Equilibrium and Kinetic Studies on Adsorption of Neutral and Ionic Species of Organic Adsorbates from Aqueous Solutions on Activated Carbon
Abstract
1. Introduction
2. Results
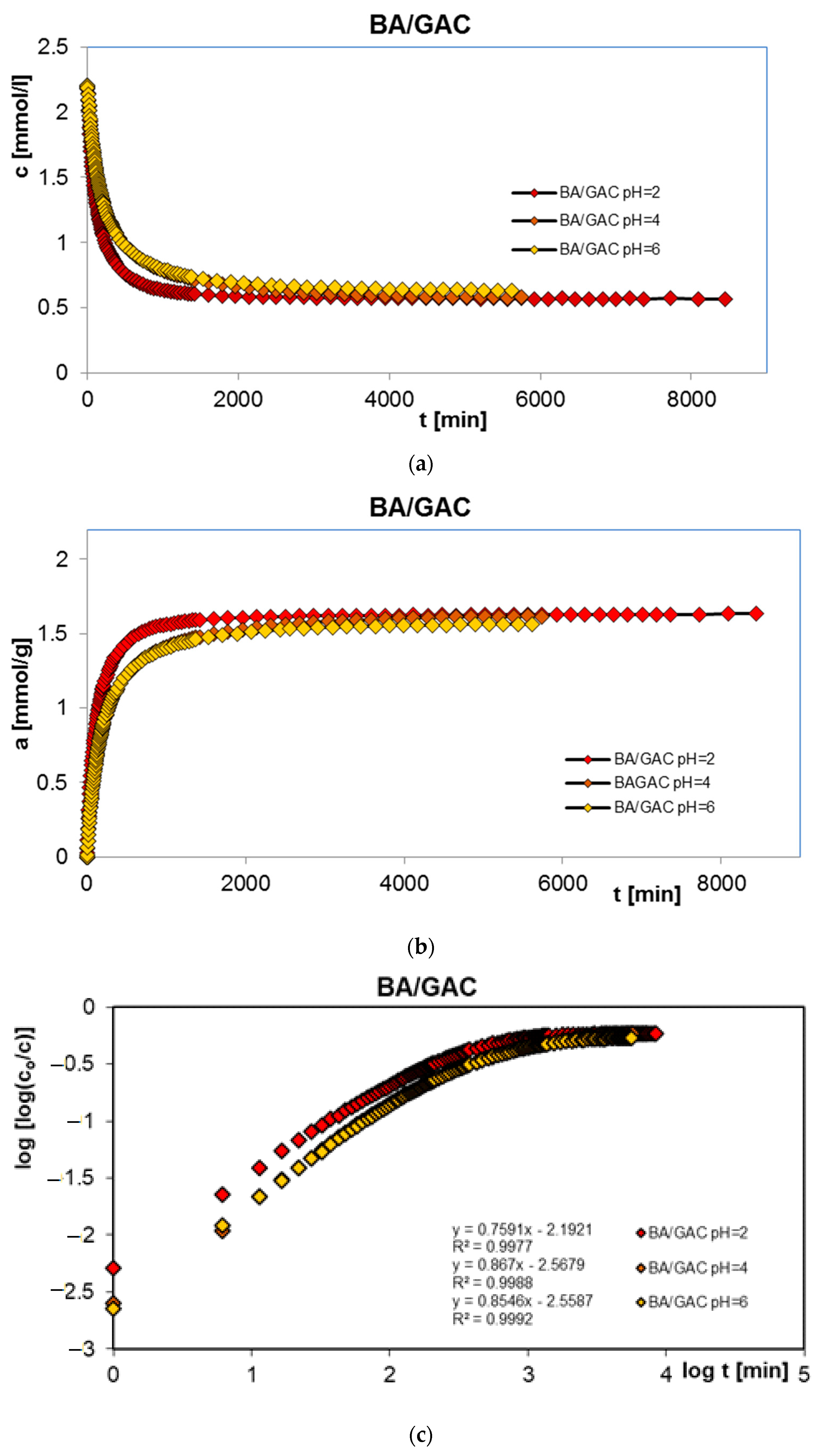
2.1. Adsorption Equilibrium
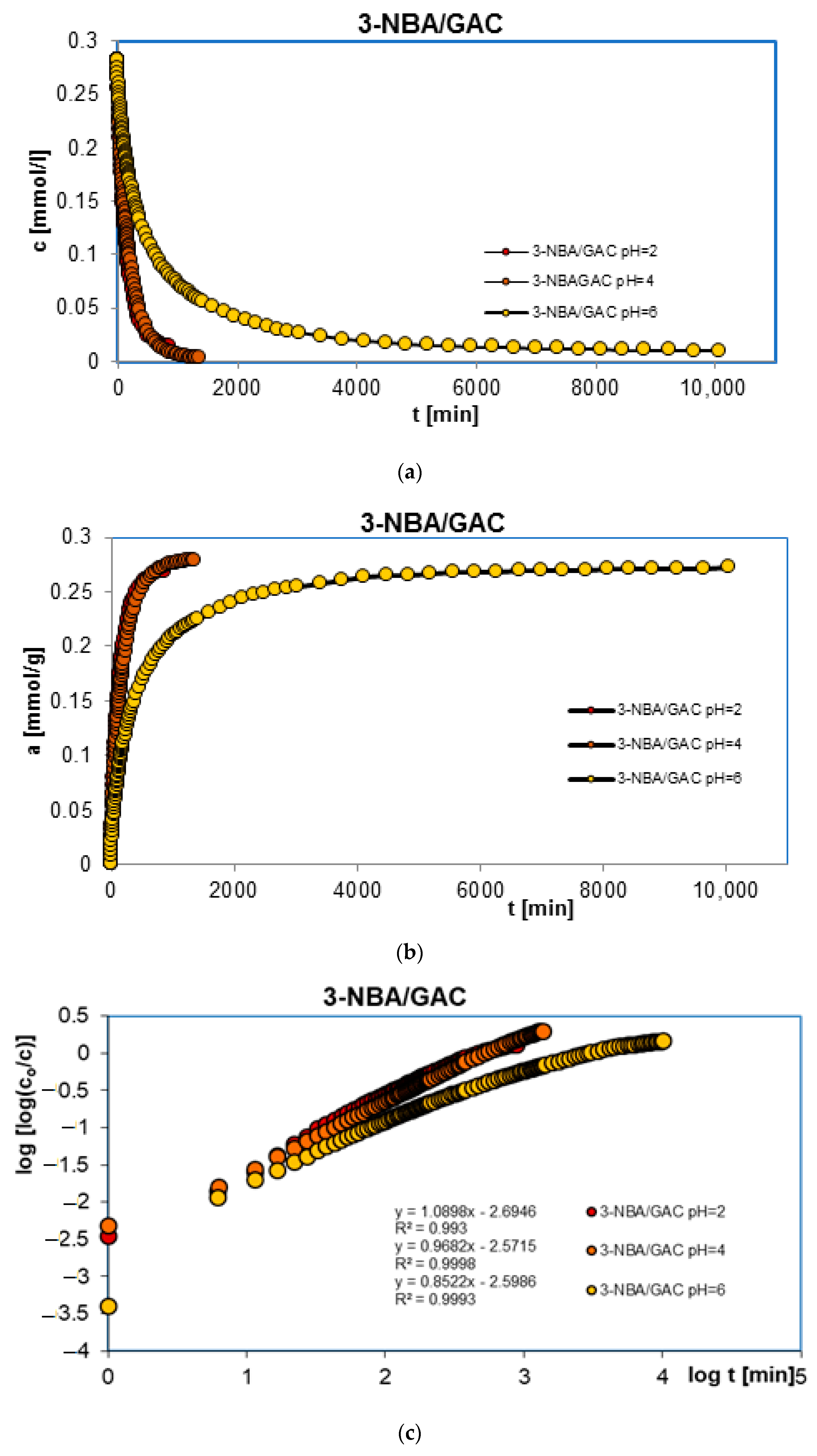
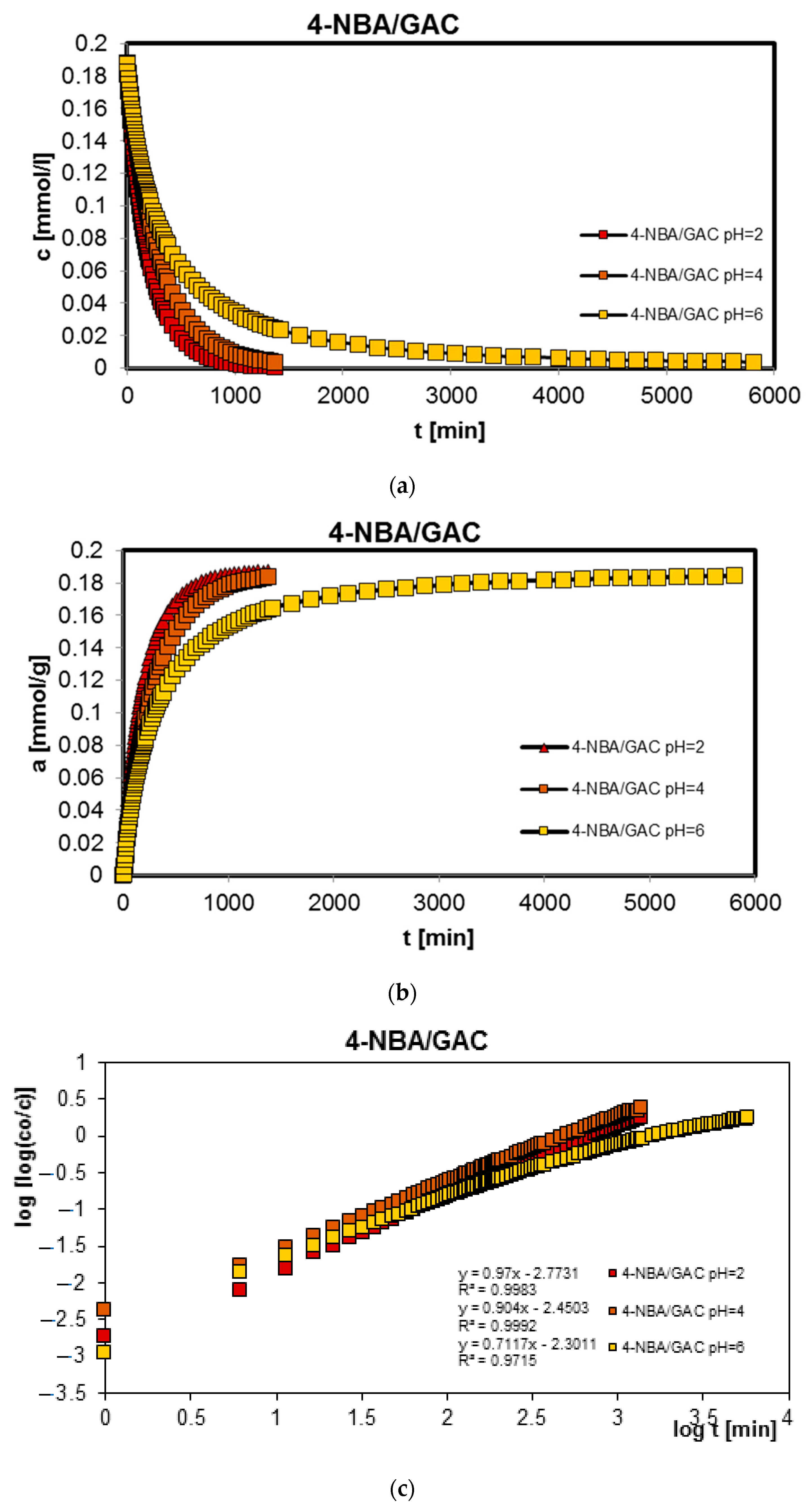
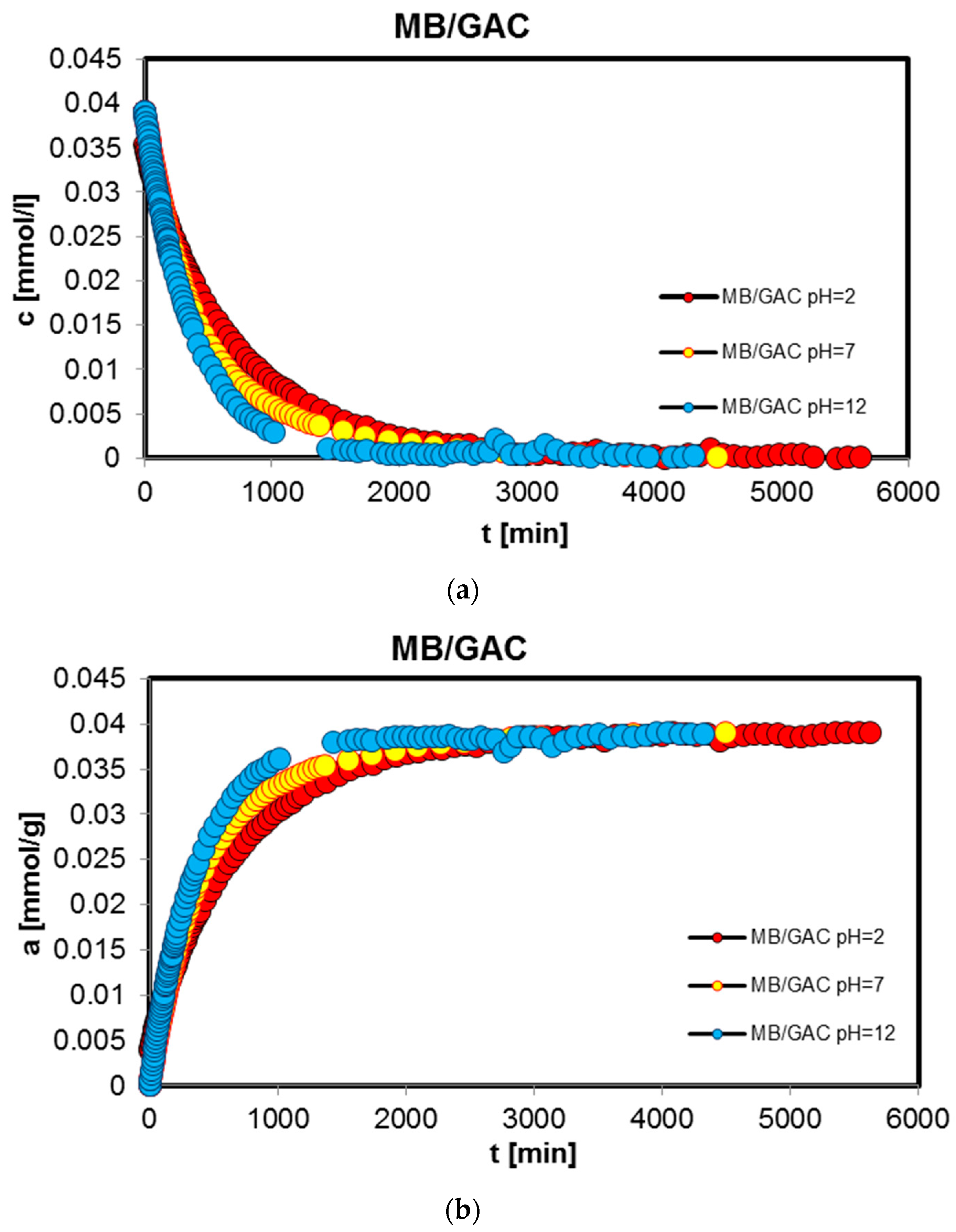
2.2. Adsorption Kinetics
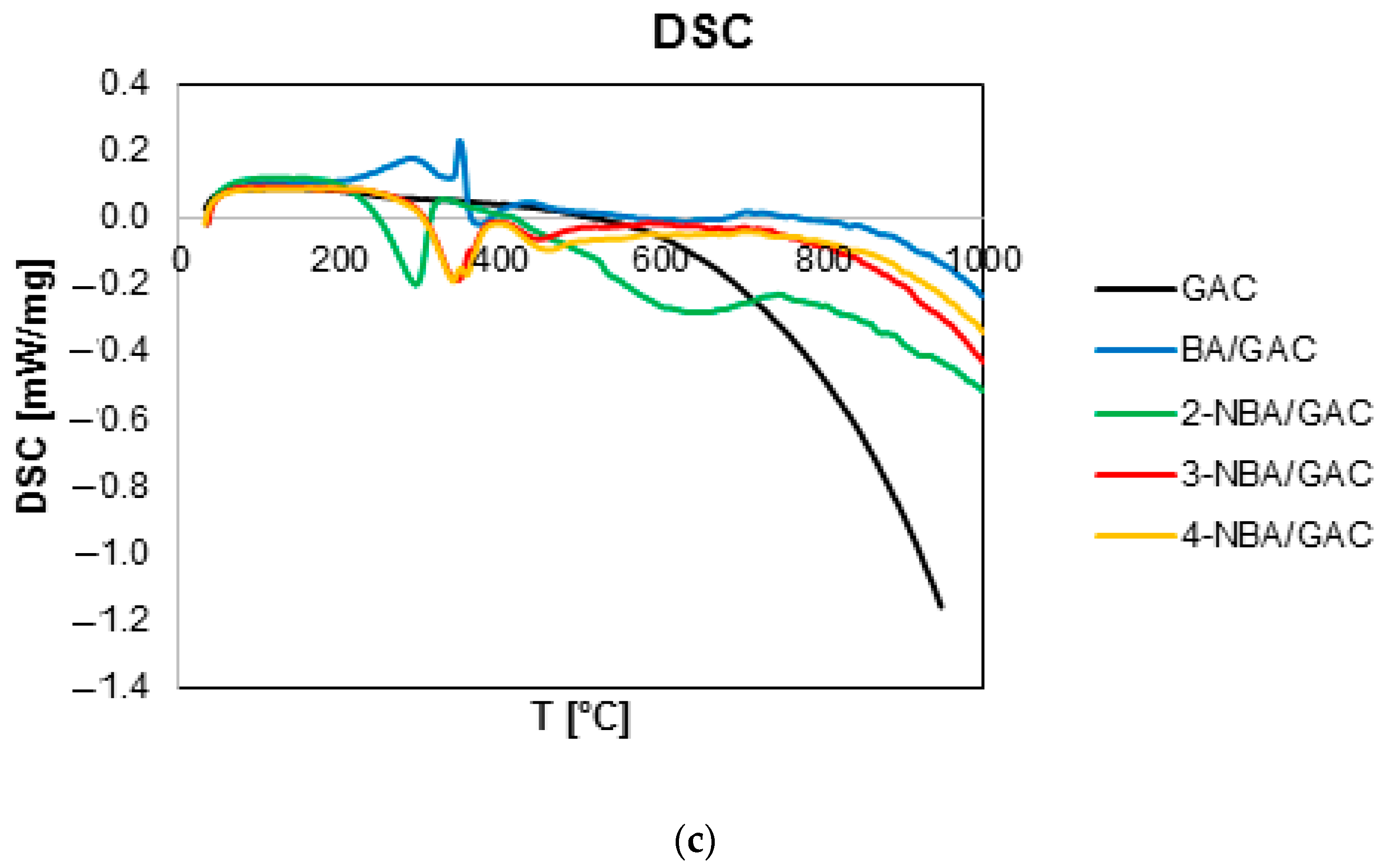
2.3. Thermal Analysis
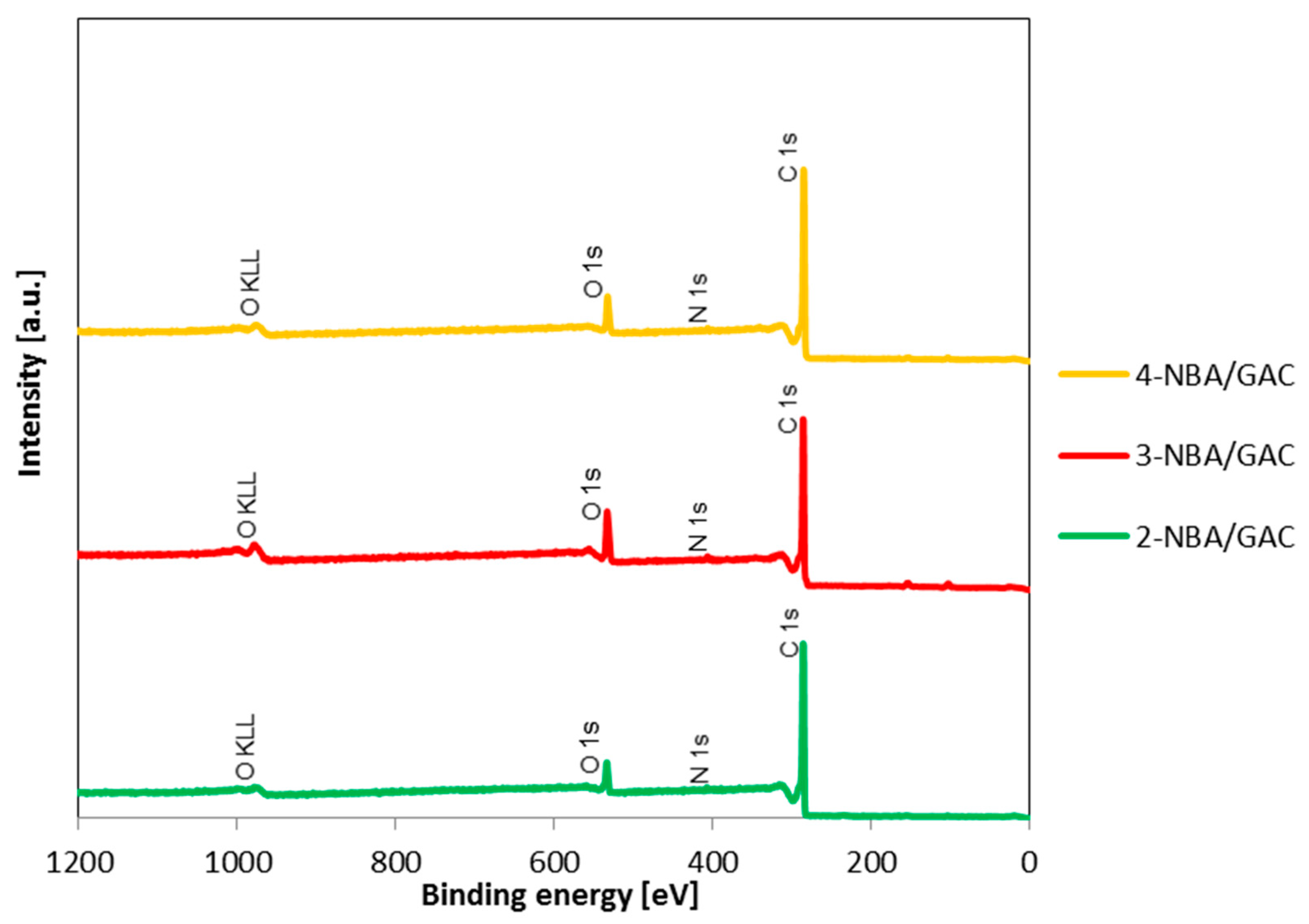
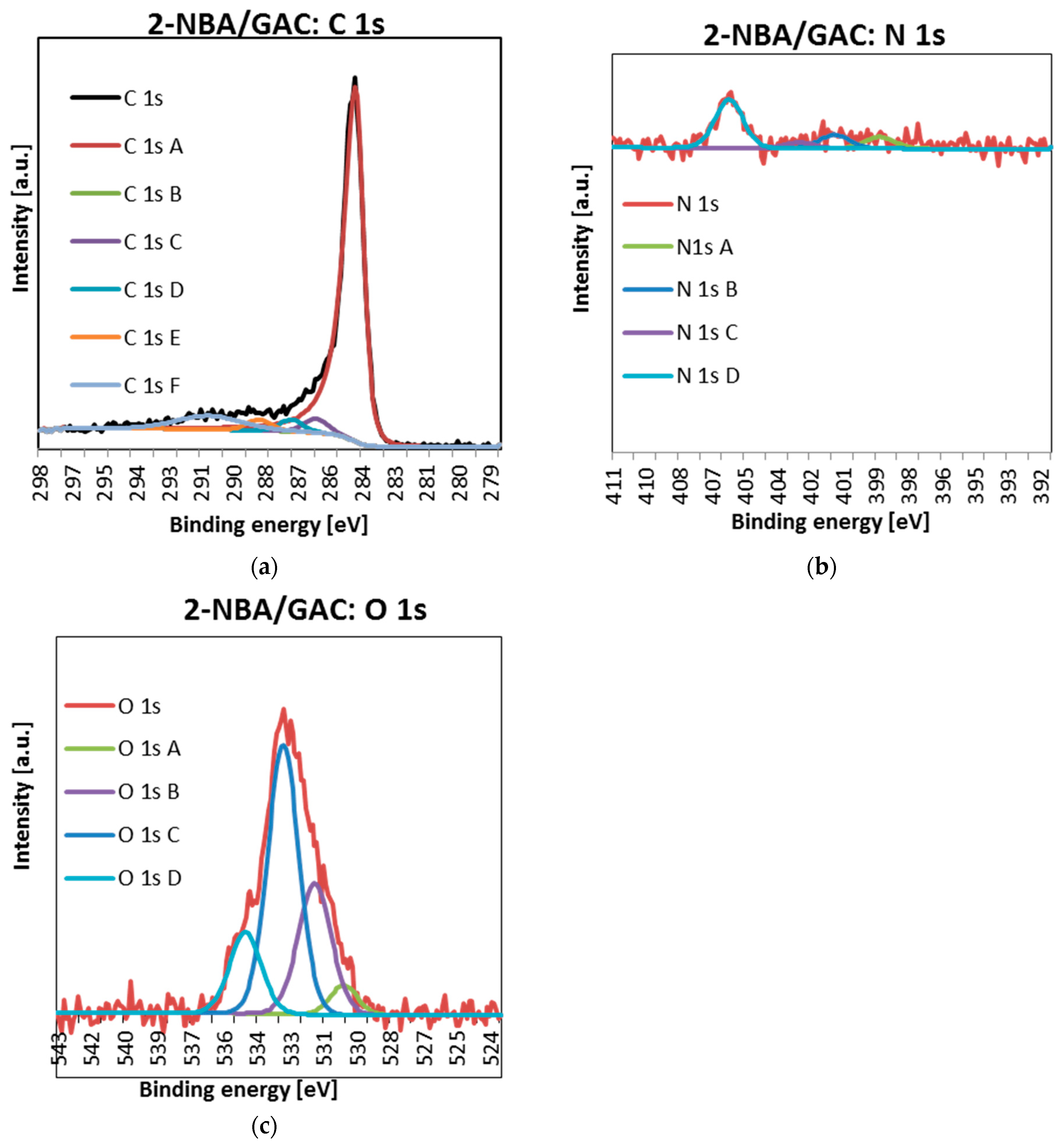
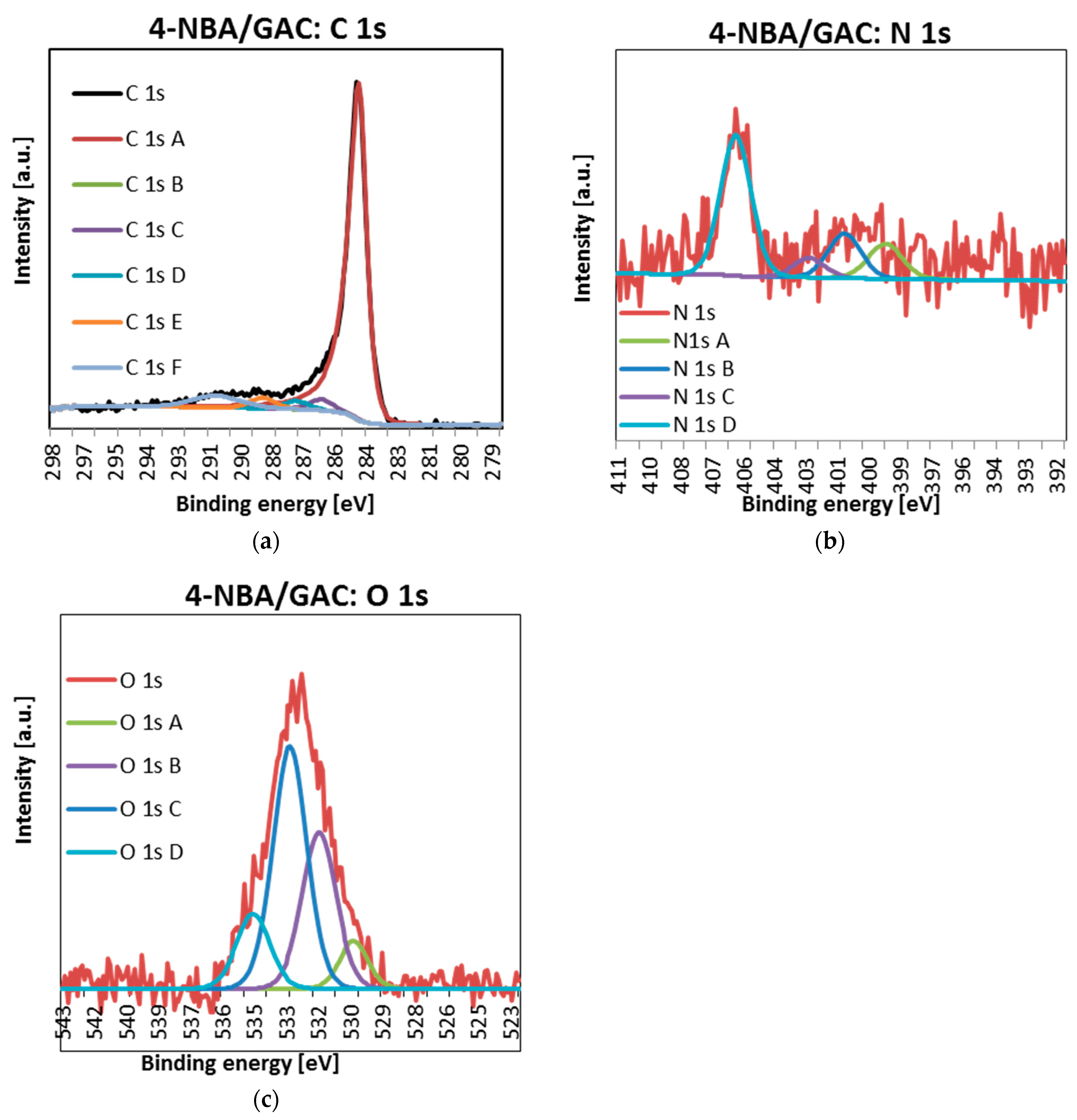
2.4. X-ray Photoelectron Spectroscopy (XPS)
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Methods
4.2.1. Adsorption Equilibrium
4.2.2. Adsorption Kinetics
4.2.3. Thermal Analysis
4.2.4. XPS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, S.; Wang, X.; Ai, Y.; Tan, X.; Hayat, T.; Hu, W.; Wang, X. Experimental and theoretical studies on competitive adsorption of aromatic compounds on reduced graphene oxides. J. Mater. Chem. A 2016, 4, 5654. [Google Scholar] [CrossRef]
- Zhao, G.X.; Jiang, L.; He, Y.D.; Li, J.X.; Dong, H.L.; Wang, X.K.; Hu, W.P. Sulfonated Graphene for Persistent Aromatic Pollutant Management. Adv. Mater. 2011, 23, 3959. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 243, Benzoic Acid” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Benzoic-Acid (accessed on 17 June 2024).
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 11087, 2-Nitrobenzoic acid” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2-Nitrobenzoic-acid (accessed on 17 June 2024).
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 8497, 3-Nitrobenzoic acid” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3-Nitrobenzoic-acid (accessed on 17 June 2024).
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 6108, 4-Nitrobenzoic acid” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4-Nitrobenzoic-acid (accessed on 17 June 2024).
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 6099, Methylene Blue” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Methylene-Blue (accessed on 17 June 2024).
- Wang, B.; Lan, J.; Bo, C.; Gong, B.; Ou, J. Adsorption of heavy metal onto biomass-derived activated carbon: Review. RSC Adv. 2023, 13, 4275. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Liang, D.; Ji, B.Y.; Wang, Y.; Li, X.; Gao, W.Y. Effect of activated carbon microstructure and adsorption mechanism on the efficient removal of chlorophyll a and chlorophyll b from Andrographis paniculata extract. Sci. Rep. 2023, 13, 21930. [Google Scholar] [CrossRef] [PubMed]
