Investigating the Promising P28 Peptide-Loaded Chitosan/Ceramic Bone Scaffolds for Bone Regeneration
Abstract
1. Introduction
2. Results
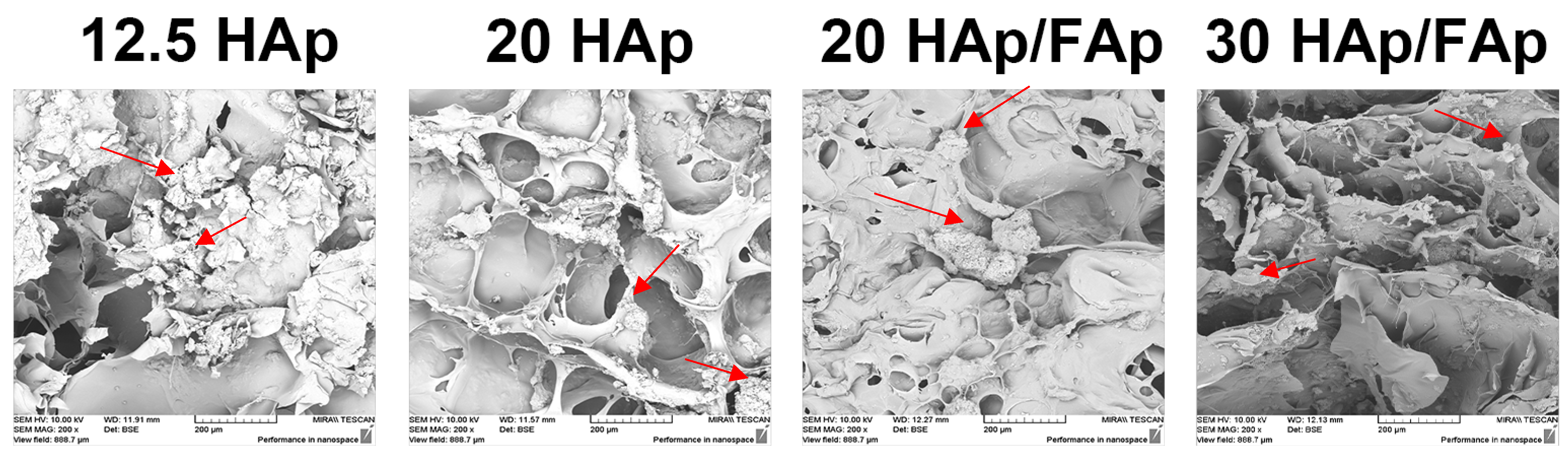
2.1. Scanning Electron Microscopy (SEM)
2.2. Antibacterial Activity
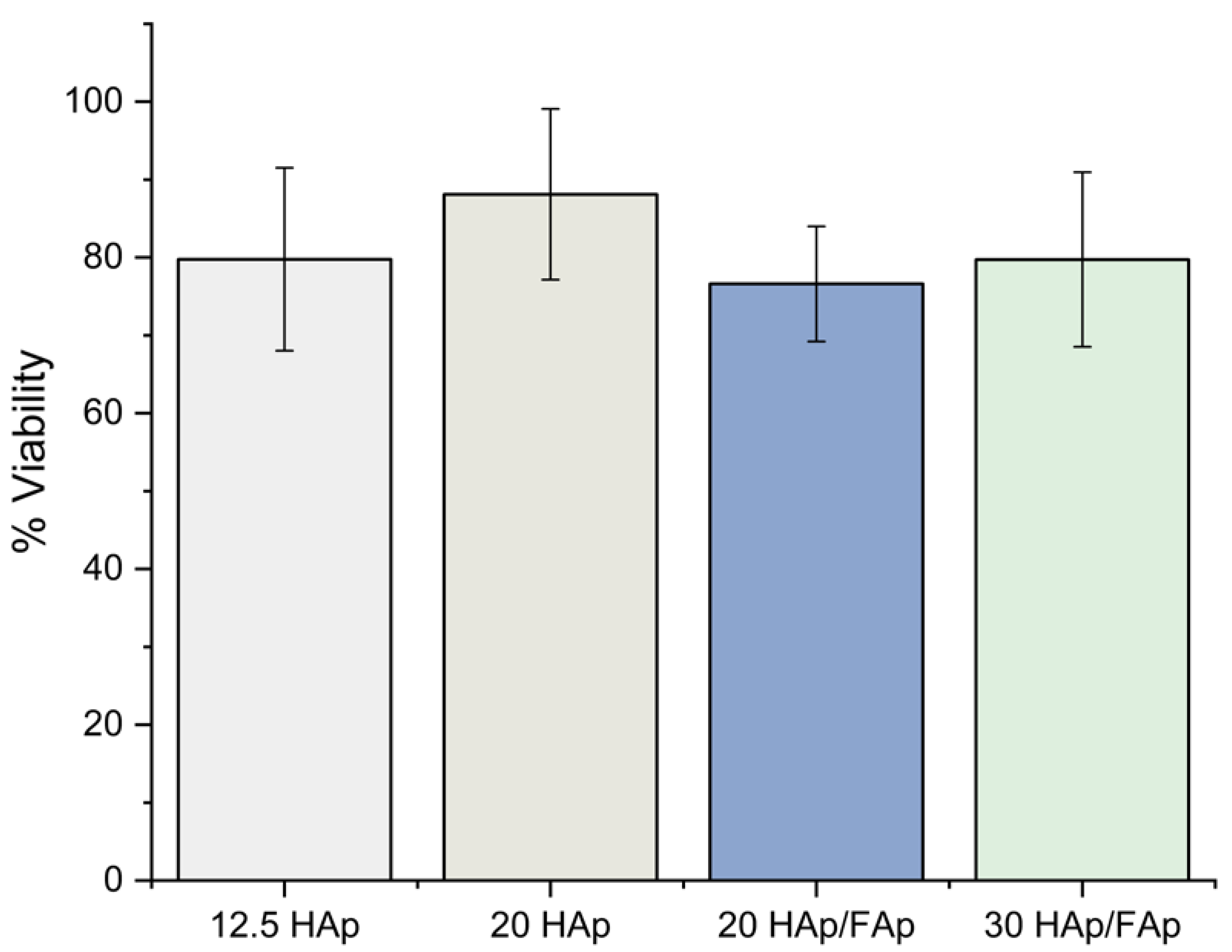
2.3. Cell Viability
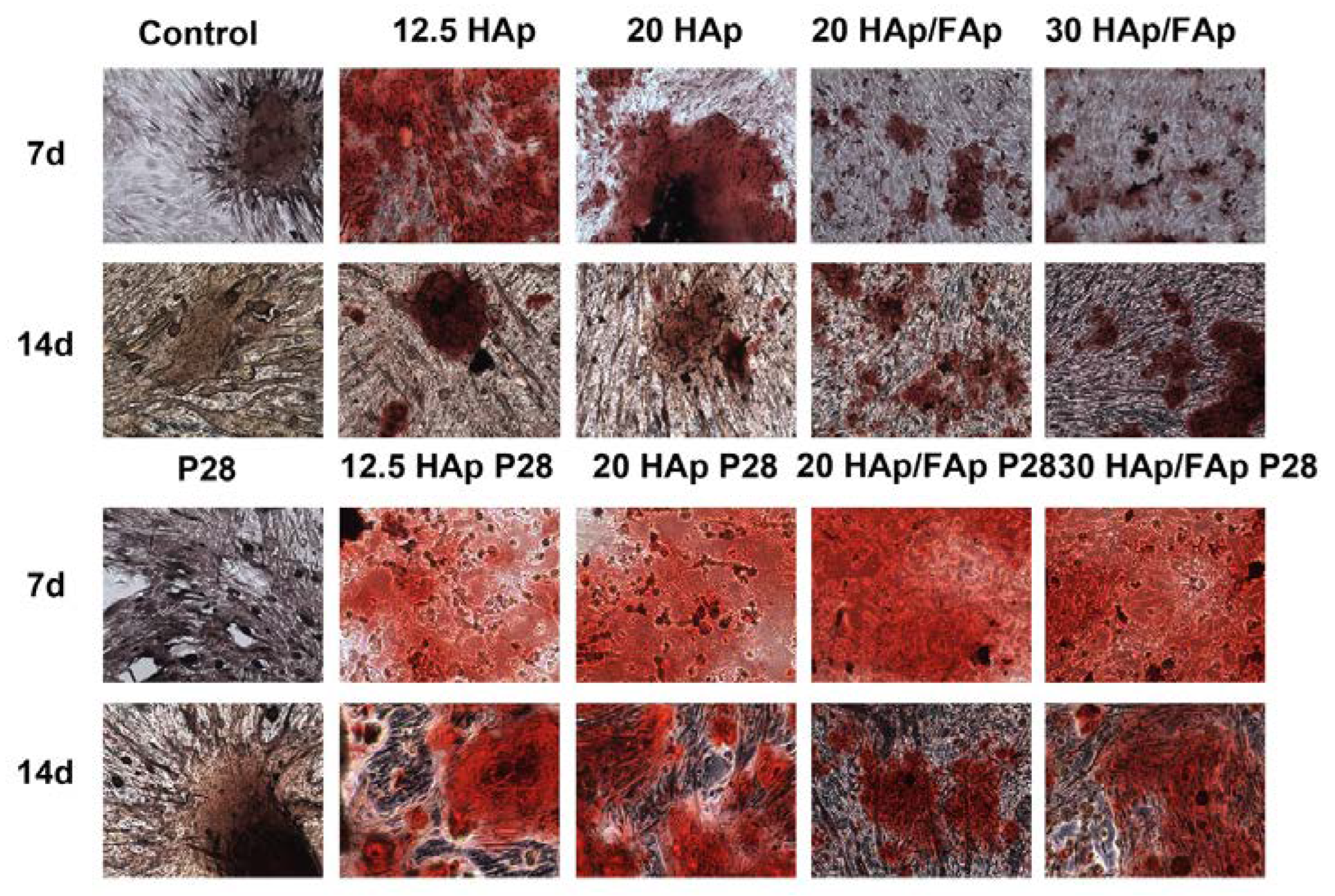
2.4. Alizarin Red S Staining
2.5. Alkaline Phosphatase (ALP) Activity
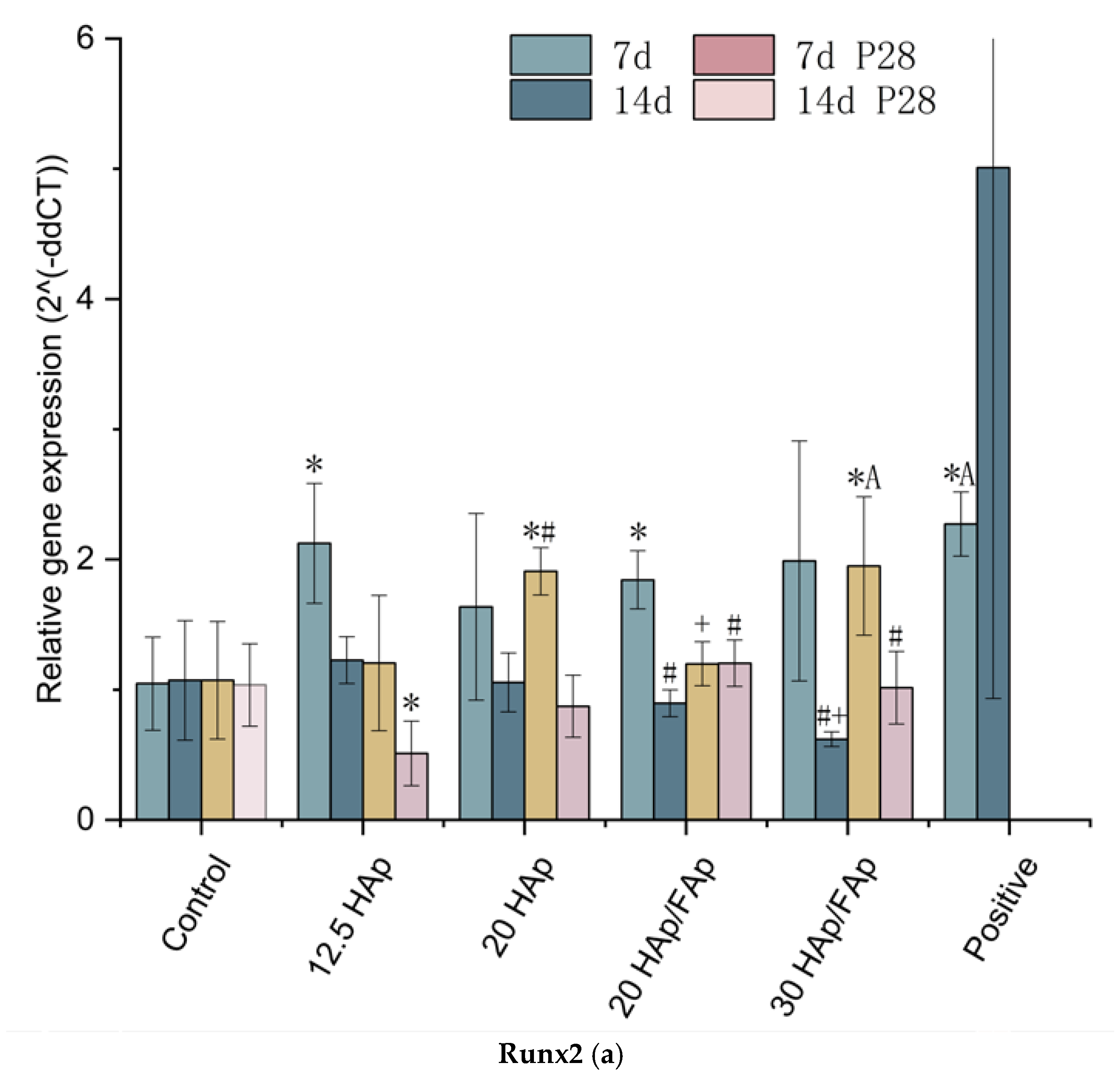
2.6. Quantitative Real-Time Polymerase Chain Reaction
3. Discussion
4. Materials and Methods
4.1. Scaffold Preparation
4.2. Peptide Loading
4.3. Scanning Electron Microscopy (SEM)
4.4. Antibacterial Test
4.5. Cell Seeding
4.6. Cytotoxicity Assay
4.7. Alizarin Red S Staining
4.8. Alkaline Phosphatase (ALP) Activity
4.9. Quantitative Real-Time Polymerase Chain Reaction
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.-T.; Zhang, Y.; Ren, H.-T.; Peng, H.-K.; Lou, C.-W.; Lin, J.-H. Two-step strategy for constructing hierarchical pore structured chitosan–hydroxyapatite composite scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 260, 117765. [Google Scholar] [CrossRef] [PubMed]
- Shaltooki, M.; Dini, G.; Mehdikhani, M. Fabrication of chitosan-coated porous polycaprolactone/strontium-substituted bioactive glass nanocomposite scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 105, 110138. [Google Scholar] [CrossRef]
- Liu, D.S.; Snyder, B.D.; Mahan, S.T. Fracture nonunion and delayed union. J. Pediatr. Orthop. Soc. N. Am. 2024, 7, 100058. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Flierl, M.A.; Smith, W.R.; Mauffrey, C.; Irgit, K.; Williams, A.E.; Ross, E.; Peacher, G.; Hak, D.J.; Stahel, P.F. Outcomes and complication rates of different bone grafting modalities in long bone fracture nonunions: A retrospective cohort study in 182 patients. J. Orthop. Surg. 2013, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Sk, S. Fracture Non-Union: A Review of Clinical Challenges and Future Research Needs. Malays. Orthop. J. 2019, 13, 1–10. [Google Scholar] [CrossRef]
- Zhao, C.Q.; Xu, X.C.; Lu, Y.J.; Wu, S.Q.; Xu, Z.Y.; Huang, T.T.; Lin, J.X. Doping lithium element to enhance compressive strength of β-TCP scaffolds manufactured by 3D printing for bone tissue engineering. J. Alloys Compd. 2020, 814, 152327. [Google Scholar] [CrossRef]
- Ekhlasmand Kermani, M.; Kheiri, A.; Amid, R.; Torshabi, M.; Houshmand, B.; Parsayan, S. Sterility and bioactivity evaluation of two types of bone graft substitutes after removing the original packaging. J. Adv. Periodontol. Implant Dent. 2023, 15, 15–21. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef] [PubMed]
