Impact of Different π-Bridges on the Photovoltaic Performance of A-D-D′-D-A Small Molecule-Based Donors
Abstract
1. Introduction
2. Results and Discussion
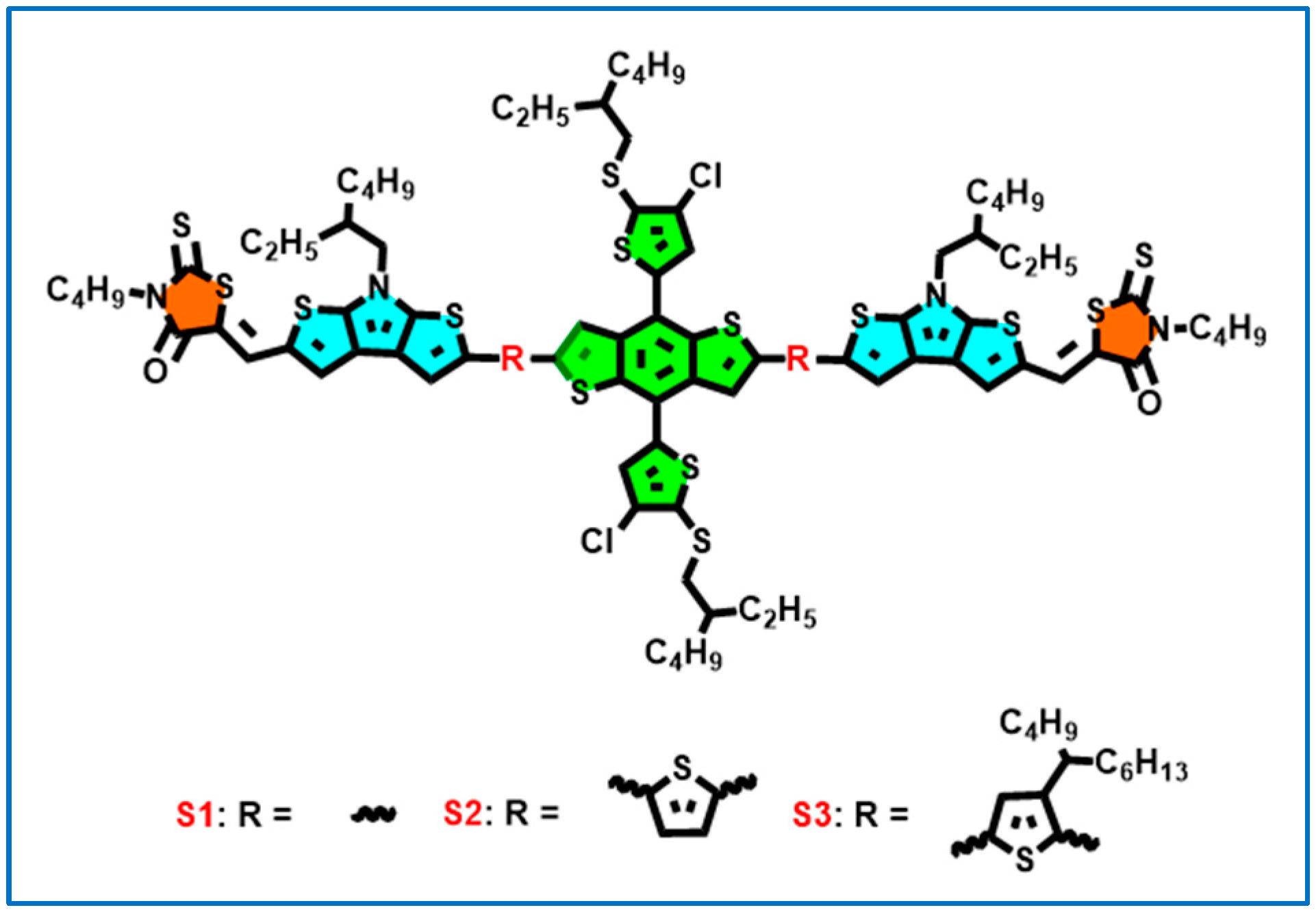
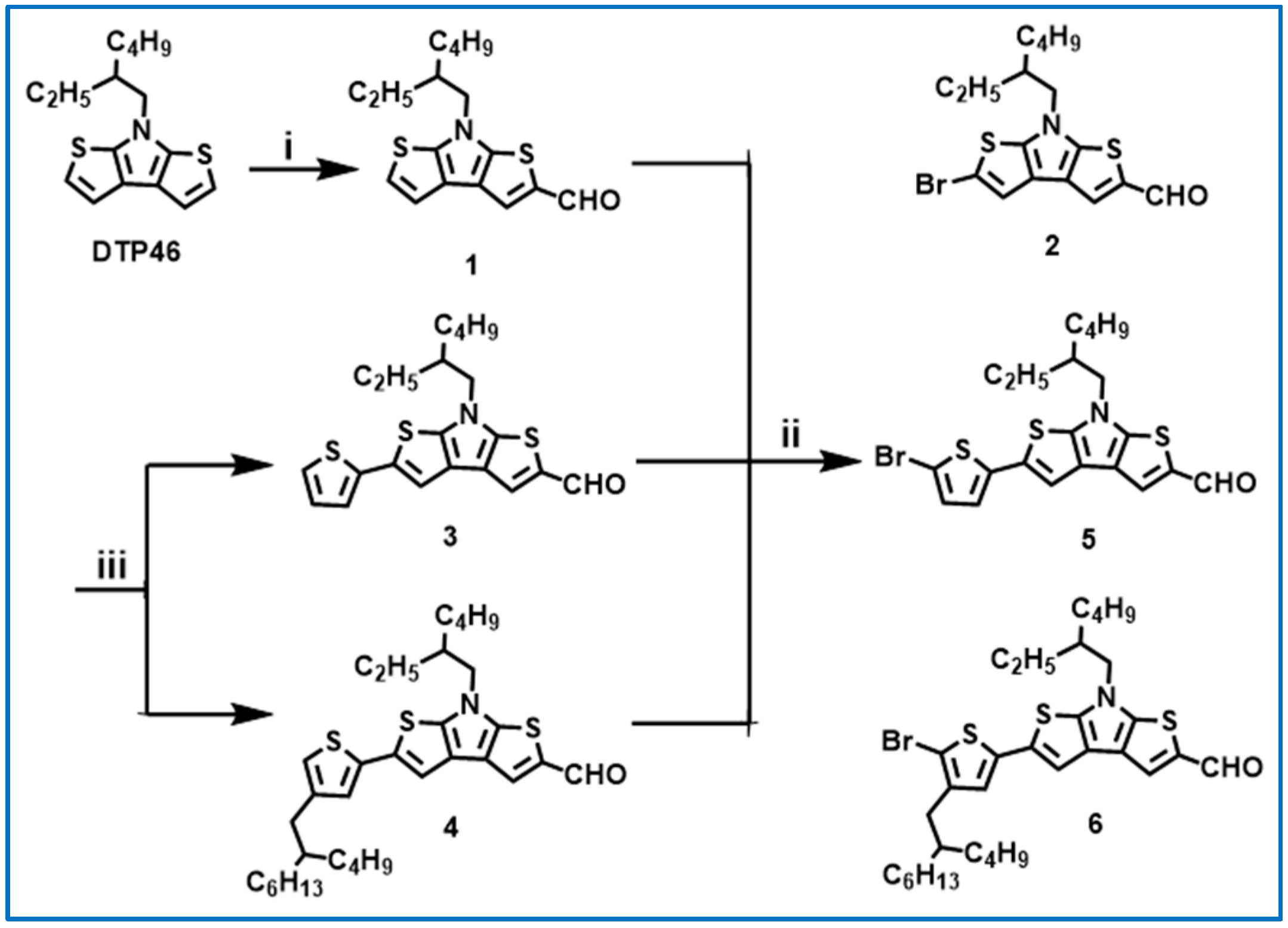
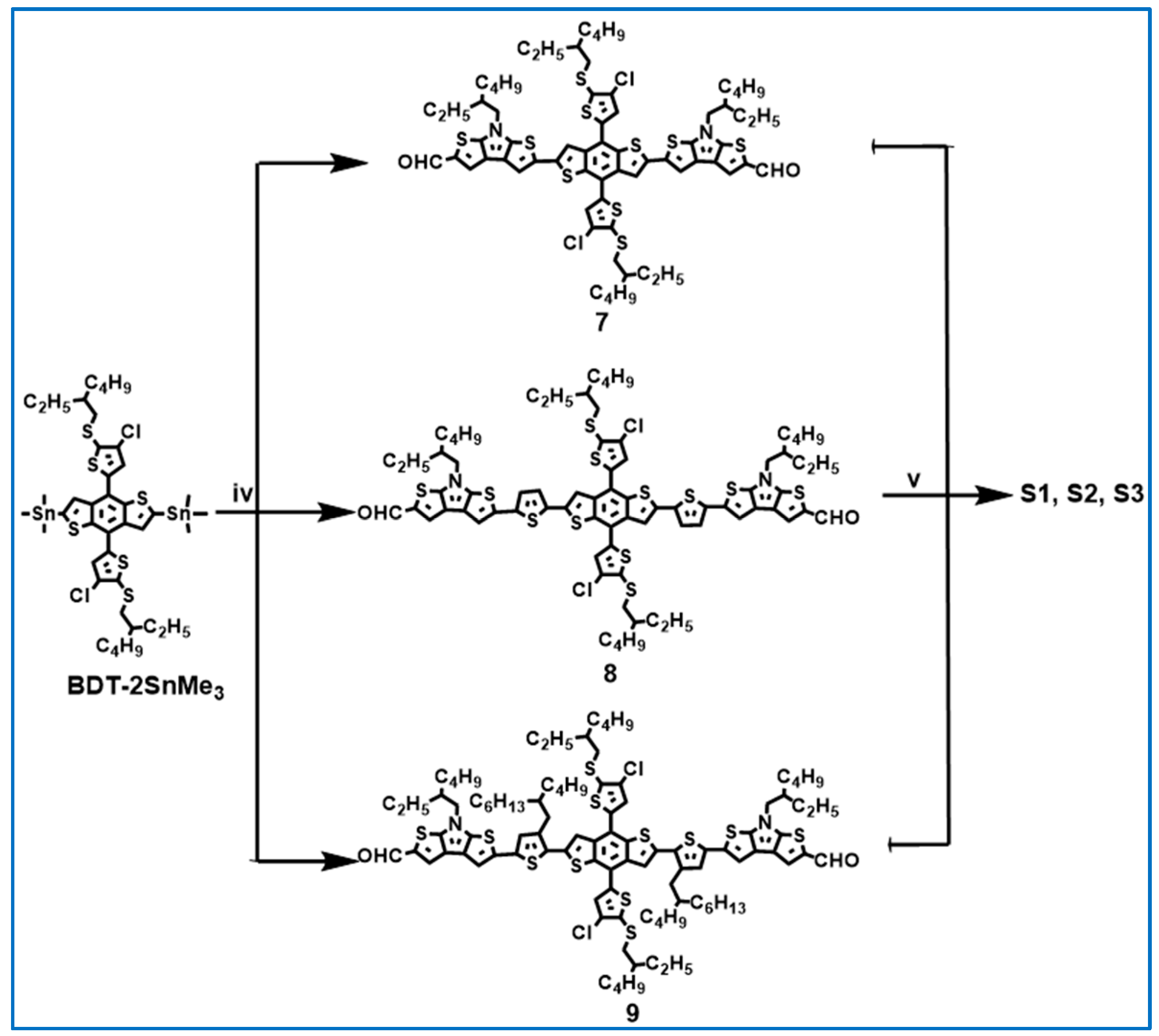
2.1. Synthesis and Characterization
2.2. Opto-Electronic Properties
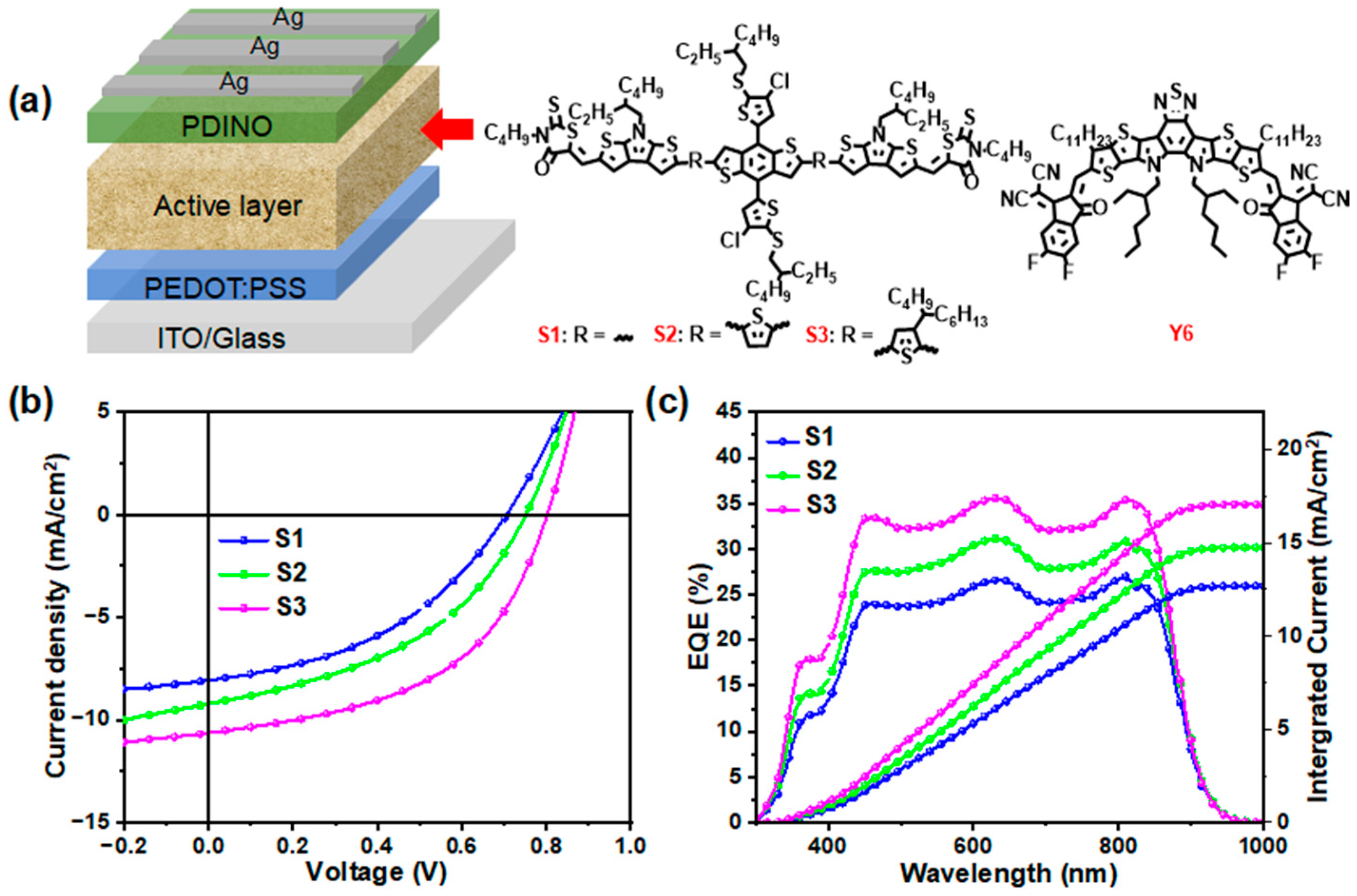
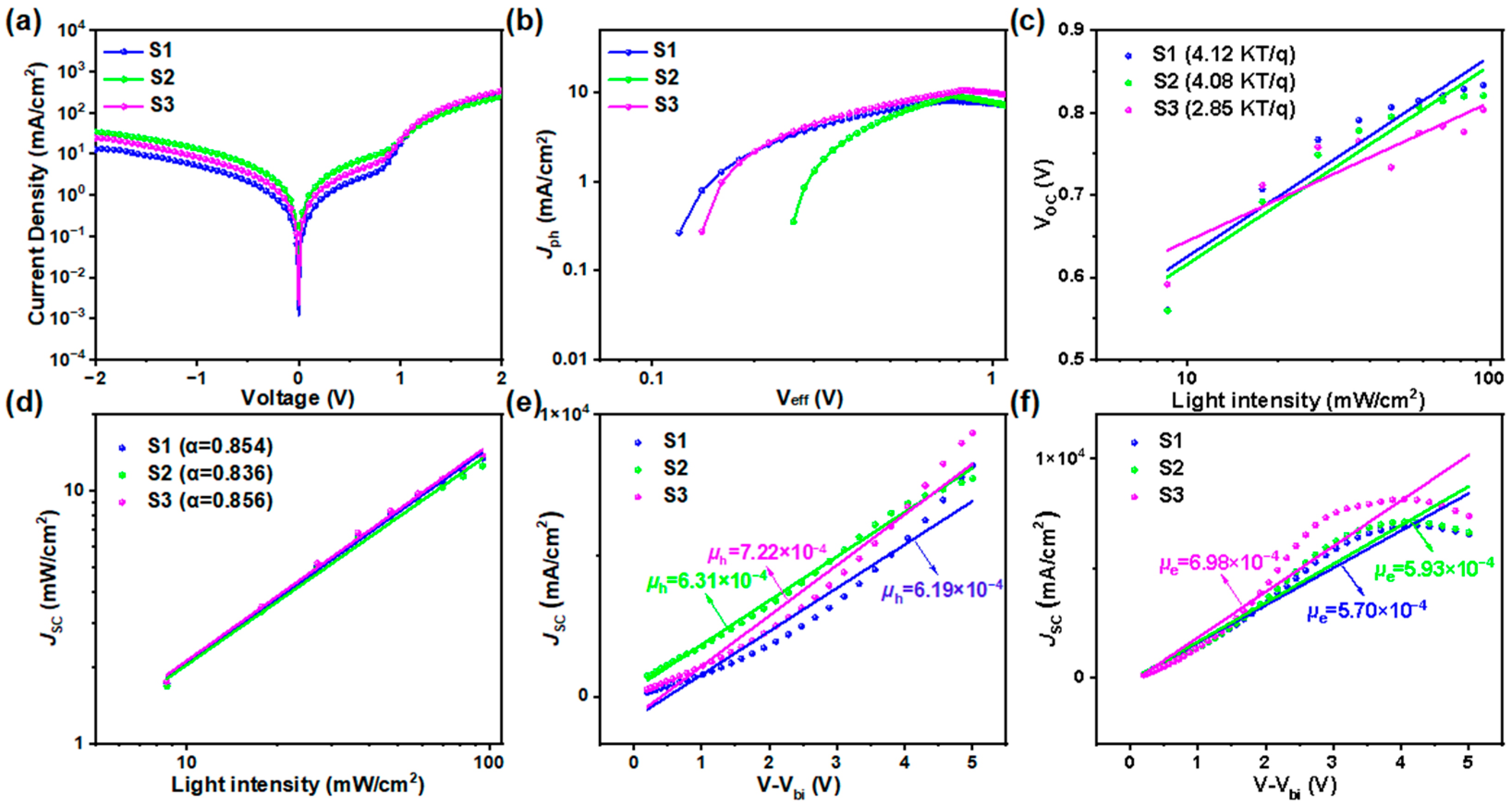
2.3. Photovoltaic Performances
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Synthesis of Compound 1 (DTP46-CHO)
3.4. Synthesis of Compound 2 (Br-DTP46-CHO)
3.5. Synthesis of Compounds 3 and 4
3.6. Synthesis of Compounds 5 and 6
3.7. Synthesis of Compounds 7, 8, and 9
3.8. Synthesis of S1, S2, and S3
3.9. Device Fabrication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Xian, K.; Zhang, T.; Hong, L.; Wang, Y.; Xu, Y.; Ma, K.; An, C.; et al. Single-junction organic photovoltaic cells with approaching 18% efficiency. Adv. Mater. 2020, 32, 1908205. [Google Scholar] [CrossRef]
- Zhu, C.; Yuan, J.; Cai, F.; Meng, L.; Zhang, H.; Chen, H.; Li, J.; Qiu, B.; Peng, H.; Chen, S.; et al. Tuning the electron-deficient core of a non-fullerene acceptor to achieve over 17% efficiency in a single-junction organic solar cell. Energy Environ. Sci. 2020, 13, 2459–2466. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Xu, S.; Zhou, Y.; Li, Z. Emerging chemistry in enhancing the chemical and photochemical stabilities of fused-ring electron acceptors in organic solar cells. Adv. Funct. Mater. 2021, 31, 2106735. [Google Scholar] [CrossRef]
- Chen, N.; Yang, L.-J.; Chen, Y.; Wu, Y.; Huang, X.-M.; Liu, H.; Xie, H.-Y.; Hu, L.; Li, Z.; Liu, S.-Y. PBDB-T accessed via direct C−H arylation polymerization for organic photovoltaic application. ACS Appl. Polym. Mater. 2022, 4, 7282–7289. [Google Scholar] [CrossRef]
- Murugan, P.; Hu, T.; Hu, X.; Chen, Y. Fused ring A–DA′D–A (Y-series) non-fullerene acceptors: Recent developments and design strategies for organic photovoltaics. J. Mater. Chem. A 2022, 10, 17968–17987. [Google Scholar] [CrossRef]
- Yang, L.-J.; Chen, N.; Huang, X.-M.; Wu, Y.; Liu, H.; Liu, P.; Hu, L.; Li, Z.-F.; Liu, S.-Y. Direct C−H arylation-derived donor polymers afford PCEs over 10% for organic solar cells. ACS Appl. Polym. Mater. 2023, 5, 7340–7349. [Google Scholar] [CrossRef]
