Si/Graphite@C Composite Fabricated by Electrostatic Self-Assembly and Following Thermal Treatment as an Anode Material for Lithium-Ion Battery
Abstract
1. Introduction
2. Results and Discussion
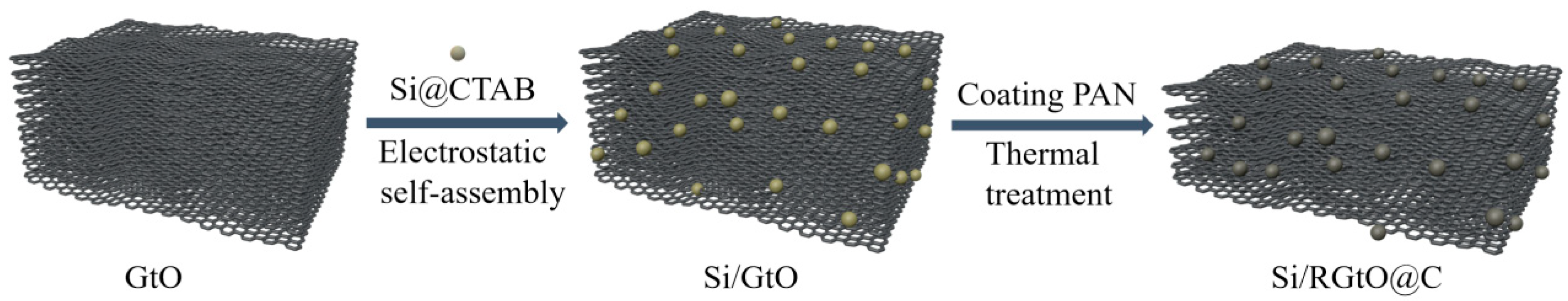
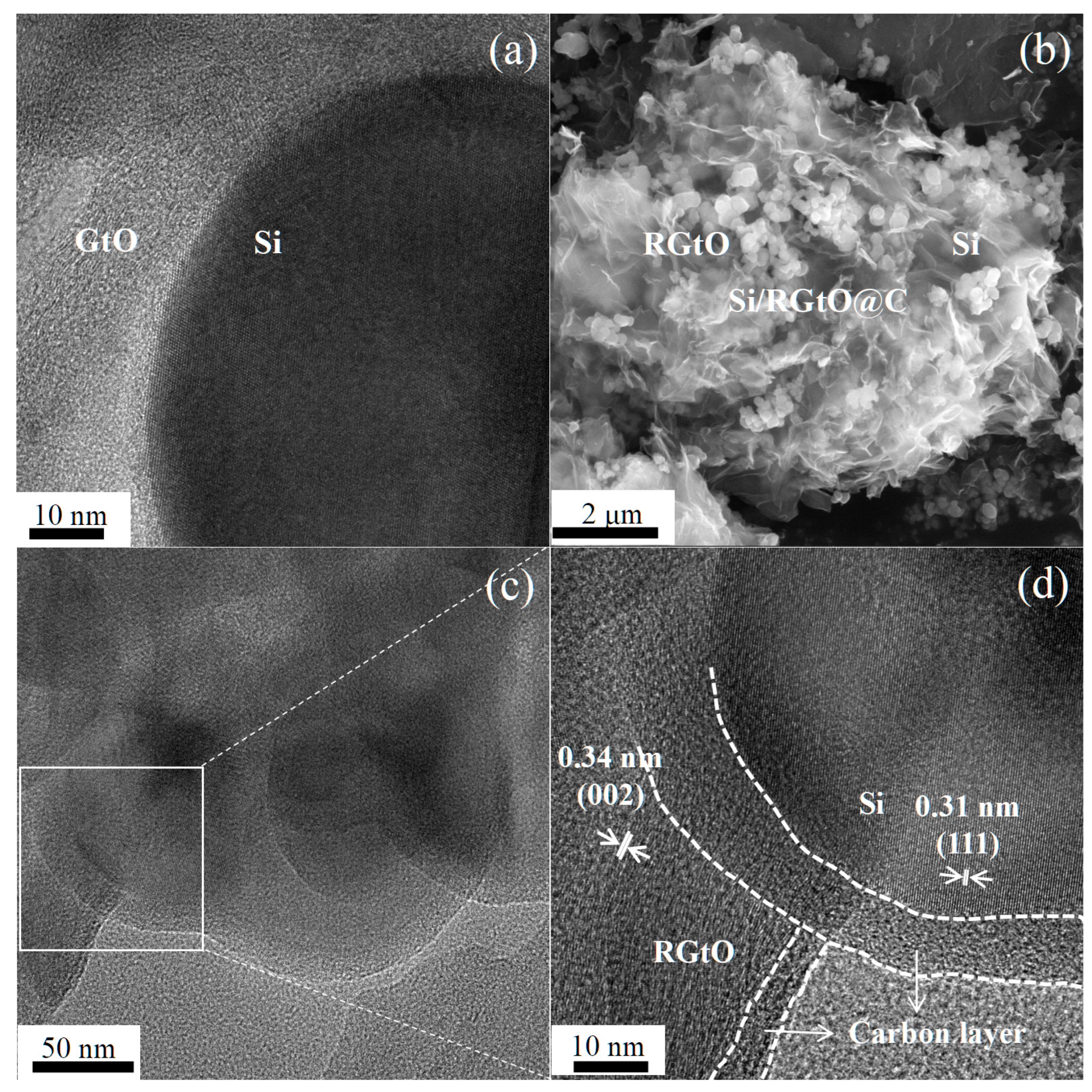
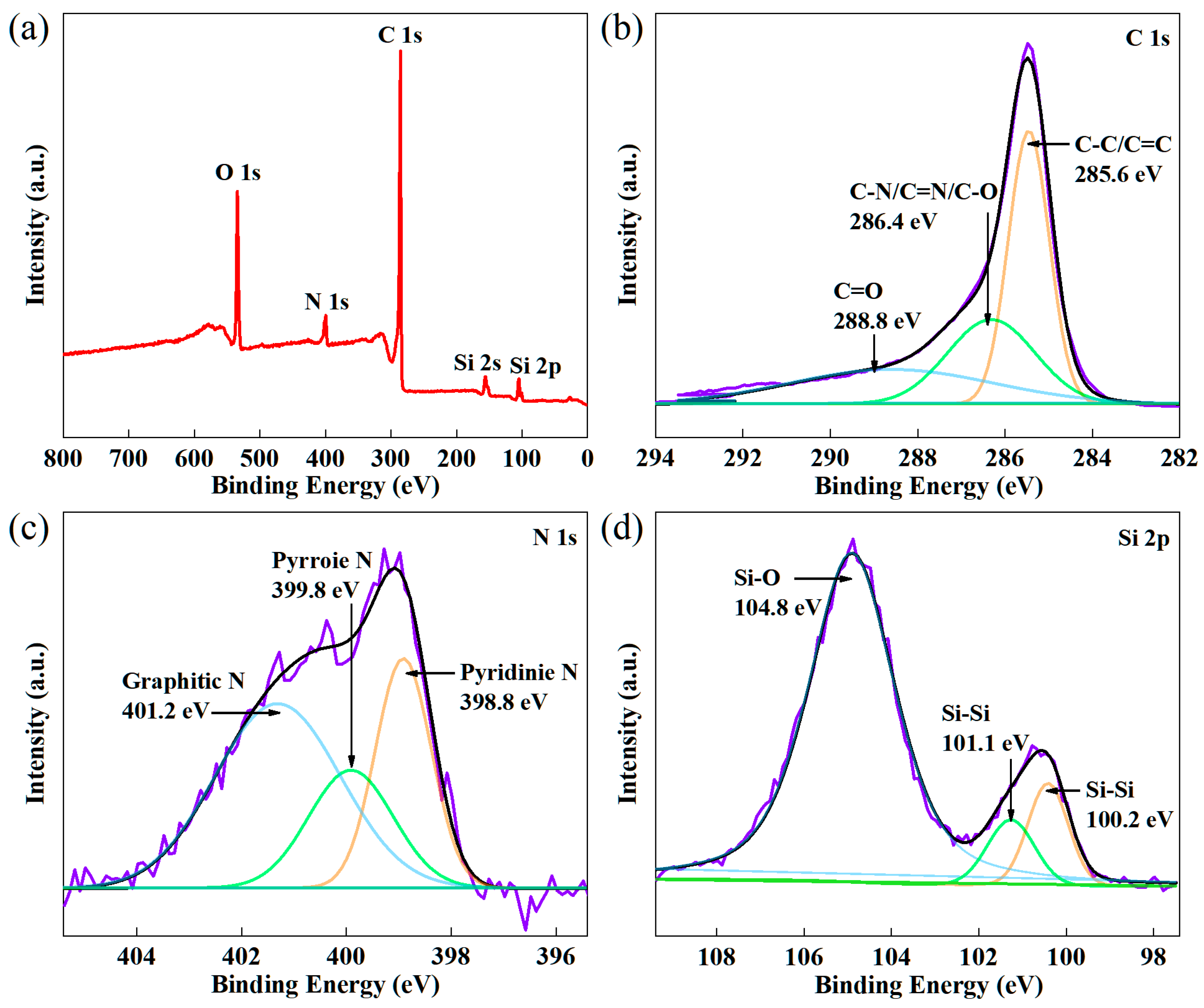
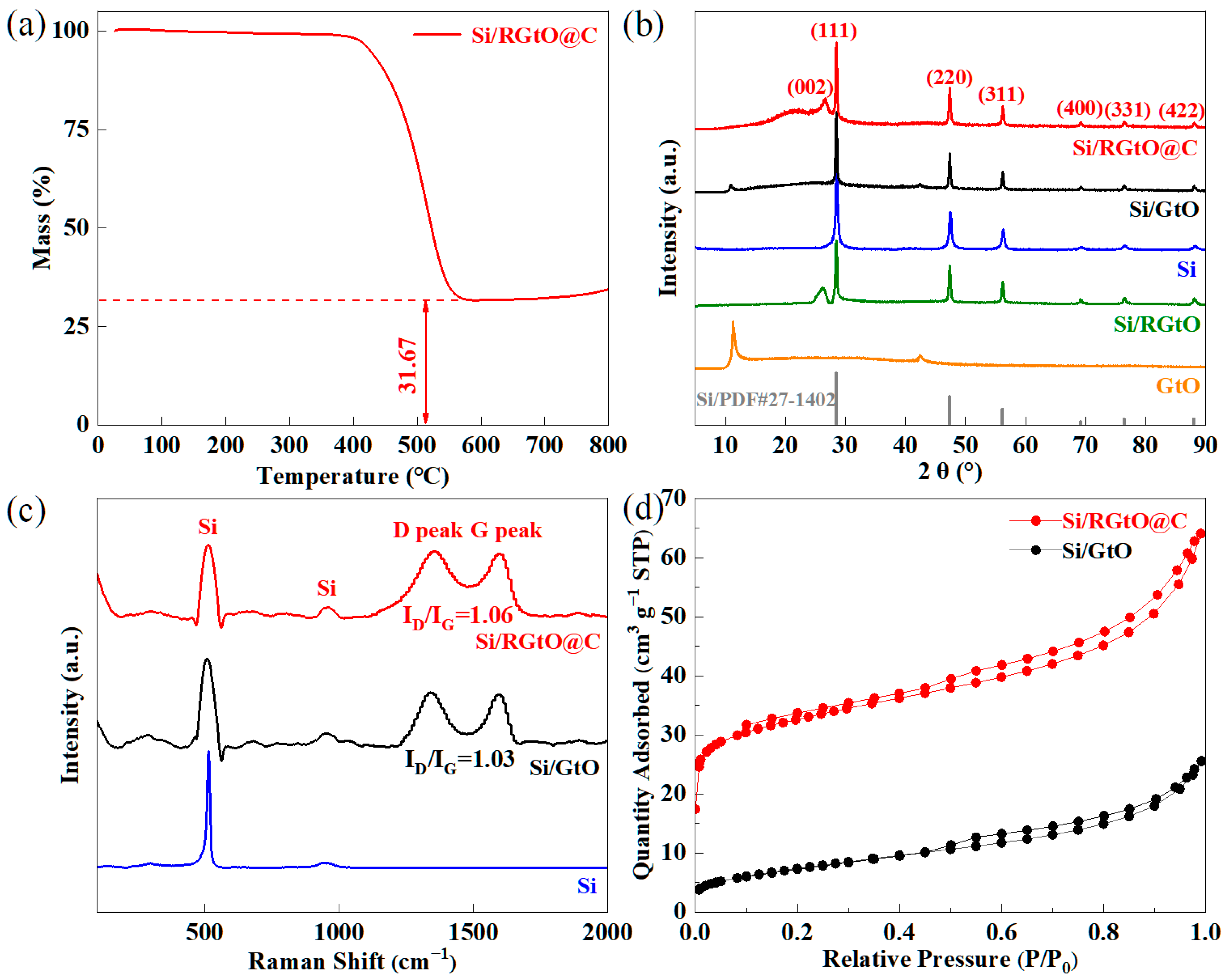
2.1. Synthesis and Structural Characterization
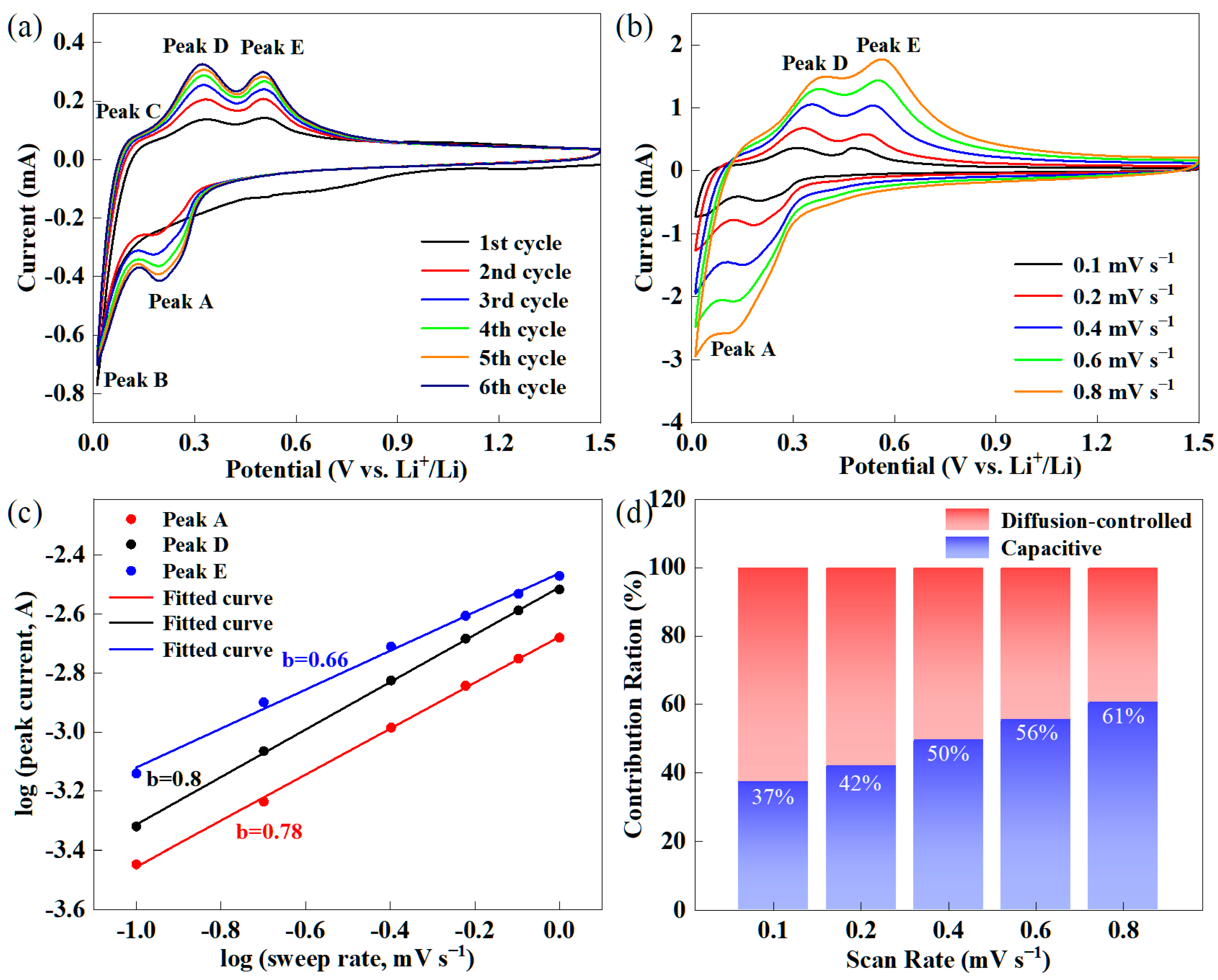
2.2. Electrochemical Properties
3. Materials and Methods
3.1. Material Preparation
3.2. Material Characterization
3.3. Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Z.J.; Wang, W.W.; Lu, Y.C. The path toward practical Li-air batteries. Joule 2022, 6, 2458–2473. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, Z.; Yang, G.; Jiang, X.; Duan, S.; Wu, Y.; Wang, M.; Ni, L.; Feng, L.; Diao, G. Regulating polysulfide transformation and stabilizing lithium anode using [PMo12O40]3− cluster@ carbon nanotube-modified separator for lithium-sulfur batteries. Batter. Supercaps 2024, 7, e202300563. [Google Scholar] [CrossRef]
- Liang, H.; Wang, L.; Wang, A.; Song, Y.; Wu, Y.; Yang, Y.; He, X. Tailoring practically accessible polymer/Inorganic composite electrolytes for all-solid-state lithium metal batteries: A review. Nano-Micro Lett. 2023, 15, 42. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Lv, X.S.; Wei, W.; Huang, B.B.; Dai, Y. Achieving high energy density for lithium-ion battery anodes by Si/C nanostructure design. J. Mater. Chem. A 2019, 7, 2165–2171. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, R.; Zhang, X.; Li, W.; Xu, R.; Diao, G.; Chen, M. Synthesis of flexible Fe3O4/C nanofibers with buffering volume expansion performance and their application in lithium-ion batteries. J. Power Sources 2017, 359, 7–16. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, R.; Wu, Q.; Li, W.; Shen, C.; Ni, L.; Yan, H.; Diao, G.; Chen, M. Petal-like MoS2 nanosheets space-confined in hollow mesoporous carbon spheres for enhanced lithium storage performance. ACS Nano 2017, 11, 8429–8436. [Google Scholar] [CrossRef]
