Abstract
Membrane filtration is an effective water recycling and purification technology to remove various pollutants in water. Inorganic membrane filtration (IMF) technology has received widespread attention because of its unique high temperature and corrosion resistance. Commonly used inorganic membranes include ceramic membranes and carbon-based membranes. As novel catalytic inorganic membrane processes, IMF coupled with advanced oxidation processes (AOPs), can realize the separation and in situ degradation of pollutants, thus mitigating membrane contamination. In this paper, the types and performance of IMF are discussed. The influencing factors of inorganic membranes in practical wastewater treatment are summarized. The applications, advantages, and disadvantages of the coupled process of IMF and AOPs are summarized and outlined. Finally, the challenges and prospects of IMF and IMF coupled with AOPs are presented, respectively. This contributes to the design and development of coupled systems of membrane filtration with inorganic materials and IMF coupled with AOPs for practical wastewater treatment.
1. Introduction
With the rapid growth and development of the global economy, large quantities of wastewater with organic pollutants are discharged into natural water through industrial and residential drainage systems every day (for example, 36,000 t/year in China) [1]. Most organic compounds have been covered by monitoring systems, but emerging pollutants were only discovered over a decade ago. This class includes personal care products, pharmaceuticals, pesticides, etc. Due to the increase in sewage discharge and the emergence of emerging pollutants, they pose serious threats to the environment and human health. [2]. To protect water resources, measures need to be taken to remove pollutants from wastewater before they are discharged into the ecosystem. Processes commonly used at this stage include adsorption, coagulation, membrane filtration, advanced oxidation processes (AOPs), and combined processes [3,4,5]. Among them, membrane filtration is considered to be an effective water recovery and purification technology that can remove various pollutants from water [6,7].
Membranes for membrane filtration can be divided into inorganic membranes, organic membranes, and organic–inorganic hybrid composite membranes based on the constituent materials [8]. Organic membranes are usually made of polymeric materials (such as cellulose acetate, polyethersulfone, polyfluoropolymers, etc.) [9,10]. Organic membranes are less expensive. During membrane filtration, the membrane is prone to blockage and contamination, requiring regular cleaning [11,12]. However, the mechanical strength and long-term stability of organic membranes are poor, which shortens their lifespan and requires regular replacement, increasing operating costs [2,13]. Bacteria present in the water can degrade organic membranes, whereas inorganic membranes are less susceptible to bacteria [14]. Compared with organic membranes, inorganic membranes have the characteristics of lower oxidation resistance, higher mechanical strength, higher hydrophilicity, more antifouling properties, more acid and alkali resistance, and higher temperature resistance [14,15,16,17]. Inorganic membranes can be applied in extreme environments, so they have received increasing attention. Membrane fouling and subsequent treatment of membrane filtration concentrate are the core issues in the membrane filtration process.
AOPs mainly include photocatalytic oxidation, Fenton oxidation, ozonation, persulfate oxidation, etc. [18,19,20]. In contrast to the physical process of membrane filtration, AOPs can utilize reactive substances generated by chemical oxidation to degrade and mineralize organic compounds [21,22]. With the help of catalysts, the technology can generate highly reactive free radicals faster and remove pollutants more quickly and completely [23]. However, in the process, nano-catalysts are often more effective, but they are difficult to fully recover [24]. Post-treatment is required after the reaction to recover the catalyst [25]. The coupling of membrane separation and AOP exhibits the synergistic effect of chemical reaction and physical separation. Membrane filtration coupled with AOPs has been widely studied in recent years.
Membrane filtration coupled with AOPs is the preparation of catalytic membranes by fixing catalyst particles onto the membrane surface or doping them onto the membrane skeleton. Catalytic membranes can avoid the agglomeration of catalysts and obtain the ability to oxidize and degrade pollutants while separating them from water [26]. The catalytic membrane not only provides effective recovery of powdered catalyst, but also enhances the mass transfer in the catalytic oxidation process [27], improving the efficiency of organic matter removal. On the other hand, the process achieves simultaneous catalytic and membrane separation, which can improve the anti-fouling performance of the membrane. Therefore, inorganic membrane filtration (IMF) coupled with AOPs is a water treatment process that can be operated continuously [28]. In practical applications, various coupling processes still face various problems, and this combination system needs further improvement.
So far, there have been numerous reviews on the preparation methods and performance of separation membranes, and the mechanism and application of AOPs, respectively. However, there have been few comprehensive analyses of membrane filtration of inorganic materials and the coupling process of IMF and AOPs. In this paper, the types and properties of inorganic membranes and the influencing factors of IMF in practical wastewater treatment are first summarized. Then, the application, advantages, and disadvantages of new catalytic IMF processes (including IMF-photocatalytic oxidation, IMF-ozonation, IMF-persulfate oxidation, and IMF-Fenton oxidation) are generalized. Finally, the challenges and future development prospects faced by the IMF and the coupled processes of IMF and AOPs are presented. The growth of membrane technology relies on developing new materials and membrane modification processes. The main research direction in the future is to develop high-throughput, high-strength, long-life, pollution resistant, and low-cost membranes.
2. Inorganic Membrane Filtration
Membrane filtration is a low technology process that utilizes the selective permeability of a membrane and applies pressure to the membrane to allow specific components to pass through and separate [29]. Based on the pore size of the membrane, pressure-driven membrane technologies can be categorized into four types: microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO). The main types of inorganic membranes include dynamic membranes, liquid membranes, silica, zeolite, carbon-based membranes (CBMs), and ceramic membranes (CMs) [30]. The two most widely used inorganic membranes for wastewater treatment, CMs and CBMs [31], have been used in a large number of applications in industrial wastewater.
2.1. Inorganic Membrane Types
2.1.1. Ceramic Membranes
CMs are generally prepared from chemically resistant metal oxides (Al2O3, TiO2, SiO2, ZrO2, etc.) or their mixtures, and are currently the most widely used inorganic films on the market [32]. Among them, Al2O3 is a widely used ceramic material for membrane fabrication. Al2O3 membranes have high strength, chemical stability, and thermal stability, and are more economical and cost-effective [31,33]. ZrO2 membranes have a monoclinic crystalline structure at room temperature and tetragonal and cubic structure at high pressure [33]. Due to their remarkable hydrophilicity and excellent heat resistance, they are suitable for oil–water separation [34,35]. TiO2 membranes contain rutile, anatase, and limonite, and have anti-fouling and anti-bacterial properties [34]. SiO2 membranes have smaller pore sizes, are less likely to be clogged during use, and can be applied to seawater desalination [30]. Ceramic membrane materials are listed according to their chemical stability from the highest strength to the lowest as follows: TiO2 > ZrO2 > Al2O3 > SiO2 [34]. The relative properties of the aluminum (Al)-, zirconium (Zr)-, silicon (Si)-, and titanium (Ti)-based ceramic membranes are presented in Figure 1a [31]. The structure of CMs is an asymmetric structure composed of multiple layers of oxides with different pore sizes and porosities. The asymmetric structure generally has 2–3 layers, including a support layer, an optional intermediate layer, and an active layer (Figure 1b). The support layer is prepared from adhesives and gelling agents, and the support layer provides mechanical support for the membrane as well as reduces the solvent permeation resistance [36,37]. The intermediate layer is thin and porous with a pore size distribution in the range of 50–500 nm, functioning to reduce the pore size and produce a smooth membrane surface [38]. The active layer, with a thickness of less than 1 μm, determines the membrane’s porosity and is used to fulfill the separation function of the membrane [13,39].
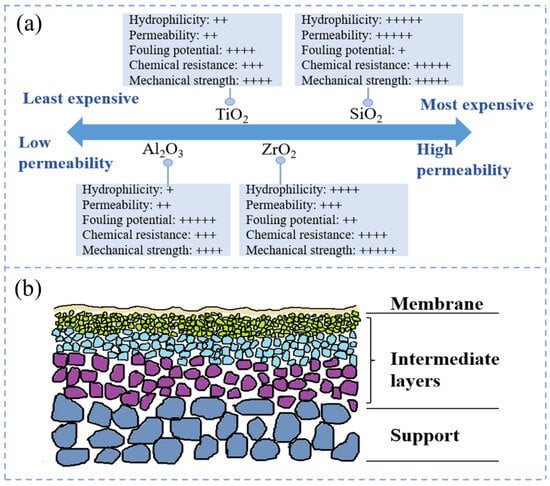
Figure 1.
(a) CM performance characteristics of different materials used for wastewater treatment [31]; (b) schematic of CM cross-section.
The steps involved in the preparation of CMs are as follows: particle suspension formation, membrane molding, membrane sintering, and precision processing [37]. Membrane molding can be further classified into slip casting, tape casting, the pressing method, freeze-casting, and direct foaming [40,41,42,43,44]. Among these, pressing is considered to be the simplest method of manufacturing CMs. In this step, the ceramic material is shaped into the desired geometry. Typically, CMs come in two main shapes, flat and tubular, and both have multiple channels. Tubular CMs have a higher specific surface area [45]. The sintering step is crucial for preparing a membrane with good mechanical strength and narrow pore size distribution. The membrane sintering process consists of three key steps, re-sintering, thermolysis, and final sintering [46]. The sintered membrane develops final mechanical strength and controls grain growth, relative density, shrinkage, and morphology. The final step is to change the membrane hydrophilicity, pore size, and surface roughness by surface modification. The physical methods involved include surface coating, layer-by-layer self-assembly, etc. [47,48]. Chemical methods include immersion, chemical vapor deposition, sol-gel, in situ reduction, etc. [37,46].
The CMs of MF and UF are widely used in surface water treatment and wastewater treatment to remove possible dissolved organic matter, suspended matter, heavy metals, bacteria, etc. [49,50,51]. Most CMs are separated based on the size exclusion of membrane pores and the electrostatic effect on the membrane surface [52]. Depending on the particle size, effective rejection (>90%) of suspended particles and bacteria could be achieved by membranes with pore sizes of >10 nm, 10–30 nm, and 10–1100 nm, respectively [53]. MF can serve as a means of pretreatment to ensure the stable operation of subsequent processes. Zhong [54] used ZrO2 membrane for MF treatment of oily wastewater generated in refineries and observed that CM exhibited better separation performance and a higher oil removal rate than organic membrane. Cui et al. [55] used a 50 nm ZrO2/Al2O3 membrane for pretreatment of seawater desalination, and the CM system was able to maintain long-term stable permeability under low-temperature (3–6 °C) conditions. Kim et al. [56] observed that ceramic UF membranes with pore sizes less than 4.0 nm can trap surfactant sodium dodecylbenzene sulfonate (SDBS). At high TMP, the substances formed by SDBS on the membrane surface caused concentration polarization, which induced a prescreening effect, increasing the retention of SDBS. The electrostatic repulsion between the surface charge of the membrane and the charged particles in the solution causes solute retention, and changes in pH value can affect the retention rate of the target substance [57]. CMs in membrane bioreactors (MBRs) allow the reactor to operate at high mixed liquor suspended solids (MLSSs) concentrations and high fluxes, so CMs can be used for larger scale wastewater treatment. In a recent study [51], the removal of micropollutants by CMBR was evaluated, with the antibiotic ofloxacin removing 98%.
The challenge of using CM can be summarized as reducing the cost of CM by reducing the number of steps in the fabrication process and lowering the sintering temperature. Metal-organic framework (MOF) membranes can be prepared at lower temperatures [14]. MOF membranes can be supported on polymeric membranes and CMs to prepare NF membranes with high selectivity [58]. Yuan et al. [59] loaded zeolite imidazolate framework (ZIF) on the substrate α-Al2O3 to prepare a novel pure ZIF-300 membrane for the removal of heavy metal ions from the aqueous environment. The prepared membranes showed a retention rate of 99.21% of CuSO4 and improved water permeability (39.2 L m−2·h−1·bar−1). The ZIF-300 membranes exhibited excellent water stability and particle size discrimination, but the affinity between the MOF layer and the ceramic substrate is low, so producing a sturdy ceramic MOF film is still a big problem. Zeolite, fly ash, kaolinite, and mullite can be used as raw material substitutes for the preparation of CMs [37,60], which can also reduce the cost. It is worth noting that commercial NF membranes of ceramics are difficult to mass produce because NFs require hard textured support and intermediate layers, and the preparation of defect-free NF membranes has high process requirements. In the future, it is necessary to further develop CMs with low cost, a long lifespan, high selectivity, and strong antifouling performance. The new membrane materials generated by the hybridization of inorganic membranes and polymer membranes are also a new research direction.
2.1.2. Carbon-Based Membranes
The emergence of several new materials, especially carbon-based nanostructures such as carbon nanotubes (CNTs), graphene, and their derivatives, has provided a new era in enabling membrane science and technology with separation properties [61]. These materials have important properties such as a large specific surface area, high permeability, homogeneous structure, tunable pore size, and strong atomic bonds [62], and thus have attracted increasing attention in solving water pollution and water scarcity problems.
- (1)
- Carbon nanotube membranes
According to the different layers and shells, CNTs can be further divided into single-walled carbon nanotubes (SWCNTs), double-walled carbon nanotubes (DWCNTs), and multi-walled carbon nanotubes (MWCNTs), which have smooth internal hydrophobic surfaces [63]. CNT membranes are prepared by incorporating CNTs into polymer matrices [61,64], and CNTs are also arranged in vertical support fillers such as epoxy resin and silicon nitride. CNT membranes are usually classified into vertically aligned CNT membranes, horizontally aligned CNT membranes, and mixed-matrix CNT membranes [64]. The proper pore size of well-arranged CNT membranes can screen contaminants of a certain size, reject salt ions, and allow water to pass through the pores [65]. Meanwhile, the hollow CNT structure provides frictionless transport of water molecules to enhance water permeability and provides excellent water flux for simple functionalization [66].
