Rapid Assessment of Metabolomic Fingerprinting of Recycled Sunflower By-Products via DART-HRMS
Abstract
1. Introduction
2. Results and Discussion
2.1. DART-HRMS Spectra
2.2. Effect of the Optimisation on the Most Informative DART-HRMS Ions
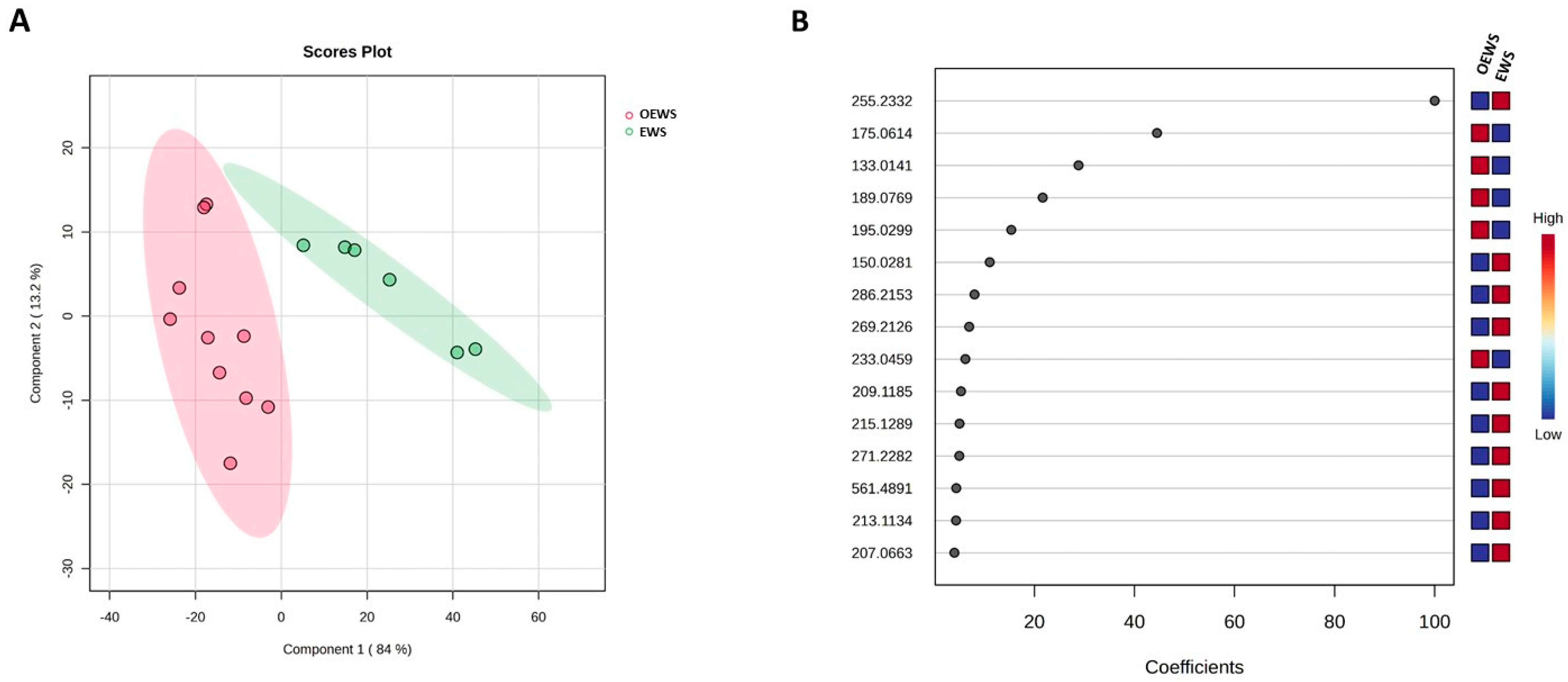
2.3. Multivariate Statistical Analysis of the DART-HRMS Fingerprintings
3. Materials and Methods
3.1. Preparation of the Sunflower Waste Processed Samples and Experimental Dataset
3.2. Fingerprinting Analysis by DART-HRMS
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakonechna, K.; Ilko, V.; Berčíková, M.; Vietoris, V.; Panovská, Z.; Doležal, M. Nutritional, Utility, and Sensory Quality and Safety of Sunflower Oil on the Central European Market. Agriculture 2024, 14, 536. [Google Scholar] [CrossRef]
- Rauf, S.; Jamil, N.; Tariq, S.A.; Khan, M.; Kausar, M.; Kaya, Y. Progress in modification of sunflower oil to expand its industrial value. J. Sci. Food Agric. 2016, 97, 1997–2006. [Google Scholar] [CrossRef]
- Petraru, A.; Ursachi, F.; Amariei, S. Nutritional characteristics assessment of sunflower seeds, oil and cake. Perspective of using sunflower oilcakes as a functional ingredient. Plants 2021, 10, 2487. [Google Scholar] [CrossRef] [PubMed]
- Goiri, I.; Zubiria, I.; Benhissi, H.; Atxaerandio, R.; Ruiz, R.; Mandaluniz, N.; Garcia-Rodriguez, A. Use of cold-pressed sunflower cake in the concentrate as a low-input local strategy to modify the milk fatty acid profile of dairy cows. Animals 2019, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Kokić, B.; Rakita, S.; Vujetić, J. Impact of using oilseed industry byproducts rich in linoleic and alpha-linolenic acid in ruminant nutrition on milk production and milk fatty acid profile. Animals 2024, 14, 539. [Google Scholar] [CrossRef] [PubMed]
- Mikus, M.; Galus, S.; Ciurzy, A. Development and characterization of novel composite films based on soy protein isolate and oilseed flours. Molecules 2021, 26, 3738. [Google Scholar] [CrossRef]
