Abstract
Fungal infections are among the most common diseases of crop plants. Various species of the Fusarium spp. are naturally prevalent and globally cause the qualitative and quantitative losses of farming commodities, mainly cereals, fruits, and vegetables. In addition, Fusarium spp. can synthesize toxic secondary metabolites—mycotoxins under high temperature and humidity conditions. Among the strategies against Fusarium spp. incidence and mycotoxins biosynthesis, the application of biological control, specifically natural plant extracts, has proved to be one of the solutions as an alternative to chemical treatments. Notably, rowanberries taken from Sorbus aucuparia are a rich source of phytochemicals, such as vitamins, carotenoids, flavonoids, and phenolic acids, as well as minerals, including iron, potassium, and magnesium, making them promising candidates for biological control strategies. The study aimed to investigate the effect of rowanberry extracts obtained by supercritical fluid extraction (SFE) under different conditions on the growth of Fusarium (F. culmorum and F. proliferatum) and mycotoxin biosynthesis. The results showed that various extracts had different effects on Fusarium growth as well as ergosterol content and mycotoxin biosynthesis. These findings suggest that rowanberry extracts obtained by the SFE method could be a natural alternative to synthetic fungicides for eradicating Fusarium pathogens in crops, particularly cereal grains. However, more research is necessary to evaluate their efficacy against other Fusarium species and in vivo applications.
1. Introduction
Sorbus aucuparia is a deciduous tree species native to most of Europe and parts of Asia and Africa, commonly cultivated as an ornamental plant, which has a few climate requirements, as it can occur in both high mountains with low temperatures and the hot south. It is a species whose fruits are edible but neglected because of their natural bitter taste and the necessity of processing them before consumption to make them palatable. In some regions, particularly in Eastern Europe and Scandinavia, it is used as an ingredient in food products such as jams or syrups [1]. Rowan is rich in organic acids, including ascorbic acid, and phenolic compounds, which have shown a wide range of biological properties [2,3]. Substances extracted from rowan fruits have proven to exhibit diuretic, anti-inflammatory, vasoconstrictor, and anti-diabetic properties [4]. The rowan tree is an important biocenotic species that provides food for many birds, e.g., song thrush (Turdus philomelos), Eurasian bullfinch (Pyrrhula pyrrhula), and mistle thrush (Turdus viscivorus) [5]. It is also a food ingredient for mammals, such as the European pine marten (Martes martes) [6], roe deer (Capreolus capreolus) [7], and red foxes (Vulpes vulpes) [8], establishing the importance of mammals as dispersers of rowan seeds [8]. Due to the constant rowanberry seed multiplication, high variability is observed for most morphological and biochemical traits of wild fruits. Therefore, it is important to learn about the characteristics of specific individuals and to introduce breeding for valuable fruits [9].
Supercritical fluid extraction is an advanced extraction method utilizing supercritical fluids, typically carbon dioxide (CO2), as a solvent. During the SFE process, the supercritical CO2 is passed through the material containing the desired compounds. The solvating properties of supercritical CO2 can be tuned by adjusting pressure and temperature, allowing selective extraction of specific compounds, which enables precise targeting of desired substances while leaving unwanted components behind. Compared to traditional solvent extraction, SFE operates at lower temperatures, preserving sensitive compounds, and uses non-toxic CO2, making it environmentally friendly and leaving no solvent residues [10]. Using SFE for Sorbus aucuparia fruits holds promise for obtaining bioactive compounds, allowing for selective extraction of beneficial components while maintaining the fruit’s nutritional integrity. The resulting extract may be a rich source of bioactive compounds for food, pharmaceutical, and biotechnological industries. Moreover, due to the variety of natural compounds, rowan fruit extracts prepared by the SFE method may have antimicrobial properties, which can be used to inhibit the growth of mycopathogens.
Up to now, a few articles have been published on the antimicrobial properties of Sorbus aucuparia fruit extracts. The antimicrobial effect is mainly attributed to the high content of sorbic acid and biphenyl phytoalexins, particularly aucuparin in rowanberries [11,12,13]. Most of the previous research concerns the effect of extracts obtained from Sorbus aucuparia fruits on bacteria growth [14,15]. In the research of Liepiņa et al. (2013), the highest inhibition on Bacillus cereus had ethanolic and aqueous extracts obtained from fresh rowanberries, which reached a percentage of inhibition equal to 10% and 9.7%, respectively [16]. A very strong antibacterial effect of acetone-derived phenolic-rich fractions of rowanberry extract against B. cereus was found [17], as well as for methanolic and aqueous extracts, which achieved an inhibition percentage above 75% [18]. Antibacterial properties of Sorbus aucuparia fruit extracts have also been observed for Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Salmonella enterica, Bacillus subtilis, Serratia marcescens, and Campylobacter jejuni [16,17,18]. Regarding the antifungal activity of Sorbus extracts, only a few studies have been conducted, mainly for Candida albicans, and their results are inconclusive [16,17,19,20,21,22,23].
Fungal infections stand as some of the most destructive agricultural diseases worldwide. Within the Fusarium spp., F. culmorum and F. proliferatum are widespread, especially in Europe, and cause substantial losses in cereals, cereal-based items, fruits, and vegetables [24,25,26]. Crop yield reduction caused by fusariosis reached from 10 to 40% [27]. These Fusarium species synthesize mycotoxins under favorable conditions, which are high temperature, humidity, and moisture, occurring pre-harvest, post-harvest, during processing, or even in storage [28]. Additionally, most mycotoxins produced by Fusarium spp. exhibit heat stability and raise serious health concerns in humans and farm animals, leading to mutagenic, teratogenic, neurotoxic, and carcinogenic effects [29]. Fusarium spp. are associated with diseases in people with localized or invasive infections who have a history of immunosuppression [30]. These infections are difficult to treat mainly due to drug-resistant isolates [31]. Due to their characteristics, Fusarium fungi become resistant to fungicide exposure very quickly [32]. The crucial thing, therefore, is the implementation of appropriate methods or fungicides [33].
This work aims to investigate the antifungal properties of Sorbus aucuparia fruit extracts obtained by supercritical fluid extraction under various temperature and pressure conditions. The antifungal activity of rowanberry fruit extracts was assessed against two Fusarium strains: F. proliferatum and F. culmorum. The effect of the extract treatments on mycelial growth and mycotoxin biosynthesis was assessed by qualitative and quantitative chromatographic analysis of ergosterol and secondary fungal metabolites.
2. Results
2.1. Preparation of the Sorbus aucuparia Fruit Extracts by Supercritical Fluid Extraction Method
Rowanberry extracts were obtained by the SFE method according to the further described procedure in four variants differing in extraction parameters such as temperature and pressure (Table 1).

Table 1.
List of the prepared extract variants (E1–E4), including extraction conditions and extraction yield.
The extraction yield ranged from 16.93 to 18.05%. The most efficient extraction conditions were demonstrated at 70 °C and 300 bar (variant E3). It has also been shown that higher pressure in the extraction process had a significant impact on the increase in extraction yield (extracts E3 or E1, p < 0.05), while lower pressure, regardless of the temperature value, resulted in a similar extraction yield.
