A Novel Ternary Catalyst PW4@MOF-808@SBA-15 for Deep Extraction Oxidation Desulfurization of Model Diesel
Abstract
1. Introduction
2. Results and Discussion
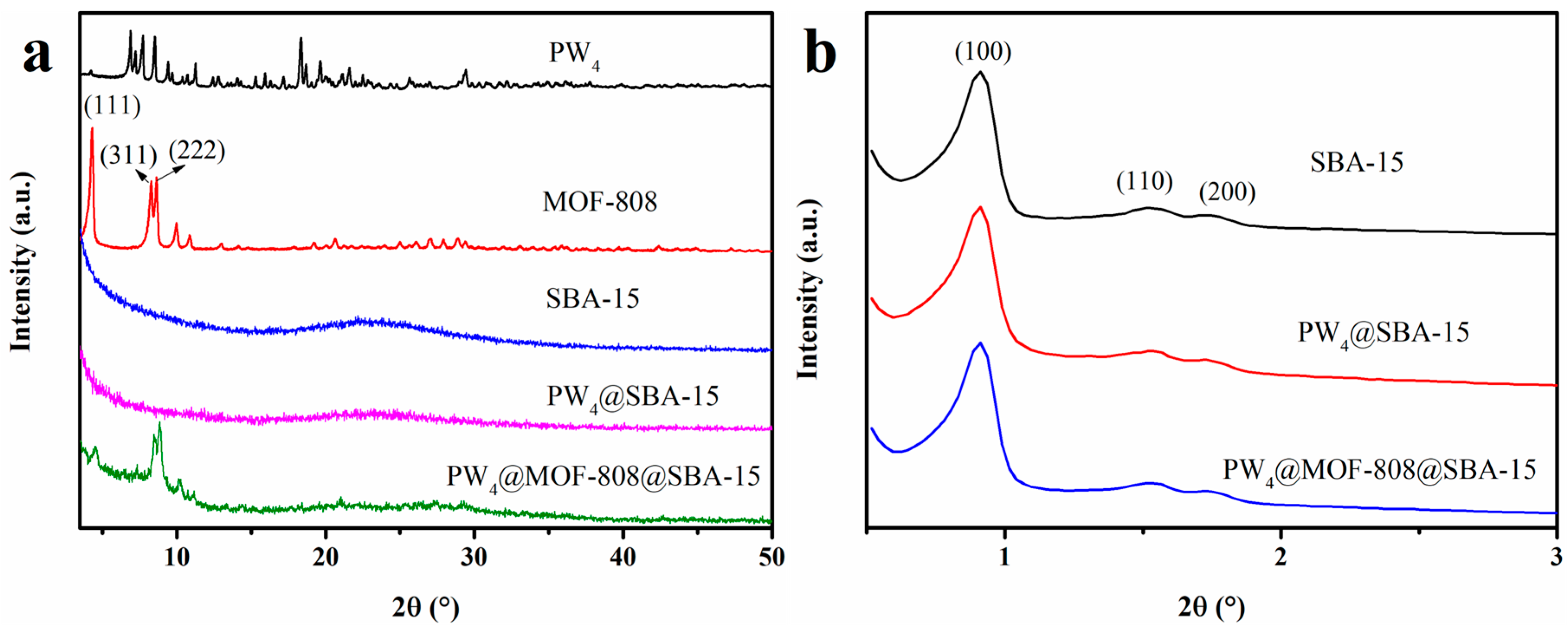
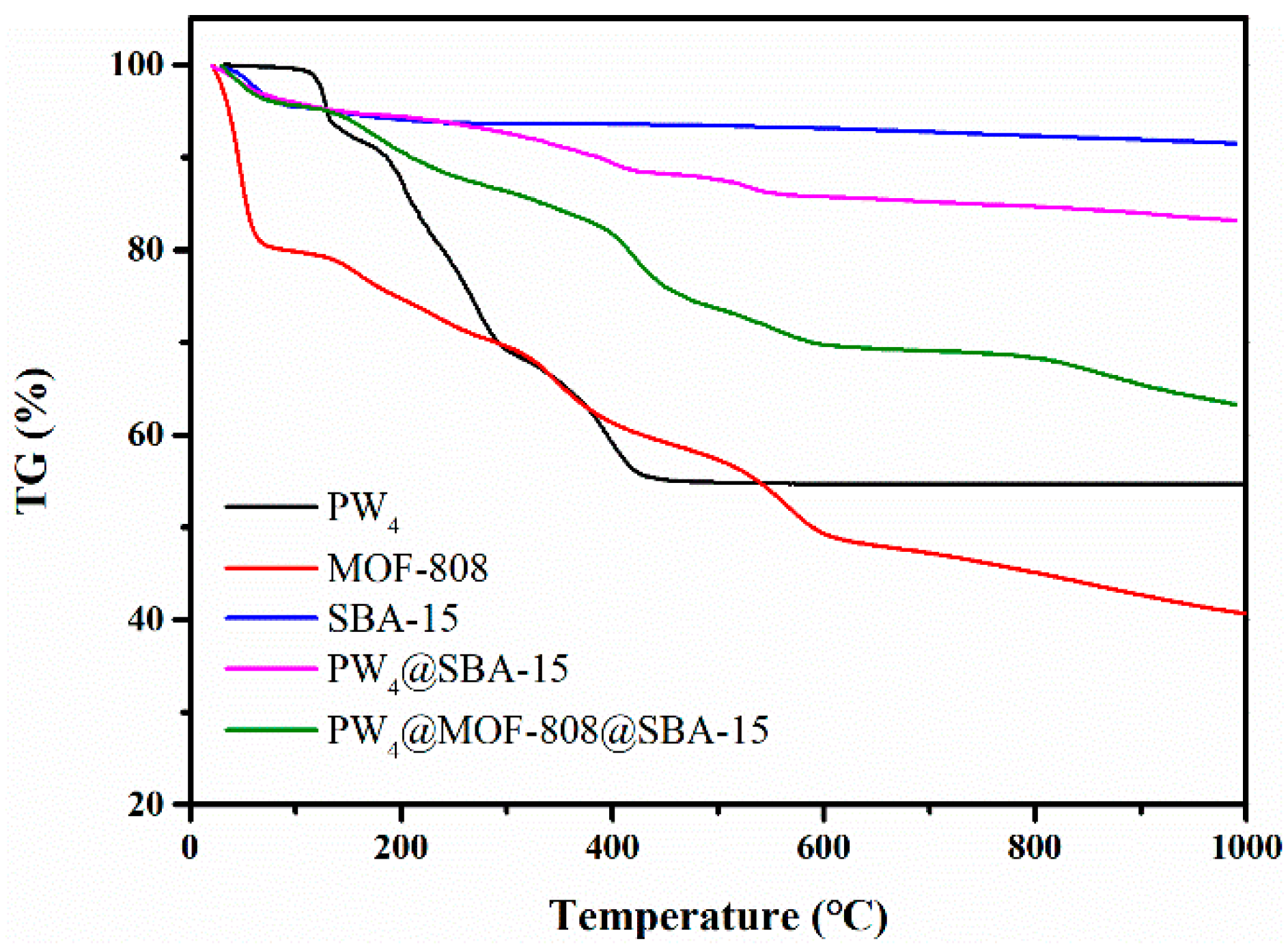
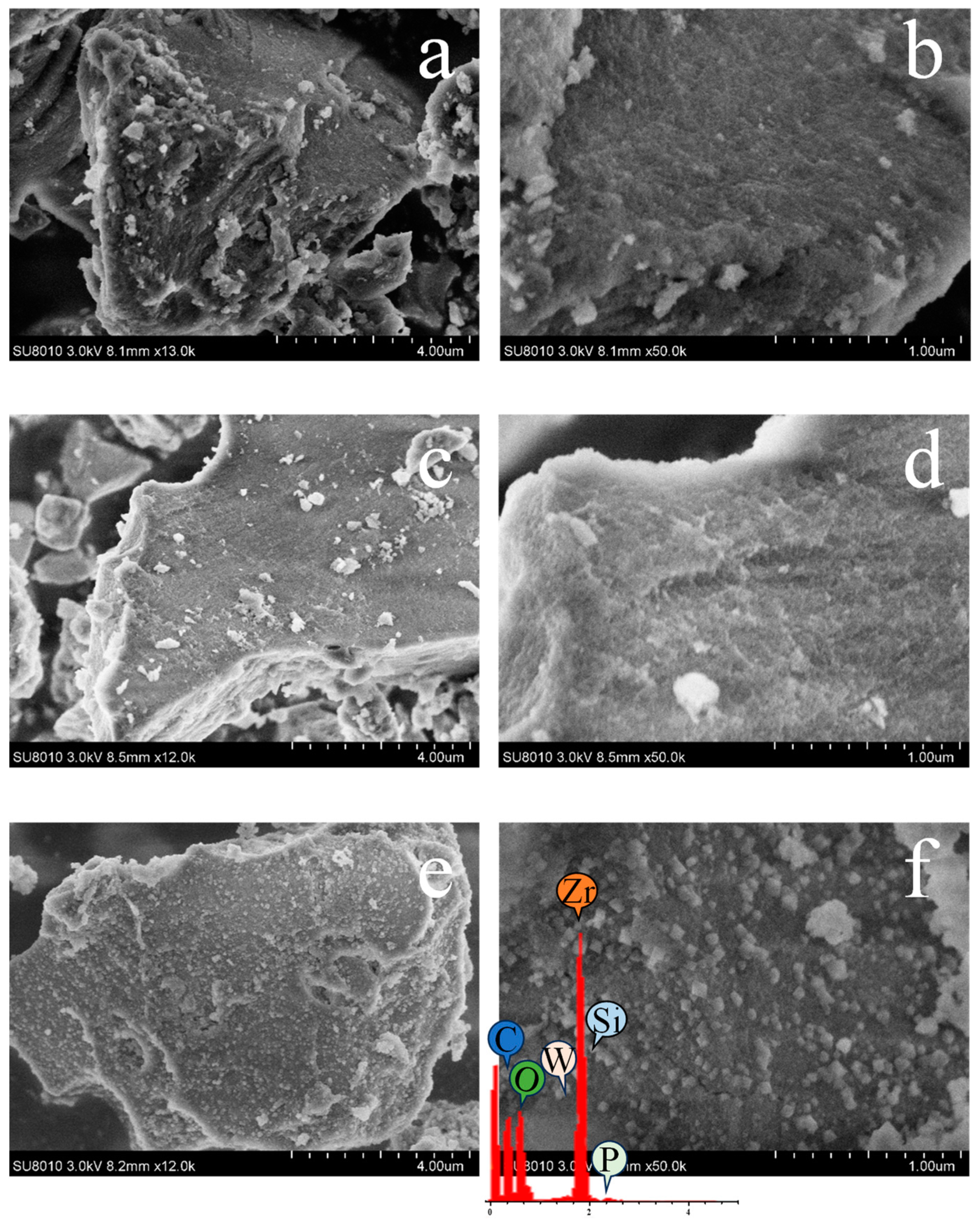
2.1. Characterization
2.2. Catalytic Performance
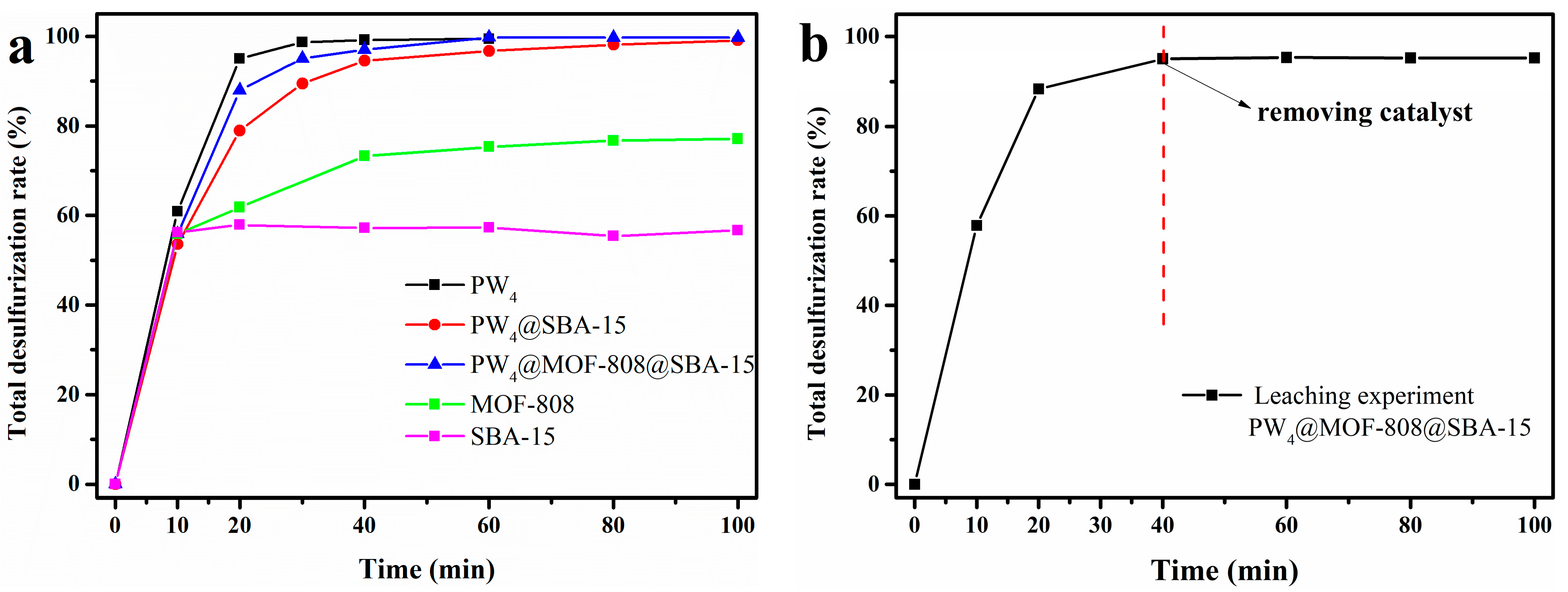
2.2.1. EODS Performance of Different Catalysts
2.2.2. Reusability
2.3. Comparison with Other Catalysts
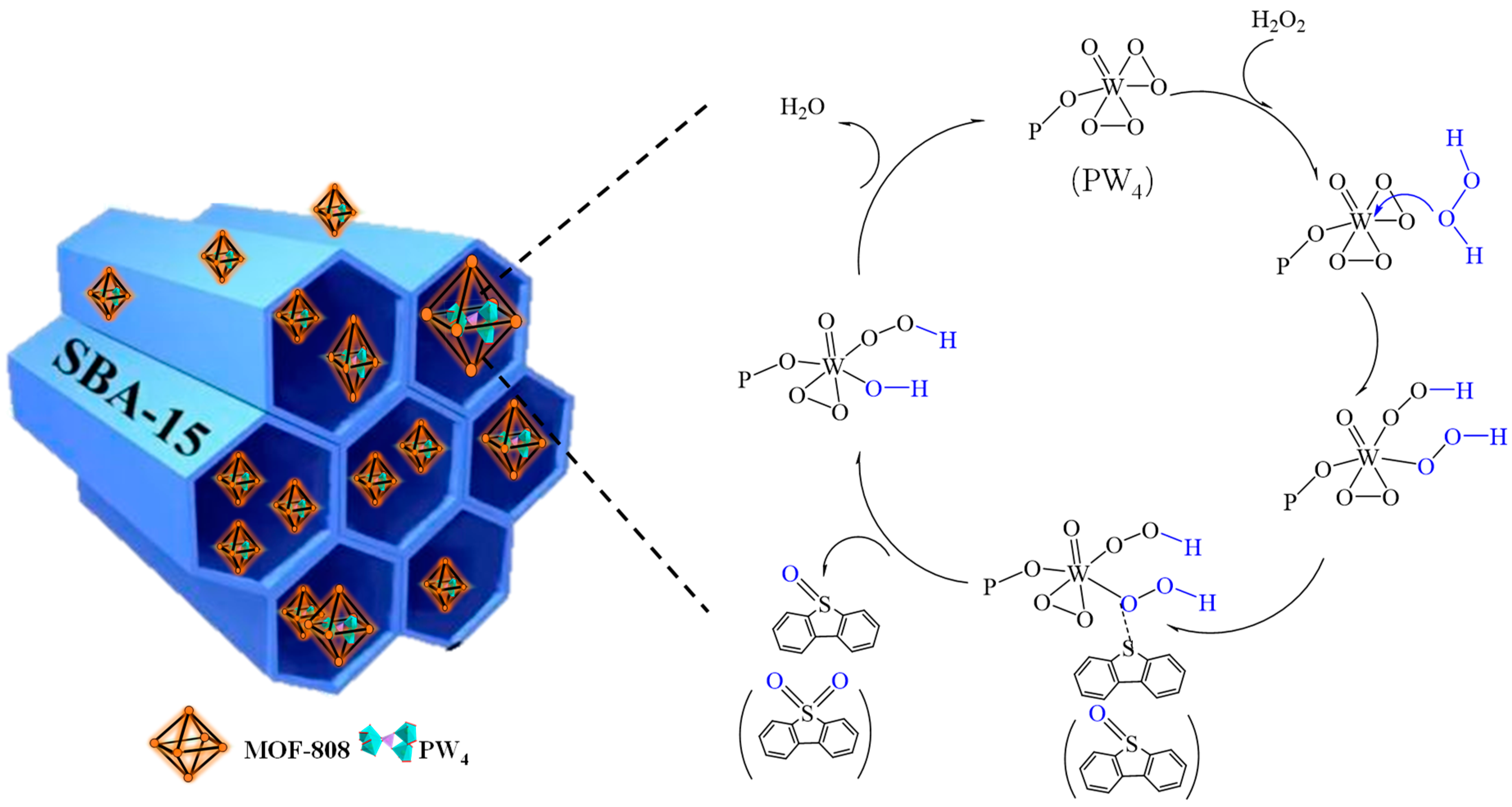
2.4. Possible Mechanism
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Catalyst Preparation
3.2.1. Synthesis of PW4@SBA-15
3.2.2. Synthesis of PW4@MOF-808@SBA-15
3.3. Catalyst Characterization
3.4. Evaluation of EODS Efficiency
3.5. Catalyst Recovery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bian, J.; Zhou, Z.; Yu, J.; Shi, X.; Huang, P.; Du, W. A comprehensive energy flow analysis method based on hydrogen energy to electric energy conversion. J. Phys. Conf. Ser. 2024, 2788, 012004. [Google Scholar] [CrossRef]
- Yildiz, I.; Caliskan, H.; Mori, K. Energy, exergy and environmental assessments of biodiesel and diesel fuels for an internal combustion engine using silicon carbide particulate filter. J. Therm. Anal. Calorim. 2021, 145, 739–750. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Peng, L.; Li, Y.; Zhou, Y.; Chai, F.; Mo, Z.; Chen, Z.; Mao, J.; Wang, W. Characterization of precipitation in the background of atmospheric pollutants reduction in Guilin: Temporal variation and source apportionment. J. Environ. Sci. 2020, 98, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-W.; Hung, H.-Y.; Sung, H.-C.; Sheu, Y.-S.; Lin, W.-L.; Wu, S.-P. Total sulfur determination in petroleum fuels for routine quality control by sector field inductively coupled plasma mass spectrometry after dilution treatment. J. Anal. At. Spectrom. 2019, 34, 570–576. [Google Scholar] [CrossRef]
