Investigating the Antimicrobial Properties of Essential Oil Constituents and Their Mode of Action
Abstract
1. Introduction
2. Results
2.1. Microbial Inactivation by Essential Oil Constituents Relative to Treatment Medium pH
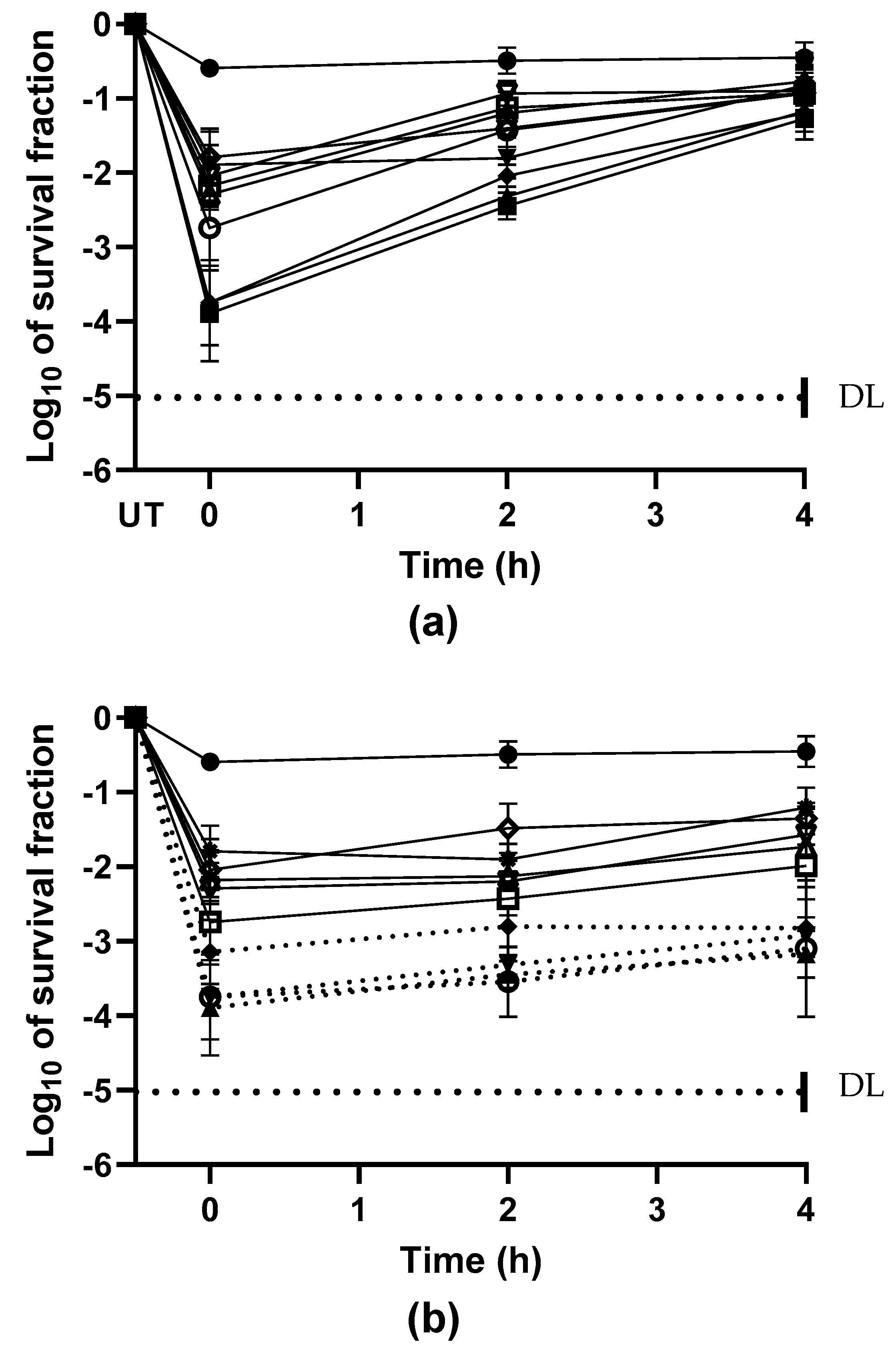
2.2. Biosynthetic Requirements for Restoring the Sublethal Injury Induced by EOCs
2.3. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Growth Conditions
4.2. Assessment of Bacterial Inactivation by EOs Constituents
4.3. Survival Counts
4.4. Measurement of Sublethal Injury
4.5. Assessment of the Biosynthetic Requirements for Repairing Sublethally Injured Cells
4.6. Protein and Ligands Retrieval and Preparation
4.7. Molecular Docking and Cluster Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hintz, T.; Matthews, K.K.; Di, R. The Use of Plant Antimicrobial Compounds for Food Preservation. BioMed Res. Int. 2015, 2015, 246264. [Google Scholar] [CrossRef] [PubMed]
- Role of Essential Oils in Food Safety: Antimicrobial and Antioxidant Applications|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S259025981930007X?token=58E1CB94946BFFA9D578E87CE0B194BA8FCD7C5198D38A6049358894E968D07787EB5EAE221FD504814EE23F99787C1D (accessed on 16 July 2020).