- Javani, R.; Maghsoudi, H.; Darvishi Gilan, S.; Majidpour, M. Study on adsorption performance of different adsorbents in nitrogen/methane separation. Sep. Sci. Technol. 2021, 56, 2562. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Rangasamy, G.; Prasannamedha, G.; Kanmani, S. A review on the applicability of adsorption techniques for remediation of recalcitrant pesticides. Chemosphere 2023, 313, 137481. [Google Scholar] [CrossRef]
- Andrunik, M.; Bajda, T. Removal of Pesticides from Waters by Adsorption: Comparison between Synthetic Zeolites and Mesoporous Silica Materials. A Review. Materials 2021, 14, 3532. [Google Scholar] [CrossRef]
- Ziółkowska, M.; Milewska-Duda, J.; Duda, J.T. Effect of adsorbate properties on adsorption mechanisms: Computational study. Adsorption 2016, 22, 589. [Google Scholar] [CrossRef][Green Version]
- Nizam, N.U.M.; Hanafiah, M.M.; Mahmoudi, E.; Halim, A.A.; Mohhamad, A.W. The removal of anionic and cationic dyes from an aqueous solution using biomass-based activated carbon. Sci. Rep. 2021, 11, 8623. [Google Scholar] [CrossRef]
- de Lima, C.R.M.; Gomes, D.N.; de Morais Filho, J.R.; Pereira, M.R.; Fonseca, J.L.C. Anionic and cationic drug sorption on interpolyelectrolyte complexes. Colloids Surf. B Biointerfaces 2018, 170, 210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, A.; Xiong, P.; Zhang, H.; Liu, Z. Experimental Study of Temperature Effect on Methane Adsorption Dynamic and Isotherm. Energies 2022, 15, 5047. [Google Scholar] [CrossRef]
- Yadav, B.S.; Dasgupta, S. Effect of time, pH, and temperature on kinetics for adsorption of methyl orange dye into the modified nitrate intercalated MgAl LDH adsorbent. Inorg. Chem. Commun. 2022, 137, 109203. [Google Scholar] [CrossRef]
- Aumeier, B.M.; Augustin, A.; Thönes, M.; Sablotny, J.; Wintgens, T.; Wessling, M. Linking the effect of temperature on adsorption from aqueous solution with solute dissociation. J. Hazard. Mater. 2022, 429, 128291. [Google Scholar] [CrossRef] [PubMed]
- Natrayan, L.; Kaliappan, S.; Naga Dheeraj Kumar Reddy, C.; Karthick, M.; Patil, P.P.; Sekar, S.; Thanappan, S. Development and Characterization of Carbon-Based Adsorbents Derived from Agricultural Wastes and Their Effectiveness in Adsorption of Heavy Metals in Waste Water. Bioinorg. Chem. Appl. 2022, 2022, 1659855. [Google Scholar] [CrossRef] [PubMed]
- Kołczyk-Siedlecka, K.; Socha, R.P.; Yang, X.; Eckert, K.; Wojnicki, M. Study on kinetics and mechanism of Re(VII) ion adsorption and desorption using commercially available activated carbon and solutions containing Se(VI) as an impurity. Hydrometallurgy 2023, 215, 105973. [Google Scholar] [CrossRef]
