Effects of Trimethylamine N-Oxide in Improving Exercise Performance in Mice: A 1H-NMR-Based Metabolomic Analysis Approach
Abstract
1. Introduction
2. Results
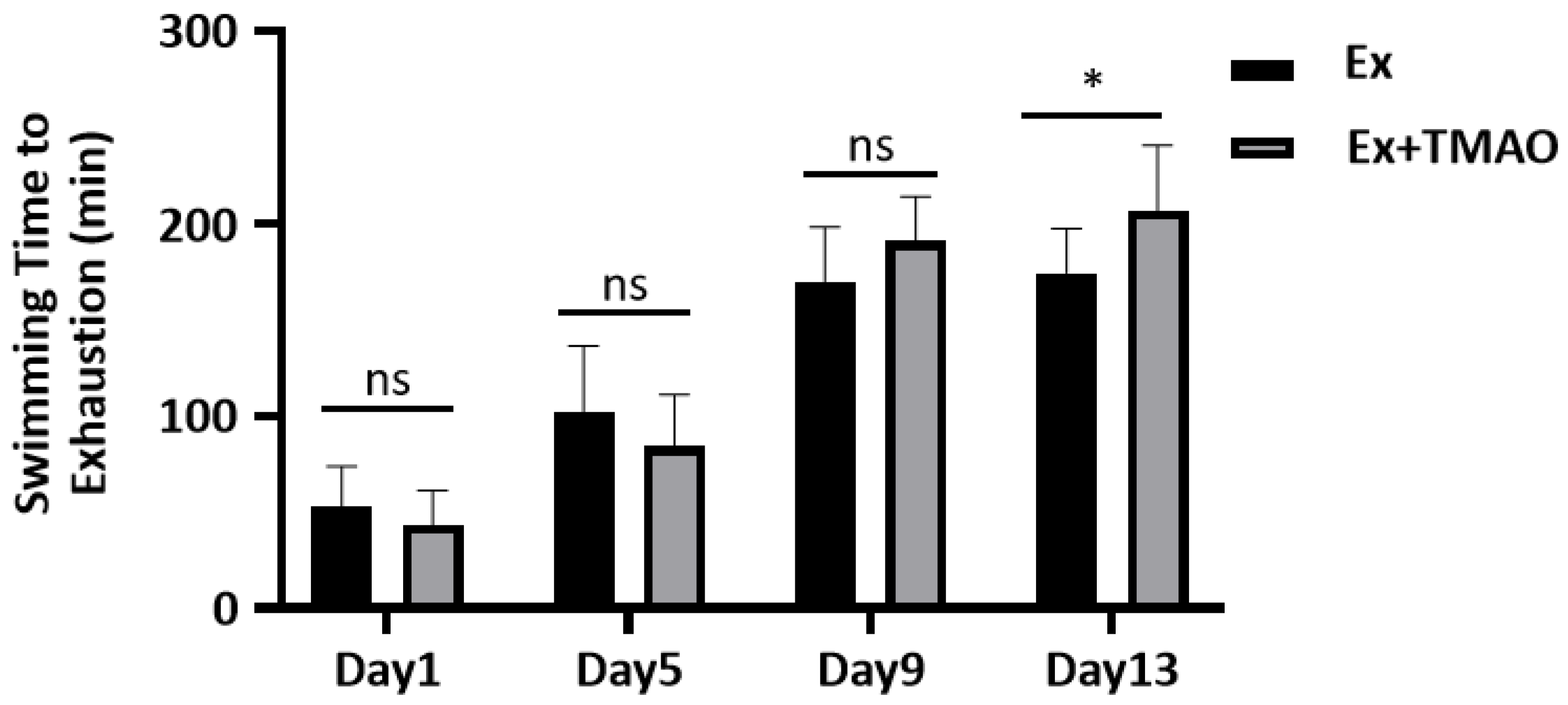
2.1. Effect of TMAO on the Swimming Exhaustive Time
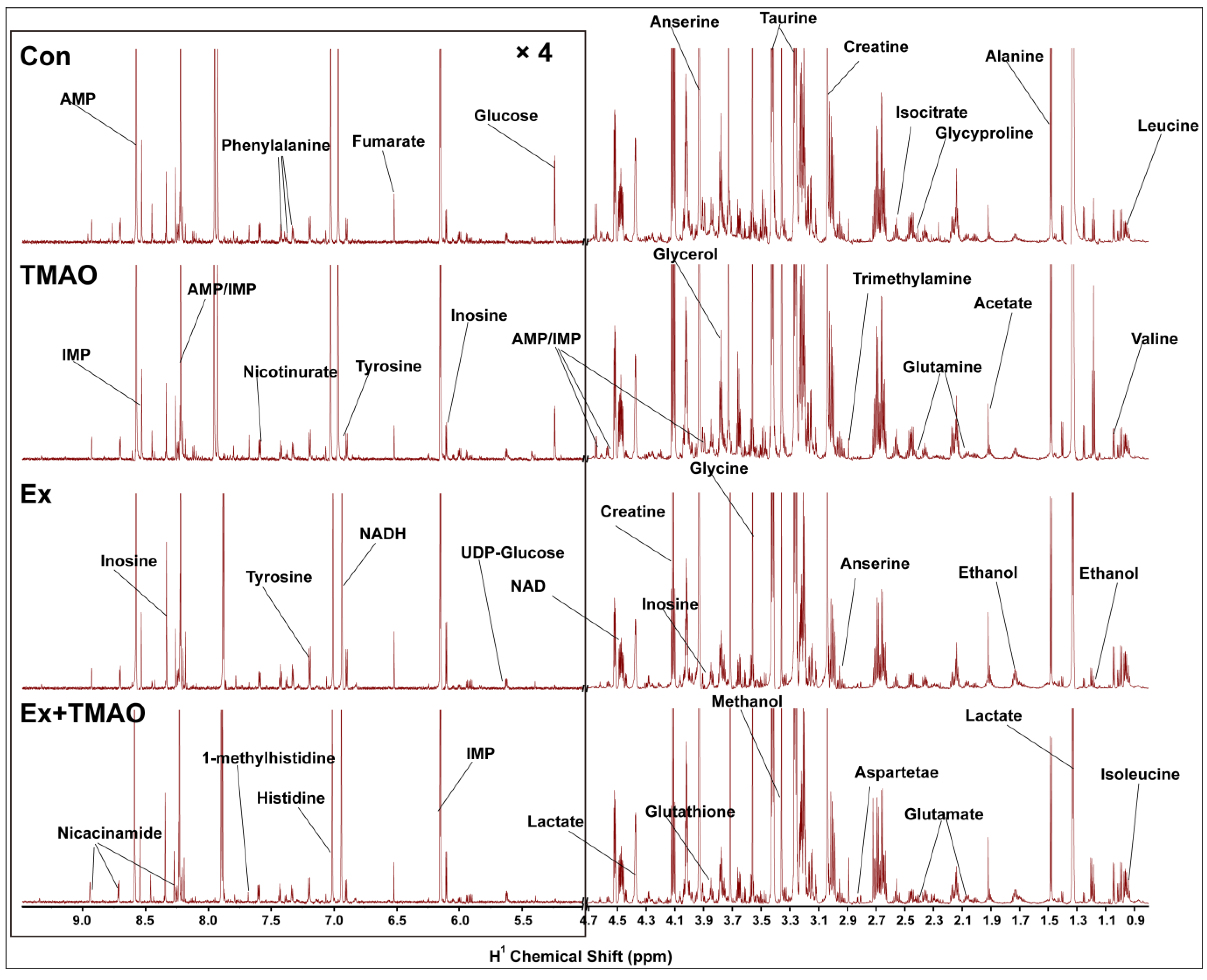
2.2. NMR Spectra of Hydrophilic Metabolites Extracted from Skeletal Muscle
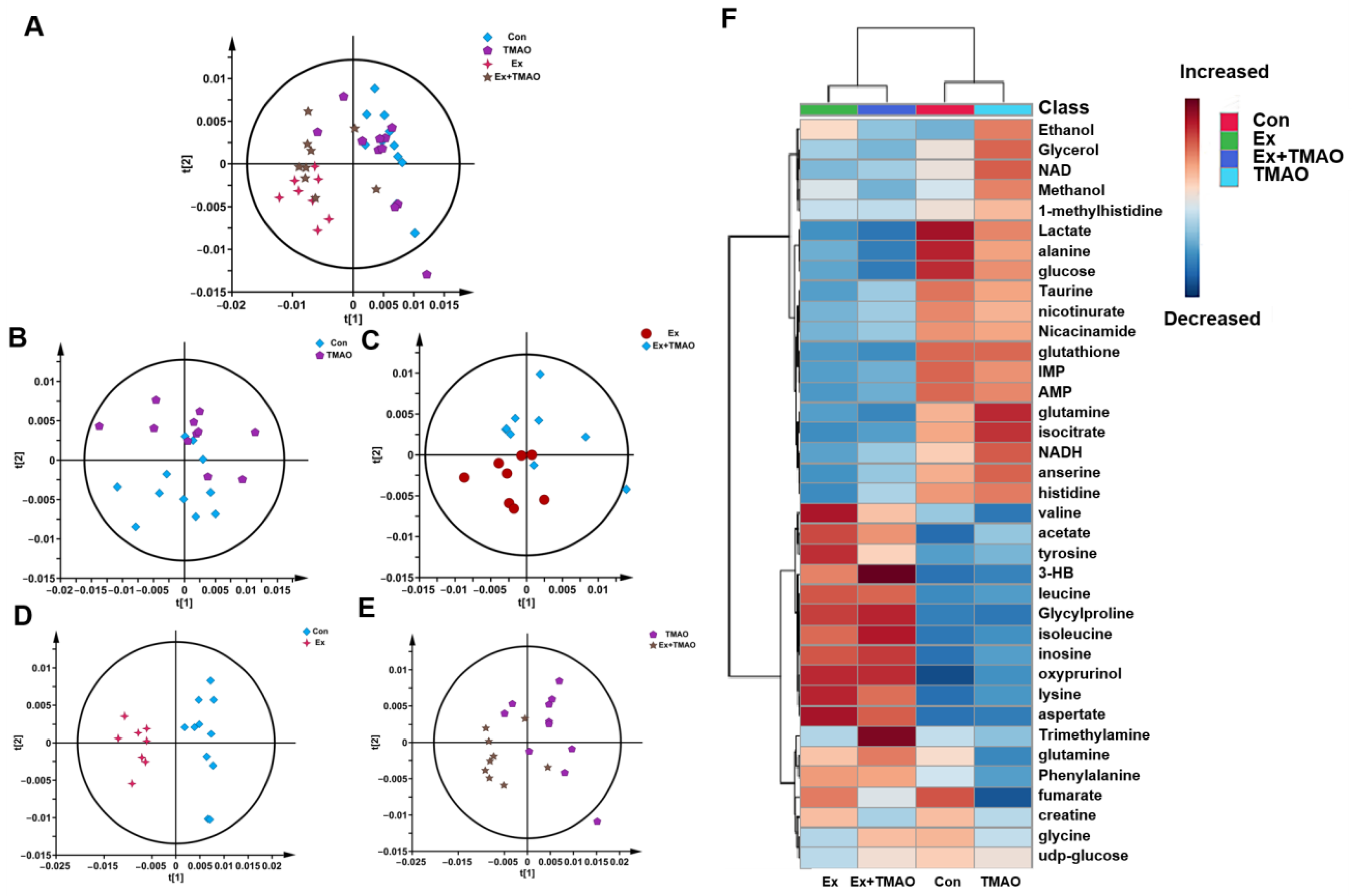
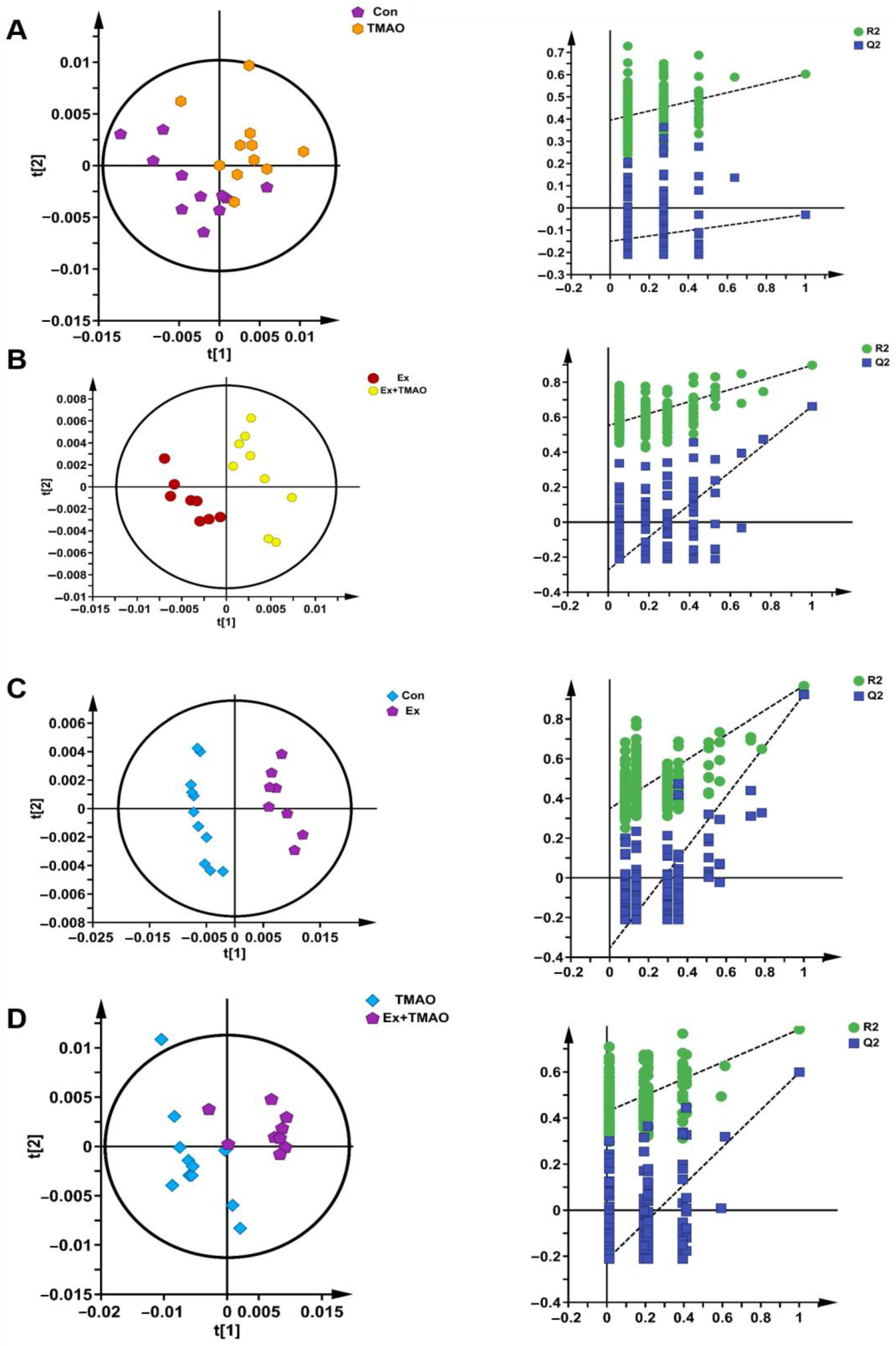
2.3. Multivariate Statistical Analysis for NMR Spectra of Metabolites Extracted from Skeletal Muscle
2.4. Identifications of Differential Metabolites of Aqueous Extracts Derived from Skeletal Muscle of Mice
2.5. Identifications of Important Metabolites of Aqueous Extracts Derived from Skeletal Muscle of Mice
2.6. Identifications of Characteristic Metabolites of Aqueous Extracts Derived from Skeletal Muscle of Mice
2.7. Identifications of Significantly Altered Metabolic Pathways of Aqueous Extracts Derived from Skeletal Muscle of Mice
3. Discussion
4. Materials and Methods
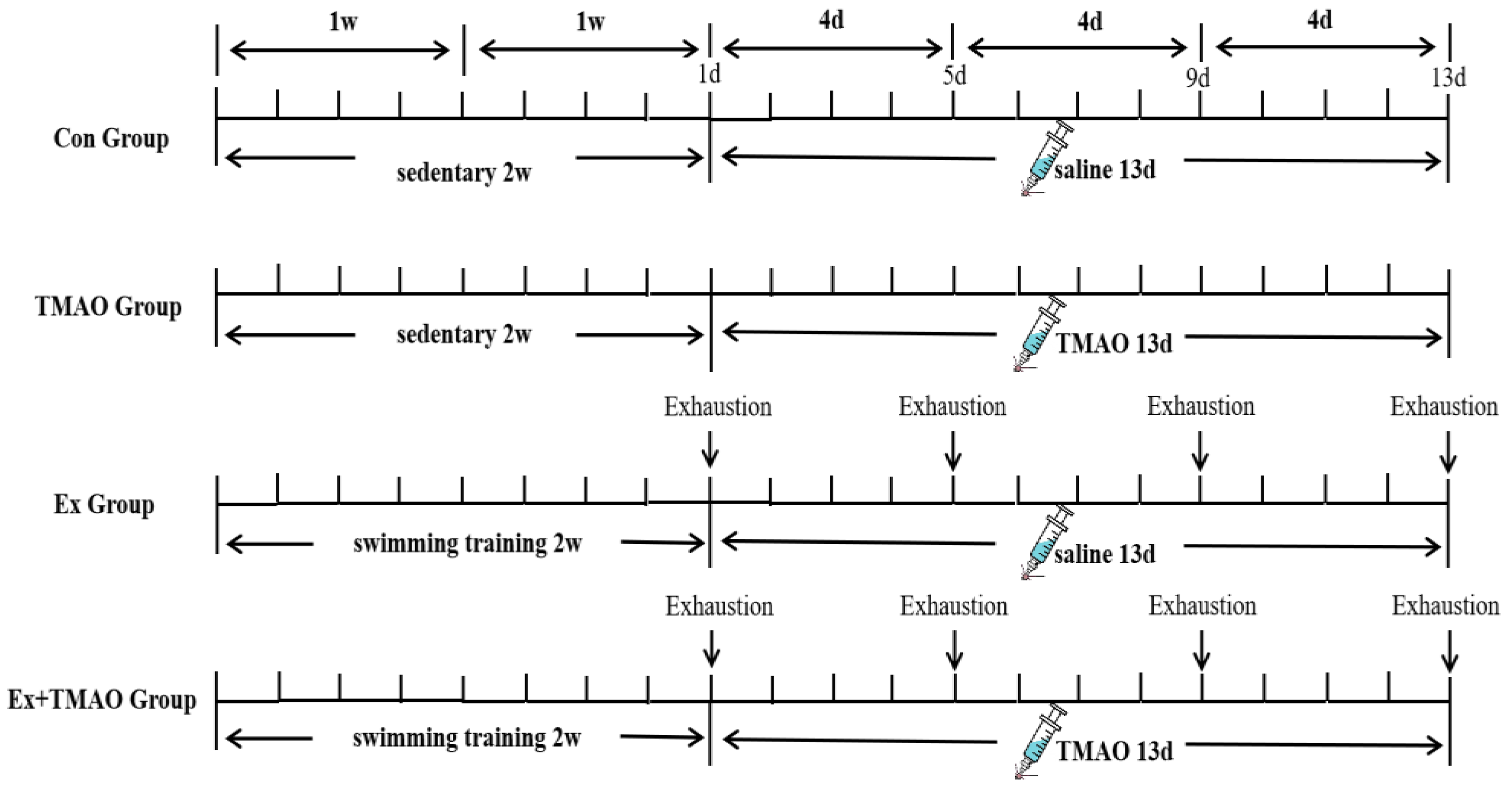
4.1. Animals and Ethics Statement
4.2. Swimming Exhaustive Test Protocol
4.3. TMAO Administration
4.4. Samples Collection
4.5. Extraction of Mice Skeletal Muscle’s Aqueous Metabolites
4.6. Preparation for NMR Sample
4.7. Measurements of Nuclear Magnetic Resonance Spectroscopy
4.8. Preprocess and Analysis of NMR Spectra
4.9. Metabolic Pathway Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Bottinelli, R.; Reggiani, C. Human skeletal muscle fibres: Molecular and functional diversity. Prog. Biophys. Mol. Biol. 2000, 73, 195–262. [Google Scholar] [CrossRef] [PubMed]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Chaki, B.; Pal, S.; Chattopadhyay, S.; Bandyopadhyay, A. High-intensity exercise-induced oxidative stress in sedentary pre-pubertal & post-pubertal boys: A comparative study. Indian J. Med. Res. 2019, 150, 167–174. [Google Scholar] [PubMed]