2.2. Total Phenolic Content of the Methanolic and Aqueous Extracts
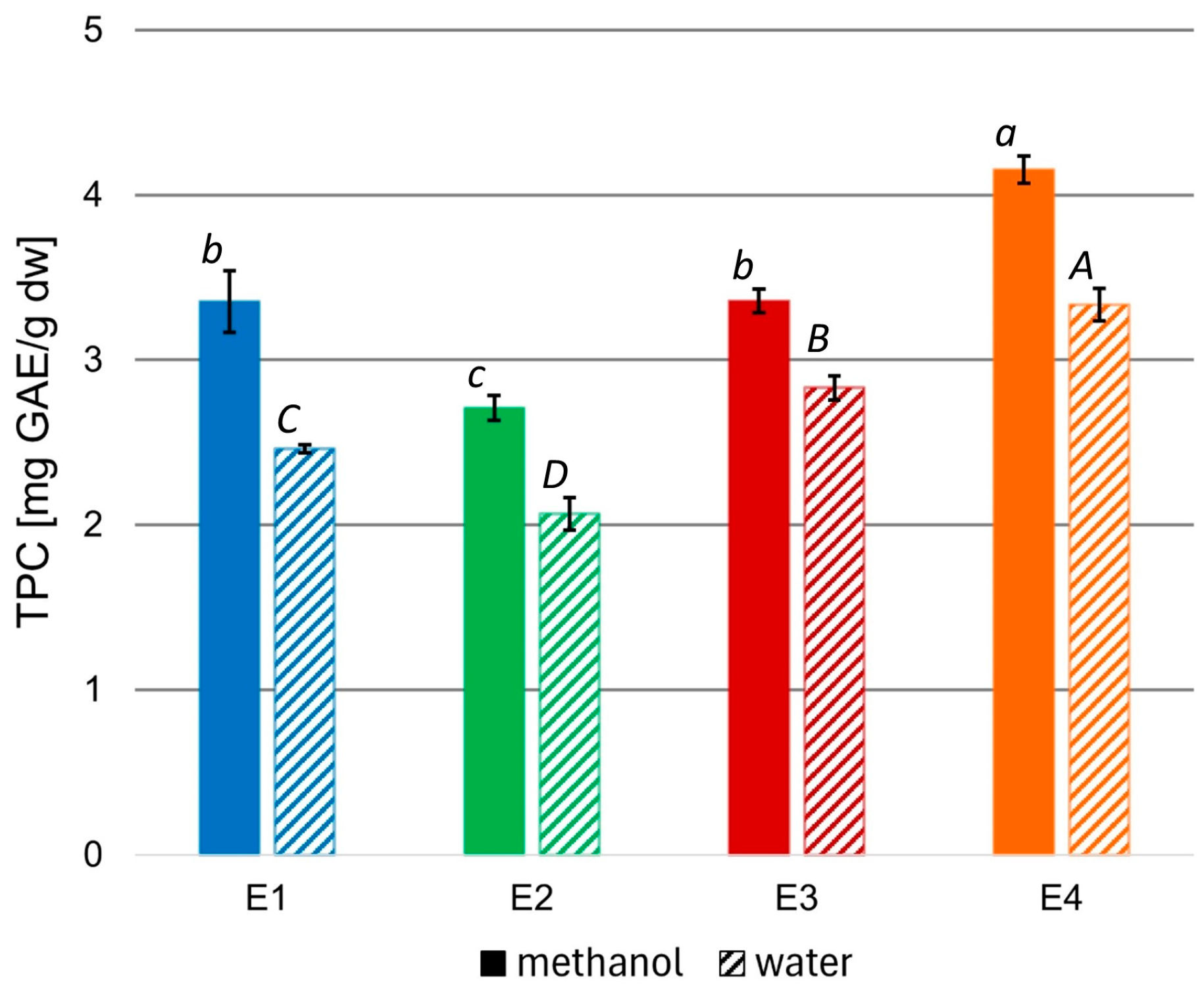
To evaluate the bioactivity of the prepared extracts, the total phenolic content (TPC) of the methanolic variants, as well as the aqueous variants obtained from the methanolic ones, were measured, and the results were expressed as mg of gallic acid (GAE) per 1 g of extract dw (Figure 1).
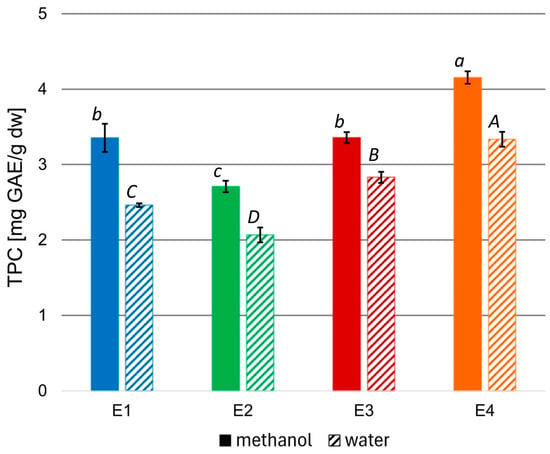
Figure 1.
TPC in methanolic and aqueous rowanberry extract variants E1–E4. All values are means of three replicates; error bars represent standard deviation. The values assigned with the superscripts of different letters are significantly different (Tukey’s HSD test, significant at p < 0.05).
The TPC of the rowanberry methanolic extracts ranged from 2.71 to 4.15 mg GAE/g dw. However, aqueous variants had significantly lower TPC than corresponding methanolic ones (p < 0.05), reaching values from 2.07 to 3.33 mg GAE/g dw. The highest TPC values characterized the extract variant E4, regardless of the solvent, while the lowest—extract variant E2.
2.3. The Inhibitory Effect of Sorbus aucuparia Fruit Extracts on Fusarium spp. Growth
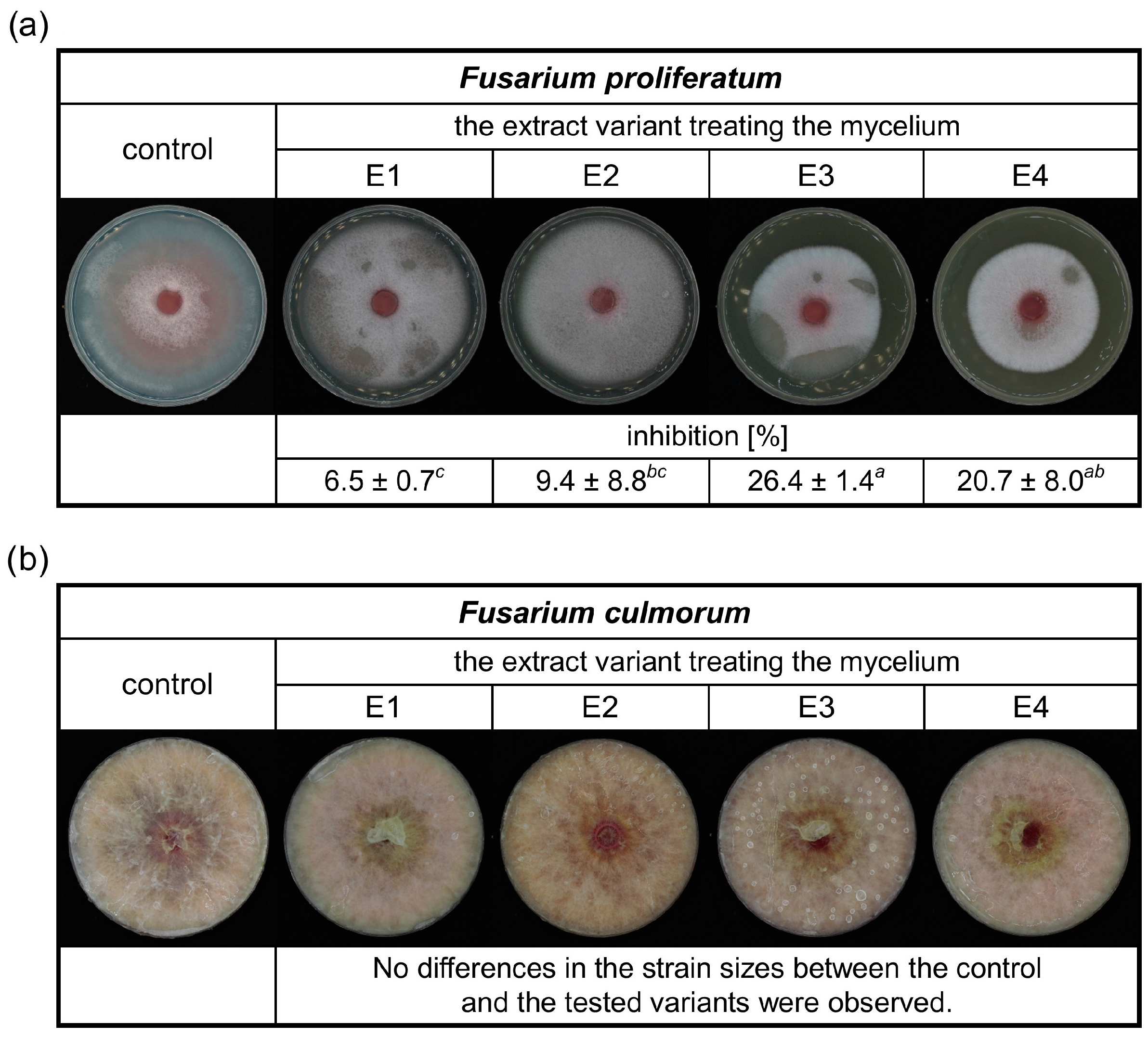
This study focused on the antifungal activity of rowanberry extracts obtained by the SFE against Fusarium spp. Therefore, the effect of the obtained aqueous extract variants (E1–E4) on the growth of F. proliferatum and F. culmorum was evaluated, and the results are presented in Figure 2.
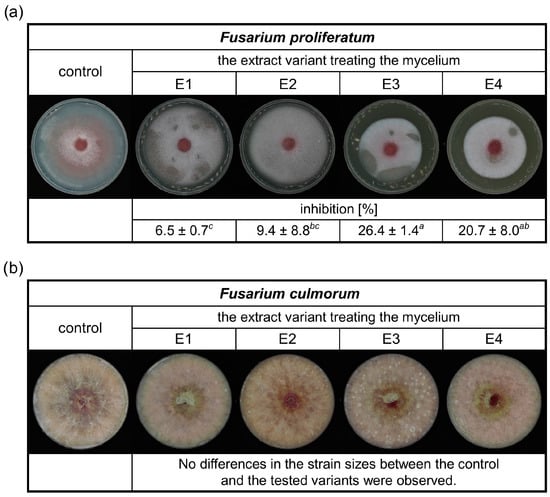
Figure 2.
The inhibitory effects of different rowanberry extracts (E1–E4) on F. proliferatum (a) and F. culmorum (b) mycelia growth in the medium. All values are means of three replicates ± standard deviation. The values assigned with the superscripts of different letters are significantly different (Tukey’s HSD test, significant at p < 0.05).
