The Effect of the Extraction Conditions on the Antioxidant Activity and Bioactive Compounds Content in Ethanolic Extracts of Scutellaria baicalensis Root
Abstract
1. Introduction
2. Results and Discussion
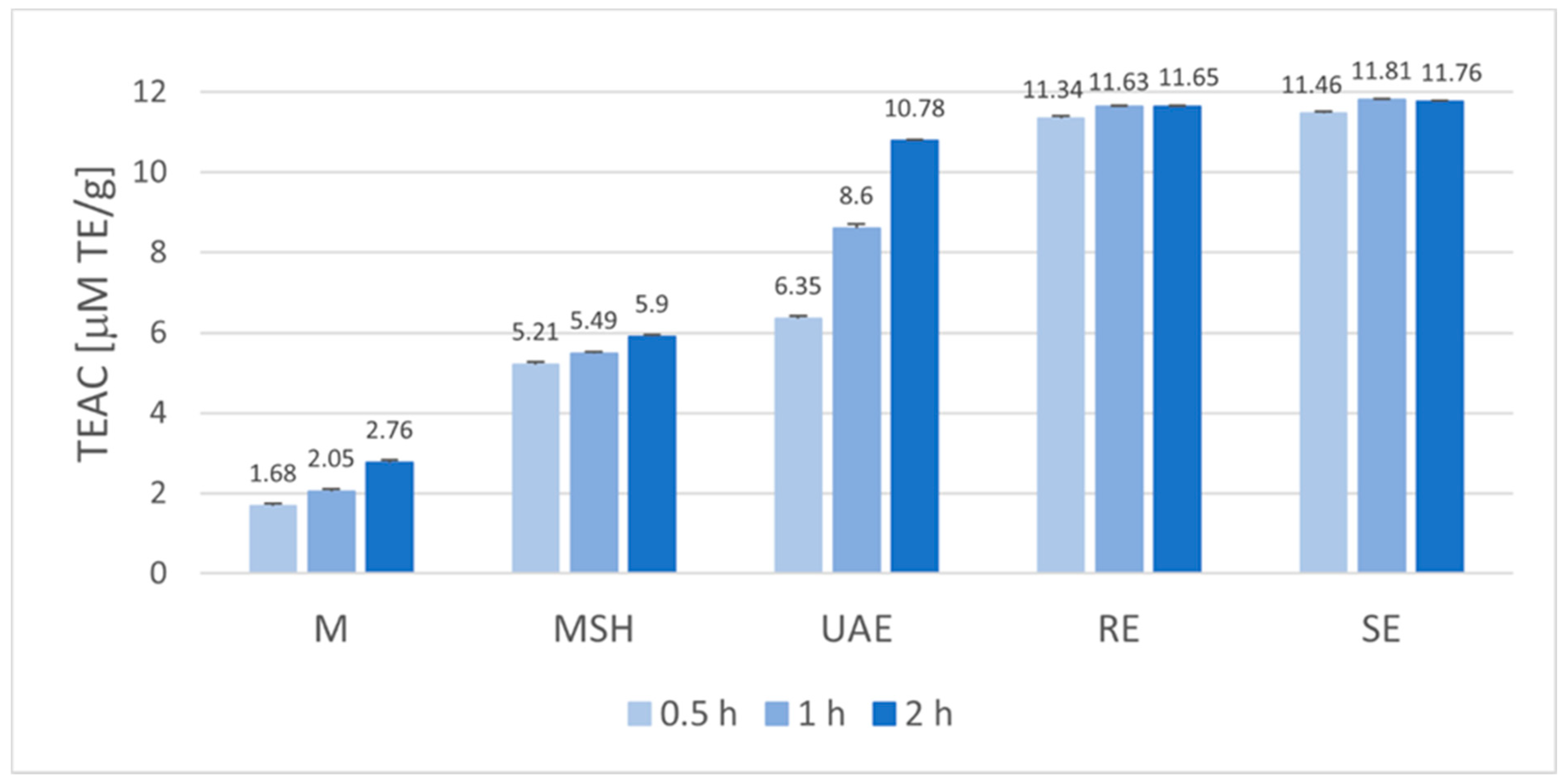
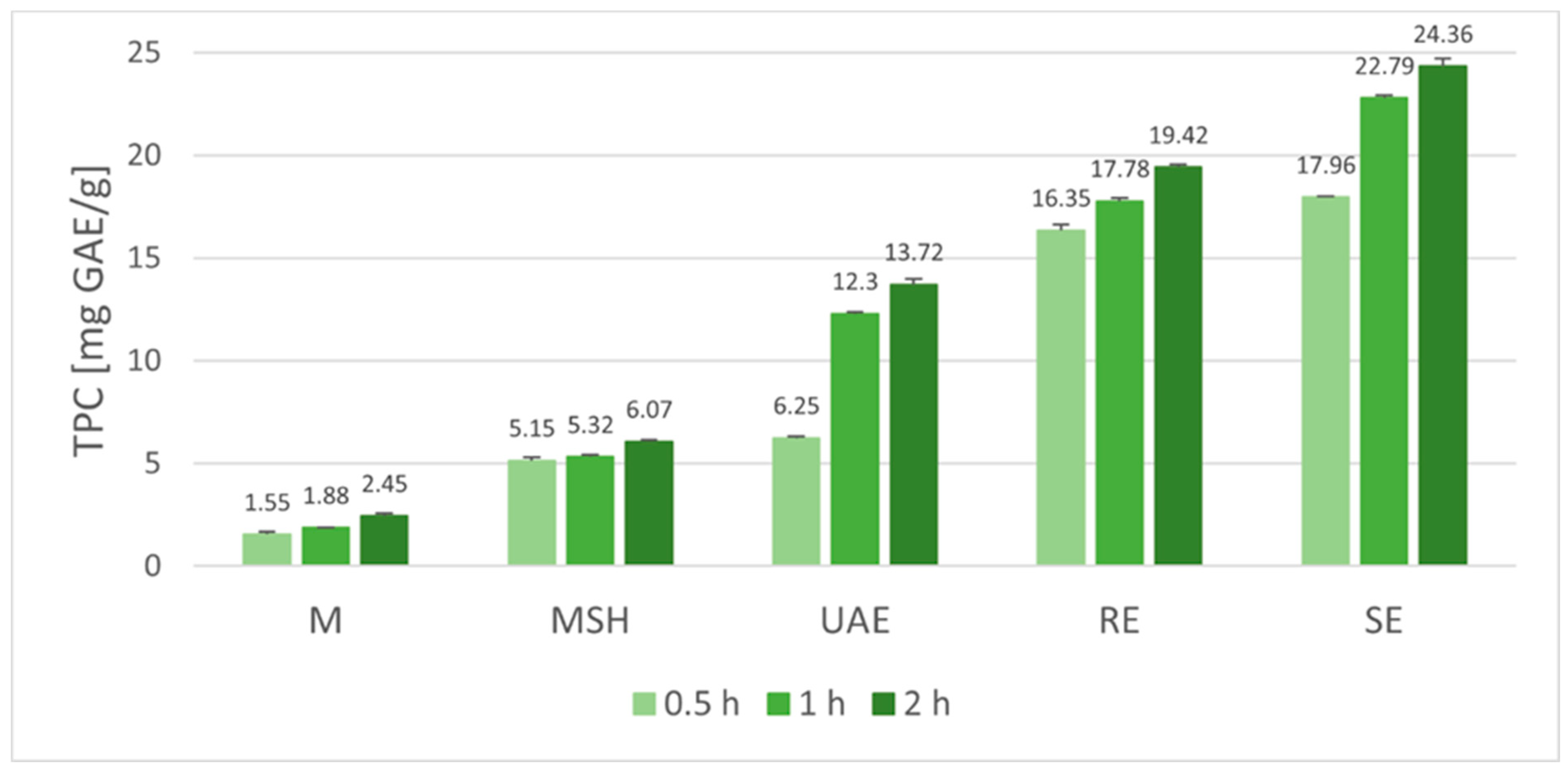
2.1. Antioxidant Potential of Baikal Skullcap Root Extracts
2.2. Statistical Analysis of Antioxidant Potential and Total Phenolic Content
2.3. Dry Extracts of Baikal Skullcap Root
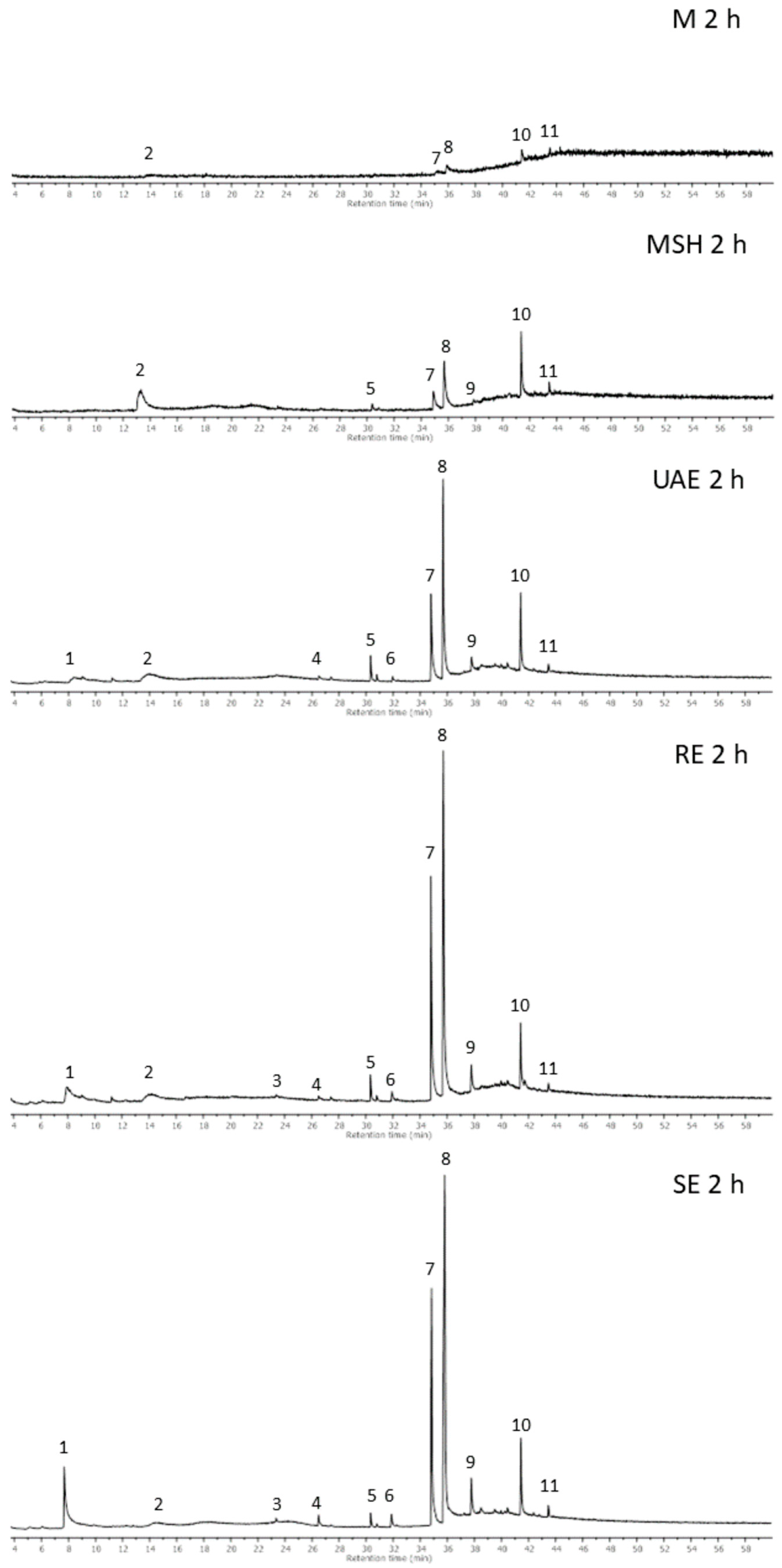
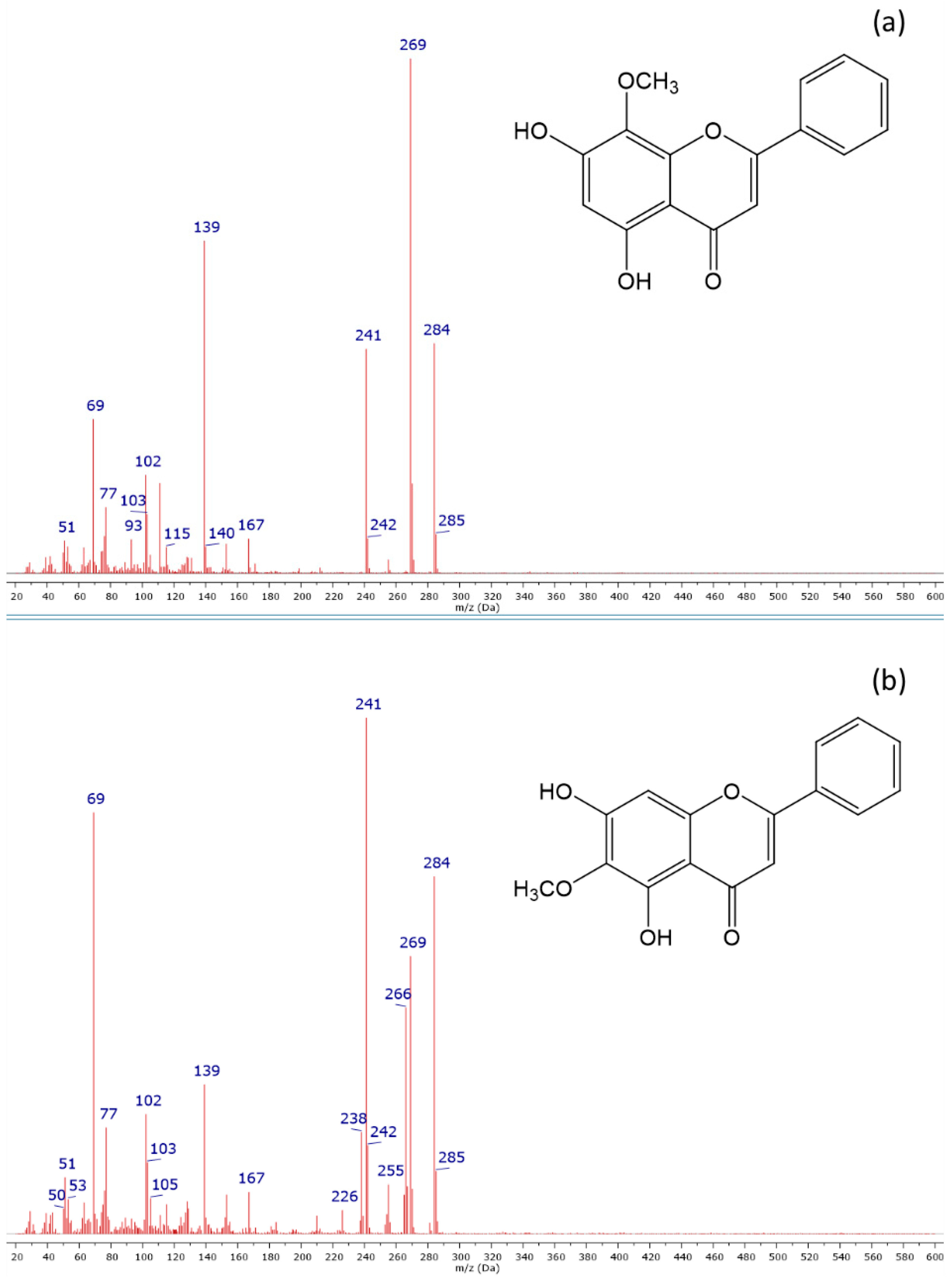
2.4. GC-MS Analysis
3. Materials and Methods
3.1. Plant Material
3.2. Extraction Techniques and Sample Preparation
3.3. Antioxidant Activity
3.4. Total Phenolic Content (TPC)
3.5. GC-MS Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kosakowska, O. Intrapopulation variability of flavonoid content in roots of baikal skullcap (Scutellaria baicalensis Georgi). Herba. Pol. 2017, 63, 23–31. [Google Scholar] [CrossRef]
- Vergun, O.; Svydenko, L.; Grygorieva, O.; Shymanska, O.; Rakhmetov, D.; Brindza, J.; Ivanišová, E. Antioxidant capacity of plant raw material of Scutellaria baicalensis Georgi. Slovak J. Food Sci./Potravinarstvo 2019, 13, 614–621. [Google Scholar] [CrossRef]
- Kosakowska, O.; Baczek, K.; Przybył, J.; Pióro-Jabrucka, E.; Węglarz, Z. Chemical variability of common skullcap (Scutellaria galericulata l.) wild growing in the area of eastern poland. Herba. Pol. 2016, 62, 7–19. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; Wang, S.; Kuang, Y.; Hu, Z.-M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef]
- Chanchal, D.K.; Singh, K.; Bhushan, B.; Chaudhary, J.S.; Kumar, S.; Varma, A.K.; Agnihotri, N.; Garg, A. An updated review of chinese skullcap (Scutellaria baicalensis): Emphasis on phytochemical constituents and pharmacological attributes. Pharmacol. Res.-Mod. Chin. Med. 2023, 9, 100326. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim. Biophys. Acta BBA-Gen. Subj. 1999, 1472, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Elkin, Y.N.; Kulesh, N.I.; Stepanova, A.Y.; Solovieva, A.I.; Kargin, V.M.; Manyakhin, A.Y. Methylated flavones of the hairy root culture Scutellaria baicalensis. J. Plant Physiol. 2018, 231, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nian, M.; Qiao, H.; Yang, X.; Wu, S.; Zheng, X. Review of bioactivity and structure-activity relationship on baicalein (5,6,7-trihydroxyflavone) and wogonin (5,7-dihydroxy-8-methoxyflavone) derivatives: Structural modifications inspired from flavonoids in Scutellaria baicalensis. Eur. J. Med. Chem. 2022, 243, 114733. [Google Scholar] [CrossRef]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. the main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef]