- Powers, S.K.; Nelson, W.B.; Hudson, M.B. Exercise-induced oxidative stress in humans: Cause and consequences. Free Radic. Biol. Med. 2011, 51, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Peternelj, T.T.; Coombes, J.S. Antioxidant supplementation during exercise training: Beneficial or detrimental? Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Wang, S.H.; Huang, C.C.; Lai, Y.C.; Song, T.Y.; Tsai, M.S. Anti-Fatigue, Antioxidation, and Anti-Inflammatory Effects of Eucalyptus Oil Aromatherapy in Swimming-Exercised Rats. Chin. J. Physiol. 2018, 61, 257–265. [Google Scholar] [CrossRef]
- Mastaloudis, A.; Morrow, J.D.; Hopkins, D.W.; Devaraj, S.; Traber, M.G. Antioxidant supplementation prevents exercise-induced lipid peroxidation, but not inflammation, in ultramarathon runners. Free Radic. Biol. Med. 2004, 36, 1329–1341. [Google Scholar] [CrossRef]
- Powers, S.K.; DeRuisseau, K.C.; Quindry, J.; Hamilton, K.L. Dietary antioxidants and exercise. J. Sports Sci. 2004, 22, 81–94. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Maisto, M.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: Also a matter of sex? Nutrition 2019, 62, 7–17.e5. [Google Scholar] [CrossRef]
- Chen, S.; Henderson, A.; Petriello, M.C.; Romano, K.A.; Gearing, M.; Miao, J.; Schell, M.; Sandoval-Espinola, W.J.; Tao, J.; Sha, B.; et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab. 2019, 30, 1141–1151. [Google Scholar] [CrossRef]
- Woltjer, R.L.; McMahan, W.; Milatovic, D.; Kjerulf, J.D.; Shie, F.S.; Rung, L.G.; Montine, K.S.; Montine, T.J. Effects of chemical chaperones on oxidative stress and detergent-insoluble species formation following conditional expression of amyloid precursor protein carboxy-terminal fragment. Neurobiol. Dis. 2007, 25, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Lupachyk, S.; Watcho, P.; Stavniichuk, R.; Shevalye, H.; Obrosova, I.G. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes 2013, 62, 944–952. [Google Scholar] [CrossRef]
- Zou, H.; Huang, C.; Zhou, L.; Lu, R.; Zhang, Y.; Lin, D. NMR-Based Metabolomic Analysis for the Effects of Trimethylamine N-Oxide Treatment on C2C12 Myoblasts under Oxidative Stress. Biomolecules 2022, 12, 1288. [Google Scholar] [CrossRef]
