Introduction of Electron Donor Groups into the Azulene Structure: The Appearance of Intense Absorption and Emission in the Visible Region
Abstract
1. Introduction
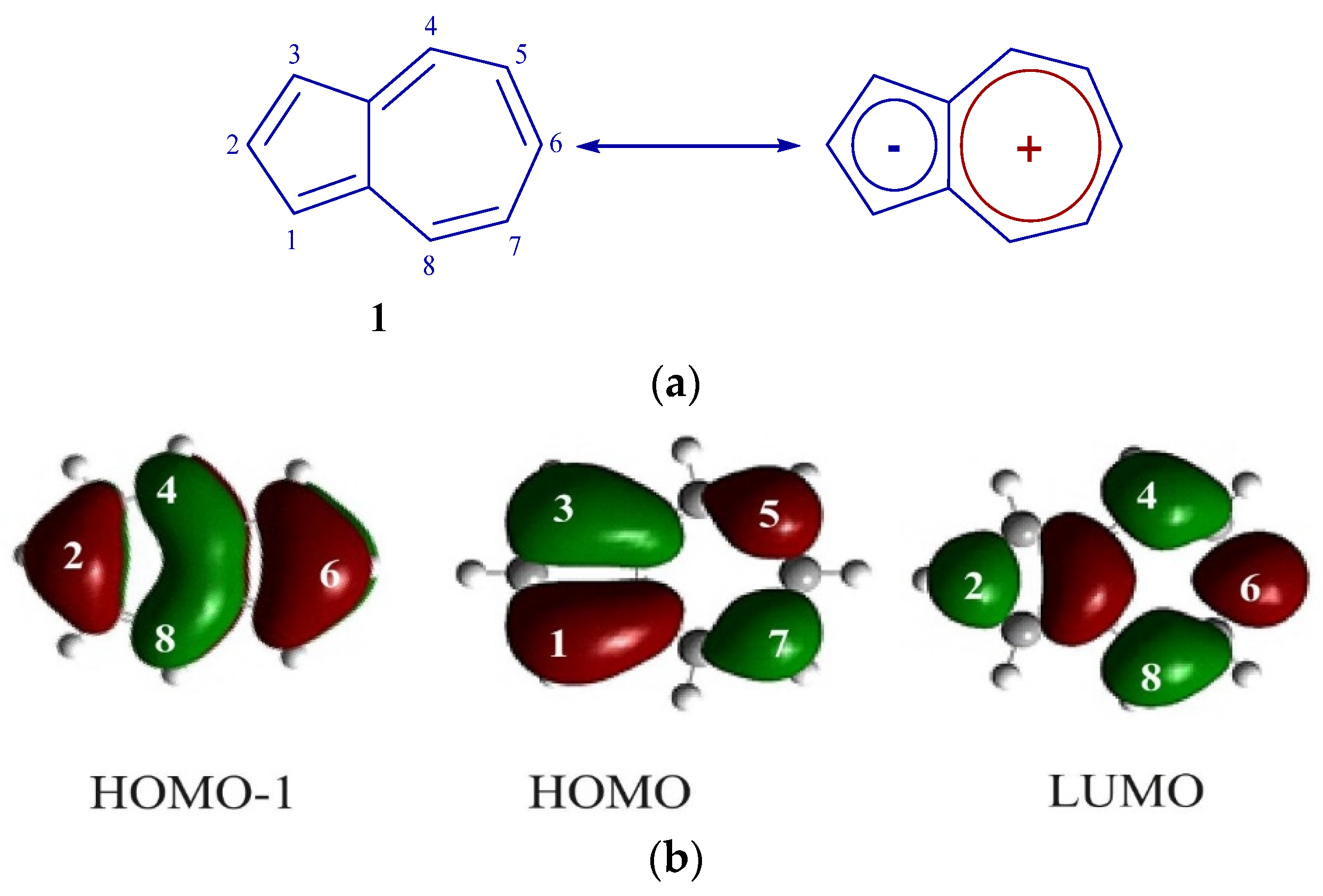
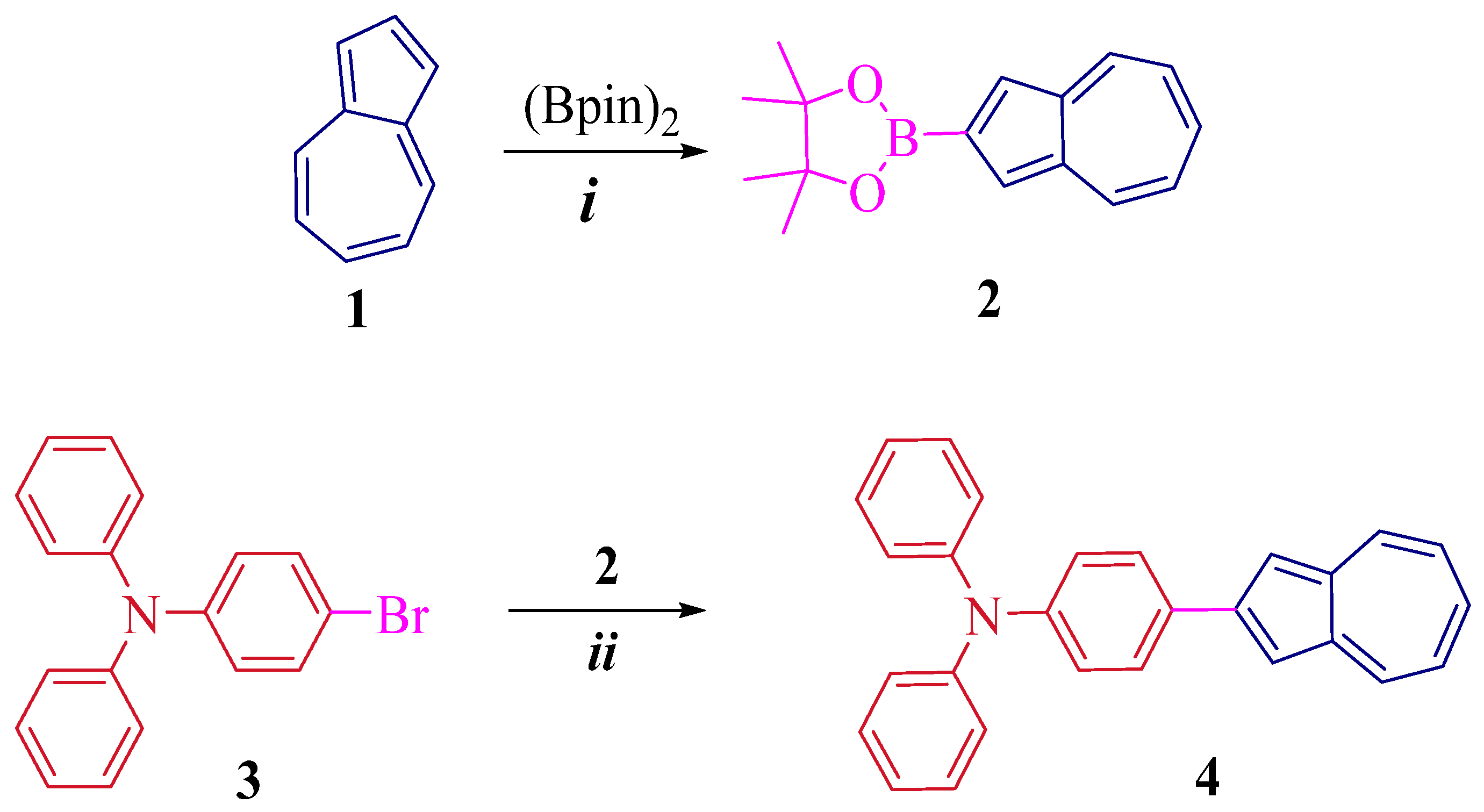
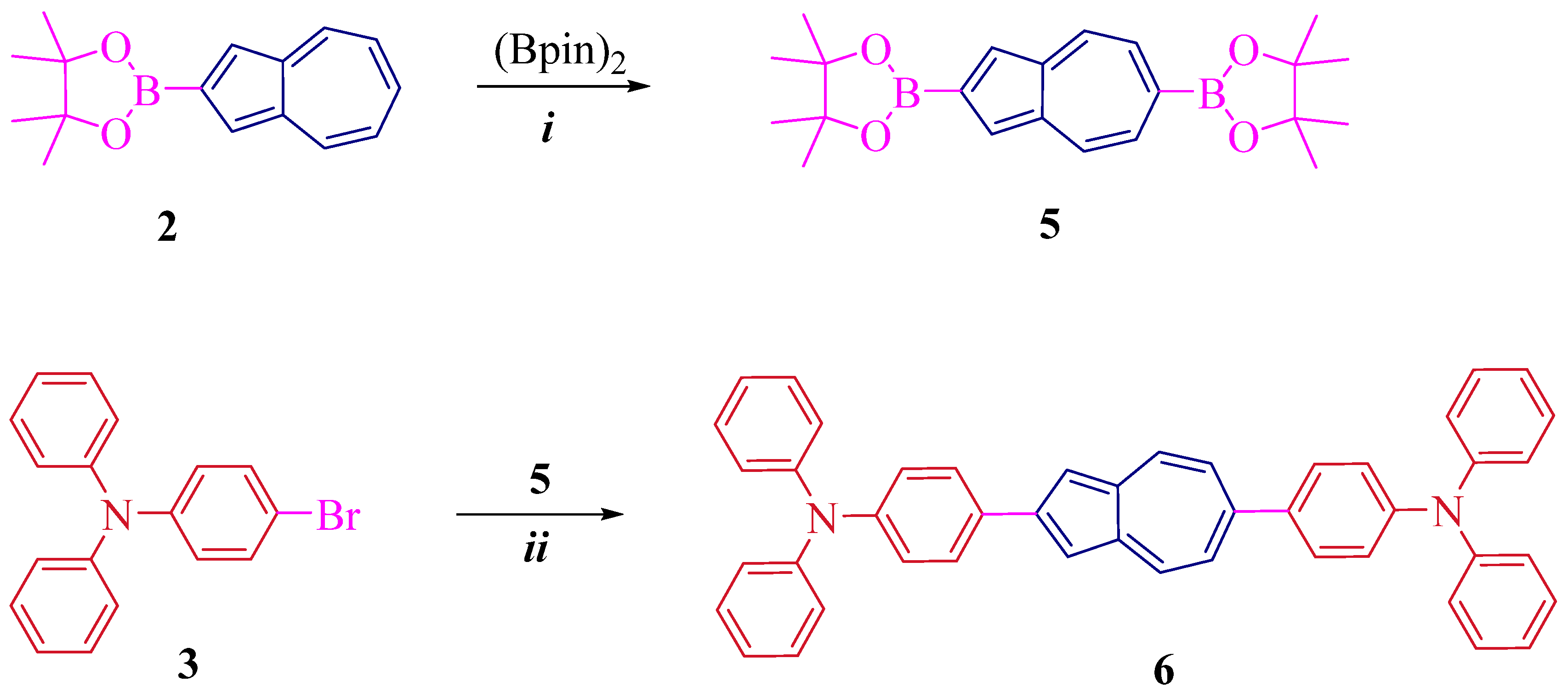
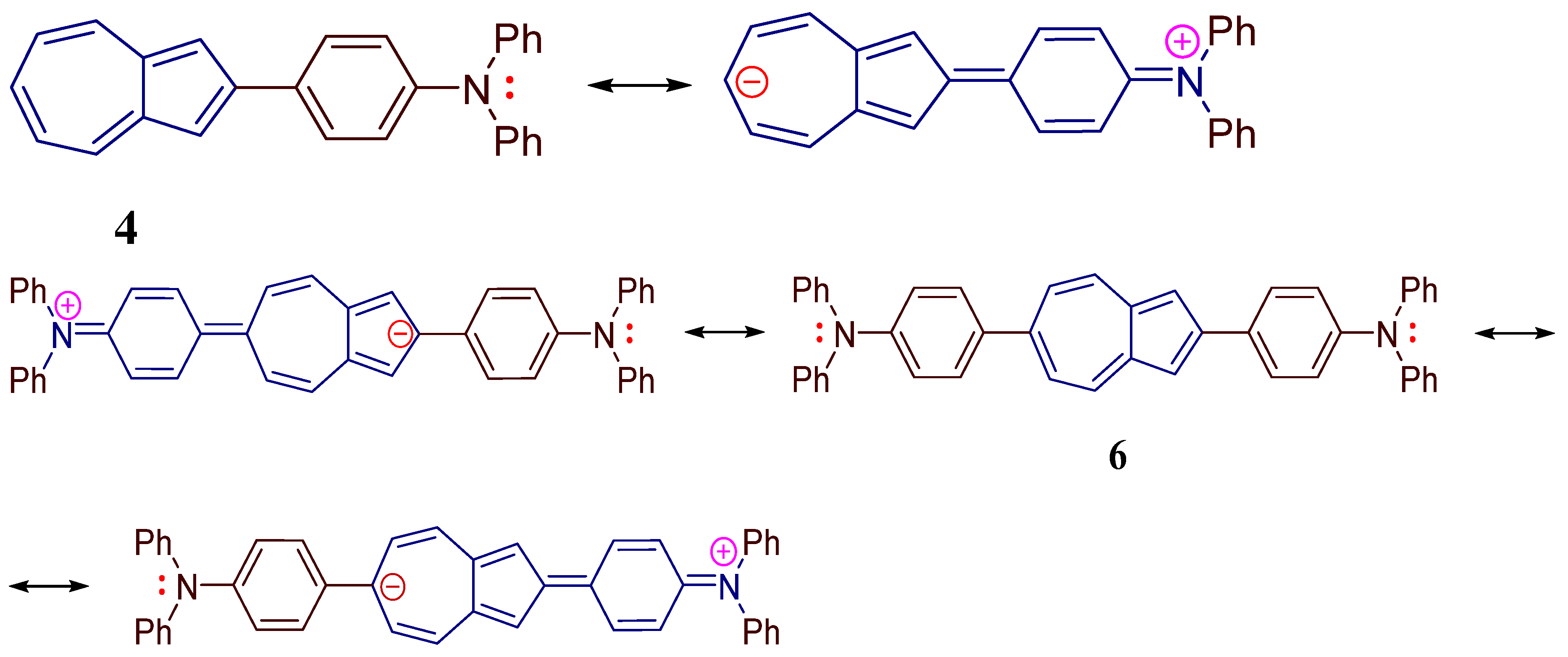
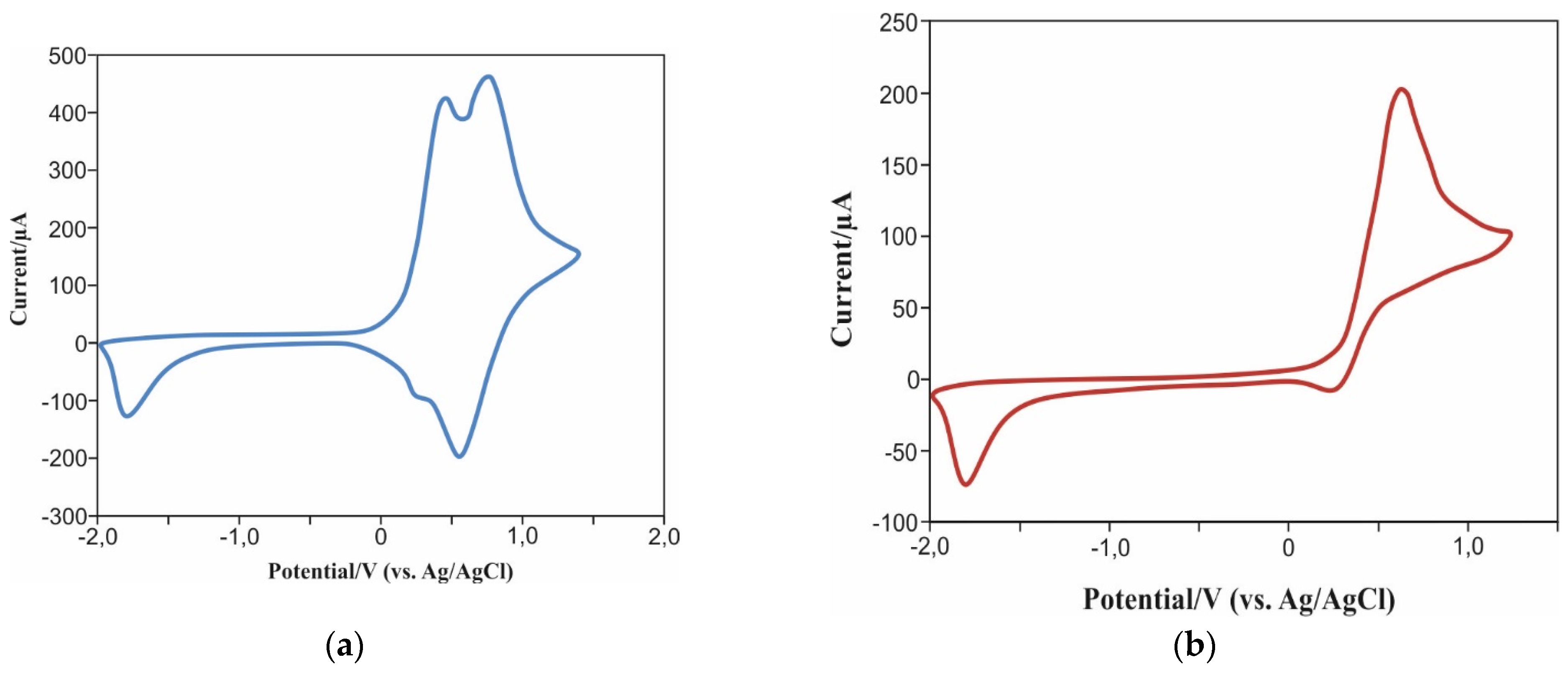
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freudenberg, J.; Jänsch, D.; Hinkel, F.; Bunz, U.H.F. Immobilization Strategies for Organic Semiconducting Conjugated Polymers. Chem. Rev. 2018, 118, 5598. [Google Scholar] [CrossRef]
- Roy, M.; Walton, J.H.; Fettinger, J.C.; Balch, A.L. Direct Crystallization of Diamine Radical Cations: Carbon-Nitrogen Bond Formation from the Reaction of Triphenylamine with TiCl4, TiBr4, or SnCl4 versus Carbon-Carbon Bond Formation with SbCl5. Chem.–Eur. J. 2022, 28, e202104631. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Yang, L.; Pan, F.-F.; Yu, G.-A.; Yin, J.; Liu, S.H. Elaborately Tuning Intramolecular Electron Transfer Through Varying Oligoacene Linkers in the Bis(diarylamino) Systems. Sci. Rep. 2016, 6, 36310. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Song, Y.; Su, Y.; Wang, X. Bis(phenothiazine)arene diradicaloids: Isolation, characterization and crystal structures. Chem. Commun. 2015, 51, 11822. [Google Scholar] [CrossRef]
