Carbon-Based Materials in Combined Adsorption/Ozonation for Indigo Dye Decolorization in Constrain Contact Time
Abstract
1. Introduction
2. Results and Discussion
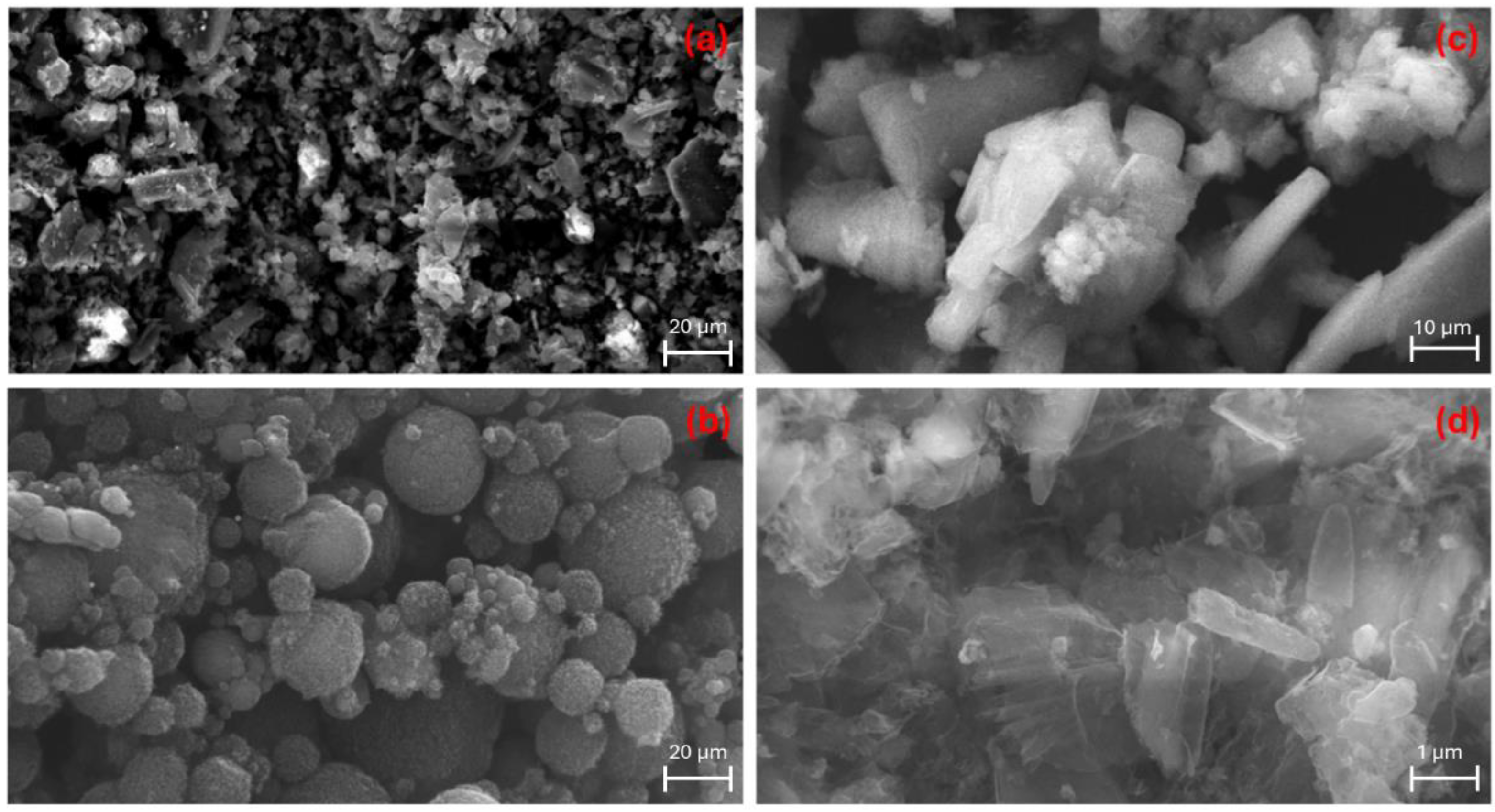
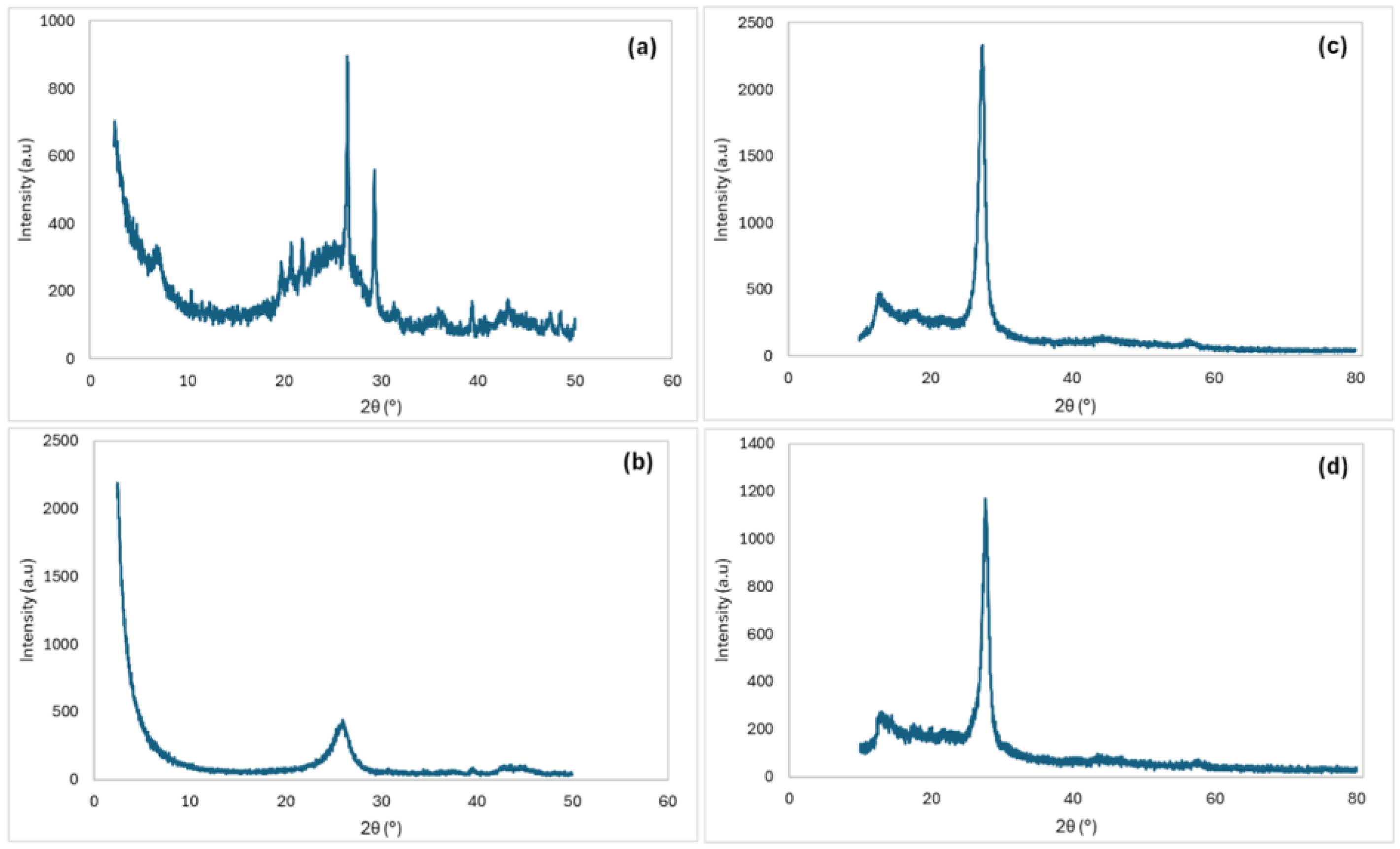
2.1. Material Characterization
2.2. Adsorption Studies
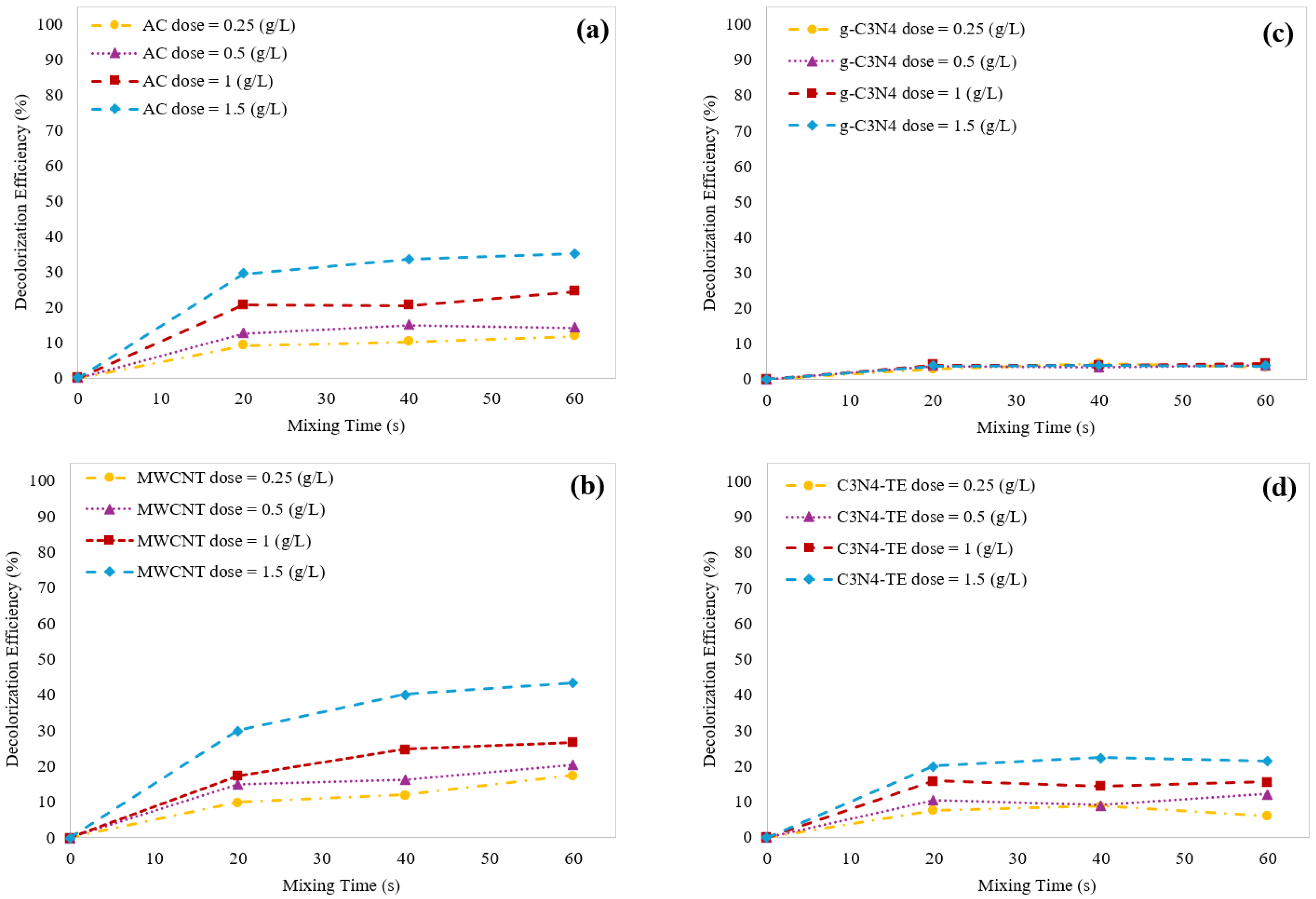
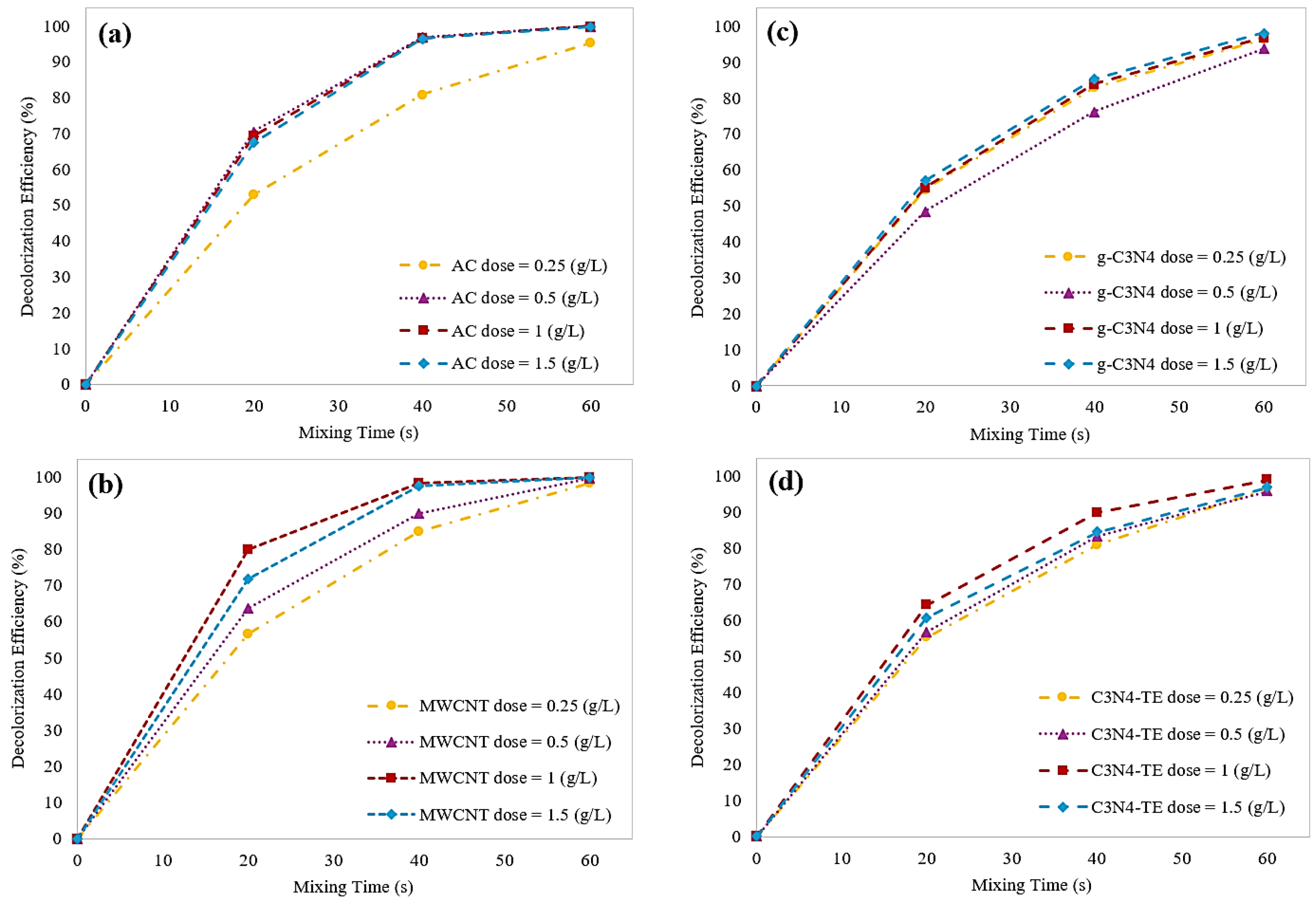
2.2.1. Effect of Adsorbent Dosage on Decolorization Efficiency
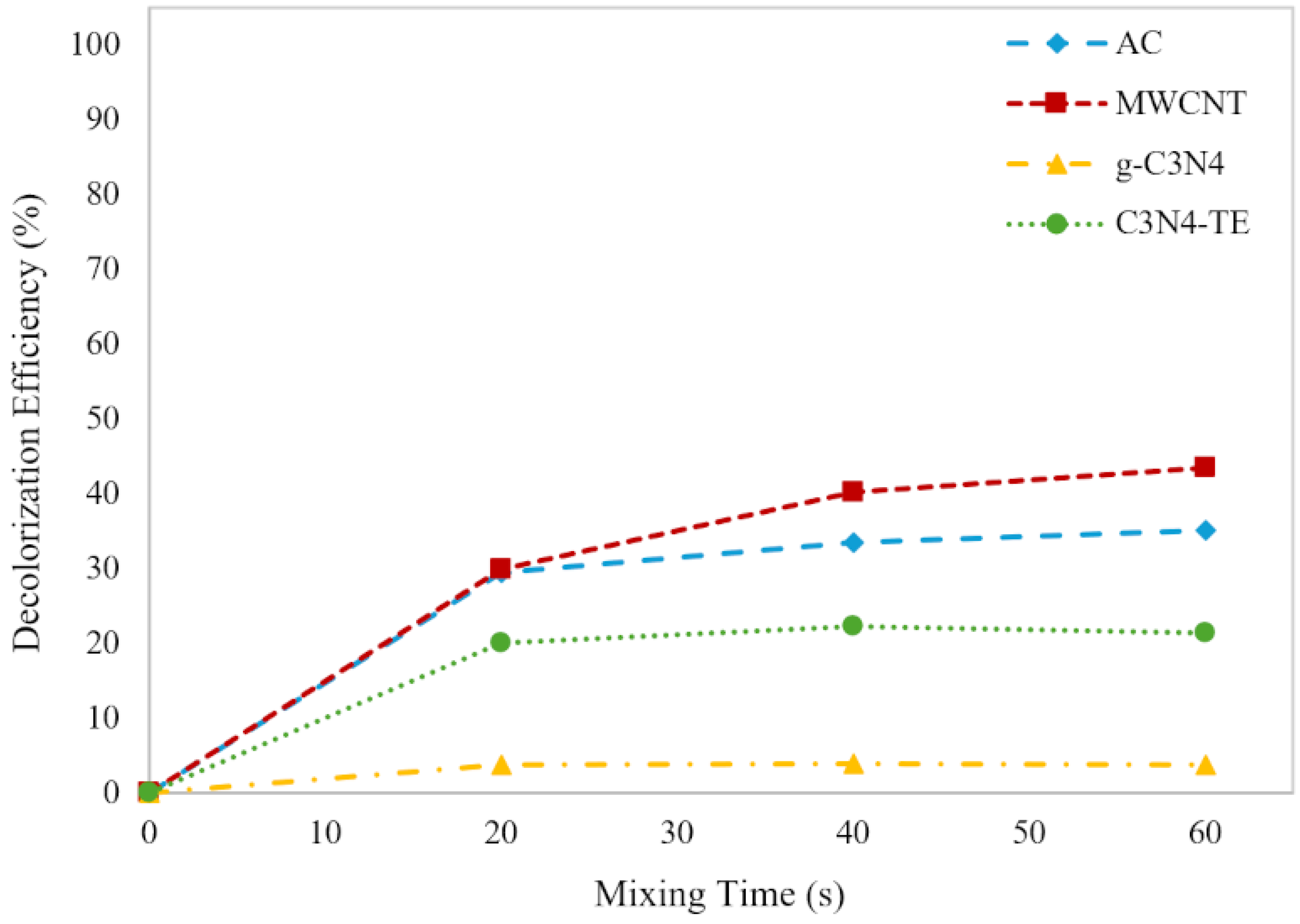
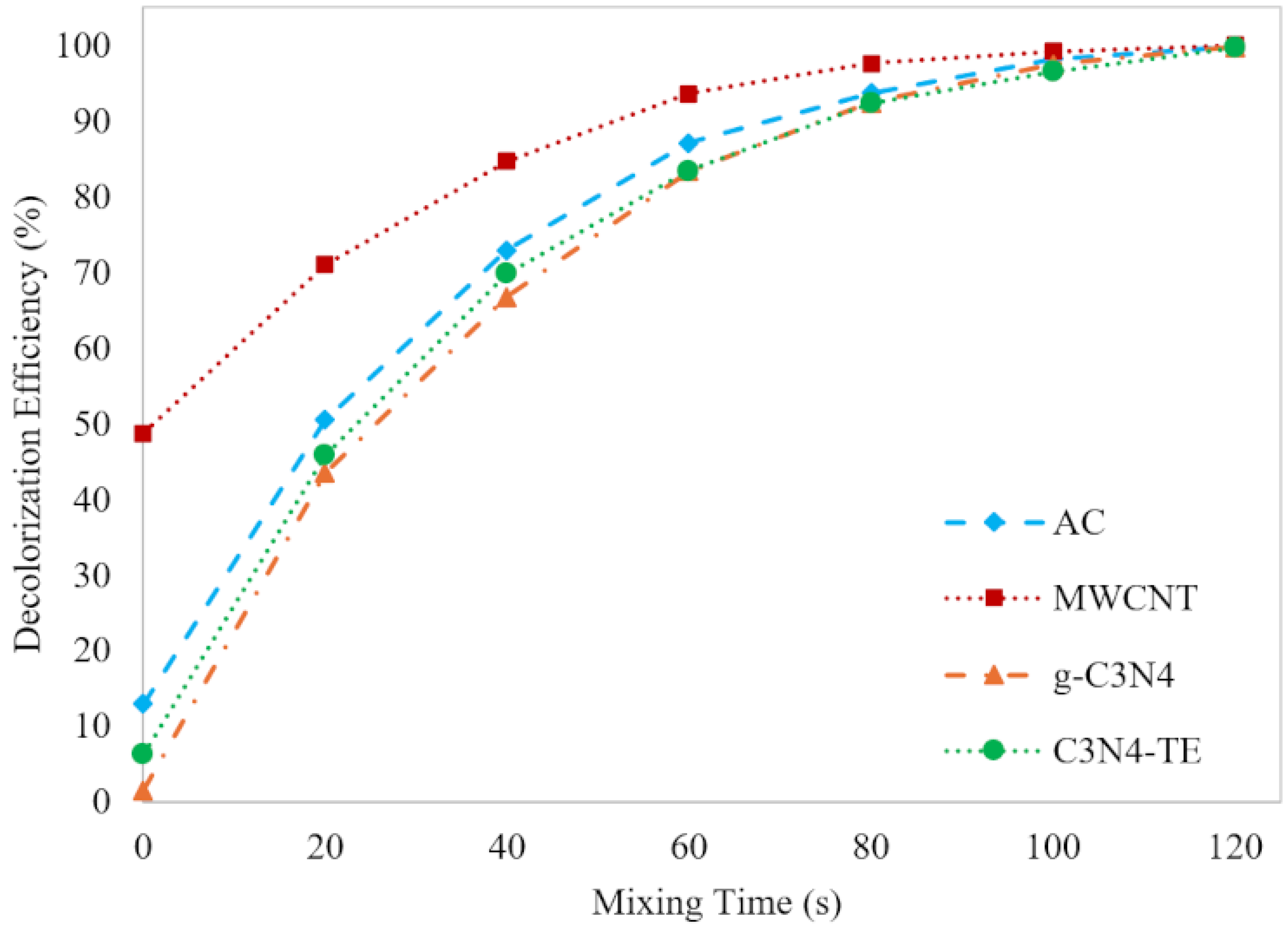
2.2.2. Adsorption Efficiency Comparison
2.3. Catalytic Ozonation Studies
2.3.1. Catalytic Ozonation Efficiency Comparison
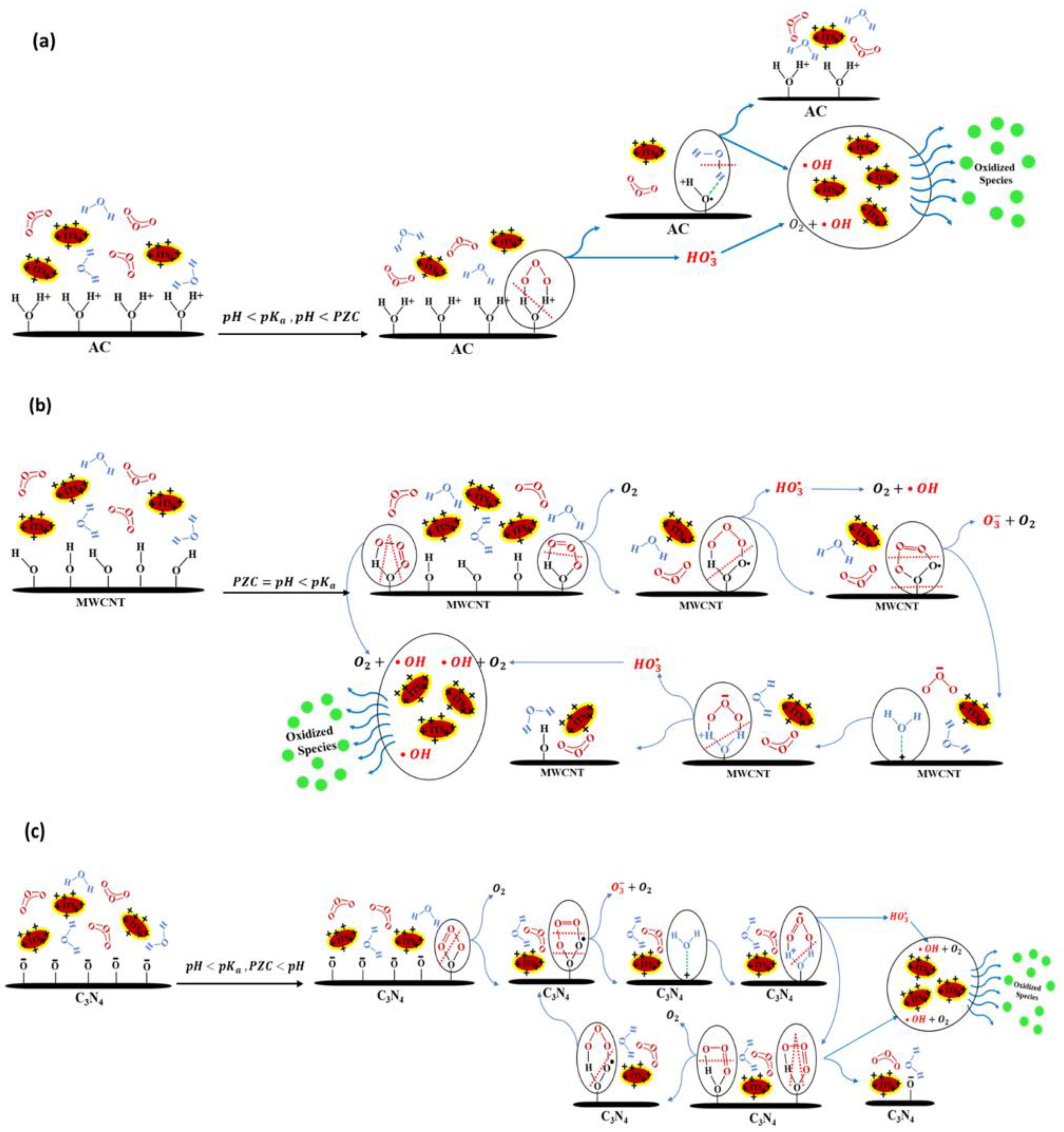
2.3.2. Catalytic Ozonation Mechanisms
2.3.3. Effect of Catalyst Dosage on Decolorization Efficiency
3. Materials and Methods
3.1. Materials
3.2. Characterization Techniques of the Catalysts
3.3. Experimental Procedures
3.3.1. Dye Solution Preparation
3.3.2. Short-Term Adsorption Experiment
3.3.3. Catalytic Ozonation Process
3.3.4. pKa Determination of ITS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McMullan, G.; Meehan, C.; Conneely, A.; Kirby, N.; Robinson, T.; Nigam, P.; Banat, I.M.; Marchant, R.; Smyth, W.F. Microbial decolourisation and degradation of textile dyes. Appl. Microbiol. Biotechnol. 2001, 56, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Roessler, A.; Jin, X. State of the art technologies and new electrochemical methods for the reduction of vat dyes. Dye. Pigment. 2003, 59, 223–235. [Google Scholar] [CrossRef]
