Novel Brønsted Acid Catalyzed C-C Bond Activation and α-Alkylation of Ketones
Abstract
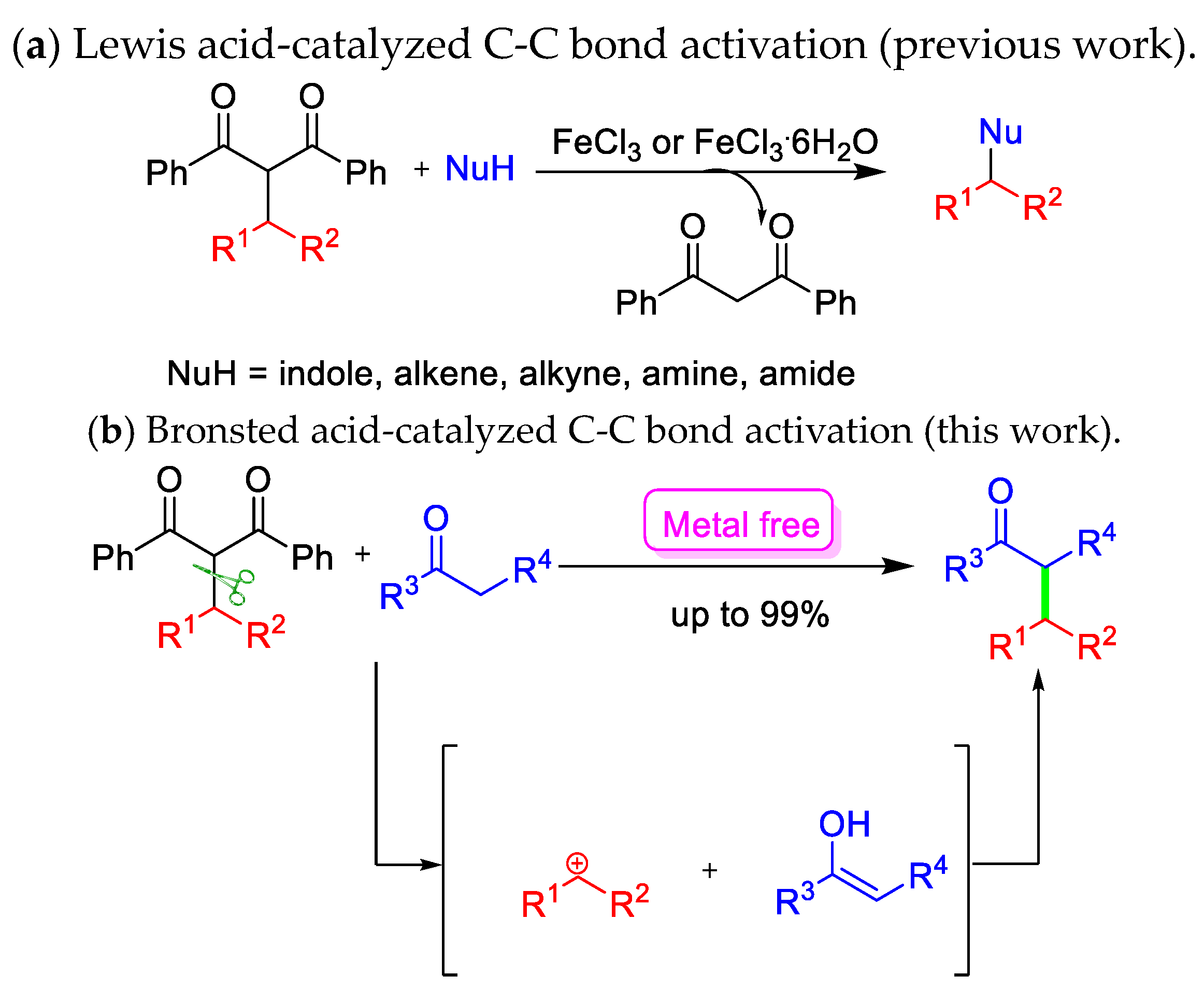
1. Introduction
2. Results and Discussion
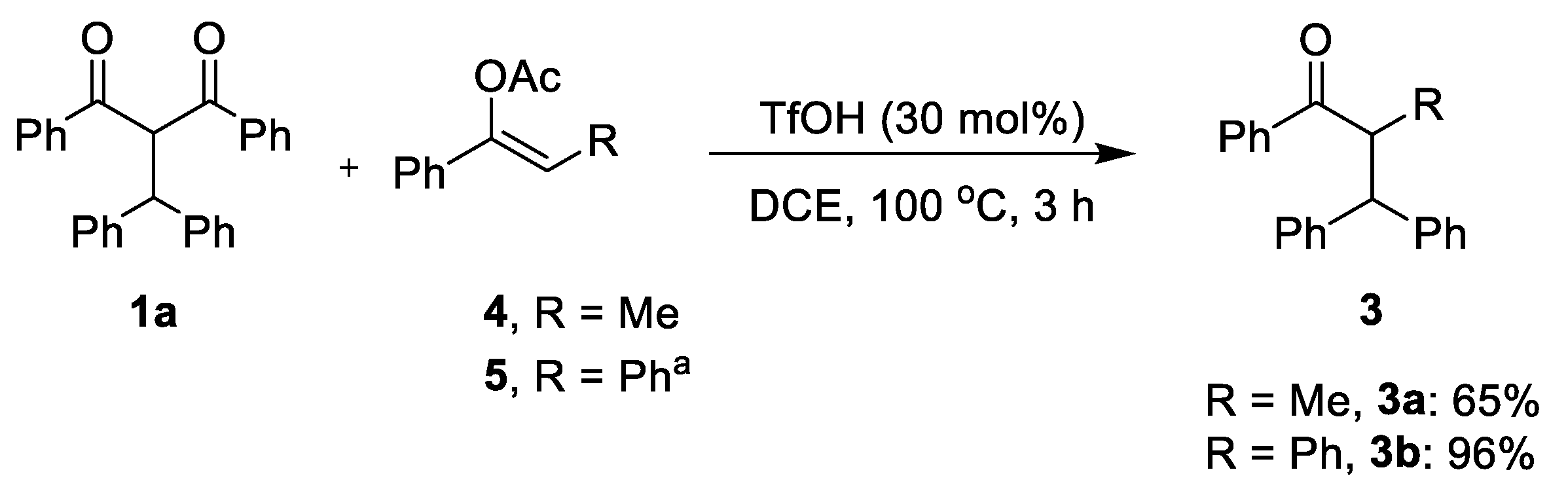
2.1. Optimization Studies
2.2. Substrate Scope Studies
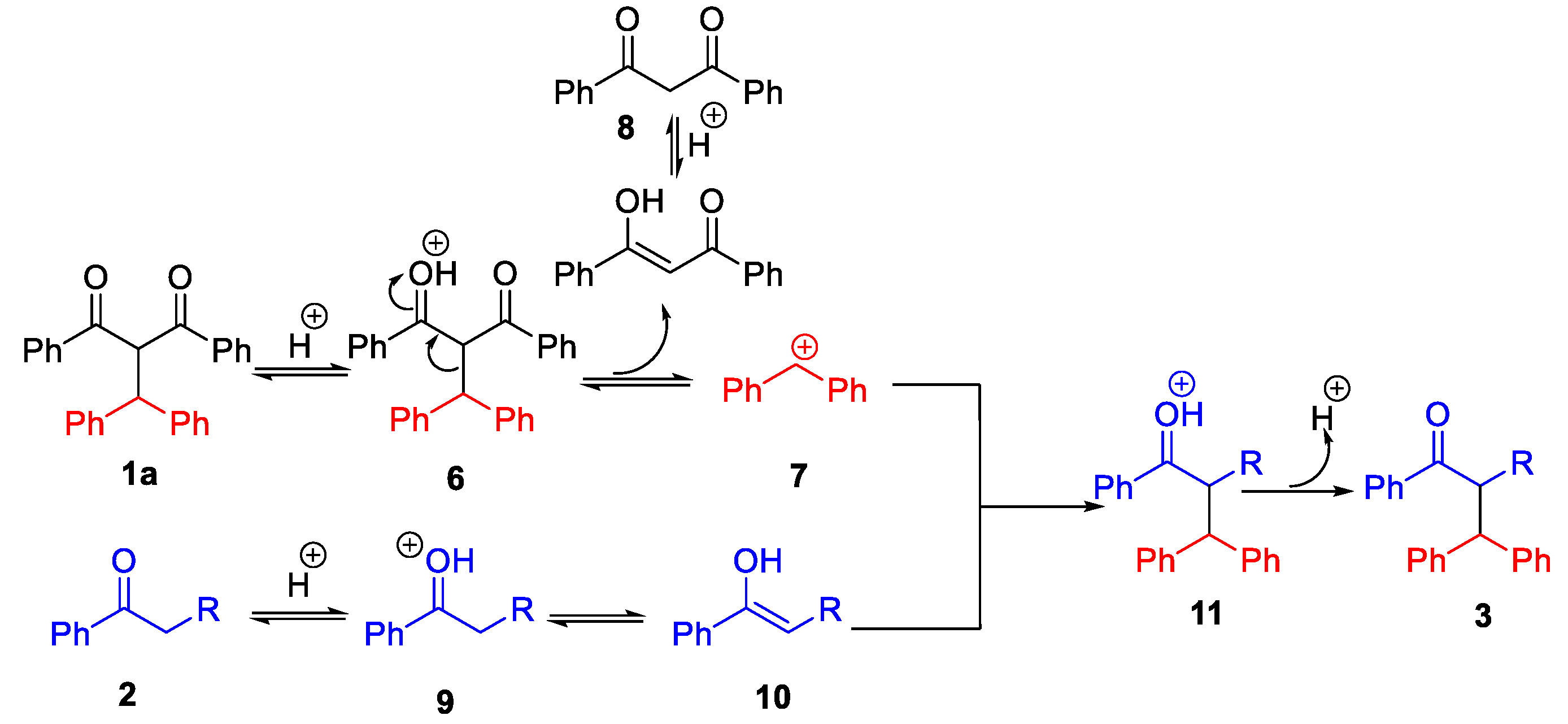
2.3. Proposed Mechanism for Brønsted Acid-Catalyzed C-C Bond Activation
3. Materials and Methods
3.1. General Information
3.2. General Experimental Procedure of Reaction of 1a and Enol Ester
3.3. General Procedure for Synthesis of Products 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, Y.J.; Park, J.-W.; Jun, C.-H. Metal-Organic Cooperative Catalysis in C-H and C-C Bond Activation and Its Concurrent Recovery. Acc. Chem. Res. 2008, 41, 222–234. [Google Scholar] [CrossRef]
- Jun, C.-H. Transition metal-catalyzed carbon-carbon bond activation. Chem. Soc. Rev. 2004, 33, 610–618. [Google Scholar] [CrossRef]
- Crabtree, R.H. The organometallic chemistry of alkanes. Chem. Rev. 1985, 85, 245–269. [Google Scholar] [CrossRef]
- Rybtchinski, B.; Milstein, D. Metal Insertion into C-C Bonds in Solution. Angew. Chem. Int. Ed. 1999, 38, 870–883. [Google Scholar] [CrossRef]
- Paul, T.; Basak, S.; Punniyamurthy, T. Weak Chelation-Assisted C4-Selective Alkylation of Indoles with Cyclopropanols via Sequential C-H/C-C Bond Activation. Org. Lett. 2022, 24, 6000–6005. [Google Scholar] [CrossRef]
- Calow, A.D.J.; Dariller, D.; Bower, J.F. Carbonylative N-Heterocyclization via Nitrogen-Directed C-C Bond Activation of Nonactivated Cyclopropanes. J. Am. Chem. Soc. 2022, 144, 11069–11074. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kaneko, Y.; Taguchi, Y.; Nakamura, A.; Okada, T.; Shiotsuki, M.; Ura, Y.; Wada, K.; Mitsudo, T. Rapid Ruthenium-Catalyzed Synthesis of Pyranopyrandiones by Reconstructive Carbonylation of Cyclopropenones Involving C-C Bond Cleavage. J. Am. Chem. Soc. 2002, 124, 6824–6825. [Google Scholar] [CrossRef]
