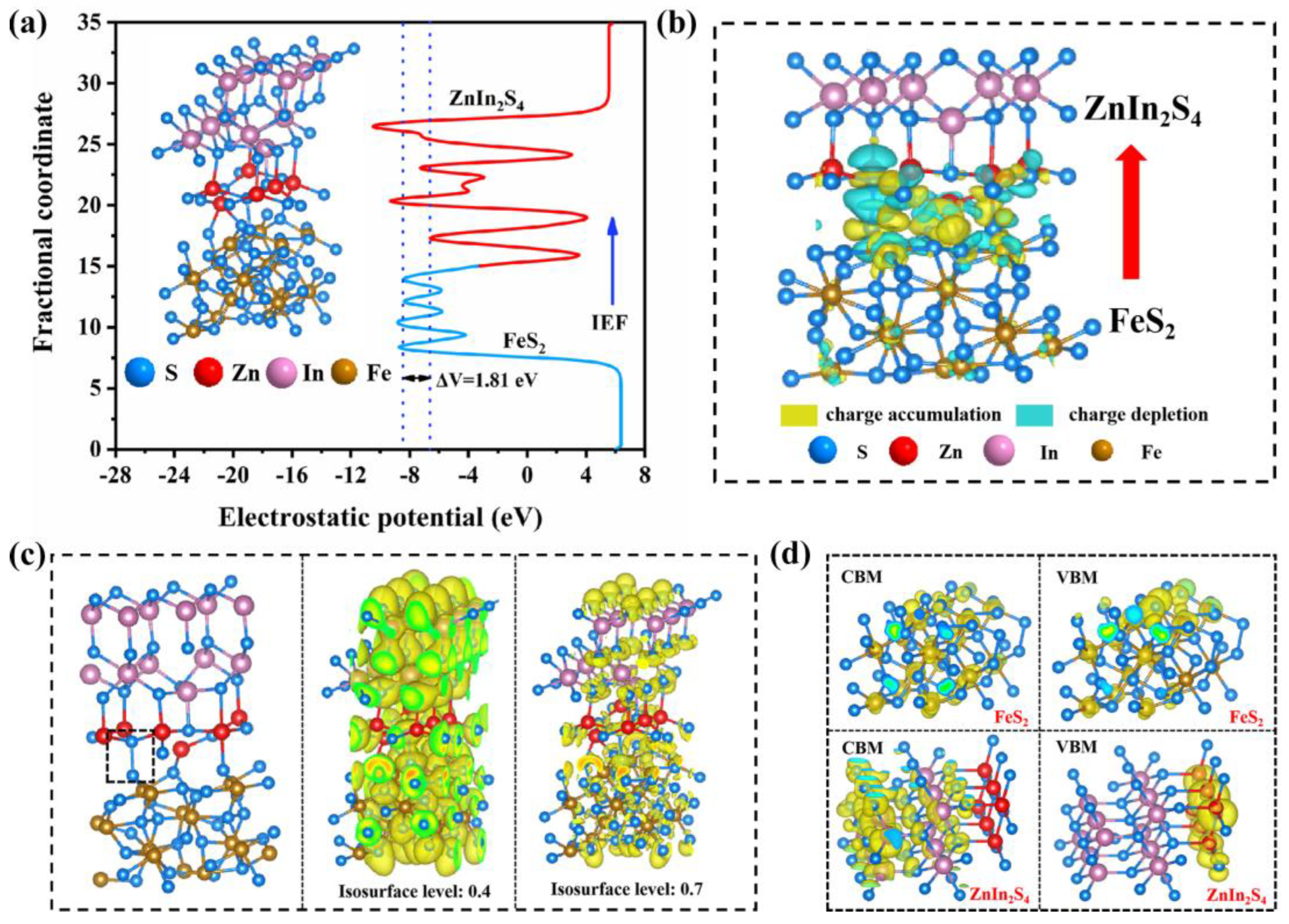
2.3. Photocatalysis Mechanism
3. Experimental Section
3.1. Synthesis of FeS2/ZIS Photocatalyst
3.2. Characterization of the Samples
3.3. Photocatalytic Activity Evaluation
3.4. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo (electro) Catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Song, H.; Luo, S.; Huang, H.; Deng, B.; Ye, J. Solar-Driven Hydrogen Production: Recent Advances, Challenges, and Future Perspectives. ACS Energy Lett. 2022, 7, 1043–1065. [Google Scholar] [CrossRef]
- Li, H.; Li, R.; Liu, G.; Zhai, M.; Yu, J. Noble Metal-Free Single-and Dual-Atom Catalysts for Artificial Photosynthesis. Adv. Mater. 2023, 36, 2301307. [Google Scholar] [CrossRef]
- Ganguly, P.; Harb, M.; Cao, Z.; Cavallo, L.; Breen, A.; Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D Nanomaterials for Photocatalytic Hydrogen Production. ACS Energy Lett. 2019, 4, 1687–1709. [Google Scholar] [CrossRef]
- Cai, J.; Shen, J.; Zhang, X.; Ng, Y.H.; Huang, J.; Guo, W.; Lin, C.; Lai, Y. Light-Driven Sustainable Hydrogen Production Utilizing TiO2 Nanostructures: A Review. Small Methods 2019, 3, 1800184. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Cao, R. Solar-to-Hydrogen Energy Conversion Based on Water Splitting. Adv. Energy Mater. 2018, 8, 1701620. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Ni, M. Hydrogen Society: From Present to Future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Tentu, R.D.; Basu, S. Photocatalytic Water Splitting for Hydrogen Production. Curr. Opin. Electrochem. 2017, 5, 56–62. [Google Scholar] [CrossRef]
- Wang, Y.; Suzuki, H.; Xie, J.; Tomita, O.; Martin, D.J.; Higashi, M.; Kong, D.; Abe, R.; Tang, J. Mimicking Natural Photosynthesis: Solar to Renewable H2 Fuel Synthesis by Z-Scheme Water Splitting Systems. Chem. Rev. 2018, 118, 5201–5241. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent Advances in Semiconductors for Photocatalytic and Photoelectrochemical Water Splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Sun, H.; Xue, W.; Fan, J.; Liu, E.; Yu, Q. Preparation of Ni12P5-Decorated Cd0.5Zn0.5S for Efficient Photocatalytic H2 Evolution. J. Alloys Compd. 2021, 854, 156951. [Google Scholar] [CrossRef]
- Li, B.; Guo, W.; Lu, X.F.; Hou, Y.; Ding, Z.; Wang, S. Position-Selected Cocatalyst Modification on a Z-Scheme Cd0.5Zn0.5S/NiTiO3 Photocatalyst for Boosted H2 Evolution. Mater. Rep. Energy 2023, 3, 100230. [Google Scholar] [CrossRef]
- Xue, X.; Dong, W.; Luan, Q.; Gao, H.; Wang, G. Novel Interfacial Lateral Electron Migration Pathway Formed by Constructing Metallized CoP2/CdS Interface for Excellent Photocatalytic Hydrogen Production. Appl. Catal. B Environ. 2023, 334, 122860. [Google Scholar] [CrossRef]