- Xu, W.; Xu, J.; Song, J.; Xiu, G. Effect of Hydrodynamic Condition on Adsorption of Sulfadiazine on Marine Sediments. J. Mar. Sci. Eng. 2023, 11, 717. [Google Scholar] [CrossRef]
- Bayuo, J.; Rwiza, M.J.; Sillanpää, M.; Mtei, K.M. Removal of heavy metals from binary and multicomponent adsorption systems using various adsorbents—A systematic review. RSC Adv. 2023, 13, 13052. [Google Scholar] [CrossRef]
- Kılıç, A.; Orhan, R. Removal of cationic dyes by adsorption in a single and binary system using activated carbon prepared from the binary mixture. Sep. Sci. Technol. 2019, 54, 2147. [Google Scholar] [CrossRef]
- Olafadehan, O.A.; Amoo, K.O.; Oyedeko, K.F.K.; Adesina, A.J. Dynamic Studies of Binary Adsorption System in Fixed Beds using Orthogonal Collocation on Finite Element Method. Int. J. Appl. Comput. Math. 2024, 10, 108. [Google Scholar] [CrossRef]
- Gugała-Fekner, D. Adenine adsorption in different pH acetate buffer. Physicochem. Probl. Miner. Process. 2022, 58, 144446. [Google Scholar] [CrossRef]
- Wei, F.; Zhu, Y.; He, T.; Zhu, S.; Wang, T.; Yao, C.; Yu, C.; Huang, P.; Li, Y.; Zhao, Q.; et al. Insights into the pH-Dependent Adsorption Behavior of Ionic Dyes on Phosphoric Acid-Activated Biochar. ACS Omega 2022, 7, 46288. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Liu, Y.; Chen, H.; Sun, X.; Wang, Q.; Hu, X.; Di, J. Adsorption properties of Pb(II) and Cd(II) in acid mine drainage by oyster shell loaded lignite composite in different morphologies. Sci. Rep. 2024, 14, 11627. [Google Scholar] [CrossRef]
- Wasilewska, M.; Derylo-Marczewska, A.; Marczewski, A.W. Comprehensive Studies of Adsorption Equilibrium and Kinetics for Selected Aromatic Organic Compounds on Activated Carbon. Molecules 2024, 29, 2038. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, M.; Marczewski, A.W.; Deryło-Marczewska, A.; Sternik, D. Nitrophenols removal from aqueous solutions by activated carbon—Temperature effect of adsorption kinetics and equilibrium. J. Environ. Chem. Eng. 2021, 9, 105459. [Google Scholar] [CrossRef]
- Aharoni, C.; Sideman, S.; Hoffer, E. Adsorption of phosphate ions by collodioncoated alumina. J. Chem. Technol. Biotechnol. 1979, 29, 404. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.H.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. A Reference Book of Standard Data for Use in X-ray Photoelectron Spectroscopy; Perkin Elmer Corporation: Eden-Prairie, MN, USA, 1979. [Google Scholar]
- Briggs, D.; Seah, M.P. Practical Surface Analysis by Augler and XPS; John Wiley & Sons: New York, NY, USA, 1983. [Google Scholar]