- Chen, L.; Shi, C.; Li, W.; Li, B.; Zhu, J.; Lin, A.; Yao, H. Palladium-Catalyzed Asymmetric C-C Bond Activation/Carbonylation of Cyclobutanones. Org. Lett. 2022, 24, 9157–9162. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Xu, Y.-J.; Wang, Y.-F. A Pd(II)-Catalyzed Ring-Expansion Reaction of Cyclic 2-Azidoalcohol Derivatives: Synthesis of Azaheterocycles. J. Am. Chem. Soc. 2009, 131, 12886–12887. [Google Scholar] [CrossRef]
- Ding, D.; Lan, Y.; Lin, Z.; Wang, C. Synthesis of gem-Difluoroalkenes by Merging Ni-Catalyzed C-F and C-C Bond Activation in Cross-Electrophile Coupling. Org. Lett. 2019, 21, 2723–2730. [Google Scholar] [CrossRef]
- Choi, S.-M.; Park, J.-U.; Kim, J.H. CoIII-Catalyzed C-H Alkenylation and Allylation with Cyclopropenes via Sequential C-H/C-C Bond Activation. Org. Lett. 2021, 23, 6674–6679. [Google Scholar]
- Shi, S.-H.; Liang, Y.; Jiao, N. Electrochemical Oxidation Induced Selective C-C Bond Cleavage. Chem. Rev. 2021, 121, 485–505. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Visible Light-Driven Radical-Mediated C-C Bond Cleavage/Functionalization in Organic Synthesis. Chem. Rev. 2021, 121, 506–561. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Ma, S. Lewis acid-catalyzed unexpected selective C-C bond cleavage: An efficient and mild construction of cyclopentenes. Chem. Commun. 2012, 48, 11784–11786. [Google Scholar] [CrossRef]
- Dieskau, A.P.; Holzwarth, M.S.; Plietker, B. Fe-Catalyzed Allylic C–C-Bond Activation: Vinylcyclopropanes As Versatile a1,a3,d5-Synthons in Traceless Allylic Substitutions and [3 + 2]-Cycloadditions. J. Am. Chem. Soc. 2012, 134, 5048–5051. [Google Scholar] [CrossRef]
