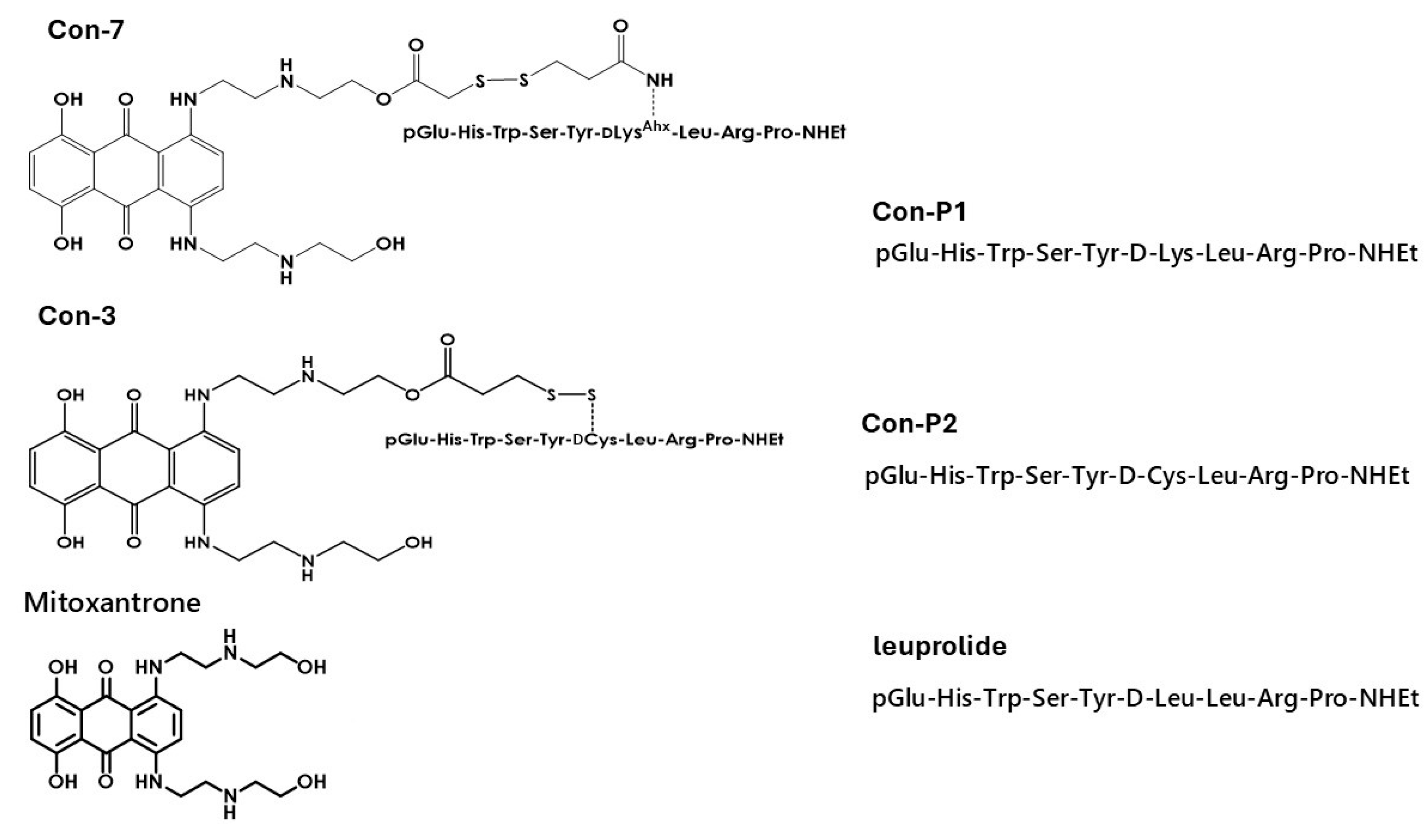
Cytotoxic Activity of Novel GnRH Analogs Conjugated with Mitoxantrone in Ovarian Cancer Cells
Abstract
1. Introduction
2. Results
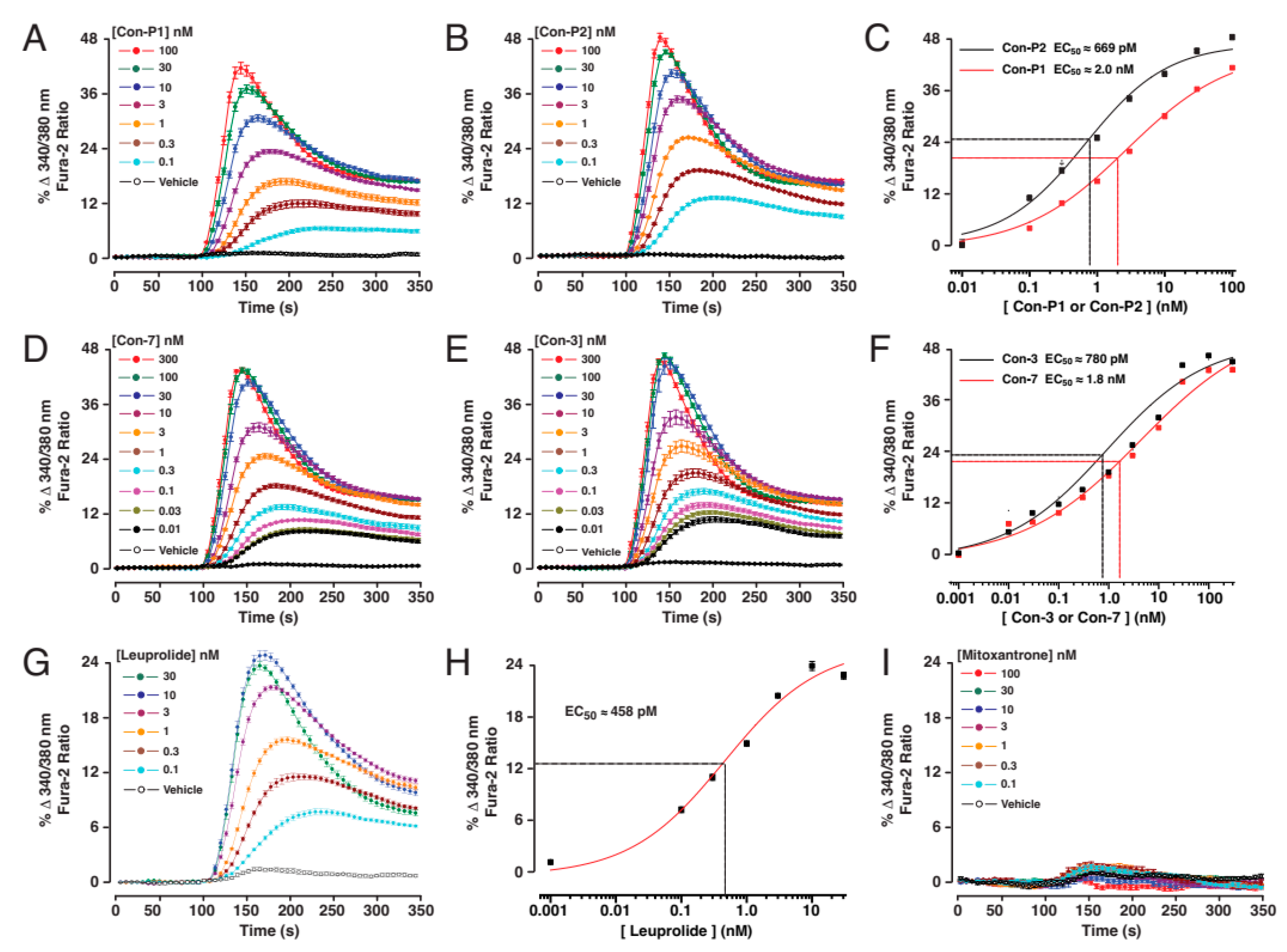
2.1. Con-3 and Con-7 Are GnRH-R Agonists
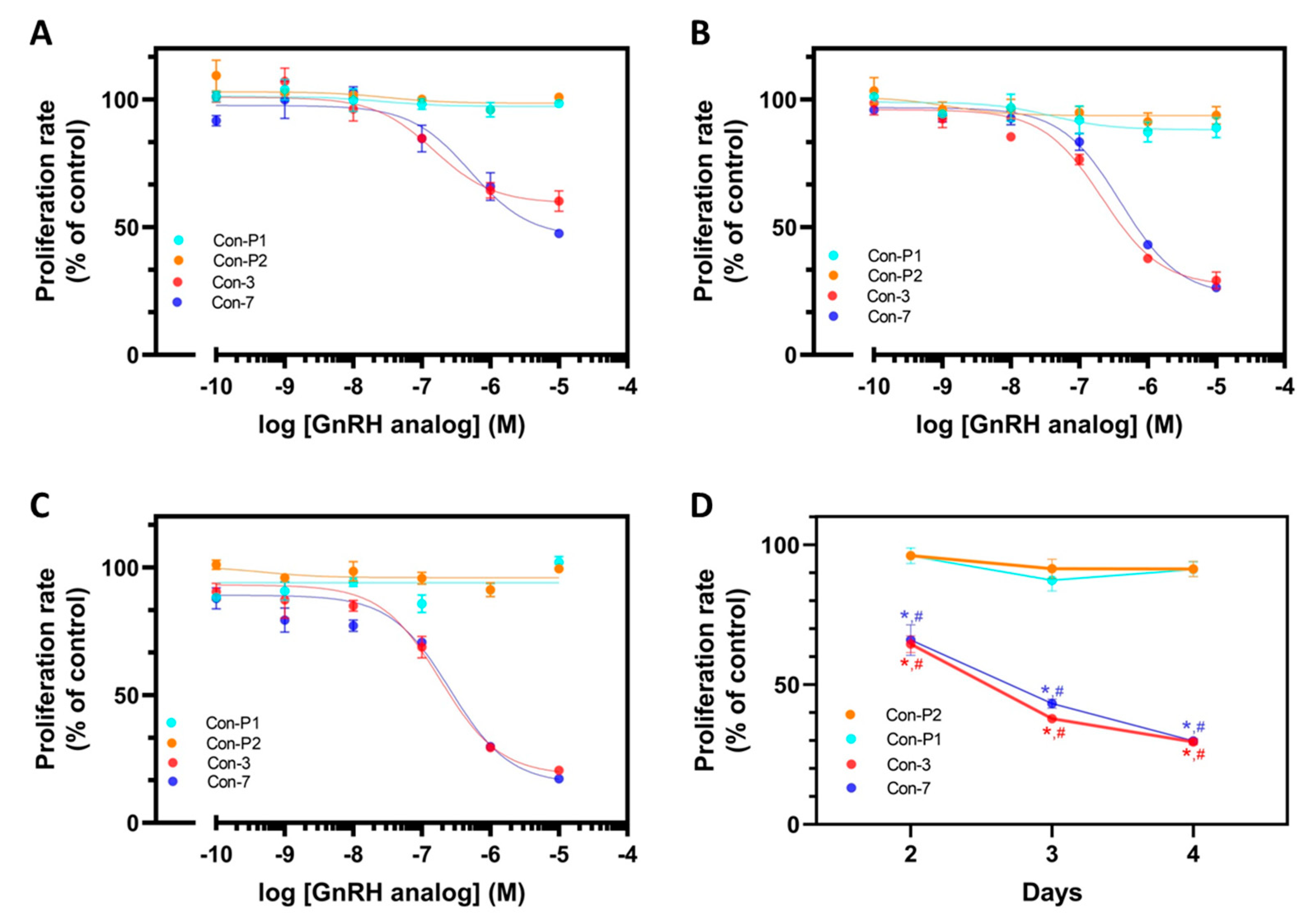
2.2. Con-3 and Con-7 Decrease the Proliferation of SKOV-3 Cells
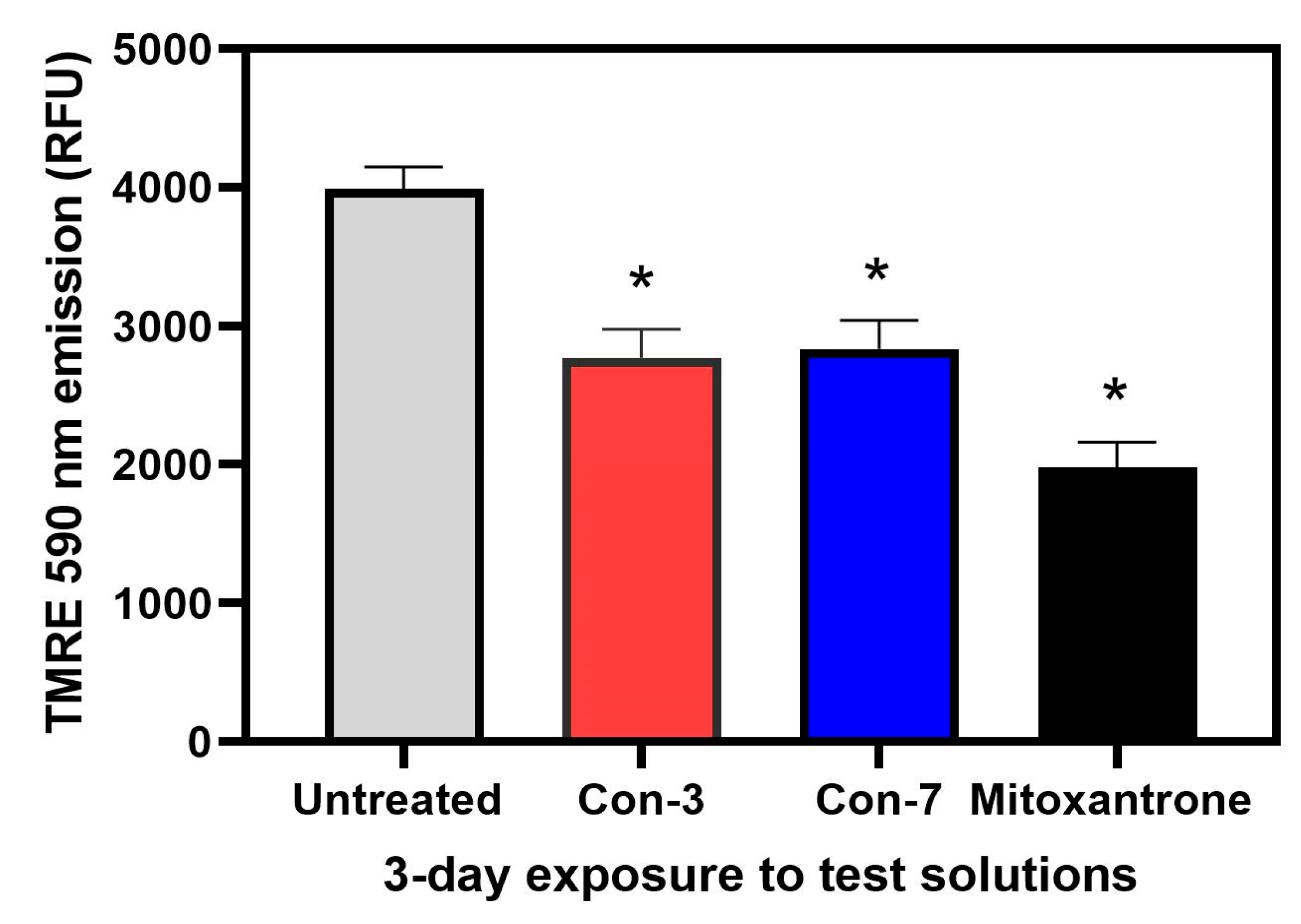
2.3. Con-3 and Con-7 Induce Apoptosis in SKOV-3 Cells
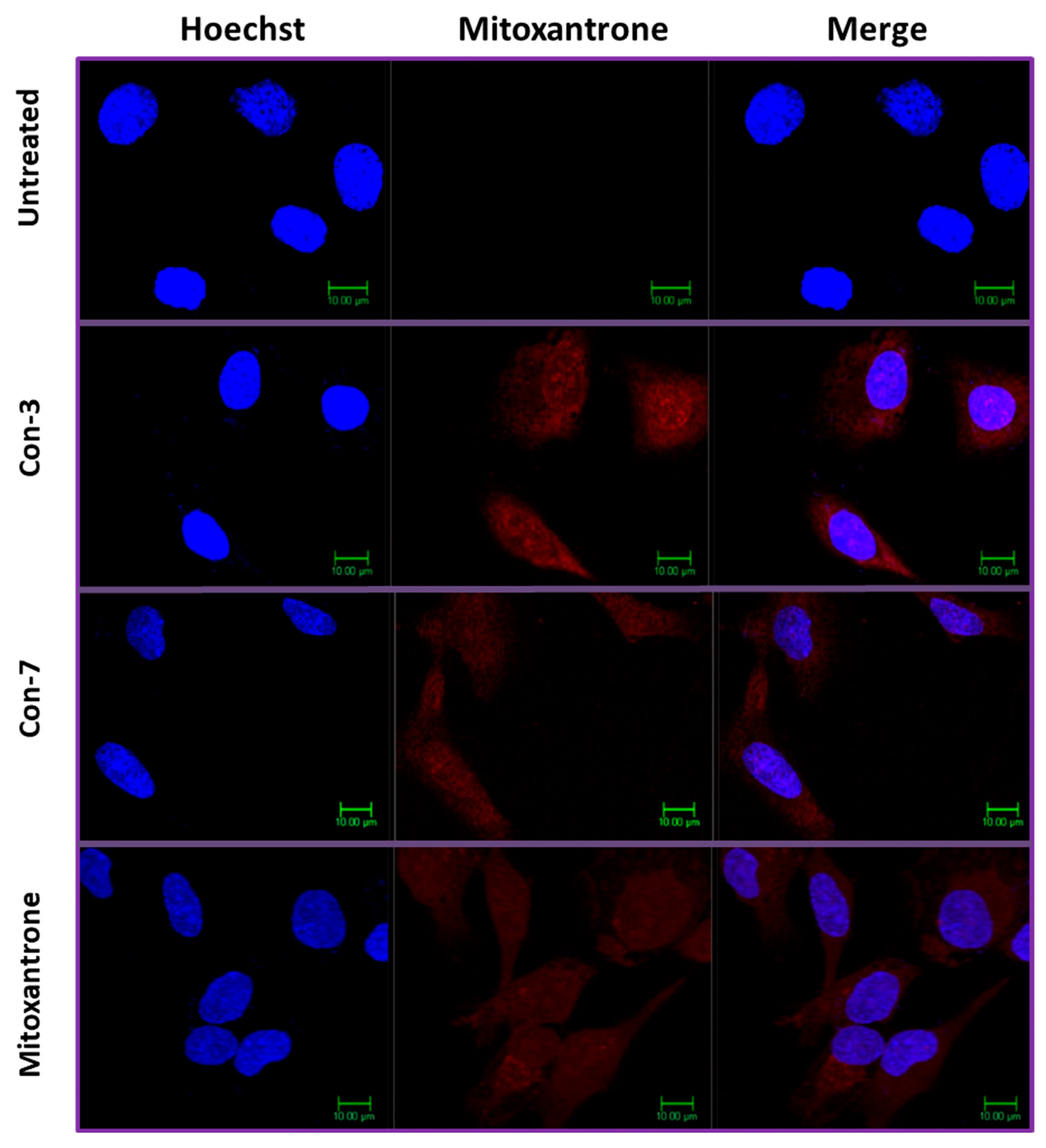
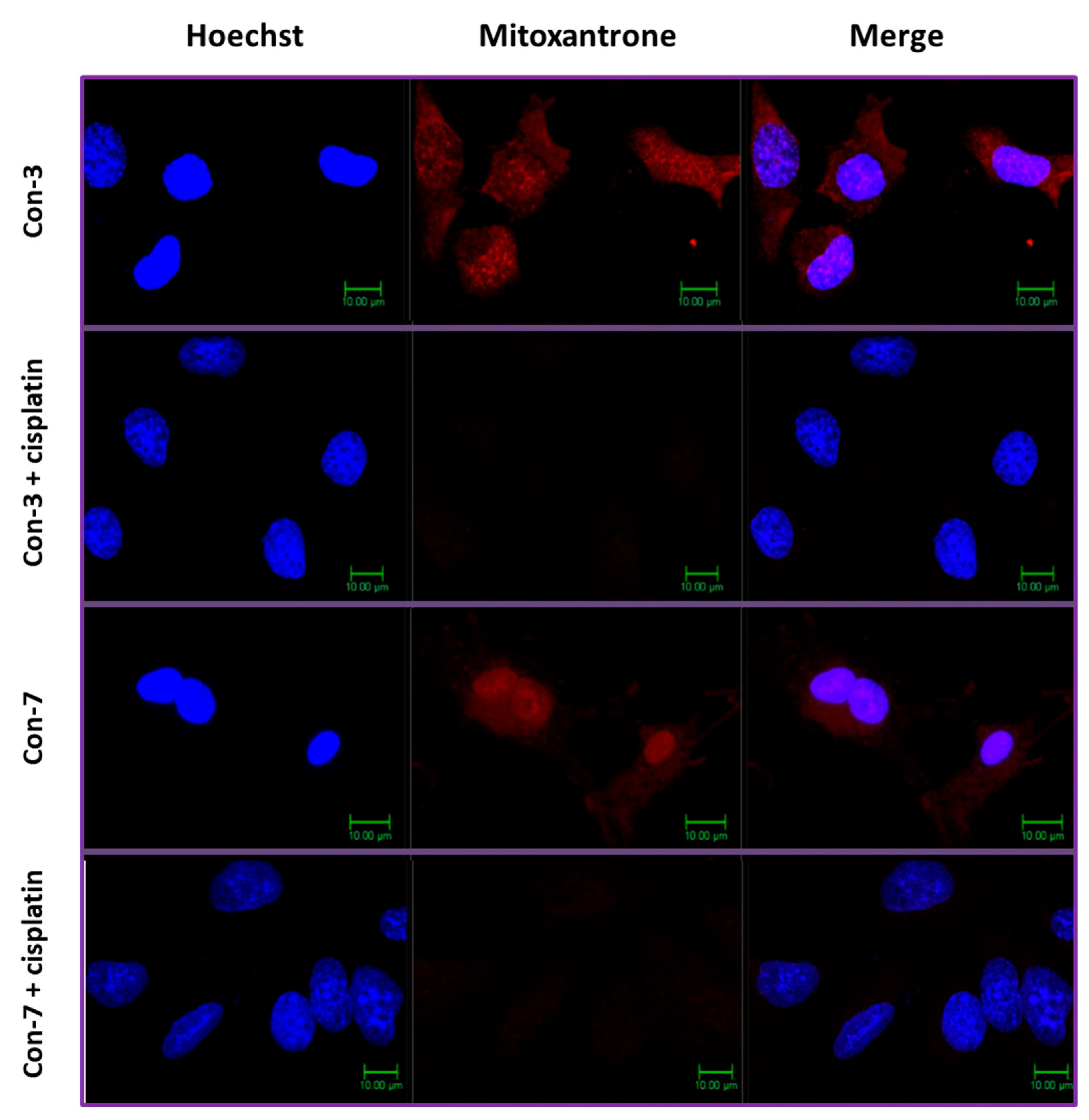
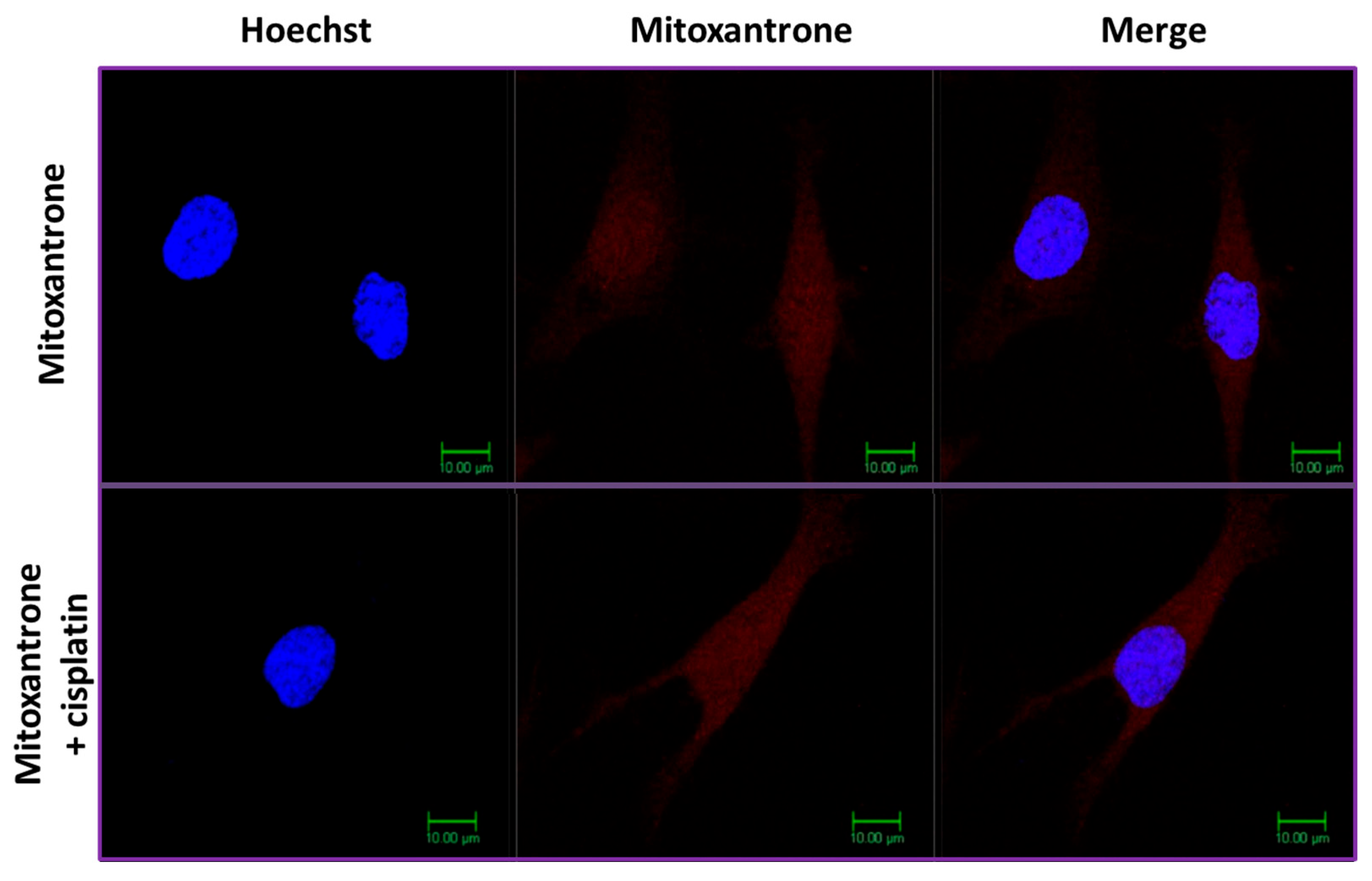
2.4. Intracellular Accumulation of Mitoxantrone after Treatment of SKOV-3 Cells with Con-3 or Con-7
2.5. Cisplatin Inhibits the Intracellular Accumulation of Mitoxantrone of Con-3 and Con-7
3. Discussion
4. Materials and Methods
4.1. Calcium-Based Kinetic Assays of GnRH-R Agonism
4.2. Cell Proliferation Assays
4.3. Cell Apoptosis Assays
4.3.1. Annexin V Assay
4.3.2. TMRE-Based Detection of Mitochondrial Membrane Potential
4.4. Confocal Microscopy
4.5. Quantification of the Fluorescence Intensity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millar, R.P.; Lu, Z.L.; Pawson, A.J.; Flanagan, C.A.; Morgan, K.; Maudsley, S.R. Gonadotropin-releasing hormone receptors. Endocr. Rev. 2004, 25, 235–275. [Google Scholar] [CrossRef] [PubMed]
- Ruf, F.; Fink, M.Y.; Sealfon, S.C. Structure of the GnRH receptor-stimulated signaling network: Insights from genomics. Front Neuroendocr. 2003, 24, 181–199. [Google Scholar] [CrossRef]
- Gründker, C.; Emons, G. Role of Gonadotropin-Releasing Hormone (GnRH) in Ovarian Cancer. Cells 2021, 10, 437. [Google Scholar] [CrossRef]
- Kang, S.K.; Choi, K.C.; Cheng, K.W.; Nathwani, P.S.; Auersperg, N.; Leung, P.C. Role of gonadotropin-releasing hormone as an autocrine growth factor in human ovarian surface epithelium. Endocrinology 2000, 141, 72–80. [Google Scholar] [CrossRef]
- Feng, Z.; Wen, H.; Bi, R.; Ju, X.; Chen, X.; Yang, W.; Wu, X. A clinically applicable molecular classification for high-grade serous ovarian cancer based on hormone receptor expression. Sci. Rep. 2016, 6, 25408. [Google Scholar] [CrossRef]
- Srkalovic, G.; Schally, A.V.; Wittliff, J.L.; Day, T.G., Jr.; Jenison, E.L. Presence and characteristics of receptors for [D-Trp6]luteinizing hormone releasing hormone and epidermal growth factor in human ovarian cancer. Int. J. Oncol. 1998, 12, 489–498. [Google Scholar] [CrossRef]
