Research on Synthesis, Structure, and Catalytic Performance of Tetranuclear Copper(I) Clusters Supported by 2-Mercaptobenz-zole-Type Ligands
Abstract
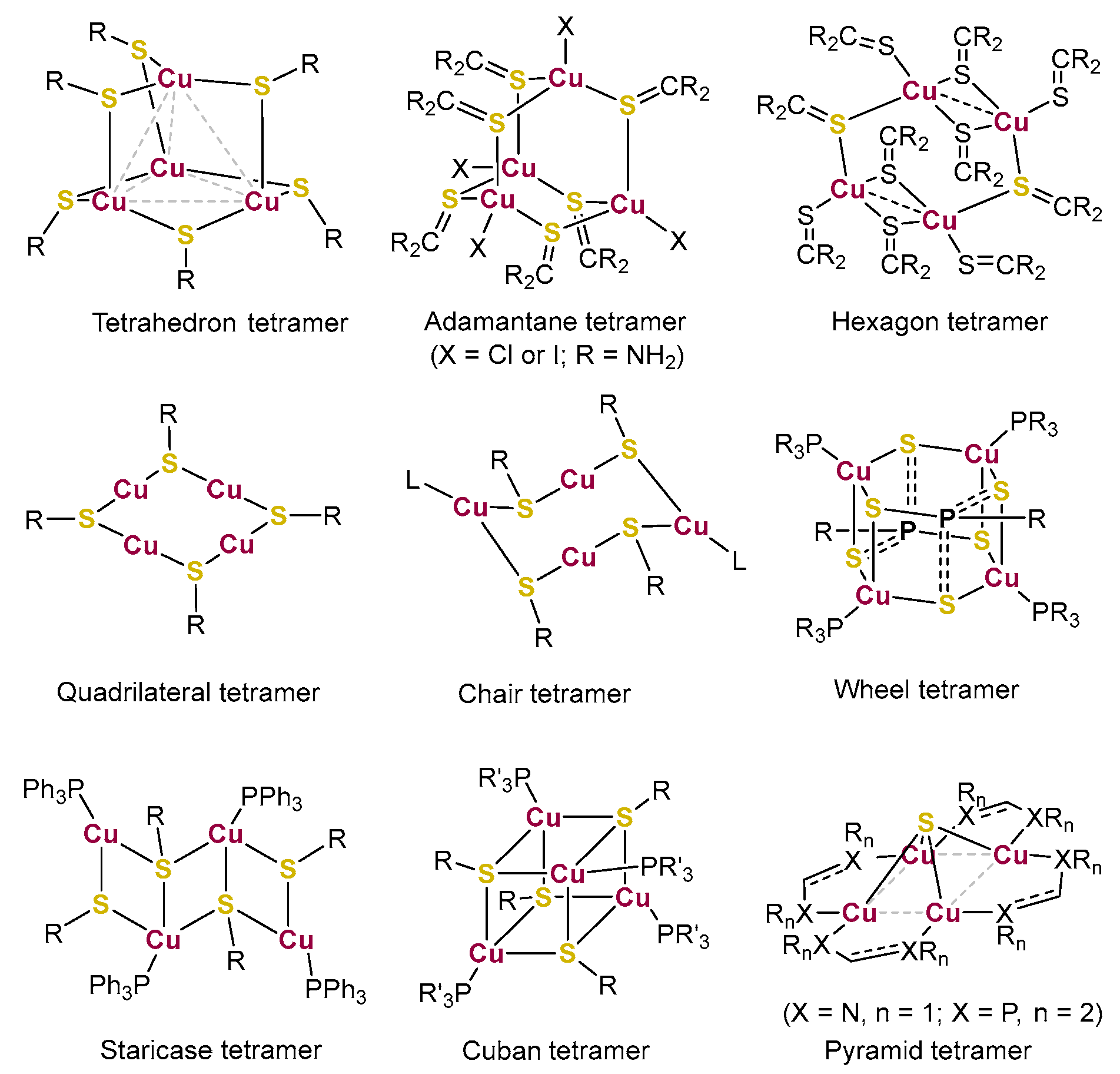
1. Introduction
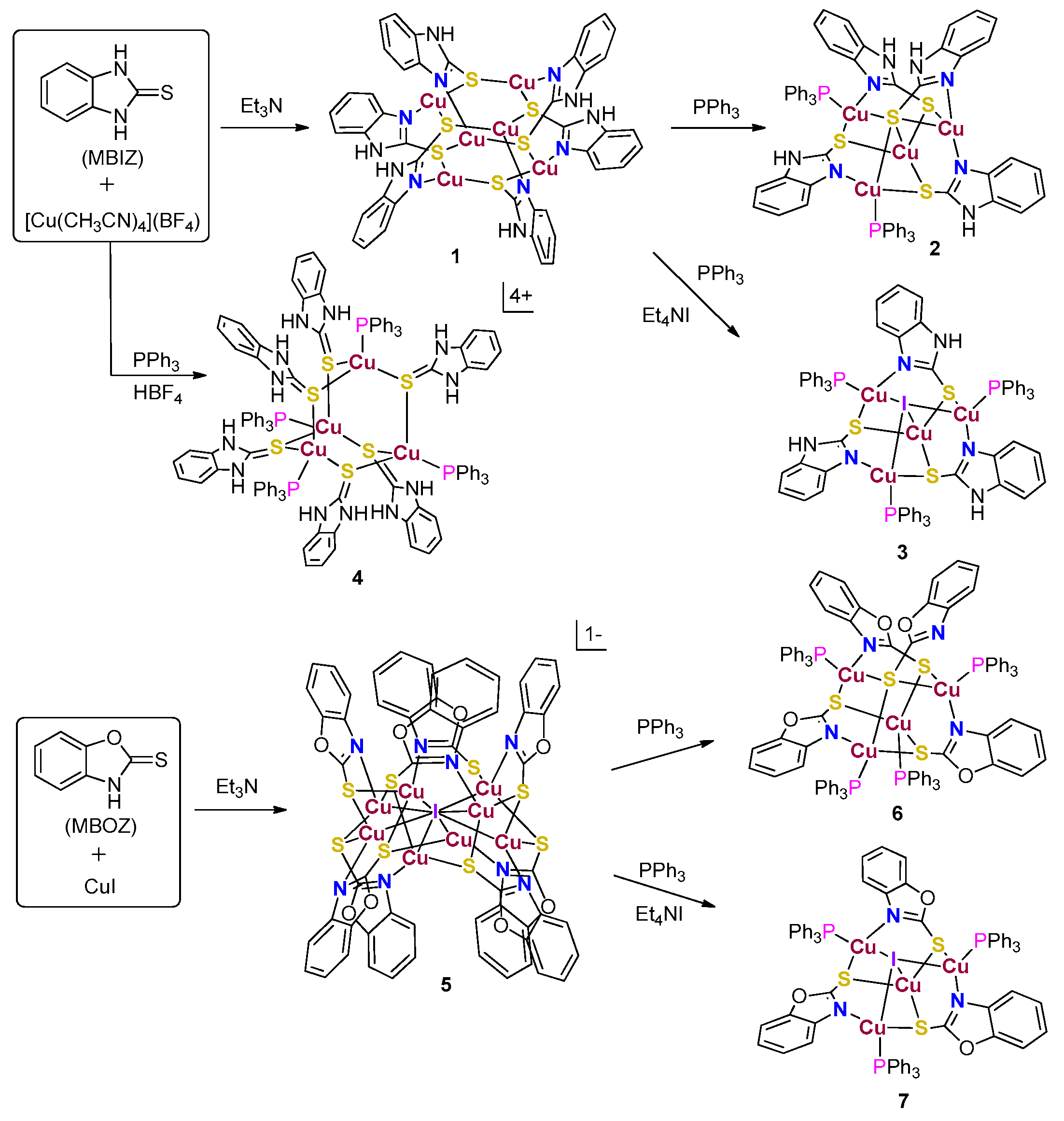
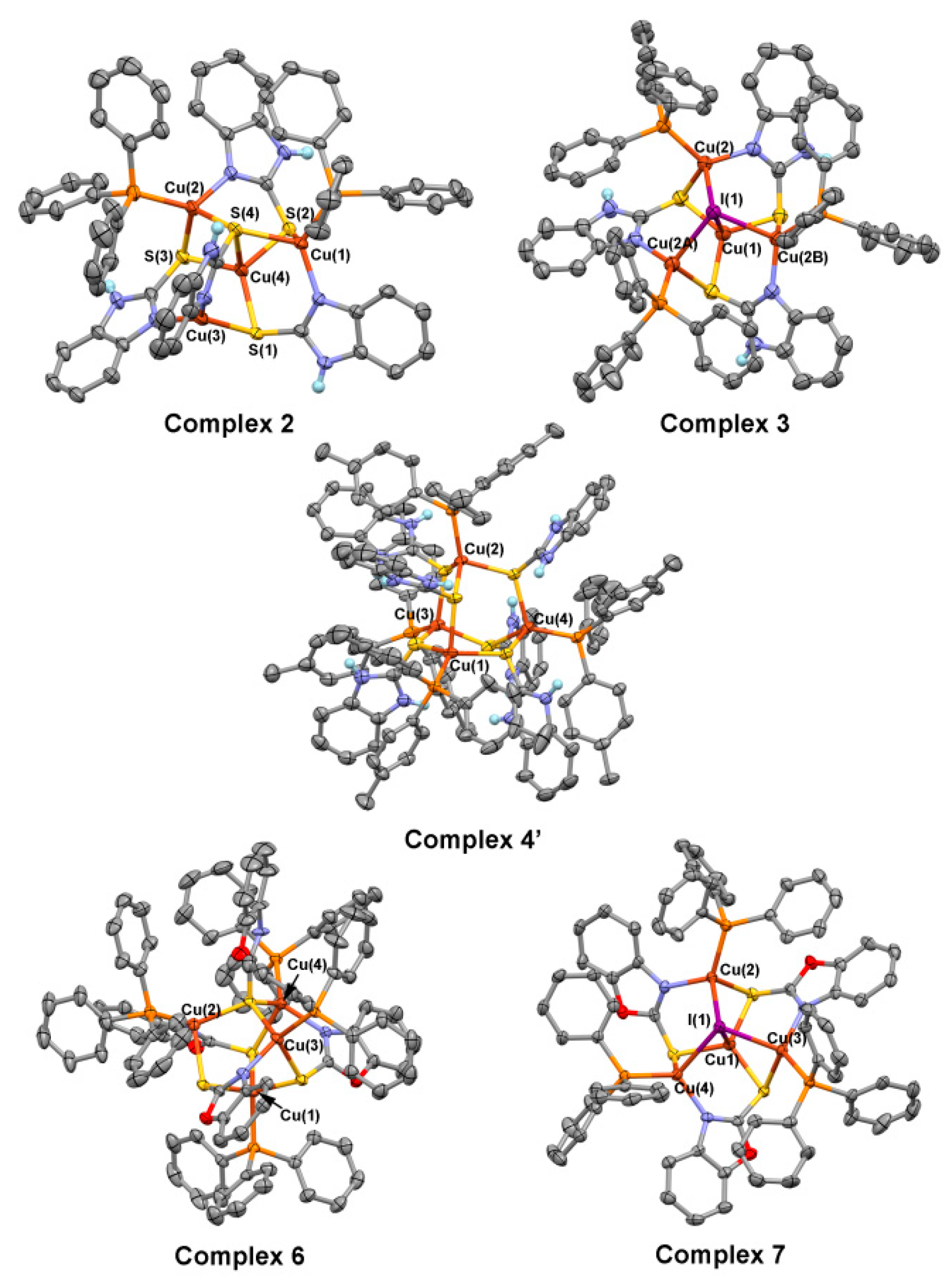
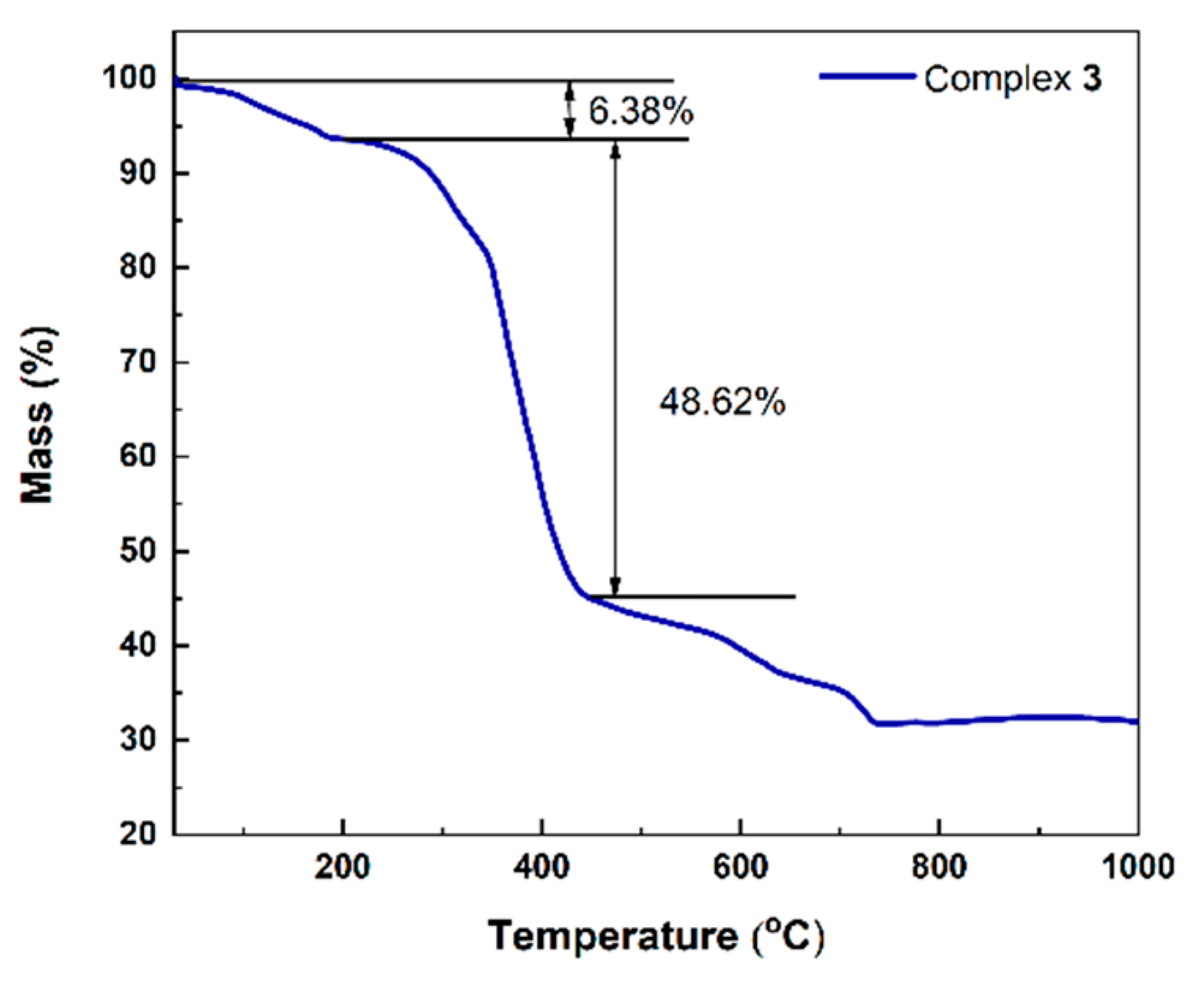
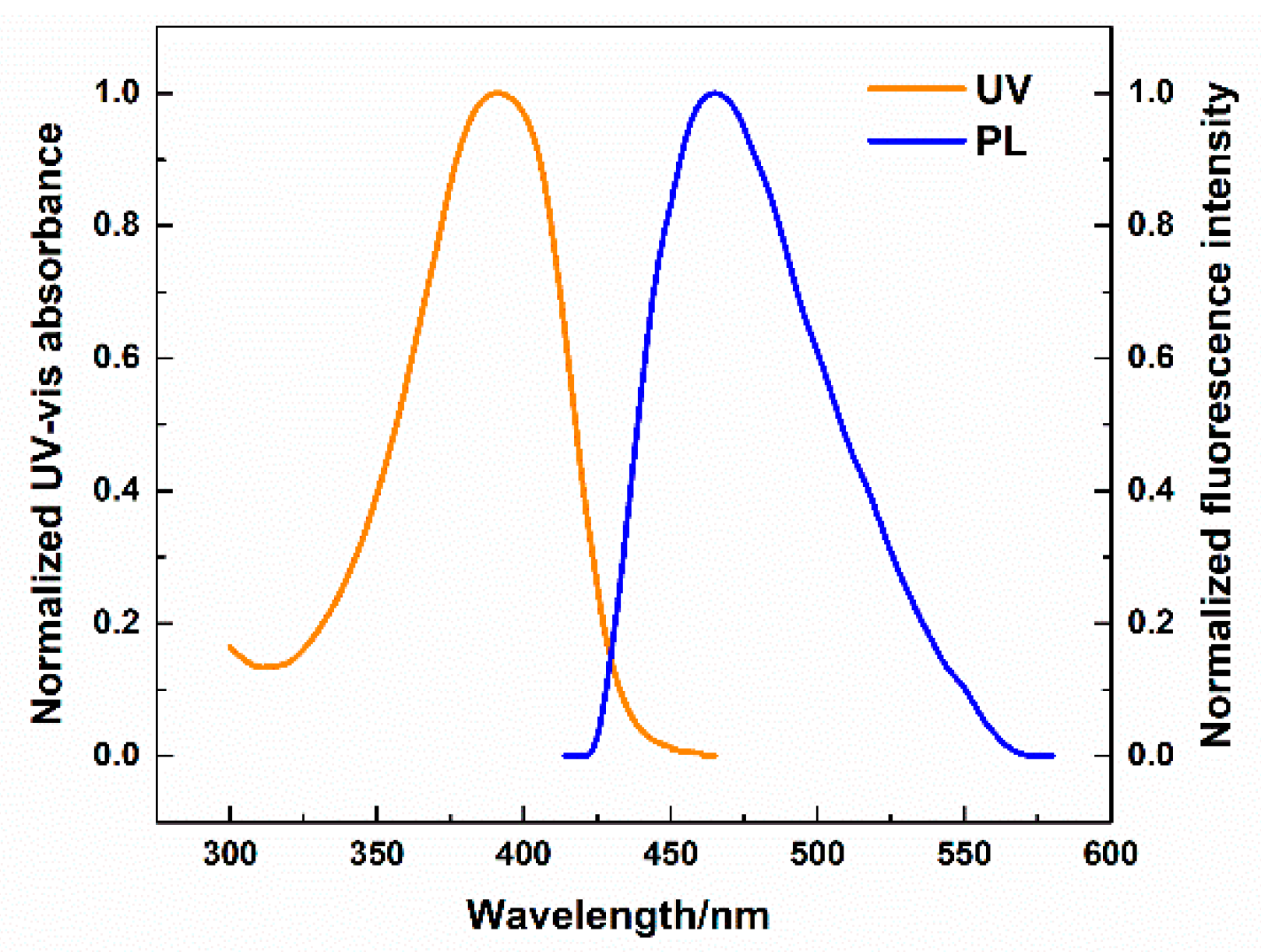
2. Results and Discussions
3. Experimental Section
3.1. General
3.2. Synthesis Procedures
3.2.1. Synthesis of Complex 2, 3, 4, 4′, 6, 7
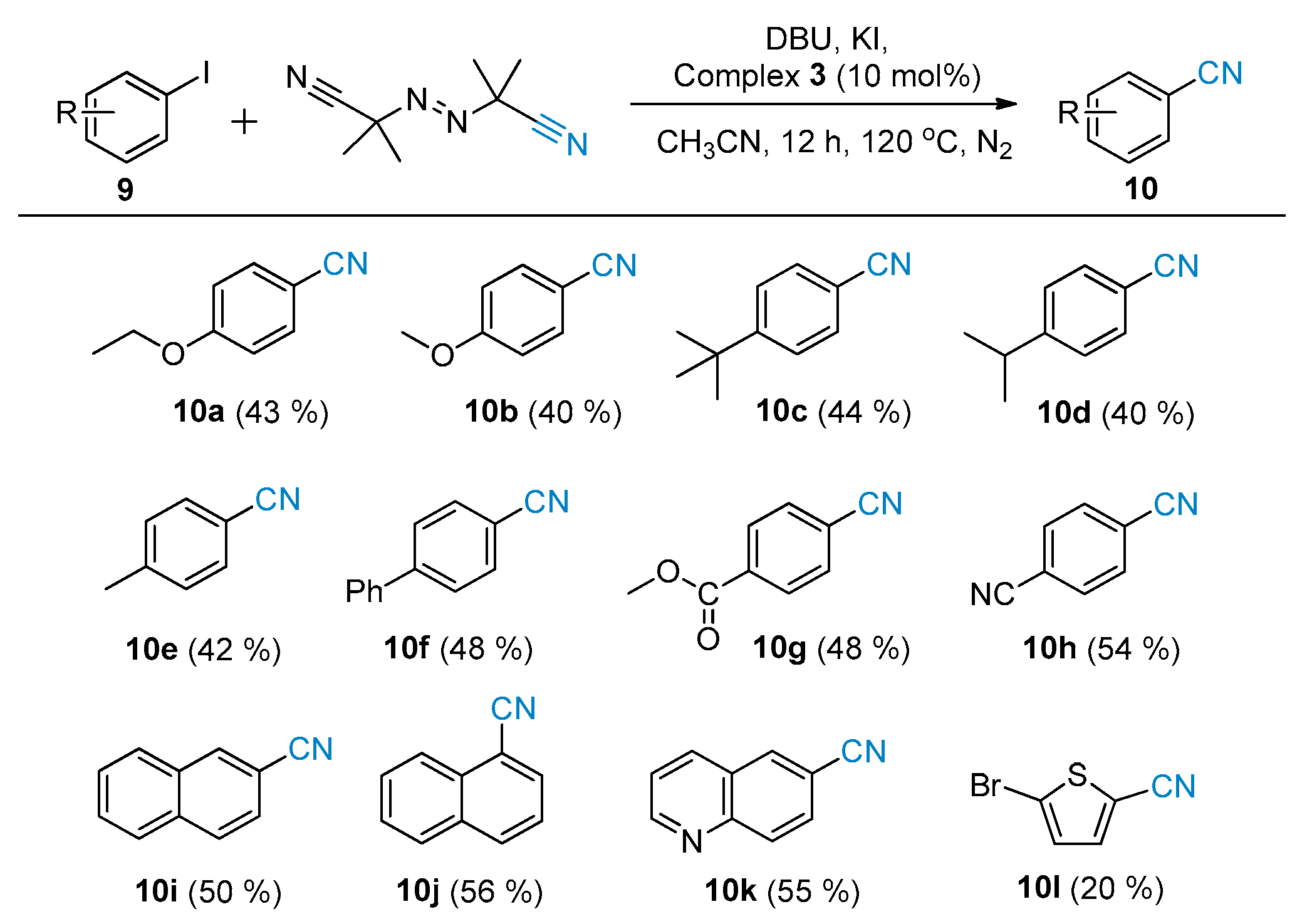
3.2.2. General Procedure for the Synthesis of Compound 10
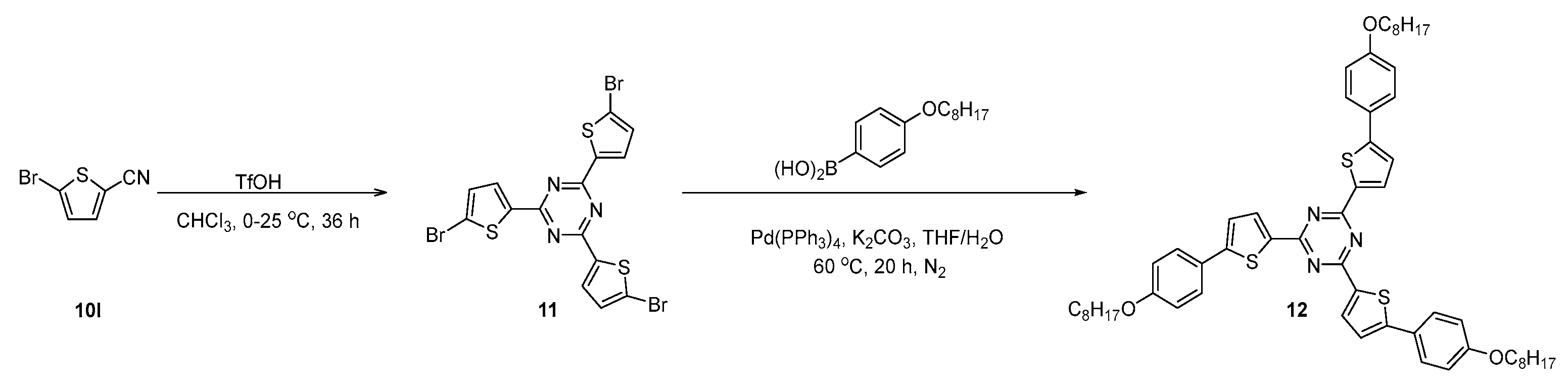
3.2.3. Experimental Procedures for the Synthesis of Compounds 11 and 12
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, S.; Braddock, D.C.; Armstrong, A.; Brennan, C.; Sale, D.; White, A.J.P.; Davies, R.P. Synthesis, characterisation and reactivity of copper(I) amide complexes and studies on their role in the modified Ullmann amination reaction. Chem. Eur. J. 2015, 21, 7179–7192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-J.; Li, H.-X.; Lia, H.-Y.; Lang, J.-P. Copper(I) 5-phenylpyrimidine-2-thiolate complexes showing unique optical properties and high visible light-directed catalytic performance. Dalton Trans. 2016, 45, 17759–17769. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.M.; Carreira, C.; Dell’Acqua, S.; Dey, S.G.; Pauleta, S.R.; Moura, I.; Solomon, E.I. Spectroscopic definition of the CuZ° intermediate in turnover of nitrous oxide reductase and molecular insight into the catalytic mechanism. J. Am. Chem. Soc. 2017, 139, 4462–4476. [Google Scholar] [CrossRef]
- Nayak, M.; Joshi, D.K.; Kumar, K.; Singh, A.S.; Tiwari, V.K.; Bhattacharya, S. Synthesis of a series of a few hydrosulfide complexes of Cu(I). A μ3-SH-Bridged rare cubane-like tetramer showing efficient catalytic activity toward azide-alkyne cycloaddition. Inorg. Chem. 2021, 60, 8075–8084. [Google Scholar] [CrossRef]
- Liu, Y.; Blanchard, V.; Danoun, G.; Zhang, Z.; Tlili, A.; Zhang, W.; Monnier, F.; Lee, A.V.D.; Mao, J.; Taillefer, M. Copper-catalyzed sonogashira reaction in water. ChemistrySelect 2017, 2, 11599–11602. [Google Scholar] [CrossRef]
- Yuan, Q.; Huang, Z.; Wu, W.; Hong, D.; Zhu, S.; Wei, J.; Zhou, S.; Wang, S. Halide-bridged tetranuclear organocopper(I) clusters supported by indolyl-based NCN pincer ligands and their catalytic activities towards the hydrophosphination of alkenes. Dalton Trans. 2022, 51, 17795–17803. [Google Scholar] [CrossRef] [PubMed]
- Doshi, A.; Sundararaman, A.; Venkatasubbaiah, K.; Zakharov, L.N.; Rheingold, A.L.; Myahkostupov, M.; Piotrowiak, P.; Jakle, F. Pentafluorophenyl copper-pyridine complexes: Synthesis, supramolecular structures via cuprophilic and π-Stacking interactions, and solid-state luminescence. Organometallics 2012, 31, 1546–1558. [Google Scholar]
- Galimova, M.F.; Zueva, E.M.; Dobrynin, A.B.; Kolesnikov, I.E.; Musin, R.R.; Musina, E.I.; Karasik, A.A. Luminescent Cu4I4-cubane clusters based on N-methyl-5,10-dihydrophenarsazines. Dalton Trans. 2021, 50, 13421–13429. [Google Scholar] [CrossRef] [PubMed]