- Grau-Fuentes, E.; Rodrigo, D.; Garzón, R.; Rossell, C.M. Understanding the marketed plant-based beverages: From ingredients technological function to their nutritional value. J. Funct. Foods 2023, 106, 105609. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Sunflower meal/cake as a sustainable protein source for global demand: Towards a zero-hunger world. Food Hydrocoll. 2024, 147, 109329. [Google Scholar] [CrossRef]
- Egea, M.B.; de Oliveira Filho, J.G.; Bertolo, M.R.V.; de Araújo, J.C.; Gautério, G.V.; Lemes, A.C. Bioactive phytochemicals from sunflower (Helianthus annuus L.) oil processing byproducts. In Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-Products-Reference Series in Phytochemistry, 1st ed.; Ramadan, H.M.F., Ed.; Springer: Cham, Switzerland, 2021; pp. 1–16. [Google Scholar] [CrossRef]
- Pedrosa, M.M.; Muzquiz, M.; García-Vallejo, C.; Burbano, C.; Cuadrado, C.; Ayet, G.; Robredo, L.M. Determination of caffeic and chlorogenic acids and their derivatives in different sunflower seeds. J. Sci. Food Agric. 2000, 80, 459–464. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, R.M.; Abd El-Hack, M.E.; Dhama, K. Review Article. Adv. Anim. Vet. Sci. 2015, 3, 634–648. [Google Scholar] [CrossRef]
- Ancuţa, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Ivanova, P.; Ivanov, I.G.; Tumbarski, Y.; Kalaydzhiev, H.; Dincheva, I.N.; Chalova, V.I. Bioactivity potential of industrial sunflower meal ethanol-wash solute obtained as waste from protein isolation process. Appl. Sci. 2021, 11, 11007. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Chalova, V.I.; Kalaydzhiev, H.R.; Mihaylova, D.; Krastanov, A.I.; Lante, A. Ultrasound-assisted extraction of antioxidant bioactive compounds from wastes of rapeseed industry and their application in delaying rapeseed oil oxidation. Environ. Technol. Innov. 2023, 30, 103081. [Google Scholar] [CrossRef]