It was observed that the prepared extracts had an inhibitory effect on the growth of F. proliferatum (Figure 2a). The lowest inhibition of the extracts was 6.5%. The most significant antifungal activity against F. proliferatum was exerted by the extract variant E3, with a reduction in mycelial growth of 26.4%. In the case of the inhibition study of rowanberry extracts on the F. culmorum growth, no differences between the control and the tested variants were observed (Figure 2b).
2.4. The Effect of Sorbus aucuparia Fruit Extracts on Ergosterol Content
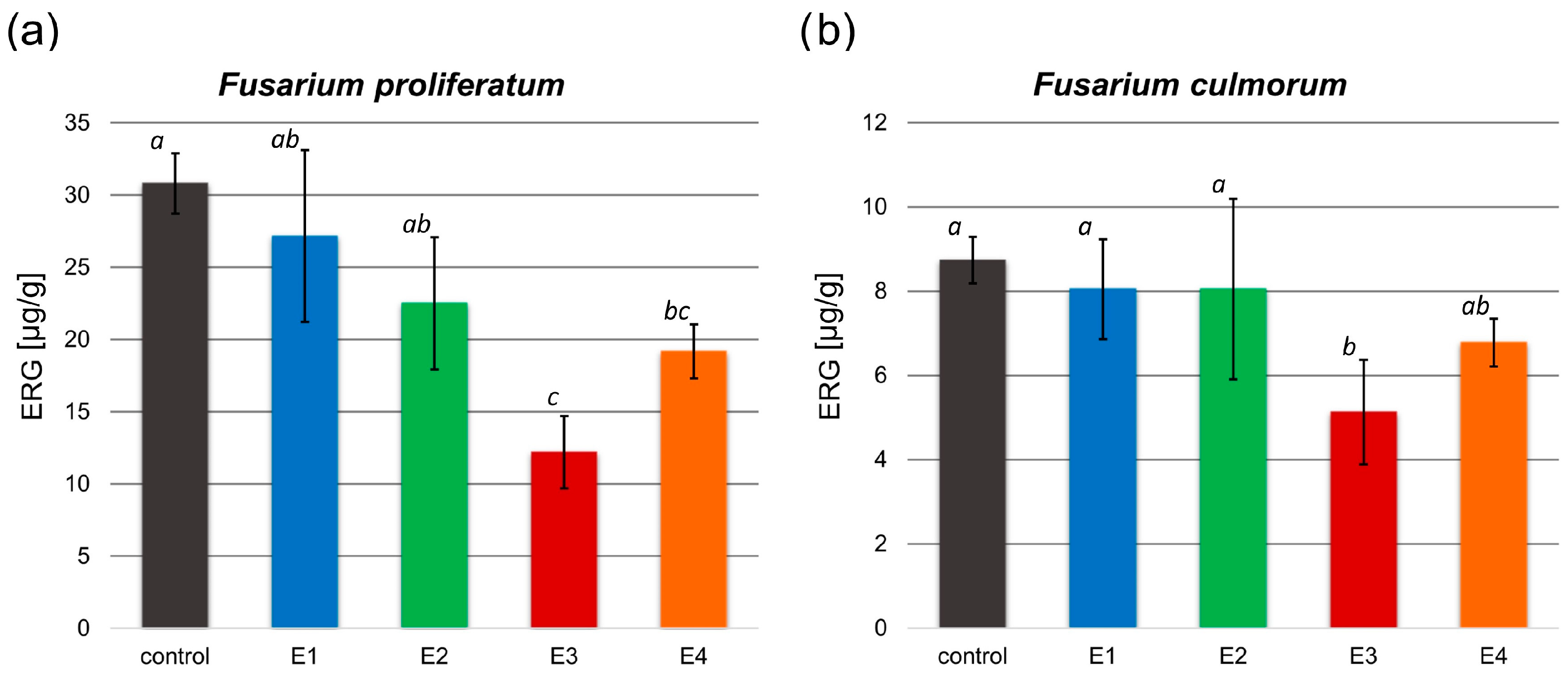
The antifungal activity of the obtained extracts on the F. proliferatum and F. culmorum growth was investigated based on the analysis of the ergosterol (ERG) content in the control and tested samples (Figure 3).
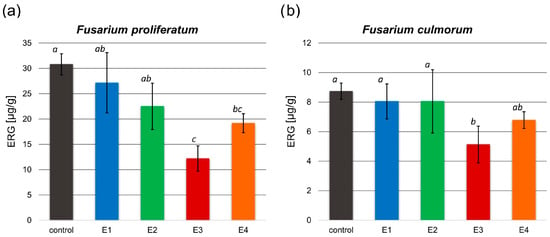
Figure 3.
Ergosterol (ERG) content [µg/g] in the control and tested samples treated with the obtained extracts (E1–E4) after inoculation with F. proliferatum (a) and F. culmorum (b). All values are means of three replicates; error bars represent standard deviation. The values assigned with the superscripts of different letters are significantly different (Tukey’s HSD test, significant at p < 0.05).
In both examined Fusarium spp. treated with the rowanberry extracts, ERG content decreased compared to the control group. A significant difference in ERG concentration was observed after treating F. proliferatum with each of the obtained extract variants, but only the E3 and E4 extract variants had a significant effect on F. culmorum (p < 0.05). The ERG content of the tested samples was in the range between 12.18 and 27.15 µg/g (30.79 µg/g control) for F. proliferatum (Figure 3a) and 5.13–8.04 µg/g (8.73 µg/g control) for F. culmorum (Figure 3b). The most significant decrease in ergosterol concentration (p < 0.05) was observed in samples treated with the extract variant E3.
The ERG reduction (Table 2) in samples with the addition of Sorbus aucuparia fruit extracts ranged from 12.41 to 60.67% for F. proliferatum and from 7.70 to 41.62% for F. culmorum, depending on the extraction variants. The greatest reduction in ergosterol content compared to the control group was observed after treatment of both Fusarium spp. with extract variant E3.

Table 2.
The percentage of ergosterol (ERG) reduction for Fusarium spp. samples treated with different variants of rowanberry extracts (E1–E4).
2.5. Mycotoxin Identification
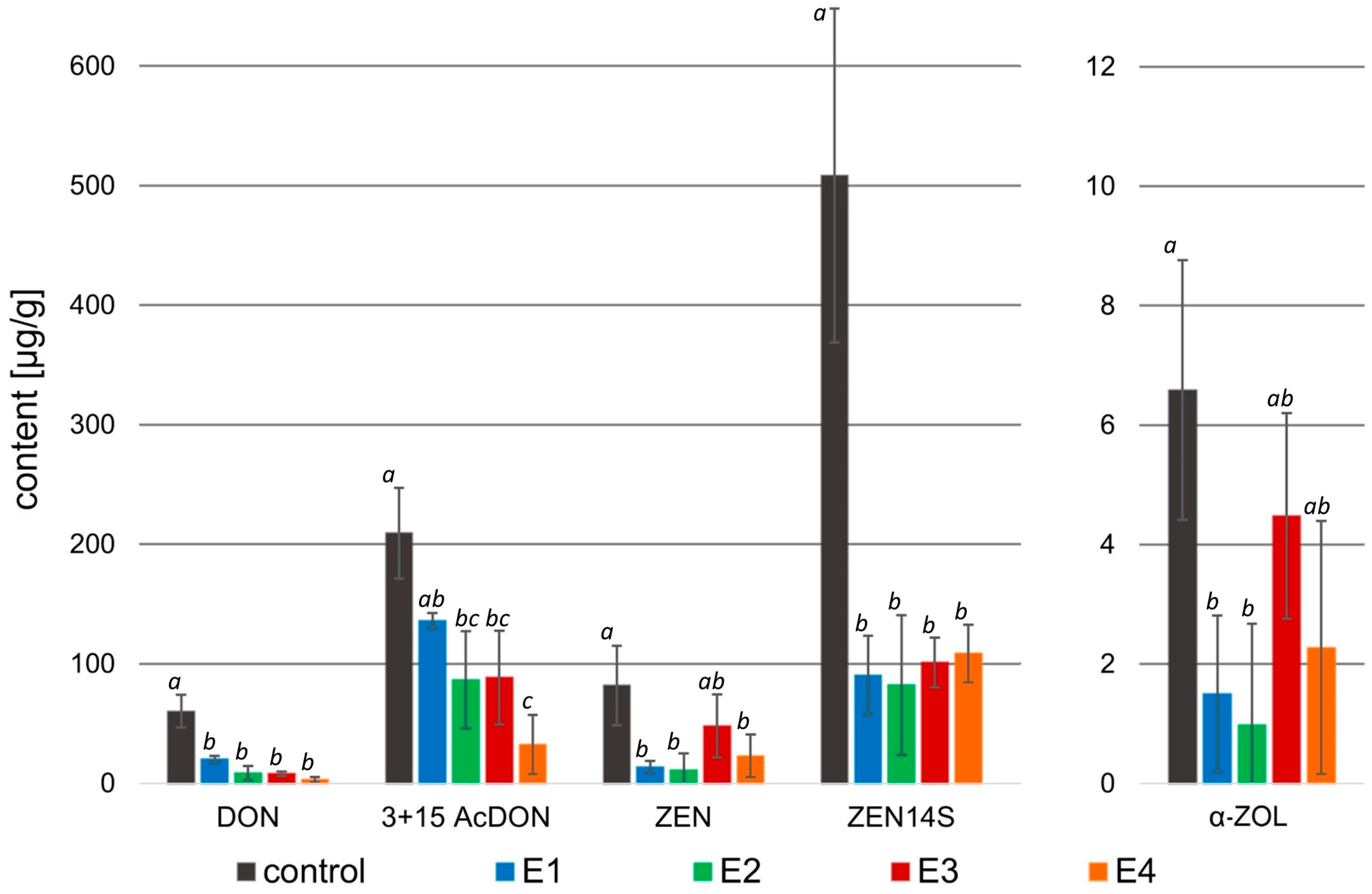
In this study, the multi-mycotoxins method was used to analyze the effect of rowanberry extracts on mycotoxin biosynthesis by Fusarium spp. For F. proliferatum, the content of fumonisins—B1 (FB1), B2 (FB2), and B3 (FB3), and beauvericin (BEA) were determined. However, in the case of F. culmorum, among the 12 analyzed mycotoxins—deoxynivalenol (DON), 3- and 15-acetyldeoxynivalenol (3- and 15-AcDON), deoxynivalenol-3-glucoside (DON-3G), nivalenol (NIV), nivalenol-3-glucoside (NIV-3G), fusarenon X (FUSX), zearalenone (ZEN), zearalenone-14-sulfate (ZEN-14S), zearalenone-14-glucoside (ZEN-14G), α-zearalenol (α-ZOL), and β-zearalenol (β-ZOL)—only six were identified and quantified: DON, 3- and 15-AcDON, ZEN, ZEN-14S, and α-ZOL (Figure 4).