- Hu, Z.; Guan, Y.; Hu, W.; Xu, Z.; Ishfaq, M. An overview of pharmacological activities of baicalin and its aglycone baicalein: New insights into molecular mechanisms and signaling pathways. Iran. J. Basic Med. Sci. 2022, 25, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, M.; Stompor-Gorący, M. Promising role of the scutellaria baicalensis root hydroxyflavone–baicalein in the prevention and treatment of human diseases. Int. J. Mol. Sci. 2023, 24, 4732. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Calway, T.D.; Wen, X.-D.; Smith, J.; Yu, C.; Wang, Y.; Mehendale, S.R.; Yuan, C.-S. Hydrophobic Flavonoids from Scutellaria baicalensis induce colorectal cancer cell apoptosis through a mitochondrial-mediated pathway. Int. J. Oncol. 2013, 42, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Bae, M.-J.; Choi, D.W.; Shon, D.-H. Skullcap (Scutellaria baicalensis) extract and its active compound, wogonin, inhibit ovalbumin-induced TH2-mediated response. Molecules 2014, 19, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhan, S.; Wang, Y.; Zhou, G.; Liang, H.; Chen, X.; Shen, H. Baicalin, the major component of traditional Chinese medicine scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomirnas. Sci. Rep. 2018, 8, 14477. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xia, T.; Yu, X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm. Res. 2015, 64, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Jelić, D.; Lower-Nedza, A.D.; Brantner, A.H.; Blažeković, B.; Bian, B.; Yang, J.; Brajša, K.; Vladimir-Knežević, S. Baicalin and baicalein inhibit Src tyrosine kinase and production of IL-6. J. Chem. 2016, 2016, 2510621. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.; Zhao, C.; Zhao, B.; Wang, J. The pharmacological efficacy of baicalin in inflammatory diseases. Int. J. Mol. Sci. 2023, 24, 9317. [Google Scholar] [CrossRef]
- Lei, L.; Zhao, J.; Liu, X.-Q.; Chen, J.; Qi, X.-M.; Xia, L.-L.; Wu, Y.-G. Wogonin alleviates kidney tubular epithelial injury in diabetic nephropathy by inhibiting PI3K/Akt/NF-κB signaling pathways. Drug Des. Devel. Ther. 2021, 15, 3131–3150. [Google Scholar] [CrossRef]
- Long, Y.; Xiang, Y.; Liu, S.; Zhang, Y.; Wan, J.; Yang, Q.; Cui, M.; Ci, Z.; Li, N.; Peng, W. Baicalin liposome alleviates lipopolysaccharide-induced acute lung injury in mice via inhibiting TLR4/JNK/ERK/NF-κB pathway. Mediators Inflamm. 2020, 2020, 8414062. [Google Scholar] [CrossRef]
