Quantitative Analysis of Isopimpinellin from Ammi majus L. Fruits and Evaluation of Its Biological Effect on Selected Human Tumor Cells
Abstract
1. Introduction
2. Results
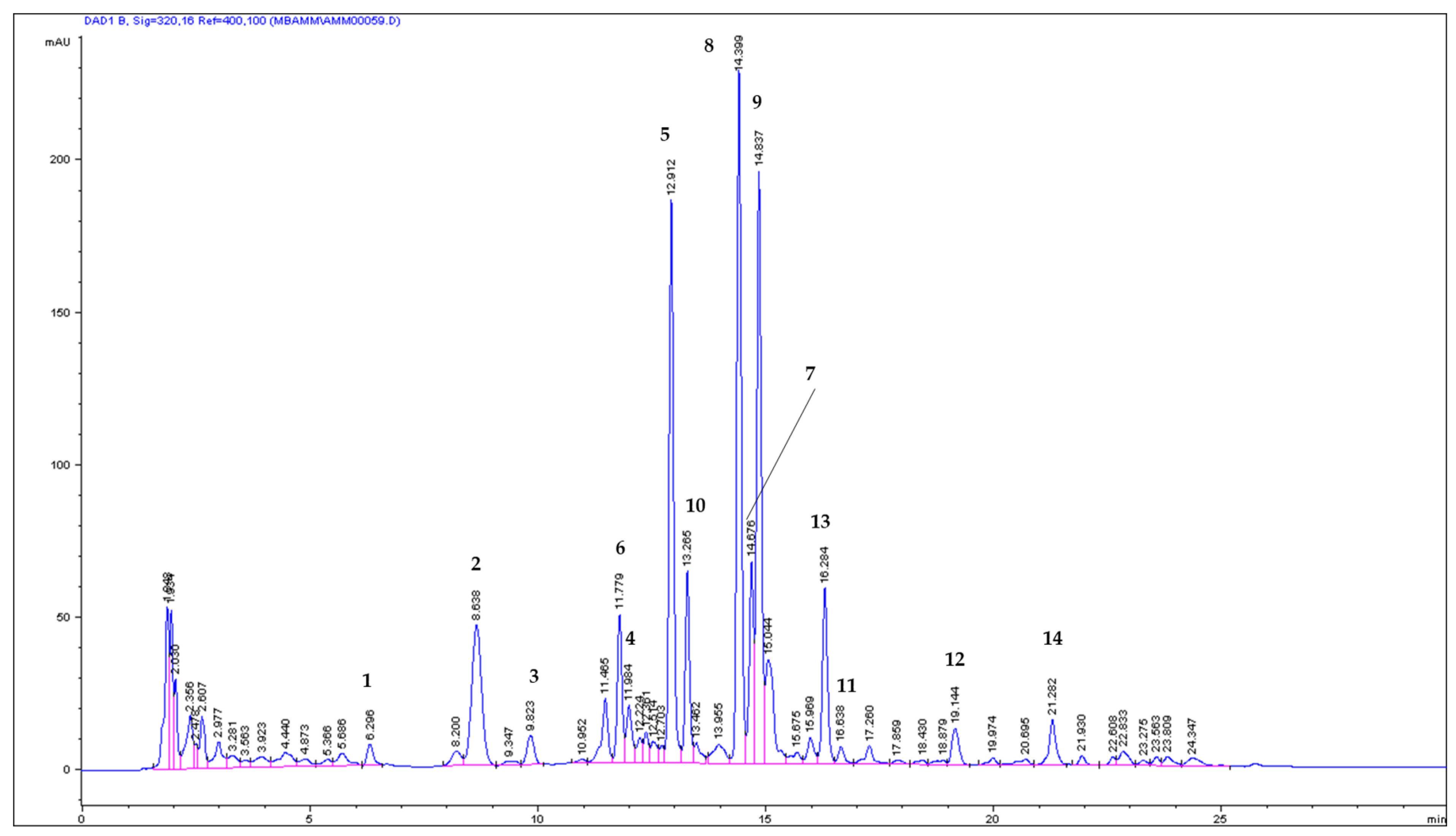
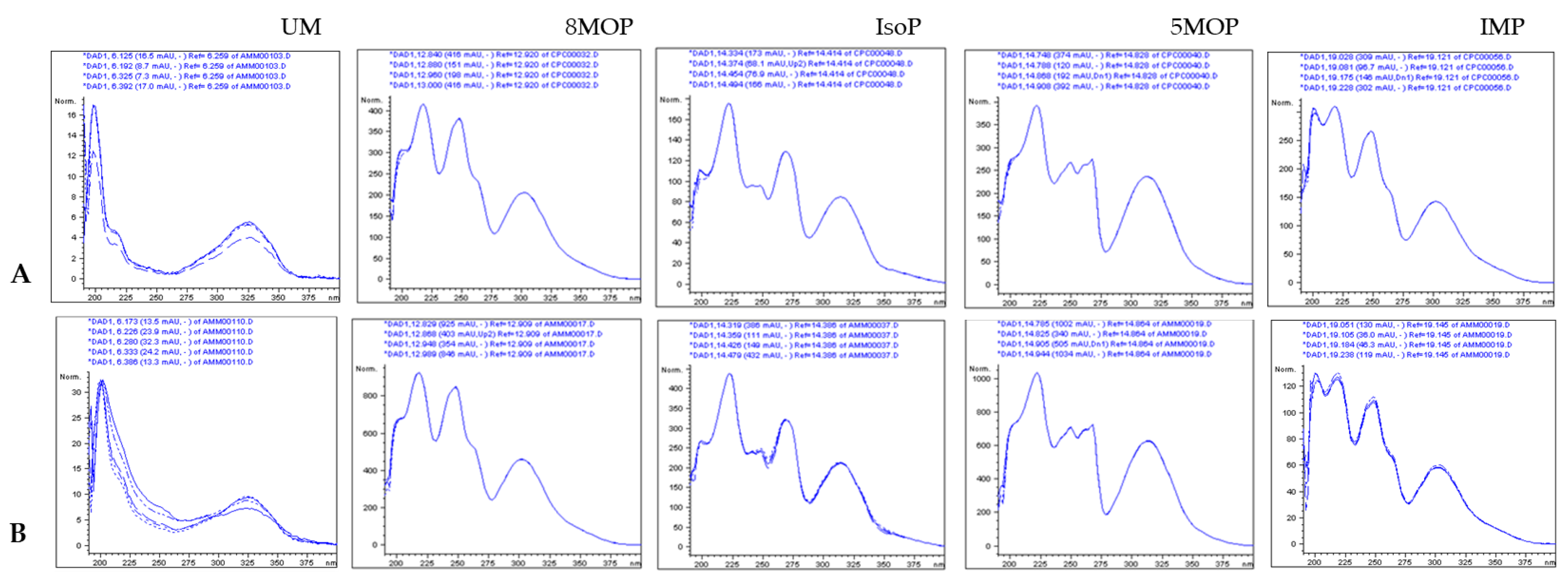
2.1. ASE/PLE Extraction Efficiency and Identification of Coumarins in the ASE Extract