- Gründker, C.; Günthert, A.R.; Westphalen, S.; Emons, G. Biology of the gonadotropin-releasing hormone system in gynecological cancers. Eur. J. Endocrinol. 2002, 146, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Limonta, P.; Montagnani Marelli, M.; Mai, S.; Motta, M.; Martini, L.; Moretti, R.M. GnRH receptors in cancer: From cell biology to novel targeted therapeutic strategies. Endocr. Rev. 2012, 33, 784–811. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Pahwa, G.S.; Brack, C.; Sturm, R.; Oberheuser, F.; Knuppen, R. Gonadotropin releasing hormone binding sites in human epithelial ovarian carcinomata. Eur. J. Cancer Clin. Oncol. 1989, 25, 215–221. [Google Scholar] [CrossRef] [PubMed]
- So, W.K.; Cheng, J.C.; Poon, S.L.; Leung, P.C. Gonadotropin-releasing hormone and ovarian cancer: A functional and mechanistic overview. FEBS J. 2008, 275, 5496–5511. [Google Scholar] [CrossRef]
- Yin, H.; Cheng, K.W.; Hwa, H.-L.; Peng, C.; Auersperg, N.; Leung, P.C.K. Expression of the messenger RNA for gonadotropin-releasing hormone and its receptor in human cancer cell lines. Life Sci. 1998, 62, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Grundker, C.; Emons, G. The Role of Gonadotropin-Releasing Hormone in Cancer Cell Proliferation and Metastasis. Front. Endocrinol. 2017, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Women’s Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Langdon, S.P.; Crew, A.J.; Ritchie, A.A.; Muir, M.; Wakeling, A.; Smyth, J.F.; Miller, W.R. Growth inhibition of oestrogen receptor-positive human ovarian carcinoma by anti-oestrogens in vitro and in a xenograft model. Eur. J. Cancer 1994, 30, 682–686. [Google Scholar] [CrossRef]
- Langdon, S.P.; Hirst, G.L.; Miller, E.P.; Hawkins, R.A.; Tesdale, A.L.; Smyth, J.F.; Miller, W.R. The regulation of growth and protein expression by estrogen in vitro: A study of 8 human ovarian carcinoma cell lines. J. Steroid Biochem. Mol. Biol. 1994, 50, 131–135. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Wang, Y.; Zhao, X.; Qi, X. Hormone therapy for ovarian cancer: Emphasis on mechanisms and applications (Review). Oncol. Rep. 2021, 46, 223. [Google Scholar] [CrossRef]
- Li, S.; Ji, X.; Wang, R.; Miao, Y. Follicle-stimulating hormone promoted pyruvate kinase isozyme type M2-induced glycolysis and proliferation of ovarian cancer cells. Arch. Gynecol. Obstet. 2019, 299, 1443–1451. [Google Scholar] [CrossRef]
- Choi, J.H.; Wong, A.S.; Huang, H.F.; Leung, P.C. Gonadotropins and ovarian cancer. Endocr. Rev. 2007, 28, 440–461. [Google Scholar] [CrossRef]
- Limonta, P.; Moretti, R.M.; Montagnani Marelli, M.; Motta, M. The biology of gonadotropin hormone-releasing hormone: Role in the control of tumor growth and progression in humans. Front Neuroendocr. 2003, 24, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, D.C.; Kim, J.W.; Choi, Y.K.; Lew, Y.O.; Kim, D.H.; Jung, J.K.; Lim, Y.A.; Namkoong, S.E. Antitumor Effect of GnRH Agonist in Epithelial Ovarian Cancer. Gynecol. Oncol. 1999, 74, 170–180. [Google Scholar] [CrossRef]