Vertically aligned CNT membranes have high water flux, which was demonstrated by Baek et al. [67] to be up to three times higher than that of typical ultrafiltration membranes. It has been reported that DWCNT membranes with pore sizes below 2 nm exhibit extremely fast water flow rates of up to 6 × 103 L cm−2 day−1 MPa−1, which is two orders of magnitude greater than that of reverse osmosis membranes (~2.6 × 102 L cm−2 day−1 MPa−1) [68]. Sadia et al. [69] studied membranes made solely from CNTs. In the experiment, H2O2 oxidants were added, and the phenol removal rate of this membrane exceeded 85% within 4 h, with an average oxidation rate of about 0.059 mol.h−1.m−2. Water molecules can be transported through CNT structures without significant impedance, thus developing some CNT membranes with excellent desalination rates and high permeability for RO systems [70,71]. Chen et al. [72] designed an asymmetric tip-functionalized CNT membrane, with one tip containing a hydrophilic group (carboxyl group) and the other tip containing a hydrophobic group (trifluoromethyl group), effectively blocking salt ions with pore sizes of 0.81 nm and 1.09 nm. Corry et al. [73] placed a series of functional groups with different charges and polarities on the tip of CNTs with a diameter of 1.1 nm. They found that eight carboxyl groups with negative charges prevented the passage of Na+ and Cl− and were able to achieve a desalination rate of 100%. Currently, research has found that modified CNTs can achieve better selectivity [74]. Zhang et al. doped the CNT-based hybrid film with Au nanoparticles. The prepared film has the multi-function of circulating catalytic degradation of soluble organic molecules and separation of oil–water lotion. The maximum flux is up to 3000 L m−2·h−1·bar−1. It decomposed 92.6% nitrophenol in oily wastewater.
- (2)
- Graphene membranes
Graphene is a material composed of the compact accumulation of sp2 hybridized carbon atoms. Graphene and graphene oxide (GO) have been widely used to construct novel membranes with layered pores [75]. Since the flux through a membrane is known to be inversely proportional to the thickness of the membrane, graphene nanosheets with single-atom thickness and two-dimensional structure offer great promise for high-throughput and energy-efficient separations [76]. Graphene materials have been recognized as the new generation of RO films, which have the advantages of a smooth surface, low roughness, few nucleation sites, and low adhesion of scaling crystals. It is more robust, thinner, chemically stronger, and ion-selective than the active layer in polymeric RO membranes [77]. Kabiri et al. [78] synthesized a thiol-functionalized graphene membrane with a unique three-dimensional porous structure for the removal of mercury ions (Hg2+) from water. The results showed that the removal rate was almost 100% for low (4 mg/L) and high (120 mg/L) concentrations of Hg2+. Graphene membranes have a size exclusion effect. O’Hern et al. [79] reported that they controlled the formation of high-density sub-nanopores on graphene membranes, which transported salts and removed larger organic molecules. While graphene oxide (GO) is prepared by oxidizing graphite with strong acids or oxidants and has hydroxyl, epoxide, and carboxyl groups [80], GO has better water dispersibility than graphene and can be well dispersed in water and other organic solvents. Good water dispersibility is beneficial for preparing GO-based membranes [81,82]. Li et al. [83] prepared porous Al2O3 tube-loaded GO composite membranes with sub-micrometer thickness and improved mechanical strength using a press-filtration deposition method, which can achieve efficient dehydration of organic solvents. Zhao et al. [84] prepared a GO membrane with adjustable interlayer spacing using a simple thermal reduction method. The prepared membrane had relatively high permeability (17.1 LMH/bar) and a high removal rate for heavy metal ions (i.e., the removal rates for Cu2+, Pb2+, Cd2+, and Ni2+ are 98.6%, 97.2%, 99.1%, and 97.2%, respectively). Chang et al. [85] reported that carboxylation could enhance the hydrophilicity of GO membranes, thereby improving dye removal efficiency. Nowadays, GO membranes are also commonly used to remove oil from wastewater. Gao et al. [86] made a pressure-driven membrane from sulfonated graphene oxide (SGO) nanosheets and hierarchical nanostructured TiO2 spheres. The flexible and super hydrophilic SGO-TiO2 membrane can effectively separate oil–water lotion stabilized by surfactants.
Despite the rapid growth and development of CNT-related research at the laboratory scale, the commercial application of CNT membranes in water treatment has proceeded at a slow pace, mainly due to the high production cost of high-quality and reproducible carbon nanotubes. Common CBMs also include coal-based carbon membranes, phenolic resin-based carbon membranes, and carbon fiber membranes. The low price of coal and phenolic resin can reduce the manufacturing cost of CBMs. Meanwhile, it was found that SWCNT was bio-persistent and could be observed to induce inflammation and lung cell proliferation in rat lungs. Risk assessment is crucial before applying these carbon nanomaterials.
2.2. Influences
The retention effect of IMF on contaminants varies considerably. The feed water conditions of the influent solution have an important influence on the retention process, making the retention effect different under different circumstances. Different inorganic membranes have widely varying properties in terms of pore size, hydrophilicity of the material, roughness, etc., and can lead to differences in contaminant removal. The presence of membrane contamination can complicate the retention process, and the retention rate may appear to increase or decrease.
2.2.1. Coexisting Substances in Water
Coexisting substances in water include temperature, pH value, inorganic ion concentration, natural organic matter (NOM), filtration pressure, etc.
Temperature is related to the permeate water viscosity, which affects pure water flux. Akhondi et al. [87] found that when the operating temperature was varied from 21 ± 1 °C to 29 ± 1 °C, the fouling resistance was reduced by 25% at a driving force of 40 mbar and 21% at 100 mbar. Changes in pH value can cause changes in the surface charge of NF membranes as well as dissociation of target substances, altering the electrostatic interaction between the membrane and the target substance, thereby altering the retention rate of the target substance. In Figure 2a, Wang et al. [88] studied the effect of different pH values on the retention efficiency of HA. When pH value is lower, the size of pollutants is smaller, the electrostatic force between the surface charges of the film is weakened, and the hydrophobicity of the film is increased. At pH 4, the flocs formed had a small particle size and were prone to membrane clogging due to concentration polarization. Under neutral conditions, the floc structure was loose, and the main cause of membrane clogging was pore clogging. Kramer et al. [89] used ceramic NF membranes with a pore size of about 0.9 nm to retain phosphate. Phosphate was present in the form of H2PO4− and HPO42− with hydrodynamic radii of 0.302 nm and 0.327 nm, respectively. Depending on the pore size of the membrane and the hydrodynamic radius of the phosphate, phosphate would not be retained. But in practice, the phosphate retention rate increased from 76% to 99% as the pH value increased in the range of 5–9. The addition of inorganic ions cannot increase the permeation flux, as salt can lead to an increase in viscosity in the pores and bulk solution [90].
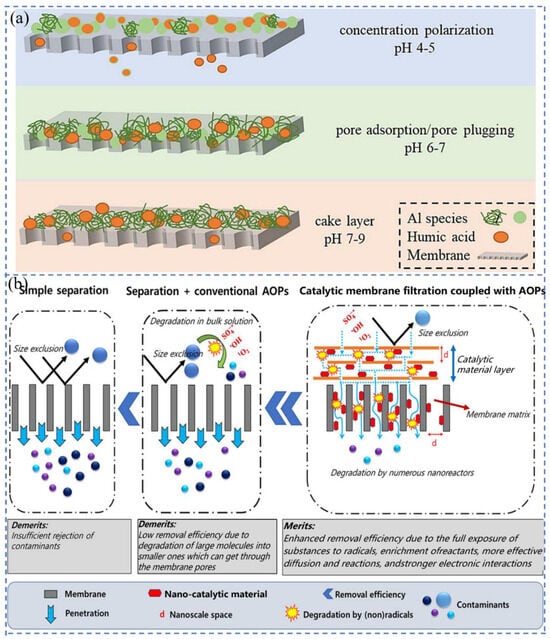
Figure 2.
(a) The main membrane-fouling mechanisms for the three coagulants [88]; (b) schematic diagram of individual separation, separation and conventional AOPs, and catalytic membrane filtration coupled with AOPs [27].
The effect of the presence of NOM on contaminant retention is not well established. Zazouli et al. [91] used two commercial composite nanofiltration membranes (SR2 and SR3 membranes) with different properties. The SR2 membrane had a larger pore size and higher membrane flux. In the absence of NOM, the removal of PhACs by SR3 membranes was greater than that of SR2 membranes (except for cefadroxil). Alginate was used as a NOM model, and the removal of indomethacin and tetracycline increased in both SR2 and SR3 membranes when 25 mg/L alginate was added. And acetaminophen removal decreased. NOM was completely retained by the membrane and formed a fouling layer on the membrane surface, thereby increasing the permeate flow resistance [92]. The increased removal of both indomethacin and tetracycline can be explained because the alginate fouling layer not only offers charge repulsion between the carboxylic acid group of the drug and the alginate group, but also enhances size exclusion [93]. The log P of acetaminophen is moderate and will accumulate in the polar alginate layer, making it easier to diffuse to the permeate side through the membrane barrier, thereby reducing the interception effect. During filtration, Ca2+ and Mg2+ accumulate on the membrane surface until precipitation is formed [94,95]. Over time, these precipitates form microcrystals, leading to inorganic fouling on the nanofiltration membrane, which reduces the membrane flux [96]. Su et al. [97] titrated different concentrations of Al3+, Fe3+, and Cu2+ solutions in NOM samples. When the concentration of the titrated Cu2+ solution was 5 μm, its complexation ability with NOM was strong, which resulted in a decrease in the membrane flux. When the concentration of the titrated Al3+ and Fe3+ solution was 20 μm, the complexation ability of ions with NOM was relatively weak, and micro flocs would be formed to block the nanofiltration membrane. Laitinen et al. [98] investigated the UF-influencing factors of membrane filtration. At a cross-flow velocity of 3 m/s, the pressure increased from 0.7 bar to 2.3 bar, the flux increased by 22 LMH (23%), and the pressure–flux curve started to level off after 1 bar. This is because as the pressure increases, the fouling layer becomes thicker/denser, making it more resistant to osmotic flow. As the cross-flow velocity increases, the increase in flux becomes greater due to the reduction of the pollution layer.
2.2.2. Inorganic Membrane Properties
The properties of inorganic membranes, such as pore size, surface roughness, hydrophilicity, or hydrophobicity, have a significant impact on membrane filtration performance.
Due to the size exclusion effect, filtration membranes are able to remove particulate matter larger than the membrane pore size [99]. Therefore, smaller pores exert a stronger interception effect [100]. The reduction of membrane pore size can improve the selectivity of the membrane and may prevent the occurrence of internal scaling. As the pore size of the membrane increases, the pure water flux of the membrane also increases. A higher pure water flux means an increase in membrane filtration rate, but a decrease in pollutant removal efficiency. In GDM filtration [101], the water flux of flat plate membranes with pore sizes of 0.22 and 0.45 μm was 227 LMH, and 679 LMH, respectively. Ideal separation membranes should have both good selectivity and high permeability. A membrane with high permeability reduces the required membrane area for water treatment, ultimately leading to a decrease in the cost of membrane filtration [63]. The roughness of the membrane determines the separation efficiency, as it affects the membrane’s anti-fouling properties [102]. Sun et al. [103] developed a smooth ceramic-based graphene seawater desalination membrane, confirming that graphene membranes exhibit more stable water flux and almost complete desalination rate (>99.9%) for high saltwater treatment. Hydrophilic membranes (water contact angle < 90°) can form a water molecule layer on the surface to prevent contact with contaminants in solution [61]. Different types of wastewater use membranes with different properties. For example, in oil–water separation, the membrane can be hydrophobic and oleophilic, or hydrophilic and oleophobic [104]. Hydrophobic membranes are preferred over hydrophilic membranes in membrane distillation [105]. Membranes can be modified to alter the membrane surface properties. Mallya et al. [106] used hydroxyl-functionalized molybdenum disulfide (OH-MoS2) nanosheets as nanofillers to design NF membranes. After 6 h of experiment, the retention rate of SA remained at 93%, while the control group only had 74%. The modified membrane had higher hydrophilicity, a negative charge, and rougher membrane morphology, which could support the formation of a water hydration layer, thus improving the antifouling and NOM removal performance. Coelho et al. [107] fabricated ceramic UF membranes with ZrO2 slurry dip-coated with porous SiC carriers; the membrane pure water permeation flux was up to 360 L/(m2·h·bar), and the pilot test of olive oil/water emulsion removed 99.91% of the oil.
Membrane contamination is complex, diverse, and unavoidable. Fouling is caused by the accumulation of organic and inorganic pollutants during the membrane filtration process, and the permeation flux decreases over time [21]. Four possible models of membrane fouling include complete pore blocking, intermediate pore blocking, cake filtration, and standard pore blocking [31]. In applications, to safeguard the effectiveness of membrane filtration, various influencing factors will be controlled as much as possible to provide a suitable environment, but this can only slow down the time for membrane contamination to occur. Pretreatment and cleaning of membranes can restore membrane flux [108,109,110,111,112,113]. Ghadimkhani et al. [114] added HA to the membrane unit system under alkaline conditions to simulate organic pollutants. After the membrane was completely scaled, air nanobubbles were introduced into the CM support for backwashing. After 6 h, the permeation flux of CM basically recovered to 99%. Although membrane cleaning can remove reversible fouling, it is still difficult to restore performance if irreversible membrane contamination occurs. Moreover, frequent cleaning operations will reduce the service life of the membrane and increase the operating cost. Scholars have found that inorganic membranes can be modified to improve their performance and reduce the occurrence of irreversible pollution [106,107,115,116]. The techniques for membrane contamination mitigation are summarized in Table 1 [107,114,117,118,119,120,121]. Materials that can provide high permeability, high selectivity, and low energy consumption will become the most effective materials for incorporating membranes. Using AOPs to degrade contaminants in wastewater before filtering improves removal efficiency but adds additional process steps [27]. However, membrane filtration coupled with AOPs can simultaneously degrade and remove pollutants (Figure 2b) [27], which accelerates mass transfer of organic pollutants and oxidants from the native solution to the catalyst surface, increasing the production rate of oxidized substances. This facilitates the interaction between the contaminants and the generated free radicals, thereby increasing the reaction rate and removal efficiency [122,123,124].

Table 1.
Effect of different strategies on membrane fouling mitigation during wastewater treatment.
3. Inorganic Membrane Filtration Coupled with AOPs
The intercepted pollutants in membrane separation still exist, which restricts its application in water treatment. AOPs can be divided into hydroxyl radical (•OH)-based AOPs (HR AOPs) and sulfate radical ()-based AOPs (SR AOPs). By coupling membrane filtration with AOPs technology and utilizing catalysts to produce highly oxidizing active substances, organic pollutants in water can be mineralized and decomposed in situ. Polymeric membranes cannot be exposed to free radicals for a long period, and prolonged contact with oxidizing agents will cause wear and tear and reduce the service life. Therefore, inorganic membranes are more suitable for coupling with AOPs [31,125]. Catalytic separation membranes will be a new generation of functional membrane products and have a promising application in future water treatment.