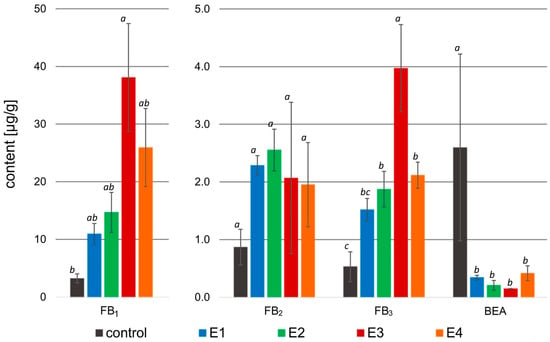
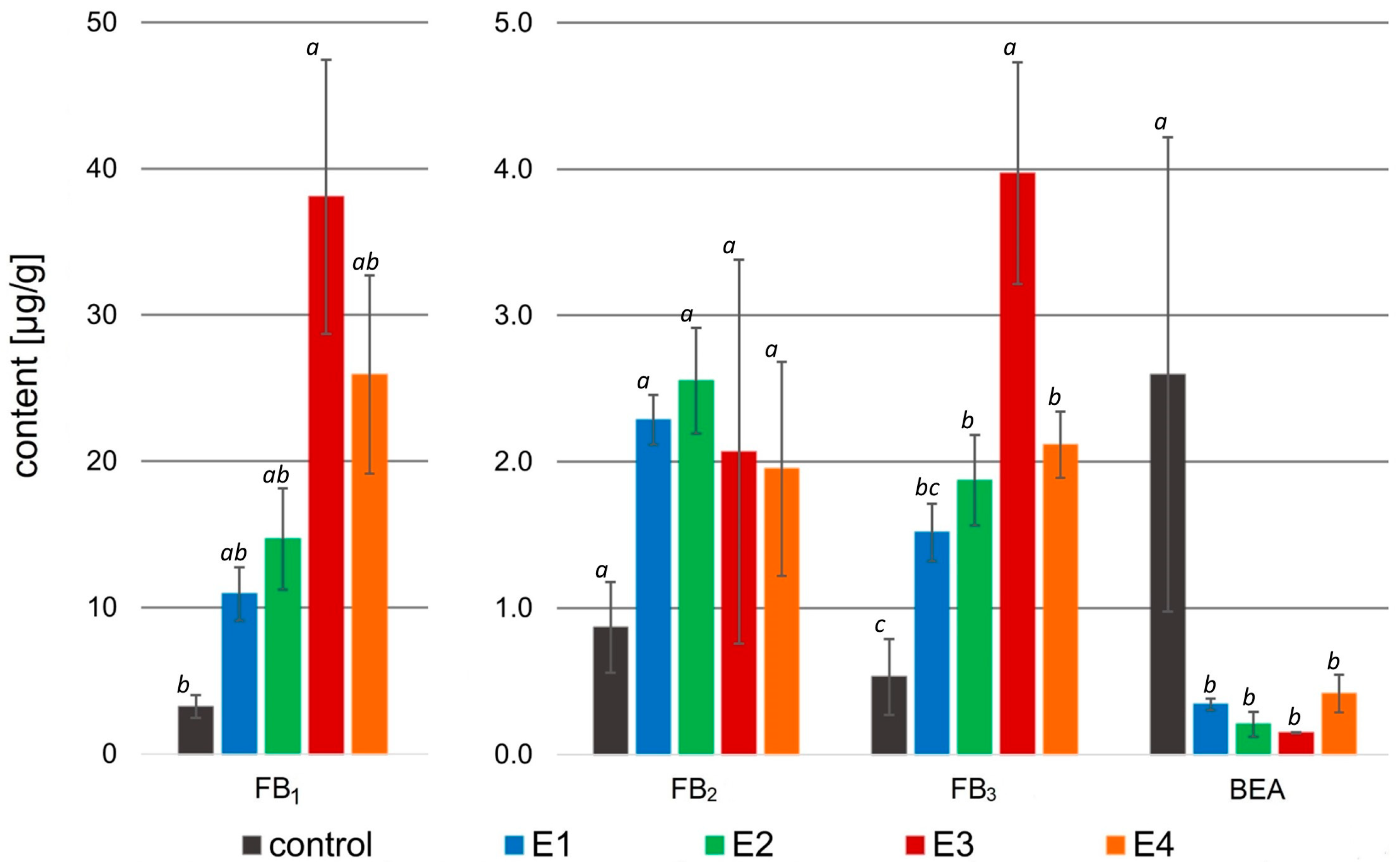
Figure 4.
Mycotoxin content [µg/g] in the samples treated with rowanberry extracts (four variants, E1–E4) after inoculation with Fusarium proliferatum. FB1—fumonisin B1, FB2—fumonisin B2, FB3—fumonisin B3, BEA—beauvericin. All values are means of three replicates; error bars represent standard deviation. The values assigned with the superscripts of different letters are significantly different (Tukey’s HSD test, significant at p < 0.05).
The application of rowanberry extracts to Fusarium proliferatum resulted in an intriguing effect on mycotoxin biosynthesis. A significant decrease in BEA content in the samples treated with the rowanberry extracts was observed, ranging from 0.15 to 0.42 to μg/g compared to the control group—2.60 μg/g (p < 0.05). However, fumonisins were biosynthesized by F. proliferatum more prominently in the samples exposed to the prepared extracts. The highest concentration of FB1 (38.08 μg/g) and FB3 (3.97 μg/g) in the prepared samples was connected with the addition of extract variant E3 (control: 3.23 μg/g, and 0.53 μg/g, respectively). However, the highest content of FB2 (2.55 μg/g) was observed after the addition of the extract variant E2 (control: 0.87 μg/g), whereas the differences in the production of this fumonisin by each extract were not statistically significant (p > 0.05). The total content of the fumonisins in the tested samples with F. proliferatum treated with the rowanberry extracts was: 14.72, 19.09, 44.11, and 29.98 µg/g for E1, E2, E3, and E4 variants, respectively (control: 4.63 µg/g). The most significant difference in the content of fumonisins was observed between the control sample and the sample treated with the E3 extract variant (p < 0.05).
In the case of the Fusarium proliferatum, the percentage of BEA reduction by prepared fruit extracts reached the highest value of 92.1% after treatment with variant E3, whereas this result is statistically similar to other extract variants (p > 0.05, Table 3). As previously mentioned, no reduction was observed in the content of fumonisins—in fact, their biosynthesis exhibited an increase compared to the control group. In the case of variant E3, a significant increase in the production of FB1 (1017.9%) and FB3 (716.1%) was observed (p < 0.05). However, the increase in FB2 production was at a similar level (123.1–208.5%), regardless of the used extract variant.

Table 3.
The percentage of mycotoxin increase or decrease in tested samples of F. proliferatum treated with different variants of rowanberry extracts (E1–E4).
Instead, the biosynthesis of all mycotoxins by F. culmorum after treatment with the rowanberry extracts was significantly lower compared to the control group (p < 0.05 for all determined mycotoxins; Figure 5).