- Chen, C.L.; Cheung, L.W.; Lau, M.T.; Choi, J.H.; Auersperg, N.; Wang, H.S.; Wong, A.S.; Leung, P.C. Differential role of gonadotropin-releasing hormone on human ovarian epithelial cancer cell invasion. Endocrine 2007, 31, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.B.; Hahne, J.C.; Häusler, S.F.; Meyer, S.; Segerer, S.E.; Diessner, J.; Dietl, J.; Honig, A. Peptidomimetic GnRH antagonist AEZS-115 inhibits the growth of ovarian and endometrial cancer cells. Anticancer Res. 2012, 32, 2063–2068. [Google Scholar]
- Zhang, N.; Qiu, J.; Zheng, T.; Zhang, X.; Hua, K.; Zhang, Y. Goserelin promotes the apoptosis of epithelial ovarian cancer cells by upregulating forkhead box O1 through the PI3K/AKT signaling pathway. Oncol. Rep. 2018, 39, 1034–1042. [Google Scholar] [CrossRef]
- Imai, A.; Ohno, T.; Ohsuye, K.; Tamaya, T. Expression of gonadotropin-releasing hormone receptor in human epithelial ovarian carcinoma. Ann. Clin. Biochem. 1994, 31 Pt 6, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Limonta, P.; Marelli, M.M.; Moretti, R.; Marzagalli, M.; Fontana, F.; Maggi, R. GnRH in the Human Female Reproductive Axis. Vitam. Horm. 2018, 107, 27–66. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Ortmann, O.; Becker, M.; Irmer, G.; Springer, B.; Laun, R.; Hölzel, F.; Schulz, K.D.; Schally, A.V. High affinity binding and direct antiproliferative effects of LHRH analogues in human ovarian cancer cell lines. Cancer Res. 1993, 53, 5439–5446. [Google Scholar]
- Irmer, G.; Bürger, C.; Müller, R.; Ortmann, O.; Peter, U.; Kakar, S.S.; Neill, J.D.; Schulz, K.D.; Emons, G. Expression of the messenger RNAs for luteinizing hormone-releasing hormone (LHRH) and its receptor in human ovarian epithelial carcinoma. Cancer Res. 1995, 55, 817–822. [Google Scholar]
- Emons, G.; Muller, V.; Ortmann, O.; Grossmann, G.; Trautner, U.; Stuckrad, B.; Schulz, K.; Schally, A. Luteinizing hormone-releasing hormone agonist triptorelin antagonizes signal transduction and mitogenic activity of epidermal growth factor in human ovarian and endometrial cancer cell lines. Int. J. Oncol. 1996, 9, 1129–1137. [Google Scholar] [CrossRef]
- Gründker, C.; Völker, P.; Emons, G.n. Antiproliferative Signaling of Luteinizing Hormone-Releasing Hormone in Human Endometrial and Ovarian Cancer Cells through G Proteinα I-Mediated Activation of Phosphotyrosine Phosphatase. Endocrinology 2001, 142, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Schally, A.V.; Armatis, P.; Szepeshazi, K.; Halmos, G.; Kovacs, M.; Zarandi, M.; Groot, K.; Miyazaki, M.; Jungwirth, A.; et al. Cytotoxic analogs of luteinizing hormone-releasing hormone containing doxorubicin or 2-pyrrolinodoxorubicin, a derivative 500-1000 times more potent. Proc. Natl. Acad. Sci. USA 1996, 93, 7269–7273. [Google Scholar] [CrossRef]
