Proposing an Affordable Plasma Device for Polymer Surface Modification and Microbial Inactivation
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Affordable Plasma Device
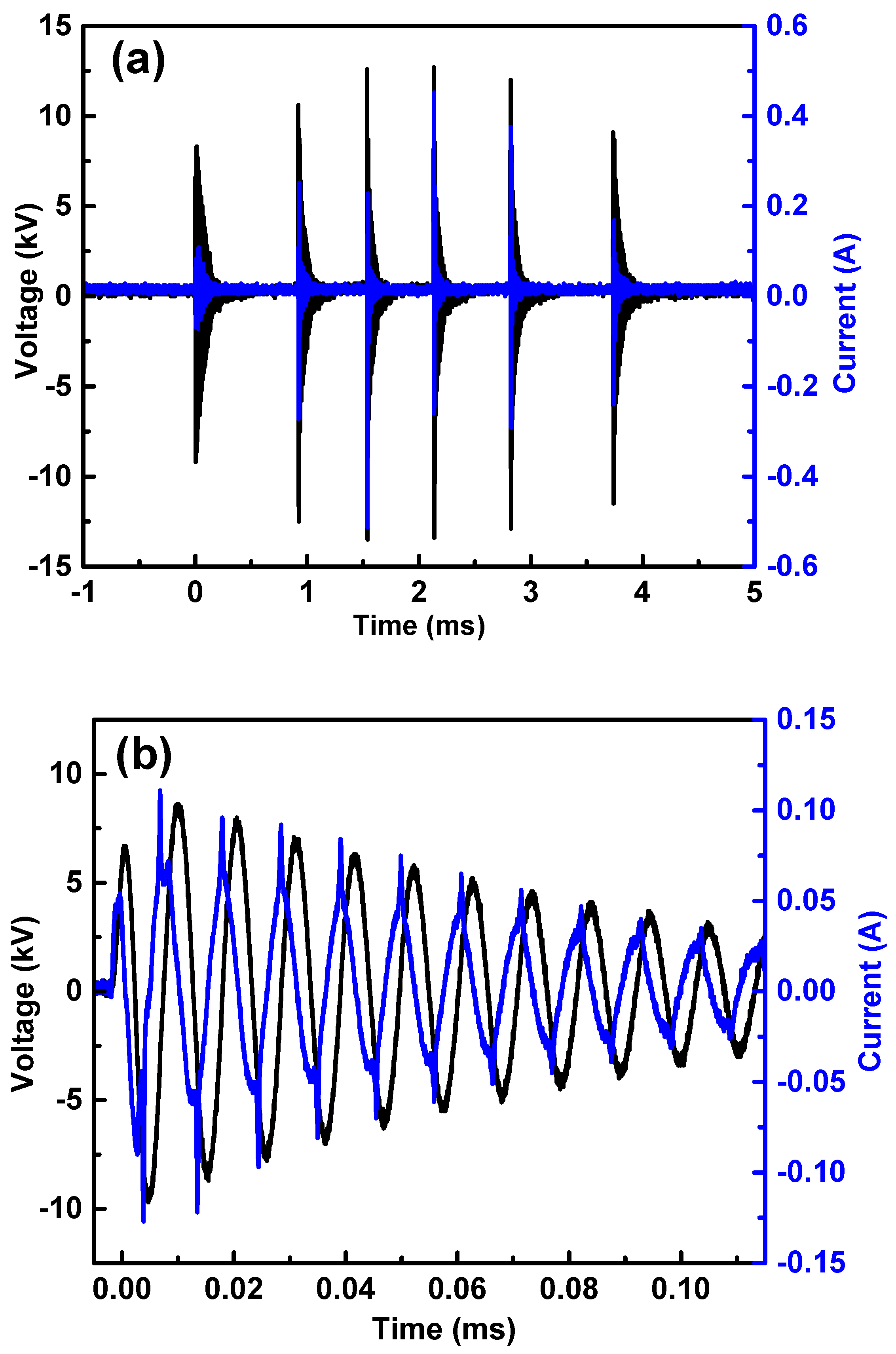
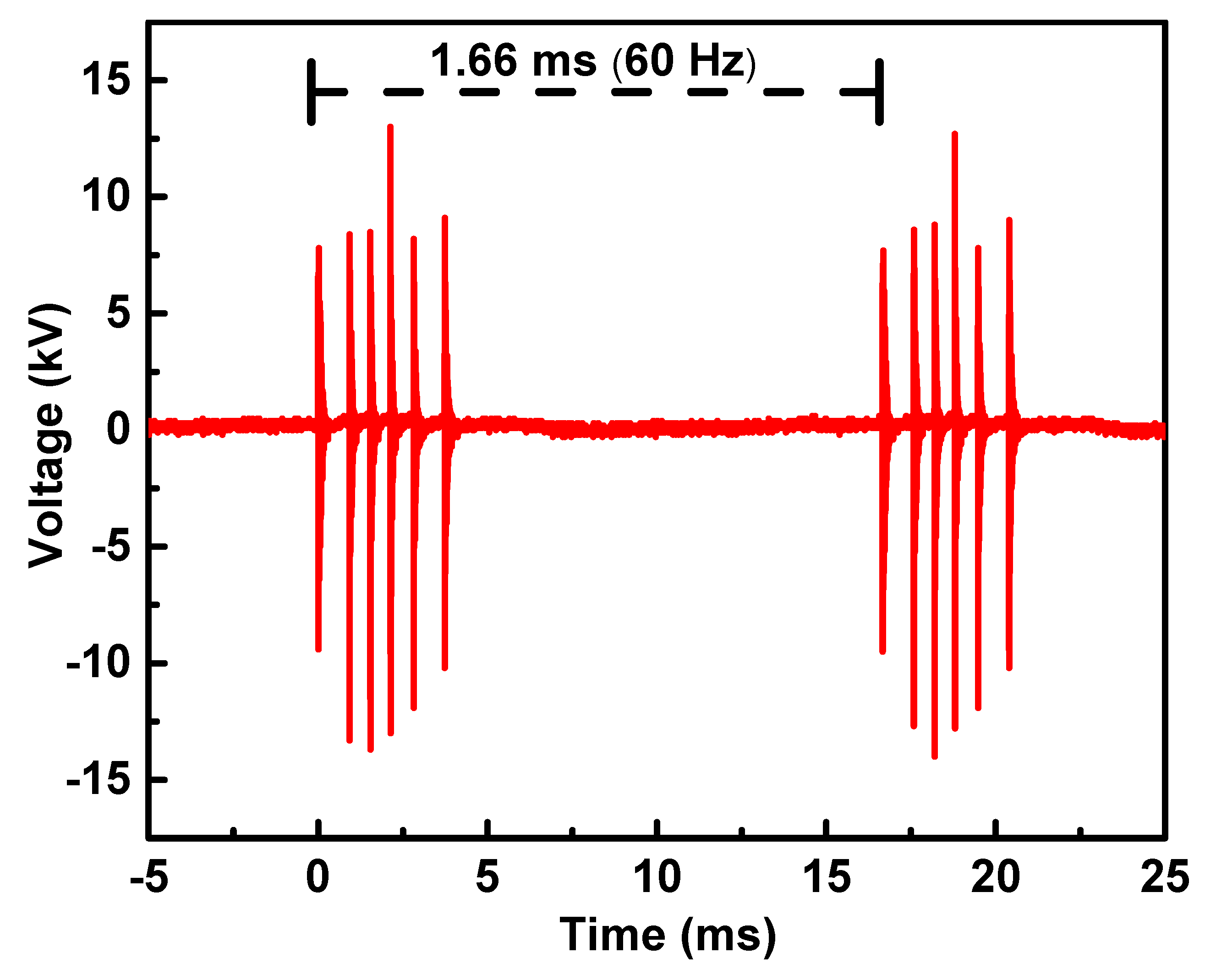
2.1.1. Electrical Characterization
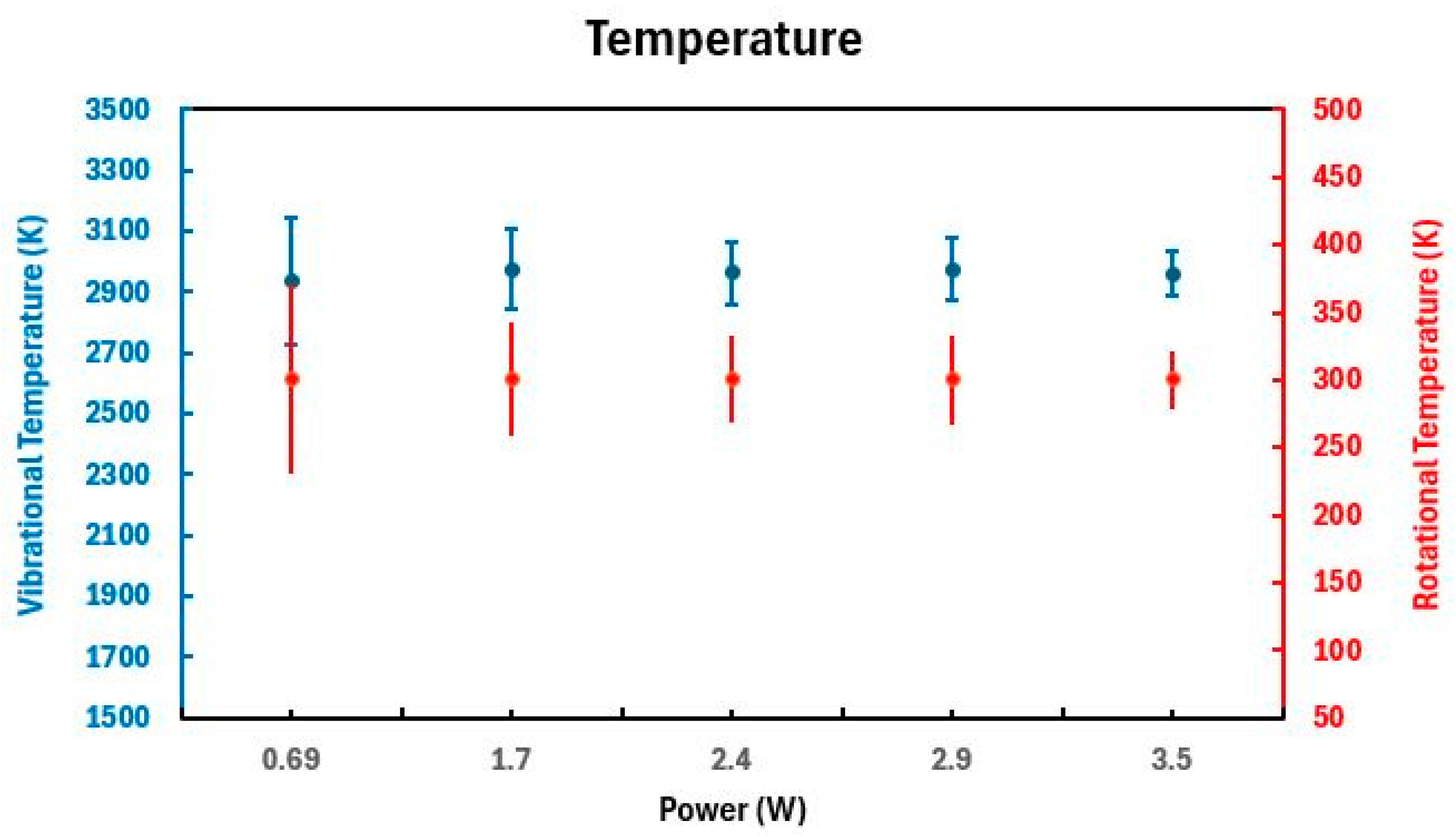
2.1.2. Spectroscopic Emissions and Thermal Parameters
2.2. Polyethylene Surface Modification
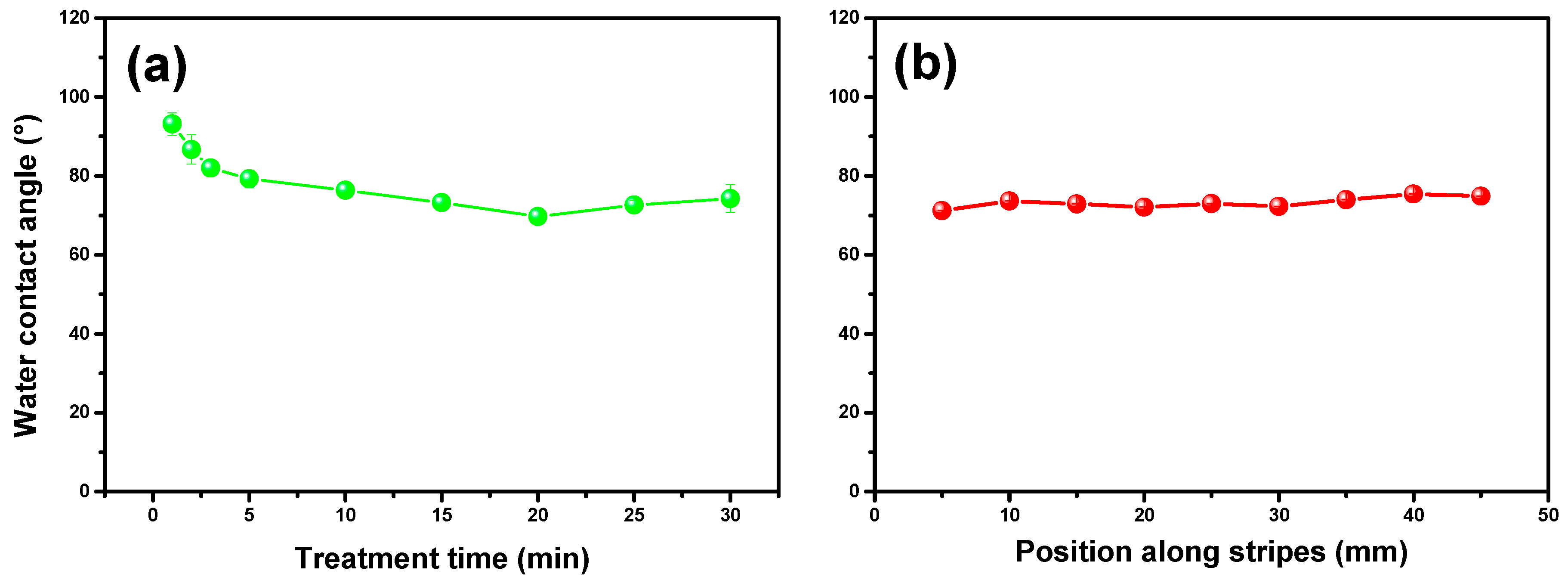
2.2.1. Characterization via Water Contact Angle and Fourier-Transform Infrared Spectroscopy
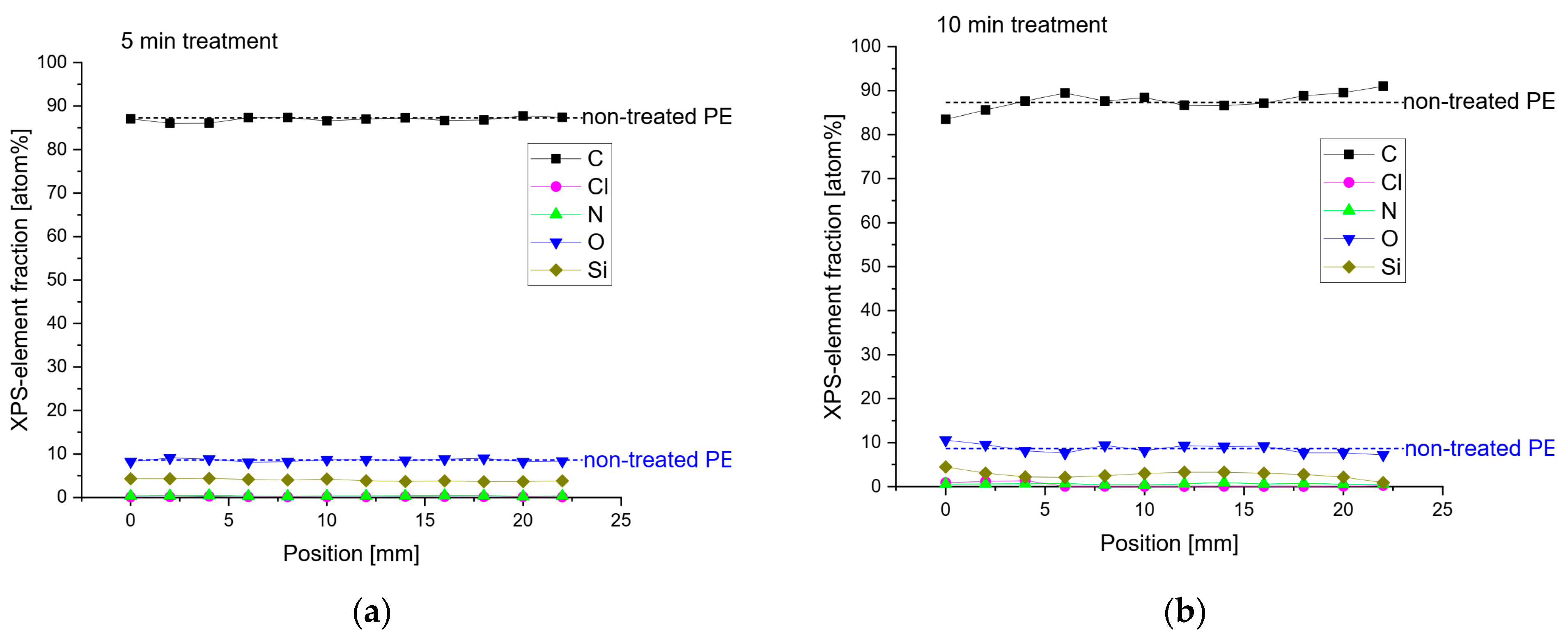
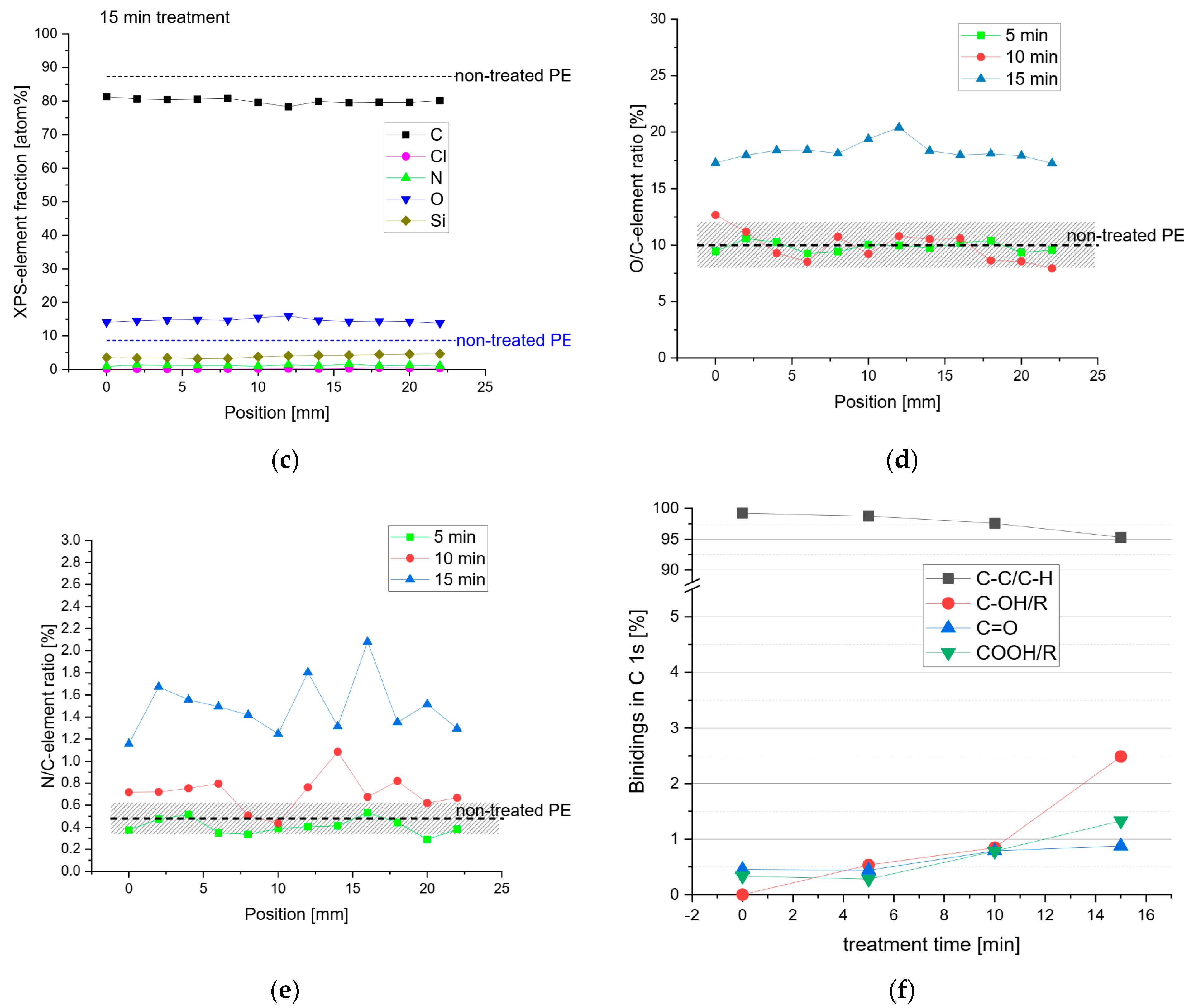
2.2.2. X-ray Photoelectron Spectroscopy
2.3. Cytotoxicity and Antimicrobial Activity
3. Materials and Methods
3.1. Affordable Plasma Device
3.2. Polymer Surface Modification
3.2.1. Polyethylene Sample Preparation
3.2.2. Characterization of Polyethylene Samples
3.3. Microbial Inactivation
3.3.1. Cytotoxicity Assay
3.3.2. Antimicrobial Activity Assay
- (i)
- Negative controls: Plates with microbial cultures but no plasma treatment were included to assess the natural growth of C. albicans and S. aureus.
- (ii)
- Positive controls: Plates treated with a known antimicrobial agent were included to assess the efficacy of the plasma treatment.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishime, T.M.C.; Wagner, R.; Kostov, K.G. Study of Modified Area of Polymer Samples Exposed to a He Atmospheric Pressure Plasma Jet Using Different Treatment Conditions. Polymers 2020, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Kodaira, F.V.d.P.; Leal, B.H.S.; Tavares, T.F.; Quade, A.; Hein, L.R.d.O.; Chiappim, W.; Kostov, K.G. Simultaneous Treatment of Both Sides of the Polymer with a Conical-Shaped Atmospheric Pressure Plasma Jet. Polymers 2023, 15, 461. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Vajpayee, M.; Ledwani, L. Eco-Friendly Surface Modification of Natural Fibres to Improve Dye Uptake Using Natural Dyes and Application of Natural Dyes in Fabric Finishing: A Review. Mater. Today Proc. 2021, 43, 2868–2871. [Google Scholar] [CrossRef]
- Dufay, M.; Jimenez, M.; Degoutin, S. Effect of Cold Plasma Treatment on Electrospun Nanofibers Properties: A Review. ACS Appl. Bio Mater. 2020, 3, 4696–4716. [Google Scholar] [CrossRef]
- Nascimento, L.; Gasi, F.; Landers, R.; da Silva Sobrinho, A.; Aragão, E.; Fraga, M.; Petraconi, G.; Chiappim, W.; Pessoa, R. Physicochemical Studies on the Surface of Polyamide 6.6 Fabrics Functionalized by DBD Plasmas Operated at Atmospheric and Sub-Atmospheric Pressures. Polymers 2020, 12, 2128. [Google Scholar] [CrossRef] [PubMed]
- Tropea, A. Microbial Contamination and Public Health: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7441. [Google Scholar] [CrossRef]
- Coque, T.M.; Cantón, R.; Pérez-Cobas, A.E.; Fernández-de-Bobadilla, M.D.; Baquero, F. Antimicrobial Resistance in the Global Health Network: Known Unknowns and Challenges for Efficient Responses in the 21st Century. Microorganisms 2023, 11, 1050. [Google Scholar] [CrossRef]