- Wang, G.; Dmitrieva, E.; Kohn, B.; Scheler, U.; Liu, Y.; Tkachova, V.; Yang, L.; Fu, Y.; Ma, J.; Zhang, P. An Efficient Rechargeable Aluminium–Amine Battery Working Under Quaternization Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202116194. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, X.; Johnson, L.R.; Bruce, P.G. Kinetics of lithium peroxide oxidation by redox mediators and consequences for the lithium–oxygen cell. Nat. Commun. 2018, 9, 1. [Google Scholar] [CrossRef]
- Mayer, D.C.; Manzi, A.; Medishetty, R.; Winkler, B.; Schneider, C.; Kieslich, G.; Po, A.; Feldmann, J.; Fischer, R.A. Controlling Multiphoton Absorption Efficiency by Chromophore Packing in Metal-Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 11594. [Google Scholar] [CrossRef]
- Wu, C.; Djurovich, P.I.; Thompson, M.E. Study of energy transfer and triplet exciton diffusion in hole-transporting host materials. Adv. Funct. Mater. 2009, 19, 3157. [Google Scholar] [CrossRef]
- Taniguchi, R.; Noto, N.; Tanaka, S.; Takahashi, K.; Sarkar, S.K.; Oyama, R.; Abe, M.; Koike, T.; Akita, M. Simple generation of various α-monofluoroalkyl radicals by organic photoredox catalysis: Modular synthesis of β-monofluoroketones. Chem. Commun. 2021, 57, 2609. [Google Scholar] [CrossRef]
