SF6 Negative Ion Formation in Charge Transfer Experiments
Abstract
1. Introduction
2. Results
3. Discussion
3.1. SF6− Formation
3.2. SF5− and F− Formation
3.3. SF3−, F2−, and S− Formation
3.4. Energy Loss Data
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravishankara, A.R.; Solomon, S.; Turnipseed, A.A. Atmospheric Lifetimes of Long-Lived Halogenated Species. Science 1993, 259, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.A.; Miller, T.M.; Viggiano, A.A.; Paulson, J.F.; Solomon, S.; Reid, G. Effects of Electron and Ion Reactions on Atmospheric Lifetimes of Fully Fluorinated Compounds. J. Geophys. Res. 1995, 100, 1287–1294. [Google Scholar] [CrossRef]
- Mason, N.J.; Dawes, A.; Mukerji, R.; Drage, E.A.; Vasekova, E.; Webb, S.M.; Limão-Vieira, P. Atmospheric Chemistry with Synchrotron Radiation. J. Phys. B At. Mol. Opt. Phys. 2005, 38, S893–S911. [Google Scholar] [CrossRef]
- Climate Change 1995, The Science of Climate Change; Houghton, J.T., Filho, L.G.M., Callander, B.A., Harris, N., Kattenberg, A., Maskell, K., Eds.; Cambridge University Press: Cambridge, UK, 1996; ISBN 9781259584749. [Google Scholar]
- Christophorou, L.G.; Olthoff, J.K. Electron Interactions with SF6. J. Phys. Chem. Ref. Data 2000, 29, 267–330. [Google Scholar] [CrossRef]
- Braun, M.; Ruf, M.W.; Hotop, H.; Allan, M. Low-Energy Electron Attachment to SF6 Molecules: Vibrational Structure in the Cross-Section for SF5- Formation up to 1 EV. Chem. Phys. Lett. 2006, 419, 517–522. [Google Scholar] [CrossRef]
- Klar, D.; Ruf, M.W.; Hotop, H. Attachment of Electrons to Molecules at MeV Resolution. Aust. J. Phys. 1992, 45, 263–291. [Google Scholar] [CrossRef]
- Gauyacq, J.P.; Herzenberg, A. The Attachment of Very Slow Electrons to Polyatomic Molecules. J. Phys. B At. Mol. Opt. Phys. 1984, 17, 1155–1171. [Google Scholar] [CrossRef]
- Gerchikov, L.G.; Gribakin, G.F. Electron Attachment to SF6 and Lifetimes of SF 6- Negative Ions. Phys. Rev. A 2008, 77, 042724. [Google Scholar] [CrossRef]
- Matejcik, S.; Eichberger, P.; Plunger, B.; Kiendler, A.; Stamatovic, A.; Märk, T.D. Dissociative Electron Attachment to SF6: Production of SF5- at Temperatures below 300 K. Int. J. Mass Spectrom. Ion Proces. 1995, 144, L13–L17. [Google Scholar] [CrossRef]
- Odom, R.W.; Smith, D.L.; Futrell, J.H. A Study of Electron Attachment to SF6 and Auto-Detachment and Stabilization of SF6-. J. Phys. B At. Mol. Opt. Phys. 1975, 8, 1349–1366. [Google Scholar] [CrossRef]
- Smith, D.; Španěl, P.; Matejcik, S.; Stamatovic, A.; Märk, T.D.; Jaffke, T.; Illenberger, E. Formation of SF5- in Electron Attachment to SF6; Swarm and Beam Results Reconciled. Chem. Phys. Lett. 1995, 240, 481–488. [Google Scholar] [CrossRef]
- Spence, D.; Schulz, G.J. Temperature Dependence of Electron Attachment at Low Energies for Polyatomic Molecules. J. Chem. Phys. 1973, 58, 1800–1803. [Google Scholar] [CrossRef]
- Brion, C.E. Negative Ion Formation in the Hexafluorides of Sulphur, Selenium and Tellurium. Int. J. Mass Spectrom. Ion Proces. 1969, 3, 197–202. [Google Scholar] [CrossRef]
- Chen, C.L.; Chantry, P.J. Photon-Enhanced Dissociative Electron Attachment in SF6 and Its Isotopic Selectivity. J. Chem. Phys. 1979, 71, 3897–3907. [Google Scholar] [CrossRef]
- Christophorou, L.G.; Olthoff, J.K. Electron Attachment Cross Sections and Negative Ion States of SF6. Int. J. Mass Spectrom. 2001, 205, 27–41. [Google Scholar] [CrossRef]
- Compton, R.N.; Christophorou, L.G.; Hurst, G.S.; Reinhardt, P.W. Nondissociative Electron Capture in Complex Molecules and Negative-Ion Lifetimes. J. Chem. Phys. 1966, 45, 4634–4639. [Google Scholar] [CrossRef]