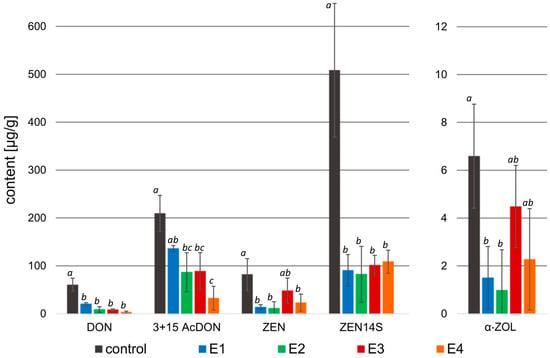
Figure 5.
Mycotoxin content [µg/g] in the samples treated with rowanberry extracts (four variants, E1–E4) after inoculation with Fusarium culmorum. DON—deoxynivalenol, 3 + 15 AcDON—3- and 15-acetyldeoxynivalenol, ZEN—zearalenone, ZEN14S—zearalenone-14-sulfate, α-ZOL—α-zearalenol. All values are means of three replicates; error bars represent standard deviation. The values assigned with the superscripts of different letters are significantly different (Tukey’s HSD test, significant at p < 0.05).
In the case of F. culmorum, the addition of all extract variants resulted in a significant decrease in the DON (3.14–20.21 μg/g), as well as 3- and 15-AcDON (32.45–136.12 μg/g) content compared to the control group (60.30 and 209.36 μg/g, respectively). The greatest decrease in the concentration of these mycotoxins was observed after the treatment of F. culmorum with the extract variant E4. However, in the case of DON, the differences in the effects of individual extracts were not significant (p > 0.05), while a significant difference was observed in the case of 3- and 15-AcDON content after treatment with E4 (p < 0.05). Moreover, all extract variants decreased in ZEN (11.10–48.01 μg/g), ZEN-14S (82.32–108.61 μg/g), and α-ZOL (0.98–4.48 μg/g) concentrations in comparison to the control (81.75, 508.53, and 6.58 μg/g, respectively). The highest decrease in these mycotoxin contents was caused by the addition of the extract variant E2 to the mycelium, whereas in the case of ZEN and α-ZOL, there were no significant differences between the activity of extract variants E1 and E2 (p > 0.05), but for the concentration of ZEN-14S, a significant difference caused by the extract variant E2 was observed (p < 0.05).
The application of rowanberry extracts on F. culmorum resulted in a reduction in all detected mycotoxins by at least 32.7%, whereas the different extract variants inhibited mycotoxin production in various grades (Table 4). A similar reduction in mycotoxins (p < 0.05) was observed regardless of the extract variant for DON: 86.6–95.1% (exception: E1: 66.1%), ZEN: 74.7–89.1% (exception E3: 43.6%), and ZEN-14S: 78.7–82.5%. The extract variant E4 reduced the synthesis of 3- and 15-AcDON by F. culmorum the most (85.5%), but the extract variant E2 reduced the synthesis of α-ZOL by 89.2% (p < 0.05).

Table 4.
The percentage of mycotoxin reduction in tested samples of F. culmorum treated with different variants of rowanberry extracts (E1–E4).
3. Discussion
Despite the considerable advancements in modern agriculture, fungal infections affecting agricultural produce, notably fusariosis, persist as a substantial threat [34]. Consequently, there is a critical need to develop innovative methodologies against pathogenic fungi. A promising approach involves utilizing biological methods to inhibit fungal development in crops, e.g., through the use of plant extracts possessing antimicrobial properties [35]. One such natural candidate is rowanberry, known for its richness in organic acids as well as phenolic and flavonoid compounds [14]. However, the imperative involves the judicious selection of an appropriate extraction method and conditions to obtain the most effective extract.
Supercritical fluid extraction is an innovative and promising method offering significant advantages in the extraction of natural compounds while aligning with the growing demand for environmentally sustainable and high-quality extraction [36]. This approach is currently widely used for the extraction of essential oils from, e.g., Lavendula genus, Pimpinella anisum, or Jasminum sambac [37,38,39]. In the case of Sorbus aucuparia, the SFE technique was used to extract pomace, seeds, or waste to obtain oil extracts [40,41,42]. The highest yield of the lipophilic fraction was received by Bobinaite et al. (2020) at 60 °C and 450 bar in 180 min [40]. However, Ivakhnov et al. (2019) reached the highest yield of the oil fraction, equal to 9.02 wt % (weight percent) at 85 °C and 329.3 bar in 72 min [42]. Testing modified extraction (CO2 expanded ethanol combined with sonication) with different co-solvents for receiving lipids from berry seeds, including rowanberries, showed that the optimal operating conditions were as follows: ethanol, 52 °C, 100 bar, and 7 min [41]. However, no research has been carried out on other (aqueous) fractions of extracts obtained, especially from Sorbus aucuparia fruits. Due to divergent data on extraction parameters, we decided to prepare four different extract variants using low and high temperatures (40 and 70 °C) at intermediate pressure values (200 and 300 bar). We used methanol as a co-solvent, which is a commonly used solvent in numerous extractions. To prepare the richest extracts, the extraction time was set to 180 min. The highest extraction yield was obtained when high temperature and pressure were used (extract variant E3), while the lowest yield was caused by the use of 200 bar, without a significant influence of temperature (E2 and E4). The extraction yield ranged from 16.90 to 18.05% (169.60–184.40 g/kg) and was similar to the yields of the typical extractions of rowanberry pomace using methanol, e.g., 20.75% [43]. In general, at a specific temperature, an increase in pressure increases the density of the solvent and the solubility of targeted compounds, promoting an increase in extraction yield. Under constant pressure, the temperature increase decreases the solvent density, reducing its solvating power but improving the vapor pressure, which increases the analyte solubility, thus increasing extraction efficiency [44]. The obtained results are consistent with these data.
To assess the quality of the obtained rowanberry extracts, the total phenolic contents were measured (see Figure 1). To date, the highest TPC values of the defatted methanol–water rowanberry fruit extract (1:1, v/v) were equal to 26.03 mg GAE/g dw and of the water residue—14.27 mg GAE/g dw [45]. However, most reports on methanolic extracts of Sorbus aucuparia fruits reported lower TPC values in a range of 2.53–10.05 mg GAE/g, depending on rowanberries genotypes, 4.35–8.19 mg GAE/g for different cultivars, and between 2.68 and 3.35 mg GAE/g [13,46,47,48]. The extract variant E4 prepared at 70 °C and 200 bar was characterized by the highest TPC (4.15 and 3.33 mg GAE/g dw for methanol and water, respectively). In comparison, the extract variant E2 obtained at 40 °C and the same pressure had the lowest TPC (2.71 and 2.07 mg GAE/g dw for methanol and water, respectively). These results suggest that higher temperatures may promote the release of phenolic compounds from the tested material due to the improvement of their solubility or acceleration of diffusion connected with the increasing temperature [49]. Aqueous solutions prepared from the corresponding methanolic extracts had significantly lower TPC values, which can be explained based on the different solubility of phenolic compounds in water and methanol. Nevertheless, the content of phenolic compounds in aqueous extracts was substantial, and due to their potential to provide a biocompatible environment given their applications as fungicides and ensuring the safety of both plants and their consumers, we chose them for microbiological experiments.
Previously published research on rowan fruit extracts has primarily concentrated on their antibacterial properties, with limited studies on their antifungal effects, which have yielded inconclusive results. No inhibitory effect on Candida albicans growth was observed after treatment with the aqueous and ethanolic, as well as acetone-derived phenolic-rich, rowanberry extracts [16,17]. However, Maliuvanchuk et al. (2023) determined an inhibition zone of ethanolic fruit extract obtained by the percolation method on C. albicans equal to 5.81 mm [23]. Further, an antifungal effect was not observed against Rhizopus stolonifer treated with ethanolic–aqueous rowanberry extract [19]. Importantly, several reports present promising results regarding the growth inhibition of various fungal species by extracts obtained from the other Sorbus spp. The extracts prepared from S. sibirica fruits were able to inhibit the growth of Aspergillus niger (fungistatic diameter: 1.51 mm) [50]. Moreover, antifungal properties of S. cashmiriana whole plant extracts were tested on various fungal species, and the most efficient inhibition was obtained against Aspergillus flavus (68%) and the lowest against Rhizoctonia solani (24%) [20]. However, acidified ethanolic extracts obtained from S. domestica fruits did not show antifungal activity against C. albicans and Saccharomyces cerevisiae [21]. In our experiment, the addition of rowanberry extracts inhibited the growth of Fusarium proliferatum from 6.5 to 26.4%, but for F. culmorum, growth inhibition was not observed (see Figure 2). However, to determine the actual antifungal activity of the obtained extracts, we measured ergosterol content in the treated mycelia. ERG, which is a sterol compound found in the cell membranes of fungi that serves as a crucial component for maintaining the structural integrity and fluidity of fungal cell membranes, is used as a specific biomarker to quantify fungal biomass [22]. A reduction in ERG content was observed for all samples, but extract variant E3 was the most effective (ERG reduction: 60.67% for F. proliferatum and 41.62% for F. culmorum). It is noteworthy that the extraction yield of this particular variant was the highest, potentially indicating an increased concentration of bioactive compounds, which exhibit antifungal properties, but this is not directly related to the total content of phenolic compounds. The extracts obtained at low temperature (40 °C) had significantly lower antifungal properties, but still, their decrease in ERG content was not lower than 7.70% against F. culmorum and 12.41% against F. proliferatum. In the case of F. proliferatum, ERG concentration reduction corresponded to the observed inhibition of mycelium growth. However, the reduction in ERG content with the absence of differences in strain sizes between the control and the tested samples of F. culmorum may be caused by the specific structure of the mycelium, for which it was not possible to observe a size decrease.