- Cheng, Z.; Tu, B.; Wu, Y.; Huang, H. High-pressure and high-temperature synthesis of black phosphorus-graphite anode material for lithium-ion batteries. Electrochim. Acta 2024, 473, 143510. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Jiang, H.; Zhang, X.L.; Cheng, F.Y.; Wu, M.H.; Zhang, H.J. Fundamental understanding and facing challenges in structural design of porous Si-based anodes for lithium-ion batteries. Adv. Funct. Mater. 2023, 33, 2301109. [Google Scholar] [CrossRef]
- Agyeman, D.A.; Song, K.; Lee, G.H.; Park, M.; Kang, Y.M. Carbon-coated Si nanoparticles anchored between reduced graphene oxides as an extremely reversible anode material for high energy-density li-ion battery. Adv. Energy Mater. 2016, 6, 1600904. [Google Scholar] [CrossRef]
- An, W.L.; He, P.; Che, Z.Z.; Xiao, C.M.; Guo, E.M.; Pang, C.L.; He, X.Q.; Ren, J.G.; Yuan, G.H.; Du, N.; et al. Scalable synthesis of pore-rich Si/C@C core-shell-structured microspheres for practical long-life lithium-ion battery anodes. ACS Appl. Mater. Interfaces 2022, 14, 10308–10318. [Google Scholar] [CrossRef]
- Müller, V.; Bernhard, R.; Wegener, J.; Pfeiffer, J.; Rössler, S.; Scurtu, R.G.; Memm, M.; Danzer, M.A.; Wohlfahrt-Mehrens, M. Evaluation of scalable porous Si-rich Si/C composites with low volume expansion in coin cells to prismatic cell formats. Energy Technol. 2020, 8, 2000217. [Google Scholar] [CrossRef]
- Feng, K.; Li, M.; Liu, W.; Kashkooli, A.G.; Xiao, X.; Cai, M.; Chen, Z. Silicon-based anodes for lithium-ion batteries: From fundamentals to practical applications. Small 2018, 14, 1702737. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Z.; Chen, H.; Luo, L.; Zhang, Q.; Chen, J.; Chen, S.; Yang, Y. Bulk boron doping and surface carbon coating enabling fast-charging and stable Si anodes: From thin film to thick Si electrodes. J. Mater. Chem. A 2021, 9, 3628–3636. [Google Scholar] [CrossRef]