- Kalia, R.; Pirzada, B.M.; Kunchala, R.K.; Naidu, B.S. Noble Metal Free Efficient Photocatalytic Hydrogen Generation by TaON/CdS Semiconductor Nanocomposites under Natural Sunlight. Int. J. Hydrogen Energy 2023, 48, 16246–16258. [Google Scholar] [CrossRef]
- Kang, E.; Kim, J.H. Highly Boosted Photocatalytic H2 Production from ZnS Particles Assisted by Cd-Cu Co-Doping. J. Environ. Chem. Eng. 2023, 11, 109833. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Liu, W.; Zhang, J.; Chen, J.; Li, Y. Copper-Doped Zinc Sulfide Nanoframes with Three-Dimensional Photocatalytic Surfaces for Enhanced Solar Driven H2 Production. Chin. J. Catal. 2022, 43, 782–792. [Google Scholar] [CrossRef]
- Zhang, R.; Jia, X.; Li, Y.; Yu, X.; Xing, Y. Oxidation Coatalyst Modified In2S3 with Efficient Interfacial Charge Transfer for Boosting Photocatalytic H2 Evolution. Int. J. Hydrogen Energy 2022, 47, 25300–25308. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Li, C.; Shi, Z.; Feng, S. Indium-Vacancy-Rich In2S3/Ni@C Photocatalyst with Chemical Bonds for Producing Hydrogen and Benzylamine Oxidation. Sep. Purif. Technol. 2023, 324, 124571. [Google Scholar] [CrossRef]
- Wang, Z.; Su, B.; Xu, J.; Hou, Y.; Ding, Z. Direct Z-scheme ZnIn2S4/LaNiO3 Nanohybrid with Enhanced Photocatalytic Performance for H2 Evolution. Int. J. Hydrogen Energy 2020, 45, 4113–4121. [Google Scholar] [CrossRef]
- Liu, J.; Chen, G.; Sun, J. Ag2S-Modified ZnIn2S4 Nanosheets for Photocatalytic H2 Generation. ACS Appl. Nano Mater. 2020, 3, 11017–11024. [Google Scholar] [CrossRef]
- Sun, G.; Tai, Z.; Li, F.; Ye, Q.; Wang, T.; Fang, Z.; Jia, L.; Liu, W.; Wang, H. Construction of ZnIn2S4/CdS/PdS S-Scheme Heterostructure for Efficient Photocatalytic H2 Production. Small 2023, 19, 2207758. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Zhang, J.; Dai, K. Metal-Sulfide-Based Heterojunction Photocatalysts: Principles, Impact, Applications, and In-Situ Characterization. Chin. J. Catal. 2023, 49, 42–67. [Google Scholar] [CrossRef]
- Di, T.; Deng, Q.; Wang, G.; Wang, S.; Wang, L.; Ma, Y. Photodeposition of CoOx and MoS2 on CdS as Dual Cocatalysts for Photocatalytic H2 Production. J. Mater. Sci. Technol. 2022, 124, 209–216. [Google Scholar] [CrossRef]
- Cha, G.; Schmuki, P.; Altomare, M. Anodic TiO2 Nanotube Membranes: Site-Selective Pt-Activation and Photocatalytic H2 Evolution. Electrochim. Acta 2017, 258, 302–310. [Google Scholar] [CrossRef]
- Kunthakudee, N.; Puangpetch, T.; Ramakul, P.; Serivalsatit, K.; Hunsom, M. Light-Assisted Synthesis of Au/TiO2 Nanoparticles for H2 Production by Photocatalytic Water Splitting. Int. J. Hydrogen Energy 2022, 47, 23570–23582. [Google Scholar] [CrossRef]