- Fu, J.; Fong, P.W.K.; Liu, H.; Huang, C.-S.; Lu, X.; Lu, S.; Abdelsamie, M.; Kodalle, T.; Sutter-Fella, C.M.; Yang, Y.; et al. 19.31% binary organic solar cell and low non-radiative recombination enabled by non-monotonic intermediate state transition. Nat. Commun. 2023, 14, 1760. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Li, Y.; Chen, Z.; Wu, H.; Lin, Y.; Gao, Y.; Wang, M.; Ding, G.; Min, J.; et al. Compromising charge generation and recombination of organic photovoltaics with mixed diluent strategy for certified 19.4% efficiency. Adv. Mater. 2023, 35, 2300400. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Bi, P.; Chen, Z.; Qiao, J.; Li, J.; Wang, W.; Zheng, Z.; Zhang, S.; Hao, X.; et al. Binary organic solar cells with 19.2% efficiency enabled by solid additive. Adv. Mater. 2023, 35, 2301583. [Google Scholar] [CrossRef]
- Wu, Y.; He, X.-Y.; Huang, X.-M.; Yang, L.-J.; Liu, P.; Chen, N.; Li, C.-Z.; Liu, S.-Y. Synthesis of long-chain oligomeric donor and acceptors via direct arylation for organic solar cells. Chin. J. Chem. 2024, 42, 523–532. [Google Scholar] [CrossRef]
- Yang, L.-J.; Wu, Y.; Murugan, P.; Liu, P.; Qiu, Z.-Y.; Peng, Y.-L.; Li, Z.-F.; Liu, S.-Y. Advancing Integration of direct C−H arylation-derived star-shaped oligomers as second acceptors for ternary organic solar cells. ACS Appl. Mater. Interfaces 2024, 16, 26348–26359. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Liu, S.; Xu, J.; Ma, R.; Peng, Z.; Peña, T.A.D.; Cui, Y.; Liang, W.; Zhou, X.; Luo, S.; et al. Over 19 % efficiency organic solar cells enabled by manipulating the intermolecular interactions through side chain fluorine functionalization. Angew. Chem. Int. Ed. 2024, 63, e202400086. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, J.; Li, C.; Lin, Y.; Li, X.; Zhang, H.; Ma, Z.; Lu, Y. Effect of nitro-substituted ending group on the photovoltaic and photocatalytic performance of non-fullerene acceptors. Chem. Eng. J. 2024, 490, 151467. [Google Scholar] [CrossRef]
- Yang, L.-J.; Chen, N.; Murugan, P.; Wu, Y.; Liu, P.; Huang, X.-M.; Li, Z.-F.; Liu, S.-Y. Direct C−H arylation vs Stille polymeri zation: Rational design, synthesis, and systematic examinations of π-conjugated polymers for organic solar cells. Mater. Today Chem. 2024, 37, 101987. [Google Scholar] [CrossRef]
- Hu, D.; Tang, H.; Chen, C.; Huang, P.; Shen, Z.; Li, H.; Liu, H.; Petoukhoff, C.E.; Jurado, J.P.; Luo, Y.; et al. Insights into preaggregation control of Y-series nonfullerene acceptors in liquid state for highly efficient binary organic solar cells. Adv. Mater. 2024, 36, 2402833. [Google Scholar] [CrossRef]
- Xu, W.; Du, L.; Du, Z.; He, W.; Li, H.; Li, G.; Yang, C.; Cheng, P.; Cao, Z.; Yu, D. Enhanced photovoltaic performance of asym metrical benzo dithiophene homopolymer donor materials in nonfullerene acceptor-based organic photovoltaics. Molecules 2024, 29, 1332. [Google Scholar] [CrossRef]