- Dou, F.; Shi, L.; Chen, G.; Zhang, D. Silicon/carbon composite anode materials for lithium-ion batteries. Electrochem. Energy Rev. 2019, 2, 149–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.S.; Fu, L.J.; Meng, J.X.; Yu, N.F.; Wang, J.; Wu, Y.P. Si/C composites as negative electrode for high energy lithium ion batteries. Chin. J. Chem. 2017, 35, 21–29. [Google Scholar] [CrossRef]
- Shi, Q.T.; Zhou, J.H.; Ullah, S.; Yang, X.Q.; Tokarska, K.; Trzebicka, B.; Ta, H.Q.; Rümmeli, M.H. A review of recent developments in Si/C composite materials for Li-ion batteries. Energy Storage Mater. 2021, 34, 735–754. [Google Scholar] [CrossRef]
- Roland, A.; Fullenwarth, J.; Ledeuil, J.-B.; Martinez, H.; Louvain, N.; Monconduit, L. How carbon coating or continuous carbon pitch matrix influence the silicon electrode/electrolyte interfaces and the performance in Li-ion batteries. Battery Energy 2022, 1, 20210009. [Google Scholar] [CrossRef]
- He, S.; Huang, S.; Wang, S.; Mizota, I.; Liu, X.; Hou, X. Considering critical factors of silicon/graphite anode materials for practical high-energy lithium-ion battery applications. Energy Fuels 2021, 35, 944–964. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zhu, Y.; Wang, J.; Ji, W.; Liu, X. Scalable synthesis of pitch-coated nanoporous si/graphite composite anodes for lithium-ion batteries. Energy Fuels 2023, 37, 4624–4631. [Google Scholar] [CrossRef]
- Zhu, S.; Lin, Y.; Yan, Z.; Jiang, J.; Yang, D.; Du, N. Novel design of uniform Si@graphite@C composite as high-performance Li-ion battery anodes. Electrochim. Acta 2021, 377, 138092. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. A step-by-step guide to perform x-ray photoelectron spectroscopy. J. Appl. Phys. 2022, 132, 011101. [Google Scholar]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Reliable determination of chemical state in X-ray photoelectron spectroscopy based on sample-work-function referencing to adventitious carbon: Resolving the myth of apparent constant binding energy of the C 1s peak. Appl. Surf. Sci. 2018, 451, 99–103. [Google Scholar] [CrossRef]
- Yi, Z.; Lin, N.; Zhao, Y.; Wang, W.; Qian, Y.; Zhu, Y.; Qian, Y. A flexible micro/nanostructured Si microsphere cross-linked by highly-elastic carbon nanotubes toward enhanced lithium ion battery anodes. Energy Storage Mater. 2019, 17, 93–100. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Zhu, Y.; Chen, Z.; Liang, J.; Zeng, Q.; Lyu, L.; Hu, C. Highly nitrogen-doped porous carbon transformed from graphitic carbon nitride for efficient metal-free catalysis. J. Hazard. Mater. 2020, 393, 121280. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Huang, X.; Liu, L.; Cai, W.; Jiajian, G.; Xuning, l.; Zhang, T.; Huang, Y.; Liu, B. Identifying active sites of nitrogen-doped carbon materials for the CO2 reduction reaction. Adv. Funct. Mater. 2018, 28, 1800499. [Google Scholar] [CrossRef]
- Weng, W.; Zeng, C.; Xiao, W. In situ pyrolysis concerted formation of Si/C hybrids during molten salt electrolysis of SiO2@polydopamine. ACS Appl. Mater. Interfaces 2019, 11, 9156–9163. [Google Scholar]
- Meng, F.; Xi, F.; Wu, J.; Ma, W.; Wei, K.; Li, S.; Chen, Z.; Ren, Y. Removal of metal impurities from diamond wire saw silicon powder by vacuum electromagnetic directional solidification. Vacuum 2024, 221, 112948. [Google Scholar] [CrossRef]
- An, Y.L.; Tian, Y.; Wei, H.; Xi, B.J.; Xiong, S.L.; Feng, J.K.; Qian, Y.T. Porosity- and graphitization-controlled fabrication of nanoporous silicon@carbon for lithium storage and Its conjugation with MXene for lithium-metal anode. Adv. Funct. Mater. 2020, 30, 1908721. [Google Scholar] [CrossRef]