- Lv, T.; Xiao, B.; Xia, F.; Chen, M.; Zhao, J.; Ma, Y.; Wu, J.; Zhang, J.; Zhang, Y.; Liu, Q. Insights Into Synergistic Effect of Pd Single Atoms and Sub-Nanoclusters on TiO2 for Enhanced Photocatalytic H2 Evolution. Chem. Eng. J. 2022, 450, 137873. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Xue, J.; Liu, H.; Zeng, S.; Feng, Y.; Zhang, Y.; Zhu, Y.; Cheng, M.; Zhang, H.; Shi, L.; Zhang, G. Bifunctional Cobalt-Doped ZnIn2S4 Hierarchical Nanotubes Endow Noble-Metal Cocatalyst-Free Photocatalytic H2 Production Coupled with Benzyl Alcohol Oxidation. Sol. RRL 2022, 6, 2101042. [Google Scholar] [CrossRef]
- Liang, Z.; Shen, R.; Ng, Y.H.; Zhang, P.; Xiang, Q.; Li, X. A review on 2D MoS2 Cocatalysts in Photocatalytic H2 Production. J. Mater. Sci. Technol. 2020, 56, 89–121. [Google Scholar] [CrossRef]
- Chang, K.; Hai, X.; Ye, J. Transition Metal Disulfides as Noble-Metal-Alternative Co-catalysts for Solar Hydrogen Production. Adv. Energy Mater. 2016, 6, 1502555. [Google Scholar] [CrossRef]
- Li, M.; Sun, J.; Chen, G.; Yao, S.; Cong, B.; Liu, P. Construction Photothermal/Pyroelectric Property of Hollow FeS2/Bi2S3 Nanostructure with Enhanced Full Spectrum Photocatalytic Activity. Appl. Catal. B Environ. 2021, 298, 120573. [Google Scholar] [CrossRef]
- Goh, M.S.; Moon, H.; Park, H.; Kim, S.; Yoon, T.; Joo, S.W.; Son, N.; Kang, M. Sustainable and Stable Hydrogen Production over Petal-shaped CdS/FeS2 S-Scheme Heterojunction by Photocatalytic Water Splitting. Int. J. Hydrogen Energy 2022, 47, 27911–27929. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Ge, L.; Chen, Y.; Mao, X.; Guan, D.; Shao, Z. High-Performance Perovskite Composite Electrocatalysts Enabled by Controllable Interface Engineering. Small 2021, 17, 2101573. [Google Scholar] [CrossRef]
- Wang, R.N.; Zeng, F.L.; Chen, X.L.; Zhu, H.L.; Qu, L.B.; Huang, X.Q.; Tang, S.; Zhao, Y.F.; Yu, B. Recyclable ZnIn2S4 Microspheres for Photocatalytic Azolation of N-Heterocycles. ACS Sustain. Chem. Eng. 2022, 10, 14212–14219. [Google Scholar] [CrossRef]
- Tan, L.; Yue, J.; Yang, Z.; Niu, X.; Yang, Y.; Zhang, J.; Zhu, Y. A Polymorphic FeS2 Cathode Enabled by Copper Current Collector Induced Displacement Redox Mechanism. ACS Nano 2021, 15, 11694–11703. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Q.; Lin, Z.; Yan, B.; Xia, C.; Yang, G. Half-Unit-Cell ZnIn2S4 Monolayer with Sulfur Vacancies for Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2019, 248, 193–201. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Ao, D. Construction of Heterostructured ZnIn2S4@ NH2-MIL-125 (Ti) Nanocomposites for Visible-Light- Driven H2 Production. Appl. Catal. B Environ. 2018, 221, 433–442. [Google Scholar] [CrossRef]
- Samad, L.; Cabán-Acevedo, M.; Shearer, M.J.; Park, K.; Hamers, R.J.; Jin, S. Direct Chemical Vapor Deposition Synthesis of Phase-Pure Iron Pyrite (FeS2) Thin Films. Chem. Mater. 2015, 27, 3108–3114. [Google Scholar] [CrossRef]