- Irimia-Vladu, M.; Głowacki, E.D.; Troshin, P.A.; Schwabegger, G.; Leonat, L.; Susarova, D.K.; Krystal, O.; Ullah, M.; Kanbur, Y.; Bodea, M.A.; et al. Indigo—A Natural Pigment for High Performance Ambipolar Organic Field Effect Transistors and Circuits. Adv. Mater. 2012, 24, 375–380. [Google Scholar] [CrossRef]
- Unlu, M.; Yukseler, H.; Yetis, U. Indigo dyeing wastewater reclamation by membrane-based filtration and coagulation processes. Desalination 2009, 240, 178–185. [Google Scholar] [CrossRef]
- Uzal, N.; Yilmaz, L.; Yetis, U. Microfiltration: A pretreatment alternative for indigo dyeing textile wastewater. Desalination 2006, 199, 515–517. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Tabrizi, M.T.F.; Glasser, D.; Hildebrandt, D. Wastewater treatment of reactive dyestuffs by ozonation in a semi-batch reactor. Chem. Eng. J. 2011, 166, 662–668. [Google Scholar] [CrossRef]
- Wang, S. A Comparative study of Fenton and Fenton-like reaction kinetics in decolourisation of wastewater. Dye. Pigment. 2008, 76, 714–720. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Tabrizi, N.S. Decolorization and aromatic ring degradation kinetics of Direct Red 80 by UV oxidation in the presence of hydrogen peroxide utilizing TiO2 as a photocatalyst. Chem. Eng. J. 2005, 112, 191–196. [Google Scholar] [CrossRef]