- Qian, D.; Ye, L.; Zhang, M.; Liang, Y.; Li, L.; Huang, Y.; Guo, X.; Zhang, S.; Tan, Z.; Hou, J.A.; et al. Design, application, and morphology study of a new photovoltaic polymer with strong aggregation in solution state. Macromolecules 2012, 45, 9611–9617. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, X.; Ma, W.; Ade, H.; Hou, J. A large-bandgap conjugated polymer for versatile photovoltaic applications with high performance. Adv. Mater. 2015, 27, 4655–4660. [Google Scholar] [CrossRef]
- Li, Z.; Chueh, C.-C.; Jen, A.K.-Y. Recent advances in molecular design of functional conjugated polymers for high-performance polymer solar cells. Prog. Polym. Sci. 2019, 99, 101175. [Google Scholar] [CrossRef]
- Tan, Z.-R.; Xing, Y.-Q.; Cheng, J.-Z.; Zhang, G.; Shen, Z.-Q.; Zhang, Y.-J.; Liao, G.; Chen, L.; Liu, S.-Y. EDOT-based conjugated polymers accessed via C−H direct arylation for efficient photocatalytic hydrogen production. Chem. Sci. 2022, 13, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, L.; Zhang, K.; Liu, S.-Y. Carbazole and diketopyrrolopyrrole-based D-A π-conjugated oligomers accessed via direct C–H arylation for opto-electronic property and performance study. Molecules 2022, 27, 9031. [Google Scholar] [CrossRef]
- Tang, H.; Xu, T.; Yan, C.; Gao, J.; Yin, H.; Lv, J.; Singh, R.; Kumar, M.; Duan, T.; Kan, Z.; et al. Donor derivative incorpo ration: An effective strategy toward high performance all-small-molecule ternary organic solar cells. Adv. Sci. 2019, 6, 1901613. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Yang, Q.; Chen, H.; Wobben, F.; Le Corre, V.M.; Singh, R.; Liu, T.; Ma, R.; Tang, H.; Koster, L.J.A.; et al. 15.34% efficiency all-small-molecule organic solar cells with an improved fill factor enabled by a fullerene additive. Energy Environ. Sci. 2020, 13, 2134–2141. [Google Scholar] [CrossRef]
- Han, D.; Wen, S.; Bi, F.; Shang, C.; Ding, X.; Saparbaev, A.; Zakhidov, E.; Kuvondikov, V.; Yang, C.; Sun, M. Small molecular donor materials based on electron withdrawing benzobisthiazole core unit enable an efficiency of 11.8% for organic solar cells. Chem. Eng. J. 2023, 463, 142400. [Google Scholar] [CrossRef]
- Yang, X.; Gao, Y.; Xu, L.-Y.; Wu, X.; Chen, X.; Shao, Y.; Xiao, B.; Liu, S.; Xia, J.; Sun, R.; et al. Efficient and stable all-small-molecule solar cells enabled by incorporating a designed giant molecule acceptor. Energy Environ. Sci. 2024, 17, 5962–5971. [Google Scholar] [CrossRef]
- Duan, T.; Gao, J.; Babics, M.; Kan, Z.; Zhong, C.; Singh, R.; Yu, D.; Lee, J.; Xiao, Z.; Lu, S. Difluorinated oligothiophenes for high-efficiency all-small-molecule organic solar cells: Positional isomeric effect of fluorine substitution on performance variations. Sol. RRL 2020, 4, 1900472. [Google Scholar] [CrossRef]