- da Silva, D.M.; Nascimento, F.D.; Milhan, N.V.M.; de Oliveira, M.A.C.; Cardoso, P.F.G.; Legendre, D.; Aoki, F.G.; Kostov, K.G.; Koga-Ito, C.Y. Cold Atmospheric Helium Plasma in the Post-COVID-19 Era: A Promising Tool for the Disinfection of Silicone Endotracheal Prostheses. Microorganisms 2024, 12, 130. [Google Scholar] [CrossRef]
- Samia, N.I.; Robicsek, A.; Heesterbeek, H.; Peterson, L.R. Methicillin-Resistant Staphylococcus aureus Nosocomial Infection Has a Distinct Epidemiological Position and Acts as a Marker for Overall Hospital-Acquired Infection Trends. Sci. Rep. 2022, 12, 17007. [Google Scholar] [CrossRef]
- Pereira, V.L.; Caramês, E.T.d.S.; Almeida, N.A.; Chiappim, W.; Pessoa, R.S.; Petraconi Filho, G.; Rocha, L.d.O. Gliding Arc Plasma Jet for Inhibiting Mycotoxin Production and Apple Brown Rot by Alternaria Alternata. Food Control 2024, 155, 110108. [Google Scholar] [CrossRef]
- Chiappim, W.; de Paula Bernardes, V.; Almeida, N.A.; Pereira, V.L.; Bragotto, A.P.A.; Cerqueira, M.B.R.; Furlong, E.B.; Pessoa, R.; Rocha, L.O. Effect of Gliding Arc Plasma Jet on the Mycobiota and Deoxynivalenol Levels in Naturally Contaminated Barley Grains. Int. J. Environ. Res. Public Health 2023, 20, 5072. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.C.; de Araújo, J.P.A.; Fusieger, A.; de Carvalho, A.F.; Nero, L.A. Microbiological Quality and Safety of Brazilian Artisanal Cheeses. Food Microbiol.-Rev. 2021, 52, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Singha, S.; Thomas, R.; Viswakarma, J.N.; Gupta, V.K. Foodborne Illnesses of Escherichia Coli O157origin and Its Control Measures. J. Food Sci. Technol. 2023, 60, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, Food Safety and Food Handling Practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef]
- Findlater, A.; Bogoch, I.I. Human Mobility and the Global Spread of Infectious Diseases: A Focus on Air Travel. Trends Parasitol. 2018, 34, 772–783. [Google Scholar] [CrossRef]
- Schinas, G.; Polyzou, E.; Spernovasilis, N.; Gogos, C.; Dimopoulos, G.; Akinosoglou, K. Preventing Multidrug-Resistant Bacterial Transmission in the Intensive Care Unit with a Comprehensive Approach: A Policymaking Manual. Antibiotics 2023, 12, 1255. [Google Scholar] [CrossRef]
- Pakdel, M.; Olsen, A.; Bar, E.M.S. A Review of Food Contaminants and Their Pathways Within Food Processing Facilities Using Open Food Processing Equipment. J. Food Prot. 2023, 86, 100184. [Google Scholar] [CrossRef]
- Wang, L.P.; Nie, L.L.; Liu, D.W.; Laroussi, M.; Lu, X.P. The Temporal Behavior of O Atom of a Nonequilibrium Atmospheric Pressure Plasma Driven by KHz Nanosecond Voltage Pulses. Plasma Process. Polym. 2023, 20, e2300038. [Google Scholar] [CrossRef]
- Trebulová, K.; Krčma, F.; Skoumalová, P.; Kozáková, Z.; Machala, Z. Effects of Different Cold Atmospheric-Pressure Plasma Sources on the Yeast Candida Glabrata. Plasma Process. Polym. 2023, 20, e202300048. [Google Scholar] [CrossRef]