- Nesbitt, H.; Bancroft, G.; Pratt, A.; Scaini, M. Sulfur and Iron Surface States on Fractured Pyrite Surfaces. Am. Mineral. 1998, 83, 1067–1076. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, F.; Zhang, B.; Xu, C.; Yang, W.; Li, Y. Fabrication of Novel FeS2 NWs/Ti3C2 Cathode for Photo-Electro-Fenton Degradation of Sulfamethazine. Chem. Eng. J. 2021, 426, 130719. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Mei, C.; Xu, J.; Zhou, S.; Wong, C.P. Pyrite FeS2 Nanobelts as High-Performance Anode Material for Aqueous Pseudocapacitor. Electrochim. Acta 2016, 222, 172–176. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Geng, M.; Peng, Y.; Zhang, Y.; Guo, X.; Yu, F.; Yang, X.; Xie, G.; Dong, W.; Liu, C.; Li, J. Hierarchical ZnIn2S4: A Promising Cocatalyst to Boost Visible-Light-Driven Photocatalytic Hydrogen Evolution of In(OH)3. Int. J. Hydrogen Energy 2019, 44, 5787–5798. [Google Scholar] [CrossRef]
- Yu, B.; Chen, X.; Huang, C.; Yao, W. 2D CdS Functionalized by NiS2-Doped Carbon Nanosheets for Photocatalytic H2 Evolution. Appl. Surf. Sci. 2022, 592, 153259. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Wang, X.; Lou, X.W. Formation of Hierarchical Co9S8@ ZnIn2S4 Heterostructured Cages as An Efficient Photocatalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2018, 140, 15145–15148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guan, D.; Gu, Y.; Xu, H.; Wang, C.; Shao, Z.; Guo, Y. Tuning Synergy between Nickel and Iron in Ruddlesden-Popper Perovskites through Controllable Crystal Dimensionalities towards Enhanced Oxygen-Evolving Activity and Stability. Carbon Energy 2024, 6, e465. [Google Scholar] [CrossRef]
- Zeng, R.; Cheng, C.; Xing, F.; Zou, Y.; Ding, K.; Huang, C. Dual Vacancies Induced Local Polarization Electric Field for High-Performance Photocatalytic H2 Production. Appl. Catal. B Environ. 2022, 316, 121680. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, J.; Lee, L.Y.S. Highly Promoted Hydrogen Production Enabled by Interfacial PN Chemical Bonds in Copper Phosphosulfide Z-Scheme Composite. Appl. Catal. B Environ. 2021, 283, 119624. [Google Scholar] [CrossRef]
- Pan, B.; Wu, Y.; Rhimi, B.; Qin, J.; Huang, Y.; Yuan, M.; Wang, C. Oxygen-Doping of ZnIn2S4 Nanosheets towards Boosted Photocatalytic CO2 Reduction. J. Energy Chem. 2021, 57, 1–9. [Google Scholar] [CrossRef]
- Qiao, F.; Liu, W.; Yang, J.; Liu, Y.; Yuan, J. Fabrication of ZnO/CuInS2 Heterojunction for Boosting Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2024, 53, 840–847. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, H.; Chao, Y.; Zhang, Q.; Zhang, W.; Lv, F.; Guo, S. Single-Atom Pt-I3 Sites on All-Inorganic Cs2SnI6 Perovskite for Efficient Photocatalytic Hydrogen Production. Nat. Commun. 2021, 12, 4412. [Google Scholar] [CrossRef]