- Petrova, D.; Lyubimenko, V.; Ivanov, E.; Gushchin, P.; Kolesnikov, I. Energy Basics of Catalytic Hydrodesulfurization of Diesel Fuels. Catalysts 2022, 12, 1301. [Google Scholar] [CrossRef]
- de Azambuja, A.O.; Bücker, F.; de Quadros, P.D.; Zhalnina, K.; Dias, R.; Vacaro, B.B.; Correa, C.; Ferrão, M.F.; de Oliveira Camargo, F.A.; Triplett, E.; et al. Microbial community composition in Brazilian stored diesel fuel of varying sulfur content, using high-throughput sequencing. Fuel 2017, 189, 340–349. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Chen, Y.; Wang, Z. Removal of sulfide from fuels by ionic liquids: Prospects for the future. Braz. J. Chem. Eng. 2023, 40, 929–963. [Google Scholar] [CrossRef]
- Duan, Z.; Bian, H.; Gao, Z.; Zhu, L.; Xia, D. Green Fuel Desulfurization with β-Cyclodextrin Aqueous Solution for Thiophenic Sulfides by Molecular Inclusion. Energy Fuels 2019, 33, 9690–9701. [Google Scholar] [CrossRef]
- Wang, E.; Yang, F.; Song, M.; Chen, G.; Zhang, Q.; Wang, F.; Bing, L.; Wang, G.; Han, D. Recent advances in the unsupported catalysts for the hydrodesulfurization of fuel. Fuel Process. Technol. 2022, 235, 107386. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Liu, T.; Jiang, Z.; Li, C. Optimizing both the CoMo/Al2O3 catalyst and the technology for selectivity enhancement in the hydrodesulfurization of FCC gasoline. Appl. Catal. A: Gen. 2019, 575, 187–197. [Google Scholar] [CrossRef]