- Hu, D.; Yang, Q.; Zheng, Y.; Tang, H.; Chung, S.; Singh, R.; Lv, J.; Fu, J.; Kan, Z.; Qin, B.; et al. 15.3% efficiency all-small-molecule organic solar cells achieved by a locally asymmetric F, Cl disubstitution strategy. Adv. Sci. 2021, 8, 2004262. [Google Scholar] [CrossRef]
- Sun, Y.; Nian, L.; Kan, Y.; Ren, Y.; Chen, Z.; Zhu, L.; Zhang, M.; Yin, H.; Xu, H.; Li, J. Rational control of sequential morphology evolution and vertical distribution toward 17.18% efficiency all-small-molecule organic solar cells. Joule 2022, 6, 2835–2848. [Google Scholar] [CrossRef]
- Wu, S.; Feng, W.; Meng, L.; Zhang, Z.; Si, X.; Chen, Y.; Wan, X.; Li, C.; Yao, Z.; Chen, Y. 15.51% efficiency all-small-molecule organic solar cells achieved by symmetric thiazolyl substitution. Nano Energy 2022, 103, 107801. [Google Scholar] [CrossRef]
- Cai, S.; Huang, P.; Cai, G.; Lu, X.; Hu, D.; Hu, C.; Lu, S. Symmetrically fluorinated benzo[1,2-b:4,5-b′]dithiophene-cored donor for high-performance all-small-molecule organic solar cells with improved active layer morphology and crystallinity. ACS Appl. Mater. Interfaces 2022, 14, 14532–14540. [Google Scholar] [CrossRef]
- Ma, X.; Wang, C.; Deng, D.; Zhang, H.; Zhang, L.; Zhang, J.; Yang, Y.; Wei, Z. Small molecule donors design rules for non-halogen solvent fabricated organic solar cells. Small 2024, 20, 2309042. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Lee, J. Progress and future potential of all-small-molecule organic solar cells based on the benzodithiophene donor material. Molecules 2023, 28, 3171. [Google Scholar] [CrossRef]
- Murugan, P.; Ravindran, E.; Sangeetha, V.; Liu, S.-Y.; Jung, J.W. Perylene-diimide for organic solar cells: Current scenario and prospects in molecular geometric, functionalization, and optoelectronic properties. J. Mater. Chem. A 2023, 11, 26393–26425. [Google Scholar] [CrossRef]
- Qiu, B.; Chen, Z.; Qin, S.; Yao, J.; Huang, W.; Meng, L.; Zhu, H.; Yang, Y.; Zhang, Z.-G.; Li, Y. Highly efficient all-small-molecule organic solar cells with appropriate active layer morphology by side chain engineering of donor molecules and thermal annealing. Adv. Mater. 2020, 32, 1908373. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Xie, L.; Peng, R.; Fanady, B.; Huang, J.; Song, W.; Yan, T.; Zhang, W.; Ge, Z. 13.34% efficiency non-fullerene all-small-molecule organic solar cells enabled by modulating the crystallinity of donors via a fluorination strategy. Angew. Chem. Int. Ed. 2020, 59, 2808–2815. [Google Scholar] [CrossRef]
