Dammarane-Type 3,4-seco-Triterpenoid from Silver Birch (Betula pendula Roth) Buds Induces Melanoma Cell Death by Promotion of Apoptosis and Autophagy
Abstract
1. Introduction
2. Results
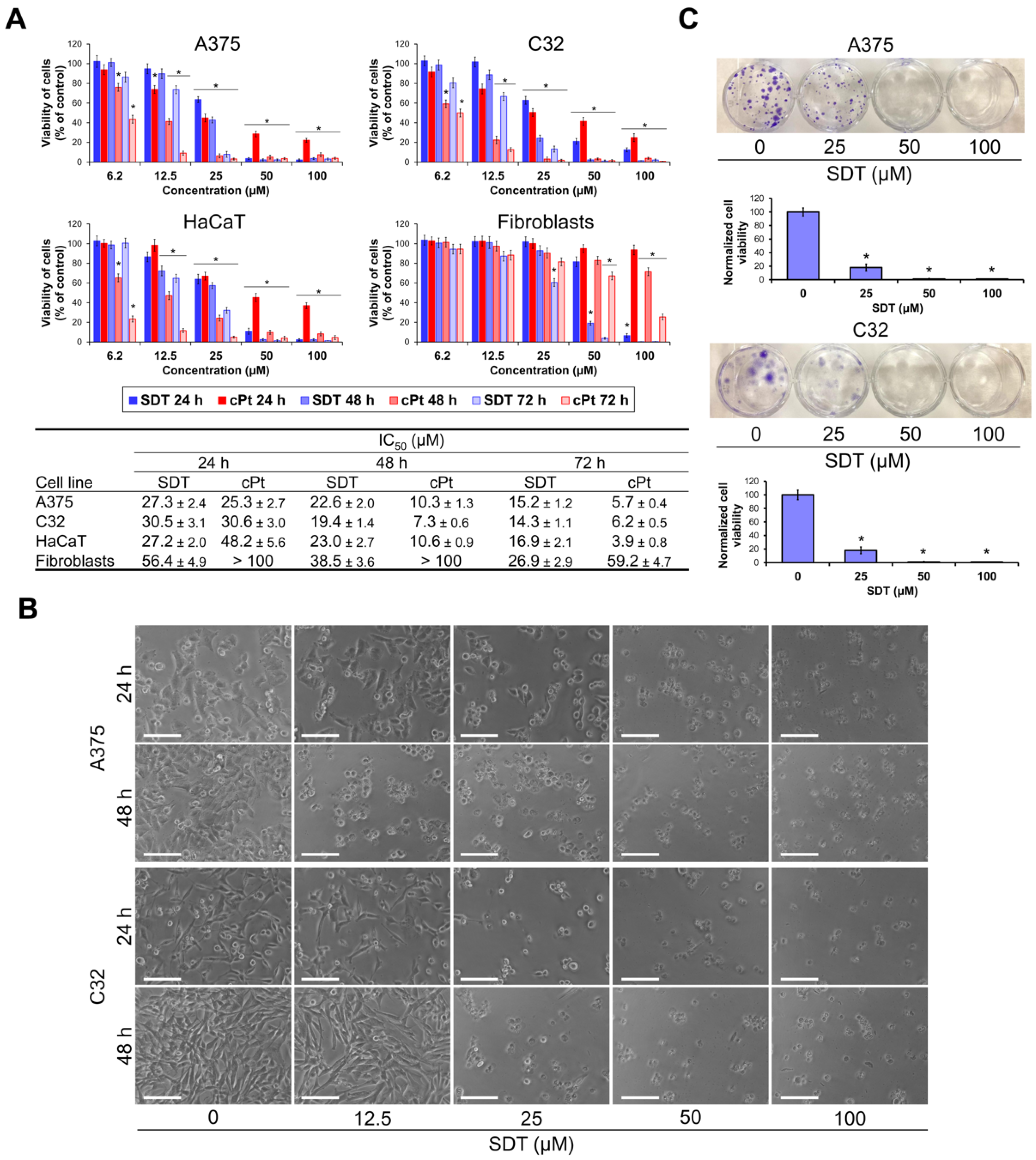
2.1. SDT Reduces Viability of Melanoma Cells
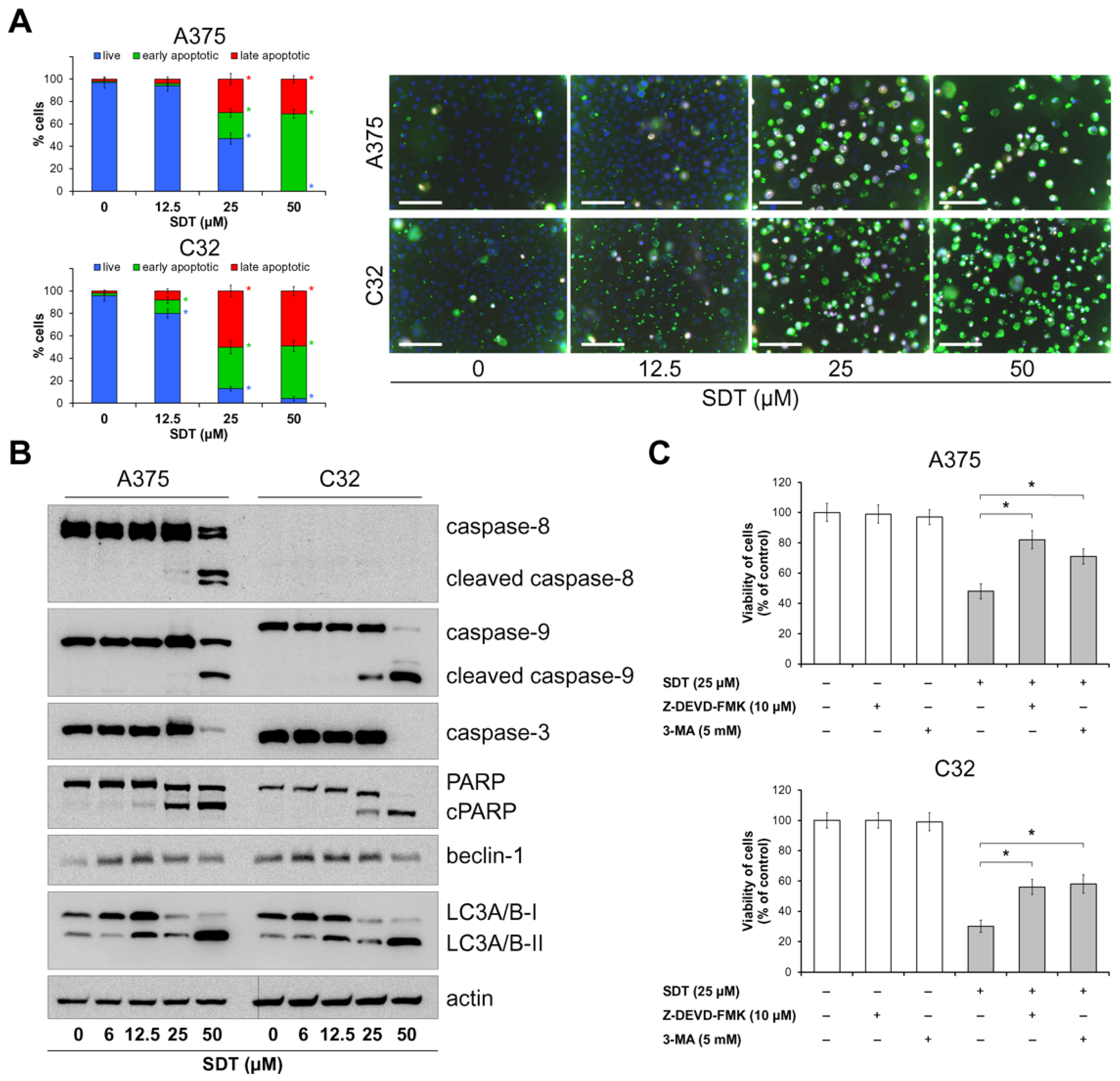
2.2. SDT Induces Death of Melanoma Cells by Apoptosis and Non-Protective Autophagy
2.3. SDT Inhibits Melanoma Cell Migration
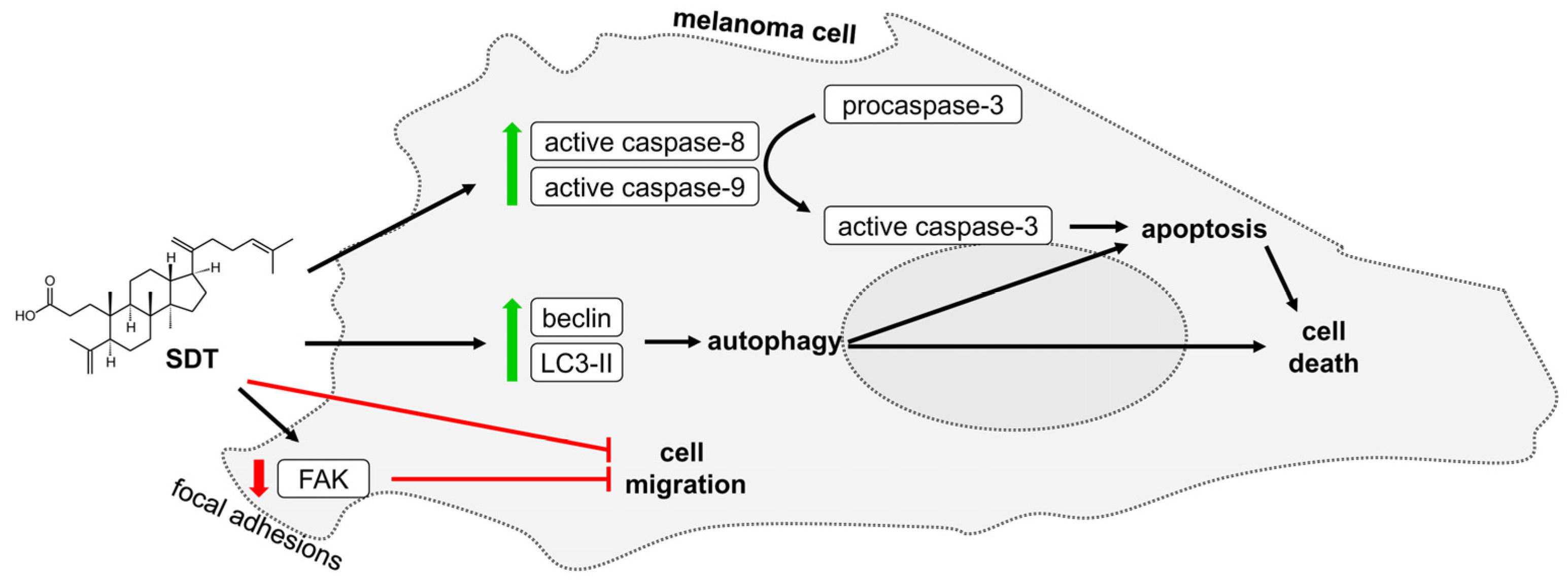
3. Discussion
4. Materials and Methods
4.1. Isolation of Triterpene Acid
4.2. Cell Culture and Treatment
4.3. Cell Viability Assay
4.4. Clonogenic Assay
4.5. Apoptosis Assay
4.6. Western Immunoblot
4.7. Wound Healing Assay
4.8. Immunofluorescence
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef]
- Xu, R.; Fazio, G.C.; Matsuda, S.P. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2020, 37, 962–998. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Z.; Wimmer, Z. Selected plant triterpenoids and their amide derivatives in cancer treatment: A review. Phytochemistry 2022, 203, 113340. [Google Scholar] [CrossRef]
- Miranda, R.S.; de Jesus, B.D.S.M.; da Silva Luiz, S.R.; Viana, C.B.; Adão Malafaia, C.R.; Figueiredo, F.S.; Carvalho, T.D.S.C.; Silva, M.L.; Londero, V.S.; da Costa-Silva, T.A.; et al. Antiinflammatory activity of natural triterpenes—An overview from 2006 to 2021. Phytother. Res. 2022, 36, 1459–1506. [Google Scholar] [CrossRef]
- Sharma, H.; Kumar, P.; Deshmukh, R.R.; Bishayee, A.; Kumar, S. Pentacyclic triterpenes: New tools to fight metabolic syndrome. Phytomedicine 2018, 50, 166–177. [Google Scholar] [CrossRef]
- Xu, G.B.; Xiao, Y.H.; Zhang, Q.Y.; Zhou, M.; Liao, S.G. Hepatoprotective natural triterpenoids. Eur. J. Med. Chem. 2018, 145, 691–716. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Wang, H.; Xiong, Y. Recent Advances in Antiviral Activities of Triterpenoids. Pharmaceuticals 2022, 15, 1169. [Google Scholar] [CrossRef]
- Sycz, Z.; Tichaczek-Goska, D.; Wojnicz, D. Anti-Planktonic and Anti-Biofilm Properties of Pentacyclic Triterpenes-Asiatic Acid and Ursolic Acid as Promising Antibacterial Future Pharmaceuticals. Biomolecules 2022, 12, 98. [Google Scholar] [CrossRef]