- Enikeeva, K.R.; Shamsieva, A.V.; Strelnik, A.G.; Fayzullin, R.R.; Zakharychev, D.V.; Kolesnikov, I.E.; Dayanova, I.R.; Gerasimova, T.P.; Strelnik, I.D.; Musina, E.I.; et al. Green emissive copper(I) coordination polymer supported by the diethylpyridylphosphine ligand as a luminescent sensor for overheating processes. Molecules 2023, 28, 706. [Google Scholar] [CrossRef]
- Dong, C.; Song, X.; Hasanov, B.E.; Yuan, Y.; Gutiérrez-Arzaluz, L.; Yuan, P.; Nematulloev, S.; Bayindir, M.; Mohammed, O.F.; Bakr, O.M. Organic−Inorganic hybrid glasses of atomically precise nanoclusters. J. Am. Chem. Soc. 2024, 146, 7373–7385. [Google Scholar] [CrossRef]
- Wang, B.; Gui, R.; Jin, H.; He, W.; Wang, Z. Red-emitting BSA-stabilized copper nanoclusters acted as a sensitive probe for fluorescence sensing and visual imaging detection of rutin. Talanta. 2018, 178, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Ren, Y.; Bick, M.; Dudek, A.; Waworuntuc, E.H.-W.; Tang, J.; Chen, J.; Chang, B. Highly fluorescent copper nanoclusters for sensing and bioimaging. Biosens. Bioelectron. 2020, 154, 112078. [Google Scholar] [CrossRef]
- Shao, C.; Li, C.; Zhang, C.; Ni, Z.; Liu, X.; Wang, Y. Novel synthesis of orange-red emitting copper nanoclusters stabilized by methionine as a fluorescent probe for norfloxacin sensing. Spectrochim. Acta Part A 2020, 236, 118334. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.d.S.; Cervo, R.; Siqueira, F.d.S.; Campos, M.M.A.d.; Lang, E.S.; Tirloni, B.; Schumacher, R.F.; Cargnelutti, R. Synthesis and antimicrobial evaluation of coordination compounds containing 2,20-bis(3-aminopyridyl) diselenide as ligand. Appl. Organomet. Chem. 2020, 34, e5750. [Google Scholar] [CrossRef]
- Ramaduraia, M.; Rajendranb, G.; Bamaa, T.S.; Prabhua, P.; Kathiravan, K. Biocompatible thiolate protected copper nanoclusters for an efficient imaging of lung cancer cells. J. Photochem. Photobiol. B 2020, 205, 111845. [Google Scholar] [CrossRef]
- Gourdon-Grünewaldt, L.; Blacque, O.; Gasser, G.; Cariou, K. Towards copper(I) clusters for photo-induced oxidation of biological thiols in living cells. ChemBioChem 2023, 24, e202300496. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Gunasekar, K.R.; Ali, S.; Sivalingam, C.; Subramanian, R.; Venkatesa, K.; Selvaraj, M.; Malakondaiah, S. Tenacious copper nanoclusters shielded by aromatic thiol for use in antibacterial, bioimaging, and dye degradation applications. J. Ind. Eng. Chem. 2024, 137, 448–454. [Google Scholar] [CrossRef]
- Liu, M.; Liang, J.; Liu, Z. Modulating the ferroelectric performance by altering halogen anions in the crystals of tetranuclear copper-clusters. New J. Chem. 2021, 45, 12091–12096. [Google Scholar] [CrossRef]
- Oh, J.; Yang, S.Y.; Kim, S.; Lee, C.; Cha, J.-H.; Jang, B.C.; Im, S.G.; Choi, S.-Y. Imidazole-based artificial synapses for neuromorphic computing: A cluster-type conductive filament via controllable nanocluster nucleation. Mater. Horiz. 2023, 10, 2035–2046. [Google Scholar] [CrossRef]