- Stella, C.; Beckwith-Hall, B.; Cloarec, O.; Holmes, E.; Lindon, J.C.; Powell, J.; van der Ouderaa, F.; Bingham, S.; Cross, A.J.; Nicholson, J.K. Susceptibility of human metabolic phenotypes to dietary modulation. J. Proteome Res. 2006, 5, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Slupsky, C.M.; Heckmann, A.B.; Christensen, B.; Peng, Y.M.; Li, X.N.; Hernell, O.; Lönnerdal, B.; Li, Z.L. Milk Fat Globule Membrane as a Modulator of Infant Metabolism and Gut Microbiota: A Formula Supplement Narrowing the Metabolic Differences between Breastfed and Formula-Fed Infants. Mol. Nutr. Food Res. 2021, 65, e2000603. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, L.; Occhiuto, C.; Molonia, M.S.; Cimino, F.; Palumbo, M.; Saija, A.; Speciale, A.; Rocco, C.; Ruberto, G.; Cristani, M. A pinitol-rich Glycyrrhiza glabra L. leaf extract as functional supplement with potential in the prevention of endothelial dysfunction through improving insulin signalling. Arch. Physiol. Biochem. 2022, 128, 1225–1234. [Google Scholar] [PubMed]
- Nicholson, J.K.; Wilson, I.D. Opinion: Understanding ‘global’ systems biology: Metabonomics and the continuum of metabolism. Nat. Rev. Drug Discov. 2003, 2, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Gao, Y.; Huang, C.; Lin, D. NMR-Based Metabolomic Analysis for the Effects of alpha-Ketoglutarate Supplementation on C2C12 Myoblasts in Different Energy States. Molecules 2021, 26, 1841. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Qi, Z.; Yang, L.; Huang, C.; Lin, D. Deciphering the Therapeutic Role of Lactate in Combating Disuse-Induced Muscle Atrophy: An NMR-Based Metabolomic Study in Mice. Molecules 2024, 29, 2216. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, J.; Liu, Z.; Chuang, C.C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Elkalaf, M.; Andel, M.; Trnka, J. Low glucose but not galactose enhances oxidative mitochondrial metabolism in C2C12 myoblasts and myotubes. PLoS ONE 2013, 8, e70772. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, N.S.; Shelar, S.B.; Jones, D.P.; Hoidal, J.R. Reductive stress impairs myogenic differentiation. Redox Biol. 2020, 34, 101492. [Google Scholar] [CrossRef]