- Noto, N.; Koike, T.; Akita, M. Visible-Light-Triggered Monofluoromethylation of Alkenes by Strongly Reducing 1,4-Bis(diphenylamino)naphthalene Photoredox Catalysis. ACS Catal. 2019, 9, 4382. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Higashibeppu, M.; Mazaki, Y. Synthesis and Properties of Twisted and Helical Azulene Oligomers and Azulene-Based Polycyclic Hydrocarbons. ChemistryOpen 2023, 12, e202100298. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Katsuoka, Y.; Yoza, K.; Sato, H.; Mazaki, Y. Stereochemistry, Stereodynamics, and Redox and Complexation Behaviors of 2,2′-Diaryl-1,1′-Biazulenes. ChemPlusChem 2019, 84, 1659. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Umemura, R.; Kaminaga, M.; Kushida, S.; Ohkubo, K.; Noro, S.I.; Mazaki, Y. Paddlewheel Complexes with Azulenes: Electronic Interaction between Metal Centers and Equatorial Ligands. ChemPlusChem 2019, 84, 655. [Google Scholar] [CrossRef]
- Konishi, A.; Yasuda, M. Breathing new life into nonalternant hydrocarbon chemistry: Syntheses and properties of polycyclic hydrocarbons containing azulene, pentalene, and heptalene frameworks. Chem. Lett. 2021, 50, 195. [Google Scholar] [CrossRef]
- Xin, H.; Hou, B.; Gao, X. Azulene-Based π-Functional Materials: Design, Synthesis, and Applications. Acc. Chem. Res. 2021, 54, 1737. [Google Scholar] [CrossRef]