- Nagy, A.; Plonowski, A.; Schally, A.V. Stability of cytotoxic luteinizing hormone-releasing hormone conjugate (AN-152) containing doxorubicin 14-O-hemiglutarate in mouse and human serum in vitro: Implications for the design of preclinical studies. Proc. Natl. Acad. Sci. USA 2000, 97, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Nagy, A.; Schally, A.V.; Lamharzi, N.; Halmos, G.; Szepeshazi, K.; Groot, K.; Armatis, P. Growth inhibition of human ovarian cancers by cytotoxic analogues of luteinizing hormone-releasing hormone. J. Natl. Cancer Inst. 1997, 89, 1803–1809. [Google Scholar] [CrossRef]
- Gunthert, A.R.; Grundker, C.; Bongertz, T.; Schlott, T.; Nagy, A.; Schally, A.V.; Emons, G. Internalization of cytotoxic analog AN-152 of luteinizing hormone-releasing hormone induces apoptosis in human endometrial and ovarian cancer cell lines independent of multidrug resistance-1 (MDR-1) system. Am. J. Obstet. Gynecol. 2004, 191, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, S.; Kotulla, G.; Kaiser, F.; Krauss, W.; Werning, G.; Elsasser, H.P.; Nagy, A.; Schulz, K.D.; Grundker, C.; Schally, A.V.; et al. Receptor mediated antiproliferative effects of the cytotoxic LHRH agonist AN-152 in human ovarian and endometrial cancer cell lines. Int. J. Oncol. 2000, 17, 1063–1069. [Google Scholar] [CrossRef]
- Allegra, J.C.; Woodcock, T.; Woolf, S.; Henderson, I.C.; Bryan, S.; Reisman, A.; Dukart, G. A randomized trial comparing mitoxantrone with doxorubicin in patients with stage IV breast cancer. Investig. New Drugs 1985, 3, 153–161. [Google Scholar] [CrossRef]
- Wang, S.L.; Lee, J.J.; Liao, A.T. Comparison of efficacy and toxicity of doxorubicin and mitoxantrone in combination chemotherapy for canine lymphoma. Can. Vet. J. 2016, 57, 271–276. [Google Scholar]
- Biniari, G.; Markatos, C.; Nteli, A.; Tzoupis, H.; Simal, C.; Vlamis-Gardikas, A.; Karageorgos, V.; Pirmettis, I.; Petrou, P.; Venihaki, M.; et al. Rational Design, Synthesis and Binding Affinity Studies of Anthraquinone Derivatives Conjugated to Gonadotropin-Releasing Hormone (GnRH) Analogues towards Selective Immunosuppression of Hormone-Dependent Cancer. Int. J. Mol. Sci. 2023, 24, 15232. [Google Scholar] [CrossRef]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (Δψm) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Bell, D.H. Characterization of the fluorescence of the antitumor agent, mitoxantrone. Biochim. Biophys. Acta 1988, 949, 132–137. [Google Scholar] [CrossRef]