- Chutjian, A.; Alajajian, S.H. S-Wave Threshold in Electron Attachment: Observations and Cross Sections in CCl4 and SF6 at Ultralow Electron Energies. Phys. Rev. A 1985, 31, 2885–2892. [Google Scholar] [CrossRef]
- Fenzlaff, M.; Gerhard, R.; Illenberger, E. Associative and Dissociative Electron Attachment by SF6 and SF5Cl. J. Chem. Phys. 1988, 88, 149–155. [Google Scholar] [CrossRef]
- Fehsenfeld, F.C. Electron Attachment to SF6. J. Chem. Phys. 1970, 53, 2000–2004. [Google Scholar] [CrossRef]
- Klar, D.; Ruf, M.W.; Hotop, H. Attachment of Electrons to Molecules at Submillielectronvolt Resolution. Chem. Phys. Lett. 1992, 189, 448–454. [Google Scholar] [CrossRef]
- Limão-Vieira, P.; Blanco, F.; Oller, J.C.; Muñoz, A.; Pérez, J.M.; Vinodkumar, M.; García, G.; Mason, N.J. Electron Scattering Cross Sections for SF6 and SF5CF3 at Intermediate and High Energies (100-10000 EV). Phys. Rev. A 2005, 71, 032720. [Google Scholar] [CrossRef]
- Cho, H.; Gulley, R.J.; Trantham, K.W.; Uhlmann, L.J.; Dedman, C.J.; Buckman, S.J. Elastic Electron Scattering from Sulfur Hexafluoride. J. Phys. B At. Mol. Opt. Phys. 2000, 33, 3531–3544. [Google Scholar] [CrossRef]
- Cho, H.; Gulley, R.J.; Buckman, S.J. The Total Elastic Cross Section for Electron Scattering from SF6. J. Phys. B At. Mol. Opt. Phys. 2000, 33, L309–L315. [Google Scholar] [CrossRef]
- Bhushan, K.G.; Rao, K.C.; Gadkari, S.C.; Yakhmi, J.V.; Gupta, S.K. Elastic Differential Cross Sections for Electron Scattering from SF6 and CS2. Phys. Rev. A 2009, 79, 012702. [Google Scholar] [CrossRef]
- Fabrikant, I.I.; Hotop, H.; Allan, M. Elastic Scattering, Vibrational Excitation, and Attachment in Low-Energy Electron- SF6 Scattering: Experiment and Effective Range Theory. Phys. Rev. A 2005, 71, 022712. [Google Scholar] [CrossRef]
- Blanco, F.; Rosado, J.; Illana, A.; García, G. Comparison of Two Screening Corrections to the Additivity Rule for the Calculation of Electron Scattering from Polyatomic Molecules. Phys. Lett. 2010, 374, 4420–4424. [Google Scholar] [CrossRef]
- Gianturco, F.A.; Lucchese, R.R. Electron Scattering from Gaseous SF6: Comparing Calculations with Experiments. J. Chem. Phys. 2001, 114, 3429–3439. [Google Scholar] [CrossRef]
- Shi, D.H.; Sun, J.F.; Liu, Y.F.; Zhu, Z.L.; Ma, H. Total Cross Sections of Electron Scattering by Several Sulfur-Containing Molecules OCS, SO2, SF4, SF6, SF 5CF3, SO2Cl2 and SO2ClF at 30-5000 EV. Eur. Phys. J. D 2009, 54, 43–50. [Google Scholar] [CrossRef]
- Harth, K.; Ruf, M.W.; Hotop, H. Electron Transfer from Laser Excited Rydberg Atoms to Molecules. Absolute Rate Constants at Low and Intermediate Principal Quantum Numbers. Z. Phys. D 1989, 14, 149–165. [Google Scholar] [CrossRef]
- Kraft, T.; Ruf, M.W.; Hotop, H. Formation of Negatively-Charged Cluster Ions in Thermal Energy Collisions with State-Selected Rydberg Atoms. Z. Phys. D 1989, 14, 179–185. [Google Scholar] [CrossRef]
- Liu, Y.; Suess, L.; Dunning, F.B. Rydberg Electron Transfer to SF6: Product Ion Lifetimes. J. Chem. Phys. 2005, 122, 214313. [Google Scholar] [CrossRef] [PubMed]
- Suess, L.; Parthasarathy, R.; Dunning, F.B. Nondissociative Low-Energy Electron Attachment to SF6, C6F6, C10F8, and c-C7F14: Negative Ion Lifetimes. J. Chem. Phys. 2002, 117, 11222–11227. [Google Scholar] [CrossRef]
- Hubers, M.M.; Los, J. Ion Pair Formation in Alkali-SF6 Collisions: Dependence on Collisional and Vibrational Energy. Chem. Phys. 1975, 10, 235–259. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry (accessed on 10 July 2024).