- Körte, L.A.; Schwabedissen, J.; Soffner, M.; Blomeyer, S.; Reuter, C.G.; Vishnevskiy, Y.V.; Neumann, B.; Stammler, H.-G.; Mitzel, N.W. Tris(perfluorotolyl)borane—A boron lewis superacid. Angew. Chem. Int. Ed. 2017, 56, 8578–8582. [Google Scholar] [CrossRef]
- Robinson, T.P.; Price, R.D.; Davidson, M.G.; Foxb, M.A.; Johnson, A.L. Why are the {Cu4N4} rings in copper(I) phosphinimide clusters [Cu{μ-N=PR3}]4 (R = NMe3 or Ph) planar? Dalton Trans. 2015, 44, 5611–5619. [Google Scholar] [CrossRef]
- Quintana, L.M.A.; Jiang, N.; Bacsa, J.; Pierre, H.S.L. Coinage metal tris(dialkylamido)imidophosphorane complexes as transmetallation reagents for cerium complexes. Dalton Trans. 2020, 49, 5420–5423. [Google Scholar] [CrossRef] [PubMed]
- Oguadinma, P.O.; Schaper, F. Bis(2-phenylethyl)-nacnac: A chiral diketiminate ligand and its copper complexes. Organometallics 2009, 28, 4089–4097. [Google Scholar] [CrossRef]
- Mothes, R.; Ruffer, T.; Shen, Y.; Jakob, A.; Walfort, B.; Petzold, H.; Schulz, S.E.; Ecke, R.; Gessnerb, T.; Lang, H. Phosphite copper(I) trifluoroacetates [((RO)3P)mCuO2CCF3] (m = 1, 2, 3): Synthesis, solid state structures and their potential use as CVD precursors. Dalton Trans. 2010, 39, 11235–11247. [Google Scholar] [CrossRef]
- Bellow, J.A.; Yousif, M.; Fang, D.; Kratz, E.G.; Cisneros, G.A.; Groysman, S. Synthesis and reactions of 3d metal complexes with the bulky alkoxide ligand [OCtBu2Ph]. Inorg. Chem. 2015, 54, 5624–5633. [Google Scholar] [CrossRef]
- Wolf, R.; Hey-Hawkins, E. Synthesis and molecular structure of the Cu4P8 cage compound [Cu4(P4Ph4)2(PCyp3)3]. Angew. Chem. Int. Ed. 2005, 44, 6241–6244. [Google Scholar] [CrossRef] [PubMed]
- Harford, P.J.; Haywood, J.; Smith, M.R.; Bhawal, B.N.; Raithby, P.R.; Uchiyamac, M.; Wheatley, A.E.H. Expanding the tools available for direct ortho cupration-targeting lithium phosphidocuprates. Dalton Trans. 2012, 41, 6148–6154. [Google Scholar] [CrossRef]
- Rathnayaka, S.C.; Lindeman, S.V.; Mankad, N.P. Multinuclear Cu(I) clusters featuring a new triply bridging coordination mode of phosphaamidinate ligands. Inorg. Chem. 2018, 57, 9439–9445. [Google Scholar] [CrossRef]
- Baslé, A.; Platsaki, S.; Dennison, C. Visualizing Biological Copper Storage: The importance of thiolate Coordinated tetranuclear clusters. Angew. Chem. 2017, 129, 8823–8826. [Google Scholar] [CrossRef]
- Trivedi, M.; Kumar, A.; Husain, A.; Rath, N.P. Copper(I) complexes containing PCP ligand catalyzed hydrogenation of carbon dioxide to formate under ambient conditions. Inorg. Chem. 2021, 60, 4385–4396. [Google Scholar] [CrossRef]
- Liang, X.-Q.; Gupta, R.K.; Li, Y.-W.; Ma, H.-Y.; Gao, L.-N.; Tung, C.-H.; Sun, D. Structural diversity of copper(I) cluster-based coordination polymers with pyrazine-2-thiol ligand. Inorg. Chem. 2020, 59, 2680–2688. [Google Scholar] [CrossRef] [PubMed]