- Bucevičius, J.; Lukinavičius, G.; Gerasimaitė, R. The Use of Hoechst Dyes for DNA Staining and Beyond. Chemosensors 2018, 6, 18. [Google Scholar] [CrossRef]
- Arner, E.S.; Holmgren, A. The thioredoxin system in cancer. Semin. Cancer Biol. 2006, 16, 420–426. [Google Scholar] [CrossRef]
- Sengupta, R.; Holmgren, A. Thioredoxin and glutaredoxin-mediated redox regulation of ribonucleotide reductase. World J. Biol. Chem. 2014, 5, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, D.T.; Ali Emadi, E.M.; Tonissen, K.F.; Clarke, F.M. The thioredoxin-thioredoxin reductase system: Over-expression in human cancer. Anticancer Res. 2003, 23, 2425–2433. [Google Scholar] [PubMed]
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Saccoccia, F.; Angelucci, F.; Boumis, G.; Carotti, D.; Desiato, G.; Miele, A.E.; Bellelli, A. Thioredoxin reductase and its inhibitors. Curr. Protein Pept. Sci. 2014, 15, 621–646. [Google Scholar] [CrossRef]
- Sazonova, E.V.; Chesnokov, M.S.; Zhivotovsky, B.; Kopeina, G.S. Drug toxicity assessment: Cell proliferation versus cell death. Cell Death Discov. 2022, 8, 417. [Google Scholar] [CrossRef]
- Khalef, L.; Lydia, R.; Filicia, K.; Moussa, B. Cell viability and cytotoxicity assays: Biochemical elements and cellular compartments. Cell Biochem. Funct. 2024, 42, e4007. [Google Scholar] [CrossRef] [PubMed]
- Suliman, R.S.; Alghamdi, S.S.; Ali, R.; Rahman, I.; Alqahtani, T.; Frah, I.K.; Aljatli, D.A.; Huwaizi, S.; Algheribe, S.; Alehaideb, Z.; et al. Distinct Mechanisms of Cytotoxicity in Novel Nitrogenous Heterocycles: Future Directions for a New Anti-Cancer Agent. Molecules 2022, 27, 2409. [Google Scholar] [CrossRef]
- Preethi, S.; Kumar, H.; Rawal, V.B.; Ajmeer, R.; Jain, V. OVERVIEW OF MITOXANTRONE-A POTENTIAL CANDIDATE FOR TREATMENT OF BREAST CANCER. Int. J. Appl. Pharm. 2022, 14, 10–22. [Google Scholar] [CrossRef]
- Evison, B.J.; Sleebs, B.E.; Watson, K.G.; Phillips, D.R.; Cutts, S.M. Mitoxantrone, More than Just Another Topoisomerase II Poison. Med. Res. Rev. 2016, 36, 248–299. [Google Scholar] [CrossRef] [PubMed]
- Enache, M.; Toader, A.M.; Enache, M.I. Mitoxantrone-Surfactant Interactions: A Physicochemical Overview. Molecules 2016, 21, 1356. [Google Scholar] [CrossRef]
- Burns, C.P.; Haugstad, B.N.; Mossman, C.J.; North, J.A.; Ingraham, L.M. Membrane lipid alteration: Effect on cellular uptake of mitoxantrone. Lipids 1988, 23, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Limbird, L.E. Identification of Receptors Using Direct Radioligand Binding Techniques. In Cell Surface Receptors: A Short Course on Theory and Methods; Springer US: Boston, MA, USA, 1996; pp. 61–122. [Google Scholar]