3.1. IMF Coupled with Photocatalytic Oxidation System
Photocatalysts can be divided into photocatalysts suspended in solution and photocatalysts fixed on a membrane (Figure 3a) [125]. In coupled systems, suspended photocatalysts are recovered by membrane energy separation, but may clog the membrane surface, resulting in decreasing permeate flux over time and increasing operating costs [126,127]. The preparation of photocatalytic membranes by immobilizing photocatalysts on membranes enables timely degradation of pollutants accumulated on the membrane surface and increases the treatment efficiency of the membrane unit [128,129,130]. UV irradiation can reduce the contact angle between the catalytic film and water, making the catalytic film super hydrophilic [131]. Under the irradiation of UV light, the electrons on the catalyst surface transition from the valence band to the conduction band, ultimately forming photogenerated electron hole pairs [132]. Photogenerated holes react with H2O to generate •OH (Figure 3b) [133,134]. These active free radicals react with organic pollutants adsorbed onto the surface of the catalytic membrane.

Figure 3.
(a) Two different states of existence of photocatalysts in PMR: A, suspended on the membrane and B, loaded on the membrane [125]; (b) photocatalytic mechanism diagram of catalytic CM [133]; (c) schematic diagram of MnO2-Co3O4-loaded CM coupled with ozonation degradation of BP-3 [135]; (d) schematic diagram of mechanism activation of PS: A, schematic diagram of free radical mechanism for metal oxide activation of PS and B, schematic diagram of the non-radical mechanism of PS-activated catalytic oxidation of organic pollutants.
The photocatalysts that can be loaded onto the membrane through various manufacturing methods are divided into precious metals, metal oxides, and non-metal oxides (Table 2) [136,137,138,139,140,141,142,143,144]. TiO2 is the most widely used catalyst in membrane filtration-coupled photocatalysis, with a high surface area, photochemical stability, and excellent excited state lifetime [136,139,140,141,145]. Choi et al. [146] prepared nanostructured TiO2/Al2O3 composite membranes with a water permeability coefficient of 6.71 L m−2·h−1·bar−1 Under UV irradiation, it can decompose methylene blue dye and creatinine. Taking precious metal Pt as an auxiliary catalyst, Kumakiri et al. [147] loaded Pt onto CM with deposited TiO2. Under UV irradiation, the deposition of Pt increased the rate of formic acid oxidation by about 2–5 times. The Pt-TiO2 catalytic membrane avoided Pt oxidation and allowed it to remain in a metallic state even in the presence of reactive oxygen species. Compared with pure Pt catalytic membranes, Pt-TiO2 catalytic membranes have a longer lifespan. The bandgap width of TiO2 is relatively large (3.0–3.2 eV), and it can only absorb UV light. Doping TiO2 catalytic membrane with impurities can expand its light absorption range to the visible light range [140,141,144]. Liu et al. [137] used sol-gel and spray pyrolysis methods to grow N-TiO2 membranes on FTO glass, and deposited P-Cu2O onto them by a hydrothermal method. The surface of the Cu2O/TiO2 thin membrane was composed of a network and large grains, which had stronger optical absorption ability than pure TiO2 thin membrane. Membrane filtration coupled with a photocatalytic oxidation system can also kill bacteria, and Ag particles enhance the antibacterial effect of photocatalytic membranes [143].

Table 2.
Summary of recent works of IMF coupled with photocatalytic oxidation in pollutant removal.
Photocatalytic membranes are used to treat low-concentration pollutants [148]. Babu et al. [149] prepared a CuO-TiO2 photocatalytic membrane for the degradation of methyl orange dye. When the initial substrate concentration was between 0.01 mM and 0.04 mM, the degradation rate of the dye was highest at 0.01 mM. As the concentration of pollutants increases, the solution becomes opaque, and the pollutants themselves absorb light, thereby reducing the efficiency of the photocatalytic process [150]. In the actual wastewater treatment process, there are suspended substances with deep chromaticity and high turbidity, which reduce the transparency of the wastewater. The light intensity transmitted to the catalytic membrane is weakened, which has a negative impact on the photocatalytic efficiency [24]. Additional energy is required to achieve artificial UV irradiation, which adds significantly to operating costs. However, the pollutant removal efficiency is greatly reduced when using sunlight [19]. In the future, it is necessary to continue developing materials that can expand the range of light absorption. How to minimize the loss of light transmission to catalytic membranes is a hot research direction. In future experimental research, it is necessary to prepare an inorganic catalytic membrane that utilizes light energy more efficiently and has a wider range of light absorption.
3.2. IMF Coupled with Ozonation System
The redox potential of O3 is 2.07 V, which allows oxidative decomposition of most of the organic pollutants in the water [151,152]. However, due to the unfavorable self-decomposition of O3 under environmental conditions, the utilization rate of O3 is low, resulting in low free radical yield. The reaction rate between O3 and organic compounds in the liquid phase is only 1.0 × 103 M−1s−1, which limits its wider application [153]. The types of catalysts used to catalyze ozonation are divided into homogeneous and non-homogeneous types. Homogeneous catalysts are generally transition metal ions, which are difficult to separate from the effluent and easily cause secondary pollution [154]. Non-homogeneous catalysts are mainly classified as metal oxides (TiO2, MnO2, Fe2O3, CeO2, etc.), noble metals, or activated carbon thereof, etc. (Table 3) [152,155,156,157,158,159,160,161,162,163,164]. Most of the non-homogeneous catalysts can be loaded onto the membrane to prepare a catalytic membrane [165,166]. O3 is activated by the catalyst on the surface of the catalytic membrane to generate •OH, and organic pollutants are efficiently degraded/mineralized into harmless inorganic compounds [167,168].

Table 3.
Summary of recent works of IMF coupled with ozonation in pollutant removal.
Compared with Mn CCM, Ce-CCM exhibits stronger mineralization ability and more effective O3 utilization during the mixing process. Li et al. [163] used a sol-gel-assisted impregnation method to prepare Mn-CCM and Ce-CCM, respectively. Under similar operating conditions, the specific O3(aq) of the mixing process of Ce-CCM consumption was 2.1 mg O3(aq) mg−1 TOC compared to 8.0 mg O3(aq) mg−1 TOC for Mn-CCM. Guo et al. [134] used a coupled system of IMF and catalytic ozonation to remove BP-3. The removal rate of BP-3 was 51.6% in membrane filtration alone, and only 47.4% in direct ozonation; the removal rate increased to 74.8%in MnO2-Co3O4@CM coupled with O3 (Figure 3c). The ion-leaching concentration was low (CMn < 2 mg/L). Under most conditions, IMF coupled with ozonation has a low utilization rate of O3 and sometimes produces disinfection byproducts (DBPs) that are more toxic than the original pollutants, which increases the harm to the environment. New catalytic membranes that can be developed in combination with ozonation can increase O3 utilization and pollutant mineralization, reduce DBP production, and reduce effluent toxicity [31,169]. Ozone can remove membrane fouling and reduce filtration resistance [170]. Making ozone purification applied to membrane cleaning research has become a hot topic in recent years. In the pilot system [164], the TMP increased to 35.9 kPa after a filtration time of 10 h only with Al2O3 membrane filtration. Under the same conditions, the catalytic TMP of the Mn-Al membrane increased to 24.2 kPa. When the ozone dosage increased to 5 mg/L, the TMP growth decreased to 4.6 kPa.
3.3. IMF Coupled with Persulfate Oxidation System
Peroxide (-O-O-) is present in persulfate molecules that have strong oxidizing properties. Its standard electrode potential is 2.5–3.1 V, exceeding O3 (+2.07 V). The generated by activating PS has a high potential and can rapidly oxidize organic pollutants like HO• [171,172]. Thermal energy, UV and visible light, alkali, transition metals, etc. can effectively activate persulfate (PS) to generate and HO• [173,174,175]. Transition metals and their oxides can be loaded onto the membranes to prepare catalytic membranes, which are coupled with persulfate oxidation for use (Table 4) [176,177,178,179,180,181,182,183,184,185]. There are usually three mechanisms to control PS-based AOPs for the degradation of organic pollutants: (i) by free radicals (in most cases, , •OH and ); (ii) non-free radicals (1O2); and (iii) direct oxidation (Figure 3d) [135,182,186,187,188].

Table 4.
Summary of recent works of IMF coupled with persulfate oxidation in pollutant removal.
Al2O3-based and SiO2-based membranes have good thermal stability [2]. Shan et al. [189] prepared a flexible copper droplet carbon/SiO2 nanofiber membrane (Cu@C/SiO2 NFM). The membrane produced could remove 95% of tetracycline hydrochloride (TCH) from wastewater. Bao et al. [178] prepared a CoFe2O4@CM catalytic membrane with a pure water permeate flux of 3000 L m−2·h−1·bar−1. They [177] also prepared Co3O4 nanocatalyst-functionalized Al2O3 CMs (CoFCM) with a honeycomb structure by using an optimized surface-nucleated heterogeneous growth of zeolitic imidazolate framework (ZIF-67) method in the same period. CoFCM and CoFe2O4@CM catalyzed the removal of over 90% SMX by persulfate. Zhao et al. [190] found that the CuFe-CM/PMS system can degrade organic matter into stable, low-molecular-weight unsaturated bonds, and the products aggregate with each other, thereby reducing the irreversible fouling of the membrane. Wu et al. [191] prepared a MnO2/Al2O3 membrane that retained a high degradation rate even after six repetitions. Quenching experiments with NaN3 and ethanol confirmed that the free radical pathway was not the dominant route of degradation. Meanwhile, studies [180] have demonstrated that Mn CMs/PMS has a non-free radical mechanism for the degradation of EDCs. The oxidation-based process of 1O2 exhibits special selectivity for the removal of phenols and bisphenols.
Carbon material membranes have great potential in SR-AOPs due to their ability to activate persulfate. Song et al. [192] designed a novel coal-based carbon catalytic membrane that could activate PDS to degrade phenol. Synergistic effects of free and non-free radicals were found to co-exist in the study, but the effect percentage was not clear. Low TMP represents energy saving and cost reduction in the filtration process. It has been found that rGO promotes TMP reduction, and GO-based composite membranes exhibit considerable antifouling ability in situ catalytic oxidation [185]. C=O groups are the active sites for CS carbon-catalyzed PMS activation, and C-OH groups are the active sites for PS activation in mesoporous carbon [193,194]. However, the intrinsic interactions between the oxidizer and the catalyst in the non-radical pathway are still unclear and need to be investigated further. Further research on the mechanism of persulfate oxidation may be a hot direction in the future, which is crucial for improving the performance of mixing processes.
3.4. IMF Coupled with Fenton and Composite Fenton Oxidation Systems
The essence of catalytic wet peroxide oxidation (CWPO) technology is to activate H2O2 to generate HO• for the degradation of organic compounds [195,196]. Current methods regarding H2O2 include Fenton, electro-Fenton (EF), and photo-Fenton (PF) [135].
3.4.1. IMF Coupled with Fenton Oxidation Systems
The pH range of Fenton technology alone is narrow (2.8~3.0), and adding a large amount of iron salts will inevitably lead to the formation of iron sludge in the wastewater treatment process. The generated iron sludge needs to be collected and processed, which increases operating costs [197,198]. Traditional solid metal oxidants are prone to agglomeration and have fewer exposed active sites [199]. The catalytic membrane-coupled Fenton system can increase the metal active sites loaded onto the surface or embedded in the membrane channels, promote the decomposition of H2O2, and enhance oxidation performance (Table 5) [200,201,202,203,204,205,206,207,208,209]. Wang et al. [201] and Shi et al. [202] immobilized MnO2 and CuFe2O4 on SiO2 nanofiber membranes (Mn-SiO2 membrane and CuFe-SiO2 membrane). Both membranes mentioned above could activate H2O2 over a wider pH range. MOF catalysts showed excellent catalytic activity with poor stability in the CWPO process [210,211]. Jiang et al. [204] optimized the preparation of UiO-66@Al2O3 ceramic tube membranes (UiO-66CT) with the Cu or Mn added. The removal rate of phenol was >90% after five cycles. This study provides a promising strategy to address the instability of MOF catalysts during the CWPO.

Table 5.
Summary of recent works of IMF coupled with Fenton, electro-Fenton, and photo-Fenton oxidation in pollutant removal.
3.4.2. IMF Coupled with Composite Fenton Oxidation Systems
The PF process simply adds sunlight or UV with appropriate wavelengths to the Fenton process [212]. Li et al. [206] combined GO and highly magnetic Fe3O4 with TiO2 components (TiO2-GO-Fe3O4) loaded onto flat CMs of SMSMPR (Figure 4a). This system destroyed -O-O- to generate HO- by UV irradiation and accelerated the reaction (Figure 4b). Degradation of amoxicillin (AMX) showed only 22.3% degradation of AMX under dark conditions and 88.5% degradation of AMX by under UV irradiation.
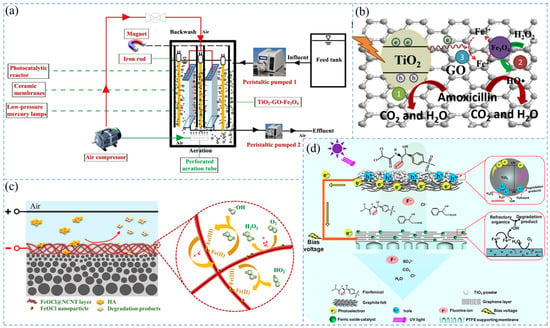
Figure 4.
Mechanism of membrane filtration coupled with composite Fenton oxidation: (a) SMSMPR SMSMPR process flow diagram [206], (b) mechanism of TiO2-GO-Fe3O4 composite membrane-coupled PF degradation AMX [206], (c) proposed EF catalytic mechanism of the FeOCl@NCNT/CM during the EF catalytic filtration [213], (d) schematic representation of the reactive oxygen species mediated mechanisms for florfenicol degradation in the PEC/EF filtration process [214].