- Castro-Muňoz, R. Emerging processes for sustainable processing of food ingredients and products. Foods 2023, 12, 3633. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Chalova, V.I.; Kalaydzhiev, H.R.; Mihaylova, D.; Krastanov, A.I.; Lante, A. Preliminary characterisation of wastes generated from the rapeseed and sunflower protein isolation process and their valorisation in delaying oil oxidation. Food Bioprocess Technol. 2021, 14, 1962–1971. [Google Scholar] [CrossRef]
- Dou, X.; Zhang, L.; Yang, R.; Wang, X.; Yu, L.; Yue, X.; Ma, F.; Mao, J.; Wang, X.; Zhang, W.; et al. Mass spectrometry in food authentication and origin traceability. Mass Spectrom. Rev. 2023, 42, 1772–1807. [Google Scholar] [CrossRef]
- Augusti, R.; Fulgêncio, A.C.C.; Nogueira, H.M.; Gomes, J.C.L.; dos Santos, L.B.; de Macedo, A.N.; Porto, B.L.S.; Sena, M.M.; Almeida, M.R. Enhancing food authentication screening through the integration of chemometrics and ambient ionization mass spectrometry: A comprehensive review. Trends Food Sci. Technol. 2024, 147, 104480. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, H.; Chingin, K.; Xiong, J.; Fang, X.; Chen, H. Ambient mass spectrometry for food science and industry. TrAC-Trends Anal. Chem. 2018, 107, 99–115. [Google Scholar] [CrossRef]
- Vaclavik, L.; Cajka, T.; Hrbek, V.; Hajslova, J. Ambient mass spectrometry employing direct analysis in real time (DART ) ion source for olive oil quality and authenticity assessment. Anal. Chim. Acta 2009, 645, 56–63. [Google Scholar] [CrossRef]
- Simas, R.C.; Eberlin, M.N.; Catharino, R.R.; Souza, V.; Alberici, R.M. Triacylglycerols oxidation in oils and fats monitored by easy ambient sonic-spray ionization mass spectrometry. J. Am. Oil Chem. Soc. 2012, 89, 1193–1200. [Google Scholar] [CrossRef]