- Kleyn, A.W.; Moutinho, A.M.C. Negative Ion Formation in Alkali-Atom–Molecule. J. Phys. B At. Mol. Opt. Phys. 2001, 4075, R1–R44. [Google Scholar] [CrossRef]
- Kleyn, A.; Los, J.; Gislason, E.A. Vibronic Coupling At Intersections of Covalent and Ionic States. Phys. Rep. 1982, 90, 1–71. [Google Scholar] [CrossRef]
- Harland, P.; Thynne, J.C.J. Ionization and Dissociation of Pentafluorosulfur Chloride by Electron Impact. J. Phys. Chem. 1969, 73, 4031–4035. [Google Scholar] [CrossRef]
- Almeida, D.; Silva, F.F.; Eden, S.; García, G.; Limão-Vieira, P. New Fragmentation Pathways in K-THF Collisions as Studied by Electron-Transfer Experiments: Negative Ion Formation. J. Phys. Chem. A 2014, 118, 690–696. [Google Scholar] [CrossRef]
- Almeida, D.; da Silva, F.F.; García, G.; Limão-Vieira, P. Dynamic of Negative Ions in Potassium-D-Ribose Collisions. J. Chem. Phys. 2013, 139, 114304. [Google Scholar] [CrossRef]
- Lozano, A.I.; Kumar, S.; Pereira, P.J.S.; Kerkeni, B.; García, G.; Limão-Vieira, P. Low-Lying Negative Ion States Probed in Potassium—Ethanol Collisions. ChemPhysChem 2024, 25, e202400314. [Google Scholar] [CrossRef]
- Silva, F.F.; Almeida, D.; Antunes, R.; Martins, G.; Nunes, Y.; Eden, S.; Garcia, G.; Limão-Vieira, P. Electron Transfer Processes in Potassium Collisions with 5-Fluorouracil and 5-Chlorouracil. Phys. Chem. Chem. Phys. 2011, 13, 21621–21629. [Google Scholar] [CrossRef]
- Silva, F.F.; Meneses, G.; Ingólfsson, O.; Limão-Vieira, P. Side Chain Effects in Reactions of the Potassium-Tyrosine Charge Transfer Complex. Chem. Phys. Lett. 2016, 662, 19–24. [Google Scholar] [CrossRef]