- Chen, Y.J.; Na, L.; Fan, J.; Zhao, J.; Hussain, N.; Jian, Y.Q.; Yuan, H.; Li, B.; Liu, B.; Choudhary, M.I.; et al. Seco-dammarane triterpenoids from the leaves of Cyclocarya paliurus. Phytochemistry 2018, 145, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Dong, L.; Appendino, G.; Bak, S. Plant triterpenoids with bond-missing skeletons: Biogenesis, distribution and bioactivity. Nat. Prod. Rep. 2020, 37, 1207–1228. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef]
- Olchowik, J.; Jankowski, P.; Suchocka, M.; Malewski, T.; Wiesiołek, A.; Hilszczańska, D. The impact of anthropogenic transformation of urban soils on ectomycorrhizal fungal communities associated with silver birch (Betula pendula Roth.) growth in natural versus urban soils. Sci. Rep. 2023, 13, 21268. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal plants of the genus Betula--traditional uses and a phytochemical-pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef]
- Krasutsky, P.A. Birch bark research and development. Nat. Prod. Rep. 2006, 23, 919–942. [Google Scholar] [CrossRef]
- Berezovska, D.; Oszako, T.; Malewski, T.; Stocki, M.; Marozau, A.; Stocka, N.; Moser, W.K.; Baggett, L.S.; Belbahri, L.; Nowakowska, J.A. Effect of Defoliation on the Defense Reactions of Silver Birch (Betula pendula) Infected with Phytophthora plurivora. Forests 2021, 12, 910. [Google Scholar] [CrossRef]
- Vedernikov, D.N.; Roshchin, V.I. Extractive components of the buds of white birch Betula pendula Roth. (Betulaceae). IV. The composition of triterpene acids, flavonoids, alcohols and esters. Khim. Rast. Syr. 2010, 4, 67–75. [Google Scholar]
- Vedernikov, D.N.; Roshchin, V.I. Extractive compounds of betulaceae family birch buds (Betula pendula Roth.): V. Composition of triterpene seco-acids. Russ. J. Bioorg. Chem. 2012, 38, 762–768. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Szoka, L.; Karna, E.; Hlebowicz-Sarat, K.; Karaszewski, J.; Boryczka, S.; Palka, J.A. Acetylenic derivative of betulin induces apoptosis in endometrial adenocarcinoma cell line. Biomed. Pharmacother. 2017, 95, 429–436. [Google Scholar] [CrossRef]