- Scire, A.; Cianfruglia, L.; Minnelli, C.; Bartolini, D.; Torquato, P.; Principato, G.; Galli, F.; Armeni, T. Glutathione compartmentalization and its role in glutathionylation and other regulatory processes of cellular pathways. Biofactors 2019, 45, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Soliman, H.A.M.; Said, R.E.M.; Martyniuk, C.J.; Osman, A.G.M.; Sayed, A.E.H. Oxidative stress, antioxidant defense responses, and histopathology: Biomarkers for monitoring exposure to pyrogallol in Clarias gariepinus. J. Environ. Manag. 2024, 351, 119845. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Hoffmann, M.K.; Howard, D.; Young, C.; Washington, J.; Unterweger, H.; Alexiou, C.; Turnbull, T.; D’Andrea, R.; Hoffmann, P.; et al. Kinetic Effects of Transferrin-Conjugated Gold Nanoparticles on the Antioxidant Glutathione-Thioredoxin Pathway. Antioxidants 2023, 12, 1617. [Google Scholar] [CrossRef]
- Spriet, L.L.; Whitfield, J. Taurine and skeletal muscle function. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 96–101. [Google Scholar] [CrossRef]
- Yang, J.; Kim, M.J.; Yoon, W.; Kim, E.Y.; Kim, H.; Lee, Y.; Min, B.; Kang, K.S.; Son, J.H.; Park, H.T.; et al. Isocitrate protects null dopaminergic cells from oxidative stress through NADP—Dependent isocitrate dehydrogenase (IDH). PLoS Genet. 2017, 13, e1006975. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hossenzadeh, H. Effects of glycine on metabolic syndrome components: A review. J. Endocrinol. Investig. 2022, 45, 927–939. [Google Scholar] [CrossRef]