The promising effects of the microbiological studies induced us to analyze secondary metabolites of Fusarium spp. F. proliferatum produced beauvericin and fumonisins from the B group. All tested rowanberry extracts significantly decreased the production of BEA, reaching reduction values from 80.6 to 92.1% (without significant differences in the degree of reduction between individual extracts, p > 0.05). Interestingly, in the case of fumonisins, there was an enormous increase in their biosynthesis in all samples after extract addition (see Figure 4). So far, this effect has been only described in a few reports [51,52]. This phenomenon can be interpreted as part of a stress response mechanism caused by the external factor, where the fungi change metabolic priorities [53]. Under stress caused by the presence of rowanberry extracts, F. proliferatum reallocates resources to prioritize the synthesis of compounds that enhance their survival, like fumonisins, which disrupt sphingolipid metabolism in host organisms [54]. Moreover, the significant increase in fumonisin production may divert metabolic resources away from beauvericin biosynthesis, or the regulatory pathways controlling BEA synthesis might be directly inhibited by the stress, further reducing its production. Furthermore, under stress conditions, fungi can release alkaline proteases, which, further, are able to break peptide bonds presented in the BEA structure [55]. Instead, Fusarium culmorum synthesized B-trichothecenes (DON, 3- and 15-AcDON) and zearalenone with derivatives (ZEN, ZEN-14S, α-ZOL). The simultaneous presence of free and modified forms can be attributed to the intricate interplay between the fungus and its host environment. Modified mycotoxins may originate from the metabolic pathways of infected plants or be synthesized de novo by the fungus, leading to their coexistence alongside their free forms [56]. The obtained results showed that F. culmorum produced ZEN-14S in high concentration (control: 508.53 μg/g) and α-ZOL in low concentration (control: 6.58 μg/g). The application of rowanberry fruit extracts resulted in a notable decrease (p < 0.05) in the biosynthesis of all tested mycotoxins, with reductions not less than 32.7%.
4. Materials and Methods
4.1. Plant Material
The plant material consisted of ripe fruits of the rowan tree Sorbus aucuparia obtained from trees growing in Poznań and Zagórów (Central Poland). The fruits were collected directly from the tree, then lyophilized and ground in liquid nitrogen. The material was stored frozen at −20 °C.
4.2. Fungal Material
Fusarium proliferatum (PEA 1) and Fusarium culmorum (KF 846) were isolated from pea seeds and wheat kernels, respectively. They were identified by molecular techniques [35,57] and preserved at the Institute of Plant Genetics, Polish Academy of Science, Poznań, Poland.
4.3. Reagents
Carbon dioxide CO2 (99.9995%, Air Products, Warszawa, Poland), methanol (99.5%, Chempur, Piekary Śląskie, Poland), and deionized water (Milipore, Burlington, MA, USA) were used to prepare rowanberry extracts. Potato dextrose agar (PDA) used for the in vitro experiment was supplied by Oxoid, Basingstoke, UK. Sodium hydroxide (≥98.8%, POCH, Gliwice, Poland), hydrochloric acid (≥37%, Sigma Aldrich, Taufkirchen, Germany), methanol (99.99%, POCH, Gliwice, Poland), and pentane (99%, POCH, Gliwice, Poland) were used to extract ergosterol or mycotoxins. For the HPLC measurements, the appropriate solvents with LC grade were used. Analytical standards purchased as ready-to-use solutions from Romer Labs (Tulln, Austria) included ERG, FB1, FB2, FB3, BEA, DON, DON-3G, 3-AcDON, 15-AcDON, NIV, NIV-3G, FUSX, ZEN, and ZEN-14G (100 µg/mL). The α- and β-ZOL concentrations were 10 µg/mL. ZEN-14S (100 µg/mL) was purchased from Aokin (Berlin, Germany).
4.4. Supercritical Fluid Extraction
The extraction was performed utilizing the MV-10ASFE extractor (Waters, Manchester, MA, USA), which included a CO2 cylinder, cooling system, fluid delivery module, column oven, back pressure regulator, heat exchanger, and fraction collection module, as well as ChromScope v1.20 software (Waters, Manchester, MA, USA). The extraction of 25 g of rowanberries conducted under various temperature (40 and 70 °C) and pressure (200 and 300 bar) conditions enabled the preparation of 4 different extract variants (E1–E4). The CO2 flow rate was 4 mL/min, and the methanol (co-solvent) flow was 1 mL/min. Each experimental run took 180 min, with the first dynamic time of 15 min, the static time of 30 min, and the second dynamic time of 135 min. The extraction yield was calculated using the following formula:
where ma is the mass of Sorbus aucuparia fruits taken for extraction, and mb is the mass of the remaining rowanberry residues after extraction.
The initial methanolic extracts obtained from Sorbus aucuparia fruits were transferred to deionized water, the remaining methanol was removed, and the aqueous extracts were concentrated using an evaporator. Finally, from the 1 g of rowanberries, 1 mL of each aqueous extract variant was prepared.
4.5. Determination of Total Phenolic Content
The total phenolic content (TPC) was determined using the modified Folin–Ciocâlteu method [58], as follows: 0.1 mL of gallic acid solutions with concentrations of 0.01, 0.05, 0.10, 0.15, 0.20, and 0.25 mg/mL, respectively, were mixed with 0.25 mL of Folin–Ciocâlteu’s reagent, and after 3 min, 3 mL of 10% calcium carbonate solution was added. After 40 min in the darkness, the absorbances of the prepared calibrating solutions were measured at 765 nm using a UV–Vis Varian Cary 300 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Then, the absorbance of appropriately diluted methanolic and aqueous extracts of rowanberries prepared analogously to the standard samples was measured similarly. TPC was determined based on the obtained calibration curve (y = 4.018x, R2 = 0.9999), and the results were expressed as mg of gallic acid per 1 g of extract dw.
4.6. Study on the Effect of Sorbus aucuparia Fruit Extracts on Fusarium Growth
The antifungal effect of rowanberries was estimated using a modified method of Uwineza et al. (2022) [56]: 15 mL of a mixture of PDA medium with 10% aqueous rowanberry extract was inoculated with a 5 mm mycelium of F. proliferatum (PEA 1) or F. culmorum (KF 846) on each Petri dish. The control group was PDA medium containing the investigated fungal strains without extracts. All samples were incubated at 25 °C in the darkness for 10 days in three replications. After 10 days of the experiment, the radial mycelial growth was measured, and the antifungal properties of each extract variant were calculated based on the following formula:
where Dc is the average diameter of the control group (PDA medium with F. proliferatum or F. culmorum), and De is the average diameter of tested samples (Fusarium spp. with PDA medium treated by rowanberry extracts).
4.7. Determination of Ergosterol Content
To 0.1 g of dried mycelium samples, 2 mL of methanol and 0.5 mL of 2 M sodium hydroxide were added. Samples were microwave-heated 3 times at 370 W for 20 s. After cooling, the samples were neutralized with 1 mL of 1 M hydrochloric acid and 2 mL of methanol. The suspensions were extracted three times with 4 mL of n-pentane, and then the solvent was air-evaporated. The dried residues were dissolved in 1 mL of methanol, filtered through 15 mm syringe filters with a 0.20 μm pore diameter (Chromafil, Macherey-Nagel, Duren, Germany), and analyzed by the UPLC-PDA technique according to Uwineza et al. (2022) [56]. The ERG content was given in µg per g of sample, and the ERG reduction percentage was calculated by the given formula:
where Ec is the ergosterol content in the control group (PDA medium with F. proliferatum or F. culmorum), and Ee is the ergosterol content in the test samples (PDA medium with Fusarium spp. treated with rowanberry extracts).
4.8. Analysis of Mycotoxins
Mycotoxins were extracted from the dried mycelium samples (0.335–0.506 g) by adding 7 mL of methanol and stirring for 24 h. Then, the samples were centrifuged at 5000 rpm for 10 min, and the supernatants were filtered using a disc filter with a 0.20 μm pore diameter (Chromafil, Macherey-Nagel, Duren, Germany). Mycotoxin detection and determination were performed with UHPLCHESI-MS/MS using a non-porous C18 Cortecs chromatographic column (100 mm × 2.1 mm × 1.6 μm, Waters, Manchester, MA, USA). The mobile phase consisted of water–methanol 90:10 (A) and methanol–water 90:10 (B); both phases had 5 mM ammonium formate and 0.2% formic acid. The separation parameters and operation of the chromatograph are given in detail in a previous publication [56]. The percentage of mycotoxin biosynthesis reduction by F. proliferatum and F. culmorum was determined using the following formula:
where Mc is the mycotoxin content in the control group (PDA medium with F. proliferatum or F. culmorum), and Me is the mycotoxin content in the tested samples (PDA medium with Fusarium spp. treated with rowanberry extracts).