- Pu, W.; Bai, R.; Zhou, K.; Peng, Y.; Zhang, M.; Hottiger, M.O.; Li, W.; Gao, X.; Sun, L. Baicalein attenuates pancreatic inflammatory injury through regulating MAPK, STAT 3 and NF-κB activation. Int. Immunopharmacol. 2019, 72, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Ishfaq, M.; Liu, Y.; Wu, Z.; Wang, J.; Li, R.; Qian, F.; Ding, L.; Li, J. Baicalin Attenuates endometritis in a rabbit model induced by infection with Escherichia coli and Staphylococcus aureus via NF-κB and JNK signaling pathways. Domest. Anim. Endocrinol. 2021, 74, 106508. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Liang, K.; An, R.; Wang, X. Baicalin protects against ethanol-induced chronic gastritis in rats by inhibiting Akt/NF-κB pathway. Life. Sci. 2019, 239, 117064. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Gao, Y.; Chen, S.; Sun, J.; Wu, J.; Meng, X. Therapeutic potential of Scutellaria baicalensis Georgi in lung cancer therapy. Phytomedicine 2022, 95, 153727. [Google Scholar] [CrossRef]
- Ahmadi, A.; Mortazavi, Z.; Mehri, S.; Hosseinzadeh, H. Protective and therapeutic effects of scutellaria baicalensis and its main active ingredients baicalin and baicalein against natural toxicities and physical hazards: A review of mechanism. DARU. J. Pharm. Sci. 2022, 30, 351–366. [Google Scholar] [CrossRef]
- Yin, H.; Huang, L.; Ouyang, T.; Chen, L. Baicalein improves liver inflammation in diabetic Db/Db mice by regulating HMGB1/TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2018, 55, 55–62. [Google Scholar] [CrossRef]
- Li, W.; Ma, F.; Zhang, L.; Huang, Y.; Li, X.; Zhang, A.; Hou, C.; Zhu, Y.; Zhu, Y. S-propargyl-cysteine exerts a novel protective effect on methionine and choline deficient diet-induced fatty liver via Akt/Nrf2/HO-1 pathway. Oxid. Med. Cell. Longev. 2016, 2016, 4690857. [Google Scholar] [CrossRef]
- Zhang, G.; Li, C.; Niu, Y.; Yu, Q.; Chen, Y.; Liu, E. Osteoprotective effect of radix scutellariae in female hindlimb-suspended sprague-dawley rats and the osteogenic differentiation effect of its major constituent. Molecules 2017, 22, 1044. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Ormsbee, L.T.; Elam, M.L.; Campbell, S.C.; Rahnama, N.; Payton, M.E.; Brummel-Smith, K.; Daggy, B.P. A combination of Scutellaria baicalensis and Acacia catechu extracts for short-term symptomatic relief of joint discomfort associated with osteoarthritis of the knee. J. Med. Food 2014, 17, 707–713. [Google Scholar] [CrossRef]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. A wogonin-rich-fraction of Scutellaria baicalensis root extract exerts chondroprotective effects by suppressing IL-1β-induced activation of AP-1 in human OA chondrocytes. Sci. Rep. 2017, 7, 43789. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, J.; Mori, T.; Smith-Powell, L.; Synold, T.W.; Chen, S.; Wen, W. Baicalin increases VEGF expression and angiogenesis by activating the ERRα/PGC-1α pathway. Cardiovasc. Res. 2011, 89, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Xiao, Y.; Ma, S.; Liu, Q.; Dang, S.; Jin, M.; Shi, Y.; Wan, B.; Zhang, Y. Inhibition of 12/15-lipoxygenase by baicalein induces microglia PPARβ/δ: A potential therapeutic role for CNS autoimmune disease. Cell. Death. Dis. 2013, 4, e569. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhong, P.; Zhu, X.; Zhou, R.; Gao, M.; Lan, Q.; Chen, J.; Chen, Y.; Zhao, W. The anti-rotavirus effect of baicalin via the gluconeogenesis-related p-JNK–PDK1–AKT–SIK2 signaling pathway. Eur. J. Pharmacol. 2021, 897, 173927. [Google Scholar] [CrossRef] [PubMed]
- Long, L.D.; Tung, N.H.; Thom, N.T.; Choi, G.J.; Hoang, V.D.; Dang, L.Q. Constituents and inhibitory effect on human pathogenic bacteria of the roots of Scutellaria baicalensis. Vietnam. J. Sci. Technol. 2019, 57, 7–14. [Google Scholar] [CrossRef][Green Version]
- Duan, C.; Matsumura, S.; Kariya, N.; Nishimura, M.; Shimono, T. In vitro antibacterial activities of Scutellaria baicalensis Georgi against cariogenic bacterial. Pediatr. Dent. J. 2007, 17, 58–64. [Google Scholar] [CrossRef]
- Jang, J.-S.; Kim, J.-H.; Kwon, M.-J. Antibacterial activity of Scutellaria baicalensis extract against antibiotic resistant bacteria. Korean J. Food Nutr. 2011, 24, 708–712. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, D.; Wang, N.; Li, S.; Tan, H.-Y.; Feng, Y. Polyphenols of chinese skullcap roots: From chemical profiles to anticancer effects. RSC Adv. 2019, 9, 25518–25532. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Liu, Y.; Wang, C.; Wang, J.; Long, C.; Guo, W.; Sun, X. Baicalein inhibits growth of epstein-barr virus-positive nasopharyngeal carcinoma by repressing the activity of EBNA1 Q-promoter. Biomed. Pharmacother. 2018, 102, 1003–1014. [Google Scholar] [CrossRef]
- Yu, X.; Yang, Y.; Li, Y.; Cao, Y.; Tang, L.; Chen, F.; Xia, J. Baicalein inhibits cervical cancer progression via downregulating long noncoding RNA BDLNR and its downstream PI3 K/Akt pathway. Int. J. Biochem. Cell Biol. 2018, 94, 107–118. [Google Scholar] [CrossRef]
- Bie, B.; Sun, J.; Guo, Y.; Li, J.; Jiang, W.; Yang, J.; Huang, C.; Li, Z. Baicalein: A review of its anti-cancer effects and mechanisms in hepatocellular carcinoma. Biomed. Pharmacother. 2017, 93, 1285–1291. [Google Scholar] [CrossRef]
