Gas Chromatography–Mass Spectrometry Analysis of Volatile Organic Compounds from Three Endemic Iris Taxa: Headspace Solid-Phase Microextraction vs. Hydrodistillation
Abstract
1. Introduction
2. Results
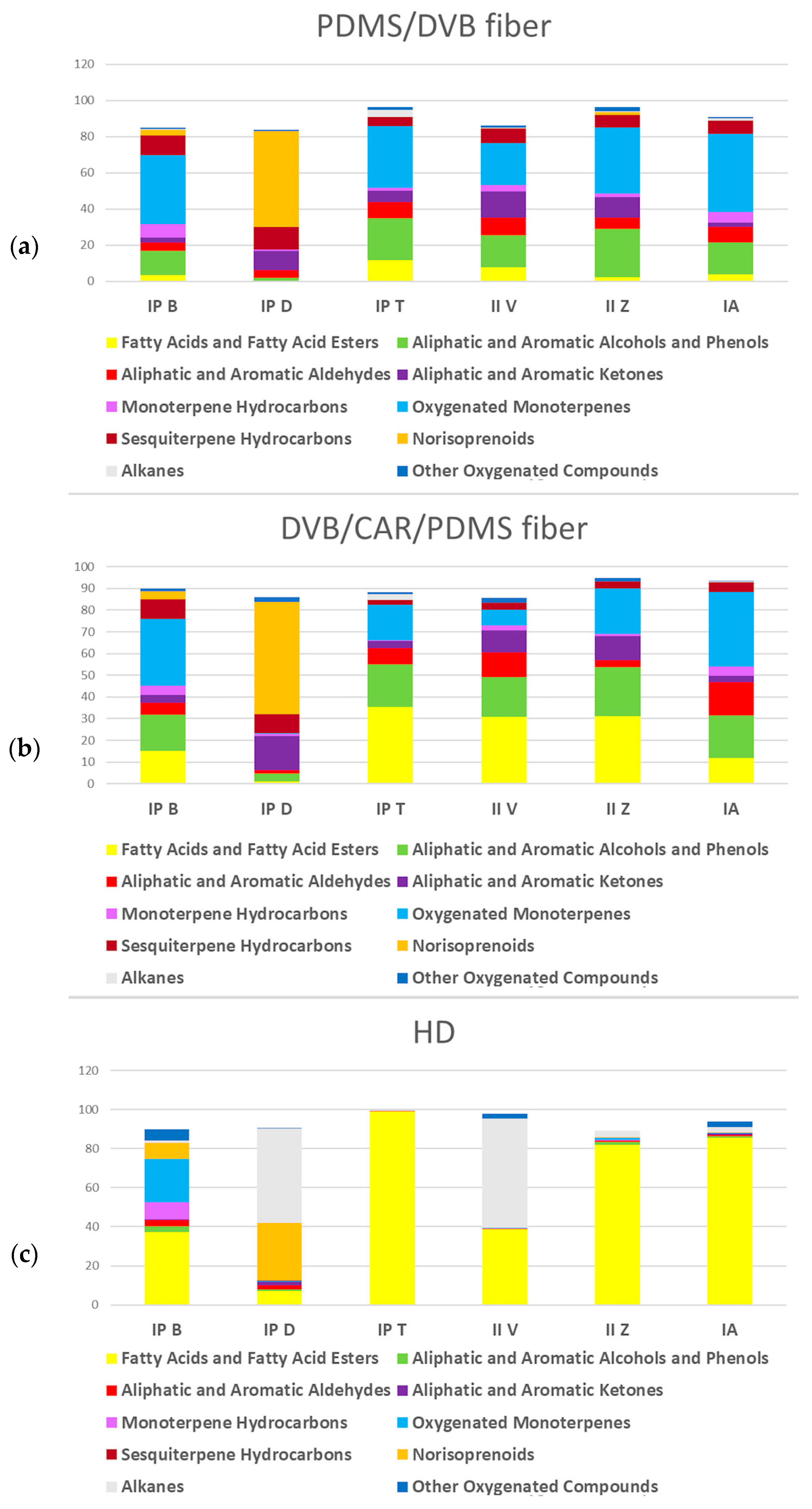
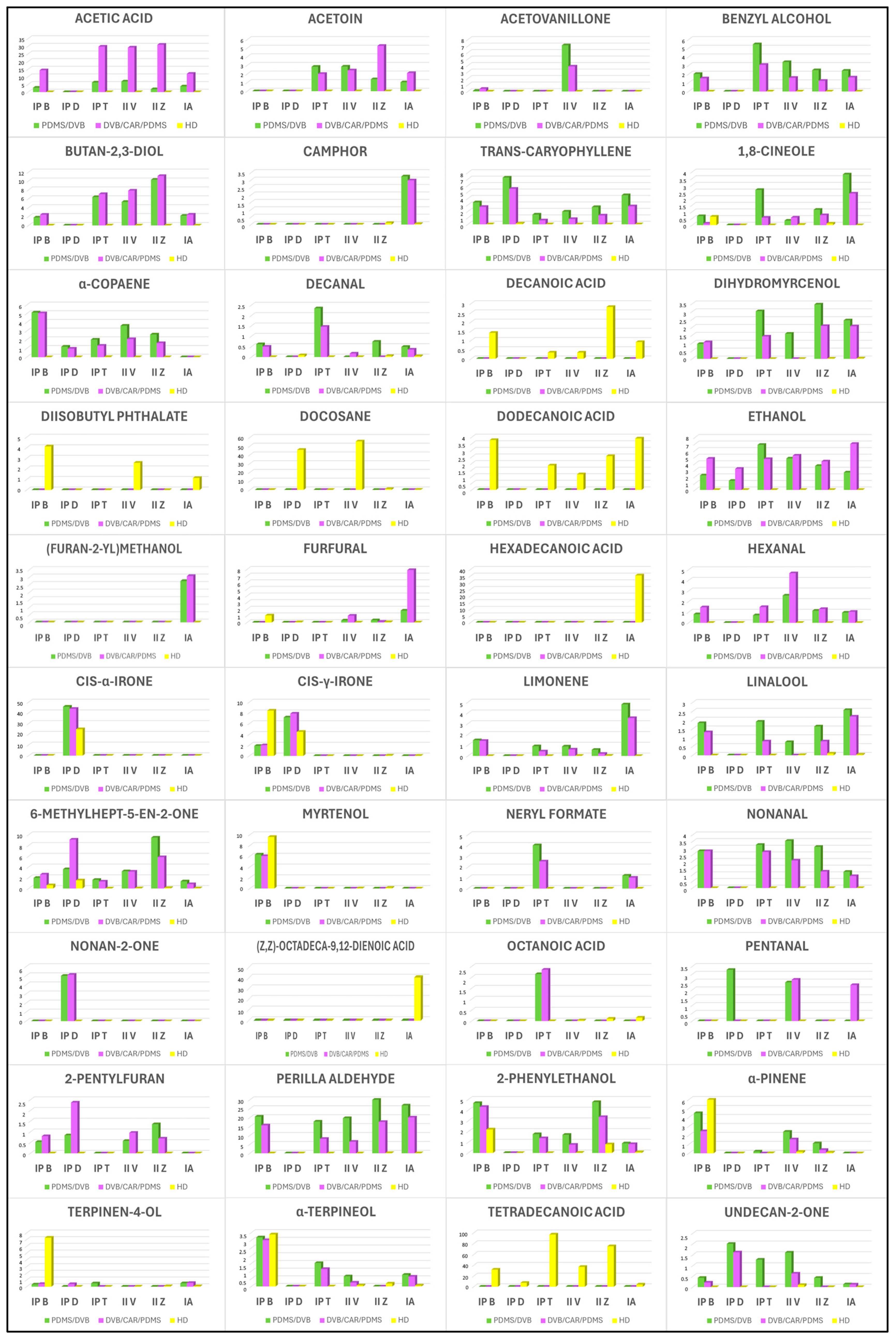
2.1. HS–SPME/GC–MS Analysis
2.1.1. PDMS/DVB Fiber
2.1.2. DVB/CAR/PDMS Fiber
2.2. HD Analysis
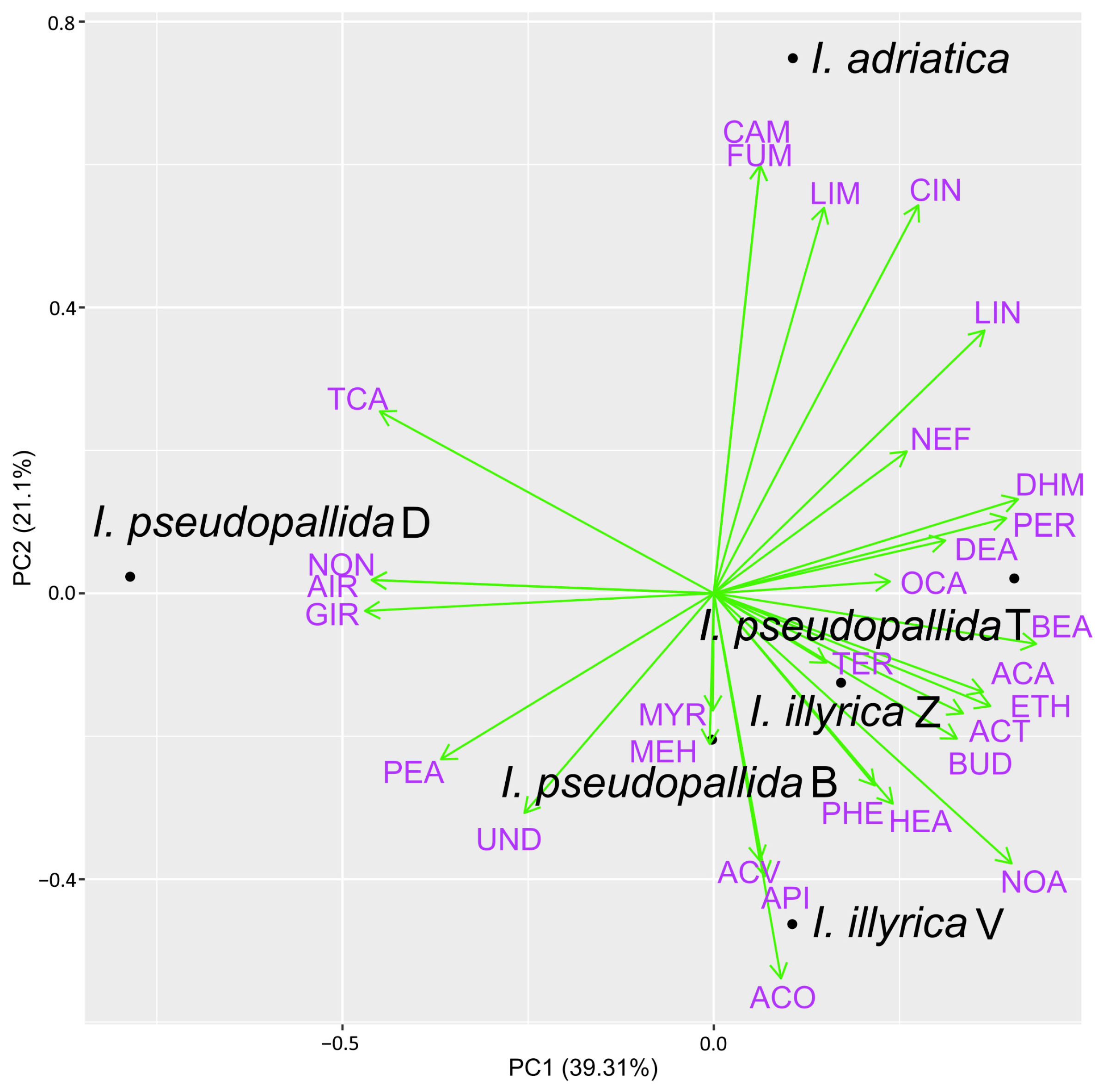
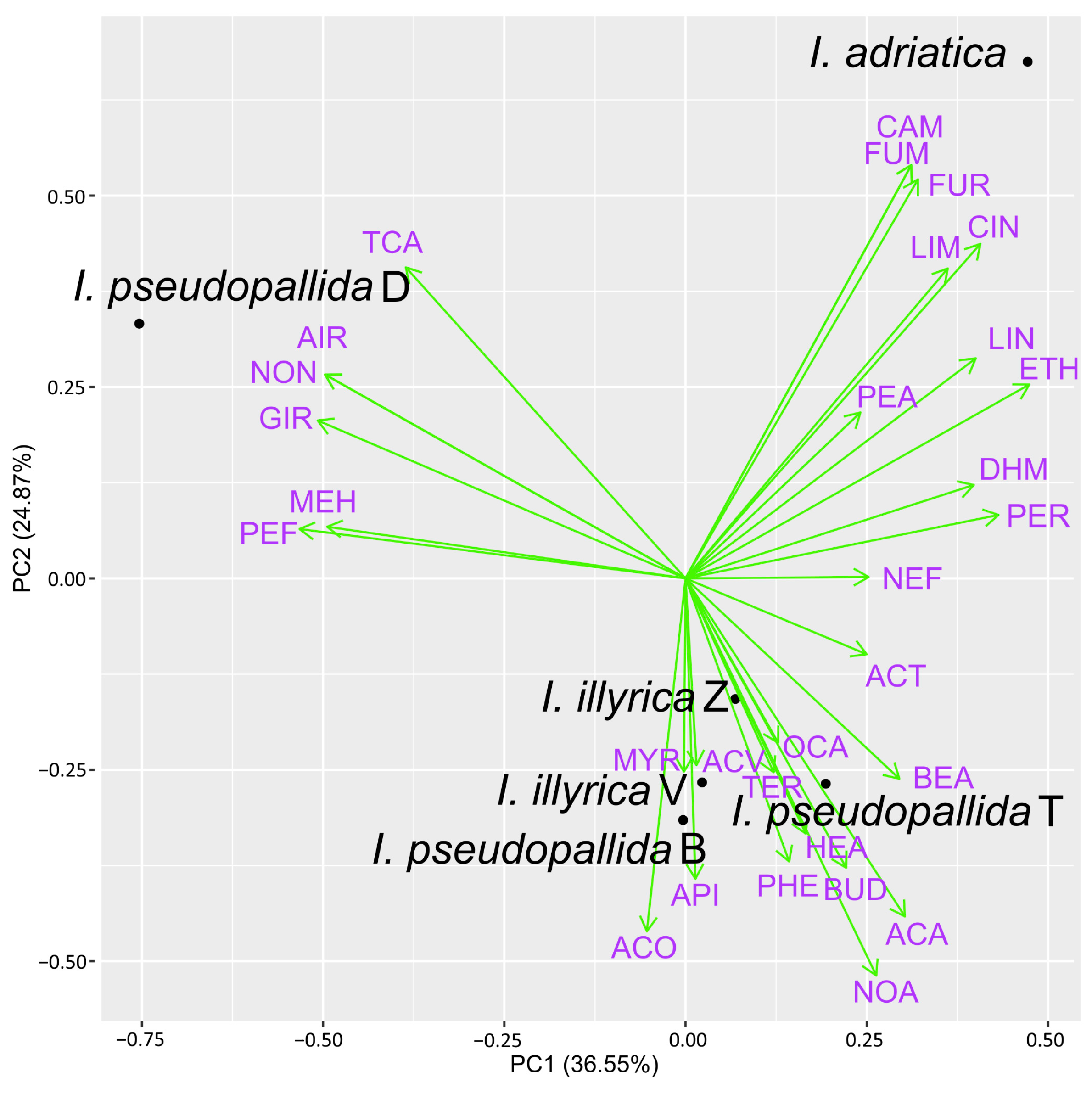
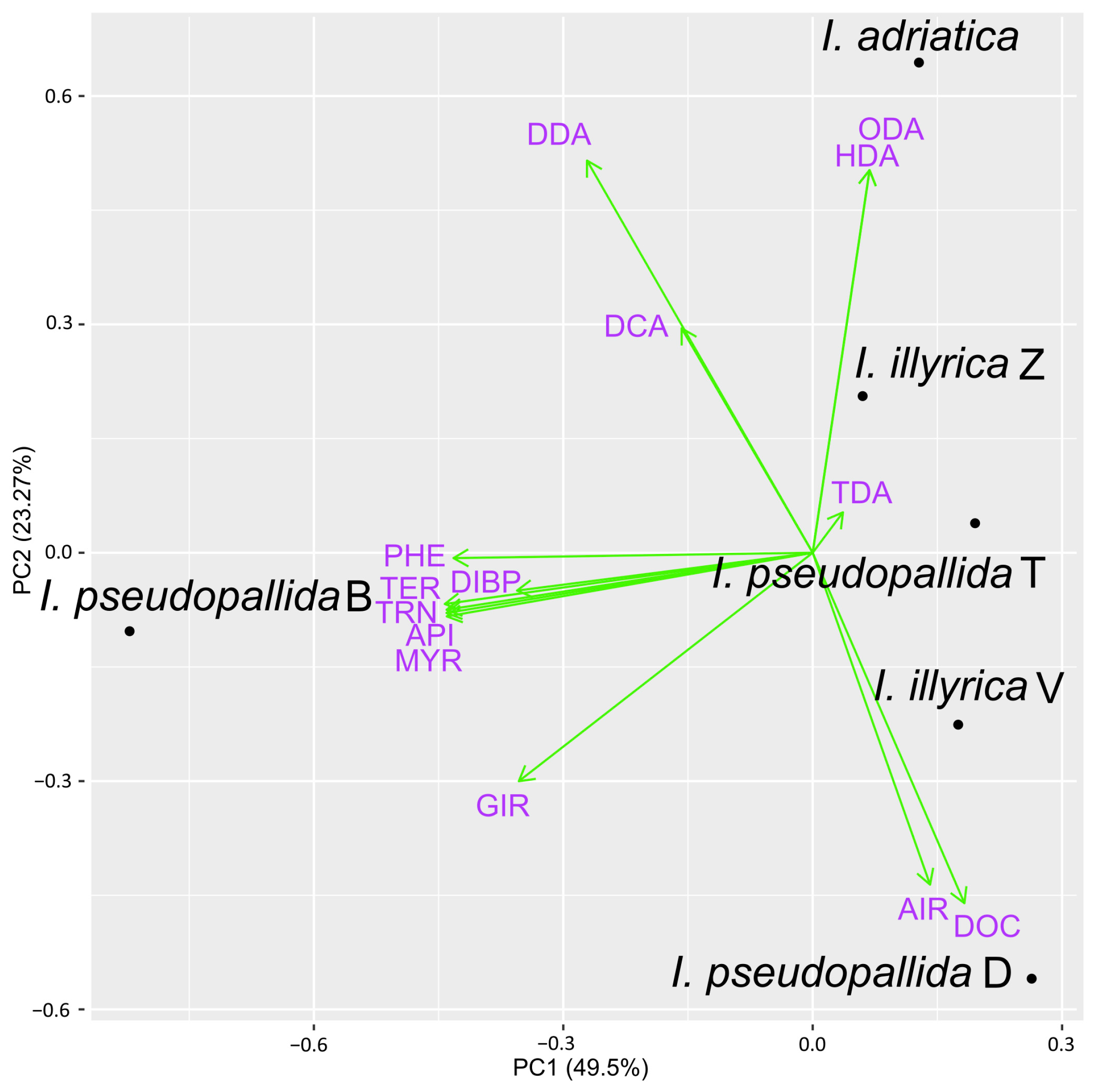
2.3. PCA Analysis of Major VOCs and EOs Constituents
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Solid-Phase Microextraction (SPME) Fibers and Extraction Procedure
4.3. Hydrodistillation (HD)
4.4. GC–MS Analysis
4.5. Principal Component Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giannenas, I.; Sidiropoulou, E.; Bonos, E.; Christaki, E.; Florou-Paneri, P. The history of herbs, medicinal and aromatic plants, and their extracts: Past, current situation and future perspectives. In Feed Additives: Aromatic Plants and Herbs in Animal Nutrition and Health; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Academic Press: London, UK, 2020; pp. 1–18. [Google Scholar]
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental chemistry of essential oils and volatile organic compounds, methods of analysis and authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef]
- Bicchi, C.; Joulain, D. A comprehensive review on essential oils and extracts from Iris rhizomes. Phytochem. Rev. 2024, 1–37. [Google Scholar] [CrossRef]
- Crişan, I.; Cantor, M. New perspectives on medicinal properties and uses of Iris sp. Hop Med. Plants 2016, 24, 24–36. [Google Scholar]
- Knothe, G.; Dunn, R.O. A comprehensive evaluation of the melting points of fatty acids and esters determined by differential scanning calorimetry. J. Am. Oil Chem. Soc. 2009, 86, 843–856. [Google Scholar] [CrossRef]
- Wajs, A.; Pranovich, A.; Reunanen, M.; Willför, S.; Holmbom, B. Characterisation of volatile organic compounds in stemwood using solid-phase microextraction. Phytochem. Anal. 2006, 17, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Pan, H.-W.; Wang, P.-H.; Yang, X.-D.; Zhai, W.-C.; Dong, Y.; Zhou, H.-L. Essential oils from Carex meyeriana Kunth: Optimization of hydrodistillation extraction by response surface methodology and evaluation of its antioxidant and antimicrobial activities. Ind. Crops Prod. 2018, 124, 669–676. [Google Scholar] [CrossRef]
- El Amine Dib, M.; Djabou, N.; Desjobert, J.-M.; Allali, H.; Tabti, B.; Muselli, A.; Costa, J. Characterization of volatile compounds of Daucus crinitus Desf. headspace solid phase microextraction as alternative technique to hydrodistillation. Chem. Cent. J. 2010, 4, 16. [Google Scholar] [CrossRef][Green Version]