- Beltrán, F.J. Ozone Reaction Kinetics for Water and Wastewater Systems; Lewis, P.A., Ed.; CRC Press: London, UK, 2004; pp. 1–358. [Google Scholar]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Wang, F.; Xiong, X.; Tian, K.; Sun, Y.; Yu, T. Application of Heterogeneous Catalytic Ozonation for Refractory Organics in Wastewater. Catalysts 2019, 9, 241. [Google Scholar] [CrossRef]
- Li, L.; Zhu, W.; Zhang, P.; Zhang, Z.; Wu, H.; Han, W. Comparison of AC/O3–BAC and O3–BAC processes for removing organic pollutants in secondary effluent. Chemosphere 2006, 62, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Alvárez, P.M.; García-Araya, J.F.; Beltrán, F.J.; Giráldez, I.; Jaramillo, J.; Gómez-Serrano, V. The influence of various factors on aqueous ozone decomposition by granular activated carbons and the development of a mechanistic approach. Carbon 2006, 44, 3102–3112. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Ma, J.; Cui, Y.-H.; Zhang, B.-P. Effect of ozonation pretreatment on the surface properties and catalytic activity of multi-walled carbon nanotube. Appl. Catal. B Environ. 2009, 92, 301–306. [Google Scholar] [CrossRef]