- Seri, P.; Nici, S.; Cappelletti, M.; Scaltriti, S.G.; Popoli, A.; Cristofolini, A.; Neretti, G. Validation of an Indirect Nonthermal Plasma Sterilization Process for Disposable Medical Devices Packed in Blisters and Cartons. Plasma Process. Polym. 2023, 20, e202300012. [Google Scholar] [CrossRef]
- Lan, C.; Zhu, H.; Wang, S.; Nie, L.; Liu, D.; Shi, Q.; Lu, X. Disinfection of Viruses with Cold Atmospheric-Pressure Plasmas: Sources, Mechanisms, and Efficacy. Plasma Process. Polym. 2023, 21, e2300183. [Google Scholar] [CrossRef]
- Bucci, C.; Tampieri, F.; Mateu-Sanz, M.; Laurita, R.; Colombo, V.; Canal, C. Oxidation of Lactate to Pyruvate Mediates the Cytotoxic Potential of Physical Plasma-Treated Saline Solutions in Ovarian Cancer. Plasma Process. Polym. 2023, 20, e202300093. [Google Scholar] [CrossRef]
- Silva, N.; Marques, J.; da Cruz, M.B.; Luís, H.; Sério, S.; Mata, A. The Applications of Cold Atmospheric Plasma in Dentistry. Plasma Process. Polym. 2023, 20, e202300067. [Google Scholar] [CrossRef]
- Murillo, D.; Huergo, C.; Gallego, B.; Rodríguez, R.; Tornín, J. Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer. Biomedicines 2023, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8, 00074. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.; Chiappim, W.; Karnopp, J.; Neto, B.; Leite, D.; da Silva Sobrinho, A.; Pessoa, R. Novel Energetic Co-Reactant for Thermal Oxide Atomic Layer Deposition: The Impact of Plasma-Activated Water on Al2O3 Film Growth. Nanomaterials 2023, 13, 3110. [Google Scholar] [CrossRef]
- Oh, J.S.; Szili, E.J.; Gaur, N.; Hong, S.H.; Furuta, H.; Kurita, H.; Mizuno, A.; Hatta, A.; Short, R.D. How to Assess the Plasma Delivery of RONS into Tissue Fluid and Tissue. J. Phys. D Appl. Phys. 2016, 49, 304005. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, C.; Liu, D.; He, T.; Guo, L.; Xu, D.; Kong, M.G. Quantifying the Concentration and Penetration Depth of Long-Lived RONS in Plasma-Activated Water by UV Absorption Spectroscopy. AIP Adv. 2019, 9, 015014. [Google Scholar] [CrossRef]
- Szili, E.J.; Oh, J.S.; Hong, S.H.; Hatta, A.; Short, R.D. Probing the Transport of Plasma-Generated RONS in an Agarose Target as Surrogate for Real Tissue: Dependency on Time, Distance and Material Composition. J. Phys. D Appl. Phys. 2015, 48, 202001. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Y.; Liu, D.; Pang, B.; Wang, S.; Zhou, C.; Zhang, H.; Xu, D.; Kong, M.G. Dynamic Analysis of Absorbance Behavior and Peak Shift of RONS in Plasma-Activated Water by UV Absorption Spectroscopy: Dependency on Gas Impurity, Pulse Polarity, and Solution PH. J. Phys. D Appl. Phys. 2021, 54, abb848. [Google Scholar] [CrossRef]
- Milhan, N.V.M.; Chiappim, W.; Sampaio, A.d.G.; da Cruz Vegian, M.R.; Pessoa, R.S.; Koga-ito, C.Y. Applications of Plasma-Activated Water in Dentistry: A Review. Int. J. Mol. Sci. 2022, 23, 4131. [Google Scholar] [CrossRef]
- Chiappim, W.; Sampaio, A.; Miranda, F.; Petraconi, G.; da Silva Sobrinho, A.; Cardoso, P.; Kostov, K.; Koga-Ito, C.; Pessoa, R. Nebulized Plasma-Activated Water Has an Effective Antimicrobial Effect on Medically Relevant Microbial Species and Maintains Its Physicochemical Properties in Tube Lengths from 0.1 up to 1.0 m. Plasma Process. Polym. 2021, 18, e202100010. [Google Scholar] [CrossRef]