- Li, J.; Yao, J.; Yu, Q.; Zhang, X.; Carabineiro, S.A.; Xiong, X.; Lv, K. Understanding the Unique Ohmic-Junction for Enhancing the Photocatalytic Activity of CoS2/MgIn2S4 towards Hydrogen Production. Appl. Catal. B 2024, 351, 123950. [Google Scholar] [CrossRef]
- Yao, B.; Wang, S.; Yao, H.; Pang, X.; Li, Y.; Sun, J. Photothermal and Pyroelectric Effects in Hollow FeS2/CdS Nanocomposites for Enhanced Photocatalytic Hydrogen Evolution. Surf. Interfaces 2024, 49, 104370. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, L.; Liu, Z.; Tang, Y.; Cheng, L.; Chen, Z.; Duo, S. Boosting the Photocatalytic Activity for H2 Production of Bi2O2Se/CdS Heterojunction. Mater. Lett. 2023, 345, 134498. [Google Scholar] [CrossRef]
- Nagakawa, H.; Ochiai, T.; Takekuma, Y.; Konuma, S.; Nagata, M. Effective Photocatalytic Hydrogen Evolution by Cascadal Carrier Transfer in the Reverse Direction. ACS Omega 2018, 3, 12770–12777. [Google Scholar] [CrossRef]
- Lu, X.; Quan, L.; Hou, H.; Qian, J.; Liu, Z.; Zhang, Q. Fabrication of 1D/2D Y-doped CeO2/ZnIn2S4 S-Scheme Photocatalyst for Enhanced Photocatalytic H2 Evolution. J. Alloys Compd. 2022, 925, 166552. [Google Scholar] [CrossRef]
- Li, F.; Liao, B.; Shen, J.; Ke, J.; Zhang, R.; Wang, Y.; Niu, Y. Enhancing Photocatalytic Activities for Sustainable Hydrogen Evolution on Structurally Matched CuInS2/ZnIn2S4 Heterojunctions. Molecules 2024, 29, 2447. [Google Scholar] [CrossRef]
- Wei, N.; Wu, Y.; Wang, M.; Sun, W.; Li, Z.; Ding, L.; Cui, H. Construction of Noble-Metal-Free TiO2 Nanobelt/ZnIn2S4 Nanosheet Heterojunction Nanocomposite for Highly Efficient Photocatalytic Hydrogen Evolution. Nanotechnology 2018, 30, 045701. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, H.; Du, R.; Zhou, S.; Wang, Q.; Ren, J.; Wang, D.; Li, H. Efficient Charge Carriers Separation and Transfer Driven by Interface Electric Field in FeS2@ZnIn2S4 Heterojunction Boost Hydrogen Evolution. Molecules 2024, 29, 4269. https://doi.org/10.3390/molecules29174269
Qiao H, Du R, Zhou S, Wang Q, Ren J, Wang D, Li H. Efficient Charge Carriers Separation and Transfer Driven by Interface Electric Field in FeS2@ZnIn2S4 Heterojunction Boost Hydrogen Evolution. Molecules. 2024; 29(17):4269. https://doi.org/10.3390/molecules29174269
Chicago/Turabian StyleQiao, Haijun, Rui Du, Sifan Zhou, Qi Wang, Jingyu Ren, Danjun Wang, and Huifeng Li. 2024. "Efficient Charge Carriers Separation and Transfer Driven by Interface Electric Field in FeS2@ZnIn2S4 Heterojunction Boost Hydrogen Evolution" Molecules 29, no. 17: 4269. https://doi.org/10.3390/molecules29174269
APA StyleQiao, H., Du, R., Zhou, S., Wang, Q., Ren, J., Wang, D., & Li, H. (2024). Efficient Charge Carriers Separation and Transfer Driven by Interface Electric Field in FeS2@ZnIn2S4 Heterojunction Boost Hydrogen Evolution. Molecules, 29(17), 4269. https://doi.org/10.3390/molecules29174269