- Dhayal, R.S.; Liao, J.-H.; Hou, H.-N.; Ervilita, R.; Liao, P.-K.; Liua, C.W. Copper(I) diselenocarbamate clusters: Synthesis, structures and single-source precursors for Cu and Se composite materials. Dalton Trans. 2015, 44, 5898–5908. [Google Scholar] [CrossRef]
- Hanau, K.; Rinn, N.; Dehnen, S. Variations in the interplay of intermetallic and metal chalcogenide units in organotin−copper selenide clusters. Inorg. Chem. 2020, 59, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, G.; Tyagi, A.; Wadawale, A.P.; Shah, A.Y.; Kedarnath, G.; Srivastava, A.P.; Singh, V. Accessing photoresponsive copper selenide nanomaterials and thin films through tetranuclear Cu(I) pyridylselenolate cluster. J. Mater. Sci. 2020, 55, 15439–15453. [Google Scholar] [CrossRef]
- Liu, H.; Bandeira, N.A.G.; Calhorda, M.J.; Drew, M.G.B.; Felix, V.; Novosad, J.; de Biani, F.F.; Zanello, P. Cu(I) and Ag(I) complexes of chalcogenide derivatives of the organometallic ligand dppf and the dppa analogue. J. Organomet. Chem. 2004, 689, 2808–2819. [Google Scholar] [CrossRef]
- Belo, D.; Figueira, M.J.; Mendonca, J.; Santos, I.C.; Almeida, M.; Henriques, R.T.; Duarte, M.T.; Rovira, C.; Veciana, J. Copper, cobalt and platinum complexes with dithiothiophene-based ligands. Eur. J. Inorg. Chem. 2005, 2005, 3337–3345. [Google Scholar] [CrossRef]
- Chen, C.; Weng, Z.; Hartwig, J.F. Synthesis of Copper(I) Thiolate Complexes in the thioetherification of aryl halides. Organometallics 2012, 31, 8031–8037. [Google Scholar] [CrossRef]
- Lane, A.C.; Vollmer, M.V.; Laber, C.H.; Melgarejo, D.Y.; Chiarella, G.M.; Fackler, J.P., Jr.; Yang, X.; Baker, G.A.; Walensky, J.R. Multinuclear Copper(I) and silver(I) amidinate complexes: Synthesis, luminescence, and CS2 insertion reactivity. Inorg. Chem. 2014, 53, 11357–11366. [Google Scholar] [CrossRef]
- Royappaa, A.T.; Papoular, R.J.; Gembicky, M.; Shepard, W.; Ross, A.D.; Stemen, A.G.; Bobbitt, J.J.; Doan, D.T.; Lapidus, S.H.; Johnston, D.H.; et al. Crystalline phase transitions and water-soluble complexes of copper(I) 2-hydroxyethanethiolate. Polyhedron 2022, 222, 115873. [Google Scholar] [CrossRef]
- Griffith, E.H.; Hunt, G.W.; Amma, E.L. The adamantane structure in polynuclear Cu4S6 cores: The crystal and molecular structures of Cu4[SC(NH2)2]6(NO3)4·4H2O and Cu4[SC(NH2)2]9(NO3)4·4H2O. J. Chem. Soc. Chem. Commun. 1976, 432–433. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Hanna, J.V.; Pakawatchai, C.; Skelton, B.W.; Thanyasirikul, Y.; White, A.H. Crystal structures and vibrational spectroscopy of copper(I) thiourea complexes. Inorg. Chem. 2009, 48, 350–368. [Google Scholar] [CrossRef]
- Raper, E.S.; Creighton, J.R.; Wilson, J.D.; Clegg, W.; Milne, A. Preparation, spectroscopy and crystal structure of a novel tetranuclear copper(I) benzimidazoline-2-thione complex: Cyclo-hexakis-μ2-(benzimidazoline-2-thione) tetrakis[benzimidazoline-2-thione copper(I)] perchlorate quatrodeca hydrate. Inorganica Chim. Acta 1988, 149, 265–271. [Google Scholar] [CrossRef]