- Tscherner, A.K.; McClatchie, T.; Kaboba, G.; Boison, D.; Baltz, J.M. Oocyte-Specific Deletion of Slc6a9 Encoding the GLYT1 Glycine Transporter Eliminates Glycine Transport in Mouse Preimplantation Embryos and Their Ability to Counter Hypertonic Stress. Cells 2023, 12, 2500. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, W.; Zhang, Q.; Xu, J.; Liu, H.; Luo, J.; Zhan, L.; Xia, Z.; Lei, S. Glycine Protects H9C2 Cardiomyocytes from High Glucose- and Hypoxia/Reoxygenation-Induced Injury via Inhibiting PKCbeta2 Activation and Improving Mitochondrial Quality. J. Diabetes Res. 2018, 2018, 9502895. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, A.P.; Culpepper, T.; Saldivar, B.; Anton, S.; Stoll, S.; Handberg, E.M.; Xu, K.; Pepine, C.; Triplett, E.W.; Aggarwal, M. A Six-Day, Lifestyle-Based Immersion Program Mitigates Cardiovascular Risk Factors and Induces Shifts in Gut Microbiota, Specifically Lachnospiraceae, Ruminococcaceae, Faecalibacterium prausnitzii: A Pilot Study. Nutrients 2021, 13, 3459. [Google Scholar] [CrossRef]
- Shepshelovich, J.; Goldstein-Magal, L.; Globerson, A.; Yen, P.M.; Rotman-Pikielny, P.; Hirschberg, K. Protein synthesis inhibitors and the chemical chaperone TMAO reverse endoplasmic reticulum perturbation induced by overexpression of the iodide transporter pendrin. J. Cell Sci. 2005, 118, 1577–1586. [Google Scholar] [CrossRef]
- Giamberardino, M.A.; Dragani, L.; Valente, R.; Di Lisa, F.; Saggini, R.; Vecchiet, L. Effects of prolonged L-carnitine administration on delayed muscle pain and CK release after eccentric effort. Int. J. Sports Med. 1996, 17, 320–324. [Google Scholar] [CrossRef]
- Volek, J.S.; Kraemer, W.J.; Rubin, M.R.; Gomez, A.L.; Ratamess, N.A.; Gaynor, P. L-Carnitine L-tartrate supplementation favorably affects markers of recovery from exercise stress. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E474–E482. [Google Scholar] [CrossRef] [PubMed]
- Spiering, B.A.; Kraemer, W.J.; Vingren, J.L.; Hatfield, D.L.; Fragala, M.S.; Ho, J.Y.; Maresh, C.M.; Anderson, J.M.; Volek, J.S. Responses of criterion variables to different supplemental doses of L-carnitine L-tartrate. J. Strength Cond. Res. 2007, 21, 259–264. [Google Scholar] [CrossRef]