- Cai, J.; Hu, Q.; He, Z.; Chen, X.; Wang, J.; Yin, X.; Ma, X.; Zeng, J. Scutellaria baicalensis Georgi and their natural flavonoid compounds in the treatment of ovarian cancer: A review. Molecules 2023, 28, 5082. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Yang, F.; Liu, J.; Jiao, Q.; Yu, H.; Nie, X.; Li, H.; Wang, X.; Xue, F. Therapeutic drug combinations against COVID-19 obtained by employing a collaborative filtering method. Heliyon 2023, 9, e14023. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Zhang, F.; Huang, C.; Yang, H.; Chen, W.; Tao, X. The effect of Scutellaria baicalensis and its active ingredients on major depressive disorder: A systematic review and meta-analysis of literature in pre-clinical research. Front. Pharmacol. 2024, 15, 1313871. [Google Scholar] [CrossRef] [PubMed]
- EghbaliFeriz, S.; Taleghani, A.; Tayarani-Najaran, Z. Central nervous system diseases and Scutellaria: A review of current mechanism studies. Biomed. Pharmacother. 2018, 102, 185–195. [Google Scholar] [CrossRef]
- Song, J.; Li, M.; Kang, N.; Jin, W.; Xiao, Y.; Li, Z.; Qi, Q.; Zhang, J.; Duan, Y.; Feng, X.; et al. Baicalein ameliorates cognitive impairment of vascular dementia rats via suppressing neuroinflammation and regulating intestinal microbiota. Brain Res. Bull. 2024, 208, 110888. [Google Scholar] [CrossRef] [PubMed]
- Yimam, M.; Burnett, B.P.; Brownell, L.; Jia, Q. Clinical and preclinical cognitive function improvement after oral treatment of a botanical composition composed of extracts from Scutellaria baicalensis and Acacia catechu. Behav. Neurol. 2016, 2016, 7240802. [Google Scholar] [CrossRef]
- Baba, M.; Subramanian, G.; Chand, J.; Wahedi, U.; Potlapati, V.; Jayanthi, K.; Azeemuddin, M.; Emran, T.; Nainu, F. Investigation of Scutellaria baicalensis for potential neuroprotective effect on the treatment of parkinson’s disease. Biointerface Res. Appl. Chem. 2024, 14, 1–15. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, C. Network pharmacology and molecular docking identify the potential mechanism and therapeutic role of Scutellaria baicalensis in alzheimer’s disease. Drug Des. Devel. Ther. 2024, 18, 1199–1219. [Google Scholar] [CrossRef]
- Ni, H.; Wu, Z.; Muhammad, I.; Lu, Z.; Li, J. Optimization of baicalin water extraction process from Scutellaria baicalensis (a traditional Chinese medicine) by using orthogonal test and HPLC. Rev. Bras. De Farmacogn. 2018, 28, 151–155. [Google Scholar] [CrossRef]
- Choi, W.; Kwon, H.-S.; Lee, H.Y. Enhancement of anti-skin inflammatory activities of scutellaria baicalensis using an alkaline reduced water extraction process. Food Sci. Biotechnol. 2014, 23, 1859–1866. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, R.-Y.; Park, E. Antioxidant and glucosidase inhibitory activities of different solvent extracts of skullcap (Scutellaria baicalensis). Food Sci. Biotechnol. 2011, 20, 1107–1112. [Google Scholar] [CrossRef]