- Gonçalves, A.G.; Órfão, J.J.M.; Pereira, M.F.R. Catalytic ozonation of sulphamethoxazole in the presence of carbon materials: Catalytic performance and reaction pathways. J. Hazard. Mater. 2012, 239–240, 167–174. [Google Scholar] [CrossRef]
- Gonçalves, A.; Órfão, J.J.; Pereira, M.F.R. Ozonation of bezafibrate promoted by carbon materials. Appl. Catal. B Environ. 2013, 140–141, 82–91. [Google Scholar] [CrossRef]
- Gonçalves, A.G.; Órfão, J.J.M.; Pereira, M.F.R. Ozonation of erythromycin over carbon materials and ceria dispersed on carbon materials. Chem. Eng. J. 2014, 250, 366–376. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Y.; Liu, C.; Xu, B.; Qi, F.; Yuan, D.; Pu, S. Insight into OH and O2.− formation in heterogeneous catalytic ozonation by delocalized electrons and surface oxygen-containing functional groups in layered-structure nanocarbons. Chem. Eng. J. 2019, 357, 655–666. [Google Scholar] [CrossRef]
- Bilińska, L.; Blus, K.; Bilińska, M.; Gmurek, M. Industrial Textile Wastewater Ozone Treatment: Catalyst Selection. Catalysts 2020, 10, 611. [Google Scholar] [CrossRef]
- Shen, Z.; Xing, X.; Pang, Z.; Wang, S.; Lv, M.; Jiang, X. Low Temperature CO Oxidation in Sintering Flue Gas Over Cu-Ce/AC Catalysts: Effect of Pretreatment with KMnO4 on Activity. Catal. Lett. 2023, 153, 1726–1737. [Google Scholar] [CrossRef]