- Derylo-Marczewska, A.; Marczewski, A.W. Nonhomogeneity Effects in Adsorption from Gas and Liquid Phases on Activated Carbons. Langmuir 1999, 15, 3981. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Marczewski, A.W. Effect of adsorbate structure on adsorption from solutions. Appl. Surf. Sci. 2002, 196, 264. [Google Scholar] [CrossRef]
- Ayranci, E.; Hoda, N.; Bayram, E. Adsorption of benzoic acid onto high specific area activated carbon cloth. J. Colloid Interface Sci. 2005, 284, 83. [Google Scholar] [CrossRef]
- Xin, X.; Si, W.; Yao, Z.; Feng, R.; Du, B.; Yan, L.; Wei, Q. Adsorption of benzoic acid from aqueous solution by three kinds of modified bentonites. J. Colloid Interface Sci. 2011, 359, 499. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Jaroniec, M. A new isotherm equation for single-solute adsorption from dilute solutions on energetically heterogeneous solids—Short communication. Monatshefte Chem. Chem. Mon. 1983, 114, 711. [Google Scholar] [CrossRef]
- Jaroniec, M.; Marczewski, A.W. Physical adsorption of gases on energetically heterogeneous solids I. Generalized Langmuir equation and its energy distribution. Monatshefte Chem. Chem. Mon. 1984, 115, 997. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Geloster Stoffe. K. Sven. Vetenskapsakademiens. Handl. 1898, 24, 1. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47. [Google Scholar] [CrossRef] [PubMed]
- Marczewski, A.W. Kinetics and equilibrium of adsorption of organic solutes on mesoporous carbons. Appl. Surf. Sci. 2007, 253, 5818. [Google Scholar] [CrossRef]
- Marczewski, A.W. Application of mixed order rate equations to adsorption of methylene blue on mesoporous carbons. Appl. Surf. Sci. 2010, 256, 5145. [Google Scholar] [CrossRef]
- Marczewski, A.W. Analysis of kinetic Langmuir model. Part I: Integrated kinetic Langmuir equation (IKL): A new complete analytical solution of the Langmuir rate equation. Langmuir 2010, 26, 15229. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Deryło-Marczewska, A.; Słota, A. Adsorption and desorption kinetics of benzene derivatives on mesoporous carbons. Adsorption 2013, 19, 391. [Google Scholar] [CrossRef]
- Haerifar, M.; Azizian, S. Fractal-like adsorption kinetics at the solid/solution interface. J. Phys. Chem. C 2012, 116, 13111. [Google Scholar] [CrossRef]
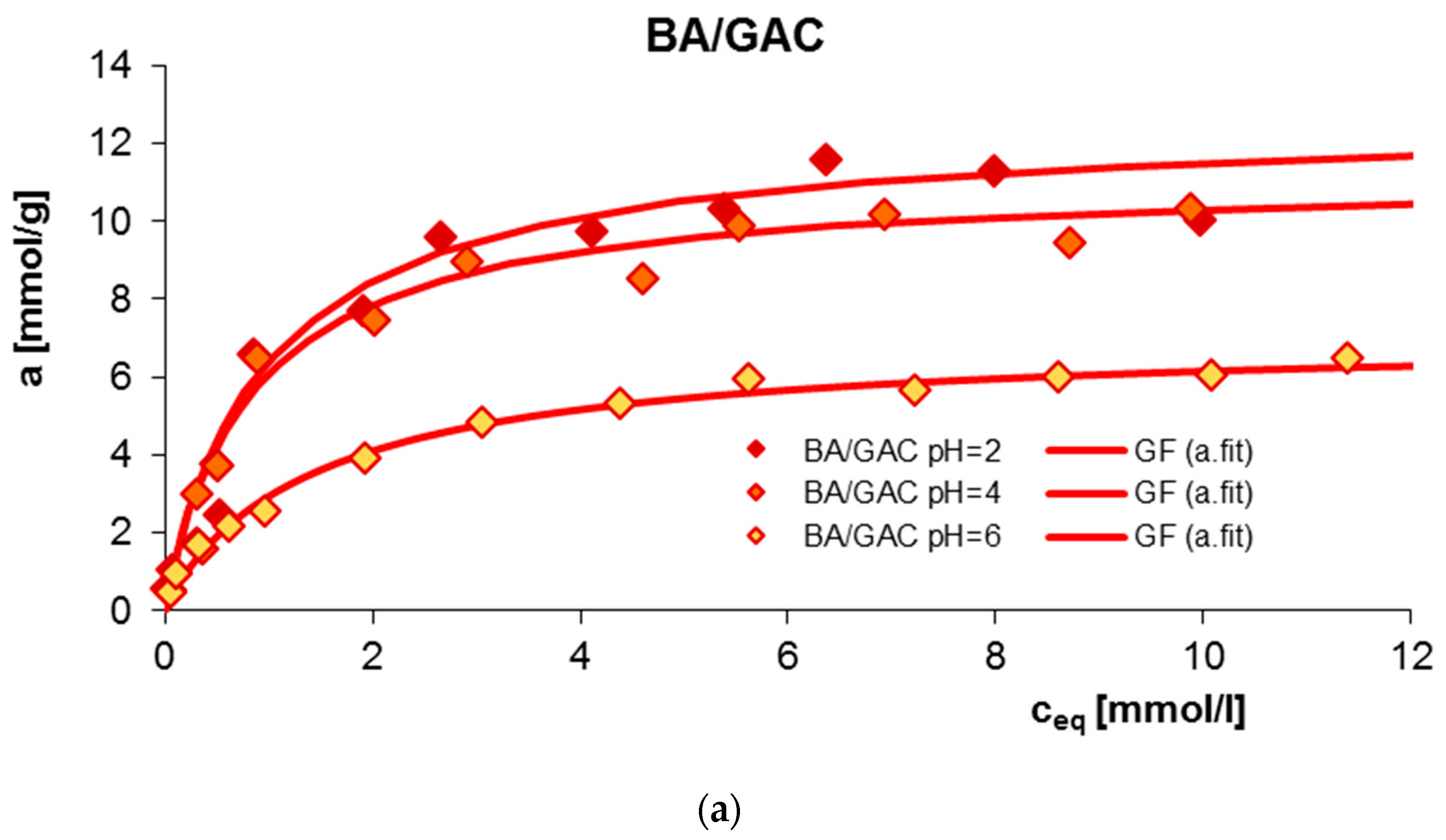
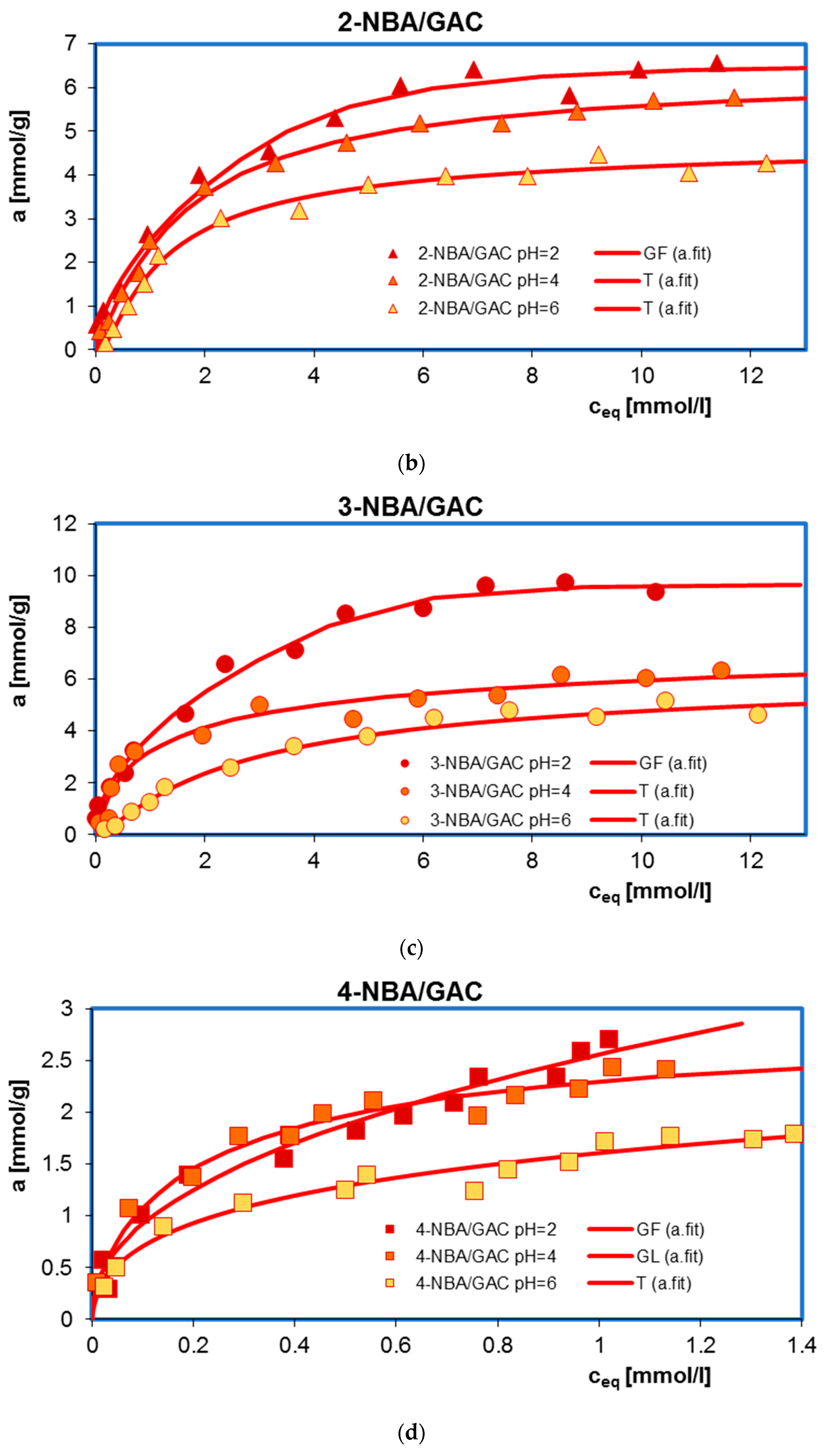
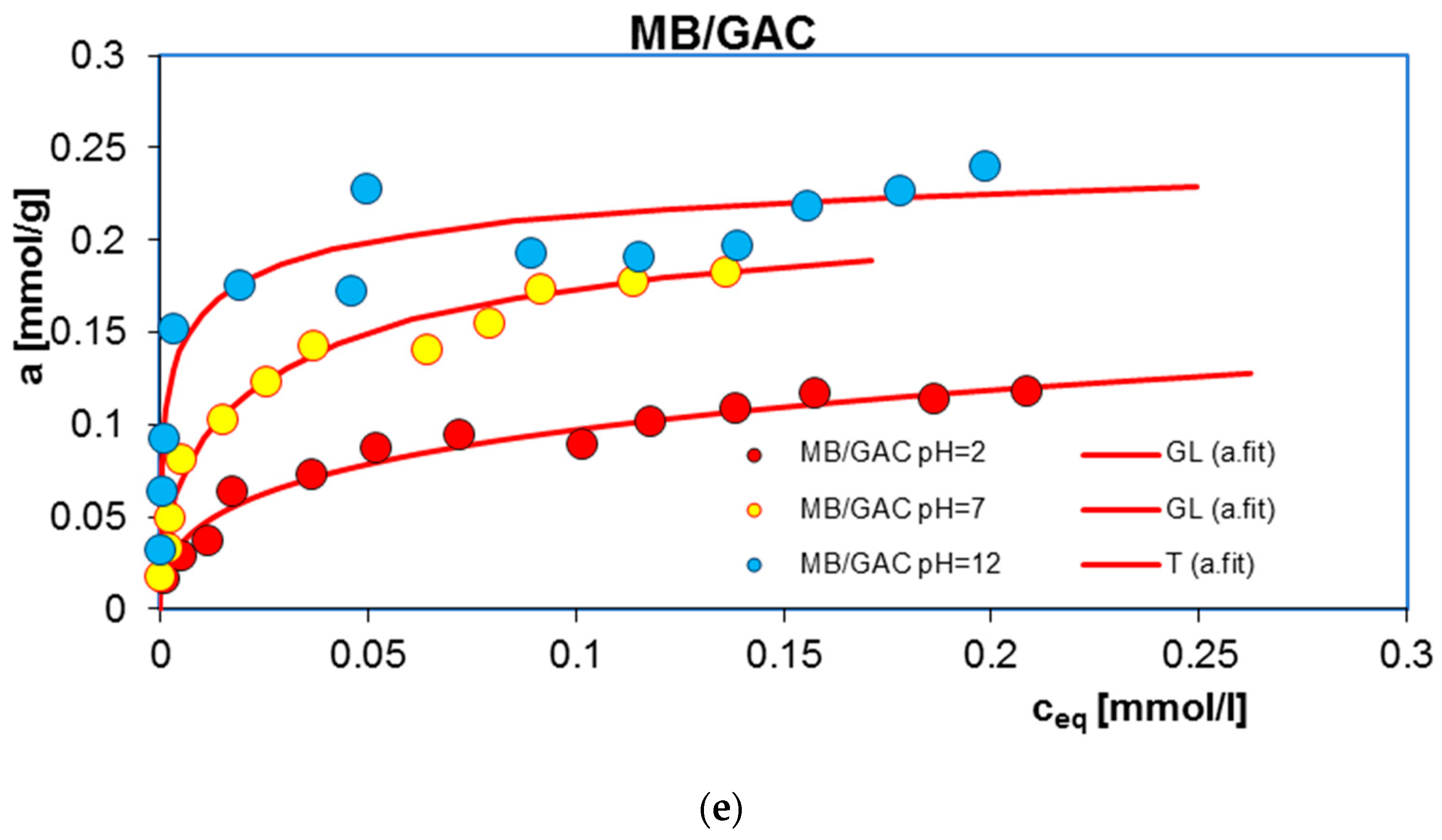

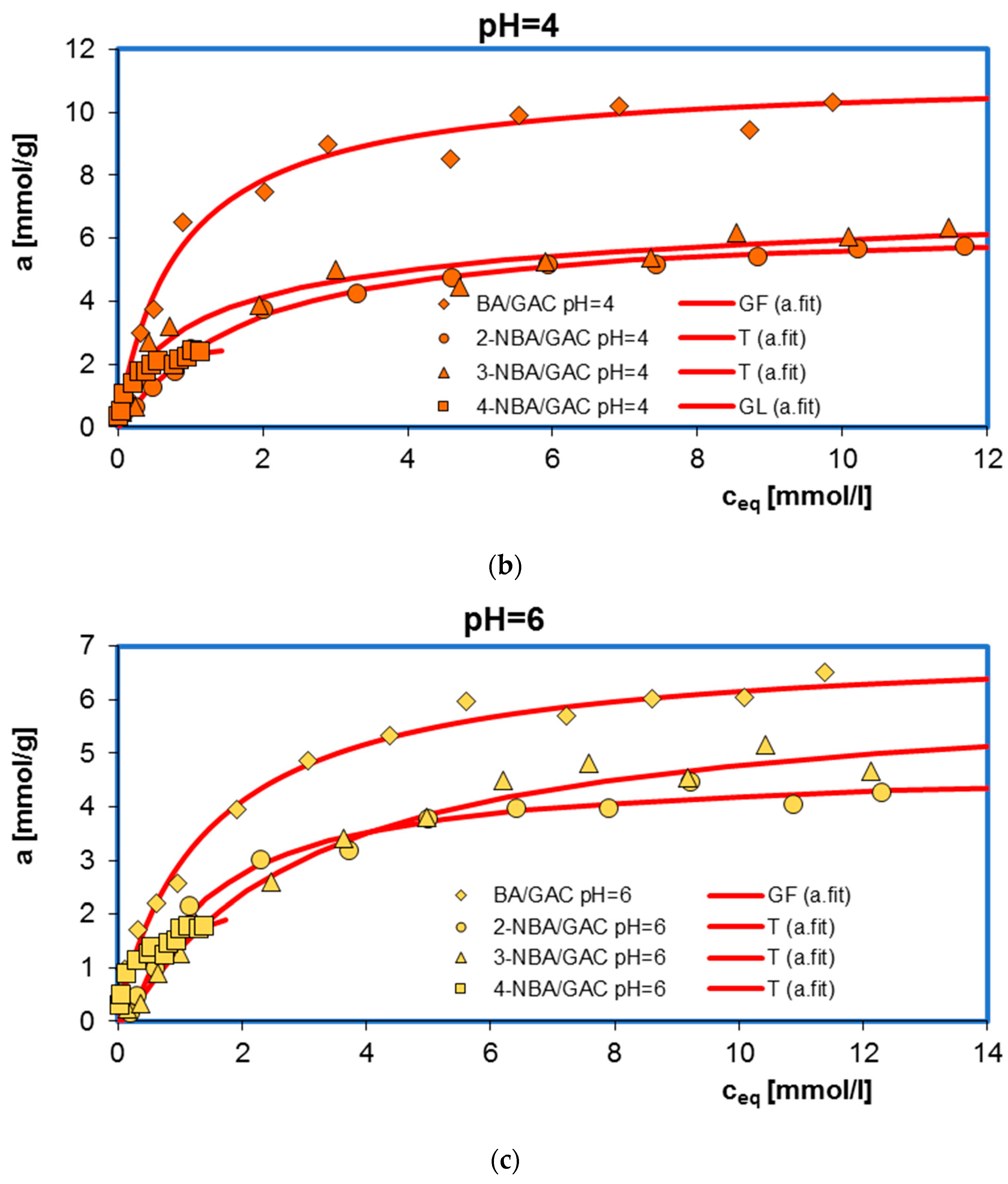





| System | Isotherm Type | am a | m b | n b | logK c | R2 d | SD(a) e |