- Laubach, M.; Hildebrand, F.; Suresh, S.; Wagels, M.; Kobbe, P.; Gilbert, F.; Kneser, U.; Holzapfel, B.M.; Hutmacher, D.W. The Concept of Scaffold-Guided Bone Regeneration for the Treatment of Long Bone Defects: Current Clinical Application and Future Perspective. J. Funct. Biomater. 2023, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Castillo, S.; Bernal-Ballén, A.; Bernal-López, C.; Segura-Puello, H.; Nieto-Mosquera, D.; Villamil-Ballesteros, A.; Muñoz-Forero, D.; Munster, L. Synthesis and Characterization of Poly(Vinyl Alcohol)-Chitosan-Hydroxyapatite Scaffolds: A Promising Alternative for Bone Tissue Regeneration. Molecules 2018, 23, 2414. [Google Scholar] [CrossRef]
- Abd-Khorsand, S.; Saber-Samandari, S.; Saber-Samandari, S. Development of nanocomposite scaffolds based on TiO 2 doped in grafted chitosan/hydroxyapatite by freeze drying method and evaluation of biocompatibility. Int. J. Biol. Macromol. 2017, 101, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Chlanda, A.; Oberbek, P.; Heljak, M.; Kijeńska-Gawrońska, E.; Bolek, T.; Gloc, M.; John, Ł.; Janeta, M.; Woźniak, M.J. Fabrication, multi-scale characterization and in-vitro evaluation of porous hybrid bioactive glass polymer-coated scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 94, 516–523. [Google Scholar] [CrossRef]
- Heidari, F.; Razavi, M.; Bahrololoom, M.E.; Tahriri, M.; Tayebi, L. Investigation of the mechanical properties and degradability of a modified chitosan-based scaffold. Mater. Chem. Phys. 2018, 204, 187–194. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, S.; Ma, Z.; Ding, C.; Chen, J.; Li, J. Chitosan-Based Scaffolds for Facilitated Endogenous Bone Re-Generation. Pharmaceuticals 2022, 15, 1023. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Sapalidis, A.; Papadopoulos, T. Hydroxyapatite/chitosan-based porous three-dimensional scaffolds with complex geometries. Mater. Today Commun. 2016, 7, 59–66. [Google Scholar] [CrossRef]
- Deepthi, S.; Venkatesan, J.; Kim, S.-K.; Bumgardner, J.D.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1338–1353. [Google Scholar] [CrossRef]