- Wang, K.; Tan, Y.; Li, P.; Wang, Y. Recycling s waste cut from diamond wire into high performance porous Si@SO2@C anodes for Li-ion battery. J. Hazard. Mater. 2021, 407, 124778. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.; Mazouzi, D.; Reyter, D.; Lestriez, B.; Moreau, P.; Guyomard, D.; Roué, L. A low-cost and high performance ball-milled Si-based negative electrode for high-energy Li-ion batteries. Energy Environ. Sci. 2013, 6, 2145–2155. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Kim, I.T.; Hur, J. Core-shell Si@c-PAN particles deposited on graphite as promising anode for lithium-ion batteries. Electrochim. Acta 2019, 297, 355–364. [Google Scholar] [CrossRef]
- Nematzadeh, M.; Nangir, M.; Massoudi, A.; Ji, X.B.; Khanlarkhani, A.; Toth, J. Electrochemical performance of nitrogen-doped graphene/silicene composite as a pseudocapacitive anode for lithium-ion battery. ChemistrySelect 2022, 7, e202104012. [Google Scholar] [CrossRef]
- Mehdi, R.; Naqvi, S.R.; Khoja, A.H.; Hussain, R. Biomass derived activated carbon by chemical surface modification as a source of clean energy for supercapacitor application. Fuel 2023, 348, 128529. [Google Scholar] [CrossRef]
- Liu, H.T.; Shan, Z.Q.; Huang, W.L.; Wang, D.D.; Lin, Z.J.; Cao, Z.J.; Chen, P.; Meng, S.X.; Chen, L. Self-Assembly of silicon@oxidized mesocarbon microbeads encapsulated in carbon as anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 4715–4725. [Google Scholar] [CrossRef]
- Xu, C.; Lindgren, F.; Philippe, B.; Gorgoi, M.; Björefors, F.; Edström, K.; Gustafsson, T. Improved performance of the silicon anode for Li-ion batteries: Understanding the surface modification mechanism of fluoroethylene carbonate as an effective electrolyte additive. Chem. Mater. 2015, 27, 2591–2599. [Google Scholar] [CrossRef]
- Zhou, X.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Facile synthesis of silicon nanoparticles inserted into graphene sheets as improved anode materials for lithium-ion batteries. Chem. Commun. 2012, 48, 2198–2200. [Google Scholar] [CrossRef]