When membrane filtration is coupled with Fenton technology with an applied current, it can catalyze at a higher and wider pH range [215,216,217]. H2O2 in EF technology can be produced by double electron reduction of dissolved oxygen on the cathode [5,218]. Due to the insulation of CM, carbon material membranes are commonly used for IMF coupling with EF. Yang et al. [219] used CNTs and porous carbon (PC) to prepare PC-CNT hollow fiber membranes and found that the efficiency of PC-CNT membranes and the coupled EF process for treating phenol was about 9.7 times higher than that of a single-membrane separation. Layered metal halide oxides have a unique two-dimensional intercalation structure and excellent electrical conductivity. Li et al. [209] developed a FeOCl functionalized reactive porous CNT electrochemical reaction filter to degrade TC. This system showed enhanced oxidation kinetics compared with conventional batch reactors. Nano FeOCl significantly promoted the production of •OH through the highly efficient cycling of Fe3+/Fe2+ (Figure 4c) [213]. Zhang et al. [213] prepared a FeOCl@NCNT/CM to control membrane fouling caused by HA. The FeOCl@NCNT/CM exhibited excellent fouling resistance. At an applied voltage of −2.2 V, the water flux of the membrane was 1.79 times higher than that without voltage. The catalytic membrane/EF system has excellent performance, but the cathode material has strict requirements for hydrophilicity, porosity, and surface area [64,220]. The external power supply may accelerate the corrosion of the membrane material and shorten the service life. To put the catalytic membrane/EF system into practical applications, it is necessary to design better composite membranes for continuous simulation experiments.
In addition, Jiang et al. [214] proposed a novel UV-driven electro-Fenton catalytic membrane (UV-EFCM) filtration system for the first time (Figure 4d). UV-EFCM used a TiO2-modified graphite felt filter (TiO2/GF) as a photoanode to treat a low concentration of florfenicol (14 μM), achieving almost complete degradation and high mineralization (78.4 ± 9.1%). This integrated system utilizes photovoltaic electronics to save energy requirements and is expected to be a hot research direction in the future.
3.5. Comparison of Different IMF Coupled with AOPs
In Section 3.1, Section 3.2, Section 3.3 and Section 3.4, the relevant applications of IMF coupled with the AOP process were introduced and discussed. We summarized the advantages and disadvantages of the above IMF coupled with photocatalytic oxidation, IMF coupled with ozonation, IMF coupled with persulfate oxidation, and IMF coupled with Fenton oxidation (Table 6). The combination of electrochemistry and membrane filtration is another advanced wastewater technology [221]. In the presence of an electric field, various types of fouling with different charging characteristics can migrate outward from the membrane surface by electrostatic repulsion or electrophoresis, or even be eliminated electrochemically by oxidation or reduction reactions [222]. CBMs have good conductivity and are often coupled with electrocatalysis. This system improves the fouling ability of the membrane and has a high removal rate for pollutants with molecular sizes smaller than the membrane pore size [223,224]. Li et al. [225] prepared dynamic electrodeposited CuO/carbon membranes by depositing CuO nanoparticles uniformly on the surface and pore wall of a coal-based carbon membrane. The removal rates of rhodamine B (RhB) and COD by this membrane coupled with electrocatalysis were 99.96% and 71.82%, which were 20 times and 1.8 times higher than those of the original carbon membrane and conventional electrodeposited CuO/carbon membrane, respectively. However, membrane filtration coupled with electrocatalysis increases energy consumption and cost accordingly. The total process energy consumption needs to be carefully considered to verify the sustainability of using additional electric fields. More research on coupling electrochemistry with membrane filtration can be conducted in the future.

Table 6.
Comparison of different IMFs coupled with AOPs.
4. Challenges and Prospects
Membrane technology is a cost-effective and simple process widely used in different applications in the separation industry, especially in water treatment and water treatment applications. This review focused on different types of inorganic membranes in membrane filtration for water treatment applications, including CMs and CBMs, which have been a hotspot of interest in recent years. Compared with organic membranes, inorganic membranes are superior in terms of permeate flux, mechanical stability, and thermal stability. Inorganic membranes are more competitive, so the research on inorganic membrane separation technology in wastewater treatment has been gradually increasing. So far, most of the reported CMs and CBMs have superior performance, but the manufacturing costs are high and not as economical as polymer membranes. The preparation of catalytic membranes can change the membrane performance to reduce membrane contamination. It is known that AOP processes are effective, but they can cause accelerated formation of degradation products. The use of both AOPs and membranes can be a good solution and effective in combating transformation products. The coupling of IMF with AOPs can not only efficiently separate pollutants, but also directly degrade organic pollutants in situ and improve the effluent water quality. However, the coupling process is susceptible to the influence of other factors, rarely used in practice. And for the additional oxidant and energy cannot be fully utilized.
There are still problems in the development and production of IMF and IMF coupled with AOPs, mainly including the following six aspects.
- (1)
- Higher cost of inorganic membranes. Despite the longer service life of inorganic membranes, both ceramic and carbon material membranes have high manufacturing costs, which limits the practical application of inorganic membranes. For CM, cost-effective natural materials such as kaolin, pyroxene, and dolomite can be used as raw materials [230,231]. Carbon nanomaterials can be used in combination with other materials to reduce the proportion of carbon materials while ensuring membrane performance [14,232]. All of these can be used as a means to reduce costs. The use of natural materials will inevitably bring other components to inorganic membranes, leading to membrane defects [14]. In the future, it will be necessary to optimize the preparation method of inorganic membranes and adjust the material ratios to create more cost-effective membrane materials.
- (2)
- Membrane fouling affects the service life of the membrane. Some IMFs are prone to membrane fouling. Reversible membrane fouling could be removed by cleaning the membrane, but excessive cleaning times will inevitably reduce the service life of the membrane. Irreversible membrane fouling cannot be eliminated [115] and will inevitably affect the permeation flux of the membrane. The membrane can be modified by loading other materials to alter its surface properties [64,233], such as roughness, hydrophilicity, stability, membrane surface charge, etc. Currently, many materials have been applied to the preparation of catalytic membranes. Materials that can provide high permeability and selectivity as well as low energy consumption, will be one of the most effective materials for doping membranes.
- (3)
- Low catalytic membrane recovery. The use of O3 or the use of tiny electric field cleaning can effectively recover membranes during wastewater treatment [120,133,234]. But research is only at the laboratory stage and practical water treatment applications are not always feasible. The toxic waste generated after cleaning requires specialized treatment and disposal. Therefore, new environmentally friendly cleaning agents should be prepared to achieve sustainable long-term membrane operation.
- (4)
- The application examples of IMF-AOPs are scarce [31]. In the literature on using membrane filtration coupling with the AOP process to remove pollutants, most of them are laboratory-simulated water samples instead of real wastewater samples. Real wastewater environments are very complex. In order to better apply catalytic membranes to long-term real-world processes, the stability and effectiveness of these catalytic processes need to be explored using real water quality.
- (5)
- In the future, it is necessary to further improve the performance of the IMF coupled with -AOPs system through optimal design. The utilization rate of oxidizer and the removal efficiency of pollutant can be improved in the coupling system while maintaining low energy consumption. And it is best to minimize the membrane pollution and improve the reuse performance of the membrane [197]. CBMs have high electrical conductivity [64,235], and catalytic CMs with electrical conductivity can reduce internal resistance due to their large thickness. The application of inorganic membranes to electrocatalysis is a future direction [220,236]. On the other hand, multifunctional catalytic membranes can be developed in the future, considering the trade-off between processing efficiency and energy consumption. The developed catalytic membranes can be applied to the removal of different pollutants under one or even multiple AOP systems.
- (6)
- The mechanism of interaction between membranes and contaminants in the IMF coupled with AOPs process has not been clearly described so far. The current mechanism of the membrane filtration and AOP coupling process is based on the removal of pollutants by AOPs. The mechanisms of synergism and interaction between contaminant degradation intermediates and membrane skeleton surfaces remain unclear. Elucidating the dynamic application of inorganic membranes in contaminant degradation can provide a clearer understanding of the membrane fouling mechanism, thus effectively preventing membrane contamination and improving membrane lifetime.
5. Conclusions
This review aimed to explore the research progress of IMF technology in wastewater treatment. According to a systematic review of existing literature, inorganic membranes are superior to polymer membranes in terms of service life, permeation flux, and cleaning efficiency, making them more competitive. The research on IMF in wastewater treatment has been gradually increasing. CMs and CBMs are currently the most popular inorganic membranes for research. Membrane filtration technology achieves wastewater purification through a screening mechanism, and the accumulated pollutants require subsequent treatment. The properties of inorganic membrane surfaces can be changed by membrane modification to provide superior separation capability. At present, coupling IMF with AOPs (including IMF–photocatalysis oxidation, IMF–ozonation, IMF–persulfate oxidation, and IMF–Fenton oxidation) has received widespread attention as a novel catalytic inorganic membrane filtration process. These combined processes can degrade pollutants while separating them, but further exploration through detailed technical and economic analysis is needed for a comprehensive application. With more and more emerging ideas and strategies to improve processing techniques and minimize defects, inorganic membranes have broad potential in practical applications. In the future, more efforts should be made to reduce the production cost and practical application of ceramic membranes.
Author Contributions
C.Z.: investigation, methodology, data curation, software, formal analysis, writing—original draft, writing—review and editing. R.Y.: conceptualization, funding acquisition, supervision, writing—review and editing. B.Z. (Beihai Zhou): writing—review and editing. H.C.: writing—review and editing. Z.C.: writing—review and editing. B.Z. (Boyun Zhu): writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
“Interdisciplinary Program for Young Teachers” of University of Science and Technology Beijing (FRF-IDRY-23-040) and National Key Research and Development Project of China (No. 2019YFD1100204).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, M.; Xu, Z.; Hou, D.; Gao, B.; Cao, X.; Ok, Y.S.; Rinklebe, J.; Bolan, N.S.; Tsang, D.C.W. Waste-derived biochar for water pollution control and sustainable development. Nat. Rev. Earth Environ. 2022, 3, 444–460. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Al-Khateeb, L.A.; Almotiry, S.; Salam, M.A. Adsorption of pharmaceutical pollutants onto graphene nanoplatelets. Chem. Eng. J. 2014, 248, 191–199. [Google Scholar] [CrossRef]
- Bukusoglu, E.; Koku, H.; Çulfaz-Emecen, P.Z. Addressing challenges in the ultrafiltration of biomolecules from complex aqueous environments. Curr. Opin. Colloid Interface Sci. 2020, 46, 52–64. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Hu, X.; Hua, T. Electrochemical advanced oxidation processes coupled with membrane filtration for degrading antibiotic residues: A review on its potential applications, advances, and challenges. Sci. Total Environ. 2021, 784, 146912. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. Review: Is interplay between nanomaterial and membrane technology the way forward for desalination? J. Chem. Technol. Biotechnol. 2014, 90, 971–980. [Google Scholar] [CrossRef]