- Yadav, L.R.; Chandran, S.V.; Lavanya, K.; Selvamurugan, N. Chitosan-based 3D-printed scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2021, 183, 1925–1938. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Wessely-Szponder, J.; Palka, K.; Barylyak, A.; Zinchenko, V.; Przekora, A. Hydroxyapatite or Fluorapatite—Which Bioceramic Is Better as a Base for the Production of Bone Scaffold?—A Comprehensive Comparative Study. Int. J. Mol. Sci. 2023, 24, 5576. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, L.; Przekora, A.; Belcarz, A.; Palka, K.; Jojczuk, M.; Lukasiewicz, P.; Nogalski, A.; Ginalska, G. Highly Porous Fluorapatite/β-1,3-Glucan Composite for Bone Tissue Regeneration: Characterization and In-Vitro Assessment of Biomedical Potential. Int. J. Mol. Sci. 2021, 22, 10414. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Tang, Y.; Lozito, T.P.; Oyster, N.; Wang, B.; Tuan, R.S. Efficient in vivo bone formation by BMP-2 engineered human mesenchymal stem cells encapsulated in a projection stereolithographically fabricated hydrogel scaffold. Stem Cell Res. Ther. 2019, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Sampath, T.K.; Vukicevic, S. Biology of bone morphogenetic protein in bone repair and regeneration: A role for autologous blood coagulum as carrier. Bone 2020, 141, 115602. [Google Scholar] [CrossRef] [PubMed]
- Azaman, F.A.; Daubiné, F.; Lebatard, A.; Brennan Fournet, M.E.; Devine, D.M. Chitosan/Hydroxyapatite Scaffolds with P28 as a Promising Osteoinductive Scaffold for Bone Healing Applications. Micro 2023, 3, 118–142. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Q.; Yang, L.; Wang, K.; Sun, T.; Ji, Y.; Liu, L.; Yu, W.; Qu, Y.; Wang, J.; et al. Sustained Delivery of BMP-2-Related Peptide from the True Bone Ceramics/Hollow Mesoporous Silica Nanoparticles Scaffold for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 211–221. [Google Scholar] [CrossRef]
- Xiong, Z.; Cui, W.; Sun, T.; Teng, Y.; Qu, Y.; Yang, L.; Zhou, J.; Chen, K.; Yao, S.; Shao, Z.; et al. Sustained delivery of PlGF-2 123-144* -fused BMP2-related peptide P28 from small intestinal submucosa/polylactic acid scaffold material for bone tissue regeneration. RSC Adv. 2020, 10, 7289–7300. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J.; Zhang, Q.; Li, Y.; Wang, Z.; Hu, Q. Preparation and characterization of bionic bone structure chitosan/hydroxyapatite scaffold for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2014, 25, 61–74. [Google Scholar] [CrossRef]
- Killion, J.A.; Kehoe, S.; Geever, L.M.; Devine, D.M.; Sheehan, E.; Boyd, D.; Higginbotham, C.L. Hydrogel/bioactive glass composites for bone regeneration applications: Synthesis and characterisation. Mater. Sci. Eng. C 2013, 33, 4203–4212. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.M.; Jang, J.E.; Kim, C.M.; Kim, E.Y.; Lee, D.; Khang, G. Osteogenic Differentiation of Bone Marrow Stem Cell in Poly(Lactic-co-Glycolic Acid) Scaffold Loaded Various Ratio of Hydroxyapatite. Int. J. Stem Cells 2013, 6, 67–74. [Google Scholar] [CrossRef]