- Jerković, I.; Marijanović, Z.; Radonić, A.; Zekić, M.; Kranjac, M. The application of headspace solid-phase microextraction as a preparation approach for gas chromatography with mass spectrometry. Kem. Ind. 2020, 69, 515–520. [Google Scholar] [CrossRef]
- Aramrueang, N.; Asavasanti, S.; Khanunthong, A. Leafy vegetables. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Academic Press: London, UK, 2019; pp. 245–272. [Google Scholar]
- Choi, B.; Weiss-Schneeweiss, H.; Temsch, E.M.; So, S.; Myeong, H.-H.; Jang, T.-S. Genome size and chromosome number evolution in Korean Iris L. species (Iridaceae Juss.). Plants 2020, 9, 1284. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Sieniawska, E.; Widelski, J.; Urjin, O.; Głowniak, P.; Skalicka-Woźniak, K. Major secondary metabolites of Iris spp. Phytochem. Rev. 2015, 14, 51–80. [Google Scholar] [CrossRef]
- Adams, M.; Berset, C.; Kessler, M.; Hamburger, M. Medicinal herbs for the treatment of rheumatic disorders—A survey of European herbals from the 16th and 17th century. J. Ethnopharmacol. 2009, 121, 343–359. [Google Scholar] [CrossRef]
- Khatib, S.; Faraloni, C.; Bouissane, L. Exploring the use of Iris species: Antioxidant properties, phytochemistry, medicinal and industrial applications. Antioxidants 2022, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Singab, A.N.B.; Ayoub, I.M.; El-Shazly, M.; Korinek, M.; Wu, T.-Y.; Cheng, Y.-B.; Chang, F.-R.; Wu, Y.-C. Shedding the light on Iridaceae: Ethnobotany, phytochemistry and biological activity. Ind. Crops Prod. 2016, 92, 308–335. [Google Scholar] [CrossRef]
- Alperth, F.; Mitić, B.; Mayer, S.; Maleš, Ž.; Kunert, O.; Hruševar, D.; Bucar, F. Metabolic profiling of rhizomes of native populations of the strictly endemic Croatian species Iris adriatica. Plant Biosyst. 2019, 153, 317–324. [Google Scholar] [CrossRef]
- Duka, I.; Maleš, Ž.; Bojić, M.; Hruševar, D.; Mitić, B. Chemical fingerprinting, total phenolics and antioxidant activity of some Iris taxa. Croat. Chem. Acta 2020, 93, 49–56. [Google Scholar] [CrossRef]
- Basgedik, B.; Ugur, A.; Sarac, N. Antimicrobial, antioxidant, antimutagenic activities, and phenolic compounds of Iris germanica. Ind. Crops Prod. 2014, 61, 526–530. [Google Scholar] [CrossRef]
- Ullah, F.; Ayaz, M.; Sadiq, A.; Hussain, A.; Ahmad, S.; Imran, M.; Zeb, A. Phenolic, flavonoid contents, anticholinesterase and antioxidant evaluation of Iris germanica var. florentina. Nat. Prod. Res. 2016, 30, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Başer, K.H.C.; Demirci, B.; Orhan, I.E.; Kartal, M.; Sekeroglu, N.; Sener, B. Composition of volatiles from three Iris species of Turkey. J. Essent. Oil Res. 2011, 23, 66–71. [Google Scholar] [CrossRef]