- Li, Z.; Cao, L.; Li, C.-J. FeCl2-Catalyzed Selective C-C Bond Formation by Oxidative Activation of a Benzylic C-H Bond. Angew. Chem. Int. Ed. 2007, 46, 6505–6507. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Liu, W.; He, Z.; Li, Z. An Efficient and General Iron-Catalyzed C-C Bond Activation with 1,3-Dicarbonyl Units as a Leaving Groups. Angew. Chem. Int. Ed. 2011, 50, 2975–2978. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, X.; Li, Z. Iron-Catalyzed C-C Bond Cleavage and C-N Bond Formation. Adv. Synth. Catal. 2013, 355, 181–190. [Google Scholar] [CrossRef]
- Casiraghi, G.; Battistini, L.; Curti, C.; Rassu, G.; Zanardi, F. The Vinylogous Aldol and Related Addition Reactions: Ten Years of Progress. Chem. Rev. 2011, 111, 3076–3154. [Google Scholar] [CrossRef]
- Palomo, C.; Oiarbide, M.; Garcıa, J.M. Current progress in the asymmetric aldol addition reaction. Chem. Soc. Rev. 2004, 33, 65–75. [Google Scholar] [CrossRef]
- Mlynarski, J.; Gut, B. Organocatalytic synthesis of carbohydrates. Chem. Soc. Rev. 2012, 41, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Magano, J.; Dunetz, J.R. Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef] [PubMed]
- Popp, F.D.; McEwen, W.E. Polyphosphoric Acids As A Reagent In Organic Chemistry. Chem. Rev. 1958, 58, 321–401. [Google Scholar] [CrossRef]
- Linsk, J. Rearrangement of 4-Methylcyclohexene during Sulfuric Acid-catalyzed Reaction with Benzene. J. Am. Chem. Soc. 1950, 72, 4257–4260. [Google Scholar] [CrossRef]
- de Los Ríos, C.; Hegedus, L.S. Reaction of Optically Active α-Aminoallenylstannanes with Aldehydes Formed in Situ from the Lewis-Acid-Catalyzed Rearrangement of Epoxides. J. Org. Chem. 2005, 70, 6541–6543. [Google Scholar] [CrossRef]