- Sampaio, A.d.G.; Chiappim, W.; Milhan, N.V.M.; Botan Neto, B.; Pessoa, R.; Koga-Ito, C.Y. Effect of the PH on the Antibacterial Potential and Cytotoxicity of Different Plasma-Activated Liquids. Int. J. Mol. Sci. 2022, 23, 13893. [Google Scholar] [CrossRef] [PubMed]
- Keidar, M.; Shashurin, A.; Volotskova, O.; Ann Stepp, M.; Srinivasan, P.; Sandler, A.; Trink, B. Cold Atmospheric Plasma in Cancer Therapy. Phys. Plasmas 2013, 20, 057101. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold Plasma Selectivity and the Possibility of a Paradigm Shift in Cancer Therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, L.; Cheng, X.; Gjika, E.; Keidar, M. Effects of Cold Atmospheric Plasma Generated in Deionized Water in Cell Cancer Therapy. Plasma Process. Polym. 2016, 13, 1151–1156. [Google Scholar] [CrossRef]
- Adil, B.H.; Al-Shammari, A.M.; Murbat, H.H. Breast Cancer Treatment Using Cold Atmospheric Plasma Generated by the FE-DBD Scheme. Clin. Plasma Med. 2020, 19–20, 100103. [Google Scholar] [CrossRef]
- Subramanian, P.S.G.; Jain, A.; Shivapuji, A.M.; Sundaresan, N.R.; Dasappa, S.; Rao, L. Plasma-Activated Water from a Dielectric Barrier Discharge Plasma Source for the Selective Treatment of Cancer Cells. Plasma Process. Polym. 2020, 17, e201900260. [Google Scholar] [CrossRef]
- Xu, D.; Wang, S.; Li, B.; Qi, M.; Feng, R.; Li, Q.; Zhang, H.; Chen, H.; Kong, M.G. Effects of Plasma-Activated Water on Skin Wound Healing in Mice. Microorganisms 2020, 8, 1091. [Google Scholar] [CrossRef]
- Bekeschus, S.; Kramer, A.; Schmidt, A. Gas Plasma-Augmented Wound Healing in Animal Models and Veterinary Medicine. Molecules 2021, 26, 5682. [Google Scholar] [CrossRef] [PubMed]
- Laurita, R.; Barbieri, D.; Gherardi, M.; Colombo, V.; Lukes, P. Chemical Analysis of Reactive Species and Antimicrobial Activity of Water Treated by Nanosecond Pulsed DBD Air Plasma. Clin. Plasma Med. 2015, 3, 53–61. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T.G. The Reactive Oxygen Species Singlet Oxygen, Hydroxy Radicals, and the Superoxide Radical Anion—Examples of Their Roles in Biology and Medicine. Oxygen 2021, 1, 77–95. [Google Scholar] [CrossRef]
- Kondeti, V.S.S.K.; Phan, C.Q.; Wende, K.; Jablonowski, H.; Gangal, U.; Granick, J.L.; Hunter, R.C.; Bruggeman, P.J. Long-Lived and Short-Lived Reactive Species Produced by a Cold Atmospheric Pressure Plasma Jet for the Inactivation of Pseudomonas Aeruginosa and Staphylococcus aureus. Free Radic. Biol. Med. 2018, 124, 275–287. [Google Scholar] [CrossRef]
- Nie, L.-H.; Shi, C.; Xu, Y.; Wu, Q.-H.; Zhu, A.-M. Atmospheric Cold Plasmas for Synthesizing Nanocrystalline Anatase TiO2 Using Dielectric Barrier Discharges. Plasma Process. Polym. 2007, 4, 574–582. [Google Scholar] [CrossRef]
- Károly, Z.; Kalácska, G.; Zsidai, L.; Mohai, M.; Klébert, S. Improvement of Adhesion Properties of Polyamide 6 and Polyoxymethylene-Copolymer by Atmospheric Cold Plasma Treatment. Polymers 2018, 10, 1380. [Google Scholar] [CrossRef]