- Oracz, J.; Kowalski, S.; Żyżelewicz, D.; Kowalska, G.; Gumul, D.; Kulbat-Warycha, K.; Rosicka-Kaczmarek, J.; Brzozowska, A.; Grzegorczyk, A.; Areczuk, A. The influence of microwave-assisted extraction on the phenolic compound profile and biological activities of extracts from selected Scutellaria species. Molecules 2023, 28, 3877. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Joerger, R.; Wu, C. Study of the chemical composition and antimicrobial activities of ethanolic extracts from roots of Scutellaria baicalensis Georgi. J. Agric. Food Chem. 2011, 59, 10934–11094. [Google Scholar] [CrossRef] [PubMed]
- Oomen, W.W.; Begines, P.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvent extraction of flavonoids of Scutellaria baicalensis as a replacement for conventional organic solvents. Molecules 2020, 25, 617. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-C.; Tsai, M.-J.; Wen, K.-C. Supercritical fluid extraction of flavonoids from scutellariae radix. J. Chromatogr. A 1999, 830, 387–395. [Google Scholar] [CrossRef]
- Olech, M.; Kasprzak, K.; Wójtowicz, A.; Oniszczuk, T.; Nowak, R.; Waksmundzka-Hajnos, M.; Combrzyński, M.; Gancarz, M.; Kowalska, I.; Krajewska, A.; et al. Polyphenol composition and antioxidant potential of instant gruels enriched with Lycium barbarum L. fruit. Molecules 2020, 25, 4538. [Google Scholar] [CrossRef] [PubMed]
- Ouamnina, A.; Alahyane, A.; Elateri, I.; Boutasknit, A.; Abderrazik, M. Relationship between phenolic compounds and antioxidant activity of some moroccan date palm fruit varieties (Phoenix dactylifera L.): A two-year study. Plant 2024, 13, 1119. [Google Scholar] [CrossRef]
- Gokhale, M.; Khanna, A.; Gautam, D. Ameliorative effect of Oroxylum Indicum [L.] Vent. metabolites on tobacco extract induced cell damage in human lymphocytes. Int. J. Biol. Pharm. Res. 2015, 6, 860–866. [Google Scholar]
- Gao, B.; Zhu, H.; Liu, Z.; He, X.; Sun, J.; Li, Y.; Wu, X.; Pehrsson, P.; Zhang, Y.; Yu, L. Chemical compositions of Scutellaria Baicalensis Georgi. (huangqin) extracts and their effects on ACE2 binding of SARS-CoV-2 spike protein, ACE2 activity, and free radicals. Int. J. Mol. Sci. 2024, 25, 2045. [Google Scholar] [CrossRef]
- Aliev, G.; Kaminsky, Y.G.; Bragin, V.; Kosenko, E.A.; Klochkov, S.G.; Bachurin, S.O.; Benberin, V.V. Flavones from the root of Scutellaria Baicalensis Georgi–drugs of the future in neurodegeneration and neuroprotection? In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 2305–2323. ISBN 978-3-642-30018-9. [Google Scholar]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From preclinical evidence to potential clinical applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar] [CrossRef]
- Lv, H.; Chen, S.; Xu, X.; Zhu, M.; Zhao, W.; Liu, K.; Liu, K. Isolation of linoleic acid from sambucus williamsii seed oil extracted by high pressure fluid and its antioxidant, antiglycemic, hypolipidemic activities. Int. J. Food Eng. 2015, 11, 383–391. [Google Scholar] [CrossRef]