- Christlieb, M.; Cowley, A.R.; Dilworth, J.R.; Donnelly, P.S.; Paterson, B.M.; Struthers, H.S.R.; White, J.M. New bimetallic compounds based on the bis(thiosemicarbazonato) motif. Dalton Trans. 2007, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Bianketti, S.; Blake, A.J.; Wilson, C.; Hubberstey, P.; Champness, N.R.; Schroder, M. Second-sphere hydrogen-bonding in heteroditopic mercaptopyridinium copper(I) frameworks. CrystEngComm 2009, 11, 763–769. [Google Scholar] [CrossRef]
- Singh, S.; Bhattacharya, S. Studies of structural diversity due to inter-/intra-molecular hydrogen bonding and photoluminescent properties in thiocarboxylate Cu(i) and Ag(i) complexes. RSC Adv. 2014, 4, 49491–49500. [Google Scholar] [CrossRef]
- Limberg, C.; Frank, N.; Dallmann, A.; Braun-Cula, B.; Herwig, C. Mercaptothiacalixarenes steer 24 copper(I) centers to form a Hollow-Sphere structure featuring Cu2S2 motifs with exceptionally short Cu···Cu distances. Angew. Chem. Int. Ed. 2020, 59, 6735–6739. [Google Scholar]
- Pichota, A.; Pregosin, P.S.; Valentini, M.; Worle, M.; Seebach, D. X-Ray, molecular diffusion, and NOESY NMR studies of chiral, tetranuclear CuI catalysts based on monodentate thiol analogues of TADDOL. Angew. Chem. Int. Ed. 2000, 39, 153–156. [Google Scholar] [CrossRef]
- Komuro, T.; Kawaguchi, H.; Tatsumi, K. Synthesis and Reactions of triphenylsilanethiolato complexes of manganese(II), iron(II), cobalt(II), and nickel(II). Inorg. Chem. 2002, 41, 5083–5090. [Google Scholar] [CrossRef]
- Kuckmann, T.I.; Hermsen, M.; Bolte, M.; Wagner, M.; Lerner, H.-W. Silylchalcogenolates MESiRtBu2 (M = Na, Cu, Zn, Fe; E = S, Se, Te; R = tBu, Ph) and disilyldichalcogenides tBu2RSiE−ESiRtBu2 (E = S, Se, Te; R = tBu, Ph): synthesis, properties, and structures. Inorg. Chem. 2005, 44, 3449–3458. [Google Scholar] [CrossRef]
- Melzer, M.M.; Mossin, S.; Cardenas, A.J.P.; Williams, K.D.; Zhang, S.; Meyer, K.; Warren, T.H. A Copper(II) Thiolate from Reductive Cleavage of an S-Nitrosothiol. Inorg. Chem. 2012, 51, 8658–8660. [Google Scholar] [CrossRef]
- Jana, A.; Jash, M.; Dar, W.A.; Roy, J.; Chakraborty, P.; Paramasivam, G.; Lebedkin, S.; Kirakci, K.; Manna, S.; Antharjanam, S.; et al. Carborane-thiol protected copper nanoclusters: Stimuli-responsive materials with tunable phosphorescence. Chem. Sci. 2023, 14, 1613–1626. [Google Scholar]
- Dance, I.G.; Fitzpatrick, L.J.; Craig, D.C.; Scudder, M.L. Monocyclic copper and silver tertiary alkanethiolates: Formation and molecular structures of (CuSBu-tert)4(Ph3P)2, (AgSCMeEt2)8(Ph3P)2 and (AgSBu-tert)14(Ph3P)4 and structural principles. Inorg. Chem. 1989, 28, 1853–1861. [Google Scholar] [CrossRef]
- Schneider, S.; Dzudza, A.; Raudasch-Sieber, G.; Marks, T.J. Copper(I) tert-Butylthiolato clusters as single-source precursors for high-quality chalcocite thin films: precursor chemistry in solution and the solid state. Chem. Mater. 2007, 19, 2768–2779. [Google Scholar]
- Li, Z.; Li, X.; Lin, P.; Du, S. Self-assembly of 1D coordination polymers containing copper(I) tert-butylthiolato clusters: Structural characterization and properties. J. Cluster Sci. 2008, 19, 357–366. [Google Scholar] [CrossRef]