- Srour, H.; Devers, E.; Mekki-Berrada, A.; Toufaily, J.; Hamieh, T.; Batiot-Dupeyrat, C.; Pinard, L. Regeneration of an aged hydrodesulfurization catalyst: Conventional thermal vs non-thermal plasma technology. Fuel 2021, 306, 121674. [Google Scholar] [CrossRef]
- Zaidi, Z.; Gupta, Y.; Gayatri, S.L.; Singh, A. A comprehensive discussion on fuel combustion and desulfurization technologies. Inorg. Chem. Commun. 2023, 154, 110964. [Google Scholar] [CrossRef]
- Adhami, M.; Movahedirad, S.; Sobati, M.A. Novel Method for Desulfurization of Mixed Fuel via Microbubble Oxidation Followed by Microtube Extraction. Energy Fuels 2024, 38, 2153–2166. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Yin, J.; Liu, X.; Qiu, W.; He, J.; Jiang, W.; Zhu, L.; Li, H.; Li, H. Mo-MOF-based ionanofluids for highly efficient extraction coupled catalytic oxidative desulfurization. Sep. Purif. Technol. 2025, 353, 128289. [Google Scholar] [CrossRef]
- Sun, S.; Yu, X.; Guo, Y.; Chen, L.; Wang, X.; Jiang, Z. Temperature-Responsive Polyoxometalate Catalysts for DBT Desulfurization in One-Pot Oxidation Combined with Extraction. Catal. Surv. Asia 2016, 20, 98–108. [Google Scholar] [CrossRef]
- Mohammed, W.T.; Almilly, R.F.K.; Al-Ali, S.B.A. Desulfurization of Diesel Fuel by Oxidation and Solvent Extraction. J. Eng. 2015, 21, 87–102. [Google Scholar] [CrossRef]
- Dou, S.-Y.; Wang, R. High-efficient utilization of gaseous oxidants in oxidative desulfurization based on sulfonated carbon materials. Fuel Process. Technol. 2022, 235, 107372. [Google Scholar] [CrossRef]
- Chen, L.; Ren, J.-T.; Wang, H.-Y.; Sun, M.-L.; Yuan, Z.-Y. Engineering a Local Hydrophilic Environment in Fuel Oil for Efficient Oxidative Desulfurization with Minimum H2O2 Oxidant. ACS Catal. 2023, 13, 12125–12133. [Google Scholar] [CrossRef]