- Silva, F.F.; Mendes, M.; García, G.; Limão-Vieira, P. Radiation in Bioanalysis, Spectroscopic Techniques and Theoretical Methods; Pereira, A.S., Tavares, P., Limão-Vieira, P., Eds.; Springer: Cham, Switzerland, 2019; pp. 329–348. [Google Scholar]
- Silva, F.F.; Cunha, T.; Rebelo, A.; Gil, A.; Calhorda, M.J.; García, G.; Ingólfsson, O.; Limão-Vieira, P. Electron-Transfer-Induced Side-Chain Cleavage in Tryptophan Facilitated through Potassium-Induced Transition-State Stabilization in the Gas Phase. J. Phys. Chem. A 2021, 125, 2324–2333. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Pamplona, B.; Kumar, S.; da Silva, F.F.; Aguilar, A.; García, G.; Bacchus-Montabonel, M.C.; Limão-Vieira, P. Ion-Pair Formation in Neutral Potassium-Neutral Pyrimidine Collisions: Electron Transfer Experiments. Front. Chem. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meneses, G.; Widmann, C.; Cunha, T.; Gil, A.; Silva, F.F.; Calhorda, M.J.; Limão-Vieira, P. Unravelling the Dissociation Pathways of Acetic Acid upon Electron Transfer in Potassium Collisions: Experimental and Theoretical Studies. Phys. Chem. Chem. Phys. 2017, 19, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.; Silva, F.F.; Kopyra, J.; García, G.; Limão-Vieira, P. Anion Formation in Gas-Phase Potassium-Uridine Collisions. Int. J. Mass Spectrom. 2014, 365–366, 243–247. [Google Scholar] [CrossRef]
- Almeida, D.; Kinzel, D.; Ferreira da Silva, F.; Puschnigg, B.; Gschliesser, D.; Scheier, P.; Denifl, S.; García, G.; González, L.; Limão-Vieira, P. N-Site de-Methylation in Pyrimidine Bases as Studied by Low Energy Electrons and Ab Initio Calculations. Phys. Chem. Chem. Phys. 2013, 15, 11431. [Google Scholar] [CrossRef]