- Gavahian, M.; Cullen, P.J. Cold Plasma as an Emerging Technique for Mycotoxin-Free Food: Efficacy, Mechanisms, and Trends. Food Rev. Int. 2020, 36, 193–214. [Google Scholar] [CrossRef]
- Misra, N.N.; Yadav, B.; Roopesh, M.S.; Jo, C. Cold Plasma for Effective Fungal and Mycotoxin Control in Foods: Mechanisms, Inactivation Effects, and Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 106–120. [Google Scholar] [CrossRef]
- Sarangapani, C.; Patange, A.; Bourke, P.; Keener, K.; Cullen, P.J. Recent Advances in the Application of Cold Plasma Technology in Foods. Annu. Rev. Food Sci. Technol. 2018, 9, 609–629. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Patange, A.; Sun, D.W.; Tiwari, B. Plasma-Activated Water: Physicochemical Properties, Microbial Inactivation Mechanisms, Factors Influencing Antimicrobial Effectiveness, and Applications in the Food Industry. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3951–3979. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Xie, H.; Jiang, J.; Li, C.; Li, W.; Li, L.; Liu, X.; Lin, F. Inactivation Effects of Plasma-Activated Water on Fusarium Graminearum. Food Control 2022, 134, 108683. [Google Scholar] [CrossRef]
- Cullen, P.J.; Lalor, J.; Scally, L.; Boehm, D.; Milosavljević, V.; Bourke, P.; Keener, K. Translation of Plasma Technology from the Lab to the Food Industry. Plasma Process. Polym. 2018, 15, e201700085. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y.; Xu, C.; Bai, Y. Effect of Plasma-Activated Water on Microbial Quality and Physicochemical Characteristics of Mung Bean Sprouts. Innov. Food Sci. Emerg. Technol. 2019, 52, 49–56. [Google Scholar] [CrossRef]
- López, M.; Calvo, T.; Prieto, M.; Múgica-Vidal, R.; Muro-Fraguas, I.; Alba-Elías, F.; Alvarez-Ordóñez, A. A Review on Non-Thermal Atmospheric Plasma for Food Preservation: Mode of Action, Determinants of Effectiveness, and Applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef]
- Subramanian, P.S.; Rao, H.; Shivapuji, A.M.; Girard-Lauriault, P.L.; Rao, L. Plasma-Activated Water from DBD as a Source of Nitrogen for Agriculture: Specific Energy and Stability Studies. J. Appl. Phys. 2021, 129, 093303. [Google Scholar] [CrossRef]
- Miranda, F.S.; Tavares, V.K.F.; Gomes, M.P.; Neto, N.F.A.; Chiappim, W.; Petraconi, G.; Pessoa, R.S.; Koga-Ito, C.Y. Physicochemical Characteristics and Antimicrobial Efficacy of Plasma-Activated Water Produced by an Air-Operated Coaxial Dielectric Barrier Discharge Plasma. Water 2023, 15, 4045. [Google Scholar] [CrossRef]
- Anuntagool, J.; Srangsomjit, N.; Thaweewong, P.; Alvarez, G. A Review on Dielectric Barrier Discharge Nonthermal Plasma Generation, Factors Affecting Reactive Species, and Microbial Inactivation. Food Control 2023, 153, 109913. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, J.-S.; Lee, J.-H.; Jeong, B.-H. Space Sterilization Effect Through High-Density Plasma Ozone Using DBD Device. J. Electr. Eng. Technol. 2022, 17, 2771–2778. [Google Scholar] [CrossRef]