- Adhami, M.; Movahedirad, S.; Sobati, M.A. Oxidative desulfurization of fuels using gaseous oxidants: A review. J. Sulfur Chem. 2022, 43, 685–712. [Google Scholar] [CrossRef]
- Ye, G.; Yang, Z.; Shi, G.; Huang, R.; Liu, X.; Zhang, Q.; Song, R. Synthesis of amorphous titanium isophthalic acid catalyst with hierarchical porosity for efficient oxidative desulfurization of model feed with minimum oxidant. Sep. Purif. Technol. 2025, 354, 128669. [Google Scholar] [CrossRef]
- Bertleff, B.; Claußnitzer, J.; Korth, W.; Wasserscheid, P.; Jess, A.; Albert, J. Extraction Coupled Oxidative Desulfurization of Fuels to Sulfate and Water-Soluble Sulfur Compounds Using Polyoxometalate Catalysts and Molecular Oxygen. ACS Sustain. Chem. Eng. 2017, 5, 4110–4118. [Google Scholar] [CrossRef]
- Zou, J.; Lin, Y.; Wu, S.; Wu, M.; Yang, C. Construction of bifunctional 3-D ordered mesoporous catalyst for oxidative desulfurization. Sep. Purif. Technol. 2021, 264, 118434. [Google Scholar] [CrossRef]
- Rajendran, A.; Cui, T.-Y.; Fan, H.-X.; Yang, Z.-F.; Feng, J.; Li, W.-Y. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Mehrvarz, E.; Taghipour, A. Polyoxometalate as an effective catalyst for the oxidative desulfurization of liquid fuels: A critical review. Rev. Chem. Eng. 2020, 36, 831–858. [Google Scholar] [CrossRef]
- Julião, D.; Mirante, F.; Ribeiro, S.O.; Gomes, A.C.; Valença, R.; Ribeiro, J.C.; Pillinger, M.; de Castro, B.; Gonçalves, I.S.; Balula, S.S. Deep oxidative desulfurization of diesel fuels using homogeneous and SBA-15-supported peroxophosphotungstate catalysts. Fuel 2019, 241, 616–624. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Duarte, B.; De Castro, B.; Granadeiro, C.M.; Balula, S.S. Improving the Catalytic Performance of Keggin [PW12O40]3− for Oxidative Desulfurization: Ionic Liquids versus SBA-15 Composite. Materials 2018, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Sun, M.; Fan, J.; Wang, P.M.; Ma, W.J.; Wei, J.F. Deep oxidative desulfurization of benzothiophene and dibenzothiophene with a peroxophosphotungstate–ionic liquid brush assembly. Appl. Organomet. Chem. 2015, 29, 633–637. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Geyer, F.; Schneemann, A.; Kment, Š.; Otyepka, M.; Zboril, R.; Vollmer, D.; Fischer, R.A. Hydrophobic Metal–Organic Frameworks. Adv. Mater. 2019, 31, 1900820. [Google Scholar] [CrossRef]
- Chakraborty, A.; Maji, T.K. Mg-MOF-74@SBA-15 hybrids: Synthesis; characterization, and adsorption properties. APL Mater. 2014, 2, 124107. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Luz, I.; Soukri, M.; Van, C.; Vankelecom, I.F.J.; Lail, M.; De, D.E. Boosting the Catalytic Performance of Metal–Organic Frameworks for Steroid Transformations by Confinement within a Mesoporous Scaffold. Angew. Chem. Int. Ed. 2017, 56, 13302–13306. [Google Scholar] [CrossRef]
- Marcucci, S.M.P.; Zanin, G.M.; Arroyo, P.A. Synthesis of SBA-15 and pore-expanded SBA-15 and surface modification with tin for covalent lipase immobilization. Microporous Mesoporous Mater. 2022, 337, 111951. [Google Scholar] [CrossRef]
- Liu, H.; Ding, W.; Lei, S.; Tian, X.; Zhou, F. Selective Adsorption of CH4/N2 on Ni-based MOF/SBA-15 Composite Materials. Nanomaterials 2019, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; He, X.; Li, W.; Zhao, L.; Fang, W.; Chen, H.; Zhang, F.; Zhang, W.; Wang, W. Zr-MOFs based on Keggin-type polyoxometalates for photocatalytic hydrogen production. J. Mater. Sci. 2018, 53, 12016–12029. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Zhang, Z.; Zhang, B.; Yang, X.; Chang, X.; Zhou, Z.; Wang, D.-H.; Zhang, M.-H.; Bu, X.-H. Synergistic effect of Zr-MOF on phosphomolybdic acid promotes efficient oxidative desulfurization. Appl. Catal. B Environ. 2019, 256, 117804. [Google Scholar] [CrossRef]
- Fernandes, S.C.; Leo, P.; Santos-Vieira, I.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Improving Oxidative Catalytic Efficiency for Fuels Desulfurization Using Hybrid Materials Based in MOF-808@SBA-15. ChemCatChem 2024, 16, e202400355. [Google Scholar] [CrossRef]
- Li, S.-W.; Yang, Z.; Gao, R.-M.; Zhang, G.; Zhao, J.-S. Direct synthesis of mesoporous SRL-POM@MOF-199@MCM-41 and its highly catalytic performance for the oxidesulfurization of DBT. Appl. Catal. B: Environ. 2018, 221, 574–583. [Google Scholar] [CrossRef]
- Li, S.-W.; Zhang, H.-Y.; Dong, S.-M.; Zhao, J.-S.; Li, R.-X. Highly efficient preformed heteropolyacid catalysts for the deep oxidative desulfurization: MOF as a bridge role under nucleation theory. J. Environ. Chem. Eng. 2022, 10, 107298. [Google Scholar] [CrossRef]
- Haruna, A.; Merican, Z.M.A.; Musa, S.G.; Rahman, M.B.A. MOF-808(Zr)-supported with Keggin Polyoxometalates as an efficient oxidative desulfurization catalyst. J. Taiwan Inst. Chem. Eng. 2023, 147, 104919. [Google Scholar] [CrossRef]
- Mirante, F.; Ribeiro, S.O.; de Castro, B.; Granadeiro, C.M.; Balula, S.S. Sustainable Desulfurization Processes Catalyzed by Titanium-Polyoxometalate@TM-SBA-15. Top. Catal. 2017, 60, 1140–1150. [Google Scholar] [CrossRef]