- Porcari, A.M.; Fernandes, G.D.; Barrera-Arellano, D.; Eberlin, M.N.; Alberici, R.M. Food quality and authenticity screening via easy ambient sonic-spray ionization mass spectrometry. Analyst 2016, 141, 1172. [Google Scholar] [CrossRef] [PubMed]
- Alberici, R.M.; Fernandes, G.D.; Porcari, A.M.; Eberlin, M.N.; Barrera-Arellano, D.; Fernández, F.M. Rapid fingerprinting of sterols and related compounds in vegetable and animal oils and phytosterol enriched-margarines by transmission mode direct analysis in real time mass spectrometry. Food Chem. 2016, 211, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.; Pico, Y.; Barcelo, D. Direct analysis in real-time high-resolution mass spectrometry as a valuable tool for polyphenols profiling in olive oil. Anal. Methods 2019, 11, 472–482. [Google Scholar] [CrossRef]
- Lara-Ortega, F.J.; Beneito-Cambra, M.; Robles-Molina, J.; García-Reyes, J.F. Direct olive oil analysis by mass spectrometry: A comparison of different ambient ionization methods. Talanta 2018, 180, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.H. Direct analysis in real time—A critical review on DART-MS. Anal. Bioanal. Chem. 2014, 406, 63–80. [Google Scholar] [CrossRef]
- Wang, Y. Recent advances in the application of direct analysis in real time-mass spectrometry (DART-MS) in food analysis. Food Res. Int. 2024, 188, 114488. [Google Scholar] [CrossRef]
- Zacometti, C.; Khazzar, S.; Massaro, A.; Tata, A.; Riuzzi, G.; Piro, R.; Novelli, E.; Segato, S.; Balzan, S. DART-HRMS reveals metabolic changes of whey through microparticulation and fermentations. Appl. Food Res. 2024, 4, 100443. [Google Scholar] [CrossRef]
- Tata, A.; Massaro, A.; Riuzzi, G.; Lanza, I.; Bragolusi, M.; Negro, A.; Novelli, E.; Piro, R.; Gottardo, F.; Segato, S. Ambient mass spectrometry for rapid authentication of milk from Alpine or lowland forage. Sci. Rep. 2022, 12, 7360. [Google Scholar] [CrossRef]