- Plant Antimicrobials and the Food Industry: Part 3—Phytochemicals. Available online: https://www.thermofisher.com/blog/food/plant-antimicrobials-and-the-food-industry-part-3-phytochemicals/ (accessed on 16 July 2020).
- Shah, M.A.; Mir, S.A. Chapter 6—Plant Extracts as Food Preservatives. In Plant Extracts: Applications in the Food Industry; Mir, S.A., Manickavasagan, A., Shah, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 127–141. ISBN 978-0-12-822475-5. [Google Scholar]
- Mir, S.A.; Shah, M.A.; Manickavasagan, A. Chapter 1—Sources of Plant Extracts. In Plant Extracts: Applications in the Food Industry; Mir, S.A., Manickavasagan, A., Shah, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–22. ISBN 978-0-12-822475-5. [Google Scholar]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ouazzou, A.; Cherrat, L.; Espina, L.; Lorán, S.; Rota, C.; Pagán, R. The Antimicrobial Activity of Hydrophobic Essential Oil Constituents Acting Alone or in Combined Processes of Food Preservation. Innov. Food Sci. Emerg. Technol. 2011, 12, 320–329. [Google Scholar] [CrossRef]
- Espina, L.; Pagán, R.; López, D.; García-Gonzalo, D. Individual Constituents from Essential Oils Inhibit Biofilm Mass Production by Multi-Drug Resistant Staphylococcus Aureus. Molecules 2015, 20, 11357–11372. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.Q.; do Nascimento, L.D.; da Costa, F.A.M.; da Costa, K.S.; de Andrade, E.H.A. In Vitro Antibacterial Activity and in Silico Analysis of the Bioactivity of Major Compounds Obtained from the Essential Oil of Virola surinamensis Warb (Myristicaceae). J. Food Qual. 2022, 2022, e5275805. [Google Scholar] [CrossRef]
- Espina, L.; Gelaw, T.K.; de Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. Mechanism of Bacterial Inactivation by (+)-Limonene and Its Potential Use in Food Preservation Combined Processes. PLoS ONE 2013, 8, e56769. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Pacheco, M.M.; Torres-Moreno, H.; Flores-Lopez, M.L.; Velázquez Guadarrama, N.; Ayala-Zavala, J.F.; Ortega-Ramírez, L.A.; López-Romero, J.C. Mechanisms and Applications of Citral’s Antimicrobial Properties in Food Preservation and Pharmaceuticals Formulations. Antibiotics 2023, 12, 1608. [Google Scholar] [CrossRef] [PubMed]
- Shoeib, N.A.; Al-Madboly, L.A.; Ragab, A.E. In Vitro and in Silico β-Lactamase Inhibitory Properties and Phytochemical Profile of Ocimum Basilicum Cultivated in Central Delta of Egypt. Pharm. Biol. 2022, 60, 1969–1980. [Google Scholar] [CrossRef]
- Somolinos, M.; García, D.; Condón, S.; Mackey, B.; Pagán, R. Inactivation of Escherichia coli by Citral. J. Appl. Microbiol. 2010, 108, 1928–1939. [Google Scholar] [CrossRef]
- Shanaida, M.; Hudz, N.; Białoń, M.; Kryvtsowa, M.; Svydenko, L.; Filipska, A.; Paweł Wieczorek, P. Chromatographic Profiles and Antimicrobial Activity of the Essential Oils Obtained from Some Species and Cultivars of the Mentheae Tribe (Lamiaceae). Saudi J. Biol. Sci. 2021, 28, 6145–6152. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ouazzou, A.; Espina, L.; Gelaw, T.K.; de Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. New Insights in Mechanisms of Bacterial Inactivation by Carvacrol. J. Appl. Microbiol. 2013, 114, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Aouf, A.; Bouaouina, S.; Abdelgawad, M.A.; Abourehab, M.A.S.; Farouk, A. In Silico Study for Algerian Essential Oils as Antimicrobial Agents against Multidrug-Resistant Bacteria Isolated from Pus Samples. Antibiotics 2022, 11, 1317. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.-H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M.M. Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef]