- Antunes, R.; Almeida, D.; Martins, G.; Mason, N.J.; Garcia, G.; Maneira, M.J.P.; Nunes, Y.; Limão-Vieira, P. Negative Ion Formation in Potassium–Nitromethane Collisions. Phys. Chem. Chem. Phys. 2010, 12, 12513–12519. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kilich, T.; Łabuda, M.; García, G.; Limão-Vieira, P. Anionic States of C6Cl6 Probed in Electron Transfer Experiments. Phys. Chem. Chem. Phys. 2022, 24, 366–374. [Google Scholar] [CrossRef]
- Kumar, S.; Chouikha, I.B.; Kerkeni, B.; García, G.; Limão-Vieira, P. Enhanced Radiosensitisation of Nimorazole upon Charge Transfer. Molecules 2022, 27, 4134. [Google Scholar] [CrossRef]
- Kumar, S.; Hoshino, M.; Kerkeni, B.; García, G.; Limão-Vieira, P. Isotope Effect in D2O Negative Ion Formation in Electron Transfer Experiments: DO−D Bond Dissociation Energy. J. Phys. Chem. Lett. 2023, 14, 5362–5369. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pereira, P.J.S.; García, G.; Limão-Vieira, P. Cl-Kinetic-Energy Release Distributions from Chlorobenzene and Related Molecules in Electron Transfer Experiments. Eur. Phys. J. D 2021, 75, 294. [Google Scholar] [CrossRef]
- Lozano, A.I.; Kumar, S.; Kerkeni, B.; García, G.; Limão-Vieira, P. Methanol Negative Ion Fragmentation Probed in Electron Transfer Experiments. J. Phys. Chem. A 2022, 126, 1076–1084. [Google Scholar] [CrossRef]
- Mendes, M.; García, G.; Bacchus-Montabonel, M.C.; Limão-Vieira, P. Electron Transfer Induced Decomposition in Potassium–Nitroimidazoles Collisions: An Experimental and Theoretical Work. Int. J. Mol. Sci. 2019, 20, 6170. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.J.; Matus, M.H.; Switzer, J.R.; Dixon, D.A.; Francisco, J.S.; Christe, K.O. Bond Dissociation Energies in Second-Row Compounds. J. Phys. Chem. A 2008, 112, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Babcock, L.M.; Streit, G.E. Ion–Molecule Reactions of SF6: Determination of I.P.(SF5), A.P. (SF5+/SF6), and D(SF5-F). J. Chem. Phys. 1981, 74, 5700–5706. [Google Scholar] [CrossRef]
- Kerr, J.A. Bond Dissociation Energies by Kinetic Methods. Chem. Rev. 1966, 66, 465–500. [Google Scholar] [CrossRef]
- Menk, S.; Das, S.; Blaum, K.; Froese, M.W.; Lange, M.; Mukherjee, M.; Repnow, R.; Schwalm, D.; Von Hahn, R.; Wolf, A. Vibrational Autodetachment of Sulfur Hexafluoride Anions at Its Long-Lifetime Limit. Phys. Rev. A 2014, 89, 022502. [Google Scholar] [CrossRef]
- Rosa, A.; Brüning, F.; Kumar, S.V.K.; Illenberger, E. Dissociative Attachment to Excited SF6: Selective IR Excitation versus Thermal Activation. Chem. Phys. Lett. 2004, 391, 361–365. [Google Scholar] [CrossRef]
- Kleyn, A.W.; Hubers, M.M.; Los, J. Ion-Pair Formation in Alkali Atom-Oxygen Molecule Collsions. Chem. Phys. 1978, 34, 55–63. [Google Scholar] [CrossRef]
- Hitchcock, A.P.; Van Der Wiel, M.J. Absolute Oscillator Strengths (5-63 EV) for Photoabsorption and Ionic Fragmentation of SF6. J. Phys. B At. Mol. Opt. Phys. 1979, 12, 2153–2169. [Google Scholar] [CrossRef]
- Yencha, A.J.; Thompson, D.B.; Cormack, A.J.; Cooper, D.R.; Zubek, M.; Bolognesi, P.; King, G.C. Threshold Photoelectron Spectroscopy of SF6. Chem. Phys. 1997, 216, 227–241. [Google Scholar] [CrossRef]
- Kumar, S.; Romero, J.; Probst, M.; Maihom, T.; García, G.; Limão-Vieira, P. Sensing the Ortho Positions in C6Cl6 and C6H4Cl2 from Cl2− Formation upon Molecular Reduction. Molecules 2022, 27, 4820. [Google Scholar] [CrossRef]