- Akopyan, A.; Polikarpova, P.; Gul, O.; Anisimov, A.; Karakhanov, E. Catalysts Based on Acidic SBA-15 for Deep Oxidative Desulfurization of Model Fuels. Energy Fuels 2020, 34, 14611–14619. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, P.; Chao, Y.; Li, H.; Zou, F.; Xun, S.; Zhu, F.; Zhao, Z. A Novel Reaction-Controlled Foam-Type Polyoxometalate Catalyst for Deep Oxidative Desulfurization of Fuels. Ind. Eng. Chem. Res. 2013, 52, 17399–17406. [Google Scholar] [CrossRef]
- Zhu, B.; Yan, L.-K.; Guan, W.; Su, Z.-M. DFT characterization on the mechanism of sulfoxidation with H2O2 catalyzed by tetranuclear peroxotungstates [XO4{WO(O2)2}4]n− (X = SiIV, PV, SVI, AsV, and SeVI). Dalton Trans. 2015, 44, 9063–9070. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, G.; Barozzino-Consiglio, G.; Robeyns, K.; Devillers, M.; Gaigneaux, E.M. Formation; Reactivity, and Catalytic Behavior of a Keggin Polyoxometalate/Bipyridine Hybrid in the Epoxidation of Cyclooctene with H2O2. Inorg. Chem. 2023, 62, 8576–8588. [Google Scholar] [CrossRef]
- Gao, Y.; Jia, S.; Zhou, H.; Chen, Z.; Yan, M.; Zhao, J. In situ encapsulation of peroxophosphotungstate in ZIF-8: A highly active, reusable and structurally stable catalyst for desulfurization. Fuel Process. Technol. 2024, 254, 108033. [Google Scholar] [CrossRef]

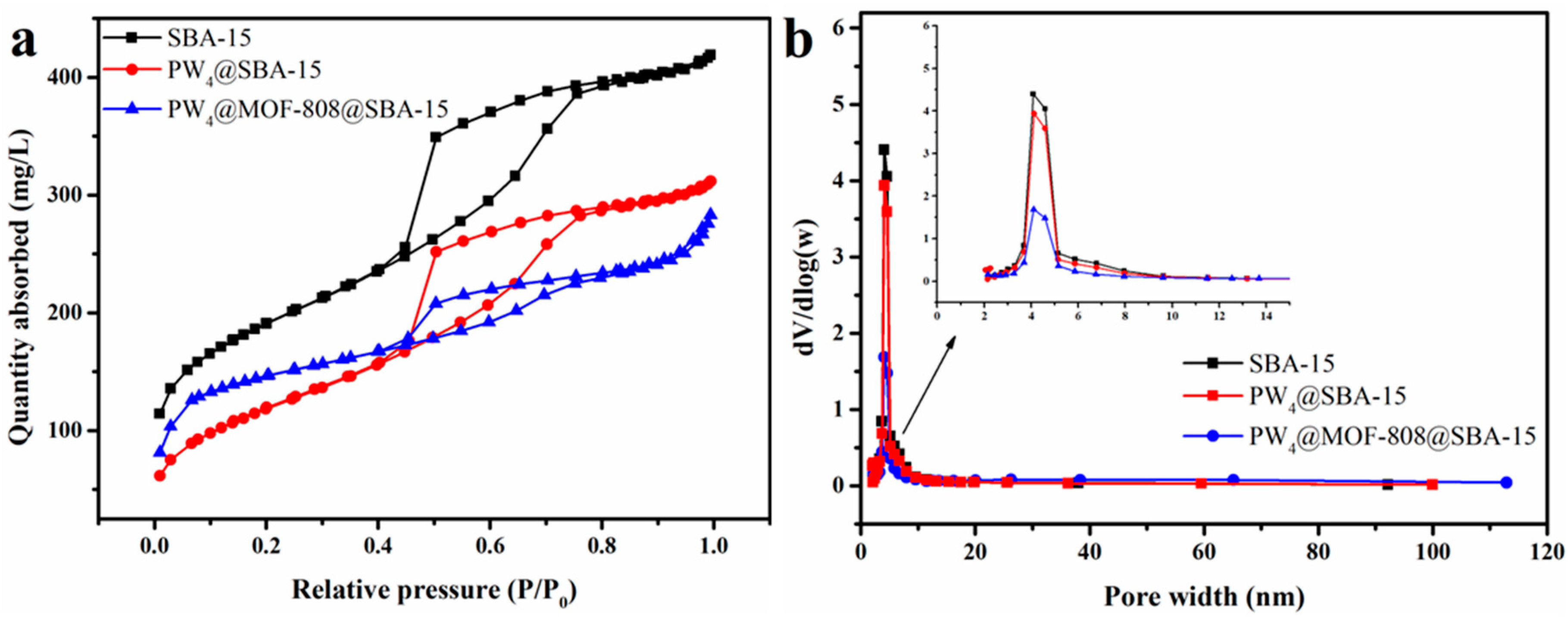
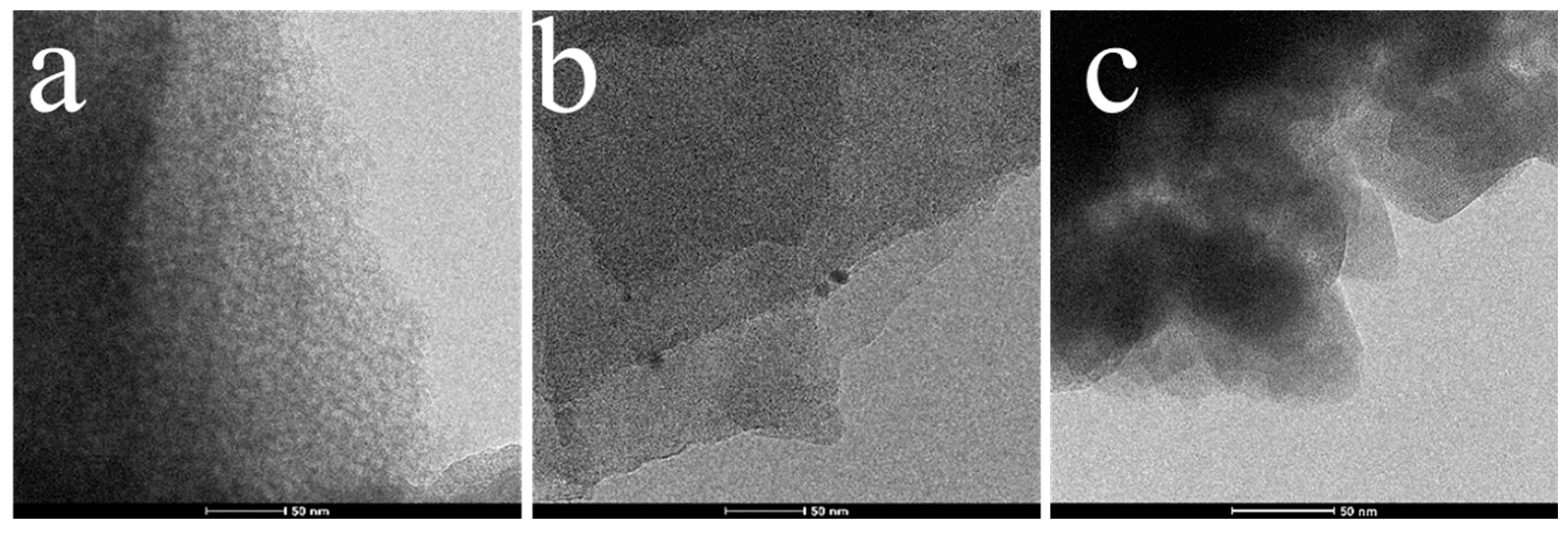
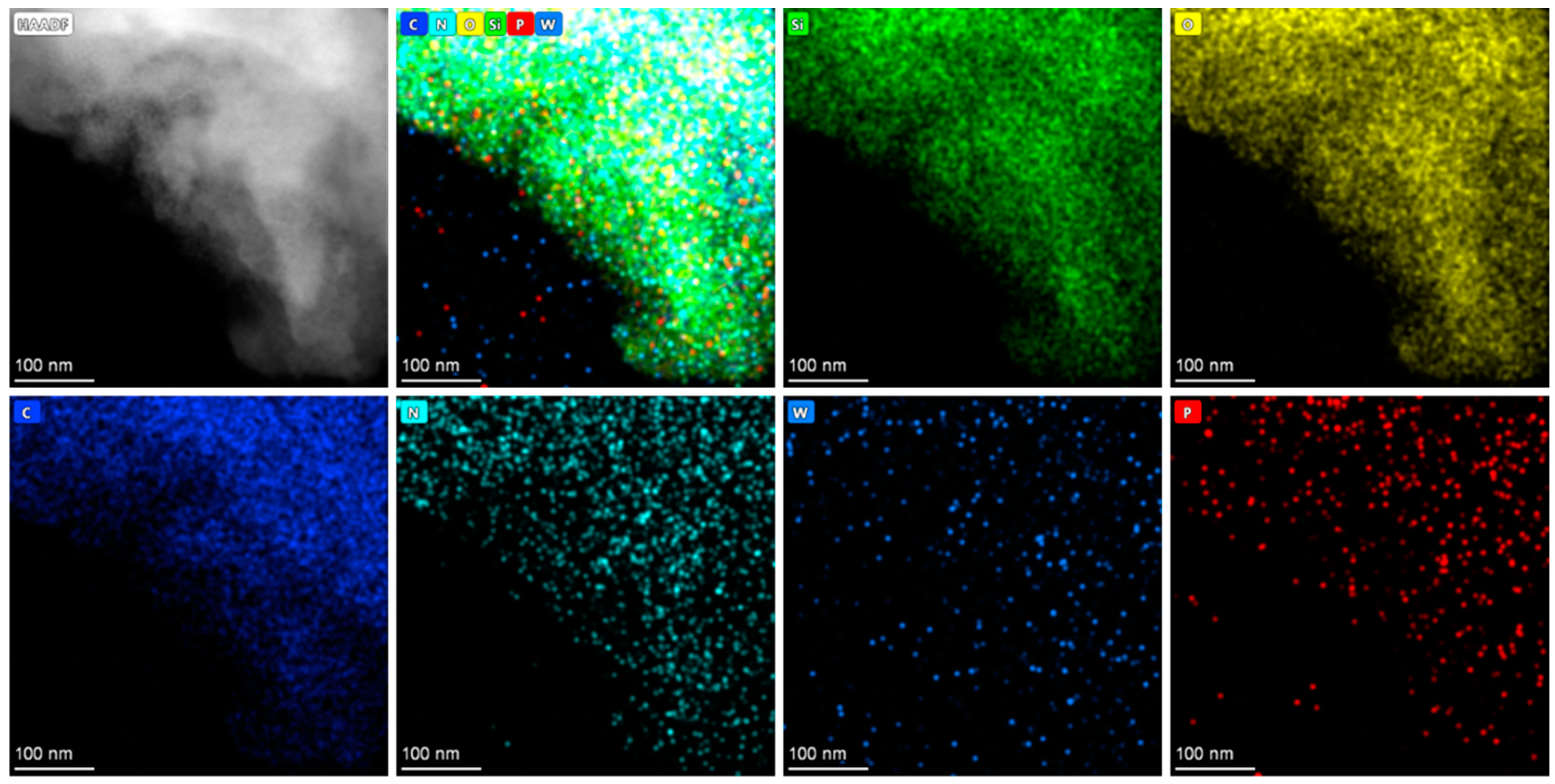
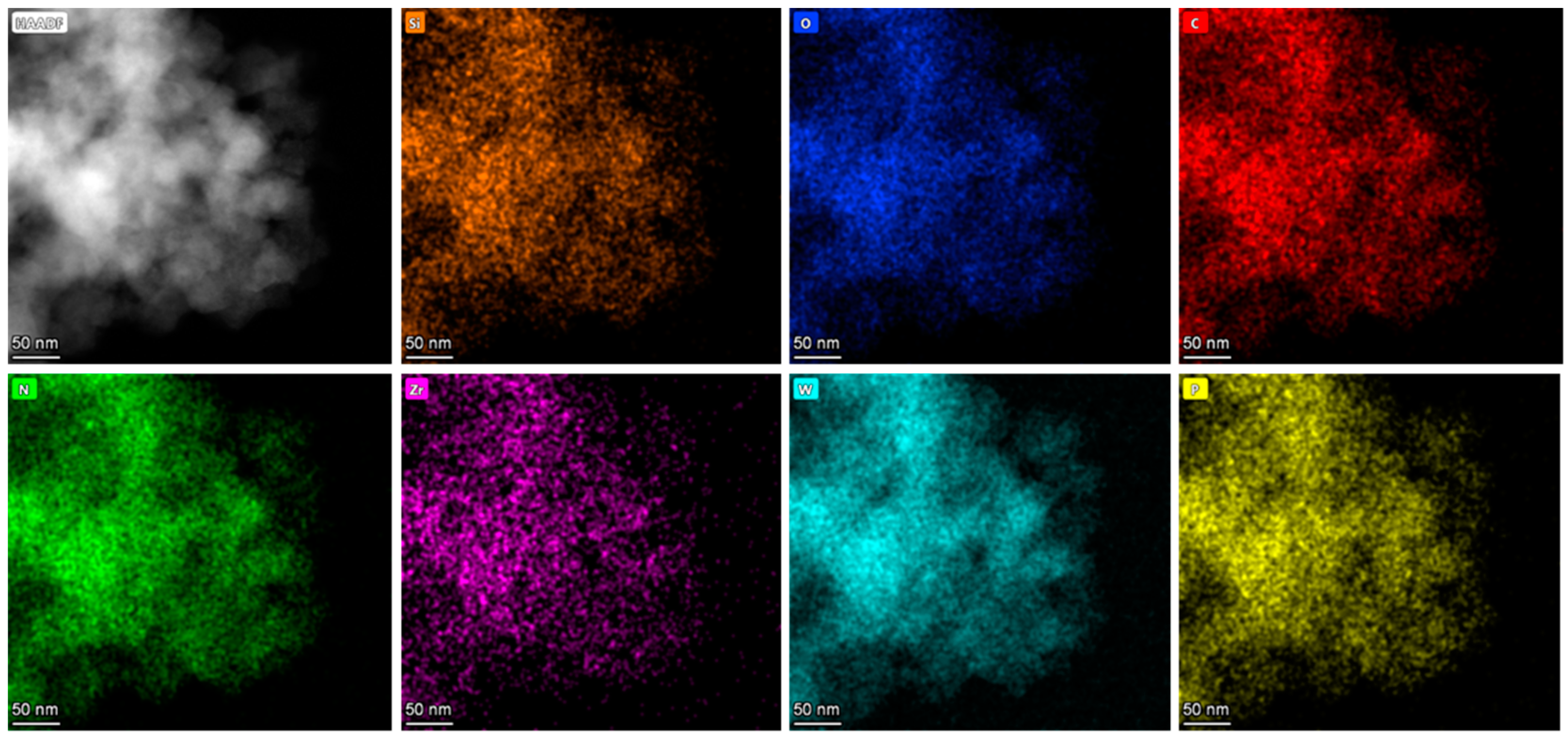
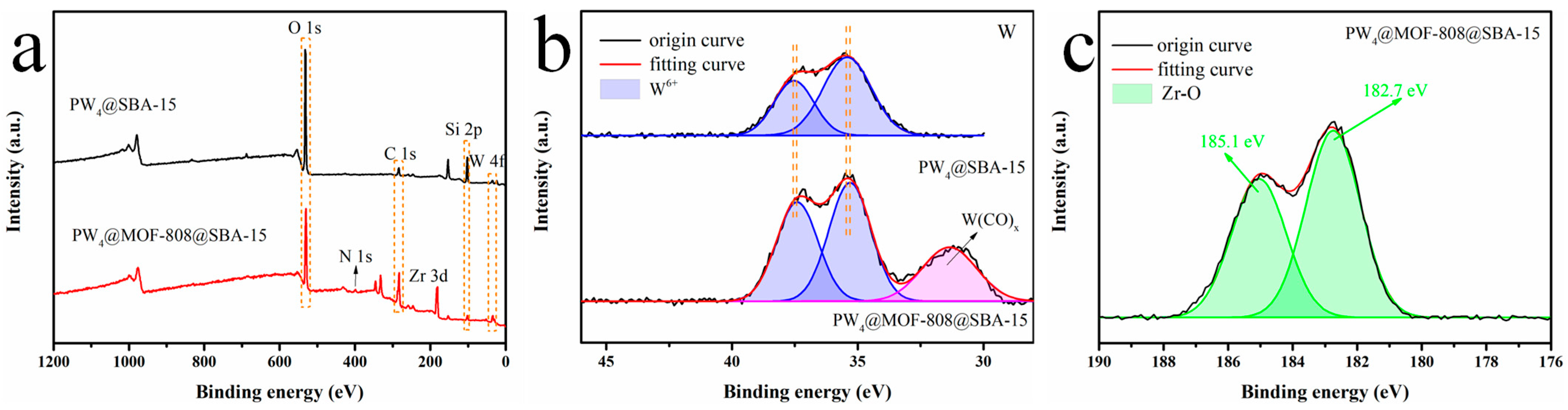


| Sample | SBET (m²/g) | V (cm3/g) | D (nm) |
|---|---|---|---|
| SBA-15 | 647.16 | 0.64 | 3.86 |
| PW4@SBA-15 | 416.29 | 0.47 | 4.36 |
| PW4@MOF-808@SBA-15 | 477.05 | 0.40 | 3.37 |