- Koozehchian, M.S.; Daneshfar, A.; Fallah, E.; Agha-Alinejad, H.; Samadi, M.; Kaviani, M.; Kaveh, B.M.; Jung, Y.P.; Sablouei, M.H.; Moradi, N.; et al. Effects of nine weeks L-Carnitine supplementation on exercise performance, anaerobic power, and exercise-induced oxidative stress in resistance-trained males. J. Exerc. Nutr. Biochem. 2018, 22, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Canlet, C.; Delplanque, B.; Agnani, G.; Lairon, D.; Gottardi, G.; Bencharif, K.; Gripois, D.; Thaminy, A.; Paris, A. 1H NMR metabonomics can differentiate the early atherogenic effect of dairy products in hyperlipidemic hamsters. Atherosclerosis 2009, 206, 127–133. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Xin, F.Z.; Zhou, D.; Xue, Y.Q.; Liu, X.L.; Yang, R.X.; Pan, Q.; Fan, J.G. Trimethylamine N-oxide attenuates high-fat high-cholesterol diet-induced steatohepatitis by reducing hepatic cholesterol overload in rats. World J. Gastroenterol. 2019, 25, 2450–2462. [Google Scholar] [CrossRef]
- Huc, T.; Drapala, A.; Gawrys, M.; Konop, M.; Bielinska, K.; Zaorska, E.; Samborowska, E.; Wyczalkowska-Tomasik, A.; Paczek, L.; Dadlez, M.; et al. Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1805–H1820. [Google Scholar] [CrossRef]
- Enea, C.; Seguin, F.; Petitpas-Mulliez, J.; Boildieu, N.; Boisseau, N.; Delpech, N.; Diaz, V.; Eugene, M.; Dugue, B. (1)H NMR-based metabolomics approach for exploring urinary metabolome modifications after acute and chronic physical exercise. Anal. Bioanal. Chem. 2010, 396, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanis, A.; Kostidis, S.; Saraslanidis, P.; Petridou, A.; Tsalis, G.; Mougios, V.; Gika, H.G.; Mikros, E.; Theodoridis, G.A. (1)H NMR-based metabonomic investigation of the effect of two different exercise sessions on the metabolic fingerprint of human urine. J. Proteome Res. 2010, 9, 6405–6416. [Google Scholar] [CrossRef]
- Pechlivanis, A.; Papaioannou, K.G.; Tsalis, G.; Saraslanidis, P.; Mougios, V.; Theodoridis, G.A. Monitoring the Response of the Human Urinary Metabolome to Brief Maximal Exercise by a Combination of RP-UPLC-MS and (1)H NMR Spectroscopy. J. Proteome Res. 2015, 14, 4610–4622. [Google Scholar] [CrossRef]