- Chodurek, E.; Orchel, A.; Gwiazdoń, P.; Kaps, A.; Paduszyński, P.; Jaworska-Kik, M.; Chrobak, E.; Bębenek, E.; Boryczka, S.; Kasperczyk, J. Antiproliferative and Cytotoxic Properties of Propynoyl Betulin Derivatives against Human Ovarian Cancer Cells: In Vitro Studies. Int. J. Mol. Sci. 2023, 24, 16487. [Google Scholar] [CrossRef]
- Szoka, Ł.; Isidorov, V.; Nazaruk, J.; Stocki, M.; Siergiejczyk, L. Cytotoxicity of Triterpene Seco-Acids from Betula pubescens Buds. Molecules 2019, 24, 4060. [Google Scholar] [CrossRef] [PubMed]
- Esimone, C.O.; Eck, G.; Nworu, C.S.; Hoffmann, D.; Uberla, K.; Proksch, P. Dammarenolic acid, a secodammarane triterpenoid from Aglaia sp. shows potent anti-retroviral activity in vitro. Phytomedicine 2010, 17, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Ukiya, M.; Kikuchi, T.; Tokuda, H.; Tabata, K.; Kimura, Y.; Arai, T.; Ezaki, Y.; Oseto, O.; Suzuki, T.; Akihisa, T. Antitumor-promoting effects and cytotoxic activities of dammar resin triterpenoids and their derivatives. Chem. Biodivers. 2010, 7, 1871–1884. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Nazaruk, J.; Stocki, M.; Bakier, S. Secondary metabolites of downy birch buds (Betula pubescens Erch.). Z. Naturforsch. C J. Biosci. 2021, 77, 145–155. [Google Scholar] [CrossRef]
- Isidorov, V.; Szoka, Ł.; Nazaruk, J. Cytotoxicity of white birch bud extracts: Perspectives for therapy of tumours. PLoS ONE 2018, 13, e0201949. [Google Scholar] [CrossRef] [PubMed]
- Szoka, L.; Nazaruk, J.; Stocki, M.; Isidorov, V. Santin and cirsimaritin from Betula pubescens and Betula pendula buds induce apoptosis in human digestive system cancer cells. J. Cell. Mol. Med. 2021, 25, 11085–11096. [Google Scholar] [CrossRef]