- Regeta, K.; Kumar, S.; Cunha, T.; Mendes, M.; Lozano, A.I.; Pereira, P.J.S.; García, G.; Moutinho, A.M.C.; Bacchus-Montabonel, M.C.; Limão-Vieira, P. Combined Experimental and Theoretical Studies on Electron Transfer in Potassium Collisions with CCl4. J. Phys. Chem. A 2020, 124, 3220–3227. [Google Scholar] [CrossRef] [PubMed]
- Lozano, A.I.; Maioli, L.S.; Pamplona, B.; Romero, J.; Mendes, M.; Silva, F.F.; Kossoski, F.; Probst, M.; Süβ, D.; Bettega, M.H.F.; et al. Selective Bond Breaking of Halothane Induced by Electron Transfer in Potassium Collisions. Phys. Chem. Chem. Phys. 2020, 22, 23837–23846. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Izadi, F.; Ončák, M.; Limão-Vieira, P.; Denifl, S. Hexachlorobenzene-Negative Ion Formation in Electron Attachment Experiments. Phys. Chem. Chem. Phys. 2022, 24, 13335–13342. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian: Wallingford, CT, USA, 2016. [Google Scholar]
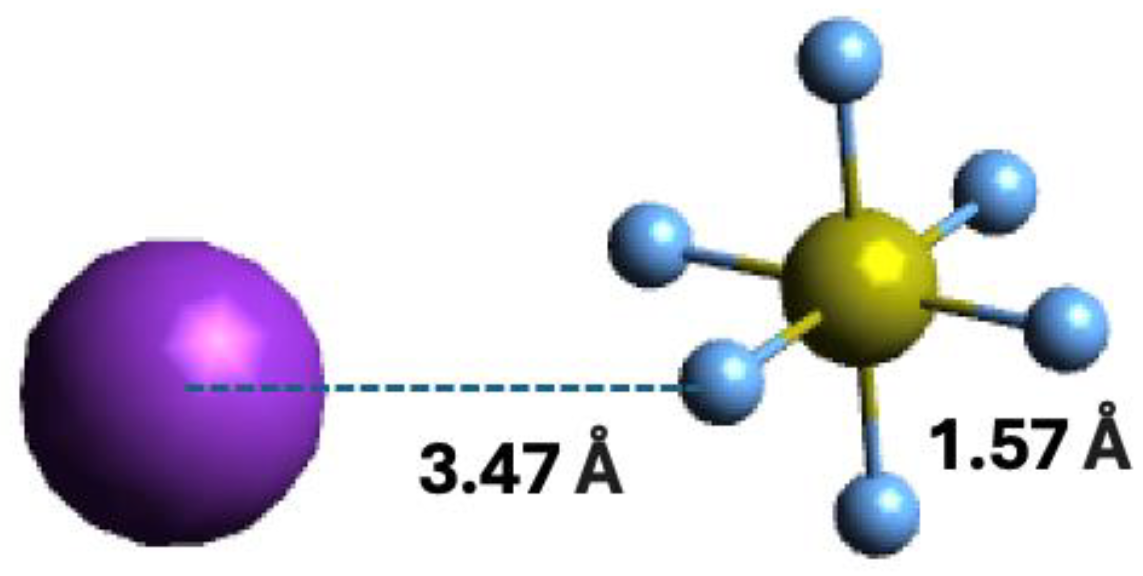

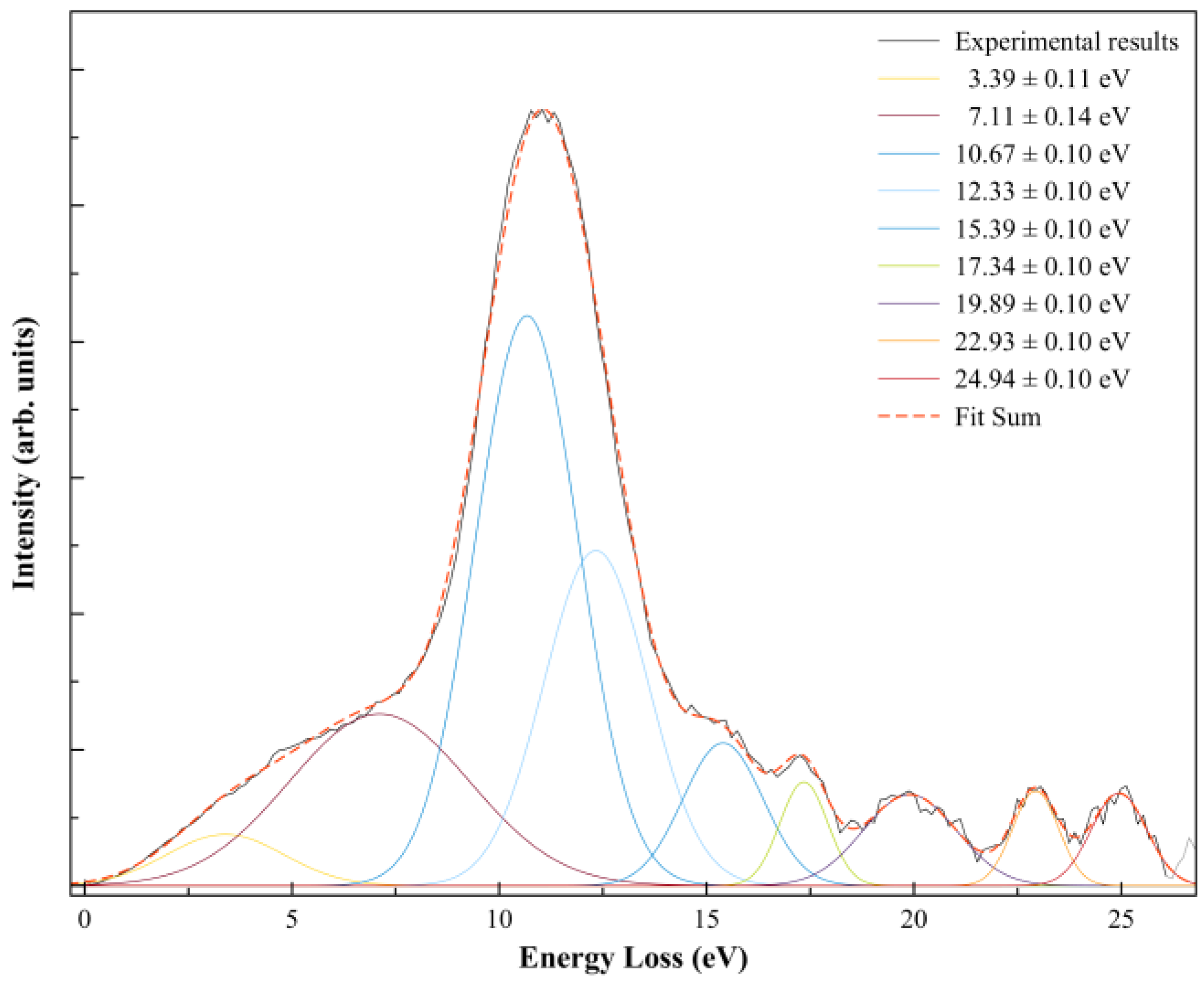
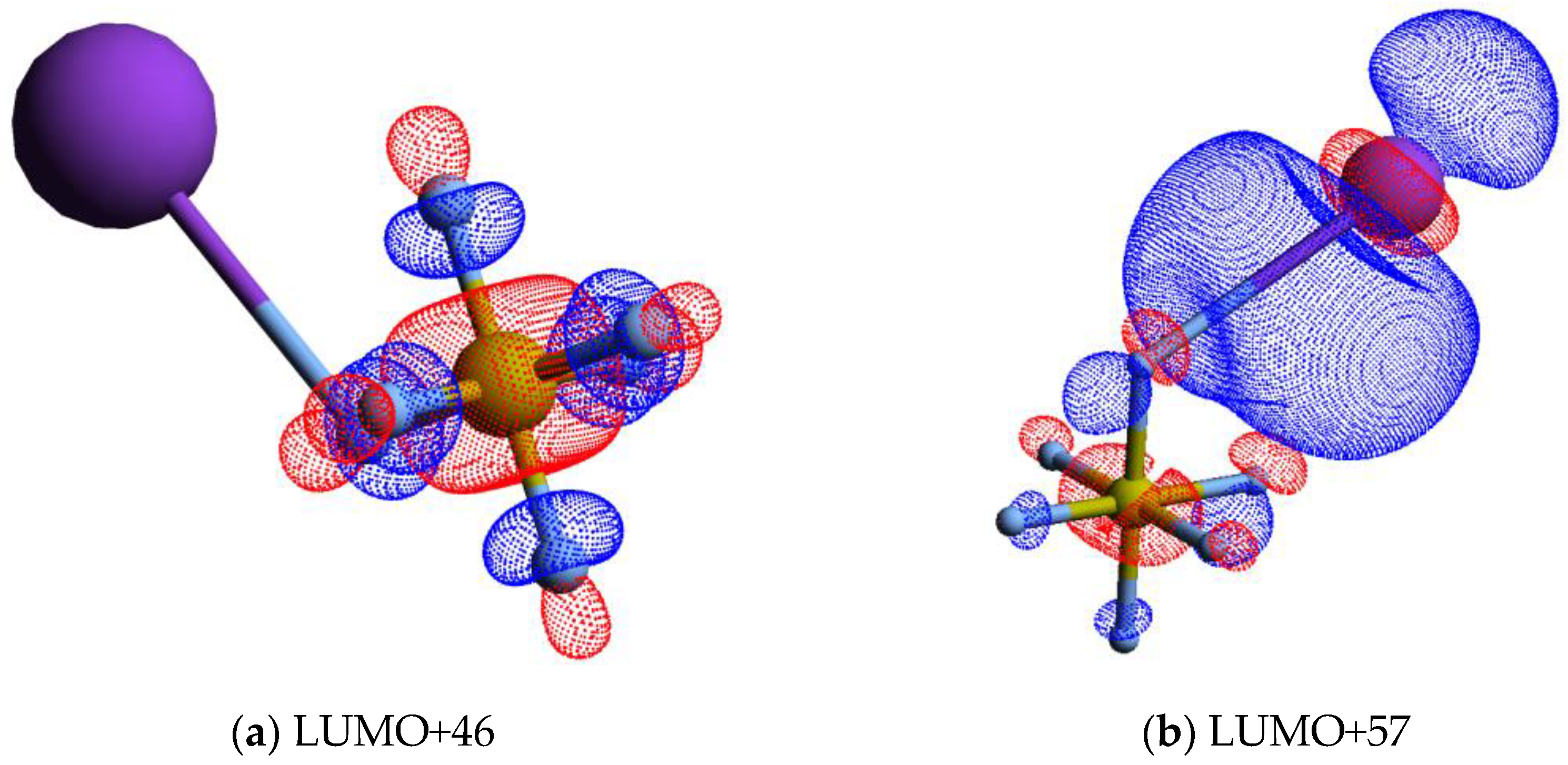
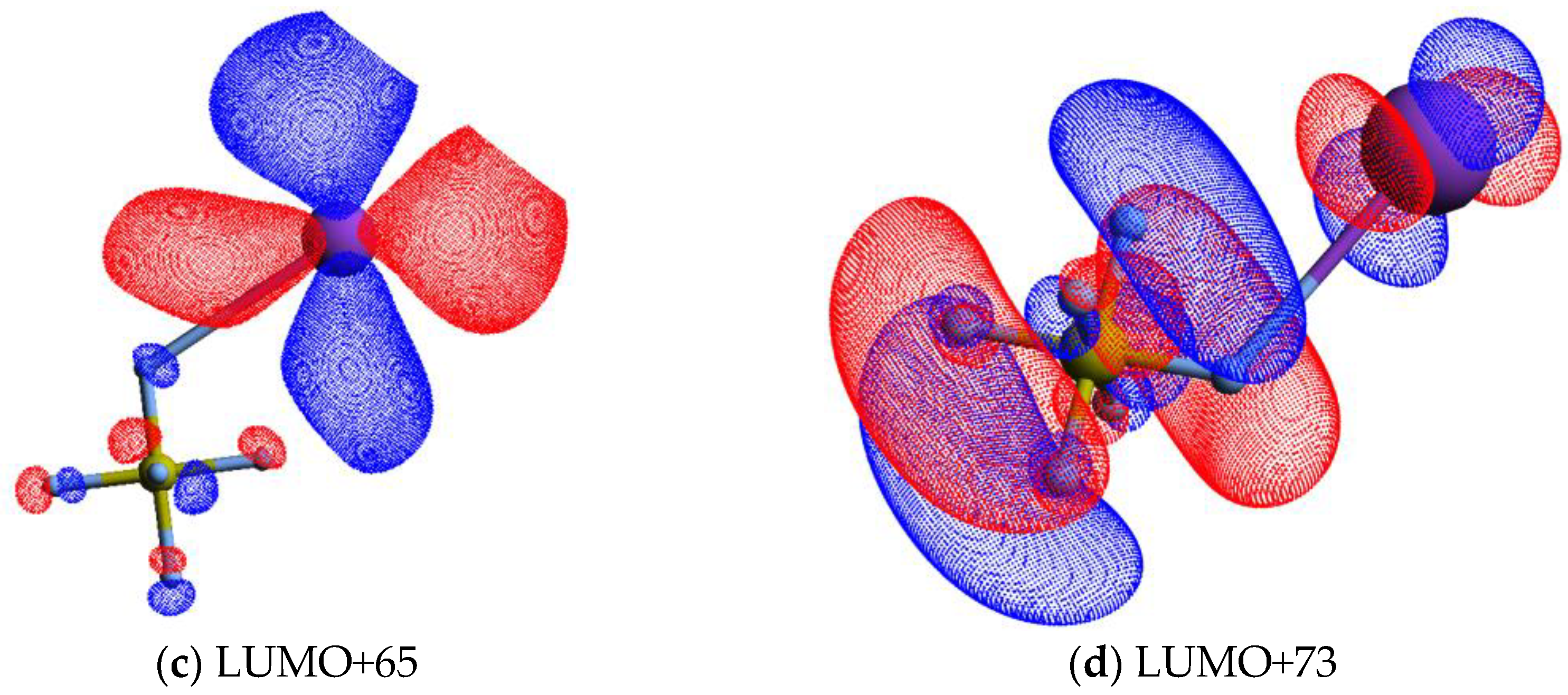
| K+ Energy Loss Feature (eV) | VEA (eV) | Calculated VE (eV) | Assignment | EA resonances (eV) [5] |
|---|---|---|---|---|
| 3.39 ± 0.11 | 0.95 ± 0.11 | – | – | – |
| 7.11 ± 0.14 | −2.77 ± 0.14 | 3.81 | LUMO+46 | 2.6 (2.8; ~2.9 [19]) |
| 10.67 ± 0.10 | −6.33 ± 0.10 | 6.30 | LUMO+57 | ~5.4; (5.7 ± 0.1 [38]) |
| 12.33 ± 0.10 | −7.99 ± 0.10 | 7.24 | LUMO+65 | 8.8 (8.9) |
| 15.39 ± 0.10 | −11.05 ± 0.10 | 11.01 | LUMO+73 | 11.3 (11.5; 11.6) |
| 17.34 ± 0.10 | −13.00 ± 0.10 | 12.89 | LUMO+81 | – |