- Koppolu, S.R.; Naveen, N.; Balamurugan, R. Triflic Acid Promoted Direct α-Alkylation of Unactivated Ketones Using Benzylic Alcohols via in Situ Formed Acetals. J. Org. Chem. 2014, 79, 6069–6078. [Google Scholar] [CrossRef]
- Hokamp, T.; Wirth, T. Hypervalent Iodine(III)-Catalysed Enantioselective α-Acetoxylation of Ketones. Chem. Eur. J. 2020, 26, 10417–10421. [Google Scholar] [CrossRef]
- Basdevant, B.; Legault, C.Y. Enantioselective Iodine(III)-Mediated Synthesis of α-Tosyloxy Ketones: Breaking the Selectivity Barrier. Org. Lett. 2015, 17, 4918–4921. [Google Scholar] [CrossRef]
- Jayamani, M.; Pant, N.; Ananthan, S.; Narayanan, K.; Pillai, C.N. Synthesis of indenes from phenylpropanones using alumina catalyst. Tetrahedron 1986, 42, 4325–4332. [Google Scholar] [CrossRef]
- Zimmerman, H.E.; Nuss, J.M.; Tantillo, A.W. Cyclopropanols and the Di-ϖ-methane Rearrangement: Mechanistic and Exploratory Organic Photochemistry. J. Org. Chem. 1988, 53, 3792–3803. [Google Scholar] [CrossRef]
- Tandiary, M.A.; Asano, M.; Hattori, T.; Takehira, S.; Masui, Y.; Onaka, M. Unprecedented alkylation of silicon enolates with alcohols via carbenium ion formations catalyzed by tin hydroxide-embedded montmorillonite. Tetrahedron Lett. 2017, 58, 1925–1928. [Google Scholar] [CrossRef]
- Yang, C.-F.; Wang, J.-Y.; Tian, S.-K. Catalytic decarboxylative alkylation of β-keto acids with sulfonamides via the cleavage of carbon–nitrogen and carbon–carbon bonds. Chem. Commun. 2011, 47, 8343–8345. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Somyo, T.; Baba, A. Direct carbon-carbon bond formation from alcohols and active methylenes, alkoxyketones, or indoles catalyzed by indium trichloride. Angew. Chem. Int. Ed. 2006, 45, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.A.; Yasuda, M.; Tsukahara, Y.; Yamauchi, T.; Wada, Y.; Baba, A. Microwave-irradiated transition-metal catalysis: Rapid and efficient dehydrative carbon-carbon coupling of alcohols with active methylenes. Synthesis 2008, 11, 1717–1724. [Google Scholar] [CrossRef]
- Sondengam, B.L.; Fomum, Z.T.; Charles, G.; Akam, T.M. A Convenient Reduction of Activated Olefens by Zinc-Copper Couple. J. Chem. Soc. 1983, 6, 1219-–1222. [Google Scholar] [CrossRef]
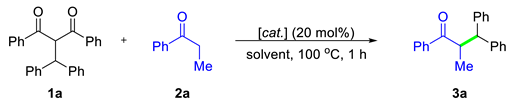 | ||||
|---|---|---|---|---|
| Entry | 2a (equivalent) | Catalyst (20 mol%) | Solvent | Yield (%) b |
| 1 | 1 | FeCl3 | DCE | 15 |
| 2 | 1 | FeCl3 + Cs2CO3 | DCE | N.D. c |
| 3 | 1 | FeCl3 + Na2CO3 | DCE | <5 |
| 4 | 1 | FeCl3 + NEt3 | DCE | N.D. c |
| 5 | 1 | FeCl3 + L-Proline | DCE | <5 |
| 6 | 1 | FeCl3 + TfOH | DCE | 18 |
| 7 | 1 | TfOH | DCE | 52 |
| 8 | 1 | MeSO3H | DCE | <5 |
| 9 | 1 | TFA | DCE | N.D. c |
| 10 | 1 | H3PO4 | DCE | N.D. c |
| 11 | 1 | AcOH | DCE | N.D. c |
| 12 | 1 | BF4H | DCE | 14 |
| 13 | 1 | PhCOOH | DCE | N.D. c |
| 14 | 2 | TfOH | DCE | 65 |
| 15 d | 2 | TfOH | DCE | 76 |
| 16 e | 2 | TfOH | DCE | 94 |
| 17 e | 2 | TfOH | THF f | <5 |
| 18 e | 2 | TfOH | DMF | N.D. c |
| 19 e | 2 | TfOH | MeCN | 60 |
| 20 e | 2 | TfOH | PhCl | 49 |
| 21 e | 2 | --- | DCE | N.D. c |
| Entry | Substrate | Product | 3 | Yield (%) b |
|---|---|---|---|---|
| 1 |  | 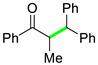 | 3a | 94 |
| 2 |  |  | 3b | 61 |
| 3 | 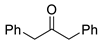 | 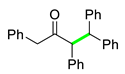 | 3c | 97 |
| 4 |  |  | 3d | 55 |
| 5 | 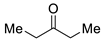 | 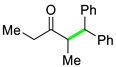 | 3e | 48 c |