- Kostov, K.G.; Rocha, V.; Koga-Ito, C.Y.; Matos, B.M.; Algatti, M.A.; Honda, R.Y.; Kayama, M.E.; Mota, R.P. Bacterial Sterilization by a Dielectric Barrier Discharge (DBD) in Air. Surf. Coat. Technol. 2010, 204, 2954–2959. [Google Scholar] [CrossRef]
- Hoppanová, L.; Kryštofová, S. Nonthermal Plasma Effects on Fungi: Applications, Fungal Responses, and Future Perspectives. Int. J. Mol. Sci. 2022, 2022, 11592. [Google Scholar] [CrossRef]
- Choi, M.S.; Jeon, E.B.; Kim, J.Y.; Choi, E.H.; Lim, J.S.; Choi, J.; Ha, K.S.; Kwon, J.Y.; Jeong, S.H.; Park, S.Y. Virucidal Effects of Dielectric Barrier Discharge Plasma on Human Norovirus Infectivity in Fresh Oysters (Crassostrea gigas). Foods 2020, 9, 1731. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, E.; Prehn, F.; Schmidt, M.; Höft, H.; Brandenburg, R.; Kettlitz, M. Indoor Air Purification by Dielectric Barrier Discharge Combined with Ionic Wind: Physical and Microbiological Investigations. J. Phys. D Appl. Phys. 2018, 51, 164003. [Google Scholar] [CrossRef]
- Das, S.P.; Dalei, G.; Barik, A. A Dielectric Barrier Discharge (DBD) Plasma Reactor: An Efficient Tool to Measure the Sustainability of Non-Thermal Plasmas through the Electrical Breakdown of Gases. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Odisha, India, 21–23 September 2018; Institute of Physics Publishing: Bristol, UK; Volume 410. [Google Scholar]
- Kim, J.Y.; Jeon, E.B.; Choi, M.S.; Choi, E.H.; Lim, J.S.; Choi, J.; Park, S.Y. The Efficiency of Atmospheric Dielectric Barrier Discharge Plasma against Escherichia Coli and Bacillus Cereus on Dried Laver (Porphyra tenera). Foods 2020, 9, 1013. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.G.; Nishime, T.M.C.; Hein, L.R.O.; Toth, A. Study of Polypropylene Surface Modification by Air Dielectric Barrier Discharge Operated at Two Different Frequencies. Surf. Coat. Technol. 2013, 234, 60–66. [Google Scholar] [CrossRef]
- Mui, T.S.M.; Mota, R.P.; Quade, A.; Hein, L.R.d.O.; Kostov, K.G. Uniform Surface Modification of Polyethylene Terephthalate (PET) by Atmospheric Pressure Plasma Jet with a Horn-like Nozzle. Surf. Coat. Technol. 2018, 352, 338–347. [Google Scholar] [CrossRef]
- Voráč, J.; Kusýn, L.; Synek, P. Deducing Rotational Quantum-State Distributions from Overlapping Molecular Spectra. Rev. Sci. Instrum. 2019, 90, 123102. [Google Scholar] [CrossRef]
- Laroussi, M.; Lu, X. Room-Temperature Atmospheric Pressure Plasma Plume for Biomedical Applications. Appl. Phys. Lett. 2005, 87, 113902. [Google Scholar] [CrossRef]
- Nascimento, F.; Petroski, K.; Kostov, K. Effects of O2 Addition on the Discharge Parameters and Production of Reactive Species of a Transferred Atmospheric Pressure Plasma Jet. Appl. Sci. 2021, 11, 6311. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS- and FTIR-Analysis of Plasma-Treated Polypropylene Film Surfaces. In Proceedings of the 12th European Conference on Applications of Surface and Interface Analysis, Brussels, Belgium, 9–14 September 2007; Surface and Interface Analysis. Wiley: Hoboken, NJ, USA, 2008; Volume 40, pp. 597–600. [Google Scholar]