- Bin, H.; Angunawela, I.; Qiu, B.; Colberts, F.J.M.; Li, M.; Dyson, M.J.; Wienk, M.M.; Ade, H.; Li, Y.; Janssen, R.A.J. Precise control of phase separation enables 12% efficiency in all small molecule solar cells. Adv. Energy Mater. 2020, 10, 2001589. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, N.; Sun, Y.; Ke, X.; Zhang, H.; Li, C.; Wan, X.; Chen, Y. All-small-molecule organic solar cells based on a fluorinated small molecule donor with high open-circuit voltage of 1.07 V. Front. Chem. 2020, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Balakirev, D.O.; Gu, C.; Peregudova, S.M.; Ponomarenko, S.A.; Liu, Z.; Luponosov, Y.N.; Min, J.; Lei, A. End group tuning in small molecule donors for non-fullerene organic solar cells. Dyes Pigments 2020, 175, 108078. [Google Scholar] [CrossRef]
- Meng, W.; Lv, J.; Duan, T.; Kan, Z.; Lu, S.; Dai, X.; Li, Z. Small molecule donor based on alkoxylated benzothiadiazole unit: Synthesis and photovoltaics properties. Mater. Chem. Phys. 2020, 247, 122874. [Google Scholar] [CrossRef]
- Wu, Q.; Deng, D.; Zhou, R.; Zhang, J.; Zou, W.; Liu, L.; Wu, S.; Lu, K.; Wei, Z. Modulation of donor alkyl terminal chains with the shifting branching point leads to the optimized morphology and efficient all-small-molecule organic solar cells. ACS Appl. Mater. Interfaces 2020, 12, 25100–25107. [Google Scholar] [CrossRef]
- Dong, X.; Yang, K.; Tang, H.; Hu, D.; Chen, S.; Zhang, J.; Kan, Z.; Duan, T.; Hu, C.; Dai, X.; et al. Improving molecular planarity by changing alky chain position enables 12.3% efficiency all-small-molecule organic solar cells with enhanced carrier lifetime and reduced recombination. Sol. RRL 2020, 4, 1900326. [Google Scholar] [CrossRef]
- Xu, T.; Lv, J.; Yang, K.; He, Y.; Yang, Q.; Chen, H.; Chen, Q.; Liao, Z.; Kan, Z.; Duan, T.; et al. 15.8% efficiency binary all-small-molecule organic solar cells enabled by a selenophene substituted sematic liquid crystalline donor. Energy Environ. Sci. 2021, 14, 5366–5376. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Miao, Y.; Jiang, X.; Wang, X.; Zhang, Z.; Lv, Z.; Liu, T.; Zou, B.; Xu, H.; et al. Synergy effect of symmetry-breaking and end-group engineering enables 16.06% efficiency for all-small-molecule organic solar cells. ACS Mater. Lett. 2024, 6, 713–719. [Google Scholar] [CrossRef]
- Rasmussen, S.C.; Evenson, S.J.; McCausland, C.B. Fluorescent thiophene-based materials and their outlook for emissive appli cations. Chem. Commun. 2015, 51, 4528–4543. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.M.; Culver, E.W.; Rasmussen, S.C. Synthesis and characterization of bis[1]benzothieno[3,2-b:2′,3′-d]pyrroles: Quantitative effects of benzannulation on dithieno[3,2-b:2′,3′-d]pyrroles. Molecules 2018, 23, 2279. [Google Scholar] [CrossRef]
- Rybakiewicz, R.; Skórka, Ł.; Gańczarczyk, R. Dithienopyrrole-based organic electroactive materials and their photovoltaic as pects. Curr. Org. Chem. 2020, 24, 2695–2736. [Google Scholar] [CrossRef]