- Amin, H.I.M.; Amin, A.A.; Tosi, S.; Mellerio, G.G.; Hussain, F.H.S.; Picco, A.M.; Vidari, G. Chemical composition and antifungal activity of essential oils from flowers, leaves, rhizomes, and bulbs of the wild Iraqi Kurdish plant Iris persica. Nat. Prod. Commun. 2017, 12, 441–444. [Google Scholar] [CrossRef]
- Almaarri, K.; Zedan, T.A.; Albatal, N. Chemical analysis of essential oils of some Syrian wild Iris species. Am. J. Biochem. Mol. Biol. 2013, 3, 38–49. [Google Scholar] [CrossRef]
- Deng, G.-B.; Zhang, H.-B.; Xue, H.-F.; Chen, S.-N.; Chen, X.-L. Chemical composition and biological activities of essential oil from the rhizomes of Iris bulleyana. Agric. Sci. China 2009, 8, 691–696. [Google Scholar] [CrossRef]
- Roger, B.; Fernandez, X.; Jeannot, V.; Chahboun, J. An alternative method for irones quantification in Iris rhizomes using headspace solid-phase microextraction. Phytochem. Anal. 2010, 21, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, Y.; Zhao, Y.; Liu, C.; Chen, X.; Li, F.; Bao, J. Identification of floral scent profiles in bearded irises. Molecules 2019, 24, 1773. [Google Scholar] [CrossRef]
- Sun, J.; Tian, K.; Jing, L.; Niu, Y.; Lou, Q.; Chen, H. Identification of characteristic aroma compounds for spicy in Iris lactea var. chinensis. Physiol. Plant. 2023, 175, e14016. [Google Scholar] [CrossRef]
- Mitić, B.; Cigić, P. Hrvatski vrt perunika i poučna botanička staza u Donjoj Stubici; Hrvatsko Botaničko Društvo: Zagreb, Croatia, 2009. [Google Scholar]
- Nikolić, T.; Milović, M.; Bogdanović, S.; Jasprica, N. Endemi u hrvatskoj flori; Alfa: Zagreb, Croatia, 2015. [Google Scholar]
- Al-Jaber, H.I. Variation in essential oil composition of Iris nigricans Dinsm. (Iridaceae) endemic to Jordan at different flowering stages. Arab. J. Chem. 2016, 9, S1190–S1196. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Rodrigues, F.; Caldeira, M.; Câmara, J.S. Development of a dynamic headspace solid-phase microextraction procedure coupled to GC–qMSD for evaluation the chemical profile in alcoholic beverages. Anal. Chim. Acta 2008, 609, 82–104. [Google Scholar] [CrossRef]
- Pinheiro, G.P.; Galbiatti, M.I.; Carneiro, M.J.; Sawaya, A. Comparison of four different solid-phase microextraction fibers for analysis of Plectranthus amboinicus (Lour.) Spreng. leaf volatiles. Adv. Med. Plant Res. 2019, 7, 38–43. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. NIST Standard Reference Database Number 69. 2023. Available online: http://webbook.nist.gov/chemistry (accessed on 16 August 2024).