|---|---|---|---|---|---|---|---|
| BA/GAC pH = 2 | GF | 11.68 | 0.88 | 1 | −0.07 | 0.950 | 0.039 |
| BA/GAC pH = 4 | GF | 10.49 | 0.91 | 1 | 0.02 | 0.964 | 0.052 |
| BA/GAC pH = 6 | GF | 6.86 | 0.88 | 1 | −0.23 | 0.991 | 0.018 |
| 2-NBA/GAC pH = 2 | GF | 6.59 | 0.59 | 1 | −0.70 | 0.985 | 0.023 |
| 2-NBA/GAC pH = 4 | T | 5.86 | 1 | 0.11 | −0.23 | 0.995 | 0.012 |
| 2-NBA/GAC pH = 6 | T | 4.82 | 1 | 0.89 | 0.76 | 0.990 | 0.019 |
| 3-NBA/GAC pH = 2 | GF | 9.84 | 0.52 | 1 | −0.77 | 0.990 | 0.019 |
| 3-NBA/GAC pH = 4 | T | 6.74 | 1 | 0.40 | 6.34 | 0.955 | 0.043 |
| 3-NBA/GAC pH = 6 | T | 5.47 | 1 | 0.85 | −0.03 | 0.994 | 0.013 |
| 4-NBA/GAC pH = 2 | GF | 3.14 | 0.44 | 1 | −2.56 | 0.969 | 0.049 |
| 4-NBA/GAC pH = 4 | GL | 2.69 | 0.62 | 0.91 | 0.43 | 0.969 | 0.049 |
| 4-NBA/GAC pH = 6 | T | 2.03 | 1 | 0.15 | 7.59 | 0.962 | 0.056 |
| MB/GAC pH = 2 | GL | 0.17 | 0.47 | 0.27 | 0.14 | 0.979 | 0.033 |
| MB/GAC pH = 7 | GL | 0.22 | 0.43 | 0.94 | 1.15 | 0.990 | 0.019 |
| MB/GAC pH = 12 | T | 0.29 | 1 | 0.31 | 4.20 | 0.932 | 0.096 |
| System | m-exp [%] | FOE [%] | SOE [%] | MOE [%] | f-FOE [%] | f-SOE [%] | f-MOE [%] |
|---|---|---|---|---|---|---|---|
| BA/GAC pH = 2 | 0.100 | 2.348 | 0.331 | 0.089 | 0.861 | 0.217 | 0.218 |
| BA/GAC pH = 4 | 0.076 | 2.877 | 0.272 | 0.273 | 0.969 | 0.129 | 0.130 |
| BA/GAC pH = 6 | 0.163 | 2.106 | 0.965 | 0.301 | 0.519 | 0.736 | 0.739 |
| 2-NBA/GAC pH = 2 | 0.188 | 0.957 | 4.742 | 0.258 | 0.306 | 1.722 | 20.045 |
| 2-NBA/GAC pH = 4 | 0.174 | 2.183 | 1.574 | 0.381 | 0.977 | 0.477 | 18.709 |
| 2-NBA/GAC pH = 6 | 0.181 | 2.465 | 1.197 | 1.203 | 0.696 | 0.397 | 0.399 |
| 3-NBA/GAC pH = 2 | 0.437 | 0.860 | 4.097 | 0.435 | 0.545 | 1.225 | 17.176 |
| 3-NBA/GAC pH = 4 | 0.120 | 0.782 | 5.372 | 0.117 | 0.233 | 2.025 | 19.184 |
| 3-NBA/GAC pH = 6 | 0.159 | 3.940 | 0.788 | 0.792 | 0.876 | 0.633 | 0.164 |
| 4-NBA/GAC pH = 2 | 0.175 | 0.418 | 5.854 | 0.186 | 0.266 | 2.040 | 2.053 |
| 4-NBA/GAC pH = 4 | 0.083 | 0.765 | 5.602 | 0.106 | 0.150 | 2.225 | 19.236 |
| 4-NBA/GAC pH = 6 | 0.169 | 3.246 | 1.490 | 0.324 | 0.821 | 0.958 | 0.944 |
| MB/GAC pH = 2 | 0.344 | 1.180 | 4.797 | 0.704 | 0.862 | 1.225 | 1.228 |
| MB/GAC pH = 7 | 0.166 | 3.964 | 2.034 | 1.801 | 1.535 | 2.751 | 2.765 |
| MB/GAC pH = 12 | 0.298 | 0.308 | 5.832 | 0.295 | 2.085 | 4.051 | 2.914 |
| System | f1 a, log k1 b | f2 a, log k2 b | f3 a, log k3 b | ueq c | t1/2 d [min] | SD(c)/co e [%] | 1 − R2 f |
|---|---|---|---|---|---|---|---|
| BA/GAC pH = 2 | 0.306; −1.90 | 0.476; −2.44 | 0.218; −3.05 | 0.712 | 96.7 | 0.100 | 1.9 × 10−5 |
| BA/GAC pH = 4 | 0.240; −1.80 | 0.487; −2.39 | 0.273; −3.09 | 0.739 | 169.0 | 0.076 | 1.1 × 10−5 |