- Bulumulla, C.; Gunawardhana, R.; Gamage, P.L.; Miller, J.T.; Kularatne, R.N.; Biewer, M.C.; Stefan, M.C. Pyrrole-containing semiconducting materials: Synthesis and applications in organic photovoltaics and organic field-effect transistors. ACS Appl. Mater. Interfaces 2020, 12, 32209–32232. [Google Scholar] [CrossRef]
- Vogt, A.; Schwer, F.; Förtsch, S.; Lorenz, C.; Mena-Osteritz, E.; Aubele, A.; Kraus, T.; Bäuerle, P. Broadly applicable synthesis of arylated dithieno[3,2-b:2′,3′-d]pyrroles as building blocks for organic electronic materials. Chem. Eur. J. 2021, 27, 12362–12370. [Google Scholar] [CrossRef]
- Mei, S.; Shao, W.; Huang, S.; Kong, X.; Hu, Z.; Yang, M.; Wu, W.; Tan, H. Novel D-A-π-A organic dyes with phenoxazine as a donor unit for dye-sensitized solar cells: The effect of an ethynyl group on performance. Energy Fuels 2021, 35, 19748–19755. [Google Scholar] [CrossRef]
- Zheng, T.; Huang, S.; Liu, Y.; Li, Z.; Kong, X.; Qin, N.; Tan, H. Molecular engineering strategies of spectral matching and structure optimization for efficient metal-free organic dyes in dye-sensitized solar cells: A theoretical study. J. Phys. Chem. A 2024, 128, 5861–5872. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Han, L.; Wei, H.; Zhu, D.; Bao, X.; Qiao, S.; Sun, W.; Chen, W.; Yang, R. Influence of a π-bridge dependent molecular configuration on the optical and electrical characteristics of organic solar cells. J. Mater. Chem. A 2016, 4, 8784–8792. [Google Scholar] [CrossRef]
- Piradi, V.; Zhang, G.; Li, T.; Zhang, M.; Peng, Q.; Zhan, X.; Zhu, X. Side-chain engineering of benzodithiophene-bridged dimeric porphyrin donors for all-small-molecule organic solar cells. ACS Appl. Mater. Interfaces 2020, 12, 41506–41514. [Google Scholar] [CrossRef]
- Wu, L.N.; Li, M.Y.; Sui, M.Y.; Huang, J.C.; Sun, G.Y.; Cheng, L. Achieve panchromatic absorption for all-small-molecule organic solar cells based on mono-porphyrin molecules by π-bridge modification. Mater. Today Energy 2021, 20, 100658. [Google Scholar] [CrossRef]
- Shen, H.; Ren, Y.; Li, J.; Xu, Y.; Han, C.; Zou, W.; Xu, H.; Sun, Y.; Kan, Y.; Gao, K. Enhanced performance via π-bridge alteration of porphyrin-based donors for all-small-molecule organic solar Cells. Chin. J. Chem. 2023, 41, 644–650. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Han, C.; Wang, J.; Bi, F.; Zheng, N.; Yang, J.; Wang, J.; Bao, X. Rationally regulating the π-bridge of small molecule acceptors for efficient organic solar cells. J. Mater. Chem. A 2022, 10, 17808–17816. [Google Scholar] [CrossRef]