- Öztaş, F.; Türkmen, A.; Öztaş, H.; Türkmen, M. The medical properties of Iris and its usage in pharmaceutical, perfumery and cosmetic industries. In Medical Research and Its Applications; Veeramani, V.P., Ed.; BP International: London, UK, 2024; Volume 4, pp. 114–124. [Google Scholar]
- Kovačić, S. Plethora of Plants—Collections of the Botanical Garden, Faculty of Science, University of Zagreb (3): Iris (Iridaceae) collection. Nat. Croat. 2019, 28, 483–514. [Google Scholar] [CrossRef]
- Crișan, I.; Vidican, R.; Olar, L.; Stoian, V.; Morea, A.; Ștefan, R. Screening for changes on Iris germanica L. rhizomes following inoculation with arbuscular mycorrhiza using Fourier transform infrared spectroscopy. Agronomy 2019, 9, 815. [Google Scholar] [CrossRef]
- Gooderham, N.J.; Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Rosol, T.J.; Davidsen, J.M.; Harman, C.L.; et al. FEMA GRAS assessment of natural flavor complexes: Sage oil, orris root extract and tagetes oil and related flavoring ingredients. Food Chem. Toxicol. 2023, 179, 113940. [Google Scholar] [CrossRef]
- Li, F.; Sun, Y.; Liu, C.; Yuan, Y.; Zheng, L.; Chen, X.; Bao, J. Genetic diversity and population structure in bearded iris cultivars derived from Iris × germanica L. and its related species I. pumila L., I. variegata L., I. pallida Lam. Genet. Resour. Crop Evol. 2020, 67, 2161–2172. [Google Scholar] [CrossRef]
- Radanova, S.S. Plants in the national symbolism of European countries: A link among countries, cultures, and religions. Asian J. Res. Bot. 2023, 6, 158–171. [Google Scholar]
- Pheko-Ofitlhile, T.; Makhzoum, A. Impact of hydrodistillation and steam distillation on the yield and chemical composition of essential oils and their comparison with modern isolation techniques. J. Essent. Oil Res. 2024, 36, 105–115. [Google Scholar] [CrossRef]
- Kara, N.; Baydar, H. Scent components in essential oil, resinoids and absolute of Iris (Iris florentina L.). Anadolu Tarim Bilim. Derg. 2014, 29, 70–74. [Google Scholar] [CrossRef]
- Mykhailenko, O. Composition of volatile oil of Iris pallida Lam. from Ukraine. Turk. J. Pharm. Sci. 2018, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Isaev, D.I.; Mikhailenko, O.A.; Gurbanov, G.M.; Kovalev, V.N. Constituents of essential oils from Azerbaijan Iris medwedewii and I. carthaliniae rhizomes. Chem. Nat. Compd. 2016, 52, 748–750. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Kovalyov, V.; Orlova, T. Chemical composition of the essential oil of several Iris species. Thai J. Pharm. Sci. 2020, 44, 179–185. [Google Scholar]
- Zang, J.; Xia, G.; Xueming, L. Physiochemical properties of Ma Lin Zi (seed of I. pallasii) oil and identification of its fatty acids. Zhongcaoyao 1983, 14, 103–105. [Google Scholar]
- Luan, Z.-J.; Li, P.-P.; Li, D.; Meng, X.-P.; Sun, J. Optimization of supercritical-CO2 extraction of Iris lactea seed oil: Component analysis and antioxidant activity of the oil. Ind. Crops Prod. 2020, 152, 112553. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Gudžinskas, Z.; Romanova, S.; Orlova, T.; Kozyra, S.; Harna, S.; Volochai, V. The comparative analysis of carboxylic acid composition of four Iris species from Ukraine. Chem. Biodivers. 2021, 18, e2000969. [Google Scholar] [CrossRef] [PubMed]
- Isayev, J.I.; Mykhailenko, O.O.; Kovalyov, V.M.; Gurbanov, G.M.; Suleymanov, M.Y. Gas chromatography-mass spectrometry studies of the component composition of carboxylic acids of the rhizomes of Iris medwedewii and Iris carthaliniae (Iridaceae). Ceska Slov. Farm. 2017, 66, 9–14. [Google Scholar] [PubMed]
- Chikhi, I.; Allali, H.; Dib, M.E.A.; Halla, N.; Muselli, A.; Tabti, B.; Costa, J. Free radical scavenging and antibacterial activity of essential oil and solvent extracts of Iris planifolia (Mill) from Algeria. J. Med. Plants Res. 2012, 6, 1961–1968. [Google Scholar]
- Yang, M.; Zhou, M.; Song, L. A review of fatty acids influencing skin condition. J. Cosmet. Dermatol. 2020, 19, 3199–3204. [Google Scholar] [CrossRef]
- Agnihotri, S.; Yin, D.M.; Mahboubi, A.; Sapmaz, T.; Varjani, S.; Qiao, W.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. A glimpse of the world of volatile fatty acids production and application: A review. Bioengineered 2022, 13, 1249–1275. [Google Scholar] [CrossRef]
- Kudlejova, L.; Risticevic, S.; Vuckovic, D. Solid-phase microextraction method development. In Handbook of Solid Phase Microextraction; Pawliszyn, J., Ed.; Elsevier: Oxford, UK, 2012; pp. 201–249. [Google Scholar]
- Gianelli, M.P.; Flores, M.; Toldrá, F. Optimisation of solid phase microextraction (SPME) for the analysis of volatile compounds in dry-cured ham. J. Sci. Food Agric. 2002, 82, 1703–1709. [Google Scholar] [CrossRef]
- Marco, A.; Navarro, J.L.; Flores, M. Volatile compounds of dry-fermented sausages as affected by solid-phase microextraction (SPME). Food Chem. 2004, 84, 633–641. [Google Scholar] [CrossRef]
- Yu, A.-N.; Sun, B.-G.; Tian, D.-T.; Qu, W.-Y. Analysis of volatile compounds in traditional smoke-cured bacon (CSCB) with different fiber coatings using SPME. Food Chem. 2008, 110, 233–238. [Google Scholar] [CrossRef]
- Mariano, A.P.X.; Ramos, A.L.C.C.; de Oliveira Júnior, A.H.; García, Y.M.; de Paula, A.C.C.F.F.; Silva, M.R.; Augusti, R.; de Araújo, R.L.B.; Melo, J.O.F. Optimization of extraction conditions and characterization of volatile organic compounds of Eugenia klotzschiana O. Berg fruit pulp. Molecules 2022, 27, 935. [Google Scholar] [CrossRef]
- Marić, T.; Friščić, M.; Marijanović, Z.; Maleš, Ž.; Jerković, I. Comparison of volatile organic compounds of Sideritis romana L. and Sideritis montana L. from Croatia. Molecules 2021, 26, 5968. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Zhang, W.; Dong, J.-H.; Wu, H.; Wang, Y.-H.; Xiao, H.-X. Optimization of SPME–GC–MS and characterization of floral scents from Aquilegia japonica and A. amurensis flowers. BMC Chem. 2021, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Ban, Z.; Xu, H.; Chen, W.; Jia, W.; Zhu, Y.; Chen, H. Analysis of floral scent component of three Iris species at different stages. Horticulturae 2024, 10, 153. [Google Scholar] [CrossRef]
- Weber, T.; Jakše, J.; Sladonja, B.; Hruševar, D.; Landeka, N.; Brana, S.; Bohanec, B.; Milović, M.; Vladović, D.; Mitić, B.; et al. Molecular study of selected taxonomically critical taxa of the genus Iris L. from the broader Alpine-Dinaric area. Plants 2020, 9, 1229. [Google Scholar] [CrossRef] [PubMed]
- You, C.-X.; Wang, Y.; Zhang, W.-J.; Yang, K.; Wu, Y.; Geng, Z.-F.; Chen, H.-P.; Jiang, H.-Y.; Du, S.-S.; Deng, Z.-W.; et al. Chemical constituents and biological activities of the purple Perilla essential oil against Lasioderma serricorne. Ind. Crops Prod. 2014, 61, 331–337. [Google Scholar] [CrossRef]
- Stevens, M.A. Relationship between polyene-carotene content and volatile compound composition of tomatoes. J. Am. Soc. Hortic. Sci. 1970, 95, 461–464. [Google Scholar] [CrossRef]
- Machado, G.; Leon, S.; Santos, F.; Lourega, R.; Dullius, J.; Mollmann, M.E.; Eichler, P. Literature review on furfural production from lignocellulosic biomass. Nat. Resour. 2016, 7, 115–129. [Google Scholar] [CrossRef]
- Maleš, I.; Dragović-Uzelac, V.; Jerković, I.; Zorić, Z.; Pedisić, S.; Repajić, M.; Garofulić, I.E.; Dobrinčić, A. Non-volatile and volatile bioactives of Salvia officinalis L., Thymus serpyllum L. and Laurus nobilis L. extracts with potential use in the development of functional beverages. Antioxidants 2022, 11, 1140. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Roje, M.; Jokić, S. Chemical diversity of headspace and volatile oil composition of two brown algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea. Molecules 2019, 24, 495. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 29 July 2024).
- Tang, Y.; Horikoshi, M.; Li, W. ggfortify: Unified interface to visualize statistical results of popular R packages. R J. 2016, 8, 474. [Google Scholar] [CrossRef]
- Horikoshi, M.; Tang, Y. ggfortify: Data Visualization Tools for Statistical Analysis Results. 2016. Available online: https://CRAN.R-project.org/package=ggfortify (accessed on 29 July 2024).