- Gambo, S.B.; Mukhtar, A.A.; Labaran, H.B.; Labaran, H.B.; Mustapha, A.; Ibrahim, S.I.; Ali, M. Chemistry, Mode of Action, Bacterial Resistance, Classification and Adverse Effects of Beta-Lactam Antibiotics: A Review. Int. J. Dermatol. Res. 2023, 5, 11–16. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between Essential Oil Components and Antibiotics: A Review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Matagne, A.; Dubus, A.; Galleni, M.; Frère, J.-M. The β-Lactamase Cycle: A Tale of Selective Pressure and Bacterial Ingenuity. Nat. Prod. Rep. 1999, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, R. Growing Group of Extended-Spectrum β-Lactamases: The CTX-M Enzymes. Antimicrob. Agents Chemother. 2004, 48, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tomanicek, S.J.; Wang, K.K.; Weiss, K.L.; Blakeley, M.P.; Cooper, J.; Chen, Y.; Coates, L. The Active Site Protonation States of Perdeuterated Toho-1 β-Lactamase Determined by Neutron Diffraction Support a Role for Glu166 as the General Base in Acylation. FEBS Lett. 2011, 585, 364–368. [Google Scholar] [CrossRef]
- Bois, S.K.D.; Marriott, M.S.; Amyes, S.G.B. TEM- and SHV-Derived Extended-Spectrum β-Lactamases: Relationship between Selection, Structure and Function. J. Antimicrob. Chemother. 1995, 35, 7–22. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Toleman, M.A. The Emergence of Pan-Resistant Gram-Negative Pathogens Merits a Rapid Global Political Response. J. Antimicrob. Chemother. 2012, 67, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Combination of Mild Heat and Plant Essential Oil Constituents to Inactivate Resistant Variants of Escherichia coli in Buffer and in Coconut Water|Request PDF. Available online: https://www.researchgate.net/publication/337539315_Combination_of_mild_heat_and_plant_essential_oil_constituents_to_inactivate_resistant_variants_of_Escherichia_coli_in_buffer_and_in_coconut_water (accessed on 23 July 2020).
- Inhibitory Effects of Citrus Essential Oils and Their Components on the Formation of N -Nitrosodimethylamine|Request PDF. Available online: https://www.researchgate.net/publication/51352833_Inhibitory_Effects_of_Citrus_Essential_Oils_and_Their_Components_on_the_Formation_of_N_-Nitrosodimethylamine (accessed on 28 May 2022).
- Zengin, H.; Baysal, A.H. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a Natural Monoterpene: A Review of Its Biological Properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Somolinos, M.; García, D.; Pagán, R.; Mackey, B. Relationship between Sublethal Injury and Microbial Inactivation by the Combination of High Hydrostatic Pressure and Citral or Tert-Butyl Hydroquinone. Appl. Environ. Microbiol. 2008, 74, 7570–7577. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, C.; Yin, Z.; Jia, R.; Peng, L.; Kang, S.; Li, Z. Antibacterial Activity of α-Terpineol May Induce Morphostructural Alterations in Escherichia coli. Braz. J. Microbiol. 2015, 45, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Kumar, S. Alpha-Terpinyl Acetate: A Natural Monoterpenoid from Elettaria cardamomum as Multi-Target Directed Ligand in Alzheimer’s Disease. J. Funct. Foods 2020, 68, 103892. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of Four Monoterpenes Contained in Essential Oils with Model Membranes: Implications for Their Antibacterial Activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
- Mączka, W.; Duda-Madej, A.; Grabarczyk, M.; Wińska, K. Natural Compounds in the Battle against Microorganisms—Linalool. Molecules 2022, 27, 6928. [Google Scholar] [CrossRef]
- Vaičiulytė, V.; Ložienė, K.; Švedienė, J.; Raudonienė, V.; Paškevičius, A. α-Terpinyl Acetate: Occurrence in Essential Oils Bearing Thymus pulegioides, Phytotoxicity, and Antimicrobial Effects. Molecules 2021, 26, 1065. [Google Scholar] [CrossRef]
- Brul, S. Preservative Agents in Foods Mode of Action and Microbial Resistance Mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.M.; Munoz, B.; West, S.K.; Rubin, G.S.; Fried, L.P. 2002—Recherche Google. Available online: https://www.google.com/search?q=freidman+et+al+2002&oq=freidman+et+al+2002&aqs=chrome..69i57.11586j0j7&sourceid=chrome&ie=UTF-8 (accessed on 31 May 2024).