- Akhmad, R.I.S.; Sari, A.A.; Asmara, A.A.; Saefumillah, A. Preparation and application of activated carbon from black liquor sludge for removing of tripolyphosphate ion. AIP Conf. Proc. 2020, 2242, 040035. [Google Scholar] [CrossRef]
- Kumar, N.B.R.; Crasta, V.; Praveen, B.M.; Kumar, M. Studies on structural, optical and mechanical properties of MWCNTs and ZnO nanoparticles doped PVA nanocomposites. Nanotechnol. Rev. 2015, 4, 457–467. [Google Scholar] [CrossRef]
- Nazir, A.; Yu, H.; Wang, L.; He, Y.; Chen, Q.; Amin, B.U.; Shen, D. Electromagnetic interference shielding properties of ferrocene-based polypyrrole/carbon material composites. Appl. Phys. A 2020, 126, 749. [Google Scholar] [CrossRef]
- Marcì, G.; García-López, E.; Pomilla, F.; Palmisano, L.; Zaffora, A.; Santamaria, M.; Krivtsov, I.; Ilkaeva, M.; Barbieriková, Z.; Brezová, V. Photoelectrochemical and EPR features of polymeric C3N4 and O-modified C3N4 employed for selective photocatalytic oxidation of alcohols to aldehydes. Catal. Today 2019, 328, 21–28. [Google Scholar] [CrossRef]
- Konstas, P.S.; Konstantinou, I.; Petrakis, D.; Albanis, T. Synthesis, Characterization of g-C3N4/SrTiO3 Heterojunctions and Photocatalytic Activity for Organic Pollutants Degradation. Catalysts 2018, 8, 554. [Google Scholar] [CrossRef]