| No. | Compound | RI | RIL | RIL Reference | SI | I. pseudopallida B | I. pseudopallida D | I. pseudopallida T | I. illyrica V | I. illyrica Z | I. adriatica |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aliphatic and Aromatic Alcohols and Phenols | |||||||||||
| 1 | Ethanol | <900 | 448 | [33] | 97% | 2.21 | 1.39 | 6.86 | 4.79 | 3.65 | 2.66 |
| 5 | Pentan-1-ol | <900 | 779 | [33] | 98% | 0.41 | - | - | - | - | 0.93 |
| 6 | Butan-2,3-diol | <900 | 802 | [33] | 97% | 1.78 | - | 6.38 | 5.29 | 10.27 | 2.18 |
| 9 | (Furan-2-yl)methanol | <900 | 864 | [33] | 96% | - | - | - | - | - | 2.75 |
| 10 | Hexan-1-ol | <900 | 867 | [33] | 96% | 0.18 | - | 0.38 | 0.73 | 1.14 | 1.09 |
| 11 | 2-Butoxyethanol | 912 | 912 | [33] | 98% | 0.24 | - | - | 0.58 | 0.75 | 0.95 |
| 14 | 1-Butoxypropan-2-ol | 949 | 945 | [33] | 95% | 0.34 | - | - | 0.31 | 1.27 | 1.39 |
| 17 | Phenol | 986 | 987 | [33] | 99% | - | - | 0.20 | 0.36 | 0.36 | 0.53 |
| 24 | 2-Ethylhexan-1-ol | 1035 | 1034 | [33] | 97% | 0.85 | - | 0.40 | 0.49 | 1.82 | 1.66 |
| 27 | Benzyl alcohol | 1042 | 1042 | [33] | 99% | 2.03 | - | 5.46 | 3.40 | 2.46 | 2.40 |
| 33 | Octan-1-ol | 1076 | 1076 | [33] | 98% | 0.50 | - | 0.35 | - | 0.34 | 0.47 |
| 38 | Nonan-2-ol | 1103 | 1102 | [33] | 96% | - | 0.50 | - | - | - | - |
| 41 | 2-Phenylethanol | 1120 | 1120 | [33] | 99% | 4.67 | - | 1.76 | 1.70 | 4.76 | 0.90 |
| 69 | Dodecan-1-ol | 1479 | 1478 | [33] | 97% | - | - | 1.40 | - | - | - |
| Total identified (%) | 13.21 | 1.89 | 23.19 | 17.65 | 26.82 | 17.91 | |||||
| Fatty Acids and Fatty Acid Esters | |||||||||||
| 2 | Acetic acid | <900 | 600 | [33] | 97% | 2.88 | - | 6.29 | 6.95 | 1.91 | 3.74 |
| 16 | Hexanoic (caproic) acid | 979 | 977 | [33] | 98% | 0.37 | - | 0.50 | 0.65 | - | - |
| 42 | Methyl octanoate | 1131 | 1127 | [33] | 97% | - | - | 1.00 | - | - | - |
| 46 | Octanoic (caprylic) acid | 1181 | 1180 | [33] | 97% | - | - | 2.15 | - | - | - |
| 49 | Ethyl octanoate | 1198 | 1196 | [33] | 98% | - | - | 1.05 | - | 0.36 | - |
| 63 | Methyl decanoate | 1330 | 1328 | [33] | 98% | 0.26 | - | 0.57 | - | - | - |
| Total identified (%) | 3.51 | - | 11.56 | 7.60 | 2.27 | 3.74 | |||||
| Aliphatic and Aromatic Aldehydes | |||||||||||
| 3 | Pentanal | <900 | 698 | [33] | 98% | - | 3.28 | - | 2.48 | - | - |
| 7 | Hexanal | <900 | 799 | [33] | 97% | 0.81 | - | 0.71 | 2.57 | 1.14 | 0.96 |
| 8 | Furfural | <900 | 848 | [33] | 98% | - | - | - | 0.31 | 0.34 | 1.80 |
| 15 | Benzaldehyde | 971 | 972 | [33] | 99% | 0.68 | - | 1.08 | 0.87 | 0.85 | 1.31 |
| 22 | 1H-Pyrrole-2-carboxaldehyde | 1014 | 1015 | [33] | 95% | - | - | - | - | - | 1.10 |
| 28 | Phenylacetaldehyde | 1052 | 1051 | [33] | 98% | - | 1.16 | - | - | - | 0.56 |
| 29 | 1-Ethyl-2-formyl pyrrole | 1058 | 1046 | [33] | 95% | - | - | - | - | - | 0.82 |
| 40 | Nonanal | 1109 | 1108 | [33] | 98% | 2.78 | - | 3.25 | 3.55 | 3.10 | 1.22 |
| 53 | Decanal | 1210 | 1210 | [33] | 99% | 0.62 | - | 2.35 | - | 0.74 | 0.49 |
| 62 | Undecanal | 1311 | 1309 | [33] | 98% | - | - | 0.76 | - | - | - |
| 66 | Dodecanal | 1413 | 1412 | [33] | 97% | - | - | 1.01 | - | - | - |
| Total identified (%) | 4.89 | 4.44 | 9.16 | 9.78 | 6.17 | 8.26 | |||||
| Aliphatic and Aromatic Ketones | |||||||||||
| 4 | Acetoin | <900 | 720 | [33] | 98% | - | - | 2.84 | 2.86 | 1.39 | 1.04 |
| 19 | 6-Methylhept-5-en-2-one | 992 | 991 | [33] | 99% | 1.98 | 3.59 | 1.61 | 3.21 | 9.47 | 1.33 |
| 31 | 2-Acetylpyrrole | 1068 | 1065 | [33] | 96% | - | - | 0.29 | - | - | - |
| 32 | Acetophenone | 1074 | 1072 | [33] | 96% | 0.16 | - | - | - | - | 0.06 |
| 36 | Nonan-2-one | 1091 | 1091 | [33] | 98% | - | 4.99 | - | - | - | - |
| 60 | Undecan-2-one | 1297 | 1296 | [33] | 97% | 0.43 | 2.01 | 1.28 | 1.60 | 0.43 | 0.13 |
| 70 | Acetovanillone | 1491 | 1491 | [33] | 97% | 0.11 | - | - | 7.12 | - | - |
| Total identified (%) | 2.68 | 10.59 | 6.02 | 14.79 | 11.29 | 2.56 | |||||
| Lactone | |||||||||||
| 12 | γ-Butyrolactone | 922 | 925 | [33] | 96% | 0.24 | - | 0.98 | 0.70 | 0.92 | 0.85 |
| Monoterpene Hydrocarbons | |||||||||||
| 13 | α-Pinene | 945 | 942 | [33] | 98% | 4.48 | - | 0.20 | 2.40 | 1.11 | - |
| 18 | β-Pinene | 986 | 985 | [33] | 98% | 0.75 | - | - | - | - | - |
| 20 | β-Myrcene | 996 | 997 | [33] | 97% | - | - | 0.50 | - | - | 0.65 |
| 23 | p-Cymene | 1033 | 1030 | [33] | 98% | 0.85 | 0.18 | - | 0.27 | 0.46 | 0.37 |
| 25 | Limonene | 1037 | 1035 | [33] | 98% | 1.46 | - | 0.90 | 0.88 | 0.57 | 4.79 |
| 30 | γ-Terpinene | 1067 | 1064 | [33] | 99% | - | 0.42 | - | - | - | - |
| Total identified (%) | 7.54 | 0.60 | 1.60 | 3.55 | 2.14 | 5.81 | |||||
| Furan | |||||||||||
| 21 | 2-Pentylfuran | 997 | 998 | [33] | 97% | 0.53 | 0.84 | - | 0.58 | 1.35 | - |
| Oxygenated Monoterpenes | |||||||||||
| 26 | 1,8-Cineole | 1041 | 1037 | [33] | 98% | 0.68 | - | 2.65 | 0.34 | 1.16 | 3.82 |
| 34 | Dihydromyrcenol | 1078 | 1075 | [33] | 96% | 0.95 | - | 3.03 | 1.60 | 3.46 | 2.45 |
| 35 | trans-Linalool oxide | 1080 | 1081 | [33] | 96% | 0.27 | - | - | - | - | - |
| 37 | 6-Camphenone | 1101 | 1095 | [33] | 95% | 0.43 | - | - | - | - | - |
| 39 | Linalool | 1104 | 1102 | [33] | 98% | 1.83 | - | 1.91 | 0.75 | 1.64 | 2.57 |
| 43 | trans-Pinocarveol | 1147 | 1147 | [33] | 97% | 0.85 | - | - | - | - | - |
| 44 | Camphor | 1152 | 1149 | [33] | 99% | - | - | - | - | - | 3.25 |
| 45 | Borneol | 1173 | 1172 | [33] | 99% | 0.53 | - | 0.22 | - | - | 0.47 |
| 47 | Terpinen-4-ol | 1184 | 1184 | [33] | 98% | 0.33 | - | 0.50 | - | - | 0.51 |
| 48 | α-Terpineol | 1196 | 1195 | [33] | 98% | 3.19 | - | 1.53 | 0.66 | - | 0.76 |
| 50 | Myrtenol | 1199 | 1198 | [33] | 97% | 6.33 | - | - | - | - | - |
| 54 | β-Citronellol | 1234 | 1232 | [33] | 97% | - | - | 1.35 | - | - | - |
| 55 | Carvacrol methyl ether | 1241 | 1246 | [33] | 96% | 0.72 | - | - | - | - | - |
| 56 | Neryl formate | 1262 | 1261 | [33] | 96% | - | - | 4.02 | - | - | 1.20 |
| 57 | (E)-Citral | 1276 | 1278 | [33] | 96% | 0.96 | - | 1.43 | - | - | - |
| 58 | Perilla aldehyde | 1279 | 1279 | [33] | 97% | 20.55 | - | 17.76 | 19.72 | 30.00 | 26.83 |
| 59 | Bornyl acetate | 1290 | 1288 | [33] | 98% | 0.38 | - | - | - | - | 1.38 |