| No. | Catalysts | Oxidant | Extractant | Temperature (°C) | Time (min) | Substrate | Removal Efficiency | Refs. |
|---|---|---|---|---|---|---|---|---|
| 1 | PMo12@MOF-808@SBA-15 | H2O2 | MeOH | 70 | 480 | DBT, 4-MDBT, 4,6-DMDBT * | 96.8 | [35] |
| 2 | SRL-POM@MOF-199@MCM-41 | O2 | - | 60 | 150 | DBT | 100 | [36] |
| 3 | POM-MOF@Fibercloth | O2 | - | 95 | 75 | DBT | 100 | [37] |
| 4 | PW11@MOF-808 | H2O2 | MeCN | 60 | 30 | DBT | 100 | [38] |
| 5 | (PW11Ti)2OH@TM-SBA-15 | H2O2 | MeCN | 70 | 60 | BT, DBT, 4-MDBT, 4,6-DMDBT * | 91% | [39] |
| 6 | HPMo/SBA-15 | H2O2 | - | 60 | 330 | DBT | 100 | [40] |
| 7 | HPWA-SBA-15 | t-BuOOH | - | 50 | 120 | DBT | 98.4 | [41] |
| 8 | PW4@MOF-808@SBA-15 | H2O2 | MeCN | 70 | 60 | DBT, 4-MDBT, 4,6-DMDBT * | 99.7 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Huang, S.; Han, N.; Zhao, J. A Novel Ternary Catalyst PW4@MOF-808@SBA-15 for Deep Extraction Oxidation Desulfurization of Model Diesel. Molecules 2024, 29, 4230. https://doi.org/10.3390/molecules29174230
Gao Y, Huang S, Han N, Zhao J. A Novel Ternary Catalyst PW4@MOF-808@SBA-15 for Deep Extraction Oxidation Desulfurization of Model Diesel. Molecules. 2024; 29(17):4230. https://doi.org/10.3390/molecules29174230
Chicago/Turabian StyleGao, Yan, Shuai Huang, Nina Han, and Jianshe Zhao. 2024. "A Novel Ternary Catalyst PW4@MOF-808@SBA-15 for Deep Extraction Oxidation Desulfurization of Model Diesel" Molecules 29, no. 17: 4230. https://doi.org/10.3390/molecules29174230
APA StyleGao, Y., Huang, S., Han, N., & Zhao, J. (2024). A Novel Ternary Catalyst PW4@MOF-808@SBA-15 for Deep Extraction Oxidation Desulfurization of Model Diesel. Molecules, 29(17), 4230. https://doi.org/10.3390/molecules29174230