4.9. Statistical Analysis
Experimental data were statistically evaluated using the Statistica 14 software package (TIBCO Software Inc., Palo Alto, CA, USA). A one-way analysis of variance (ANOVA) was used to calculate the means and standard deviations and to assess the significance of the differences in the effects of individual extracts. Subsequently, the post hoc Tukey’s honest significant difference (HSD) test was used for paired comparisons (p = 0.05).
5. Conclusions
This study aimed to determine the influence of Sorbus aucuparia fruit extracts obtained by a supercritical fluid extraction method under different conditions on Fusarium proliferatum and F. culmorum growth and mycotoxin biosynthesis. It was observed that the obtained extracts inhibited the growth of only F. proliferatum. However, the decrease in ergosterol concentrations showed that the growth of both Fusarium species was reduced, but this effect was more evident for F. proliferatum than F. culmorum. Rowanberry extract obtained at 70 °C and 300 bars showed the most significant antifungal activity, with an ERG reduction of 60.67% for F. proliferatum and 41.62% for F. culmorum. Despite a significant decrease in ERG content in both Fusarium spp., which indicated fungi reduction, there was a diversification in their biosynthesis of secondary metabolites. All tested extract variants reduced the production of BEA by F. proliferatum. However, fumonisins showed substantial increases in their biosynthesis compared to the control group. This phenomenon can be attributed to the fungal survival mechanism that stimulates mycotoxin production under specific stress conditions. Instead, extracts obtained from rowan fruits consistently decreased the production of all tested mycotoxins by F. culmorum. All prepared extract variants similarly reduced the content of DON, ZEN, and ZEN-14S. However, the most significant reduction in 3- and 15-AcDON content occurred when samples were treated with the extract prepared at 40 °C and 200 bar, and in the case of α-ZOL, with the extract prepared at 70 °C and 200 bar. The obtained results are important for practically assessing the effectiveness of Sorbus aucuparia fruit extracts against Fusarium pathogens in cereal crops. Future studies on the gene expression of the tested Fusarium spp. are planned to understand the mechanisms of their growth inhibition and the different mycotoxin biosynthesis effects. The current research shows great potential for advancing sustainable agricultural practices, addressing the challenges of fungal diseases in cereal crops, and enhancing food safety.
Author Contributions
Conceptualization, S.R. and A.W.; methodology, S.R., N.G.-W., M.U., M.T. and A.W.; software, A.W.; validation, S.R., Ł.S. and A.W.; formal analysis, S.R. and A.W.; investigation, S.R., N.G.-W., M.U., M.B. and A.W.; resources, A.W., M.B., M.T. and Ł.S.; data curation, A.W.; writing—original draft preparation, S.R.; writing—review and editing, S.R., N.G.-W., M.U., Ł.S. and A.W.; visualization, S.R. and A.W.; supervision, A.W.; project administration, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by Polish Minister of Science and Education, under the program “Regional Initiative of Excellence” in 2019–2022 (Grant No. 008/RID/2018/19).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moskalets, V.; Hulko, B.; Rozhko, I.; Moroz, V.; Ivankiv, M. Morpho-Physiological Characteristics of Plants and Biochemical Parameters of Rowan Berries, Common Rowan, and Domestic Rowan Grown in the Conditions of the Northern Forest-Steppe of Ukraine. Sci. Horiz. 2023, 26, 78–92. [Google Scholar] [CrossRef]
- Huang, S.; Yang, N.; Liu, Y.; Gao, J.; Huang, T.; Hu, L.; Zhao, J.; Li, Y.; Li, C.; Zhang, X. Grape Seed Proanthocyanidins Inhibit Colon Cancer-Induced Angiogenesis through Suppressing the Expression of VEGF and Angl. Int. J. Mol. Med. 2012, 30, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and Anticancer Properties of Berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal Plants of the Russian Pharmacopoeia; Their History and Applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef]
- Raspé, O.; Findlay, C.; Jacquemart, A. Sorbus aucuparia L. J. Ecol. 2000, 88, 910–930. [Google Scholar] [CrossRef]
- Jędrzejewski, W.; Zalewski, A.; Jędrzejewska, B. Foraging by Pine Marten Martes Martes in Relation to Food Resources in Białowieża National Park, Poland. Acta Theriol. 1993, 38, 405–426. [Google Scholar] [CrossRef]
- Krasnov, V.; Shelest, Z.; Boiko, S.; Gulik, I.; Sieniawski, W. The Diet of the Roe Deer (Capreolus capreolus) in the Forest Ecosystems of Zhytomirske Polesie of the Ukraine. For. Res. Pap. 2015, 76, 184–190. [Google Scholar] [CrossRef][Green Version]
- Guitián, J.; Munilla, I. Responses of Mammal Dispersers to Fruit Availability: Rowan (Sorbus aucuparia) and Carnivores in Mountain Habitats of Northern Spain. Acta Oecologica 2010, 36, 242–247. [Google Scholar] [CrossRef]
- Bozhuyuk, M.R.; Ercisli, S.; Ayed, R.B.; Jurikova, T.; Fidan, H.; Ilhan, G.; Ozkan, G.; Sagbas, H.I. Compositional Diversity in Fruits of Rowanberry (Sorbus aucuparia L.) Genotypes Originating from Seeds. Genetika 2020, 52, 55–65. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical Fluid Extraction: Recent Advances and Applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef]
- Brunner, U. Some Antifungal Properties of Sorbic Acid Extracted from Berries of Rowan (Sorbus aucuparia). J. Biol. Educ. 1985, 19, 41–47. [Google Scholar] [CrossRef]
- Kokubun, T.; Harborne, J.B.; Eagles, J.; Waterman, P.G. Antifungal Biphenyl Compounds Are the Phytoalexins of the Sapwood of Sorbus aucuparia. Phytochemistry 1995, 40, 57–59. [Google Scholar] [CrossRef]
- Qiu, X.; Lei, C.; Huang, L.; Li, X.; Hao, H.; Du, Z.; Wang, H.; Ye, H.; Beerhues, L.; Liu, B. Endogenous Hydrogen Peroxide Is a Key Factor in the Yeast Extract-Induced Activation of Biphenyl Biosynthesis in Cell Cultures of Sorbus aucuparia. Planta 2012, 235, 217–223. [Google Scholar] [CrossRef]
- Arvinte, O.M.; Senila, L.; Becze, A.; Amariei, S. Rowanberry—A Source of Bioactive Compounds and Their Biopharmaceutical Properties. Plants 2023, 12, 3225. [Google Scholar] [CrossRef]
- Sołtys, A.; Galanty, A.; Podolak, I. Ethnopharmacologically Important but Underestimated Genus Sorbus: A Comprehensive Review; Springer: Dordrecht, The Netherlands, 2020; Volume 19, ISBN 0123456789. [Google Scholar]
- Liepiņa, I.; Nikolajeva, V.; Jākobsone, I. Antimicrobial Activity of Extracts from Fruits of Aronia melanocarpa and Sorbus aucuparia. Environ. Exp. Biol. 2013, 11, 195–199. [Google Scholar]
- Nohynek, L.J.; Alakomi, H.L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.M.; Puupponen-Pimiä, R.H. Berry Phenolics: Antimicrobial Properties and Mechanisms of Action against Severe Human Pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Krisch, J.; Galgóczy, L.; Tölgyesì, M.; Papp, T.; Vágvölgyi, C. Effect of Fruit Juices and Pomace Extracts on the Growth of Gram-Positive and Gram-Negative Bacteria. Acta Biol. Szeged. 2008, 52, 267–270. [Google Scholar]
- Bazarnova, Y.G.; Ivanchenko, O.B. Investigation of the Composition of Biologically Active Substances in Extracts of Wild Plants. Vopr. Pitan. 2016, 85, 100–107. [Google Scholar]
- Khan, S.; Kazmi, M.H.; Fatima, I.; Malik, A.; Inamullah, F.; Farheen, S.; Abbas, T. Cashmirins A and B, New Antifungal and Urease Inhibitory Prenylated Coumarins from Sorbus cashmiriana. Braz. J. Pharm. Sci. 2022, 58, e21493. [Google Scholar] [CrossRef]