| BA/GAC pH = 6 | 0.137; −1.33 | 0.579; −2.09 | 0.284; −2.7 | 0.739 | 257.8 | 0.163 | 5.5 × 10−5 |
| 2-NBA/GAC pH = 2 | 0.112; −1.82 | 0.846; −2.41 | 0.041; −3.43 | 1 | 144.0 | 0.188 | 3.2 × 10−5 |
| 2-NBA/GAC pH = 4 | 0.324; −1.95 | 0.509; −2.41 | 0.167; −2.98 | 0.962 | 160.6 | 0.174 | 3.2 × 10−5 |
| 2-NBA/GAC pH = 6 | 0.248; −1.76 | 0.403; −2.58 | 0.349; −3.56 | 0.456 | 310.0 | 0.181 | 2.1 × 10−4 |
| 3-NBA/GAC pH = 2 | 0.218; −1.83 | 0.782; −2.25 | - | 0.957 | 99.2 | 0.437 | 2.1 × 10−3 |
| 3-NBA/GAC pH = 4 | 0.046; −1.69 | 0.439; −2.16 | 0.515; −2.45 | 0.994 | 132.9 | 0.120 | 1.3 × 10−5 |
| 3-NBA/GAC pH = 6 | 0.129; −1.78 | 0.463; −2.48 | 0.408; −3.19 | 0.962 | 311.9 | 0.159 | 2.5 × 10−5 |
| 4-NBA/GAC pH = 2 | 0.319; −2.27 | 0.681; −2.56 | - | 0.996 | 133.5 | 0.175 | 2.9 × 10−5 |
| 4-NBA/GAC pH = 4 | 0.01; −1.03 | 0.238; −2.01 | 0.752; −2.39 | 0.997 | 199.9 | 0.083 | 6.3 × 10−6 |
| 4-NBA/GAC pH = 6 | 0.185; −1.91 | 0.508; −2.52 | 0.307; −3.11 | 0.982 | 249.3 | 0.169 | 2.7 × 10−5 |
| MB/GAC pH = 2 | 0.731; −2.53 | 0.269; −3.04 | - | 1 | 395.5 | 0.344 | 1.02 × 10−4 |
| MB/GAC pH = 7 | 0.099; 0.53 | 0.837; −2.88 | 0.064; −2.17 | 0.997 | 307.2 | 0.166 | 3.51 × 10−5 |
| MB/GAC pH = 12 | 0.017; −2.1 | 0.983; −2.58 | - | 0.992 | 260.2 | 0.298 | 7.54 × 10−5 |
| System | f2 a | log k1 b | ueq c | t1/2 d [min] | SD(c)/co e [%] | 1 − R2 f |
|---|---|---|---|---|---|---|
| BA/GAC pH = 2 | 0.884 | −3.16 | 0.713 | 97.0 | 0.089 | 1.6 × 10−5 |
| BA/GAC pH = 4 | 0.998 | −4.87 | 0.756 | 158.0 | 0.273 | 1.4 × 10−4 |
| BA/GAC pH = 6 | 0.827 | −2.78 | 0.738 | 181.3 | 0.301 | 1.9 × 10−4 |
| 2-NBA/GAC pH = 2 | 0.355 | −2.49 | 0.981 | 140.0 | 0.258 | 3.2 × 10−5 |
| 2-NBA/GAC pH = 4 | 0.722 | −2.76 | 0.952 | 155.6 | 0.381 | 1.6 × 10−4 |
| 2-NBA/GAC pH = 6 | 0.997 | −2.44 | 0.407 | 274.6 | 1.203 | 9.6 × 10−3 |
| 3-NBA/GAC pH = 2 | 0.302 | −2.26 | 0.958 | 99.4 | 0.435 | 2.1 × 10−3 |
| 3-NBA/GAC pH = 4 | 0.302 | −2.40 | 0.992 | 132.6 | 0.117 | 1.4 × 10−5 |
| 3-NBA/GAC pH = 6 | 0.997 | −5.0 | 0.997 | 342.7 | 0.792 | 6.3 × 10−4 |
| 4-NBA/GAC pH = 2 | 0.174 | −2.52 | 0.992 | 134.0 | 0.186 | 3.3 × 10−5 |
| 4-NBA/GAC pH = 4 | 0.295 | −2.4 | 0.998 | 198.8 | 0.106 | 1.1 × 10−5 |
| 4-NBA/GAC pH = 6 | 0.889 | −3.38 | 0.992 | 254.2 | 0.324 | 1.0 × 10−4 |
| MB/GAC pH = 2 | 0.323 | −2.77 | 0.984 | 443.1 | 0.704 | 4.4 × 10−4 |
| MB/GAC pH = 7 | 0.423 | −2.99 | 1 | 304.9 | 1.801 | 7.9 × 10−5 |
| MB/GAC pH = 12 | 0.038 | −2.59 | 0.994 | 260.2 | 0.295 | 7.6 × 10−5 |
| Sample | TG | DTG | DSC | ||
|---|---|---|---|---|---|