| 6 | 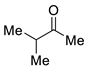 |  | 3f | 42 c |
| 7 | 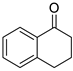 |  | 3g | 43 |
| 8 |  | 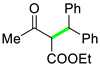 | 3h | 97 |
| 9 | 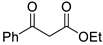 | 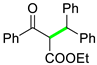 | 3i | 90 |
| 10 | 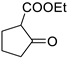 | 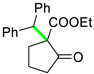 | 3j | 57 |
| 11 | 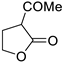 | 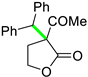 | 3k | 57 |
| 12 | 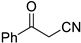 | 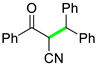 | 3l | 96 |
| 13 |  | 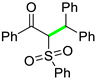 | 3m | 85 |
| 14 | 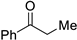 | 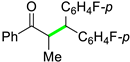 | 3n | 87 |
| 15 |  | 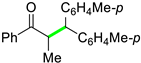 | 3o | 90 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Cheng, H.; Han, H.; Li, L.; Liu, X.; Chu, X.; Li, X. Novel Brønsted Acid Catalyzed C-C Bond Activation and α-Alkylation of Ketones. Molecules 2024, 29, 4266. https://doi.org/10.3390/molecules29174266
Li W, Cheng H, Han H, Li L, Liu X, Chu X, Li X. Novel Brønsted Acid Catalyzed C-C Bond Activation and α-Alkylation of Ketones. Molecules. 2024; 29(17):4266. https://doi.org/10.3390/molecules29174266
Chicago/Turabian StyleLi, Wenjuan, Huihang Cheng, Huabo Han, Lu Li, Xinming Liu, Xianxu Chu, and Xiaopei Li. 2024. "Novel Brønsted Acid Catalyzed C-C Bond Activation and α-Alkylation of Ketones" Molecules 29, no. 17: 4266. https://doi.org/10.3390/molecules29174266
APA StyleLi, W., Cheng, H., Han, H., Li, L., Liu, X., Chu, X., & Li, X. (2024). Novel Brønsted Acid Catalyzed C-C Bond Activation and α-Alkylation of Ketones. Molecules, 29(17), 4266. https://doi.org/10.3390/molecules29174266