- Huang, C.C.; Lin, W.T.; Hsu, F.L.; Tsai, P.W.; Hou, C.C. Metabolomics investigation of exercise-modulated changes in metabolism in rat liver after exhaustive and endurance exercises. Eur. J. Appl. Physiol. 2010, 108, 557–566. [Google Scholar] [CrossRef]
- Miccheli, A.; Marini, F.; Capuani, G.; Miccheli, A.T.; Delfini, M.; Di Cocco, M.E.; Puccetti, C.; Paci, M.; Rizzo, M.; Spataro, A. The Influence of a Sports Drink on the Postexercise Metabolism of Elite Athletes as Investigated by NMR-Based Metabolomics. J. Am. Coll. Nutr. 2009, 28, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Duggan, G.E.; Hittel, D.S.; Sensen, C.W.; Weljie, A.M.; Vogel, H.J.; Shearer, J. Metabolomic response to exercise training in lean and diet-induced obese mice. J. Appl. Physiol. 2011, 110, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Gluick, T.C.; Yadav, S. Trimethylamine N-oxide stabilizes RNA tertiary structure and attenuates the denaturating effects of urea. J. Am. Chem. Soc. 2003, 125, 4418–4419. [Google Scholar] [CrossRef] [PubMed]
- Meersman, F.; Bowron, D.; Soper, A.K.; Koch, M.H.J. Counteraction of urea by trimethylamine N-oxide is due to direct interaction (vol 97, pg 2559, 2009). Biophys. J. 2010, 98, 174. [Google Scholar]
- Bennion, B.J.; Daggett, V. Counteraction of urea-induced protein denaturation by trimethylamine N-oxide: A chemical chaperone at atomic resolution. Proc. Natl. Acad. Sci. USA 2004, 101, 6433–6438. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Jing, Z.; Ordovas, J.; Wang, J.; Shen, L. viaAnti-fatigue and anti-oxidant effects of curcumin supplementation in exhaustive swimming mice Nrf2/Keap1 signal pathway. Curr. Res. Food Sci. 2022, 5, 1148–1157. [Google Scholar] [CrossRef]
- Hu, M.; Du, J.; Du, L.; Luo, Q.; Xiong, J. Anti-fatigue activity of purified anthocyanins prepared from purple passion fruit (P. edulis Sim) epicarp in mice. J. Funct. Foods 2020, 65, 103725. [Google Scholar] [CrossRef]


| Metabolites | Multiple Comparisons | ||