| ΔT [°C] | Mass Loss [%] | Total Mass Loss [%] | Tmin [°C] | endo/exo | |
| GAC | 30–180 | 0.48 | 3.03 | 52 | endo |
| 180–1000 | 2.55 | 292.3 697.5 | exo exo | ||
| BA/GAC | 30–180 | 0.36 | 18.77 | 110.4 | endo |
| 180–500 | 11.02 | 299.4 | endo | ||
| 500–1000 | 7.37 | 733.6 | endo | ||
| 2-NBA/GAC | 30–180 | 0.44 | 23.39 | 119.4 | endo |
| 180–500 | 7.53 | 294.4 444.9 | exo exo | ||
| 500–1000 | 15.37 | 711.3 | exo | ||
| 3-NBA/GAC | 30–180 | 0.42 | 15.40 | 104.9 | endo |
| 180–500 | 7.69 | 339.9 444.9 | exo exo | ||
| 500–1000 | 7.25 | 719.9 | exo | ||
| 4-NBA/GAC | 30–180 | 0.38 | 17.38 | 121.3 | endo |
| 180–500 | 8.17 | 341.3 410.3 | exo exo | ||
| 500–1000 | 8.81 | 741.3 | exo |
| Sample | |||
|---|---|---|---|
| GAC | BA/GAC | 2-, 3-, 4-NBA/GAC | |
| m/z | 17 | 17 | 17 |
| 18 | 18 | 18 | |
| 44 | 39 | 30 | |
| 78 | 44 | 39 | |
| 45 | 44 | ||
| 50 | 45 | ||
| 51 | 46 | ||
| 65 | 50 | ||
| 75 | 51 | ||
| 78 | 65 | ||
| 105 | 75 | ||
| 122 | 78 | ||
| Sample | Name | Peak Position [eV] | Full Width at Half Maximum (FWHM) | Atomic Concentration [at.%] |
|---|---|---|---|---|
| 2-NBA/GAC | C 1s | 284.8 | 2.5 | 92.5 |
| N 1s | 407.1 | 2.5 | 0.8 | |
| O 1s | 533.1 | 3.4 | 6.6 | |
| 3-NBA/GAC | C 1s | 284.8 | 2.5 | 86.3 |
| N 1s | 406.3 | 2.6 | 1.5 | |
| O 1s | 533.1 | 4.6 | 12.2 | |
| 4-NBA/GAC | C 1s | 284.8 | 2.5 | 91.3 |
| N 1s | 405.6 | 2.1 | 1.2 | |
| O 1s | 532.3 | 3.8 | 7.5 |
| Sample | Name | Peak Position [eV] | Full Width at Half Maximum (FWHM) | Atomic Concentration [at.%] | Suggested Binding |
|---|---|---|---|---|---|
| 2-NBA/GAC | C 1s A | 284.5 | 0.80 | 90.6 | C=C sp2 |
| C 1s B | 285.00 | 1.24 | 0.0 | C-C/C-H | |
| C 1s C | 286.20 | 1.24 | 3.6 | C-OH/C-O-C | |
| C 1s D | 287.30 | 1.24 | 3.0 | C=O | |
| C 1s E | 288.70 | 1.24 | 2.8 | O=C-O- | |
| O 1s A | 530.32 | 1.45 | 5.1 | quinones | |
| O 1s B | 531.63 | 1.75 | 27.1 | O=C/O=C-O-R | |
| O 1s C | 533.05 | 1.64 | 51.9 | Ar-OH | |
| O 1s D | 534.76 | 1.67 | 15.9 | O=C-O-R | |
| N 1s A | 399.06 | 1.59 | 15.3 | organic matrix | |
| N 1s B | 401.12 | 1.59 | 18.0 | organic matrix | |
| N 1s C | 402.75 | 1.58 | 7.6 | N-oxide | |
| N 1s D | 405.93 | 1.48 | 59.1 | nitro group | |
| 3-NBA/GAC | C 1s A | 284.50 | 0.78 | 90.8 | C=C sp2 |
| C 1s B | 285.00 | 1.32 | 0.0 | C-C/C-H | |
| C 1s C | 286.20 | 1.32 | 4.0 | C-OH/C-O-C | |
| C 1s D | 287.30 | 1.32 | 2.2 | C=O | |
| C 1s E | 288.70 | 1.32 | 3.0 | O=C-O- | |
| O 1s A | 530.14 | 1.75 | 11.3 | quinones | |
| O 1s B | 531.69 | 1.75 | 27.6 | O=C/O=C-O-R | |
| O 1s C | 533.08 | 1.72 | 50.2 | Ar-OH | |
| O 1s D | 534.65 | 1.75 | 10.9 | O=C-O-R | |