- Mandolfino, C.; Lertora, E.; Gambaro, C. Influence of Cold Plasma Treatment Parameters on the Mechanical Properties of Polyamide Homogeneous Bonded Joints. Surf. Coat. Technol. 2017, 313, 222–229. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Polyethylene Characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- De Souza, I.A.; Do Nascimento Neto, A.B.; De Queiroz, J.C.A.; Matamoros, E.P.; De Carvalho Costa, T.H.; Feitor, M.C.; De Souza, J.M.L.; Camara, N.T.; Da Silva Severiano Sobrinho, V. Study of the Influence of Variation in Distances between Electrodes in Spectral DBD Plasma Excitation. Mater. Res. 2016, 19, 202–206. [Google Scholar] [CrossRef]
- Mahindrakar, J.N.; Patil, Y.S.; Salunkhe, P.H.; Ankushrao, S.S.; Kadam, V.N.; Ubale, V.P.; Ghanwat, A.A. Optically Transparent, Organosoluble Poly(Ether-Amide)s Bearing Triptycene Unit; Synthesis and Characterization. J. Macromol. Sci. Part A Pure Appl. Chem. 2018, 55, 658–667. [Google Scholar] [CrossRef]
- Desai, S.M.; Singh, R.P. Surface Modification of Polyethylene; Springer: Berlin/Heidelberg, Germany, 2004; pp. 231–294. [Google Scholar]
- Booth, J.P.; Mozetič, M.; Nikiforov, A.; Oehr, C. Foundations of Plasma Surface Functionalization of Polymers for Industrial and Biological Applications. Plasma Sources Sci. Technol. 2022, 31, 103001. [Google Scholar] [CrossRef]
- Gerber, I.C.; Mihai, C.T.; Gorgan, L.; Ciorpac, M.; Nita, A.; Pohoata, V.; Mihaila, I.; Topala, I. Viability and Cell Biology for HeLa and Vero Cells after Exposure to Low-Temperature Air Dielectric Barrier Discharge Plasma; Begell House Inc.: Danbury, CT, USA, 2017; Volume 7. [Google Scholar]
- Borges, A.C.; de Morais Gouvêa Lima, G.; Mayumi Castaldelli Nishime, T.; Vidal Lacerda Gontijo, A.; Kostov, K.G.; Koga-Ito, C.Y. Amplitude-Modulated Cold Atmospheric Pressure Plasma Jet for Treatment of Oral Candidiasis: In Vivo Study. PLoS ONE 2018, 13, e0199832. [Google Scholar] [CrossRef]
- Maisch, T.; Shimizu, T.; Isbary, G.; Heinlin, J.; Karrer, S.; Klämpfl, T.G.; Li, Y.F.; Morfill, G.; Zimmermann, J.L. Contact-Free Inactivation of Candida albicans Biofilms by Cold Atmospheric Air Plasma. Appl. Environ. Microbiol. 2012, 78, 4242–4247. [Google Scholar] [CrossRef]
- Khosravi, S.; Jafari, S.; Zamani, H.; Nilkar, M. Inactivation of Staphylococcus aureus and Escherichia Coli Biofilms by Air-Based Atmospheric-Pressure DBD Plasma. Appl. Biochem. Biotechnol. 2021, 193, 3641–3650. [Google Scholar] [CrossRef]
- Gupta, T.T.; Matson, J.S.; Ayan, H. Antimicrobial Effectiveness of Regular Dielectric-Barrier Discharge (DBD) and Jet DBD on the Viability of Pseudomonas Aeruginosa. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 68. [Google Scholar] [CrossRef]
- Andrés Juan, C.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E.; Reinbothe, S. Molecular Sciences The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci 2021, 22, 4642. [Google Scholar] [CrossRef]
- Poiata, A.; Motrescu, I.; Nastuta, A.; Creanga, D.E.; Popa, G. Microorganism Response to Atmospheric Pressure Helium Plasma DBD Treatment. J. Electrost. 2010, 68, 128–131. [Google Scholar] [CrossRef]