- Lu, X.Q.; Xu, P.; Song, W.J.; Zhou, P.; Liao, M.D.; Zeng, G.; Hu, X.B.; Li, J.X.; Zhang, M.Y.; Huang, Q.Z.; et al. Dual carbon boosts silicon-based anodes for excellent initial coulombic efficiency and cycling stability of lithium-ion batteries. J. Alloys Compd. 2023, 938, 168646. [Google Scholar] [CrossRef]
- Yin, L.; Gao, Y.J.; Jeon, I.; Yang, H.; Kim, J.P.; Jeong, S.Y.; Cho, C.R. Rice-panicle-like γ-Fe2O3@C nanofibers as high-rate anodes for superior lithium-ion batteries. Chem. Eng. J. 2019, 356, 60–68. [Google Scholar] [CrossRef]
- Su, D.; Huang, M.; Zhang, J.; Guo, X.; Chen, J.; Xue, Y.; Yuan, A.; Kong, Q. High N-doped hierarchical porous carbon networks with expanded interlayers for efficient sodium storage. Nano Res. 2020, 13, 2862–2868. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, C.; Li, F.; Jin, H.; Li, B.; Ding, F.; Yang, Y.; Ha, M.N.; Tran, D.L.; Yuan, F. Reinforced interfacial interaction between Si and graphite to improve the cyclic stability of lithium-ion batteries. ACS Appl. Mater. Interfaces 2024, 16, 23416–23425. [Google Scholar] [CrossRef]
- Elomari, G.; Hdidou, L.; Larhlimi, H.; Aqil, M.; Makha, M.; Alami, J.; Dahbi, M. Sputtered silicon-coated graphite electrodes as high cycling stability and improved kinetics anodes for lithium ion batteries. ACS Appl. Mater. Interfaces 2024, 16, 2193–2203. [Google Scholar] [CrossRef]
- Li, Q.L.; Chen, D.Q.; Li, K.; Wang, J.; Zhao, J.B. Electrostatic self-assembly bmSi@C/rGO composite as anode material for lithium ion battery. Electrochim. Acta 2016, 202, 140–146. [Google Scholar] [CrossRef]
- Jiao, Y.C.; Mukhopadhyay, A.; Ma, Y.; Yang, L.; Hafez, A.M.; Zhu, H.L. Ion transport nanotube assembled with vertically aligned metallic MoS2 for high rate lithium-ion batteries. Adv. Energy Mater. 2018, 8, 1702779. [Google Scholar] [CrossRef]