- Groysman, S.; Holm, R.H. A series of mononuclear quasi-two-coordinate copper(I) complexes employing a sterically demanding thiolate ligand. Inorg. Chem. 2009, 48, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Najafabadi, B.K.; Corrigan, J.F. N-heterocyclic carbenes as effective ligands for the preparation of stabilized copper- and silver-t-butylthiolate clusters. Dalton Trans. 2014, 43, 2104–2111. [Google Scholar] [CrossRef]
- Shi, W.; Shafaei-Fallah, M.; Anson, C.E.; Rothenberger, A. A strategy for the build-up of transition-metal complexes containing tripodal [ArPOS2]2− and [ArPS3]2−ligands (Ar = 4-anisyl). Dalton Trans. 2005, 3909–3912. [Google Scholar] [CrossRef]
- Shi, W.; Shafaei-Fallah, M.; Anson, C.E.; Rothenberger, A. Metal thiophosphonates and related compounds: An emerging area of supramolecular coordination chemistry. Dalton Trans. 2006, 3257–3262. [Google Scholar] [CrossRef]
- Shafaei-Fallah, M.; Shi, W.; Fenske, D.; Rothenberger, A. Syntheses and structures of transition metal complexes with dithiophosphinato and trithiophosphinato ligands. Z. Anorg. Allg. Chem. 2006, 632, 1091–1096. [Google Scholar] [CrossRef]
- Dance, I.G.; Scudder, M.L.; Fitzpatrick, L.J. Preparation, distorted step structure, and topological analysis of (.mu.3-SPh)2(.mu.-SPh)2(CuPPh3)4(tol)2 (tol = toluene). Inorg. Chem. 1985, 24, 2547–2550. [Google Scholar] [CrossRef]
- Deivaraj, T.C.; Lai, G.X.; Vittal, J.J. Chemistry of thiocarboxylates: synthesis and structures of neutral copper(I) thiocarboxylates with triphenylphosphine. Inorg. Chem. 2000, 39, 1028–1034. [Google Scholar] [CrossRef]
- Fernandez-Recio, L.; Fenske, D.; Fuhr, O. Copper Chalcogenide Cluster Compounds with Nitro-functionalized Ligand Shell. Z. Anorg. Allg. Chem. 2008, 634, 2853–2857. [Google Scholar] [CrossRef]
- Langer, R.; Wunsche, L.; Fenske, D.; Fuhr, O. Copper chalcogenide cluster compounds with bromo-functionalized ligand shell. Z. Anorg. Allg. Chem. 2009, 635, 2488–2494. [Google Scholar] [CrossRef]
- Langer, R.; Yadav, M.; Weinert, B.; Fenske, D.; Fuhr, O. Luminescence in functionalized copper thiolate clusters-synthesis and structural effects. Eur. J. Inorg. Chem. 2013, 2013, 3623–3631. [Google Scholar] [CrossRef]
- Johnson, B.J.; Antholine, W.E.; Lindeman, S.V.; Mankad, N.P. A Cu4S model for the nitrous oxide reductase active sites supported only by nitrogen ligands. Chem. Commun. 2015, 51, 11860–11863. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.J.; Antholine, W.E.; Lindeman, S.V.; Graham, M.J.; Mankad, N.P. A one-hole Cu4S cluster with N2O reductase activity: A structural and functional model for CuZ*. J. Am. Chem. Soc. 2016, 138, 13107–13110. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Rathnayaka, S.C.; Islam, S.M.; MacMillan, S.N.; Mankad, N.P. N2O reductase activity of a [Cu4S] cluster in the 4CuI redox state modulated by hydrogen bond donors and proton relays in the secondary coordination sphere. Angew. Chem. Int. Ed. 2020, 132, 637–641. [Google Scholar] [CrossRef]