- Fedorova, I.; Hashimoto, A.; Fecik, R.A.; Hedrick, M.P.; Hanus, L.O.; Boger, D.L.; Rice, K.C.; Basile, A.S. Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems. J. Pharmacol. Exp. Ther. 2001, 299, 332–342. [Google Scholar]
- Tamanna, N.; Mahmood, N. Food processing and maillard reaction products: Effect on human health and nutrition. Int. J. Food Sci. 2015, 2015, 526762. [Google Scholar] [CrossRef] [PubMed]
- Bakhiya, N.; Monien, B.; Frank, H.; Seidel, A.; Glatt, H. Renal organic anion transporters OAT1 and OAT3 mediate the cellular accumulation of 5-sulfooxymethylfurfural, a reactive, nephrotoxic metabolite of the maillard product 5-hydroxymethylfurfural. Biochem. Pharmacol. 2009, 78, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X. In vitro antioxidant activity of 5-HMF isolated from marine red alga laurencia undulata in free-radical-mediated oxidative systems. J. Microbiol. Biotechnol. 2009, 19, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 1–47. [Google Scholar] [CrossRef]
| Extraction Technique | Extraction Time [h] | RSA [%] (Mean ± SD *) |
|---|---|---|
| Maceration (M) | 0.5 | 15.46 ± 0.50 |
| 1 | 18.40 ± 0.43 | |
| 2 | 24.04 ± 0.52 | |
| Maceration with shaking (MSH) | 0.5 | 43.42 ± 0.47 |
| 1 | 45.65 ± 0.27 | |
| 2 | 48.85 ± 0.46 | |
| Ultrasound-assisted extraction (UAE) | 0.5 | 52.41 ± 0.53 |
| 1 | 70.19 ± 0.80 | |
| 2 | 87.46 ± 0.22 | |
| Reflux extraction (RE) | 0.5 | 91.88 ± 0.50 |
| 1 | 94.16 ± 0.25 | |
| 2 | 94.30 ± 0.13 | |
| Soxhlet extraction (SE) | 0.5 | 92.82 ± 0.43 |
| 1 | 95.59 ± 0.18 | |
| 2 | 95.22 ± 0.17 |
| Variables (n = 45) | Mean | Median | Min. | Max. | Lower Quartile | Upper Quartile | Standard Deviation |
| RSA [%] | 64.656 | 70.500 | 14.936 | 95.763 | 43.921 | 93.978 | 29.942 |
| TEAC [µM TE/g] | 7.898 | 8.637 | 1.610 | 11.832 | 5.275 | 11.606 | 3.786 |
| TPC [mg GAE/g] | 11.535 | 12.260 | 1.414 | 24.630 | 5.191 | 17.937 | 7.702 |
| Extraction Technique | TEAC vs. Time | TPC vs. Time | TPC vs. TEAC |
|---|---|---|---|
| Maceration (M) | 0.9937 * | 0. 9715 * | 0.9869 * |
| Maceration with shaking (MSH) | 0.9849 * | 0.9579 * | 0.9641 * |
| Ultrasound-assisted extraction (UAE) | 0.9798 * | 0.8597 * | 0.9427 * |
| Reflux extraction (RE) | 0.7651 * | 0.9663 * | 0.8515 * |
| Soxhlet extraction (SE) | 0.6570 | 0.8871 * | 0.9166 * |
| Extraction Conditions | Yield [%] | C [mg/mL] | RSA [%] | RSA(y) vs. C(x) Equation | R2 | IC50 [mg/mL] |
|---|---|---|---|---|---|---|
| RE 2 h | 17.0 | 0.1 0.2 0.3 0.4 0.5 | 12.21 25.35 38.58 52.10 63.28 | y = 128.89x − 0.363 | 0.9990 | 0.39 |