- Liu, W.; Zhong, Y.M.; Yang, S.Y.; Zhang, S.S.; Yu, X.Y.; Wang, H.Q.; Li, Q.Y.; Li, J.; Cai, X.; Fang, Y.P. Electrospray synthesis of nano-Si encapsulated in graphite/carbon microplates as robust anodes for high performance lithium-ion batteries. Sustain. Energy Fuels 2018, 2, 679–687. [Google Scholar] [CrossRef]
- Wang, H.; Xie, J.; Zhang, S.C.; Cao, G.S.; Zhao, X.B. Scalable preparation of silicon@graphite/carbon microspheres as high-performance lithium-ion battery anode materials. RSC Adv. 2016, 6, 69882–69888. [Google Scholar] [CrossRef]
- Chen, M.X.; Cao, W.Y.; Wang, L.C.; Ma, X.; Han, K. Chessboard-like silicon/graphite anodes with high cycling stability toward practical lithium-ion batteries. ACS Appl. Energy Mater. 2021, 4, 775–783. [Google Scholar] [CrossRef]
- Li, M.-Q.; Qu, M.-Z.; He, X.-Y.; Yu, Z.-L. Effects of electrolytes on the electrochemical performance of Si/graphite/disordered carbon composite anode for lithium-ion batteries. Electrochim. Acta 2009, 54, 4506–4513. [Google Scholar] [CrossRef]
- Wang, Z.L.; Mao, Z.M.; Lai, L.F.; Okubo, M.; Song, Y.H.; Zhou, Y.J.; Liu, X.; Huang, W. Sub-micron silicon/pyrolyzed carbon@natural graphite self-assembly composite anode material for lithium-ion batteries. Chem. Eng. J. 2017, 313, 187–196. [Google Scholar] [CrossRef]
- Hou, J.; Gong, B.L.; Hou, C.P.; Wang, B.P.; Yang, D.; Wang, X.W. Facile synthesis of nano-Si modified graphite composite as anode material for lithium ion batteries. Int. J. Electrochem. Sci. 2019, 14, 3455–3464. [Google Scholar]
- Parekh, M.H.; Sediako, A.D.; Naseri, A.; Thomson, M.J.; Pol, V.G. In situ mechanistic elucidation of superior Si-C-Graphite Li-ion battery anode formation with thermal safety aspects. Adv. Energy Mater. 2020, 10, 1902799. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.; Li, S.Y.; Chen, X.H.; Xi, F.S.; Wan, X.H.; Ma, W.-H.; Deng, R. Scalable synthesis of N-doped Si/G@voids@C with porous structures for high-performance anode of lithium-ion batteries. Rare Met. 2023, 42, 4091–4102. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, W.; Qian, Y.; Liu, X.; Lin, N.; Qian, Y. Mechanical pressing route for scalable preparation of microstructured/nanostrutured Si/graphite composite for lithium ion battery anodes. ACS Sustain. Chem. Eng. 2018, 6, 14230–14238. [Google Scholar] [CrossRef]
- Ma, Z.; Hang, H.; Wu, Z.; Jiang, X.; Ni, L.; Diao, G.; Yang, Q.; Fu, Y. LFO modified separator for the initial anode irreversibility compensation in lithium-ion rechargeable full cells. Electrochim. Acta 2024, 490, 144268. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Zhu, G.; Huang, J.; Meng, X.; Hao, M.; Zhu, S.; Wu, Z.; Kong, F.; Zhou, Y.; Li, Q.; et al. Si/Graphite@C Composite Fabricated by Electrostatic Self-Assembly and Following Thermal Treatment as an Anode Material for Lithium-Ion Battery. Molecules 2024, 29, 4108. https://doi.org/10.3390/molecules29174108
Yao J, Zhu G, Huang J, Meng X, Hao M, Zhu S, Wu Z, Kong F, Zhou Y, Li Q, et al. Si/Graphite@C Composite Fabricated by Electrostatic Self-Assembly and Following Thermal Treatment as an Anode Material for Lithium-Ion Battery. Molecules. 2024; 29(17):4108. https://doi.org/10.3390/molecules29174108
Chicago/Turabian StyleYao, Jintao, Guangzhao Zhu, Jingrui Huang, Xiaoru Meng, Maolong Hao, Shoupu Zhu, Zhen Wu, Fanxu Kong, Yue Zhou, Qi Li, and et al. 2024. "Si/Graphite@C Composite Fabricated by Electrostatic Self-Assembly and Following Thermal Treatment as an Anode Material for Lithium-Ion Battery" Molecules 29, no. 17: 4108. https://doi.org/10.3390/molecules29174108
APA StyleYao, J., Zhu, G., Huang, J., Meng, X., Hao, M., Zhu, S., Wu, Z., Kong, F., Zhou, Y., Li, Q., & Diao, G. (2024). Si/Graphite@C Composite Fabricated by Electrostatic Self-Assembly and Following Thermal Treatment as an Anode Material for Lithium-Ion Battery. Molecules, 29(17), 4108. https://doi.org/10.3390/molecules29174108