- Azaman, F.A.; Zhou, K.; Blanes-Martínez, M.D.M.; Brennan Fournet, M.; Devine, D.M. Bioresorbable Chitosan-Based Bone Regeneration Scaffold Using Various Bioceramics and the Alteration of Photoinitiator Concentration in an Extended UV Photocrosslinking Reaction. Gels 2022, 8, 696. [Google Scholar] [CrossRef] [PubMed]
- Chacon, E.L.; Bertolo, M.R.V.; De Guzzi Plepis, A.M.; Da Conceição Amaro Martins, V.; Dos Santos, G.R.; Pinto, C.A.L.; Pelegrine, A.A.; Teixeira, M.L.; Buchaim, D.V.; Nazari, F.M.; et al. Collagen-chitosan-hydroxyapatite composite scaffolds for bone repair in ovariectomized rats. Sci. Rep. 2023, 13, 28. [Google Scholar] [CrossRef]
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow cytometry: Basic principles and applications. Crit. Rev. Biotechnol. 2017, 37, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yelverton, C.J.; Barnard, T.G. Rapid Quantification of the Total Viable Bacterial Population on Human Hands Using Flow Cytometry with SYBR® Green I. Cytometry B Clin. Cytom. 2019, 96, 397–403. [Google Scholar] [CrossRef]
- Metsemakers, W.-J.; Morgenstern, M.; Senneville, E.; Borens, O.; Govaert, G.A.M.; Onsea, J.; Depypere, M.; Richards, R.G.; Trampuz, A.; Verhofstad, M.H.J.; et al. General treatment principles for fracture-related infection: Recommendations from an international expert group. Arch. Orthop. Trauma Surg. 2020, 140, 1013–1027. [Google Scholar] [CrossRef]
- Winkler, H. Treatment of chronic orthopaedic infection. EFORT Open Rev. 2017, 2, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Barandehfard, F.; Kianpour Rad, M.; Hosseinnia, A.; Khoshroo, K.; Tahriri, M.; Jazayeri, H.E.; Moharamzadeh, K.; Tayebi, L. The addition of synthesized hydroxyapatite and fluorapatite nanoparticles to a glass-ionomer cement for dental restoration and its effects on mechanical properties. Ceram. Int. 2016, 42, 17866–17875. [Google Scholar] [CrossRef]
- Seyedmajidi, S.; Rajabnia, R.; Seyedmajidi, M. Evaluation of antibacterial properties of hydroxyapatite/bioactive glass and fluorapatite/bioactive glass nanocomposite foams as a cellular scaffold of bone tissue. J. Lab. Physicians 2018, 10, 265–270. [Google Scholar] [CrossRef]
- Mobini, S.; Solati-Hashjin, M.; Peirovi, H.; Osman, N.A.A.; Gholipourmalekabadi, M.; Barati, M.; Samadikuchaksaraei, A. Bioactivity and Biocompatibility Studies on Silk-Based Scaffold for Bone Tissue Engineering. J. Med. Biol. Eng. 2013, 33, 207. [Google Scholar] [CrossRef]