- Elwahy, A.H.; Hafner, K. Alkynylazulenes as Building Blocks for Highly Unsaturated Scaffolds. Asian J. Org. Chem. 2021, 10, 2010. [Google Scholar] [CrossRef]
- Bakun, P.; Czarczynska-Goslinska, B.; Goslinski, T.; Lijewski, S. In vitro and in vivo biological activities of azulene derivatives with potential applications in medicine. Med. Chem. Res. 2021, 30, 834. [Google Scholar] [CrossRef]
- Lvov, A.G.; Bredihhin, A. Azulene as an ingredient for visible-light- and stimuli-responsive photoswitches. Org. Biomol. Chem. 2021, 19, 4460. [Google Scholar] [CrossRef]
- Murfin, L.C.; Lewis, S.E. Azulene—A Bright Core for Sensing and Imaging. Molecules 2021, 26, 353. [Google Scholar] [CrossRef]
- Shoji, T.; Ito, S.; Yasunami, M. Synthesis of Azulene Derivatives from 2H-Cyclohepta[b]furan-2-ones as Starting Materials: Their Reactivity and Properties. Int. J. Mol. Sci. 2021, 22, 10686. [Google Scholar] [CrossRef]
- Anderson, A.G.; Steckler, B.M. Azulene. VIII. A Study of the Visible Absorption Spectra and Dipole Moments of Some 1- and 1,3-Substituted Azulenes. J. Am. Chem. Soc. 1959, 81, 4941–4946. [Google Scholar] [CrossRef]
- Tomin, V.I.; Włodarkiewicz, A. Anti-Kasha behavior of DMABN dual fluorescence. J. Lumin. 2018, 198, 220–225. [Google Scholar] [CrossRef]