| SE 2 h | 21.7 | 0.1 0.2 0.3 0.4 0.5 | 14.19 25.35 38.58 52.10 63.28 | y = 127.95x + 2.393 | 0.9982 | 0.37 |
| No. | Compound | RT [min] | RI | Identification Methods | MS Signals *, m/z | Presence in Extract ** | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [M●]+ | Characteristic Fragment Ions | M | MSH | UAE | RE | SE | |||||
| 1 | 5-Hydroxy-methylfurfural | 7.72 | 1251 | MS-NIST | 126 | 97, 41, 126, 69, 29 | nd | nd | tr | + | ++ |
| 2 | unidentified | 14.05 | 1524 | - | 164(?) | 31,29,57, 43, 73 | tr | + | + | + | tr |
| 3 | Palmitic acid | 23.36 | 1964 | RI, MS-NIST | 256 | 43,73, 60, 41, 57 | nd | nd | nd | tr | tr |
| 4 | Linoleic acid | 26.49 | 2133 | RI, MS-NIST | 280 | 67,81, 82, 95, 55 | nd | nd | nd | tr | + |
| 5 | (Z)-9-Octadecen-amide | 30.35 | 2360 | MS-NIST | 281 | 59,72,55, 41, 43 | nd | tr | + | + | + |
| 6 | unidentified | 31.90 | 2456 | - | 286(?) | 167, 69, 182, 78, 103 | nd | nd | tr | tr | + |
| 7 | Oroxylin A | 34.83 | 2649 | MS-LIT | 284 | 241, 69, 269, 266, 139 | tr | + | ++ | +++ | +++ |
| 8 | Wogonin | 35.80 | 2716 | MS-NIST | 284 | 269, 139, 241, 69, 167 | tr | ++ | +++ | +++ | +++ |
| 9 | Dihydroxydimethoxyflavone | 37.78 | 2858 | MS-analysis | 314 | 299, 271, 169, 69, 102 | nd | tr | + | + | ++ |
| 10 | Dihydroxytetramethoxyflavone | 41.44 | 3139 | MS-analysis | 374 | 359,211,183,69, 127 | tr | ++ | ++ | ++ | ++ |
| 11 | β-Sitosterol | 43.47 | 3306 | RI, MS-NIST | 414 | 43,57,55,41,145 | tr | tr | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzięcioł, M.; Wala, K.; Wróblewska, A.; Janda-Milczarek, K. The Effect of the Extraction Conditions on the Antioxidant Activity and Bioactive Compounds Content in Ethanolic Extracts of Scutellaria baicalensis Root. Molecules 2024, 29, 4153. https://doi.org/10.3390/molecules29174153
Dzięcioł M, Wala K, Wróblewska A, Janda-Milczarek K. The Effect of the Extraction Conditions on the Antioxidant Activity and Bioactive Compounds Content in Ethanolic Extracts of Scutellaria baicalensis Root. Molecules. 2024; 29(17):4153. https://doi.org/10.3390/molecules29174153
Chicago/Turabian StyleDzięcioł, Małgorzata, Klaudia Wala, Agnieszka Wróblewska, and Katarzyna Janda-Milczarek. 2024. "The Effect of the Extraction Conditions on the Antioxidant Activity and Bioactive Compounds Content in Ethanolic Extracts of Scutellaria baicalensis Root" Molecules 29, no. 17: 4153. https://doi.org/10.3390/molecules29174153
APA StyleDzięcioł, M., Wala, K., Wróblewska, A., & Janda-Milczarek, K. (2024). The Effect of the Extraction Conditions on the Antioxidant Activity and Bioactive Compounds Content in Ethanolic Extracts of Scutellaria baicalensis Root. Molecules, 29(17), 4153. https://doi.org/10.3390/molecules29174153