- Ressler, A.; Ródenas-Rochina, J.; Ivanković, M.; Ivanković, H.; Rogina, A.; Gallego Ferrer, G. Injectable chitosan-hydroxyapatite hydrogels promote the osteogenic differentiation of mesenchymal stem cells. Carbohydr. Polym. 2018, 197, 469–477. [Google Scholar] [CrossRef]
- Przekora, A.; Ginalska, G. Chitosan/β-1,3-glucan/hydroxyapatite bone scaffold enhances osteogenic differentiation through TNF-α-mediated mechanism. Mater. Sci. Eng. C 2017, 73, 225–233. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. Enhanced osteogenic differentiation of stem cells by 3D printed PCL scaffolds coated with collagen and hydroxyapatite. Sci. Rep. 2022, 12, 12359. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, M.; Yao, S.; Ji, Y.; Shi, L.; Tang, K.; Xiong, Z.; Yang, F.; Chen, K.; Guo, X. Guided osteoporotic bone regeneration with composite scaffolds of mineralized ECM/heparin membrane loaded with BMP2-related peptide. Int. J. Nanomed. 2018, 13, 791–804. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, Z.; Liu, M.; Yang, L.; Yao, S.; Chen, K.; Yu, K.; Qu, Y.; Sun, T.; Guo, X. Creation of Bony Microenvironment with Extracellular Matrix Doped-Bioactive Ceramics to Enhance Osteoblast Behavior and Delivery of Aspartic Acid-Modified BMP-2 Peptides. Int. J. Nanomed. 2020, 15, 8465–8478. [Google Scholar] [CrossRef] [PubMed]
- Djošić, M.; Janković, A.; Stevanović, M.; Stojanović, J.; Vukašinović-Sekulić, M.; Kojić, V.; Mišković-Stanković, V. Hydroxyapatite/poly(vinyl alcohol)/chitosan coating with gentamicin for orthopedic implants. Mater. Chem. Phys. 2023, 303, 127766. [Google Scholar] [CrossRef]
- Kong, L.; Gao, Y.; Lu, G.; Gong, Y.; Zhao, N.; Zhang, X. A study on the bioactivity of chitosan/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Eur. Polym. J. 2006, 42, 3171–3179. [Google Scholar] [CrossRef]
- Abazari, M.F.; Nasiri, N.; Nejati, F.; Kohandani, M.; Hajati-Birgani, N.; Sadeghi, S.; Piri, P.; Soleimanifar, F.; Rezaei-Tavirani, M.; Mansouri, V. Acceleration of osteogenic differentiation by sustained release of BMP2 in PLLA /graphene oxide nanofibrous scaffold. Polym. Adv. Technol. 2021, 32, 272–281. [Google Scholar] [CrossRef]