- Mankad, N.P. Triazenide-supported [Cu4S] structural mimics of CuZ that mediate N2O disproportionation rather than reduction. Chem. Sci. 2024, 15, 1820–1828. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Singh, N.; Jang, D.O. A 2-mercaptobenzimidazole-based emissive Cu(I) complex for selective determination of iodide with large Stokes shift. Sens. Actuators B 2017, 243, 372–379. [Google Scholar] [CrossRef]
- Wei, W.; Wu, M.; Gao, Q.; Zhang, Q.; Huang, Y.; Jiang, F.; Hong, M. A novel supramolecular tetrahedron assembled from tetranuclear copper(I) cluster molecules via aryl embrace interactions. Inorg. Chem. 2009, 48, 420–422. [Google Scholar] [CrossRef]
- Hao, Z.-M.; Guo, C.-H.; Wu, H.-S.; Zhang, X.-M. Luminescent boracite-like metal–organic frameworks constructed by Cu-centered CuCu4 tetrahedra and CuCu3 triangles with an acentric cubic superlarge cell. Cryst. Eng. Comm. 2010, 12, 55–58. [Google Scholar] [CrossRef]
- Hu, S.; Yu, F.-Y.; Zhang, P.; Zhou, A.-J. An acs-type metal–organic framework with an unprecedented undecanuclear copper cluster as secondary building unit. Eur. J. Inorg. Chem. 2012, 2012, 3669–3673. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Su, C.-H.; Pang, H.-Y.; Huang, F.-P.; Yu, Q.; Bian, H.-D. 1-(2-Hydroxyethyl)-5-mercapto-1H-tetrazole as ligand in Cu(I) complexes with or without X-ions (X = Cl, Br, I): Synthesis, crystal structures and properties. J. Coord. Chem. 2017, 70, 3650–3659. [Google Scholar] [CrossRef]
- Huang, D.-Y.; Hao, H.-M.; Yao, P.-F.; Qin, X.-H.; Huang, F.-P.; Yu, Q.; Bian, H.-D. CuIX (X = Cl−, Br−, I−) inorganic networks separated and stabilized by a mercaptotetrazole ligand. Polyhedron 2015, 97, 260–267. [Google Scholar] [CrossRef]
- Yue, C.-Y.; Liu, F.-L.; Deng, W.-T.; Tao, J.; Hong, M.-C. Iodide-centered cuprous octatomic Ring: A luminescent molecular thermometer exhibiting dual-Emission character. Cryst. Growth Des. 2018, 18, 22–26. [Google Scholar] [CrossRef]
- Beswick, M.A.; Brasse, C.; Halcrow, M.A.; Raithby, P.R.; Russell, C.A.; Steiner, A.; Snaith, R.; Wright, D.S. Role of bifunctional N/S bridging in the association of metals; syntheses and structures of heterobimetallic [Ph3P)2CuL2Li(thf)2]·thf and tetrameric [{CuL(PPh3)}4](L = 1,3-benzoxazoline-2-thionate, thf = tetrahydrofuran). Dalton Trans. 1996, 3793–3797. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Xiang, S.; Zhu, Y.; Chen, C.; Huang, D. Base induced C–CN bond cleavage at room temperature: A convenient method for the activation of acetonitrile. Inorg. Chem. Front. 2019, 6, 1135–1140. [Google Scholar] [CrossRef]
- Xu, H.; Liu, P.-T.; Li, Y.-H.; Han, F.-S. Copper-mediated direct aryl C–H cyanation with azobisisobutyronitrile via a free-radical pathway. Org. Lett. 2013, 15, 3354–3357. [Google Scholar] [CrossRef]
- Rong, G.; Mao, J.; Zheng, Y.; Yao, R.; Xu, X. Cu-Catalyzed direct cyanation of terminal alkynes with AMBN or AIBN as the cyanation reagent. Chem. Commun. 2015, 51, 13822–13825. [Google Scholar] [CrossRef]