- Rakibuddin, M.; Kim, H.; Khan, M.E. Graphite-like carbon nitride (C3N4) modified N-doped LaTiO3 nanocomposite for higher visible light photocatalytic and photo-electrochemical performance. Appl. Surf. Sci. 2018, 15, 400–412. [Google Scholar] [CrossRef]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J.C. Physicochemical Properties of Activated Carbon: Their Effect on the Adsorption of Pharmaceutical Compounds and Adsorbate–Adsorbent Interactions. C-J. Carbon Res. 2018, 4, 62. [Google Scholar] [CrossRef]
- Fallah, N.; Bloise, E.; Santoro, D.; Mele, G. State of Art and Perspectives in Catalytic Ozonation for Removal of Organic Pollutants in Water: Influence of Process and Operational Parameters. Catalysts 2023, 13, 324. [Google Scholar] [CrossRef]
- Pan, X.; Fan, Z.; Chen, W.; Ding, Y.; Luo, H.; Bao, X. Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles. Nat. Mater. 2007, 6, 507–511. [Google Scholar] [CrossRef]
- Du, H.-Y.; Wang, C.-H.; Hsu, H.-C.; Chang, S.-T.; Yen, S.-C.; Chen, L.-C.; Viswanathan, B.; Chen, K.-H. High performance of catalysts supported by directly grown PTFE-free micro-porous CNT layer in a proton exchange membrane fuel cell. J. Mater. Chem. 2011, 21, 2512–2516. [Google Scholar] [CrossRef]
- Faria, P.; Órfão, J.; Pereira, M. Activated carbon and ceria catalysts applied to the catalytic ozonation of dyes and textile effluents. Appl. Catal. B Environ. 2009, 88, 341–350. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.S.; Aris, A. Combined adsorption and catalytic ozonation for removal of sulfamethoxazole using Fe2O3/CeO2 loaded activated carbon. Chem. Eng. J. 2011, 170, 136–144. [Google Scholar] [CrossRef]
- Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Influence of the surface chemistry of multi-walled carbon nanotubes on their activity as ozonation catalysts. Carbon 2010, 48, 4369–4381. [Google Scholar] [CrossRef]
- García-López, E.I.; Pomilla, F.R.; Bloise, E.; Lü, X.-F.; Mele, G.; Palmisano, L.; Marcì, G. C3N4 Impregnated with Porphyrins as Heterogeneous Photocatalysts for the Selective Oxidation of 5-Hydroxymethyl-2-Furfural Under Solar Irradiation. Top. Catal. 2021, 64, 758–771. [Google Scholar] [CrossRef]
- Krivtsov, I.; García-López, E.I.; Marcì, G.; Palmisano, L.; Amghouz, Z.; García, J.R.; Ordóñez, S.; Díaz, E. Selective photocatalytic oxidation of 5-hydroxymethyl-2-furfural to 2,5-furandicarboxyaldehyde in aqueous suspension of g-C3N4. Appl. Catal. B Environ. 2017, 204, 430–439. [Google Scholar] [CrossRef]
- Manu, B. Physico-chemical treatment of indigo dye wastewater. Color. Technol. 2007, 123, 197–202. [Google Scholar] [CrossRef]
- Ammar, S.; Abdelhedi, R.; Flox, C.; Arias, C.; Brillas, E. Electrochemical degradation of the dye indigo carmine at boron-doped diamond anode for wastewaters remediation. Environ. Chem. Lett. 2006, 4, 229–233. [Google Scholar] [CrossRef]