- Miranda, F.S.; Tavares, V.K.F.; Silva, D.M.; Milhan, N.V.M.; Neto, N.F.A.; Gomes, M.P.; Pessoa, R.S.; Koga-Ito, C.Y. Influence of Gas Type on Reactive Species Formation, Antimicrobial Activity, and Cytotoxicity of Plasma-Activated Water Produced in a Coaxial DBD Reactor. Plasma Chem. Plasma Process. 2024, 44, 1713–1733. [Google Scholar] [CrossRef]
- Demirors, M. The History of Polyethylene; ACS: Washington, DC, USA, 2011; pp. 115–145. [Google Scholar]
- Paxton, N.C.; Allenby, M.C.; Lewis, P.M.; Woodruff, M.A. Biomedical Applications of Polyethylene. Eur. Polym. J. 2019, 118, 412–428. [Google Scholar] [CrossRef]
- Patel, R.M. Polyethylene. In Multilayer Flexible Packaging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 17–34. [Google Scholar]
- Chiodi Borges, A.; Castaldelli Nishime, T.M.; Kostov, K.G.; de Morais Gouvêa Lima, G.; Vidal Lacerda Gontijo, A.; de Carvalho, J.N.M.M.; Yzumi Honda, R.; Koga-Ito, C.Y. Cold Atmospheric Pressure Plasma Jet Modulates Candida albicans Virulence Traits. Clin. Plasma Med. 2017, 7–8, 9–15. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.






| Percentage Reduction (%) | ||||
|---|---|---|---|---|
| 1 Min | 3 Min | 5 Min | 10 Min | |
| C. albicans | 99.992 ± 0.002 | 99.995 ± 0.003 | 99.997 ± 0.001 | 99.998 ± 0.001 |
| S. aureus | 99.980 ± 0.001 | 99.987 ± 0.003 | 99.985 ± 0.001 | 99.995 ± 0.001 |
| Microorganism | Wavelength (λ) | Optical Density (O.D.) |
|---|---|---|
| S. aureus | 490 | 0.029 |
| C. albicans | 530 | 0.138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappim, W.; Kodaira, F.V.d.P.; Castro, G.F.S.d.; Silva, D.M.d.; Tavares, T.F.; Almeida, A.C.d.P.L.; Leal, B.H.S.; Quade, A.; Koga-Ito, C.Y.; Kostov, K.G. Proposing an Affordable Plasma Device for Polymer Surface Modification and Microbial Inactivation. Molecules 2024, 29, 4270. https://doi.org/10.3390/molecules29174270
Chiappim W, Kodaira FVdP, Castro GFSd, Silva DMd, Tavares TF, Almeida ACdPL, Leal BHS, Quade A, Koga-Ito CY, Kostov KG. Proposing an Affordable Plasma Device for Polymer Surface Modification and Microbial Inactivation. Molecules. 2024; 29(17):4270. https://doi.org/10.3390/molecules29174270
Chicago/Turabian StyleChiappim, William, Felipe Vicente de Paula Kodaira, Gisele Fátima Soares de Castro, Diego Morais da Silva, Thayna Fernandes Tavares, Ana Carla de Paula Leite Almeida, Bruno Henrique Silva Leal, Antje Quade, Cristiane Yumi Koga-Ito, and Konstantin Georgiev Kostov. 2024. "Proposing an Affordable Plasma Device for Polymer Surface Modification and Microbial Inactivation" Molecules 29, no. 17: 4270. https://doi.org/10.3390/molecules29174270
APA StyleChiappim, W., Kodaira, F. V. d. P., Castro, G. F. S. d., Silva, D. M. d., Tavares, T. F., Almeida, A. C. d. P. L., Leal, B. H. S., Quade, A., Koga-Ito, C. Y., & Kostov, K. G. (2024). Proposing an Affordable Plasma Device for Polymer Surface Modification and Microbial Inactivation. Molecules, 29(17), 4270. https://doi.org/10.3390/molecules29174270