- Sun, T.; Qu, Y.; Cui, W.; Yang, L.; Ji, Y.; Yu, W.; Navinduth, R.; Shao, Z.; Yang, H.; Guo, X. Evaluation of osteogenic inductivity of a novel BMP2-mimicking peptide P28 and P28-containing bone composite. J. Biomed. Mater. Res. A 2018, 106, 210–220. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Ji, Y.; Deng, H.; Long, M.; Ge, S.; Su, Y.; Chan, S.Y.; Loh, X.J.; Zhuang, A.; et al. A non-invasive smart scaffold for bone repair and monitoring. Bioact. Mater. 2023, 19, 499–510. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Yang, K.; Yuan, Y.; Liu, C. RhBMP-2-loaded calcium silicate/calcium phosphate cement scaffold with hierarchically porous structure for enhanced bone tissue regeneration. Biomaterials 2013, 34, 9381–9392. [Google Scholar] [CrossRef] [PubMed]
- Elghazel, A.; Taktak, R.; Bouaziz, J.; Charfi, S.; Keskes, H. TCP-Fluorapatite Composite Scaffolds: Mechanical Characterization and In Vitro/In Vivo Testing. In Scaffolds in Tissue Engineering—Materials, Technologies and Clinical Applications; Baino, F., Ed.; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Signorini, L.; Marenzi, G.; Facente, A.; Marrelli, B.; Marano, R.M.; Valletta, A.; Pacifici, L.; Gasparro, R.; Sammartino, G.; Severino, M. Critical Overview on Pure Chitosan-based Scaffolds for Bone Tissue Engineering: Clinical insights in Dentistry. Int. J. Med. Sci. 2023, 20, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Azueta-Aguayo, P.H.; Chuc-Gamboa, M.G.; Aguilar-Pérez, F.J.; Aguilar-Ayala, F.J.; Rodas-Junco, B.A.; Vargas-Coronado, R.F.; Cauich-Rodríguez, J.V. Effects of Neutralization on the Physicochemical, Mechanical, and Biological Properties of Ammonium-Hydroxide-Crosslinked Chitosan Scaffolds. Int. J. Mol. Sci. 2022, 23, 14822. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z. Chitosan-calcium carbonate scaffold with high mineral content and hierarchical structure for bone regeneration. Smart Mater. Med. 2023, 4, 552–561. [Google Scholar] [CrossRef]
- Nikpour, M.R.; Rabiee, S.M.; Jahanshahi, M. Synthesis and characterization of hydroxyapatite/chitosan nanocomposite materials for medical engineering applications. Compos. Part B Eng. 2012, 43, 1881–1886. [Google Scholar] [CrossRef]
- Mohamed, K.R.; El-Rashidy, Z.M.; Salama, A.A. In vitro properties of nano-hydroxyapatite/chitosan biocomposites. Ceram. Int. 2011, 37, 3265–3271. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, G.; Wu, Q.; Zuo, J.; Qin, Y.; Wang, J. Novel Mesoporous Hydroxyapatite/Chitosan Composite for Bone Repair. J. Bionic Eng. 2012, 9, 243–251. [Google Scholar] [CrossRef]
- Tejaswini, T.; Keerthana, M.; Vidyavathi, M.; Kumar, R.V.S. Design and evaluation of atorvastatin-loaded chitosan-hydroxyapatite composite bioscaffolds for wound-healing activity. Future J. Pharm. Sci. 2020, 6, 111. [Google Scholar] [CrossRef]