- Nenov, A.; Borrego-Varillas, R.; Oriana, A.; Ganzer, L.; Segatta, F.; Conti, I.; Segarra-Marti, J.; Omachi, J.; Dapor, M.; Taioli, S.; et al. UV-Light-Induced Vibrational Coherences: The Key to Understand Kasha Rule Violation in trans-Azobenzene. J. Phys. Chem. Lett. 2018, 9, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, J.C.; Catalán, J. Kasha’s Rule: A Reappraisal. Phys. Chem. Chem. Phys. 2019, 21, 10061–10069. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Park, S.Y.; Gierschner, J. Dual Emission: Classes, Mechanisms, and Conditions. Angew. Chem. Int. Ed. 2021, 60, 22624–22638. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, D.; Ludvikova, L.; Banerjee, A.; Ottosson, H.; Slanina, T. Excited-State (Anti)Aromaticity Explains Why Azulene Disobeys Kasha’s Rule. J. Am. Chem. Soc. 2023, 145, 21569–21575. [Google Scholar] [CrossRef] [PubMed]
- Shevyakov, S.V.; Li, H.; Muthyala, R.; Asato, A.E.; Croney, J.C.; Jameson, D.M.; Liu, R.S. Orbital control of the color and excited state properties of formylated and fluorinated derivatives of azulene. J. Phys. Chem. A 2003, 107, 3295. [Google Scholar] [CrossRef]
- Kurotobi, K.; Miyauchi, M.; Takakura, K.; Murafuji, T.; Sugihara, Y. Direct Introduction of a Boryl Substituent into the 2-Position of Azulene: Application of the Miyaura and Smith Methods to Azulene. Eur. J. Org. Chem. 2003, 2003, 3663–3665. [Google Scholar] [CrossRef]