- Wang, P.; Bi, F.; Li, Y.; Han, C.; Zheng, N.; Zhang, S.; Wang, J.; Wu, Y.; Bao, X. Manipulating the intermolecular interactions through side chain engineering and unilateral π-bridge strategy for efficient small molecular photovoltaic acceptor. Adv. Funct. Mater. 2022, 32, 2200166. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, J.; Zhang, Y.; Shao, J.; Li, Y. Direct arylation synthesis of small molecular acceptors for organic solar cells. Molecules 2023, 28, 3515. [Google Scholar] [CrossRef]
- Kang, X.; Ding, X.; Du, Z.; Bi, F.; Sun, M.; Bao, X. Understanding the impact of thiazole unit sequence in π-bridge on the perfor mance of small molecule donor materials. Synthetic Met. 2024, 307, 117657. [Google Scholar] [CrossRef]
- Chen, B.-W.; Cao, K.; Wang, X.; Chen, Z.-C.; Jeong, S.Y.; Qiu, Z.-L.; Dai, L.-S.; Li, Y.-F.; Yang, K.-Y.; Yun, D.-Q.; et al. Design and performance of small-molecule donors with donor–π-acceptor architecture toward vacuum-deposited organic photovoltaics having heretofore highest short-circuit current density. Small 2024, 2403486. [Google Scholar] [CrossRef]
- Oh, S.; Ryu, D.H.; Jin, S.-M.; Kim, Y.; Kim, J.Y.; Jahankhan, M.; Lee, S.; Song, C.E.; Lee, H.K.; Shin, W.S.; et al. Effect of π-bridge ring fusion on the wide-bandgap donor and its application for high-efficiency all small-molecule tandem solar cells. ACS Appl. Energy Mater. 2024, 7, 5005–5017. [Google Scholar] [CrossRef]
- Crociani, L. The double-cross of benzotriazole-based polymers as donors and acceptors in non-fullerene organic solar cells. Molecules 2024, 29, 3625. [Google Scholar] [CrossRef]
- Ou, Z.; Qin, J.; Jin, K.; Zhang, J.; Zhang, L.; Yi, C.; Jin, Z.; Song, Q.; Sun, K.; Yang, J.; et al. Engineering of the alkyl chain branching point on a lactone polymer donor yields 17.81% efficiency. J. Mater. Chem. A 2022, 10, 3314–3320. [Google Scholar] [CrossRef]
- Shen, S.; Yang, L.; Mi, Y.; Zhou, Y.; Li, M.; Zhang, J.; Ye, L.; Song, J. Alkyl branching sites on π-spacers for dipyran-based high-efficiency organic solar cells. ACS Appl. Energy Mater. 2023, 6, 1066–1075. [Google Scholar] [CrossRef]
- Yang, D.; Li, S.; Guo, J.; Zhuo, H.; Zhou, L.; Lai, W.; Zhang, Z.; Li, X.; Meng, L.; Li, Y. Enhanced photovoltaic performance of 9,10-difluorodithieno[3,2-a:2′,3′-c]phenazine-based polymer donors by the synergistic effect of alkyl-thiophene π-bridges and halogen atom modification. ACS Appl. Polym. Mater. 2023, 5, 10315–10323. [Google Scholar] [CrossRef]
- Li, J.; Li, K.; Mei, Z.; Chen, Y.; Xie, Q.; Yu, C.; Liu, X.; Wang, Y.; Wu, Y.; Liao, Q.; et al. Designing simple non-fused terthiophene-based electron acceptors for efficient organic solar cells. J. Energy Chem. 2024, 96, 501–508. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, X.; Tang, X.; Meng, S.; Cao, J.; Zhang, Z.-G.; Weng, C.; Shen, P. Low-cost and completely non-fused small-molecule acceptors based on tetrathiophene featuring 3,5-dialkylthiophene side chain enable efficient organic solar cells. Chem. Eng. J. 2024, 484, 149584. [Google Scholar] [CrossRef]