- Hatcher, J.F.; Bryan, G.T. Factors Affecting the Mutagenic Activity of Quercetin for Salmonella typhimurium TA98: Metal Ions, Antioxidants and pH. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1985, 148, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Mackey: The Microbiological Safety and Quality of Food—Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=The+Microbiological+Safety+and+Quality+of+Food&author=B.+M.+Mackey&publication_year=2000& (accessed on 22 May 2024).
- Bernstein, F.C.; Koetzle, T.F.; Williams, G.J.B.; Meyer, E.F., Jr.; Brice, M.D.; Rodgers, J.R.; Kennard, O.; Shimanouchi, T.; Tasumi, M. The Protein Data Bank. Eur. J. Biochem. 1977, 80, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound Databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. EADock: Docking of Small Molecules into Protein Active Sites with a Multiobjective Evolutionary Optimization. Proteins Struct. Funct. Bioinform. 2007, 67, 1010–1025. [Google Scholar] [CrossRef]
- «Document.Pdf». Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=ab82608e9a44c17b60d7f908565fba628295dc72#page=44 (accessed on 30 April 2024).
 ), or selective medium added with bile salts (TSAYE-BS) (■). Data are mean ± standard deviation (error bars). The values with different superscripts (a, b, c, d, or e) in the same columns are significantly different (p< 0.05). DL: The dotted line represents the detection limit.
), or selective medium added with bile salts (TSAYE-BS) (■). Data are mean ± standard deviation (error bars). The values with different superscripts (a, b, c, d, or e) in the same columns are significantly different (p< 0.05). DL: The dotted line represents the detection limit.
 ), or selective medium added with bile salts (TSAYE-BS) (■). Data are mean ± standard deviation (error bars). The values with different superscripts (a, b, c, d, or e) in the same columns are significantly different (p< 0.05). DL: The dotted line represents the detection limit.
), or selective medium added with bile salts (TSAYE-BS) (■). Data are mean ± standard deviation (error bars). The values with different superscripts (a, b, c, d, or e) in the same columns are significantly different (p< 0.05). DL: The dotted line represents the detection limit.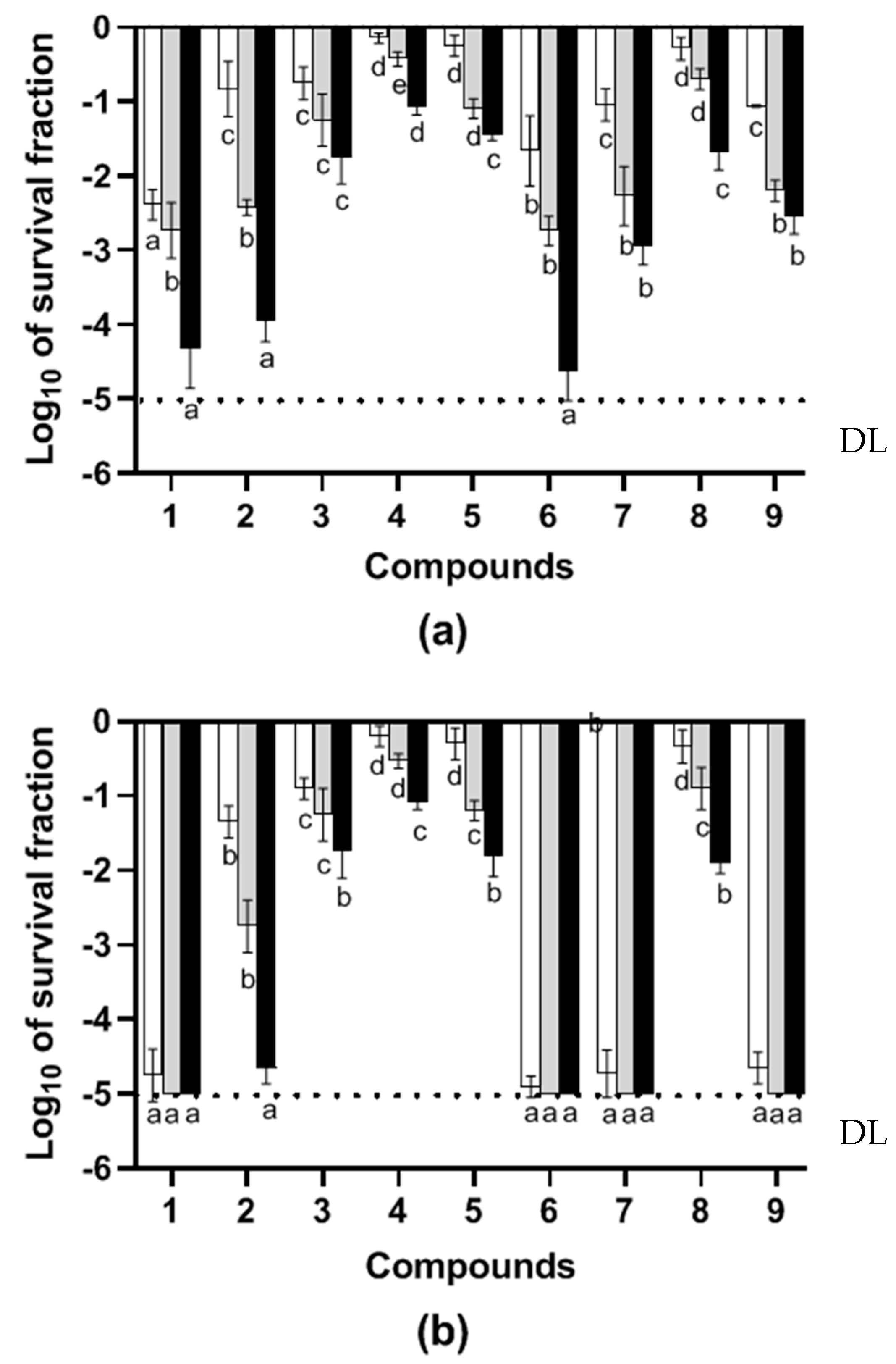

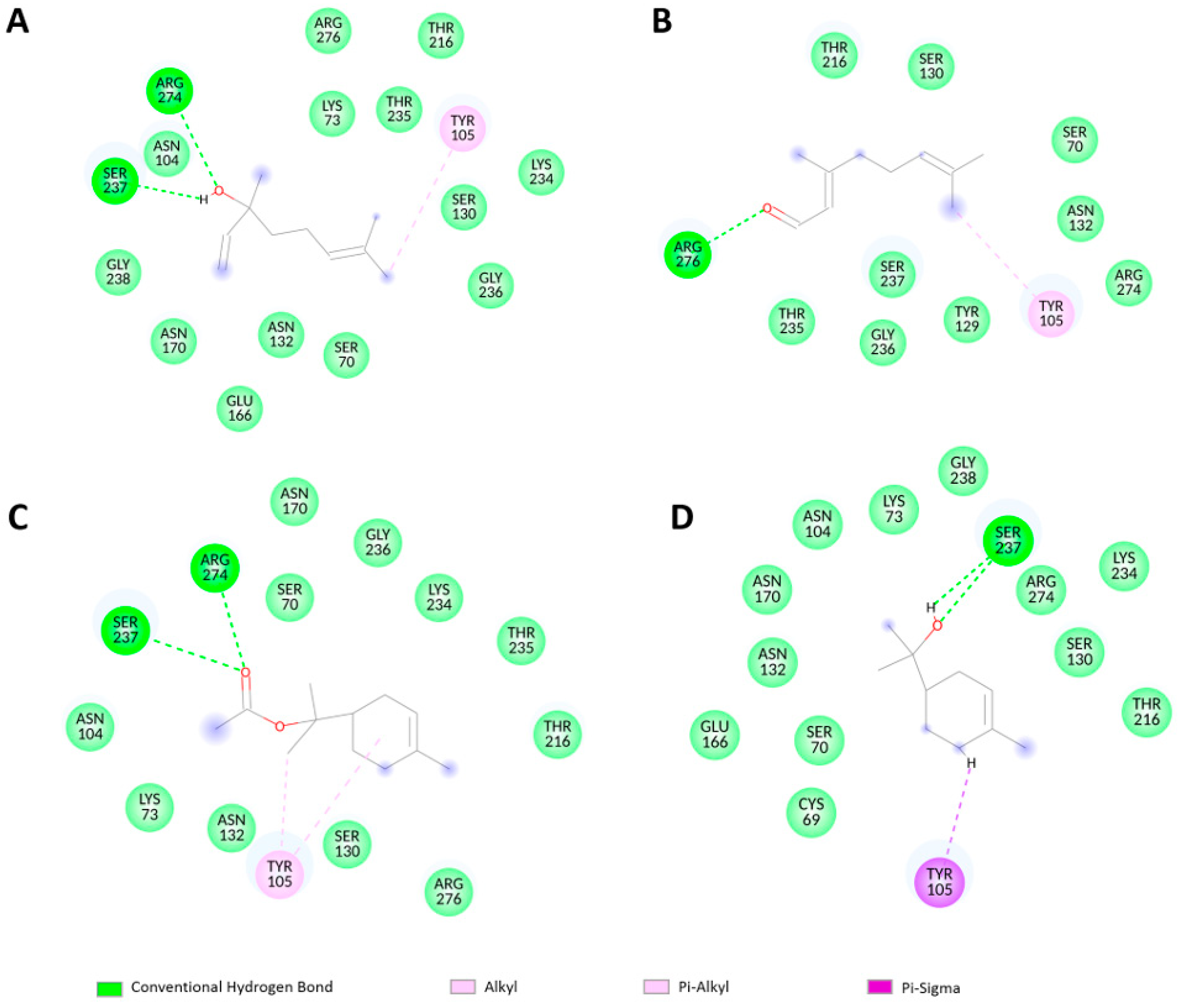