- Zhu, R.; Diaz, A.J.; Shen, Y.; Qi, F.; Chang, X.; Durkin, D.P.; Sun, Y.; Solares, S.D.; Shuai, D. Mechanism of humic acid fouling in a photocatalytic membrane system. J. Membr. Sci. 2018, 563, 531–540. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, X.; Wang, Y.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A review on oil/water emulsion separation membrane material. J. Environ. Chem. Eng. 2022, 10, 107257. [Google Scholar] [CrossRef]
- Alduraiei, F.; Kumar, S.; Liu, J.; Nunes, S.P.; Szekely, G. Rapid fabrication of fluorinated covalent organic polymer membranes for organic solvent nanofiltration. J. Membr. Sci. 2022, 648, 120345. [Google Scholar] [CrossRef]
- Lau, W.J.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Chen, J.P.; Ismail, A.F. A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Pan, Y.; Di, H.; Zeng, G.; Zhang, L.; Zhang, C. A modified mussel-inspired method to fabricate TiO2 decorated superhydrophilic PVDF membrane for oil/water separation. J. Membr. Sci. 2016, 506, 60–70. [Google Scholar] [CrossRef]
- Geise, G.M.; Lee, H.-S.; Miller, D.J.; Freeman, B.D.; McGrath, J.E.; Paul, D.R. Water purification by membranes: The role of polymer science. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1685–1718. [Google Scholar] [CrossRef]
- Ghandashtani, M.B.; Zokaee Ashtiani, F.; Karimi, M.; Fouladitajar, A. A novel approach to fabricate high performance nano-SiO2 embedded PES membranes for microfiltration of oil-in-water emulsion. Appl. Surf. Sci. 2015, 349, 393–402. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Tan, Y.H.; Abdullah, E.C.; Rahman, M.E.; Karri, R.R. A comprehensive review on micropollutants removal using carbon nanotubes-based adsorbents and membranes. J. Environ. Chem. Eng. 2021, 9, 106647. [Google Scholar] [CrossRef]
- Ghernaout, D. Advanced Oxidation Processes for Wastewater Treatment: Facts and Future Trends. OALib 2020, 07, 1–15. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. Int. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- He, Z.; Ong, J.H.; Bao, Y.; Hu, X. Chemocatalytic ceramic membranes for removing organic pollutants in wastewater: A review. J. Environ. Chem. Eng. 2023, 11, 109548. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting-The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Liu, T.; Aniagor, C.O.; Ejimofor, M.I.; Menkiti, M.C.; Tang, K.H.D.; Chin, B.L.F.; Chan, Y.H.; Yiin, C.L.; Cheah, K.W.; Ho Chai, Y.; et al. Technologies for removing pharmaceuticals and personal care products (PPCPs) from aqueous solutions: Recent advances, performances, challenges and recommendations for improvements. J. Mol. Liq. 2023, 374, 121144. [Google Scholar] [CrossRef]
- Li, N.; Lu, X.; He, M.; Duan, X.; Yan, B.; Chen, G.; Wang, S. Catalytic membrane-based oxidation-filtration systems for organic wastewater purification: A review. J. Hazard. Mater. 2021, 414, 125478. [Google Scholar] [CrossRef]
- Bao, Y.; Lee, W.J.; Wang, P.; Xing, J.; Liang, Y.N.; Lim, T.-T.; Hu, X. A novel molybdenum-based nanocrystal decorated ceramic membrane for organics degradation via catalytic wet air oxidation (CWAO) at ambient conditions. Catal. Today 2021, 364, 276–284. [Google Scholar] [CrossRef]
- Kumari, P.; Bahadur, N.; Dumée, L.F. Photo-catalytic membrane reactors for the remediation of persistent organic pollutants—A review. Sep. Purif. Technol. 2020, 230, 115878. [Google Scholar] [CrossRef]
- Ly, Q.V.; Cui, L.; Asif, M.B.; Khan, W.; Nghiem, L.D.; Hwang, Y.; Zhang, Z. Membrane-based nanoconfined heterogeneous catalysis for water purification: A critical review. Water Res. 2023, 230, 119577. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, N.; Han, Y.; Wang, X.; Liu, S.; Zhang, L.; Sun, Y.; Jiang, B. Development of polyacrylonitrile/perovskite catalytic membrane with abundant channel-assisted reaction sites for organic pollutant removal. Chem. Eng. J. 2022, 437, 135163. [Google Scholar] [CrossRef]
- Zuo, K.; Wang, K.; DuChanois, R.M.; Fang, Q.; Deemer, E.M.; Huang, X.; Xin, R.; Said, I.A.; He, Z.; Feng, Y.; et al. Selective membranes in water and wastewater treatment: Role of advanced materials. Mater. Today 2021, 50, 516–532. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Chadha, U.; Selvaraj, S.K.; Vishak Thanu, S.; Cholapadath, V.; Abraham, A.M.; Zaiyan, M.; Manikandan, M.; Paramasivam, V. A review of the function of using carbon nanomaterials in membrane filtration for contaminant removal from wastewater. Mater. Res. Express 2022, 9, 012003. [Google Scholar] [CrossRef]
- Akash, F.A.; Shovon, S.M.; Rahman, W.; Rahman, M.A.; Chakraborty, P.; Prasetya, T.A.E.; Monir, M.U. Advancements in ceramic membrane technology for water and wastewater treatment: A comprehensive exploration of current utilizations and prospective horizons. Desalination Water Treat. 2024, 319, 100569. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Da, X.; Chen, X.; Sun, B.; Wen, J.; Qiu, M.; Fan, Y. Preparation of zirconia nanofiltration membranes through an aqueous sol–gel process modified by glycerol for the treatment of wastewater with high salinity. J. Membr. Sci. 2016, 504, 29–39. [Google Scholar] [CrossRef]
- DeFriend, K.A.; Wiesner, M.R.; Barron, A.R. Alumina and aluminate ultrafiltration membranes derived from alumina nanoparticles. J. Membr. Sci. 2003, 224, 11–28. [Google Scholar] [CrossRef]
- Ewis, D.; Ismail, N.A.; Hafiz, M.; Benamor, A.; Hawari, A.H. Nanoparticles functionalized ceramic membranes: Fabrication, surface modification, and performance. Environ. Sci. Pollut. Res. 2021, 28, 12256–12281. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Z.; Sheng, M.; Qiao, Z.; Wang, J. Scaling up of defect-free flat membrane with ultra-high gas permeance used for intermediate layer of multi-layer composite membrane and oxygen enrichment. Sep. Purif. Technol. 2020, 239, 116580. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired Surfaces with Superwettability: New Insight on Theory, Design, and Applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef]
- Issaoui, M.; Limousy, L. Low-cost ceramic membranes: Synthesis, classifications, and applications. Comptes Rendus Chim. 2019, 22, 175–187. [Google Scholar] [CrossRef]
- Das, N.; Maiti, H.S. Ceramic membrane by tape casting and sol–gel coating for microfiltration and ultrafiltration application. J. Phys. Chem. Solids 2009, 70, 1395–1400. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Rahman, M.A.; Jaafar, J.; Ismail, A.F.; Amin, S.Z.M. Green silica-based ceramic hollow fiber membrane for seawater desalination via direct contact membrane distillation. Sep. Purif. Technol. 2018, 205, 22–31. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Guan, K.; Peng, C.; Wu, J. Freeze-casting of alumina ultra-filtration membranes with good performance for anionic dye separation. Ceram. Int. 2018, 44, 11901–11904. [Google Scholar] [CrossRef]
- Du, Z.; Yao, D.; Xia, Y.; Zuo, K.; Yin, J.; Liang, H.; Zeng, Y.-P. Highly porous silica foams prepared via direct foaming with mixed surfactants and their sound absorption characteristics. Ceram. Int. 2020, 46, 12942–12947. [Google Scholar] [CrossRef]
- Oun, A.; Tahri, N.; Mahouche-Chergui, S.; Carbonnier, B.; Majumdar, S.; Sarkar, S.; Sahoo, G.C.; Ben Amar, R. Tubular ultrafiltration ceramic membrane based on titania nanoparticles immobilized on macroporous clay-alumina support: Elaboration, characterization and application to dye removal. Sep. Purif. Technol. 2017, 188, 126–133. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Gopal, S.; Nambikkattu, J.; Rambabu, K.; Aboulella, A.M.; Ranil Wickramasinghe, S.; Banat, F. Recent developments in porous ceramic membranes for wastewater treatment and desalination: A review. J. Environ. Manag. 2021, 293, 112925. [Google Scholar] [CrossRef]
- Li, Y.; Richardson, J.B.; Mark Bricka, R.; Niu, X.; Yang, H.; Li, L.; Jimenez, A. Leaching of heavy metals from E-waste in simulated landfill columns. Waste Manag. 2009, 29, 2147–2150. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, S.; Wang, Z.; Yu, S. Composite NF membranes with anti-bacterial activity prepared by electrostatic self-assembly for dye recycle. J. Taiwan Inst. Chem. Eng. 2020, 106, 34–50. [Google Scholar] [CrossRef]
- Fujiwara, M.; Imura, T. Photo Induced Membrane Separation for Water Purification and Desalination Using Azobenzene Modified Anodized Alumina Membranes. ACS Nano 2015, 9, 5705–5712. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, A.; Gad, A. Chemical and microstructural analyses for heavy metals removal from water media by ceramic membrane filtration. Water Sci. Technol. 2017, 75, 439–450. [Google Scholar] [CrossRef]
- Asif, M.B.; Ren, B.; Li, C.; Maqbool, T.; Zhang, X.; Zhang, Z. Powdered activated carbon—Membrane bioreactor (PAC-MBR): Impacts of high PAC concentration on micropollutant removal and microbial communities. Sci. Total Environ. 2020, 745, 141090. [Google Scholar] [CrossRef]
- Ağtaş, M.; Dilaver, M.; Koyuncu, İ. Ceramic membrane overview and applications in textile industry: A review. Water Sci. Technol. 2021, 84, 1059–1078. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, X.; Fan, Y.; Xing, W. 1.11 Ceramic Membranes. In Comprehensive Membrane Science and Engineering, 2nd ed.; Drioli, E., Giorno, L., Fontananova, E., Eds.; Elsevier: Oxford, UK, 2017; pp. 270–297. [Google Scholar]
- Zhong, J.; Sun, X.; Wang, C. Treatment of oily wastewater produced from refinery processes using flocculation and ceramic membrane filtration. Sep. Purif. Technol. 2003, 32, 93–98. [Google Scholar] [CrossRef]
- Cui, Z.; Xing, W.; Fan, Y.; Xu, N. Pilot study on the ceramic membrane pre-treatment for seawater desalination with reverse osmosis in Tianjin Bohai Bay. Desalination 2011, 279, 190–194. [Google Scholar] [CrossRef]
- Kim, S.; Park, C. Potential of ceramic ultrafiltration membranes for the treatment of anionic surfactants in laundry wastewater for greywater reuse. J. Water Process Eng. 2021, 44, 102373. [Google Scholar] [CrossRef]
- Ji, Y.; Qian, W.; Yu, Y.; An, Q.; Liu, L.; Zhou, Y.; Gao, C. Recent developments in nanofiltration membranes based on nanomaterials. Chin. J. Chem. Eng. 2017, 25, 1639–1652. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Yuan, S.; Zhou, J.; Wang, B. Challenges and recent advances in MOF-polymer composite membranes for gas separation. Inorg. Chem. Front. 2016, 3, 896–909. [Google Scholar] [CrossRef]
- Yuan, J.; Hung, W.-S.; Zhu, H.; Guan, K.; Ji, Y.; Mao, Y.; Liu, G.; Lee, K.-R.; Jin, W. Fabrication of ZIF-300 membrane and its application for efficient removal of heavy metal ions from wastewater. J. Membr. Sci. 2019, 572, 20–27. [Google Scholar] [CrossRef]
- Cao, J.; Dong, X.; Li, L.; Dong, Y.; Hampshire, S. Recycling of waste fly ash for production of porous mullite ceramic membrane supports with increased porosity. J. Eur. Ceram. Soc. 2014, 34, 3181–3194. [Google Scholar] [CrossRef]
- Al-anzi, B.S.; Siang, O.C. Recent developments of carbon based nanomaterials and membranes for oily wastewater treatment. RSC Adv. 2017, 7, 20981–20994. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed]
- Thines, R.K.; Mubarak, N.M.; Nizamuddin, S.; Sahu, J.N.; Abdullah, E.C.; Ganesan, P. Application potential of carbon nanomaterials in water and wastewater treatment: A review. J. Taiwan Inst. Chem. Eng. 2017, 72, 116–133. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Zhang, L.; Li, S.; Yuan, Y.; Xiao, X.; Fan, X.; Song, C. Carbon-based membrane materials and applications in water and wastewater treatment: A review. Environ. Chem. Lett. 2020, 19, 1457–1475. [Google Scholar] [CrossRef]
- Corry, B. Designing carbon nanotube membranes for efficient water desalination. J. Phys. Chem. B 2008, 112, 1427–1434. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, C.; Seo, D.K.; Kim, T.; Lee, J.S.; Kim, Y.H.; Ahn, K.H.; Bae, S.S.; Lee, S.C.; Lim, J.; et al. High performance and antifouling vertically aligned carbon nanotube membrane for water purification. J. Membr. Sci. 2014, 460, 171–177. [Google Scholar] [CrossRef]
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef]
- Jame, S.A.; Zhou, Z. Electrochemical carbon nanotube filters for water and wastewater treatment. Nanotechnol. Rev. 2016, 5, 41–50. [Google Scholar] [CrossRef]
- Sarkar, B.; Mandal, S.; Tsang, Y.F.; Kumar, P.; Kim, K.H.; Ok, Y.S. Designer carbon nanotubes for contaminant removal in water and wastewater: A critical review. Sci. Total Environ. 2018, 612, 561–581. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, J.; Zhu, C.; Zeng, X.C.; Francisco, J.S. Water desalination through rim functionalized carbon nanotubes. J. Mater. Chem. A 2019, 7, 3583–3591. [Google Scholar] [CrossRef]
- Chen, Q.; Meng, L.; Li, Q.; Wang, D.; Guo, W.; Shuai, Z.; Jiang, L. Water transport and purification in nanochannels controlled by asymmetric wettability. Small 2011, 7, 2225–2231. [Google Scholar] [CrossRef]
- Corry, B. Water and ion transport through functionalised carbon nanotubes: Implications for desalination technology. Energy Environ. Sci. 2011, 4, 751–759. [Google Scholar] [CrossRef]