- Fujinaga, M.; Murafuji, T.; Kurotobi, K.; Sugihara, Y. Polyborylation of azulenes. Tetrahedron 2009, 65, 7115–7121. [Google Scholar] [CrossRef]
- Rahimi, K.; Botiz, I.; Agumba, J.O.; Motamen, S.; Stingelin, N.; Reiter, G. Light absorption of poly(3-hexylthiophene) single crystals. RSC Adv. 2014, 4, 11121. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Koumura, N.; Cui, Y.; Takahashi, M.; Sekiguchi, H.; Mori, A.; Kubo, T.; Furube, A.; Hara, K. Hexylthiophene-Functionalized Carbazole Dyes for Efficient Molecular Photovoltaics: Tuning of Solar-Cell Performance by Structural Modification. Chem. Mater. 2008, 20, 3993. [Google Scholar] [CrossRef]
- Shirota, Y.; Kageyama, H. Charge Carrier Transporting Molecular Materials and Their Applications in Devices. Chem. Rev. 2007, 107, 953. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Habibi, A.; Ni, P.; Nahdi, H.; Bouanis, F.Z.; Bourcier, S.; Clavier, G.; Frigoli, M.; Yassar, A. Synthesis and characterization of solution-processed indophenine derivatives for function as a hole transport layer for perovskite solar cells. Dye. Pigment. 2023, 213, 111136. [Google Scholar] [CrossRef]
- Ren, S.; Wang, Z.; Zhang, W.; Ding, Y.; Yi, Z. Donor-acceptor-based organic polymer semiconductor materials to achieve high hole mobility in organic field-effect transistors. Polymers 2023, 15, 3713. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.R.; Fréchet, J.M.J. Organic Semiconducting Oligomers for Use in Thin Film Transistors. Chem. Rev. 2007, 107, 1066. [Google Scholar] [CrossRef] [PubMed]