- Sagdic, O.; Polat, B.; Yetim, H. Bioactivities of Some Wild Fruits Grown in Turkey. Erwerbs-Obstbau 2022, 64, 299–305. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of Antifungal and Anti-Aflatoxigenic Properties of Essential Oil Derived from Turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maliuvanchuk, S.; Grytsyk, A.; Melnyk, M.; Kutsyk, R.; Yurkiv, K.; Raal, A.; Koshovyi, O. Sorbus Aucuparia L. Fruit Extract and Its Cosmetics—As Promising Agents for Prophylactic and Treatment of Pyodermitis: Phytochemical and Microbiological Research. Open Agric. J. 2023, 17, e18743315268063. [Google Scholar] [CrossRef]
- Shi, W.; Tan, Y.; Wang, S.; Gardiner, D.M.; De Saeger, S.; Liao, Y.; Wang, C.; Fan, Y.; Wang, Z.; Wu, A. Mycotoxigenic Potentials of Fusarium Species in Various Culture Matrices Revealed by Mycotoxin Profiling. Toxins 2017, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- El Chami, J.; El Chami, E.; Tarnawa, Á.; Kassai, K.M.; Kende, Z.; Jolánkai, M. Effect of Fusarium Infection on Wheat Quality Parameters. Cereal Res. Commun. 2023, 51, 179–187. [Google Scholar] [CrossRef]
- Tava, V.; Prigitano, A.; Cortesi, P.; Esposto, M.C.; Pasquali, M. Fusarium Musae from Diseased Bananas and Human Patients: Susceptibility to Fungicides Used in Clinical and Agricultural Settings. J. Fungi 2021, 7, 784. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium Species and Mycotoxins Associated with Head Blight in Small-Grain Cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Saadullah, A.A.M. Studies on Teratogenic and Maternal Effects of Trichothecene (TCT) Extracted from Fusarium and trichoderma Culture on Pregnant Albino Mice. Bionatura 2023, 8, 1–4. [Google Scholar] [CrossRef]
- Dabas, Y.; Bakhshi, S.; Xess, I. Fatal Cases of Bloodstream Infection by Fusarium solani and Review of Published Literature. Mycopathologia 2016, 181, 291–296. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.S.; Bonifaz, A.; Ranque, S.; de Hoog, G.S.; Verweij, P.E.; Meis, J.F. Current Antifungal Treatment of Fusariosis. Int. J. Antimicrob. Agents 2018, 51, 326–332. [Google Scholar] [CrossRef]
- Ribas e Ribas, A.D.; Spolti, P.; Del Ponte, E.M.; Donato, K.Z.; Schrekker, H.; Fuentefria, A.M. Is the Emergence of Fungal Resistance to Medical Triazoles Related to Their Use in the Agroecosystems? A Mini Review. Braz. J. Microbiol. 2016, 47, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Batista, B.G.; de Chaves, M.A.; Reginatto, P.; Saraiva, O.J.; Fuentefria, A.M. Human Fusariosis: An Emerging Infection That Is Difficult to Treat. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200013. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef] [PubMed]
- Uwineza, P.A.; Urbaniak, M.; Stępień, Ł.; Gramza-Michałowska, A.; Waśkiewicz, A. Lamium album Flower Extracts: A Novel Approach for Controlling Fusarium Growth and Mycotoxin Biosynthesis. Toxins 2023, 15, 651. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical Fluid Extraction and Fractionation of Natural Matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Vairinhos, J.; Miguel, M.G. Essential Oils of Spontaneous Species of the Genus Lavandula from Portugal: A Brief Review. Z. Naturforsch. C 2020, 75, 233–245. [Google Scholar] [CrossRef]
- Rodrigues, V.M.; Rosa, P.T.V.; Marques, M.O.M.; Petenate, A.J.; Meireles, M.A.A. Supercritical Extraction of Essential Oil from Aniseed (Pimpinella anisum L.) Using CO2: Solubility, Kinetics, and Composition Data. J. Agric. Food Chem. 2003, 51, 1518–1523. [Google Scholar] [CrossRef]
- Ye, Q.; Jin, X.; Wei, S.; Zheng, G.; Li, X. Effect of Subcritical Fluid Extraction on the High Quality of Headspace Oil from Jasminum sambac (L.) Aiton. J. AOAC Int. 2016, 99, 725–729. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Kraujalis, P.; Tamkutė, L.; Urbonavičienė, D.; Viškelis, P.; Venskutonis, P.R. Recovery of Bioactive Substances from Rowanberry Pomace by Consecutive Extraction with Supercritical Carbon Dioxide and Pressurized Solvents. J. Ind. Eng. Chem. 2020, 85, 152–160. [Google Scholar] [CrossRef]
- Al-Hamimi, S.; Turner, C. A Fast and Green Extraction Method for Berry Seed Lipid Extraction Using CO2 Expanded Ethanol Combined with Sonication. Eur. J. Lipid Sci. Technol. 2020, 122, 1900283. [Google Scholar] [CrossRef]
- Ivakhnov, A.D.; Sadkova, K.S.; Sobashnikova, A.S.; Skrebets, T.E. Optimization of Oil Extraction from Rowanberry Waste in Alcoholic Beverage Production. Russ. J. Phys. Chem. B 2019, 13, 1135–1138. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Grootaert, C.; Van Camp, J.; Šarkinas, A.; Liaudanskas, M.; Žvikas, V.; Viškelis, P.; Rimantas Venskutonis, P. Chemical Composition, Antioxidant, Antimicrobial and Antiproliferative Activities of the Extracts Isolated from the Pomace of Rowanberry (Sorbus aucuparia L.). Food Res. Int. 2020, 136, 109310. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Rutkowska, M.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Zakrzewska, A.; Magiera, A.; Olszewska, M.A. Novel Insight into Biological Activity and Phytochemical Composition of Sorbus aucuparia L. Fruits: Fractionated Extracts as Inhibitors of Protein Glycation and Oxidative/Nitrative Damage of Human Plasma Components. Food Res. Int. 2021, 147, 110526. [Google Scholar] [CrossRef]
- Sarv, V.; Venskutonis, P.R.; Rätsep, R.; Aluvee, A.; Kazernavičiūtė, R.; Bhat, R. Antioxidants Characterization of the Fruit, Juice, and Pomace of Sweet Rowanberry (Sorbus aucuparia L.) Cultivated in Estonia. Antioxidants 2021, 10, 1779. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Rop, O.; Jurikova, T.; Sochor, J.; Fisera, M.; Balla, S.; Baron, M.; Hrabe, J. Bioactive Compounds in Sweet Rowanberry Fruits of Interspecific Rowan Crosses. Cent. Eur. J. Biol. 2014, 9, 1078–1086. [Google Scholar] [CrossRef]
- Wolf, J.; Göttingerová, M.; Kaplan, J.; Kiss, T.; Venuta, R.; Nečas, T. Determination of the Pomological and Nutritional Properties of Selected Plum Cultivars and Minor Fruit Species. Hortic. Sci. 2020, 47, 181–193. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Wei, J.; Shi, J.; Gao, J.; Zhou, Z.; Fan, J. Biological Activities of Extract Prepared from Sorbus sibirica Fruit. J. Chem. Pharm. Res. 2014, 6, 1369–1372. [Google Scholar]
- Ferrochio, L.; Cendoya, E.; Farnochi, M.C.; Massad, W.; Ramirez, M.L. Evaluation of Ability of Ferulic Acid to Control Growth and Fumonisin Production of Fusarium verticillioides and Fusarium proliferatum on Maize Based Media. Int. J. Food Microbiol. 2013, 167, 215–220. [Google Scholar] [CrossRef]
- Bodoira, R.; Velez, A.; Maestri, D.; Herrera, J. Bioactive Compounds Obtained from Oilseed By-Products with Subcritical Fluids: Effects on Fusarium verticillioides Growth. Waste Biomass Valorization 2020, 11, 5913–5924. [Google Scholar] [CrossRef]
- Reynoso, M.M.; Torres, A.M.; Ramirez, M.L.; Rodríguez, M.I.; Chulze, S.N.; Magan, N. Efficacy of Antioxidant Mixtures on Growth, Fumonisin Production and Hydrolytic Enzyme Production by Fusarium verticillioides and F. proliferatum in Vitro on Maize-Based Media. Mycol. Res. 2002, 106, 1093–1099. [Google Scholar] [CrossRef]
- Reynoso, M.M.; Torres, A.M.; Chulze, S.N. Fusaproliferin, Beauvericin and Fumonisin Production by Different Mating Populations among the Gibberella Fujikuroi Complex Isolated from Maize. Mycol. Res. 2004, 108, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Urbaniak, M.; Bryła, M.; Stepien, Ł.; Modrzewska, M.; Waśkiewicz, A. In Vitro Effects of Lemon Balm Extracts in Reducing the Growth and Mycotoxins Biosynthesis of Fusarium culmorum and F. proliferatum. Toxins 2022, 14, 355. [Google Scholar] [CrossRef]
- Perincherry, L.; Urbaniak, M.; Pawłowicz, I.; Kotowska, K.; Waśkiewicz, A.; Stępień, Ł. Dynamics of Fusarium Mycotoxins and Lytic Enzymes during Pea Plants’ Infection. Int. J. Mol. Sci. 2021, 22, 9888. [Google Scholar] [CrossRef]
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of Total Phenolic Content Using the Folin-C Assay: Single-Laboratory Validation. J. AOAC Int. 2019, 102, 320–321. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).