| Total identified (%) | 38.00 | - | 34.40 | 23.07 | 36.26 | 43.24 | |||||
| Alkanes | |||||||||||
| 51 | Dodecane | 1200 | 1200 | [33] | 98% | - | - | 1.72 | - | - | - |
| 61 | Tridecane | 1300 | 1300 | [33] | 97% | - | - | 1.00 | - | - | - |
| 65 | Tetradecane | 1400 | 1400 | [33] | 98% | 0.34 | - | 1.20 | 0.25 | 0.25 | 0.52 |
| 72 | Pentadecane | 1500 | 1500 | [33] | 97% | - | - | - | - | - | 0.52 |
| Total identified (%) | 0.34 | - | 3.92 | 0.25 | 0.25 | 1.04 | |||||
| Norisoprenoids | |||||||||||
| 52 | Safranal | 1204 | 1205 | [33] | 98% | 1.46 | - | - | - | 1.69 | - |
| 75 | cis-α-Irone | 1544 | 1546 | [33] | 97% | - | 45.76 | - | - | - | - |
| 76 | cis-γ-Irone * | 1551 | - | [24] | 95% | 1.87 | 7.17 | - | - | - | - |
| Total identified (%) | 3.33 | 52.93 | - | - | 1.69 | - | |||||
| Sesquiterpene Hydrocarbons | |||||||||||
| 64 | α-Copaene | 1381 | 1376 | [33] | 96% | 5.14 | 1.21 | 2.02 | 3.62 | 2.61 | - |
| 67 | trans-Caryophyllene | 1424 | 1423 | [33] | 97% | 3.40 | 7.24 | 1.51 | 1.97 | 2.67 | 4.51 |
| 68 | α-Humulene | 1459 | 1459 | [33] | 98% | 0.73 | 0.27 | 0.42 | 0.66 | 0.80 | 1.13 |
| 71 | α-Farnesene | 1499 | 1503 | [33] | 97% | 0.81 | 1.09 | 1.19 | 1.99 | 0.98 | 1.94 |
| 73 | α-Muurolene | 1504 | 1505 | [33] | 96% | 0.62 | 1.73 | - | - | - | - |
| 74 | δ-Cadinene | 1520 | 1519 | [33] | 98% | - | 1.17 | - | - | - | - |
| Total identified (%) | 10.70 | 12.71 | 5.14 | 8.24 | 7.06 | 7.58 | |||||
| Aromatic Ester | |||||||||||
| 77 | Benzyl benzoate | 1769 | 1770 | [33] | 97% | - | - | 0.23 | - | - | - |
| Total amount of identified compounds (%) | 84.97 | 84.00 | 96.20 | 86.21 | 96.22 | 90.99 | |||||
| No. | Compound | RI | RIL | RIL Reference | SI | I. pseudopallida B | I. pseudopallida D | I. pseudopallida T | I. illyrica V | I. illyrica Z | I. adriatica |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aliphatic and Aromatic Alcohols and Phenols | |||||||||||
| 1 | Ethanol | <900 | 448 | [33] | 97% | 4.75 | 3.22 | 4.70 | 5.23 | 4.34 | 7.01 |
| 5 | Pentan-1-ol | <900 | 779 | [33] | 97% | - | - | - | 1.70 | - | 0.82 |
| 6 | Butan-2,3-diol | <900 | 802 | [33] | 97% | 2.41 | - | 7.05 | 7.83 | 11.11 | 2.43 |
| 9 | (Furan-2-yl)methanol | <900 | 864 | [33] | 96% | - | - | - | - | - | 3.08 |
| 10 | Hexan-1-ol | <900 | 867 | [33] | 96% | 0.96 | - | 0.14 | 1.29 | 0.30 | 0.89 |
| 11 | 2-Butoxyethanol | 912 | 912 | [33] | 97% | 0.43 | - | 0.18 | - | 0.42 | 0.38 |
| 14 | 1-Butoxypropan-2-ol | 949 | 945 | [33] | 95% | 0.29 | - | - | - | 0.81 | 0.80 |
| 17 | Phenol | 986 | 987 | [33] | 99% | 0.58 | - | - | - | - | - |
| 23 | 2-Ethylhexan-1-ol | 1035 | 1034 | [33] | 97% | 0.82 | - | - | - | 1.13 | 1.36 |
| 26 | Benzyl alcohol | 1042 | 1042 | [33] | 99% | 1.52 | - | 5.08 | 1.58 | 1.23 | 1.62 |
| 30 | Octan-1-ol | 1076 | 1076 | [33] | 98% | 0.59 | - | 0.28 | - | - | 0.27 |
| 35 | Nonan-2-ol | 1103 | 1102 | [33] | 98% | - | 0.51 | - | - | - | - |
| 38 | 2-Phenylethanol | 1120 | 1120 | [33] | 99% | 4.31 | - | 1.38 | 0.77 | 3.36 | 0.83 |
| 65 | Dodecan-1-ol | 1479 | 1478 | [33] | 97% | - | - | 0.93 | - | - | - |
| Total identified (%) | 16.66 | 3.73 | 19.74 | 18.40 | 22.70 | 19.49 | |||||
| Fatty Acids and Fatty Acid Esters | |||||||||||
| 2 | Acetic acid | <900 | 600 | [33] | 96% | 14.29 | - | 29.76 | 29.20 | 31.02 | 12.00 |
| 16 | Hexanoic (caproic) acid | 979 | 977 | [33] | 98% | 0.52 | - | 0.67 | 1.64 | - | - |
| 39 | Methyl octanoate | 1131 | 1127 | [33] | 97% | - | 0.43 | 1.27 | - | - | - |
| 42 | Octanoic (caprylic) acid | 1181 | 1180 | [33] | 96% | - | - | 2.35 | - | - | - |
| 45 | Ethyl octanoate | 1198 | 1196 | [33] | 97% | - | - | 0.61 | - | - | - |
| 58 | Methyl decanoate | 1330 | 1328 | [33] | 98% | 0.30 | 0.51 | 0.67 | - | - | - |
| Total identified (%) | 15.11 | 0.94 | 35.33 | 30.84 | 31.02 | 12.00 | |||||
| Aliphatic and Aromatic Aldehydes | |||||||||||
| 3 | Pentanal | <900 | 698 | [33] | 98% | - | - | - | 2.65 | - | 2.31 |
| 7 | Hexanal | <900 | 799 | [33] | 97% | 1.46 | - | 1.49 | 4.67 | 1.30 | 1.04 |
| 8 | Furfural | <900 | 848 | [33] | 98% | - | - | - | 1.03 | 0.11 | 7.91 |
| 15 | Benzaldehyde | 971 | 972 | [33] | 99% | 0.93 | 0.67 | 1.11 | 0.88 | 0.68 | 1.01 |
| 21 | 1H-Pyrrole-2-carboxaldehyde | 1014 | 1015 | [33] | 96% | - | - | - | - | - | 0.51 |
| 27 | Phenylacetaldehyde | 1052 | 1051 | [33] | 98% | - | 0.99 | - | - | - | 0.38 |
| 28 | 1-Ethyl-2-formyl pyrrole | 1058 | 1046 | [33] | 95% | - | - | - | - | - | 0.92 |
| 37 | Nonanal | 1109 | 1108 | [33] | 97% | 2.78 | - | 2.71 | 2.07 | 1.24 | 0.90 |
| 49 | Decanal | 1210 | 1210 | [33] | 99% | 0.50 | - | 1.46 | 0.17 | - | 0.36 |
| 61 | Dodecanal | 1413 | 1412 | [33] | 98% | - | - | 0.58 | - | - | - |
| Total identified (%) | 5.67 | 1.66 | 7.35 | 11.47 | 3.33 | 15.34 | |||||
| Aliphatic and Aromatic Ketones | |||||||||||
| 4 | Acetoin | <900 | 720 | [33] | 98% | - | - | 2.01 | 2.42 | 5.27 | 2.11 |
| 18 | 6-Methylhept-5-en-2-one | 992 | 991 | [33] | 99% | 2.61 | 9.10 | 1.30 | 3.14 | 5.83 | 0.81 |
| 29 | Acetophenone | 1074 | 1072 | [33] | 97% | 0.11 | - | - | - | - | - |
| 33 | Nonan-2-one | 1091 | 1091 | [33] | 96% | - | 5.11 | - | - | - | - |
| 56 | Undecan-2-one | 1297 | 1296 | [33] | 97% | 0.21 | 1.61 | - | 0.63 | - | 0.12 |
| 66 | Acetovanillone | 1491 | 1491 | [33] | 98% | 0.41 | - | - | 3.85 | - | - |
| Total identified (%) | 3.34 | 15.82 | 3.31 | 10.04 | 11.10 | 3.04 | |||||
| Lactone | |||||||||||
| 12 | γ-Butyrolactone | 922 | 925 | [33] | 96% | 0.41 | - | 0.91 | 1.34 | 0.89 | 0.24 |
| Monoterpene Hydrocarbons | |||||||||||
| 13 | α-Pinene | 945 | 942 | [33] | 96% | 2.46 | - | - | 1.55 | 0.37 | - |
| 19 | β-Myrcene | 996 | 997 | [33] | 97% | - | - | - | - | - | 0.69 |
| 22 | p-Cymene | 1033 | 1030 | [33] | 98% | 0.68 | 0.67 | - | - | 0.17 | - |
| 24 | Limonene | 1037 | 1035 | [33] | 97% | 1.40 | - | 0.42 | 0.60 | 0.20 | 3.52 |
| Total identified (%) | 4.54 | 0.67 | 0.42 | 2.15 | 0.74 | 4.21 | |||||
| Furan | |||||||||||
| 20 | 2-Pentylfuran | 997 | 998 | [33] | 96% | 0.80 | 2.37 | - | 0.96 | 0.69 | - |
| Oxygenated Monoterpenes | |||||||||||
| 25 | 1,8-Cineole | 1041 | 1037 | [33] | 98% | 0.12 | - | 0.56 | 0.57 | 0.75 | 2.38 |
| 31 | Dihydromyrcenol | 1078 | 1075 | [33] | 96% | 1.06 | - | 1.42 | - | 2.09 | 2.07 |