- Zaumseil, J.; Sirringhaus, H. Electron and Ambipolar Transport in Organic Field-Effect Transistors. Chem. Rev. 2007, 107, 1296. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Huang, F.; Cao, Y. Recent development of push–pull conjugated polymers for bulk-heterojunction photovoltaics: Rational design and fine tailoring of molecular structures. J. Mater. Chem. 2012, 22, 10416. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Hamano, T.; Inoue, M.; Nakamura, T.; Wakamiya, A.; Mazaki, Y. Intense absorption of azulene realized by molecular orbital inversion. Chem. Commun. 2023, 59, 10604–10607. [Google Scholar] [CrossRef]
- Ren, S.; Wang, Z.; Zhang, W.; Yassar, A.; Chen, J.; Wang, S. Incorporation of diketopyrrolopyrrole into polythiophene for the preparation of organic polymer transistors. Molecules 2024, 29, 260. [Google Scholar] [CrossRef]
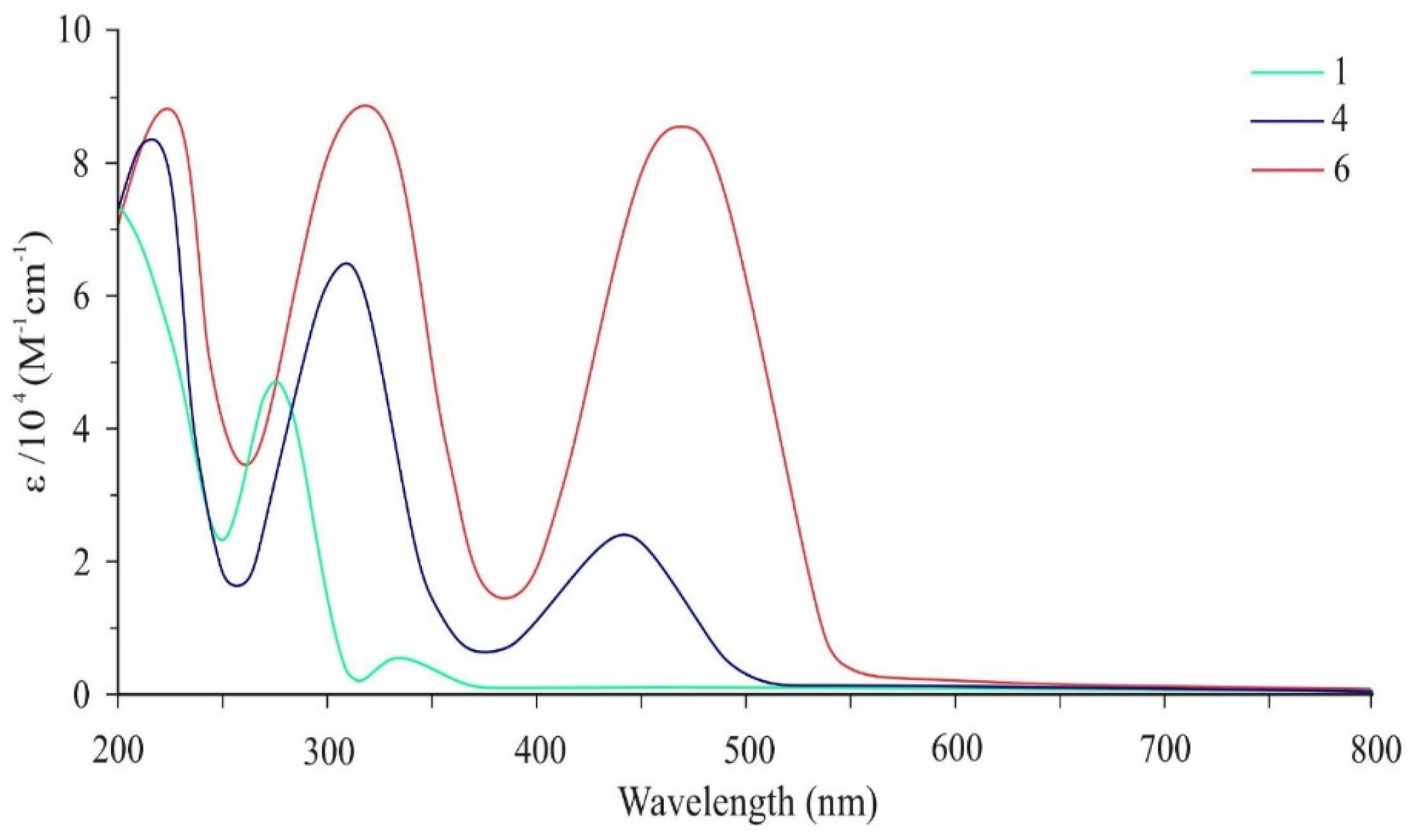
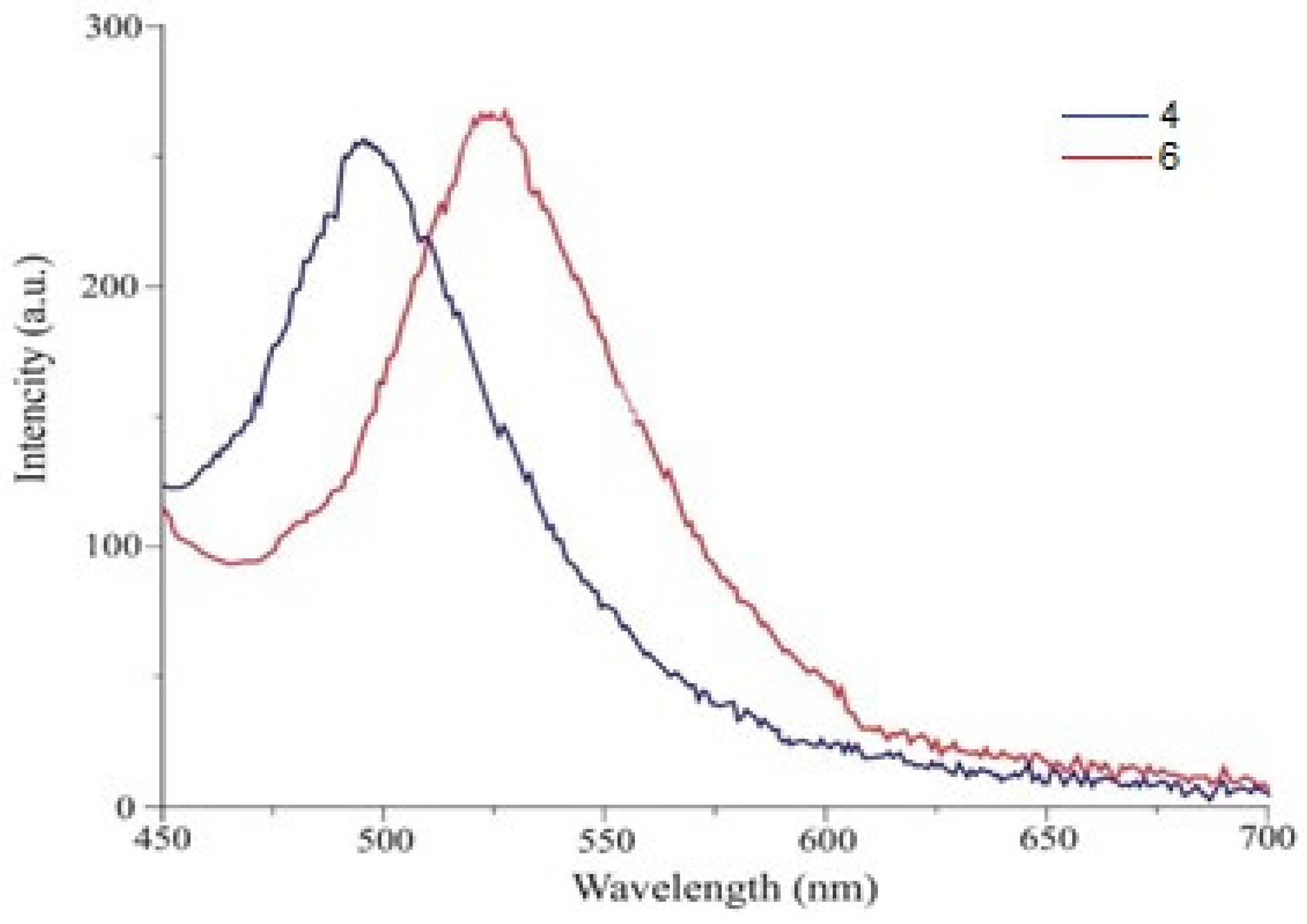

| Compound | Solvent | Absorption | Fluorescence a | ||
|---|---|---|---|---|---|
| λabs, nm | ε M−1 cm−1 | λem, nm | Intensity, a.u | ||
| 4 | Dichloromethane | 215 | 83,695 | 495 | 257 |
| 306 | 64,130 | ||||
| 436 | 24,637 | ||||