- Ren, J.; Gao, S. Effect of an external electric field on the ordered structures of blended donor polymers in solar cells. J. Phys. Chem. C 2022, 126, 11318–11329. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, X.; Hartnagel, P.; Riera-Galindo, S.; Beket, G.; Österberg, T.; Gao, F.; Kirchartz, T.; Inganäs, O. Air processing of thick and semitransparent laminated polymer: Non-fullerene acceptor blends introduce asymmetric current-voltage characteristics. Adv. Funct. Mater. 2023, 33, 2301192. [Google Scholar] [CrossRef]
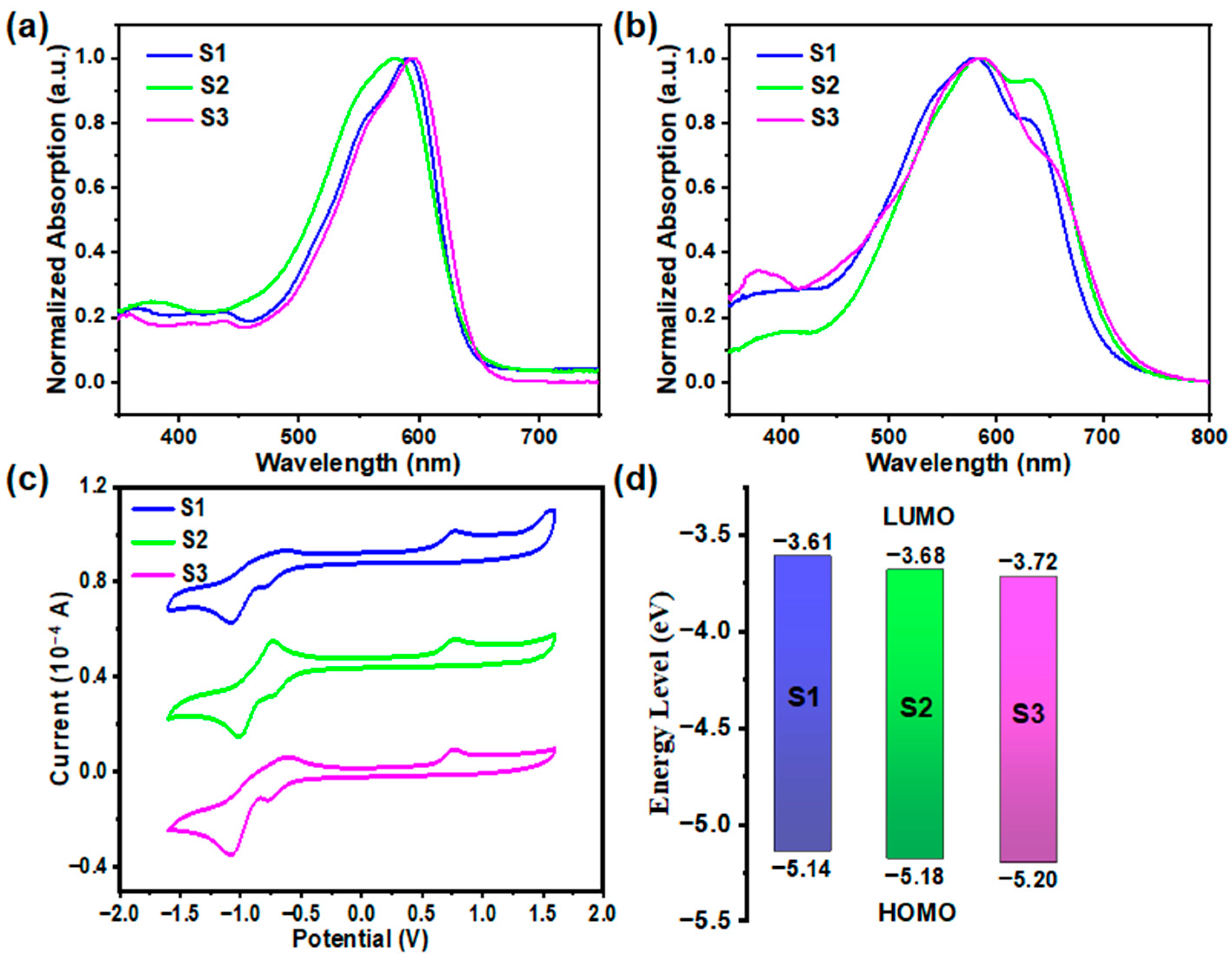
| λmax s (nm) a | λmax f (nm) b | λonset (nm) b | Eg (eV) c | EHOMO (eV) d | ELUMO (eV) d | |
|---|---|---|---|---|---|---|
| S1 | 591 | 580 | 708 | 1.75 | −5.14 | −3.61 |
| S2 | 580 | 585 | 724 | 1.71 | −5.18 | −3.68 |
| S3 | 595 | 586 | 732 | 1.69 | −5.20 | −3.72 |
| VOC (V) | JSC (mA/cm2) | JSC a (mA/cm2) | FF (%) | PCE b (%) | |
|---|---|---|---|---|---|
S1:Y6 | 0.71 (0.70 ± 0.005) | 8.08 (7.46 ± 0.48) | 7.93 | 42.16 (41.25 ± 0.89) | 2.42 (2.27 ± 0.14) |
S2:Y6 | 0.75 (0.75 ± 0.004) | 9.23 (8.91 ± 0.33) | 9.20 | 42.93 (42.59 ± 0.46) | 3.00 (2.85 ± 0.11) |
S3:Y6 | 0.80 (0.79 ± 0.002) | 10.64 (10.17 ± 0.51) | 10.59 | 49.72 (49.62 ± 0.39) | 4.25 (4.21 ± 0.07) |
| μh (cm2 V−1 s−1) | μe (cm2 V−1 s−1) | μh/μe | |
|---|---|---|---|
| S1:Y6 | 6.19 × 10−4 | 5.70 × 10−4 | 1.09 |
| S2:Y6 | 6.31 × 10−4 | 5.93 × 10−4 | 1.06 |
| S3:Y6 | 7.22 × 10−4 | 6.98 × 10−4 | 1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Wu, Y.; Murugan, P.; Liu, P.; Peng, Y.; Qiu, Z.; Li, Z.; Yu, C.; Liu, S. Impact of Different π-Bridges on the Photovoltaic Performance of A-D-D′-D-A Small Molecule-Based Donors. Molecules 2024, 29, 4231. https://doi.org/10.3390/molecules29174231
Yang L, Wu Y, Murugan P, Liu P, Peng Y, Qiu Z, Li Z, Yu C, Liu S. Impact of Different π-Bridges on the Photovoltaic Performance of A-D-D′-D-A Small Molecule-Based Donors. Molecules. 2024; 29(17):4231. https://doi.org/10.3390/molecules29174231
Chicago/Turabian StyleYang, Lingjun, Yu Wu, Pachaiyappan Murugan, Peng Liu, Yulong Peng, Zhiyong Qiu, Zaifang Li, Changlin Yu, and Shiyong Liu. 2024. "Impact of Different π-Bridges on the Photovoltaic Performance of A-D-D′-D-A Small Molecule-Based Donors" Molecules 29, no. 17: 4231. https://doi.org/10.3390/molecules29174231
APA StyleYang, L., Wu, Y., Murugan, P., Liu, P., Peng, Y., Qiu, Z., Li, Z., Yu, C., & Liu, S. (2024). Impact of Different π-Bridges on the Photovoltaic Performance of A-D-D′-D-A Small Molecule-Based Donors. Molecules, 29(17), 4231. https://doi.org/10.3390/molecules29174231