| 32 | trans-Linalool oxide | 1080 | 1081 | [33] | 96% | 0.30 | - | - | - | - | - |
| 34 | 6-Camphenone | 1101 | 1095 | [33] | 95% | 0.30 | - | - | - | - | - |
| 36 | Linalool | 1104 | 1102 | [33] | 98% | 1.31 | - | 0.79 | - | 0.79 | 2.20 |
| 40 | Camphor | 1152 | 1149 | [33] | 99% | - | - | - | - | - | 2.98 |
| 41 | Borneol | 1173 | 1172 | [33] | 98% | 0.76 | - | 0.17 | - | - | 0.49 |
| 43 | Terpinen-4-ol | 1184 | 1184 | [33] | 97% | 0.41 | 0.39 | - | - | - | 0.56 |
| 44 | α-Terpineol | 1196 | 1195 | [33] | 98% | 3.01 | - | 1.14 | 0.25 | - | 0.64 |
| 46 | Myrtenol | 1199 | 1198 | [33] | 97% | 6.04 | - | - | - | - | - |
| 50 | β-Citronellol | 1234 | 1232 | [33] | 97% | - | - | 1.21 | - | - | - |
| 51 | Carvacrol methyl ether | 1241 | 1246 | [33] | 96% | 0.59 | - | - | - | - | - |
| 52 | Neryl formate | 1262 | 1261 | [33] | 96% | - | - | 2.52 | - | - | 1.01 |
| 53 | (E)-Citral | 1276 | 1278 | [33] | 95% | 0.82 | - | 0.52 | - | - | 0.67 |
| 54 | Perilla aldehyde | 1279 | 1279 | [33] | 98% | 15.63 | - | 8.09 | 6.47 | 17.59 | 20.08 |
| 55 | Bornyl acetate | 1290 | 1288 | [33] | 97% | 0.27 | - | - | - | - | 1.24 |
| Total identified (%) | 30.62 | 0.39 | 16.42 | 7.29 | 21.22 | 34.32 | |||||
| Alkanes | |||||||||||
| 47 | Dodecane | 1200 | 1200 | [33] | 98% | - | - | 1.37 | - | - | - |
| 57 | Tridecane | 1300 | 1300 | [33] | 99% | - | - | 0.58 | - | - | - |
| 60 | Tetradecane | 1400 | 1400 | [33] | 98% | 0.05 | - | 0.71 | - | - | 0.35 |
| Total identified (%) | 0.05 | - | 2.66 | - | - | 0.35 | |||||
| Norisoprenoids | |||||||||||
| 48 | Safranal | 1204 | 1205 | [33] | 98% | 1.72 | - | - | - | - | - |
| 70 | cis-α-Irone | 1544 | 1546 | [33] | 96% | - | 43.74 | - | - | - | - |
| 71 | cis-γ-Irone * | 1551 | - | [24] | 95% | 1.99 | 7.87 | - | - | - | - |
| Total identified (%) | 3.71 | 51.61 | - | - | - | - | |||||
| Sesquiterpene Hydrocarbons | |||||||||||
| 59 | α-Copaene | 1381 | 1376 | [33] | 96% | 5.06 | 0.98 | 1.32 | 2.08 | 1.62 | - |
| 62 | trans-Caryophyllene | 1424 | 1423 | [33] | 97% | 2.70 | 5.50 | 0.61 | 0.82 | 1.37 | 2.79 |
| 63 | trans-α-Bergamotene | 1441 | 1441 | [33] | 96% | - | - | - | - | - | 0.15 |
| 64 | α-Humulene | 1459 | 1459 | [33] | 97% | 0.59 | 0.05 | 0.32 | 0.28 | 0.22 | 0.73 |
| 67 | α-Farnesene | 1499 | 1503 | [33] | 97% | 0.33 | - | - | - | - | 0.83 |
| 68 | α-Muurolene | 1504 | 1505 | [33] | 95% | 0.39 | 1.41 | - | - | - | - |
| 69 | δ-Cadinene | 1520 | 1519 | [33] | 98% | - | 0.86 | - | - | - | - |
| Total identified (%) | 9.07 | 8.80 | 2.25 | 3.18 | 3.21 | 4.50 | |||||
| Total amount of identified compounds (%) | 89.98 | 85.99 | 88.39 | 85.67 | 94.90 | 93.49 | |||||
| No. | Compound | RI | RIL | RIL Reference | SI | I. pseudopallida B | I. pseudopallida D | I. pseudopallida T | I. illyrica V | I. illyrica Z | I. adriatica |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aliphatic and Aromatic Aldehydes | |||||||||||
| 1 | Furfural | <900 | 848 | [33] | 98% | 1.07 | 0.05 | - | 0.01 | 0.06 | 0.02 |
| 4 | Heptanal | <900 | 894 | [33] | 98% | - | 0.08 | - | - | 0.02 | 0.02 |
| 7 | Benzaldehyde | 971 | 972 | [33] | 99% | - | 0.27 | 0.02 | 0.02 | 0.08 | 0.05 |
| 13 | Octanal | 1005 | 1004 | [33] | 97% | - | 0.12 | - | - | - | 0.02 |
| 19 | Phenylacetaldehyde | 1052 | 1051 | [33] | 98% | 0.98 | 0.26 | 0.02 | 0.02 | 0.14 | 0.10 |
| 21 | 2,6-Dimethylhept-5-enal | 1059 | 1060 | [33] | 97% | 1.01 | 0.14 | - | - | 0.17 | - |
| 29 | Nonanal | 1109 | 1108 | [33] | 96% | - | - | - | 0.03 | 0.06 | 0.10 |
| 33 | (E)-Non-2-enal | 1166 | 1161 | [33] | 97% | - | - | - | 0.02 | 0.05 | - |
| 41 | Decanal | 1210 | 1210 | [33] | 99% | - | 0.09 | - | - | 0.05 | 0.04 |
| 47 | Undecanal | 1311 | 1309 | [33] | 98% | - | - | - | - | - | 0.03 |
| 54 | Dodecanal | 1413 | 1412 | [33] | 97% | - | 1.28 | - | - | 0.20 | 0.30 |
| Total identified (%) | 3.06 | 2.29 | 0.04 | 0.10 | 0.83 | 0.68 | |||||
| Alkanes | |||||||||||
| 2 | 4-Methyloctane | <900 | 864 | [33] | 95% | - | 0.05 | - | - | - | - |
| 46 | Tridecane | 1300 | 1300 | [33] | 98% | - | - | - | - | - | 0.05 |
| 59 | Pentadecane | 1500 | 1500 | [33] | 97% | - | - | - | - | 0.06 | 0.02 |
| 70 | Heneicosane | 2100 | 1600 | [33] | 98% | - | 1.06 | 0.09 | 0.09 | 0.40 | 0.48 |
| 72 | Docosane | 2200 | 2200 | [33] | 97% | - | 45.79 | - | 55.45 | 1.04 | 0.21 |
| 73 | Tricosane | 2300 | 2300 | [33] | 98% | 0.96 | 1.66 | 0.10 | 0.20 | 1.86 | 1.84 |
| Total identified (%) | 0.96 | 48.56 | 0.19 | 55.74 | 3.36 | 2.60 | |||||
| Aliphatic and Aromatic Alcohols and Phenols | |||||||||||
| 3 | Hexan-1-ol | <900 | 867 | [33] | 96% | - | - | - | 0.01 | - | 0.02 |
| 6 | 1-Butoxypropan-2-ol | 949 | 945 | [33] | 95% | - | - | - | - | 0.02 | 0.01 |
| 9 | Phenol | 986 | 987 | [33] | 99% | - | - | - | 0.02 | 0.04 | - |
| 15 | 2-Ethylhexan-1-ol | 1035 | 1034 | [33] | 97% | - | - | - | - | 0.02 | 0.03 |
| 18 | Benzyl alcohol | 1042 | 1042 | [33] | 99% | - | 0.09 | - | - | - | 0.04 |
| 24 | Octan-1-ol | 1076 | 1076 | [33] | 98% | - | 0.11 | - | 0.01 | 0.02 | 0.04 |
| 27 | 2-Methoxyphenol | 1093 | 1092 | [33] | 98% | - | 0.11 | - | - | 0.07 | 0.03 |
| 31 | 2-Phenylethanol | 1120 | 1120 | [33] | 99% | 2.20 | - | - | 0.02 | 0.81 | 0.07 |
| 35 | Nonan-1-ol | 1176 | 1175 | [33] | 95% | - | - | - | - | - | 0.05 |
| 48 | 2-Methoxy-4-vinylphenol | 1318 | 1317 | [33] | 99% | 0.96 | 0.12 | 0.02 | 0.08 | 0.33 | 0.08 |
| 57 | Dodecan-1-ol | 1479 | 1478 | [33] | 97% | - | 0.07 | - | - | 0.13 | 0.30 |
| Total identified (%) | 3.16 | 0.50 | 0.02 | 0.14 | 1.44 | 0.70 | |||||
| Monoterpene Hydrocarbons | |||||||||||
| 5 | α-Pinene | 945 | 942 | [33] | 98% | 5.98 | - | 0.01 | 0.16 | 0.08 | - |
| 10 | β-Pinene | 986 | 985 | [33] | 97% | 0.80 | - | - | - | - | - |
| 14 | p-Cymene | 1033 | 1030 | [33] | 98% | 0.50 | - | - | 0.01 | 0.02 | - |
| 16 | Limonene | 1037 | 1035 | [33] | 97% | - | - | - | 0.01 | 0.02 | - |
| 20 | (E)-β-ocymene | 1055 | 1054 | [33] | 98% | - | - | - | - | - | - |
| 22 | γ-Terpinene | 1067 | 1064 | [33] | 99% | 1.18 | - | - | - | - | - |
| Total identified (%) | 8.46 | - | 0.01 | 0.18 | 0.12 | - | |||||
| Fatty Acids and Fatty Acid Esters | |||||||||||