- Szoka, L.; Nazaruk, J.; Giegiel, J.; Isidorov, V. Prolidase-proline oxidase axis is engaged in apoptosis induction by birch buds flavonol santin in endometrial adenocarcinoma cell line. Front. Mol. Biosci. 2023, 10, 1247536. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Q.; Wu, J.; Yang, Y.; Yang, Y.; Xie, Q.; Liu, L.; Wang, B.; Qiu, Y.; Yu, H.; et al. Qingqianliusus A-N, 3,4-seco-dammarane triterpenoids from the leaves of Cyclocarya paliurus and their biological activities. Arab. J. Chem. 2023, 16, 104441. [Google Scholar] [CrossRef]
- Koren, E.; Fuchs, Y. Modes of Regulated Cell Death in Cancer. Cancer Discov. 2021, 11, 245–265. [Google Scholar] [CrossRef]
- Grudzińska, M.; Stachnik, B.; Galanty, A.; Sołtys, A.; Podolak, I. Progress in Antimelanoma Research of Natural Triterpenoids and Their Derivatives: Mechanisms of Action, Bioavailability Enhancement and Structure Modifications. Molecules 2023, 28, 7763. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, H.; Rayburn, E.R.; Zhao, Y.; Hill, D.L.; Zhang, R. 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol, a novel natural product for prostate cancer therapy: Activity in vitro and in vivo and mechanisms of action. Br. J. Cancer 2008, 98, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W.; Gamal-Eldeen, A.M.; El-Desouky, S.K.; Kim, Y.K.; Huefner, A.; Saf, R. Induction of caspase-8 and death receptors by a new dammarane skeleton from the dried fruits of Forsythia koreana. Z. Naturforsch. C J. Biosci. 2013, 68, 29–38. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, K.C.; Lee, E.E.; Tookmanian, E.M.; Kesserwan, C.A.; Manfredi, J.J.; Hatton, J.N.; Loukissas, J.K.; Zavadil, J.; Zhou, L.; Olivier, M.; et al. The TP53 Database: Transition from the International Agency for Research on Cancer to the US National Cancer Institute. Cell. Death Differ. 2022, 29, 1071–1073. [Google Scholar] [CrossRef]
- Heliawati, L.; Khatimah, H.; Hermawati, E.; Syah, Y.M. Four Dammarane Triterpenes and Their Inhibitory Properties Against Eight Receptor Tyrosine Kinases. Nat. Prod. Sci. 2020, 26, 345–350. [Google Scholar] [CrossRef]