| 6 | Dichloromethane | 224 | 87,977 | 525 | 264 |
| 315 | 87,888 | ||||
| 468 | 86,888 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merkhatuly, N.; Iskanderov, A.; Abeuova, S.; Iskanderov, A.; Zhokizhanova, S. Introduction of Electron Donor Groups into the Azulene Structure: The Appearance of Intense Absorption and Emission in the Visible Region. Molecules 2024, 29, 3354. https://doi.org/10.3390/molecules29143354
Merkhatuly N, Iskanderov A, Abeuova S, Iskanderov A, Zhokizhanova S. Introduction of Electron Donor Groups into the Azulene Structure: The Appearance of Intense Absorption and Emission in the Visible Region. Molecules. 2024; 29(14):3354. https://doi.org/10.3390/molecules29143354
Chicago/Turabian StyleMerkhatuly, Nurlan, Ablaykhan Iskanderov, Saltanat Abeuova, Amantay Iskanderov, and Saltanat Zhokizhanova. 2024. "Introduction of Electron Donor Groups into the Azulene Structure: The Appearance of Intense Absorption and Emission in the Visible Region" Molecules 29, no. 14: 3354. https://doi.org/10.3390/molecules29143354
APA StyleMerkhatuly, N., Iskanderov, A., Abeuova, S., Iskanderov, A., & Zhokizhanova, S. (2024). Introduction of Electron Donor Groups into the Azulene Structure: The Appearance of Intense Absorption and Emission in the Visible Region. Molecules, 29(14), 3354. https://doi.org/10.3390/molecules29143354