- El-sayed, B.; Ibrahim, I.A.; Mohamed, W.A.A.; Ahmed, M.A.M. Synthesis and Characterization of Crystalline Nano TiO2 and ZnO and their Effects on the Photodegradation of Indigo Carmine Dye. Int. J. Adv. Eng. Nano Technol. 2015, 2, 2347–6389. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallah, N.; Bloise, E.; García-López, E.I.; Mele, G. Carbon-Based Materials in Combined Adsorption/Ozonation for Indigo Dye Decolorization in Constrain Contact Time. Molecules 2024, 29, 4144. https://doi.org/10.3390/molecules29174144
Fallah N, Bloise E, García-López EI, Mele G. Carbon-Based Materials in Combined Adsorption/Ozonation for Indigo Dye Decolorization in Constrain Contact Time. Molecules. 2024; 29(17):4144. https://doi.org/10.3390/molecules29174144
Chicago/Turabian StyleFallah, Naghmeh, Ermelinda Bloise, Elisa I. García-López, and Giuseppe Mele. 2024. "Carbon-Based Materials in Combined Adsorption/Ozonation for Indigo Dye Decolorization in Constrain Contact Time" Molecules 29, no. 17: 4144. https://doi.org/10.3390/molecules29174144
APA StyleFallah, N., Bloise, E., García-López, E. I., & Mele, G. (2024). Carbon-Based Materials in Combined Adsorption/Ozonation for Indigo Dye Decolorization in Constrain Contact Time. Molecules, 29(17), 4144. https://doi.org/10.3390/molecules29174144