- Sadowska, J.M.; Power, R.N.; Genoud, K.J.; Matheson, A.; González-Vázquez, A.; Costard, L.; Eichholz, K.; Pitacco, P.; Hallegouet, T.; Chen, G.; et al. A Multifunctional Scaffold for Bone Infection Treatment by Delivery of microRNA Therapeutics Combined With Antimicrobial Nanoparticles. Adv. Mater. 2024, 36, 2307639. [Google Scholar] [CrossRef]
- Gama E Silva, G.L.; Sato De Souza Bustamante Monteiro, M.; Dos Santos Matos, A.P.; Santos-Oliveira, R.; Kenechukwu, F.C.; Ricci-Júnior, E. Nanofibers in the treatment of osteomyelitis and bone regeneration. J. Drug Deliv. Sci. Technol. 2022, 67, 102999. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef]
- Lukman Hekiem, N.L.; Md Ralib, A.A.; Mohd Hatta, M.A.; Ahmad, F.B.; Nordin, A.N.; Ab Rahim, R.; Za’bah, N.F. Effect of chitosan dissolved in different acetic acid concentration towards VOC sensing performance of quartz crystal microbalance overlay with chitosan. Mater. Lett. 2021, 291, 129524. [Google Scholar] [CrossRef]
- Nasker, P.; Mukherjee, M.; Kant, S.; Tripathy, S.; Sinha, A.; Das, M. Fluorine substituted nano hydroxyapatite: Synthesis, bio-activity and antibacterial response study. Ceram. Int. 2018, 44, 22008–22013. [Google Scholar] [CrossRef]
- Sirait, M.; Sinulingga, K.; Siregar, N.; Doloksaribu, M.E. Amelia Characterization of hydroxyapatite by cytotoxicity test and bending test. J. Phys. Conf. Ser. 2022, 2193, 012039. [Google Scholar] [CrossRef]
- Kim, H.J.; Hong, S.J.; Lee, S.; Park, J.M.; Park, J.; Park, J.S.; Shim, S.H.; Park, K. Induction of Bone Formation by 3D Biologically Active Scaffolds Containing RGD-NPs, BMP2, and NtMPCs. Adv. Ther. 2021, 4, 2000245. [Google Scholar] [CrossRef]
- Zhao, X.; Han, Y.; Li, J.; Cai, B.; Gao, H.; Feng, W.; Li, S.; Liu, J.; Li, D. BMP-2 immobilized PLGA/hydroxyapatite fibrous scaffold via polydopamine stimulates osteoblast growth. Mater. Sci. Eng. C 2017, 78, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Chatzinikolaidou, M.; Pontikoglou, C.; Terzaki, K.; Kaliva, M.; Kalyva, A.; Papadaki, E.; Vamvakaki, M.; Farsari, M. Recombinant human bone morphogenetic protein 2 (rhBMP-2) immobilized on laser-fabricated 3D scaffolds enhance osteogenesis. Colloids Surf. B Biointerfaces 2017, 149, 233–242. [Google Scholar] [CrossRef]
- Kannan, S.; Ghosh, J.; Dhara, S.K. Osteogenic differentiation potential of porcine bone marrow mesenchymal stem cell subpopulations selected in different basal media. Biol. Open 2020, 9, bio.053280. [Google Scholar] [CrossRef]