- Kim, E.S.; Hwang, G.; Gamal El-Din, M.; Liu, Y. Development of nanosilver and multi-walled carbon nanotubes thin-film nanocomposite membrane for enhanced water treatment. J. Membr. Sci. 2012, 394, 37–48. [Google Scholar] [CrossRef]
- Singh, R.; Samuel, M.S.; Ravikumar, M.; Ethiraj, S.; Kirankumar, V.S.; Kumar, M.; Arulvel, R.; Suresh, S. Processing of Carbon-Based Nanomaterials for the Removal of Pollutants from Water/Wastewater Application. Water 2023, 15, 3003. [Google Scholar] [CrossRef]
- Kim, H.W.; Yoon, H.W.; Yoon, S.M.; Yoo, B.M.; Ahn, B.K.; Cho, Y.H.; Shin, H.J.; Yang, H.; Paik, U.; Kwon, S.; et al. Selective gas transport through few-layered graphene and graphene oxide membranes. Science 2013, 342, 91–95. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; Grossman, J.C. Nanoporous graphene as a reverse osmosis membrane: Recent insights from theory and simulation. Desalination 2015, 366, 59–70. [Google Scholar] [CrossRef]
- Kabiri, S.; Tran, D.N.H.; Cole, M.A.; Losic, D. Functionalized three-dimensional (3D) graphene composite for high efficiency removal of mercury. Environ. Sci. Water Res. Technol. 2016, 2, 390–402. [Google Scholar] [CrossRef]
- O’Hern, S.C.; Boutilier, M.S.H.; Idrobo, J.-C.; Song, Y.; Kong, J.; Laoui, T.; Atieh, M.; Karnik, R. Selective Ionic Transport through Tunable Subnanometer Pores in Single-Layer Graphene Membranes. Nano Lett. 2014, 14, 1234–1241. [Google Scholar] [CrossRef]
- An, Y.-C.; Gao, X.-X.; Jiang, W.-L.; Han, J.-L.; Ye, Y.; Chen, T.-M.; Ren, R.-Y.; Zhang, J.-H.; Liang, B.; Li, Z.-L.; et al. A critical review on graphene oxide membrane for industrial wastewater treatment. Environ. Res. 2023, 223, 115409. [Google Scholar] [CrossRef]
- Makhija, G.; Sharma, V.; Singh, S.; Sharma, N.; Vyas, R.; Sachdev, K. Investigation on the suitability of water/polyethylene glycol solutions for GO layer deposition in GO/Ag/GO films for transparent conducting electrode. Appl. Nanosci. 2019, 9, 1671–1683. [Google Scholar] [CrossRef]
- Zhang, D.; Dai, F.; Zhang, P.; An, Z.; Zhao, Y.; Chen, L. The photodegradation of methylene blue in water with PVDF/GO/ZnO composite membrane. Mater. Sci. Eng. C 2019, 96, 684–692. [Google Scholar] [CrossRef]
- Li, G.; Shi, L.; Zeng, G.; Zhang, Y.; Sun, Y. Efficient dehydration of the organic solvents through graphene oxide (GO)/ceramic composite membranes. RSC Adv. 2014, 4, 52012–52015. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, H.; Wang, H.; Rassu, P.; Wang, Z.; Song, P.; Rao, D. Free-standing graphene oxide membrane with tunable channels for efficient water pollution control. J. Hazard. Mater. 2019, 366, 659–668. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, X.; Wei, Y.; Wang, X.; Wang, J.; Zhang, Y.; Gao, C. Enhanced desalination performance of carboxyl functionalized graphene oxide nanofiltration membranes. Desalination 2017, 405, 29–39. [Google Scholar] [CrossRef]
- Gao, P.; Liu, Z.; Sun, D.D.; Ng, W.J. The efficient separation of surfactant-stabilized oil–water emulsions with a flexible and superhydrophilic graphene–TiO2composite membrane. J. Mater. Chem. A 2014, 2, 14082–14088. [Google Scholar] [CrossRef]
- Akhondi, E.; Wu, B.; Sun, S.; Marxer, B.; Lim, W.; Gu, J.; Liu, L.; Burkhardt, M.; McDougald, D.; Pronk, W.; et al. Gravity-driven membrane filtration as pretreatment for seawater reverse osmosis: Linking biofouling layer morphology with flux stabilization. Water Res. 2015, 70, 158–173. [Google Scholar] [CrossRef]
- Wang, W.; Li, R.; Bu, F.; Gao, Y.; Gao, B.; Yue, Q.; Yang, M.; Li, Y. Coagulation and membrane fouling mechanism of Al species in removing humic acid: Effect of pH and a dynamics process analysis. Sep. Purif. Technol. 2023, 309, 123130. [Google Scholar] [CrossRef]
- Kramer, F.C.; Shang, R.; Rietveld, L.C.; Heijman, S.J.G. Influence of pH, multivalent counter ions, and membrane fouling on phosphate retention during ceramic nanofiltration. Sep. Purif. Technol. 2019, 227, 115675. [Google Scholar] [CrossRef]
- Luo, J.; Wan, Y. Effects of pH and salt on nanofiltration—A critical review. J. Membr. Sci. 2013, 438, 18–28. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Susanto, H.; Nasseri, S.; Ulbricht, M. Influences of solution chemistry and polymeric natural organic matter on the removal of aquatic pharmaceutical residuals by nanofiltration. Water Res. 2009, 43, 3270–3280. [Google Scholar] [CrossRef]
- Wang, L.-F.; He, D.-Q.; Chen, W.; Yu, H.-Q. Probing the roles of Ca2+ and Mg2+ in humic acids-induced ultrafiltration membrane fouling using an integrated approach. Water Res. 2015, 81, 325–332. [Google Scholar] [CrossRef]
- Verliefde, A.R.D.; Cornelissen, E.R.; Heijman, S.G.J.; Verberk, J.Q.J.C.; Amy, G.L.; Van der Bruggen, B.; van Dijk, J.C. The role of electrostatic interactions on the rejection of organic solutes in aqueous solutions with nanofiltration. J. Membr. Sci. 2008, 322, 52–66. [Google Scholar] [CrossRef]
- Al-Amoudi, A.S. Factors affecting natural organic matter (NOM) and scaling fouling in NF membranes: A review. Desalination 2010, 259, 1–10. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, W. Efficient faulty variable selection and parsimonious reconstruction modelling for fault isolation. J. Process Control 2016, 38, 31–41. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, Y.; Xu, X.; Peng, X.; Niu, Y.; Xu, P.; Li, T. Research on the factors influencing nanofiltration membrane fouling and the prediction of membrane fouling. J. Water Process Eng. 2024, 59, 104876. [Google Scholar] [CrossRef]
- Su, Z.; Liu, T.; Li, X.; Graham, N.J.D.; Yu, W. Tracking metal ion-induced organic membrane fouling in nanofiltration by adopting spectroscopic methods: Observations and predictions. Sci. Total Environ. 2020, 708, 135051. [Google Scholar] [CrossRef]
- Laitinen, N.; Michaud, D.; Piquet, C.; Teilleria, N.; Luonsi, A.; Levänen, E.; Nyström, M. Effect of filtration conditions and backflushing on ceramic membrane ultrafiltration of board industry wastewaters. Sep. Purif. Technol. 2001, 24, 319–328. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.; Huang, S.; Mai, S.; Yang, C.; Wang, X.; Zhou, Z. Effects of various factors on critical flux in submerged membrane bioreactors for municipal wastewater treatment. Sep. Purif. Technol. 2008, 62, 56–63. [Google Scholar] [CrossRef]
- Ji, X.; Huang, J.; Teng, L.; Li, S.; Li, X.; Cai, W.; Chen, Z.; Lai, Y. Advances in particulate matter filtration: Materials, performance, and application. Green Energy Environ. 2023, 8, 673–697. [Google Scholar] [CrossRef]
- Wu, B.; Hochstrasser, F.; Akhondi, E.; Ambauen, N.; Tschirren, L.; Burkhardt, M.; Fane, A.G.; Pronk, W. Optimization of gravity-driven membrane (GDM) filtration process for seawater pretreatment. Water Res. 2016, 93, 133–140. [Google Scholar] [CrossRef]
- Mao, Y.; Huang, Q.; Meng, B.; Zhou, K.; Liu, G.; Gugliuzza, A.; Drioli, E.; Jin, W. Roughness-enhanced hydrophobic graphene oxide membrane for water desalination via membrane distillation. J. Membr. Sci. 2020, 611, 118364. [Google Scholar] [CrossRef]
- Sun, C.; Lin, B.; Zheng, X.; Dong, Y.; Zhao, M.; Tang, C.Y. Robust ceramic-based graphene membrane for challenging water treatment with enhanced fouling and scaling resistance. Water Res. 2023, 243, 120348. [Google Scholar] [CrossRef]
- Baig, U.; Faizan, M.; Sajid, M. Multifunctional membranes with super-wetting characteristics for oil-water separation and removal of hazardous environmental pollutants from water: A review. Adv. Colloid Interface Sci. 2020, 285, 102276. [Google Scholar] [CrossRef]
- Wae AbdulKadir, W.A.F.; Ahmad, A.L.; Seng, O.B.; Che Lah, N.F. Biomimetic hydrophobic membrane: A review of anti-wetting properties as a potential factor in membrane development for membrane distillation (MD). J. Ind. Eng. Chem. 2020, 91, 15–36. [Google Scholar] [CrossRef]
- Mallya, D.S.; Yang, G.; Lei, W.; Muthukumaran, S.; Baskaran, K. Tuning nanofiltration membrane performance: OH–MoS2 nanosheet engineering and divalent cation influence on fouling and organic removal. Discov. Nano 2023, 18, 131. [Google Scholar] [CrossRef]
- Bortot Coelho, F.E.; Kaiser, N.N.; Magnacca, G.; Candelario, V.M. Corrosion resistant ZrO2/SiC ultrafiltration membranes for wastewater treatment and operation in harsh environments. J. Eur. Ceram. Soc. 2021, 41, 7792–7806. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Reverse osmosis pretreatment technologies and future trends: A comprehensive review. Desalination 2019, 452, 159–195. [Google Scholar] [CrossRef]
- Duan, J.; Wilson, F.; Graham, N.; Tay, J.H. Adsorption of humic acid by powdered activated carbon in saline water conditions. Desalination 2003, 151, 53–66. [Google Scholar] [CrossRef]
- Huang, H.; Schwab, K.; Jacangelo, J.G. Pretreatment for low pressure membranes in water treatment: A review. Environ. Sci. Technol. 2009, 43, 3011–3019. [Google Scholar] [CrossRef]
- Huang, H.; Young, T.A.; Jacangelo, J.G. Chlorine-induced permeability recovery for low-pressure membrane filtration of natural waters. J. Membr. Sci. 2008, 325, 50–57. [Google Scholar] [CrossRef]
- Sakamoto, H.; Hafuka, A.; Tsuchiya, T.; Kimura, K. Intensive routine cleaning for mitigation of fouling in flat-sheet ceramic membranes used for drinking water production: Unique characteristics of resulting foulants. Sep. Purif. Technol. 2022, 301, 121950. [Google Scholar] [CrossRef]
- Hube, S.; Hauser, F.; Burkhardt, M.; Brynjólfsson, S.; Wu, B. Ultrasonication-assisted fouling control during ceramic membrane filtration of primary wastewater under gravity-driven and constant flux conditions. Sep. Purif. Technol. 2023, 310, 123083. [Google Scholar] [CrossRef]
- Ghadimkhani, A.; Zhang, W.; Marhaba, T. Ceramic membrane defouling (cleaning) by air Nano Bubbles. Chemosphere 2016, 146, 379–384. [Google Scholar] [CrossRef]
- Omar, N.M.A.; Othman, M.H.D.; Tai, Z.S.; Milad, M.; Sokri, M.N.M.; Puteh, M.H. Recent progress and technical improvement strategies for mitigating ceramic membrane bottlenecks in water purification processes: A review. Int. J. Appl. Ceram. Technol. 2023, 20, 3327–3356. [Google Scholar] [CrossRef]
- Wu, H.; Sun, C.; Huang, Y.; Zheng, X.; Zhao, M.; Gray, S.; Dong, Y. Treatment of oily wastewaters by highly porous whisker-constructed ceramic membranes: Separation performance and fouling models. Water Res. 2022, 211, 118042. [Google Scholar] [CrossRef]
- Sun, H.; Liu, H.; Han, J.; Zhang, X.; Cheng, F.; Liu, Y. Chemical cleaning-associated generation of dissolved organic matter and halogenated byproducts in ceramic MBR: Ozone versus hypochlorite. Water Res. 2018, 140, 243–250. [Google Scholar] [CrossRef]
- Shang, R.; Verliefde, A.R.; Hu, J.; Zeng, Z.; Lu, J.; Kemperman, A.J.; Deng, H.; Nijmeijer, K.; Heijman, S.G.; Rietveld, L.C. Tight ceramic UF membrane as RO pre-treatment: The role of electrostatic interactions on phosphate rejection. Water Res. 2014, 48, 498–507. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Liu, Z.; Zhang, X. Integration of ferrate (VI) pretreatment and ceramic membrane reactor for membrane fouling mitigation in reclaimed water treatment. J. Membr. Sci. 2018, 552, 315–325. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, H.; Ding, A.; Tang, X.; Liu, B.; Zhu, X.; Gan, Z.; Wu, D.; Li, G. Ferrous iron/peroxymonosulfate oxidation as a pretreatment for ceramic ultrafiltration membrane: Control of natural organic matter fouling and degradation of atrazine. Water Res. 2017, 113, 32–41. [Google Scholar] [CrossRef]
- Mao, H.; Qiu, M.; Chen, X.; Verweij, H.; Fan, Y. Fabrication and in-situ fouling mitigation of a supported carbon nanotube/γ-alumina ultrafiltration membrane. J. Membr. Sci. 2018, 550, 26–35. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, T.; Chang, Q.; Ma, C.; Yang, Y.; Wang, S.; Pan, Z.; Sun, Y.; Ding, G. Performance Stability and Regeneration Property of Catalytic Membranes Coupled with Advanced Oxidation Process: A Comprehensive Review. Sustainability 2023, 15, 7556. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Ait Akbour, R.; Nidheesh, P.V.; Hamdani, M. Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes. Chem. Eng. Process. Process Intensif. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Chang, Q.; Zhou, J.-E.; Wang, Y.; Liang, J.; Zhang, X.; Cerneaux, S.; Wang, X.; Zhu, Z.; Dong, Y. Application of ceramic microfiltration membrane modified by nano-TiO2 coating in separation of a stable oil-in-water emulsion. J. Membr. Sci. 2014, 456, 128–133. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Ghalamchi, L.; Vatanpour, V.; Khataee, A. Photocatalytic-membrane technology: A critical review for membrane fouling mitigation. J. Ind. Eng. Chem. 2021, 93, 101–116. [Google Scholar] [CrossRef]
- Vatanpour, V.; Karami, A.; Sheydaei, M. Central composite design optimization of Rhodamine B degradation using TiO 2 nanoparticles/UV/PVDF process in continuous submerged membrane photoreactor. Chem. Eng. Process. Process Intensif. 2017, 116, 68–75. [Google Scholar] [CrossRef]
- Khataee, A.; Arefi-Oskoui, S.; Abdollahi, B.; Hanifehpour, Y.; Joo, S.W. Synthesis and characterization of Pr x Zn1−x Se nanoparticles for photocatalysis of four textile dyes with different molecular structures. Res. Chem. Intermed. 2015, 41, 8425–8439. [Google Scholar] [CrossRef]