- Ru, H.; Yan, L.; Ong, A.; Xu, Y.; Zhang, X.; Zhou, W. Atmospheric solids analysis probe-mass spectrometry (ASAP-MS) as a rapid fingerprinting technique to differentiate the harvest seasons of Tieguanyin oolong teas. Food Chem. 2023, 408, 135135. [Google Scholar] [CrossRef]
- Sajid, A.; von Holst, C. Analysis of feed additives by DART mass spectrometry: Method optimisation and applications for product traceability in the European Union focusing on coccidiostats and carotenoids. Food Addit. Contam. Part A 2023, 40, 1183–1197. [Google Scholar] [CrossRef]
- Guo, S.; Ge, Y.; Jom, K.N. A review of phytochemistry, metabolite changes, and medicinal uses of the common sunflower seed and sprouts (Helianthus annuus L.). Chem. Cent. J. 2017, 11, 95. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L.; Anastasi, U. Sowing time and irrigation scheduling effects on seed yield and fatty acids profile of sunflower in semi-arid climate. Int. J. Plant Prod. 2017, 11, 17–32. [Google Scholar]
- Sun, T.; Zhang, H.; Dong, Z.; Liu, Z.; Zheng, M. Ultrasonic-promoted enzymatic preparation, identification and multi-active studies of nature-identical phenolic acid glycerol derivatives. RSC Adv. 2020, 10, 11139–11147. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F. Pheno-Phospholipids and Lipo-Phenolics: Novel Structured Antioxidants, 1st ed.; Springer-Nature: Cham, Switzerland, 2021; pp. 1–60. [Google Scholar]
- Marinova, E.M.; Yanishlieva, N.V. Antioxidant activity and mechanism of action of some phenolic acids at ambient and high temperatures. Food Chem. 2003, 81, 189–197. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Bartel, I.; Mandryk, I.; Horbańczuk, J.O.; Wierzbicka, A.; Koszarska, M. Nutraceutical properties of syringic acid in civilization diseases—Review. Nutrients 2024, 16, 10. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Sova, M. Activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Arzola-Rodríguez, S.I.; Muñoz-Castellanos, L.N.; López-Camarillo, C.; Salas, E. Phenolipids, amphipilic phenolic antioxidants with modified properties and their spectrum of applications in development: A review. Biomolecules 2022, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.A.; McClements, D.J.; Decker, E.A. Challenges of utilizing healthy fats in foods. Adv. Nutr. 2015, 6, 309S–317S. [Google Scholar] [CrossRef]
- Gandova, V.; Ivanova, P.; Kalaydzhiev, H.; Perifanova-Nemska, M.; Chalova, V.I. Dissolution and surface tension properties of ethanol-wash solute obtained from industrial sunflower meal. Biointerface Res. Appl. Chem. 2021, 11, 11284–11292. [Google Scholar] [CrossRef]
- Gibb, S.; Strimmer, K. MALDIquant: A versatile R package for the analysis of mass spectrometry data. Bioinformatics 2012, 28, 2270–2271. [Google Scholar] [CrossRef] [PubMed]
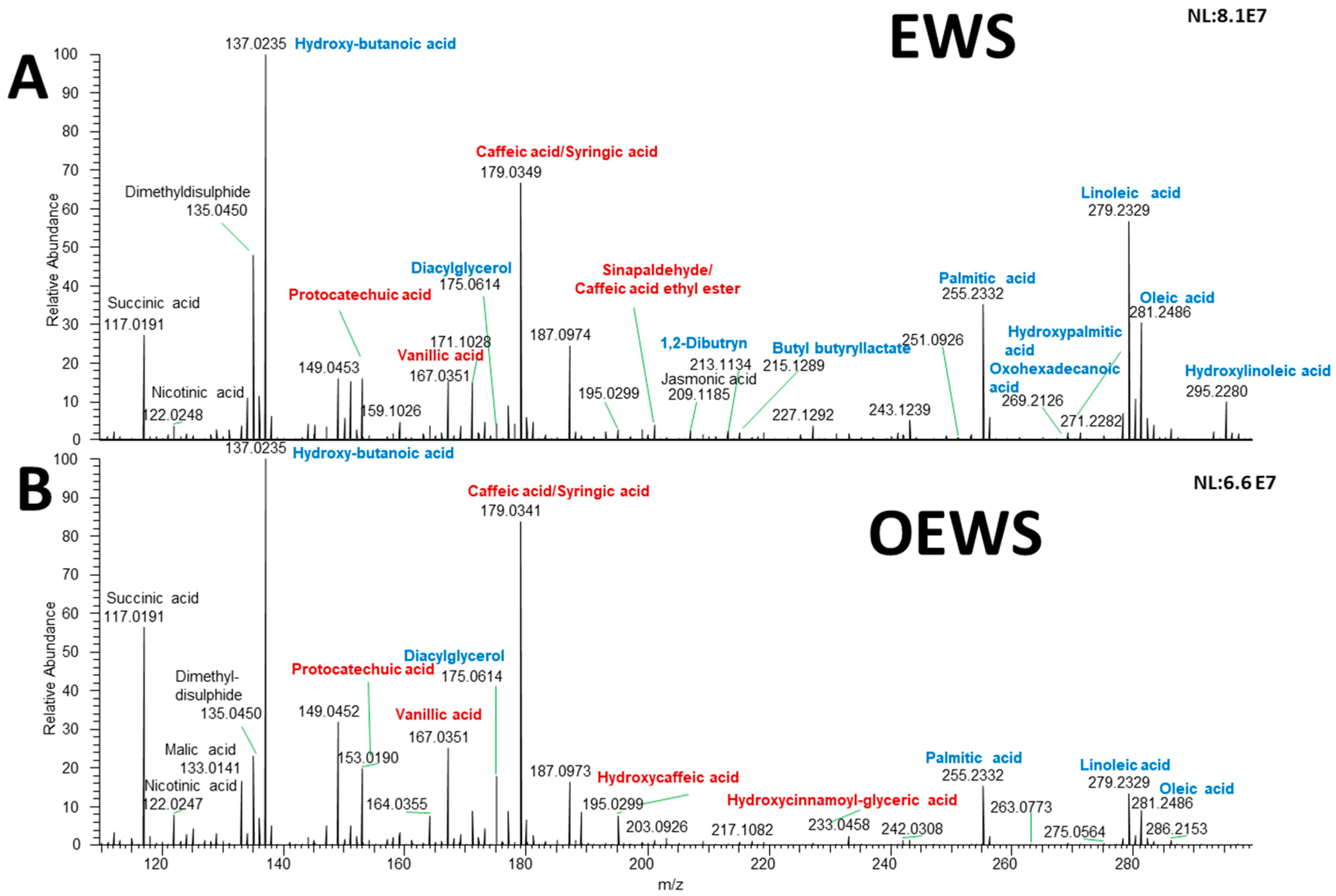
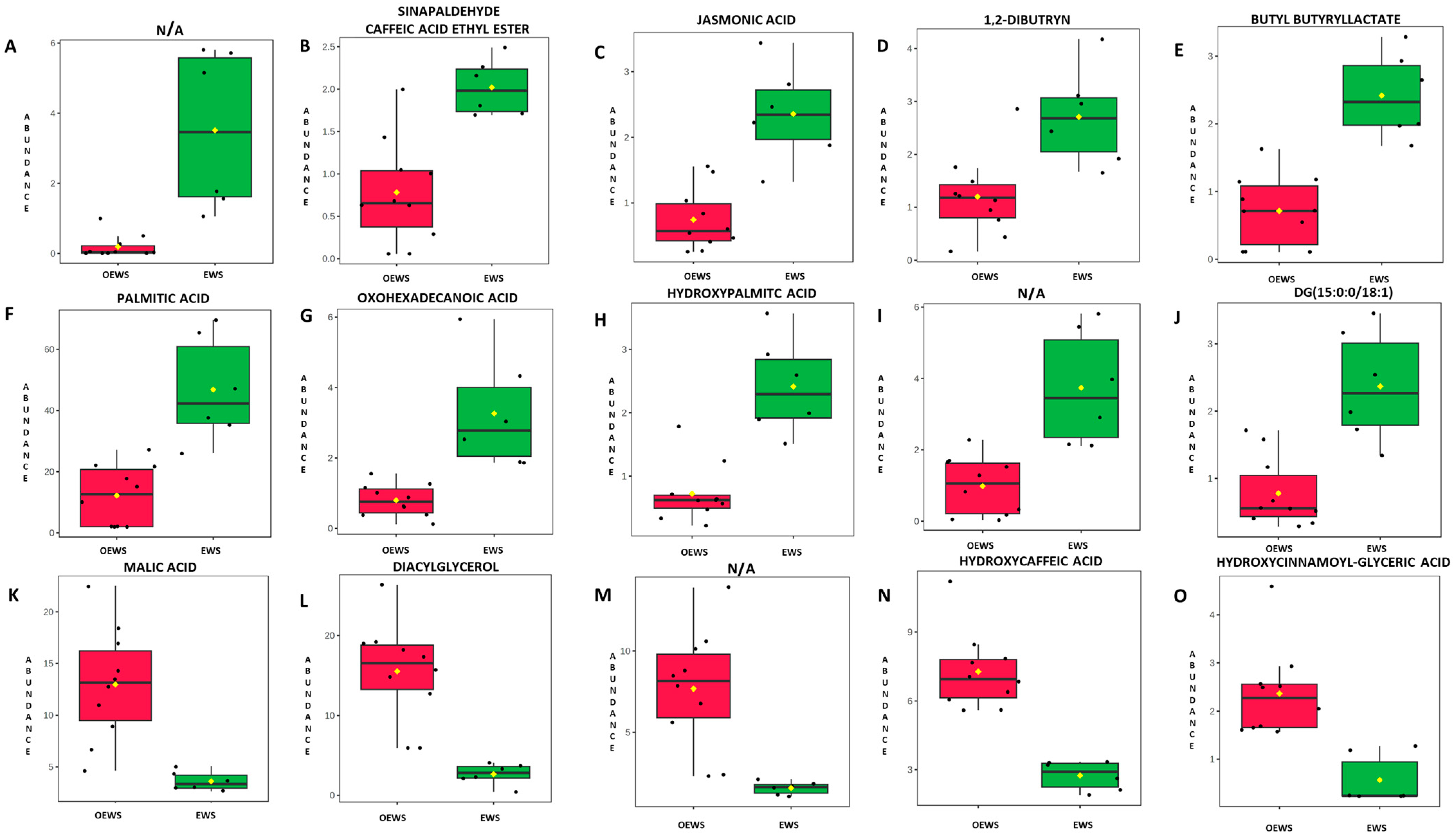
| m/z | m/z Theoretical Mass | Error (ppm) | Elemental Formula | Type of Ion | log2(FC) | Padj | Tentative Assignment |
|---|---|---|---|---|---|---|---|
| EWS | |||||||
| 150.0281 | − | − | C8H8O2 | [M − H]− | −4.197 | 0.023 | N/A |
| 207.0663 | 207.0663 | 0 | C11H12O4 | [M − H]− | −1.368 | 0.046 | Sinapaldehyde/Caffeic acid ethyl ester |
| 209.1185 | 209.1183 | 0.95 | C12H18O3 | [M − H]− | −1.660 | 0.023 | Jasmonic acid |
| 213.1134 | 213.1127 | − | C11H20O5 | [M – H − H2O]− | −1.175 | 0.036 | 1,2-Dibutyrin |
| 215.1289 | 215.1289 | 0 | C11H20O4 | [M − H]− | −1.760 | 0.023 | Butyl butyryllactate |
| 255.2332 | 255.233 | 0.78 | C16H32O2 | [M − H]− | −1.942 | 0.028 | Palmitic acid |
| 269.2126 | 269.2122 | 1.5 | C16H30O3 | [M − H]− | −2.029 | 0.015 | Oxohexadecanoic acid |
| 271.2282 | 271.2279 | 1.10 | C16H32O3 | [M − H]− | −1.741 | 0.022 | Hydroxypalmitic acid |
| 286.2153 | − | − | C7H6O3 | [M − H]− | −1.927 | 0.036 | N/A |