- Hu, S.; Chen, H.; Zhou, X.; Chen, G.; Hu, K.; Cheng, Y.; Wang, L.; Zhang, F. Thermally induced self-agglomeration 3D scaffolds with BMP-2-loaded core–shell fibers for enhanced osteogenic differentiation of rat adipose-derived stem cells. Int. J. Nanomed. 2018, 13, 4145–4155. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Song, H.; Zhen, J.; Qiu, Y.; Liu, X.; Xu, W.; Zhang, S. Study on the bone morphogenetic protein 2 loaded synergistic hierarchical porous silk/carbon nanocage scaffold for the repair of bone defect. Mater. Des. 2020, 196, 109105. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, K.; Liu, M.; Guo, X.; Qu, Y.; Cui, W.; Shao, Z.; Zhang, X.; Xu, S. Loading of BMP-2-related peptide onto three-dimensional nano-hydroxyapatite scaffolds accelerates mineralization in critical-sized cranial bone defects. J. Tissue Eng. Regen. Med. 2018, 12, 864–877. [Google Scholar] [CrossRef] [PubMed]





| Scaffold ID | CS (g) | HAp (g) | FAp (g) | Volume of Acetic Acid/(mL) |
|---|---|---|---|---|
| 12.5 HAp | 1.5 | 1.5 | 0 | 12.5 |
| 20 HAp | 1.5 | 1.5 | 0 | 20 |
| 20 HAp/FAp | 1.5 | 0.75 | 0.75 | 20 |
| 30 HAp/FAp | 1.5 | 0.75 | 0.75 | 30 |
| Gene | Primer Sequences Forward/Reverse |
|---|---|
| Bglap | 5′-GACACCATGAGGACCATCTTTC-3′/5′-CATGAAGGCTTTGTCAGACTCA-3′ |
| Col1a1 | 5′-CCAATGGTGCTCCTGGTATT-3′/5′-GGTTCACCACTGTTACCCTT-3′ |
| Runx2 | 5′-CTCTGATCGCCTCAGTGATTT-3′/5′-CTGCCTGGGATCTGTAATCTG-3′ |
| Tbp | 5′-AGTGCCCAGCATCACTATTT-3′/5′-GGTCCATGATTCTCCCTTTCTT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, K.; Simonassi-Paiva, B.; Fehrenbach, G.; Yan, G.; Portela, A.; Pogue, R.; Cao, Z.; Fournet, M.B.; Devine, D.M. Investigating the Promising P28 Peptide-Loaded Chitosan/Ceramic Bone Scaffolds for Bone Regeneration. Molecules 2024, 29, 4208. https://doi.org/10.3390/molecules29174208
Zhou K, Simonassi-Paiva B, Fehrenbach G, Yan G, Portela A, Pogue R, Cao Z, Fournet MB, Devine DM. Investigating the Promising P28 Peptide-Loaded Chitosan/Ceramic Bone Scaffolds for Bone Regeneration. Molecules. 2024; 29(17):4208. https://doi.org/10.3390/molecules29174208
Chicago/Turabian StyleZhou, Keran, Bianca Simonassi-Paiva, Gustavo Fehrenbach, Guangming Yan, Alexandre Portela, Robert Pogue, Zhi Cao, Margaret Brennan Fournet, and Declan M. Devine. 2024. "Investigating the Promising P28 Peptide-Loaded Chitosan/Ceramic Bone Scaffolds for Bone Regeneration" Molecules 29, no. 17: 4208. https://doi.org/10.3390/molecules29174208
APA StyleZhou, K., Simonassi-Paiva, B., Fehrenbach, G., Yan, G., Portela, A., Pogue, R., Cao, Z., Fournet, M. B., & Devine, D. M. (2024). Investigating the Promising P28 Peptide-Loaded Chitosan/Ceramic Bone Scaffolds for Bone Regeneration. Molecules, 29(17), 4208. https://doi.org/10.3390/molecules29174208