- Adeleke, J.T.; Theivasanthi, T.; Thiruppathi, M.; Swaminathan, M.; Akomolafe, T.; Alabi, A.B. Photocatalytic degradation of methylene blue by ZnO/NiFe2O4 nanoparticles. Appl. Surf. Sci. 2018, 455, 195–200. [Google Scholar] [CrossRef]
- Ghalamchi, L.; Aber, S.; Vatanpour, V.; Kian, M. Development of an antibacterial and visible photocatalytic nanocomposite microfiltration membrane incorporated by Ag3PO4/CuZnAl NLDH. Sep. Purif. Technol. 2019, 226, 218–231. [Google Scholar] [CrossRef]
- Fan, H.; Li, G.; Yang, F.; Yang, L.; Zhang, S. Photodegradation of cellulose under UV light catalysed by TiO2. J. Chem. Technol. Biotechnol. 2011, 86, 1107–1112. [Google Scholar] [CrossRef]
- Huang, T.; Huang, W.; Zhou, C.; Situ, Y.; Huang, H. Superhydrophilicity of TiO2/SiO2 thin films: Synergistic effect of SiO2 and phase-separation-induced porous structure. Surf. Coat. Technol. 2012, 213, 126–132. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Chen, M.; Heijman, S.G.J.; Rietveld, L.C. State-of-the-Art Ceramic Membranes for Oily Wastewater Treatment: Modification and Application. Membranes 2021, 11, 888. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Lai, C.; Wang, D.; Liu, S.; Li, X.; Zhou, X.; Yi, H.; Li, B.; Zhang, M.; Li, L.; et al. In situ chemical oxidation: Peroxide or persulfate coupled with membrane technology for wastewater treatment. J. Mater. Chem. A 2021, 9, 11944–11960. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, B.; Qi, F. A novel ceramic membrane coated with MnO2–Co3O4 nanoparticles catalytic ozonation for benzophenone-3 degradation in aqueous solution: Fabrication, characterization and performance. Chem. Eng. J. 2016, 287, 381–389. [Google Scholar] [CrossRef]
- Szymański, K.; Morawski, A.W.; Mozia, S. Humic acids removal in a photocatalytic membrane reactor with a ceramic UF membrane. Chem. Eng. J. 2016, 305, 19–27. [Google Scholar] [CrossRef]
- Liu, X.; Cao, L.; Sun, W.; Zhou, Z.; Yang, J. A P/N type compounded Cu2O/TiO2 photo-catalytic membrane for organic pollutant degradation. Res. Chem. Intermed. 2016, 42, 6289–6300. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, X.; Chen, S.; Zhao, H.; Zhao, Y. The removal of sodium dodecylbenzene sulfonate surfactant from water using silica/titania nanorods/nanotubes composite membrane with photocatalytic capability. Appl. Surf. Sci. 2006, 252, 8598–8604. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T.; Dona-Rodriguez, J.M.; Romanos, G.E.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef]
- Wang, Z.-b.; Guan, Y.-j.; Chen, B.; Bai, S.-l. Retention and separation of 4BS dye from wastewater by the N-TiO2 ceramic membrane. Desalination Water Treat. 2015, 57, 16963–16969. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Baker, M.A.; Grilli, R.; Lozzi, L.; Di Camillo, D.; Mamane, H. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 2016, 310, 98–107. [Google Scholar] [CrossRef]
- Kandy, M.M.; Gaikar, V.G. Photocatalytic reduction of CO2 using CdS nanorods on porous anodic alumina support. Mater. Res. Bull. 2018, 102, 440–449. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, J.; Kang, P.; Tang, Q.; Sun, Q.; Ma, M. Ag nanocrystals decorated g-C3N4/Nafion hybrid membranes: One-step synthesis and photocatalytic performance. Mater. Lett. 2018, 213, 218–221. [Google Scholar] [CrossRef]
- Isari, A.A.; Payan, A.; Fattahi, M.; Jorfi, S.; Kakavandi, B. Photocatalytic degradation of rhodamine B and real textile wastewater using Fe-doped TiO2 anchored on reduced graphene oxide (Fe-TiO2/rGO): Characterization and feasibility, mechanism and pathway studies. Appl. Surf. Sci. 2018, 462, 549–564. [Google Scholar] [CrossRef]
- Kwon, B.G. Characterization of the hydroperoxyl/superoxide anion radical (HO2/O2−) formed from the photolysis of immobilized TiO2 in a continuous flow. J. Photochem. Photobiol. A Chem. 2008, 199, 112–118. [Google Scholar] [CrossRef]
- Choi, H.; Stathatos, E.; Dionysiou, D.D. Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination 2007, 202, 199–206. [Google Scholar] [CrossRef]
- Kumakiri, I.; Diplas, S.; Simon, C.; Nowak, P. Photocatalytic Membrane Contactors for Water Treatment. Ind. Eng. Chem. Res. 2011, 50, 6000–6008. [Google Scholar] [CrossRef]
- Kirk, C.H.; Wang, P.; Chong, C.Y.D.; Zhao, Q.; Sun, J.; Wang, J. TiO2 photocatalytic ceramic membranes for water and wastewater treatment: Technical readiness and pathway ahead. J. Mater. Sci. Technol. 2024, 183, 152–164. [Google Scholar] [CrossRef]
- Babu, S.G.; Karthik, P.; John, M.C.; Lakhera, S.K.; Ashokkumar, M.; Khim, J.; Neppolian, B. Synergistic effect of sono-photocatalytic process for the degradation of organic pollutants using CuO-TiO2/rGO. Ultrason. Sonochemistry 2019, 50, 218–223. [Google Scholar] [CrossRef]
- Khader, E.H.; Mohammed, T.J.; Albayati, T.M.; Harharah, H.N.; Amari, A.; Saady, N.M.C.; Zendehboudi, S. Current trends for wastewater treatment technologies with typical configurations of photocatalytic membrane reactor hybrid systems: A review. Chem. Eng. Process. Process Intensif. 2023, 192, 109503. [Google Scholar] [CrossRef]
- Jagadevan, S.; Graham, N.J.; Thompson, I.P. Treatment of waste metalworking fluid by a hybrid ozone-biological process. J Hazard. Mater. 2013, 244, 394–402. [Google Scholar] [CrossRef]
- Kim, J.; Davies, S.H.R.; Baumann, M.J.; Tarabara, V.V.; Masten, S.J. Effect of ozone dosage and hydrodynamic conditions on the permeate flux in a hybrid ozonation–ceramic ultrafiltration system treating natural waters. J. Membr. Sci. 2008, 311, 165–172. [Google Scholar] [CrossRef]
- Chiang, Y.P.; Liang, Y.Y.; Chang, C.N.; Chao, A.C. Differentiating ozone direct and indirect reactions on decomposition of humic substances. Chemosphere 2006, 65, 2395–2400. [Google Scholar] [CrossRef]
- Ghuge, S.P.; Saroha, A.K. Catalytic ozonation for the treatment of synthetic and industrial effluents—Application of mesoporous materials: A review. J Environ. Manage. 2018, 211, 83–102. [Google Scholar] [CrossRef]
- Byun, S.; Davies, S.H.; Alpatova, A.L.; Corneal, L.M.; Baumann, M.J.; Tarabara, V.V.; Masten, S.J. Mn oxide coated catalytic membranes for a hybrid ozonation–membrane filtration: Comparison of Ti, Fe and Mn oxide coated membranes for water quality. Water Res. 2011, 45, 163–170. [Google Scholar] [CrossRef]
- Park, H.; Kim, Y.; An, B.; Choi, H. Characterization of natural organic matter treated by iron oxide nanoparticle incorporated ceramic membrane-ozonation process. Water Res. 2012, 46, 5861–5870. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Quan, X.; Chen, S.; Zhang, Y. Ceramic membrane separation coupled with catalytic ozonation for tertiary treatment of dyestuff wastewater in a pilot-scale study. Chem. Eng. J. 2016, 301, 19–26. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, H.; Qu, F.; Ding, A.; Chang, H.; Liu, B.; Tang, X.; Wu, D.; Li, G. Fabrication of Mn oxide incorporated ceramic membranes for membrane fouling control and enhanced catalytic ozonation of p -chloronitrobenzene. Chem. Eng. J. 2017, 308, 1010–1020. [Google Scholar] [CrossRef]
- Chen, S.; Yu, J.; Wang, H.; Yu, H.; Quan, X. A pilot-scale coupling catalytic ozonation–membrane filtration system for recirculating aquaculture wastewater treatment. Desalination 2015, 363, 37–43. [Google Scholar] [CrossRef]
- Guo, Y.; Song, Z.; Xu, B.; Li, Y.; Qi, F.; Croue, J.P.; Yuan, D. A novel catalytic ceramic membrane fabricated with CuMn(2)O(4) particles for emerging UV absorbers degradation from aqueous and membrane fouling elimination. J Hazard. Mater. 2018, 344, 1229–1239. [Google Scholar] [CrossRef]
- Lee, W.J.; Bao, Y.; Hu, X.; Lim, T.-T. Hybrid catalytic ozonation-membrane filtration process with CeOx and MnOx impregnated catalytic ceramic membranes for micropollutants degradation. Chem. Eng. J. 2019, 378, 121670. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, K.; Wang, H.; Wang, X.-m.; Zhang, X.-h.; Huang, X. Incorporating catalytic ceramic membrane into the integrated process of in situ ozonation, membrane filtration and biological degradation: Enhanced performance and underlying mechanisms. J. Membr. Sci. 2022, 652, 120509. [Google Scholar] [CrossRef]
- Li, P.; Miao, R.; Wang, P.; Sun, F.; Li, X.-y. Bi-metal oxide-modified flat-sheet ceramic membranes for catalytic ozonation of organic pollutants in wastewater treatment. Chem. Eng. J. 2021, 426, 131263. [Google Scholar] [CrossRef]
- Szymański, K.; Mozia, S.; Ayral, A.; Brosillon, S.; Mendret, J. Hybrid system coupling ozonation and nanofiltration with functionalized catalytic ceramic membrane for ibuprofen removal. Environ. Sci. Pollut. Res. 2023, 30, 69042–69053. [Google Scholar] [CrossRef]
- Sui, M.; Sheng, L.; Lu, K.; Tian, F. FeOOH catalytic ozonation of oxalic acid and the effect of phosphate binding on its catalytic activity. Appl. Catal. B Environ. 2010, 96, 94–100. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, H.; Peng, P.; Bo, H. Degradation and transformation of atrazine under catalyzed ozonation process with TiO2 as catalyst. J Hazard. Mater. 2014, 279, 444–451. [Google Scholar] [CrossRef]
- Liu, J.; He, K.; Zhang, J.; Li, C.; Zhang, Z. Coupling ferrate pretreatment and in-situ ozonation/ceramic membrane filtration for wastewater reclamation: Water quality and membrane fouling. J. Membr. Sci. 2019, 590, 117310. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Jiang, P.; Wang, G.; Zhang, J.; Zhang, C. ZnAl2O4 as a novel high-surface-area ozonation catalyst: One-step green synthesis, catalytic performance and mechanism. Chem. Eng. J. 2015, 260, 623–630. [Google Scholar] [CrossRef]
- Masten, S.J.; Tarabara, V.V.; Corneal, L.M.; Byun, S.; Baumann, M.J.; Davies, S.H. Fabrication of catalytic ceramic membranes for water filtration. Water Supply 2010, 10, 81–86. [Google Scholar] [CrossRef]
- Chen, X.; Ma, J.; Chen, J.; Wang, Z. Ceramic membrane filtration coupled with ozonation for water purification: Principles, applications and perspectives. J. Water Process Eng. 2023, 55, 104127. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M.; Gohari, F. Degradation of 2,4,6-trichlorophenol in aqueous solutions using peroxymonosulfate/activated carbon/UV process via sulfate and hydroxyl radicals. J. Water Process Eng. 2016, 9, 22–28. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Xiao, H.; Hong, C.; Zhu, F.; Yao, Y.; Xue, Z. Accelerated TiO2 photocatalytic degradation of Acid Orange 7 under visible light mediated by peroxymonosulfate. Chem. Eng. J. 2012, 193, 290–295. [Google Scholar] [CrossRef]
- Furman, O.S.; Teel, A.L.; Ahmad, M.; Merker, M.C.; Watts, R.J. Effect of Basicity on Persulfate Reactivity. J. Environ. Eng. 2011, 137, 241–247. [Google Scholar] [CrossRef]
- Zou, J.; Ma, J.; Chen, L.; Li, X.; Guan, Y.; Xie, P.; Pan, C. Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting Fe(III)/Fe(II) cycle with hydroxylamine. Environ. Sci. Technol. 2013, 47, 11685–11691. [Google Scholar] [CrossRef]
- Luo, X.; Liang, H.; Qu, F.; Ding, A.; Cheng, X.; Tang, C.Y.; Li, G. Free-standing hierarchical alpha-MnO(2)@CuO membrane for catalytic filtration degradation of organic pollutants. Chemosphere 2018, 200, 237–247. [Google Scholar] [CrossRef]
- Bao, Y.; Oh, W.-D.; Lim, T.-T.; Wang, R.; Webster, R.D.; Hu, X. Surface-nucleated heterogeneous growth of zeolitic imidazolate framework—A unique precursor towards catalytic ceramic membranes: Synthesis, characterization and organics degradation. Chem. Eng. J. 2018, 353, 69–79. [Google Scholar] [CrossRef]
- Bao, Y.; Lim, T.-T.; Wang, R.; Webster, R.D.; Hu, X. Urea-assisted one-step synthesis of cobalt ferrite impregnated ceramic membrane for sulfamethoxazole degradation via peroxymonosulfate activation. Chem. Eng. J. 2018, 343, 737–747. [Google Scholar] [CrossRef]
- Wang, S.; Tian, J.; Wang, Q.; Xiao, F.; Gao, S.; Shi, W.; Cui, F. Development of CuO coated ceramic hollow fiber membrane for peroxymonosulfate activation: A highly efficient singlet oxygen-dominated oxidation process for bisphenol a degradation. Appl. Catal. B Environ. 2019, 256, 117783. [Google Scholar] [CrossRef]
- Chen, L.; Maqbool, T.; Nazir, G.; Hou, C.; Yang, Y.; Guo, J.; Zhang, X. Developing the large-area manganese-based catalytic ceramic membrane for peroxymonosulfate activation: Applications in degradation of endocrine disrupting compounds in drinking water. J. Membr. Sci. 2022, 655, 120602. [Google Scholar] [CrossRef]
- Hirani, R.A.K.; Wu, H.; Asif, A.H.; Rafique, N.; Shi, L.; Zhang, S.; Wu, Z.; Zhang, L.-C.; Wang, S.; Yin, Y.; et al. Cobalt oxide functionalized ceramic membrane for 4-hydroxybenzoic acid degradation via peroxymonosulfate activation. J. Hazard. Mater. 2023, 448, 130874. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Y.; Guan, Z.; Li, Q.; Xia, D. Development of CuCo2O4 integrated ceramic membrane for peroxymonosulfate activation: An efficient oxidation process for bisphenol A degradation. J. Environ. Chem. Eng. 2023, 11, 109500. [Google Scholar] [CrossRef]