- Hislop, J.N.; Madziva, M.T.; Everest, H.M.; Harding, T.; Uney, J.B.; Willars, G.B.; Millar, R.P.; Troskie, B.E.; Davidson, J.S.; McArdle, C.A. Desensitization and internalization of human and xenopus gonadotropin-releasing hormone receptors expressed in alphaT4 pituitary cells using recombinant adenovirus. Endocrinology 2000, 141, 4564–4575. [Google Scholar] [CrossRef][Green Version]
- Millar, R.P.; Pawson, A.J.; Morgan, K.; Rissman, E.F.; Lu, Z.L. Diversity of actions of GnRHs mediated by ligand-induced selective signaling. Front. Neuroendocr. 2008, 29, 17–35. [Google Scholar] [CrossRef]
- Vrecl, M.; Heding, A.; Hanyaloglu, A.; Taylor, P.L.; Eidne, K.A. Internalization kinetics of the gonadotropin-releasing hormone (GnRH) receptor. Pflügers Arch. 2000, 439, r019–r020. [Google Scholar] [CrossRef]
- Pawson, A.; Katz, A.; Sun, Y.; Lopes, J.; Illing, N.; Millar, R.; Davidson, J. Contrasting internalization kinetics of human and chicken gonadotropin-releasing hormone receptors mediated by C-terminal tail. J. Endocrinol. 1998, 156, R9–R12. [Google Scholar] [CrossRef] [PubMed]
- Rubartelli, A.; Bajetto, A.; Allavena, G.; Wollman, E.; Sitia, R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J. Biol. Chem. 1992, 267, 24161–24164. [Google Scholar] [CrossRef]
- Lillig, C.H.; Holmgren, A. Thioredoxin and related molecules--from biology to health and disease. Antioxidants Redox Signal. 2007, 9, 25–47. [Google Scholar] [CrossRef]
- Nakamura, H.; Masutani, H.; Yodoi, J. Extracellular thioredoxin and thioredoxin-binding protein 2 in control of cancer. Semin. Cancer Biol. 2006, 16, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Tanudji, M.; Hevi, S.; Chuck, S.L. The nonclassic secretion of thioredoxin is not sensitive to redox state. Am. J. Physiol. Physiol. 2003, 284, C1272–C1279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tapeinou, A.; Giannopoulou, E.; Simal, C.; Hansen, B.E.; Kalofonos, H.; Apostolopoulos, V.; Vlamis-Gardikas, A.; Tselios, T. Design, synthesis and evaluation of an anthraquinone derivative conjugated to myelin basic protein immunodominant (MBP(85-99)) epitope: Towards selective immunosuppression. Eur. J. Med. Chem. 2018, 143, 621–631. [Google Scholar] [CrossRef]