- Sugiyama, M.G.; Fairn, G.D.; Antonescu, C.N. Akt-ing Up Just About Everywhere: Compartment-Specific Akt Activation and Function in Receptor Tyrosine Kinase Signaling. Front. Cell. Dev. Biol. 2019, 7, 70. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Hossain, M.T.; Rahaman, M.A.; Rahman, P.; Hasan, M.S.; Das, R.C.; Khan, M.K.; Sikder, M.H.; Alam, M.; Uddin, M.J.; et al. Molecular pharmacology and therapeutic advances of the pentacyclic triterpene lupeol. Phytomedicine 2022, 99, 154012. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Huang, Y.; Bai, C.; Zhang, X.; Fang, M.; Ju, Z.; Liu, B. Membrane dynamics of ATG4B and LC3 in autophagosome formation. J. Mol. Cell. Biol. 2022, 13, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, X.; Liu, Q.; Ho, I.H.; Wei, X.; Yin, T.; Zhan, Y.; Zhang, W.; Zhang, W.; Chen, B.; et al. Hederagenin potentiated cisplatin- and paclitaxel-mediated cytotoxicity by impairing autophagy in lung cancer cells. Cell Death Dis. 2020, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- El-Baba, C.; Baassiri, A.; Kiriako, G.; Dia, B.; Fadlallah, S.; Moodad, S.; Darwiche, N. Terpenoids’ anti-cancer effects: Focus on autophagy. Apoptosis 2021, 26, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, X.; Fang, Q.; Zhao, Z.; Lin, C.; Zhou, Y.; Liu, F.; Zhu, C.; Wu, A. Betulinic acid induces apoptosis of HeLa cells via ROS-dependent ER stress and autophagy in vitro and in vivo. J. Nat. Med. 2024, 78, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chen, H.Y.; Hsieh, C.P.; Huang, Y.F.; Chang, I.L. Betulin inhibits mTOR and induces autophagy to promote apoptosis in human osteosarcoma cell lines. Environ. Toxicol. 2020, 35, 879–887. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Z.; Chen, K.; Zhuo, Q.; Chen, M.; Wang, J.; Lai, X.; Wang, L. Lupeol inhibits the proliferation and migration of MDA-MB-231 breast cancer cells via a novel crosstalk mechanism between autophagy and the EMT. Food Funct. 2022, 13, 4967–4976. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, Q.; Xu, W.; Wang, H.; Che, Y.; Wu, M.; Wang, L.; Lijuan, C.; Hao, H. Total ginsenosides extract induce autophagic cell death in NSCLC cells through activation of endoplasmic reticulum stress. J. Ethnopharmacol. 2019, 243, 112093. [Google Scholar] [CrossRef]