| 19.89 ± 0.10 | −15.55 ± 0.10 | 15.68 | LUMO+97 | – |
| 22.93 ± 0.10 | −18.59 ± 0.10 | 17.60 | LUMO+101 | – |
| 24.94 ± 0.10 | −20.60 ± 0.10 | 21.56 | LUMO+102 | – |
| Compound | Electron Affinity (eV) [35] | Bond Dissociation Energy (eV) [57] |
|---|---|---|
| SF6 | 0.910 ± 0.070 1 | – |
| SF5 | 3.850 ± 0.020 | – |
| SF3 | 3.070 ± 0.020 | – |
| F2 | 3.005 ± 0.071 | – |
| F | 3.401191 ± 0.000026 | – |
| SF5–F | – | 4.00 ± 0.16 [58] |
| SF4–F | – | 2.51 ± 0.13 |
| SF3–F | – | 3.47 ± 0.56 |
| SF2–F | – | 2.64 ± 0.12 |
| SF–F | – | 3.98 ± 0.24 |
| S–F | – | 3.51 ± 0.07 |
| F–F | – | 1.627 ± 0.100 2 [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Hoshino, M.; Kerkeni, B.; García, G.; Ouerfelli, G.; Al-Mogren, M.M.; Limão-Vieira, P. SF6 Negative Ion Formation in Charge Transfer Experiments. Molecules 2024, 29, 4118. https://doi.org/10.3390/molecules29174118
Kumar S, Hoshino M, Kerkeni B, García G, Ouerfelli G, Al-Mogren MM, Limão-Vieira P. SF6 Negative Ion Formation in Charge Transfer Experiments. Molecules. 2024; 29(17):4118. https://doi.org/10.3390/molecules29174118
Chicago/Turabian StyleKumar, Sarvesh, Masamitsu Hoshino, Boutheïna Kerkeni, Gustavo García, Ghofrane Ouerfelli, Muneerah Mogren Al-Mogren, and Paulo Limão-Vieira. 2024. "SF6 Negative Ion Formation in Charge Transfer Experiments" Molecules 29, no. 17: 4118. https://doi.org/10.3390/molecules29174118
APA StyleKumar, S., Hoshino, M., Kerkeni, B., García, G., Ouerfelli, G., Al-Mogren, M. M., & Limão-Vieira, P. (2024). SF6 Negative Ion Formation in Charge Transfer Experiments. Molecules, 29(17), 4118. https://doi.org/10.3390/molecules29174118