- Castellón, E.; Clementi, M.; Hitschfeld, C.; Sánchez, C.; Benítez, D.; Sáenz, L.; Contreras, H.; Huidobro, C. Effect of Leuprolide and Cetrorelix on Cell Growth, Apoptosis, and GnRH Receptor Expression in Primary Cell Cultures from Human Prostate Carcinoma. Cancer Investig. 2006, 24, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Montagnani Marelli, M.; Moretti, R.M.; Mai, S.; Procacci, P.; Limonta, P. Gonadotropin-releasing hormone agonists reduce the migratory and the invasive behavior of androgen-independent prostate cancer cells by interfering with the activity of IGF-I. Int. J. Oncol. 2007, 30, 261–271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ravenna, L.; Salvatori, L.; Morrone, S.; Lubrano, C.; Cardillo, M.R.; Sciarra, F.; Frati, L.; Di Silverio, F.; Petrangeli, E. Effects of triptorelin, a gonadotropin-releasing hormone agonist, on the human prostatic cell lines PC3 and LNCaP. J. Androl. 2000, 21, 549–557. [Google Scholar] [CrossRef]
- Imai, A.; Takagi, A.; Horibe, S.; Takagi, H.; Tamaya, T. Fas and Fas ligand system may mediate antiproliferative activity of gonadotropin-releasing hormone receptor in endometrial cancer cells. Int. J. Oncol. 1998, 13, 97–100. [Google Scholar] [CrossRef]
- Gründker, C.; Schulz, K.; Günthert, A.R.; Emons, G.n. Luteinizing Hormone-Releasing Hormone Induces Nuclear Factorκ B-Activation and Inhibits Apoptosis in Ovarian Cancer Cells. J. Clin. Endocrinol. Metab. 2000, 85, 3815–3820. [Google Scholar] [CrossRef][Green Version]
- Sugiyama, M.; Imai, A.; Furui, T.; Tamaya, T. Gonadotropin-releasing hormone retards doxorubicin-induced apoptosis and serine/threonine phosphatase inhibition in ovarian cancer cells. Oncol. Rep. 2005, 13, 813–817. [Google Scholar] [CrossRef]
- Kleinman, D.; Douvdevani, A.; Schally, A.V.; Levy, J.; Sharoni, Y. Direct growth inhibition of human endometrial cancer cells by the gonadotropin-releasing hormone antagonist SB-75: Role of apoptosis. Am. J. Obstet. Gynecol. 1994, 170, 96–102. [Google Scholar] [CrossRef]
- Lu, Z.L.; Gallagher, R.; Sellar, R.; Coetsee, M.; Millar, R.P. Mutations remote from the human gonadotropin-releasing hormone (GnRH) receptor-binding sites specifically increase binding affinity for GnRH II but not GnRH I: Evidence for ligand-selective, receptor-active conformations. J. Biol. Chem. 2005, 280, 29796–29803. [Google Scholar] [CrossRef] [PubMed]
- Millar, R.P.; Pawson, A.J. Outside-in and inside-out signaling: The new concept that selectivity of ligand binding at the gonadotropin-releasing hormone receptor is modulated by the intracellular environment. Endocrinology 2004, 145, 3590–3593. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T.P. Chapter 2—How Different Tissues Process Drug Response. In A Pharmacology Primer, 4th ed.; Kenakin, T.P., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 21–43. [Google Scholar]