- Elliott, Q.; Alabugin, I.V. AIBN as an electrophilic reagent for cyano group transfer. J. Org. Chem. 2023, 88, 2648–2654. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Henwood, A.F.; Hall, D.; Cordes, D.B.; Slawin, A.M.Z.; Lemaur, V.; Olivier, Y.; Samuel, I.D.W.; Zysman-Colman, E. Luminescent Dinuclear Copper(I) Complexes Bearing an Imidazolylpyrimidine Bridging Ligand. Inorg. Chem. 2020, 59, 14772–14784. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Shimizu, T.; Liu, F.; Ungar, G.; Kato, T. Electro-functional octupolar π-conjugated columnar liquid crystals. J. Am. Chem. Soc. 2011, 133, 13437–13444. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Eifuku, N. Alkoxy-substituted Derivatives of π-Conjugated Acyclic Anion Receptors: Effects of Substituted Positions. Chem. Lett. 2009, 38, 208–209. [Google Scholar] [CrossRef]
- Percec, V.; Wang, S.; Huang, N.; Partridge, B.E.; Wang, X.; Sahoo, D.; Hoffman, D.J.; Malineni, J.; Peterca, M.; Jezorek, R.L.; et al. An Accelerated Modular-Orthogonal Ni-Catalyzed Methodology to Symmetric and Nonsymmetric Constitutional Isomeric AB2 to AB9 Dendrons Exhibiting Unprecedented Self-Organizing Principles. J. Am. Chem. Soc. 2021, 143, 17724–17743. [Google Scholar] [CrossRef]
- CrysAlis Pro, Oxford Diffraction: Yarnton, UK, 2010.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Gagne, R.R.; Koval, C.A.; Lisensky, G.C. Ferrocene as an internal standard for electrochemical measurements. Inorg. Chem. 1980, 19, 2854–2855. [Google Scholar] [CrossRef]
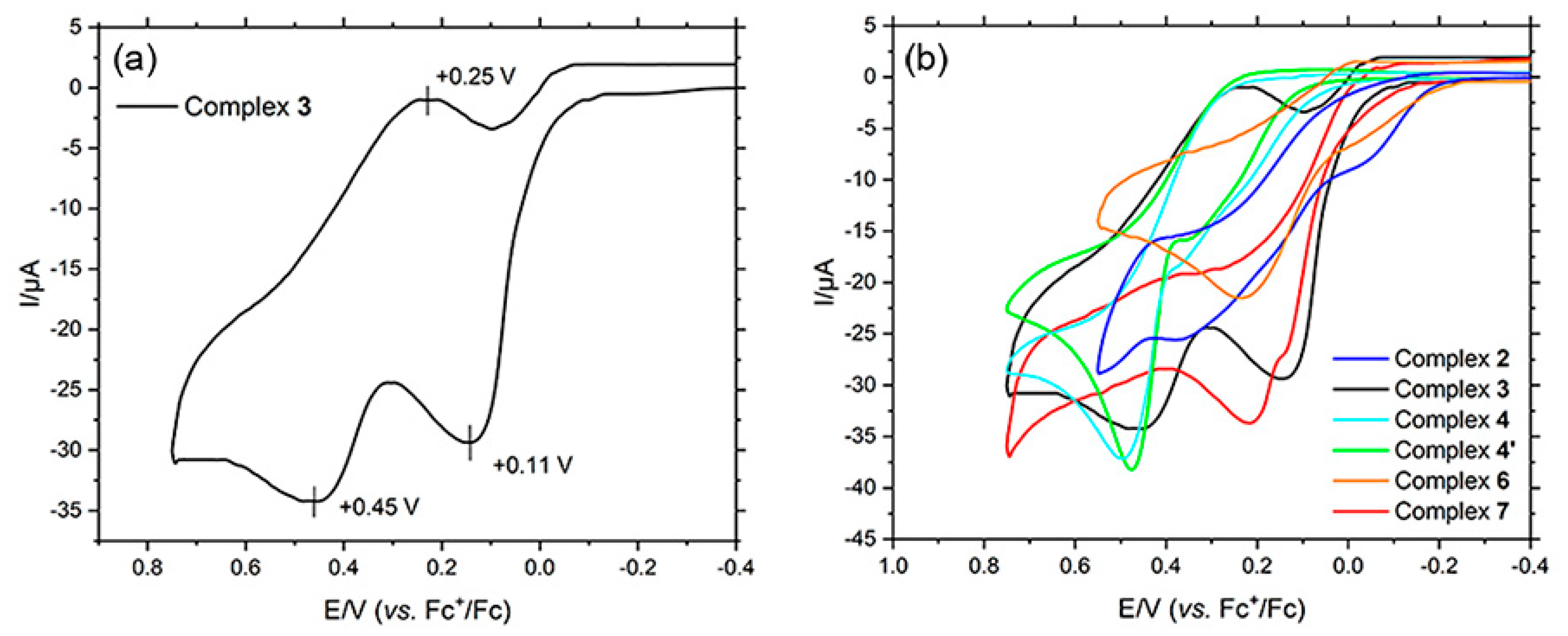
| Complex | Epa | Epc | Complex | Epa | Epc |
|---|---|---|---|---|---|
| 2 | −0.03 V, +0.36 V | - | 4′ | +0.35 V, +0.48 V | - |
| 3 | +0.11 V, +0.45 V | +0.25 V | 6 | −0.03 V, +0.22 V | - |
| 4 | +0.36 V, +0.49 V | - | 7 | +0.15 V, +0.21 V | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Zhan, W.; Fan, W.; Zhang, X. Research on Synthesis, Structure, and Catalytic Performance of Tetranuclear Copper(I) Clusters Supported by 2-Mercaptobenz-zole-Type Ligands. Molecules 2024, 29, 4228. https://doi.org/10.3390/molecules29174228
Zhu T, Zhan W, Fan W, Zhang X. Research on Synthesis, Structure, and Catalytic Performance of Tetranuclear Copper(I) Clusters Supported by 2-Mercaptobenz-zole-Type Ligands. Molecules. 2024; 29(17):4228. https://doi.org/10.3390/molecules29174228
Chicago/Turabian StyleZhu, Tingyu, Wangyuan Zhan, Weibin Fan, and Xiaofeng Zhang. 2024. "Research on Synthesis, Structure, and Catalytic Performance of Tetranuclear Copper(I) Clusters Supported by 2-Mercaptobenz-zole-Type Ligands" Molecules 29, no. 17: 4228. https://doi.org/10.3390/molecules29174228
APA StyleZhu, T., Zhan, W., Fan, W., & Zhang, X. (2024). Research on Synthesis, Structure, and Catalytic Performance of Tetranuclear Copper(I) Clusters Supported by 2-Mercaptobenz-zole-Type Ligands. Molecules, 29(17), 4228. https://doi.org/10.3390/molecules29174228