|---|---|---|---|
| Ex + TMAO vs. Ex | Ex vs. Con | Ex + TMAO vs. TMAO | |
| Leucine | ns | ↓ | ns |
| Isoleucine | ns | ↑ | ↑ |
| Valine | ↓ | ns | ns |
| Ethanol | ns | ns | ns |
| 3HB | ↑ | ↑ | ↑ |
| Alanine | ns | ↓ | ↓ |
| Lysine | ns | ↑ | ↑ |
| Acetate | ns | ↑ | ns |
| Glycylproline | ns | ↑ | ↑ |
| Glutamate | ns | ns | ns |
| Glutamine | ns | ↓ | ↓ |
| Isocitrate | ↑ | ↓ | ↓ |
| Anserine | ↑ | ↓ | ↓ |
| Aspartate | ns | ↑ | ↑ |
| TMA | ↑ | ns | ns |
| Taurine | ↑ | ↓ | ns |
| Glycine | ↑ | ↓ | ns |
| Glutathione | ↑ | ↓ | ↓ |
| Lactate | ns | ↓ | ↓ |
| NAD | ns | ns | ↓ |
| Glucose | ns | ↓ | ↓ |
| IMP | ns | ↓ | ↓ |
| Fumarate | ↓ | ns | ↑ |
| NADH | ns | ns | ↓ |
| Histidine | ns | ↓ | ↓ |
| Tyrosine | ↓ | ns | ns |
| Oxypurinol | ns | ↑ | ↑ |
| Inosine | ns | ↑ | ns |
| AMP | ns | ↓ | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, H.; Gong, L.; Wang, Z.; Huang, C.; Luo, Y.; Jia, X.; Yu, J.; Lin, D.; Zhang, Y. Effects of Trimethylamine N-Oxide in Improving Exercise Performance in Mice: A 1H-NMR-Based Metabolomic Analysis Approach. Molecules 2024, 29, 4128. https://doi.org/10.3390/molecules29174128
Zou H, Gong L, Wang Z, Huang C, Luo Y, Jia X, Yu J, Lin D, Zhang Y. Effects of Trimethylamine N-Oxide in Improving Exercise Performance in Mice: A 1H-NMR-Based Metabolomic Analysis Approach. Molecules. 2024; 29(17):4128. https://doi.org/10.3390/molecules29174128
Chicago/Turabian StyleZou, Hong, Lijing Gong, Zhiyuan Wang, Caihua Huang, Yue Luo, Xiao Jia, Jingjing Yu, Donghai Lin, and Yimin Zhang. 2024. "Effects of Trimethylamine N-Oxide in Improving Exercise Performance in Mice: A 1H-NMR-Based Metabolomic Analysis Approach" Molecules 29, no. 17: 4128. https://doi.org/10.3390/molecules29174128
APA StyleZou, H., Gong, L., Wang, Z., Huang, C., Luo, Y., Jia, X., Yu, J., Lin, D., & Zhang, Y. (2024). Effects of Trimethylamine N-Oxide in Improving Exercise Performance in Mice: A 1H-NMR-Based Metabolomic Analysis Approach. Molecules, 29(17), 4128. https://doi.org/10.3390/molecules29174128