- Wang, Z.; Nengzi, L.-c.; Zhang, X.; Zhao, Z.; Cheng, X. Novel NiCo2S4/CS membranes as efficient catalysts for activating persulfate and its high activity for degradation of nimesulide. Chem. Eng. J. 2020, 381, 122571. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, H.; Duan, X.; Sun, H.; Tan, X.; Liu, S.; Wang, S. Magnetic Ni-Co alloy encapsulated N-doped carbon nanotubes for catalytic membrane degradation of emerging contaminants. Chem. Eng. J. 2019, 362, 251–261. [Google Scholar] [CrossRef]
- Sheng, J.; Yin, H.; Qian, F.; Huang, H.; Gao, S.; Wang, J. Reduced graphene oxide-based composite membranes for in-situ catalytic oxidation of sulfamethoxazole operated in membrane filtration. Sep. Purif. Technol. 2020, 236, 116275. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Yu, S.; Chen, B.; Chen, C.; Shen, L.; Li, B.; Lin, H. The coupling of persulfate activation and membrane separation for the effective pollutant degradation and membrane fouling alleviation. Chem. Eng. J. 2023, 451, 139009. [Google Scholar] [CrossRef]
- Liangdy, A.; Lee, W.J.; Bao, Y.; Oh, W.-D.; Lim, T.-T. Hybrid process of persulfate-based advanced oxidation with MeOx-functionalized catalytic ceramic membrane for synergistic removal of micropollutants: Recent developments, new insights, and prospects. Chem. Eng. J. 2023, 466, 143280. [Google Scholar] [CrossRef]
- Shan, H.; Dong, X.; Cheng, X.; Si, Y.; Yu, J.; Ding, B. Highly flexible, mesoporous structured, and metallic Cu-doped C/SiO2 nanofibrous membranes for efficient catalytic oxidative elimination of antibiotic pollutants. Nanoscale 2019, 11, 14844–14856. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, D.; Xu, C.; Zhong, J.; Chen, M.; Xu, S.; Cao, Y.; Zhao, Q.; Yang, M.; Ma, J. Synergistic oxidation—Filtration process analysis of catalytic CuFe2O4—Tailored ceramic membrane filtration via peroxymonosulfate activation for humic acid treatment. Water Res. 2020, 171, 115387. [Google Scholar] [CrossRef]
- Wu, H.; Xu, X.; Shi, L.; Yin, Y.; Zhang, L.C.; Wu, Z.; Duan, X.; Wang, S.; Sun, H. Manganese oxide integrated catalytic ceramic membrane for degradation of organic pollutants using sulfate radicals. Water Res. 2019, 167, 115110. [Google Scholar] [CrossRef]
- Fan, X.; Li, S.; Sun, M.; Song, C.; Xiao, J.; Du, J.; Tao, P.; Sun, T.; Shao, M.; Wang, T. Degradation of phenol by coal-based carbon membrane integrating sulfate radicals-based advanced oxidation processes. Ecotoxicol. Environ. Saf. 2019, 185, 109662. [Google Scholar] [CrossRef]
- Wang, Y.; Ao, Z.; Sun, H.; Duan, X.; Wang, S. Activation of peroxymonosulfate by carbonaceous oxygen groups: Experimental and density functional theory calculations. Appl. Catal. B Environ. 2016, 198, 295–302. [Google Scholar] [CrossRef]
- Yu, C.; Xiong, Z.; Zhou, H.; Zhou, P.; Zhang, H.; Huang, R.; Yao, G.; Lai, B. Marriage of membrane filtration and sulfate radical-advanced oxidation processes (SR-AOPs) for water purification: Current developments, challenges and prospects. Chem. Eng. J. 2022, 433, 133802. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, K.; Zhou, Y.; Yu, W.; Tao, S.; Le, C.; Lu, D.; Yu, Z.; Liang, S.; Hu, J.; et al. Profiling of amino acids and their interactions with proteinaceous compounds for sewage sludge dewatering by Fenton oxidation treatment. Water Res. 2020, 175, 115645. [Google Scholar] [CrossRef]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Effect of matrix components on UV/H2O2 and UV/S2O82− advanced oxidation processes for trace organic degradation in reverse osmosis brines from municipal wastewater reuse facilities. Water Res. 2016, 89, 192–200. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J. An overview of the application of Fenton oxidation to industrial wastewaters treatment. J. Chem. Technol. Biotechnol. 2008, 83, 1323–1338. [Google Scholar] [CrossRef]
- Yang, T.; Yu, D.; Wang, D.; Yang, T.; Li, Z.; Wu, M.; Petru, M.; Crittenden, J. Accelerating Fe(III)/Fe(II) cycle via Fe(II) substitution for enhancing Fenton-like performance of Fe-MOFs. Appl. Catal. B Environ. 2021, 286, 119859. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Sun, J.; Wang, Y.; Zhao, X. Electrospun flexible self-standing Cu–Al2O3 fibrous membranes as Fenton catalysts for bisphenol A degradation. J. Mater. Chem. A 2017, 5, 19151–19158. [Google Scholar] [CrossRef]
- Wang, X.; Dou, L.; Yang, L.; Yu, J.; Ding, B. Hierarchical structured MnO2@SiO2 nanofibrous membranes with superb flexibility and enhanced catalytic performance. J. Hazard. Mater. 2017, 324, 203–212. [Google Scholar] [CrossRef]
- Shi, F.; Shan, H.; Li, D.; Yin, X.; Yu, J.; Ding, B. A general strategy to fabricate soft magnetic CuFe(2)O(4)@SiO(2) nanofibrous membranes as efficient and recyclable Fenton-like catalysts. J. Colloid Interface Sci. 2019, 538, 620–629. [Google Scholar] [CrossRef]
- Plakas, K.V.; Mantza, A.; Sklari, S.D.; Zaspalis, V.T.; Karabelas, A.J. Heterogeneous Fenton-like oxidation of pharmaceutical diclofenac by a catalytic iron-oxide ceramic microfiltration membrane. Chem. Eng. J. 2019, 373, 700–708. [Google Scholar] [CrossRef]
- Jiang, S.; Zhao, Z.; Cui, K.; Tang, Y.; Du, X.; He, B.; Li, M.; Feng, J.; Yu, B.; Xiong, W. Catalytic wet peroxide oxidation of phenolic wastewater on novel Cu/Mn-UiO-66@Al2O3 ceramic tube membrane catalysts. Chem. Eng. J. 2022, 430, 132787. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Yang, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Graphene oxide/Fe(III)-based metal-organic framework membrane for enhanced water purification based on synergistic separation and photo-Fenton processes. Appl. Catal. B Environ. 2020, 264, 118548. [Google Scholar] [CrossRef]
- Li, Q.; Kong, H.; Li, P.; Shao, J.; He, Y. Photo-Fenton degradation of amoxicillin via magnetic TiO2-graphene oxide-Fe3O4 composite with a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). J. Hazard. Mater. 2019, 373, 437–446. [Google Scholar] [CrossRef]
- Jiang, W.-L.; Xia, X.; Han, J.-L.; Ding, Y.-C.; Haider, M.R.; Wang, A.-J. Graphene Modified Electro-Fenton Catalytic Membrane for in Situ Degradation of Antibiotic Florfenicol. Environ. Sci. Technol. 2018, 52, 9972–9982. [Google Scholar] [CrossRef]
- Huong Le, T.X.; Dumée, L.F.; Lacour, S.; Rivallin, M.; Yi, Z.; Kong, L.; Bechelany, M.; Cretin, M. Hybrid graphene-decorated metal hollow fibre membrane reactors for efficient electro-Fenton— Filtration co-processes. J. Membr. Sci. 2019, 587, 117182. [Google Scholar] [CrossRef]
- Li, Z.; Shen, C.; Liu, Y.; Ma, C.; Li, F.; Yang, B.; Huang, M.; Wang, Z.; Dong, L.; Wolfgang, S. Carbon nanotube filter functionalized with iron oxychloride for flow-through electro-Fenton. Appl. Catal. B Environ. 2020, 260, 118204. [Google Scholar] [CrossRef]
- Sharma, V.K.; Feng, M. Water depollution using metal-organic frameworks-catalyzed advanced oxidation processes: A review. J. Hazard. Mater. 2019, 372, 3–16. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, S.; Xiong, W.; Liu, D.; Li, M.; He, B.; Fan, X.; Luo, D. Supported CuO catalysts on metal-organic framework (Cu-UiO-66) for efficient catalytic wet peroxide oxidation of 4-chlorophenol in wastewater. Microporous Mesoporous Mater. 2020, 291, 109703. [Google Scholar] [CrossRef]
- Ahile, U.J.; Wuana, R.A.; Itodo, A.U.; Sha’Ato, R.; Dantas, R.F. A review on the use of chelating agents as an alternative to promote photo-Fenton at neutral pH: Current trends, knowledge gap and future studies. Sci. Total Environ. 2020, 710, 134872. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Li, Y.; Chen, X.; Yao, Y.; Zhang, P.; Ren, S.; Li, M.; Luo, Y.; Chen, N.; et al. Heterogeneous electro-Fenton catalytic FeOCl@NCNT/ceramic membrane filtration for in-situ membrane fouling control: Performance and mechanism. Sep. Purif. Technol. 2023, 316, 123845. [Google Scholar] [CrossRef]
- Jiang, W.-L.; Ding, Y.-C.; Haider, M.R.; Han, J.-L.; Liang, B.; Xia, X.; Yang, L.-M.; Wang, H.-c.; Peng, Y.-Z.; Wang, A.-J. A novel TiO2/graphite felt photoanode assisted electro-Fenton catalytic membrane process for sequential degradation of antibiotic florfenicol and elimination of its antibacterial activity. Chem. Eng. J. 2020, 391, 123503. [Google Scholar] [CrossRef]
- Inchaurrondo, N.; Font, J.; Ramos, C.P.; Haure, P. Natural diatomites: Efficient green catalyst for Fenton-like oxidation of Orange II. Appl. Catal. B Environ. 2016, 181, 481–494. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, S.; Meng, X.; Yu, S. Research progress of MOF-based membrane reactor coupled with AOP technology for organic wastewater treatment. Environ. Sci. Pollut. Res. 2023, 30, 104958–104975. [Google Scholar] [CrossRef]
- Ye, Z.; Oriol, R.; Yang, C.; Sirés, I.; Li, X.-Y. A novel NH2-MIL-88B(Fe)-modified ceramic membrane for the integration of electro-Fenton and filtration processes: A case study on naproxen degradation. Chem. Eng. J. 2022, 433, 133547. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.; Wang, H.; Wang, Z.; Zeng, G.; Xu, P.; Huang, D.; Chen, M.; Song, B.; Qin, H.; et al. Metal-organic framework-derived nanomaterials in environment related fields: Fundamentals, properties and applications. Coord. Chem. Rev. 2021, 429, 213618. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, S.; Zhou, J.; Quan, X. A novel porous-carbon-based hollow fiber membrane with electrochemical reduction mediated by in-situ hydroxyl radical generation for fouling control and water treatment. Appl. Catal. B Environ. 2019, 255, 117772. [Google Scholar] [CrossRef]
- Zhu, X.; Jassby, D. Electroactive Membranes for Water Treatment: Enhanced Treatment Functionalities, Energy Considerations, and Future Challenges. Acc. Chem. Res. 2019, 52, 1177–1186. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical technologies for wastewater treatment and resource reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Li, C.; Guo, X.; Wang, X.; Fan, S.; Zhou, Q.; Shao, H.; Hu, W.; Li, C.; Tong, L.; Kumar, R.R.; et al. Membrane fouling mitigation by coupling applied electric field in membrane system: Configuration, mechanism and performance. Electrochim. Acta 2018, 287, 124–134. [Google Scholar] [CrossRef]
- Tao, P.; Xu, Y.; Song, C.; Yin, Y.; Yang, Z.; Wen, S.; Wang, S.; Liu, H.; Li, S.; Li, C.; et al. A novel strategy for the removal of rhodamine B (RhB) dye from wastewater by coal-based carbon membranes coupled with the electric field. Sep. Purif. Technol. 2017, 179, 175–183. [Google Scholar] [CrossRef]
- Sun, M.; Feng, G.; Zhang, M.; Song, C.; Tao, P.; Wang, T.; Shao, M. Enhanced removal ability of phenol from aqueous solution using coal-based carbon membrane coupled with electrochemical oxidation process. Colloids Surf. A Physicochem. Eng. Asp. 2018, 540, 186–193. [Google Scholar] [CrossRef]
- Li, C.; Feng, G.; Pan, Z.; Song, C.; Fan, X.; Tao, P.; Wang, T.; Shao, M.; Zhao, S. High-performance electrocatalytic microfiltration CuO/Carbon membrane by facile dynamic electrodeposition for small-sized organic pollutants removal. J. Membr. Sci. 2020, 601, 117913. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Mansas, C.; Mendret, J.; Brosillon, S.; Ayral, A. Coupling catalytic ozonation and membrane separation: A review. Sep. Purif. Technol. 2020, 236, 116221. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Bao, C.; Xu, X.; Li, D.; Chen, J.; Hong, M.; Peng, B.; Zhang, Q. Self-cleaning catalytic membrane for water treatment via an integration of Heterogeneous Fenton and membrane process. J. Membr. Sci. 2021, 624, 119121. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Ismail, A.F.; Rahman, M.A.; Harun, Z.; Jaafar, J.; Nomura, M. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review. Ceram. Int. 2018, 44, 4538–4560. [Google Scholar] [CrossRef]
- Jeong, Y.; Cho, K.; Kwon, E.E.; Tsang, Y.F.; Rinklebe, J.; Park, C. Evaluating the feasibility of pyrophyllite-based ceramic membranes for treating domestic wastewater in anaerobic ceramic membrane bioreactors. Chem. Eng. J. 2017, 328, 567–573. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K.; Kwiecińska-Mydlak, A. New generation of semipermeable membranes with carbon nanotubes for water and wastewater treatment: Critical review. Arch. Environ. Prot. 2021, 47, 3–27. [Google Scholar] [CrossRef]
- Yi, Q.; Li, Z.; Li, J.; Zhou, J.; Li, X.; Dai, R.; Wang, X. Enhancing oxidants activation by transition metal-modified catalytic membranes for wastewater treatment. Res. Chem. Intermed. 2023, 49, 655–678. [Google Scholar] [CrossRef]
- Dong, Z.; Shang, W.; Dong, W.; Zhao, L.; Li, M.; Wang, R.; Sun, F. Suppression of membrane fouling in the ceramic membrane bioreactor (CMBR) by minute electric field. Bioresour. Technol. 2018, 270, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiao, S.; Jin, R.; Zhou, J.; Quan, X. Novel Anaerobic Electrochemical Membrane Bioreactor with a CNTs Hollow Fiber Membrane Cathode to Mitigate Membrane Fouling and Enhance Energy Recovery. Environ. Sci. Technol. 2019, 53, 1014–1021. [Google Scholar] [CrossRef]
- Duan, W.; Ronen, A.; de Leon, J.V.; Dudchenko, A.; Yao, S.; Corbala-Delgado, J.; Yan, A.; Matsumoto, M.; Jassby, D. Treating anaerobic sequencing batch reactor effluent with electrically conducting ultrafiltration and nanofiltration membranes for fouling control. J. Membr. Sci. 2016, 504, 104–112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).