- Wirawan, E.; Vande Walle, L.; Kersse, K.; Cornelis, S.; Claerhout, S.; Vanoverberghe, I.; Roelandt, R.; De Rycke, R.; Verspurten, J.; Declercq, W.; et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010, 1, e18. [Google Scholar] [CrossRef]
- Akasaka, T.; van Leeuwen, R.L.; Yoshinaga, I.G.; Mihm, M.C., Jr.; Byers, H.R. Focal adhesion kinase (p125FAK) expression correlates with motility of human melanoma cell lines. J. Investig. Dermatol. 1995, 105, 104–108. [Google Scholar] [CrossRef]
- Acquaviva, J.; Smith, D.L.; Jimenez, J.P.; Zhang, C.; Sequeira, M.; He, S.; Sang, J.; Bates, R.C.; Proia, D.A. Overcoming acquired BRAF inhibitor resistance in melanoma via targeted inhibition of Hsp90 with ganetespib. Mol. Cancer Ther. 2014, 13, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Rodriguez, Y.A.R.; Jeong, K.; Ahn, E.E.; Lim, S.S. Targeting focal adhesion kinase in cancer cells and the tumor microenvironment. Exp. Mol. Med. 2020, 52, 877–886. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szoka, L.; Stocki, M.; Isidorov, V. Dammarane-Type 3,4-seco-Triterpenoid from Silver Birch (Betula pendula Roth) Buds Induces Melanoma Cell Death by Promotion of Apoptosis and Autophagy. Molecules 2024, 29, 4091. https://doi.org/10.3390/molecules29174091
Szoka L, Stocki M, Isidorov V. Dammarane-Type 3,4-seco-Triterpenoid from Silver Birch (Betula pendula Roth) Buds Induces Melanoma Cell Death by Promotion of Apoptosis and Autophagy. Molecules. 2024; 29(17):4091. https://doi.org/10.3390/molecules29174091
Chicago/Turabian StyleSzoka, Lukasz, Marcin Stocki, and Valery Isidorov. 2024. "Dammarane-Type 3,4-seco-Triterpenoid from Silver Birch (Betula pendula Roth) Buds Induces Melanoma Cell Death by Promotion of Apoptosis and Autophagy" Molecules 29, no. 17: 4091. https://doi.org/10.3390/molecules29174091
APA StyleSzoka, L., Stocki, M., & Isidorov, V. (2024). Dammarane-Type 3,4-seco-Triterpenoid from Silver Birch (Betula pendula Roth) Buds Induces Melanoma Cell Death by Promotion of Apoptosis and Autophagy. Molecules, 29(17), 4091. https://doi.org/10.3390/molecules29174091