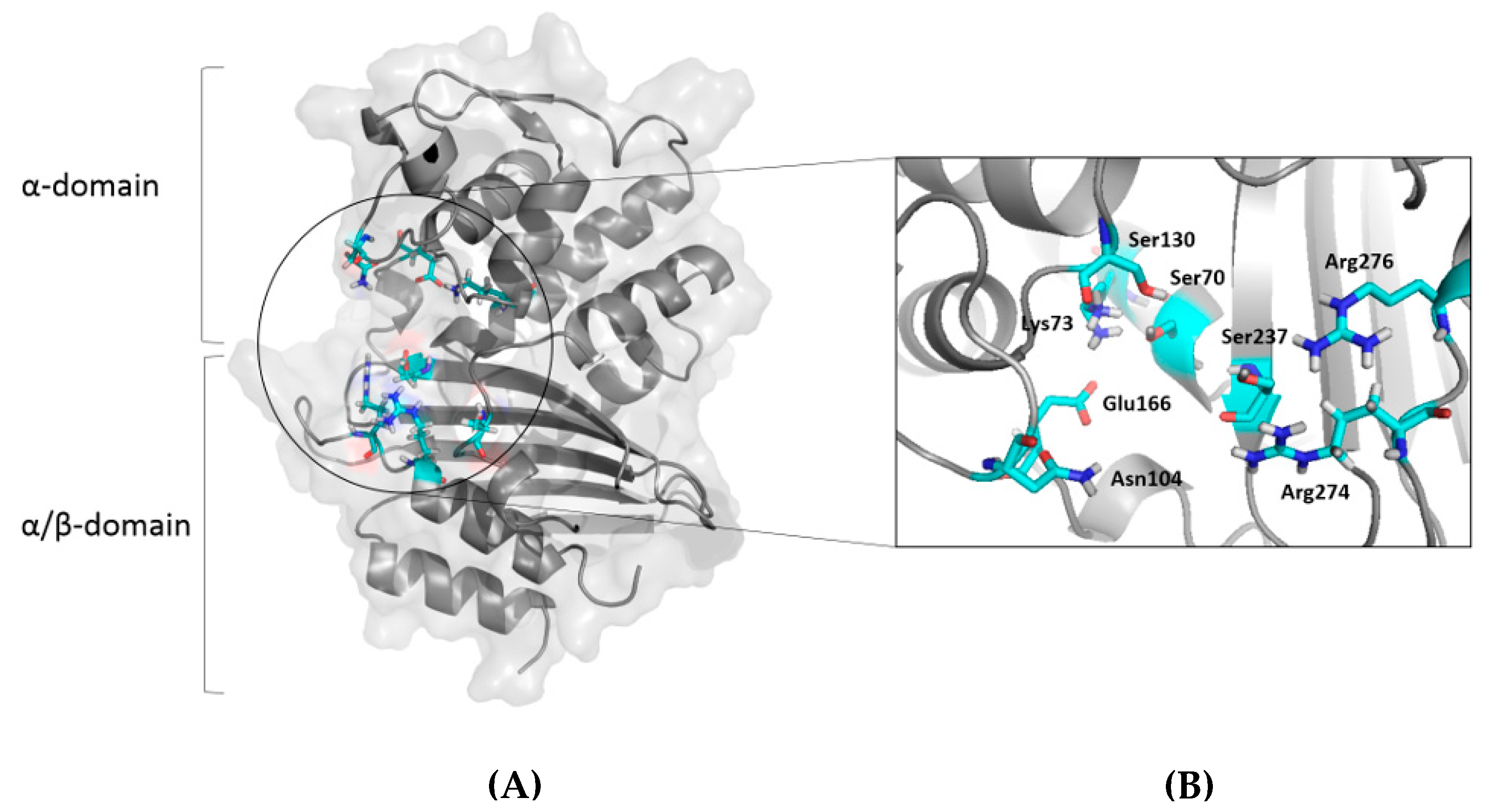
| Compound Name | Number of Poses at Toho-1 Active Site | ΔG(kcal/mol) | Fullfitness (kcal/mol) |
|---|---|---|---|
| Benzylpenicillin | 152 | −8.65 | −1162.95 |
| Linalool (+)/− | 86 | −6.13 | −1262.22 |
| Limonene (+)− | 47 | −5.82 | −1262.05 |
| Citral | 108 | −6.43 | −1257.62 |
| α-Terpinyl acetate | 88 | −6.32 | −1268.07 |
| α-Terpineol | 56 | −6.13 | −1265.05 |
| Carvacrol | 39 | −5.85 | −1261.54 |
| Thymol | 48 | −5.99 | −1261.30 |
| Eucalypto (1,8-Cineol) | 38 | −6.05 | −1248.12 |
| p-cymene | 24 | −5.75 | −1251.82 |
| Compound Name | Pubchem CID | Description |
|---|---|---|
| Benzylpenicillin | 5904 | Natural penicillin |
| Linalool (+)/− | 6549 | Plant metabolite |
| Limonene (+)− | 22311 | Natural product found in plant and other organisms |
| Citral | 638011 | Plant metabolite |
| α-Terpinyl acetate | 111037 | Natural product found in plant and other organisms |
| α-Terpineol | 17100 | Natural product found in plant and other organisms |
| Carvacrol | 10364 | Phenol derivative of cymene. Antimicrobial agent, anti-fungal agent, food additive. |
| Thymol | 6989 | Phenol derivative of cymene with antiseptic, antibacterial, and antifungal actions, |