| 561.4891 | 561.4883 | 1.4 | C36H68O5 | [M – H − H2O]− | −1.605 | 0.023 | DG (15:0/0:0/18:1) |
| OEWS | |||||||
| 133.0141 | 133.0142 | −0.7 | C4H6O5 | [M − H]− | 1.844 | 0.022 | Malic acid |
| 175.0614 | 175.0612 | 1.14 | C7H12O5 | [M − H]− | 2.546 | 0.015 | Diacylglycerol |
| 189.0769 | 189.0768 | 0.5 | C8H14O5 | [M − H]− | 2.298 | 0.015 | N/A |
| 195.0299 | 195.0299 | 0 | C9H8O5 | [M − H]− | 1.405 | 0.015 | Hydroxycaffeic acid |
| 233.0459 | 233.0450 | 3.8 | C12H10O5 | [M – H − H2O]− | 2.064 | 0.023 | Hydroxycinnamoyl-(x)-glyceric acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacometti, C.; Lante, A.; Cisneros, M.; Massaro, A.; Mihaylova, D.; Chalova, V.; Krastanov, A.; Kalaydzhiev, H.; Riuzzi, G.; Tata, A.; et al. Rapid Assessment of Metabolomic Fingerprinting of Recycled Sunflower By-Products via DART-HRMS. Molecules 2024, 29, 4092. https://doi.org/10.3390/molecules29174092
Zacometti C, Lante A, Cisneros M, Massaro A, Mihaylova D, Chalova V, Krastanov A, Kalaydzhiev H, Riuzzi G, Tata A, et al. Rapid Assessment of Metabolomic Fingerprinting of Recycled Sunflower By-Products via DART-HRMS. Molecules. 2024; 29(17):4092. https://doi.org/10.3390/molecules29174092
Chicago/Turabian StyleZacometti, Carmela, Anna Lante, Miluska Cisneros, Andrea Massaro, Dasha Mihaylova, Vesela Chalova, Albert Krastanov, Hristo Kalaydzhiev, Giorgia Riuzzi, Alessandra Tata, and et al. 2024. "Rapid Assessment of Metabolomic Fingerprinting of Recycled Sunflower By-Products via DART-HRMS" Molecules 29, no. 17: 4092. https://doi.org/10.3390/molecules29174092
APA StyleZacometti, C., Lante, A., Cisneros, M., Massaro, A., Mihaylova, D., Chalova, V., Krastanov, A., Kalaydzhiev, H., Riuzzi, G., Tata, A., & Segato, S. (2024). Rapid Assessment of Metabolomic Fingerprinting of Recycled Sunflower By-Products via DART-HRMS. Molecules, 29(17), 4092. https://doi.org/10.3390/molecules29174092