- Patel, V.A.; Longacre, A.; Hsiao, K.; Fan, H.; Meng, F.; Mitchell, J.E.; Rauch, J.; Ucker, D.S.; Levine, J.S. Apoptotic Cells, at All Stages of the Death Process, Trigger Characteristic Signaling Events That Are Divergent from and Dominant over Those Triggered by Necrotic Cells: IMPLICATIONS FOR THE DELAYED CLEARANCE MODEL OF AUTOIMMUNITY. J. Biol. Chem. 2006, 281, 4663–4670. [Google Scholar] [CrossRef] [PubMed]
- Warnes, G. A Flow Cytometric Immunophenotyping Approach to the Detection of Regulated Cell Death Processes. J. Immunol. Sci. 2018, 2, 6–12. [Google Scholar] [CrossRef][Green Version]

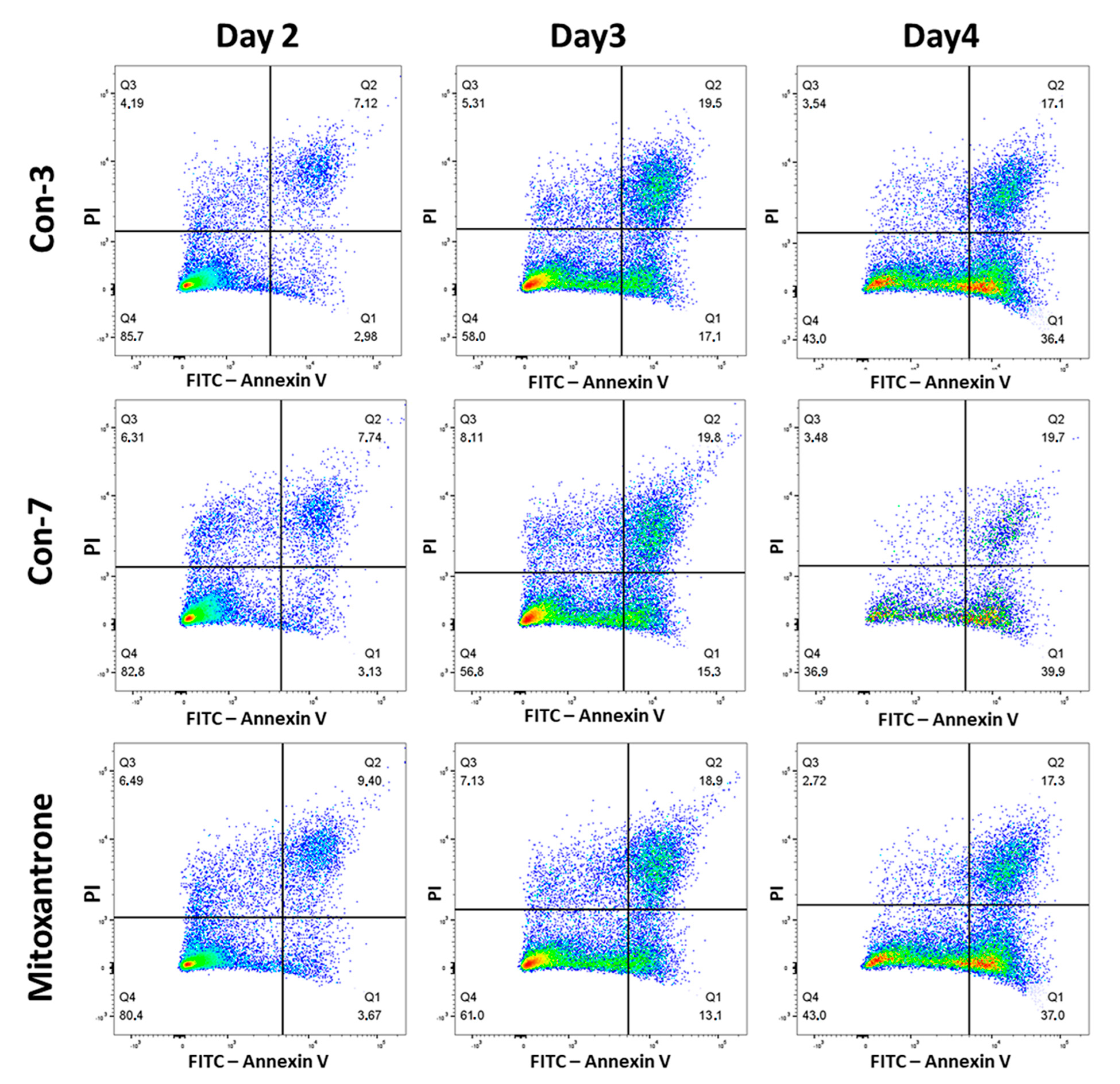
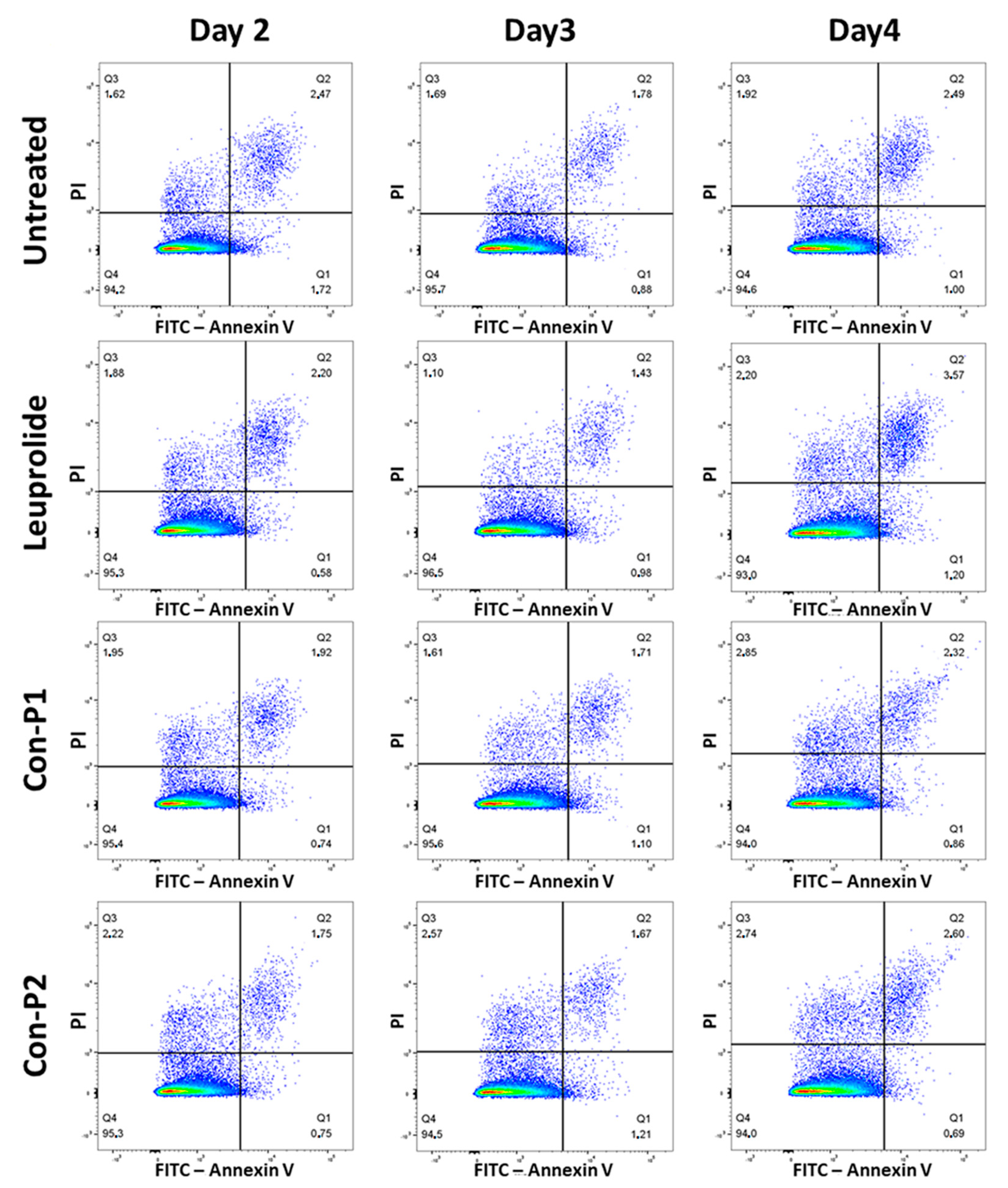
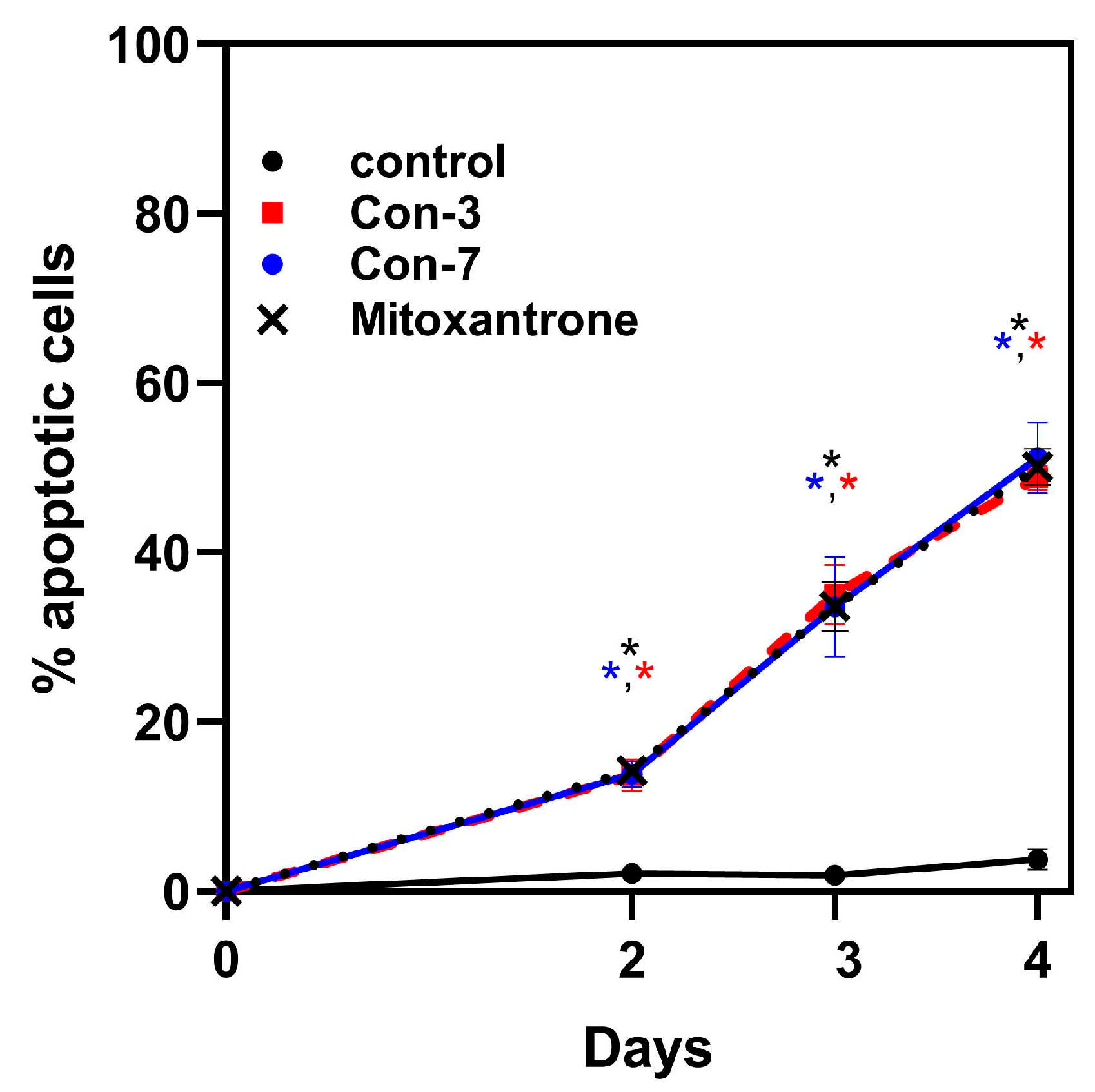
| Apoptotic Cells (%) | Control | Con-3 | Con-7 | Mitoxantrone | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Day2 | 2.12 | 0.67 | 13.68 * | 1.87 | 13.81 * | 1.58 | 14.26 * | 0.62 |
| Day3 | 1.96 | 0.35 | 35.00 * | 3.50 | 33.53 * | 5.91 | 33.60 * | 3.00 |
| Day4 | 3.69 | 0.70 | 49.50 * | 2.04 | 51.17 * | 4.22 | 50.10 * | 2.15 |
| Fluorescence Intensity | SEM | |
|---|---|---|
| Con-7 | 9738 | 398.4 |
| Con-7 + cisplatin | 3244 | 236.7 |
| Con-3 | 9015 | 243.6 |
| Con-3 + cisplatin | 2978 | 161.7 |
| Mitoxantrone | 9078 | 396.1 |
| Mitoxantrone + cisplatin | 9021 | 16.2 |
| Control | 583 | 23.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markatos, C.; Biniari, G.; Chepurny, O.G.; Karageorgos, V.; Tsakalakis, N.; Komontachakis, G.; Vlata, Z.; Venihaki, M.; Holz, G.G.; Tselios, T.; et al. Cytotoxic Activity of Novel GnRH Analogs Conjugated with Mitoxantrone in Ovarian Cancer Cells. Molecules 2024, 29, 4127. https://doi.org/10.3390/molecules29174127
Markatos C, Biniari G, Chepurny OG, Karageorgos V, Tsakalakis N, Komontachakis G, Vlata Z, Venihaki M, Holz GG, Tselios T, et al. Cytotoxic Activity of Novel GnRH Analogs Conjugated with Mitoxantrone in Ovarian Cancer Cells. Molecules. 2024; 29(17):4127. https://doi.org/10.3390/molecules29174127
Chicago/Turabian StyleMarkatos, Christos, Georgia Biniari, Oleg G. Chepurny, Vlasios Karageorgos, Nikos Tsakalakis, Georgios Komontachakis, Zacharenia Vlata, Maria Venihaki, George G. Holz, Theodore Tselios, and et al. 2024. "Cytotoxic Activity of Novel GnRH Analogs Conjugated with Mitoxantrone in Ovarian Cancer Cells" Molecules 29, no. 17: 4127. https://doi.org/10.3390/molecules29174127
APA StyleMarkatos, C., Biniari, G., Chepurny, O. G., Karageorgos, V., Tsakalakis, N., Komontachakis, G., Vlata, Z., Venihaki, M., Holz, G. G., Tselios, T., & Liapakis, G. (2024). Cytotoxic Activity of Novel GnRH Analogs Conjugated with Mitoxantrone in Ovarian Cancer Cells. Molecules, 29(17), 4127. https://doi.org/10.3390/molecules29174127