| N 1s A | 399.04 | 1.71 | 17.0 | organic matrix | |
| N 1s B | 401.06 | 1.63 | 16.4 | organic matrix | |
| N 1s C | 402.67 | 1.59 | 5.3 | N-oxide | |
| N 1s D | 406.00 | 1.52 | 61.3 | nitro group | |
| 4-NBA/GAC | C 1s A | 284.50 | 0.78 | 91.1 | C=C sp2 |
| C 1s B | 285.00 | 1.29 | 0.0 | C-C/C-H | |
| C 1s C | 286.20 | 1.29 | 3.2 | C-OH/C-O-C | |
| C 1s D | 287.30 | 1.29 | 2.4 | C=O | |
| C 1s E | 288.80 | 1.29 | 3.2 | O=C-O- | |
| O 1s A | 530.24 | 1.47 | 7.8 | quinones | |
| O 1s B | 531.75 | 1.75 | 30.5 | O=C/O=C-O-R | |
| O 1s C | 533.03 | 1.75 | 47.4 | Ar-OH | |
| O 1s D | 534.64 | 1.72 | 14.3 | O=C-O-R | |
| N 1s A | 399.24 | 1.80 | 15.9 | organic matrix | |
| N 1s B | 401.06 | 1.72 | 19.4 | organic matrix | |
| N 1s C | 402.66 | 1.64 | 8.0 | N-oxide | |
| N 1s D | 405.92 | 1.60 | 56.7 | nitro group |
| Adsorbate | Structural Formula | M a [g/mol] | cs b [g/L] | pKa c | m.p. d [°C] | b.p. e [°C] | Chemical Safety |
|---|---|---|---|---|---|---|---|
| Benzoic acid |  | 122.12 | 2.9 | 4.2 | 122.4 | 249.2 | Corrosive Health Hazard |
| 2-Nitrobenzoic acid |  | 167.12 | 6.8 | 2.17 | 147.5 | 295.7 | Irritant |
| 3-Nitrobenzoic acid | 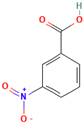 | 167.12 | 3.0 | 3.45 | 140–141 | 295.7 | Irritant |
| 4-Nitrobenzoic acid | 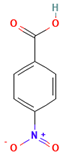 | 167.12 | 0.42 | 3.44 | 237–240 | 295.7 | Irritant |
| Methylene blue |  | 319.85 | 43.6 | >12 | 100–110 | - | Corrosive Irritant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewska, M.; Derylo-Marczewska, A.; Marczewski, A.W. Equilibrium and Kinetic Studies on Adsorption of Neutral and Ionic Species of Organic Adsorbates from Aqueous Solutions on Activated Carbon. Molecules 2024, 29, 3032. https://doi.org/10.3390/molecules29133032
Wasilewska M, Derylo-Marczewska A, Marczewski AW. Equilibrium and Kinetic Studies on Adsorption of Neutral and Ionic Species of Organic Adsorbates from Aqueous Solutions on Activated Carbon. Molecules. 2024; 29(13):3032. https://doi.org/10.3390/molecules29133032
Chicago/Turabian StyleWasilewska, Małgorzata, Anna Derylo-Marczewska, and Adam W. Marczewski. 2024. "Equilibrium and Kinetic Studies on Adsorption of Neutral and Ionic Species of Organic Adsorbates from Aqueous Solutions on Activated Carbon" Molecules 29, no. 13: 3032. https://doi.org/10.3390/molecules29133032
APA StyleWasilewska, M., Derylo-Marczewska, A., & Marczewski, A. W. (2024). Equilibrium and Kinetic Studies on Adsorption of Neutral and Ionic Species of Organic Adsorbates from Aqueous Solutions on Activated Carbon. Molecules, 29(13), 3032. https://doi.org/10.3390/molecules29133032