| Eucalypto (1,8-cineole) | 2758 | Natural product with anti-inflammatory proprieties. Flavoring agent |
| p-cymene | 7463 | Plant metabolite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noui Mehidi, I.; Ait Ouazzou, A.; Tachoua, W.; Hosni, K. Investigating the Antimicrobial Properties of Essential Oil Constituents and Their Mode of Action. Molecules 2024, 29, 4119. https://doi.org/10.3390/molecules29174119
Noui Mehidi I, Ait Ouazzou A, Tachoua W, Hosni K. Investigating the Antimicrobial Properties of Essential Oil Constituents and Their Mode of Action. Molecules. 2024; 29(17):4119. https://doi.org/10.3390/molecules29174119
Chicago/Turabian StyleNoui Mehidi, Ilham, Abdenour Ait Ouazzou, Wafa Tachoua, and Karim Hosni. 2024. "Investigating the Antimicrobial Properties of Essential Oil Constituents and Their Mode of Action" Molecules 29, no. 17: 4119. https://doi.org/10.3390/molecules29174119
APA StyleNoui Mehidi, I., Ait Ouazzou, A., Tachoua, W., & Hosni, K. (2024). Investigating the Antimicrobial Properties of Essential Oil Constituents and Their Mode of Action. Molecules, 29(17), 4119. https://doi.org/10.3390/molecules29174119