| 8 | Hexanoic (caproic) acid | 979 | 977 | [33] | 98% | - | - | - | - | 0.02 | 0.06 |
| 36 | Octanoic (caprylic) acid | 1181 | 1180 | [33] | 96% | - | - | - | 0.03 | 0.11 | 0.16 |
| 44 | Nonanoic (pelargonic) acid | 1290 | 1290 | [33] | 97% | - | - | - | - | - | 0.34 |
| 49 | Methyl decanoate | 1330 | 1328 | [33] | 95% | - | - | - | - | 0.13 | - |
| 51 | Decanoic (capric) acid | 1376 | 1377 | [33] | 98% | 1.41 | - | 0.34 | 0.34 | 2.82 | 0.91 |
| 53 | Ethyl decanoate | 1399 | 1397 | [33] | 96% | - | - | - | - | 0.42 | - |
| 63 | Dodecanoic (lauric) acid | 1570 | 1570 | [33] | 96% | 3.78 | - | 1.85 | 1.18 | 2.56 | 3.90 |
| 64 | Ethyl dodecanoate | 1599 | 1597 | [33] | 97% | - | - | - | - | 0.65 | - |
| 65 | Tetradecanoic (myristic) acid | 1780 | 1780 | [33] | 98% | 31.92 | 7.27 | 97.01 | 37.12 | 75.11 | 4.20 |
| 68 | Hexadecanoic (palmitic) acid | 1966 | 1963 | [33] | 99% | - | - | - | - | - | 35.48 |
| 71 | (Z,Z)-Octadeca-9,12-dienoic (linoleic) acid | 2150 | 2147 | [33] | 98% | - | - | - | - | - | 40.69 |
| Total identified (%) | 37.11 | 7.27 | 99.20 | 38.67 | 81.82 | 85.74 | |||||
| Aliphatic and Aromatic Ketones | |||||||||||
| 11 | 6-Methylhept-5-en-2-one | 992 | 991 | [33] | 99% | 0.61 | 1.51 | 0.02 | 0.05 | 0.11 | 0.05 |
| 23 | Acetophenone | 1074 | 1072 | [33] | 97% | - | - | - | - | - | 0.01 |
| 30 | 6-Methyl-3,5-heptadien-2-one | 1110 | 1107 | [33] | 96% | - | 0.26 | - | - | - | - |
| 45 | Undecan-2-one | 1297 | 1296 | [33] | 97% | - | - | - | 0.10 | - | - |
| 58 | Acetovanillone | 1491 | 1491 | [33] | 98% | - | - | - | - | 0.12 | - |
| 66 | Hexahydrofarnesyl acetone | 1851 | 1850 | [33] | 97% | - | - | - | - | - | 0.63 |
| Total identified (%) | 0.61 | 1.77 | 0.02 | 0.15 | 0.23 | 0.69 | |||||
| Furan | |||||||||||
| 12 | 2-Pentylfuran | 997 | 998 | [33] | 97% | - | - | - | 0.03 | - | 0.05 |
| Oxygenated Monoterpenes | |||||||||||
| 17 | 1,8-Cineole | 1041 | 1037 | [33] | 98% | 0.64 | - | - | 0.03 | 0.12 | - |
| 25 | Dihydromyrcenol | 1078 | 1075 | [33] | 96% | - | - | - | - | 0.02 | 0.03 |
| 26 | trans-Linalool oxide | 1080 | 1081 | [33] | 95% | - | 0.11 | - | - | - | - |
| 28 | Linalool | 1104 | 1102 | [33] | 98% | - | - | - | 0.02 | 0.10 | 0.04 |
| 29 | Camphor | 1152 | 1149 | [33] | 99% | - | - | - | - | 0.11 | 0.04 |
| 34 | Borneol | 1173 | 1172 | [33] | 98% | - | - | - | 0.02 | - | - |
| 37 | Terpinen-4-ol | 1184 | 1184 | [33] | 97% | 7.29 | - | - | 0.02 | 0.07 | 0.07 |
| 38 | α-Terpineol | 1196 | 1195 | [33] | 98% | 3.38 | - | - | 0.07 | 0.19 | 0.06 |
| 39 | Myrtenol | 1199 | 1198 | [33] | 97% | 9.60 | - | - | 0.04 | 0.14 | - |
| 42 | (E)-Citral | 1276 | 1278 | [33] | 95% | 1.49 | 0.15 | - | 0.06 | 0.20 | 0.03 |
| 43 | Perilla aldehyde | 1279 | 1279 | [33] | 98% | - | - | - | - | 0.16 | 0.07 |
| 50 | Eugenol | 1363 | 1365 | [33] | 98% | - | - | - | - | - | 0.02 |
| 56 | (E)-Geranylacetone | 1458 | 1458 | [33] | 98% | - | 0.21 | - | 0.02 | 0.09 | 0.15 |
| Total identified (%) | 22.40 | 0.47 | - | 0.28 | 1.20 | 0.51 | |||||
| Norisoprenoids | |||||||||||
| 40 | Safranal | 1204 | 1205 | [33] | 96% | - | - | - | - | 0.20 | - |
| 60 | trans-α-Irone | 1520 | 1504 | [33] | 97% | - | 0.26 | - | - | - | - |
| 61 | cis-α-Irone | 1544 | 1546 | [33] | 98% | - | 24.70 | - | - | 0.03 | 0.04 |
| 62 | cis-γ-Irone * | 1551 | - | [24] | 95% | 8.43 | 4.48 | - | - | 0.08 | 0.03 |
| Total identified (%) | 8.43 | 29.44 | - | - | 0.31 | 0.07 | |||||
| Sesquiterpene Hydrocarbons | |||||||||||
| 52 | α-Copaene | 1381 | 1376 | [33] | 97% | - | - | 0.02 | - | - | - |
| 55 | trans-Caryophyllene | 1424 | 1423 | [33] | 99% | - | 0.12 | - | - | - | - |
| Total identified (%) | - | 0.12 | 0.02 | - | - | - | |||||
| Aromatic Esters | |||||||||||
| 67 | Diisobutyl phthalate | 1873 | 1873 | [33] | 98% | 4.13 | - | - | 2.58 | - | 1.15 |
| 69 | Dibutyl phthalate | 1967 | 1967 | [33] | 97% | 1.51 | 0.39 | - | - | - | 1.93 |
| Total identified (%) | 5.64 | 0.39 | - | 2.58 | - | 3.08 | |||||
| Total amount of identified compounds (%) | 89.83 | 90.81 | 99.50 | 97.87 | 89.31 | 94.12 | |||||
| Taxon | Sample Code | Location | Collection Date | Latitude/Longitude |
|---|---|---|---|---|
| Iris pseudopallida | I. pseudopallida B | Bast | 28 April 2019 | 43°19′58.4″ N/ 16°59′2.4″ E |
| I. pseudopallida D | Dubrovnik | 7 April 2019 | 42°39′36.4″ N/ 18°04′01.7″ E | |
| I. pseudopallida T | Topići | 28 April 2019 | 43°21′46.0″ N/ 16°57′28.4″ E | |
| Iris illyrica | I. illyrica V | Vir | 23 April 2019 | 44°18′27.3″ N/ 15°1′52.5″ E |
| I. illyrica Z | Zaton | 19 April 2019 | 43°46′26.5″ N/ 15°50′10.8″ E | |
| Iris adriatica | I. adriatica | Brač | 4 April 2019 | 43°21′30.3″ N/ 16°35′49.6″ E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friščić, M.; Maleš, Ž.; Maleš, I.; Duka, I.; Radonić, A.; Mitić, B.; Hruševar, D.; Jurić, S.; Jerković, I. Gas Chromatography–Mass Spectrometry Analysis of Volatile Organic Compounds from Three Endemic Iris Taxa: Headspace Solid-Phase Microextraction vs. Hydrodistillation. Molecules 2024, 29, 4107. https://doi.org/10.3390/molecules29174107
Friščić M, Maleš Ž, Maleš I, Duka I, Radonić A, Mitić B, Hruševar D, Jurić S, Jerković I. Gas Chromatography–Mass Spectrometry Analysis of Volatile Organic Compounds from Three Endemic Iris Taxa: Headspace Solid-Phase Microextraction vs. Hydrodistillation. Molecules. 2024; 29(17):4107. https://doi.org/10.3390/molecules29174107
Chicago/Turabian StyleFriščić, Maja, Željan Maleš, Ivanka Maleš, Ivan Duka, Ani Radonić, Božena Mitić, Dario Hruševar, Sandra Jurić, and Igor Jerković. 2024. "Gas Chromatography–Mass Spectrometry Analysis of Volatile Organic Compounds from Three Endemic Iris Taxa: Headspace Solid-Phase Microextraction vs. Hydrodistillation" Molecules 29, no. 17: 4107. https://doi.org/10.3390/molecules29174107
APA StyleFriščić, M., Maleš, Ž., Maleš, I., Duka, I., Radonić, A., Mitić, B., Hruševar, D., Jurić, S., & Jerković, I. (2024). Gas Chromatography–Mass Spectrometry Analysis of Volatile Organic Compounds from Three Endemic Iris Taxa: Headspace Solid-Phase Microextraction vs. Hydrodistillation. Molecules, 29(17), 4107. https://doi.org/10.3390/molecules29174107