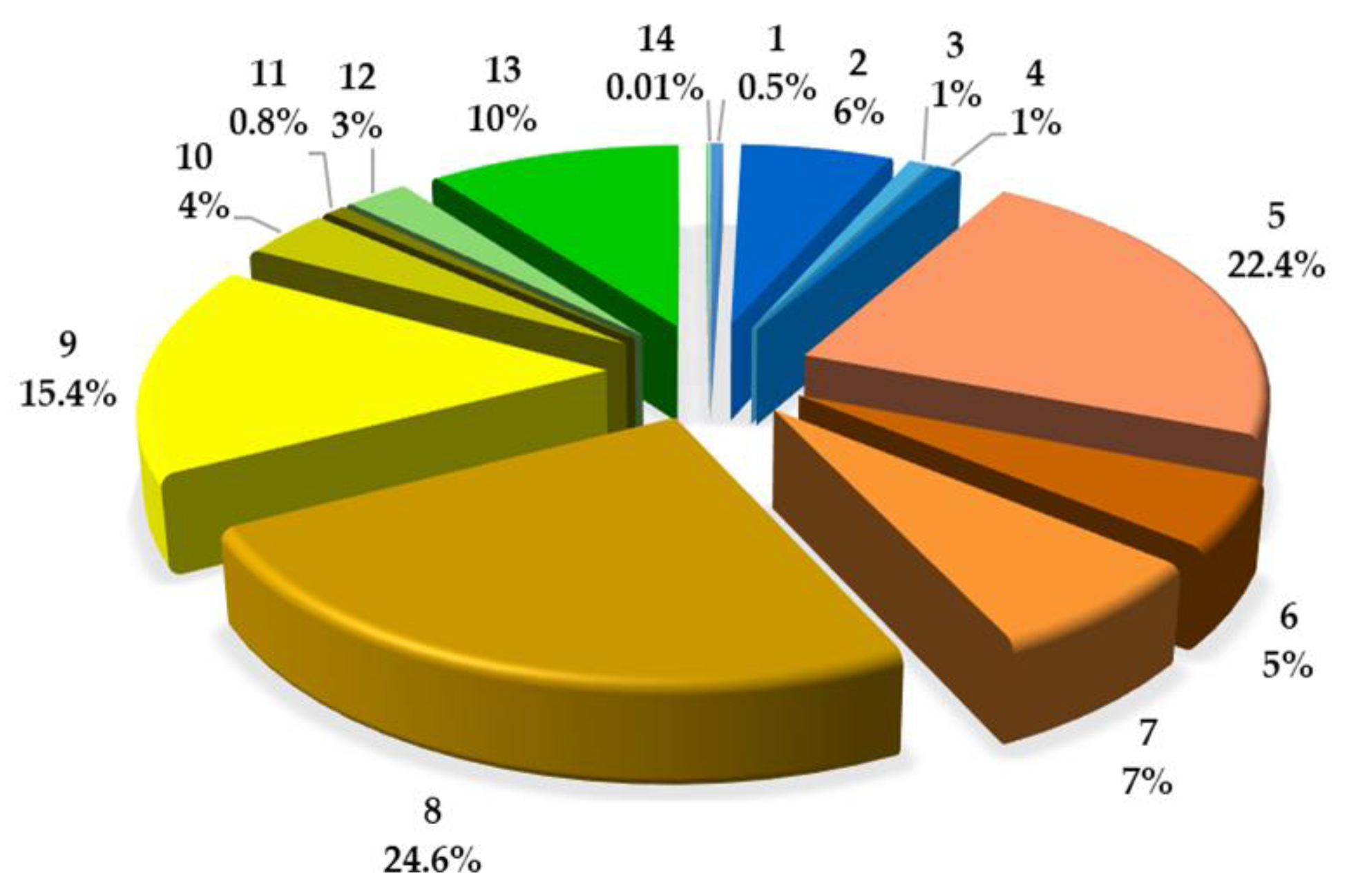
2.2. Quantitative Analysis of Coumarins in PLE/ASE Extracts
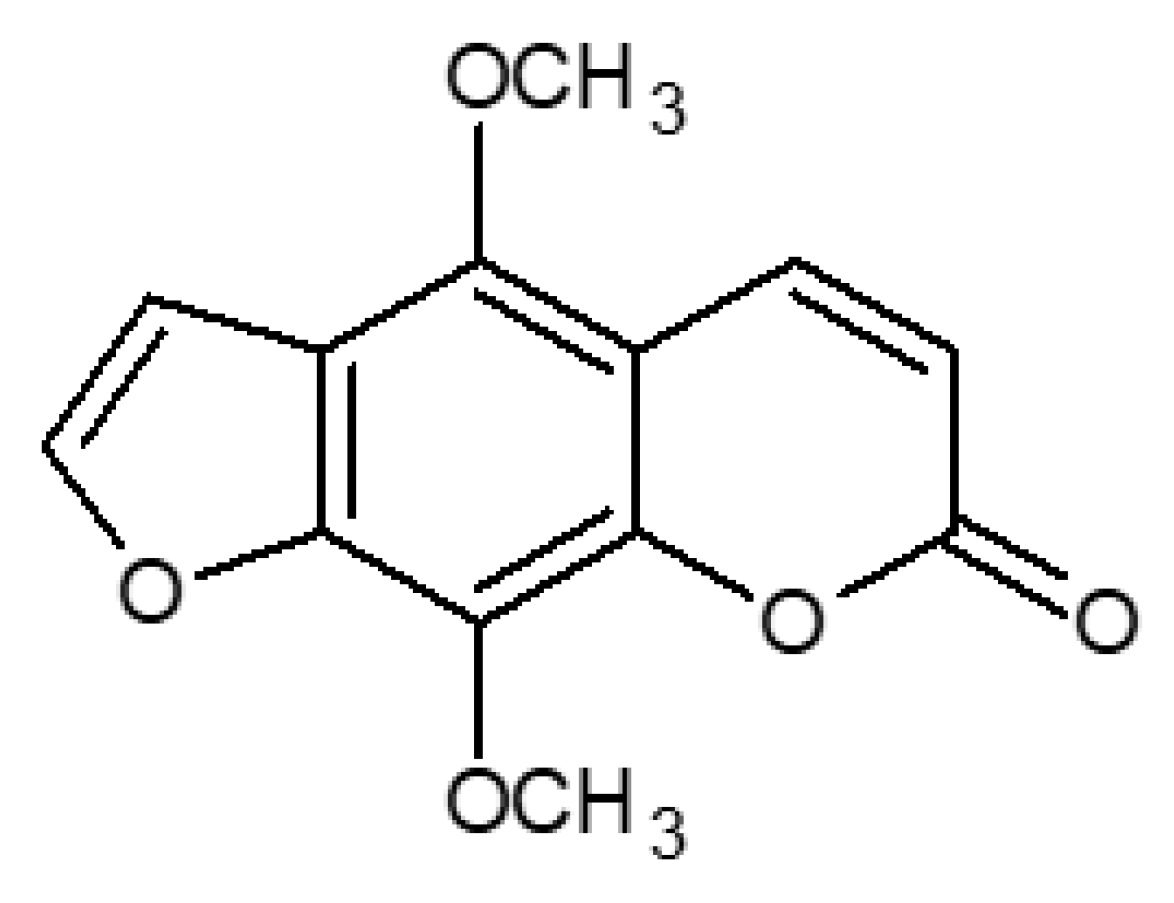
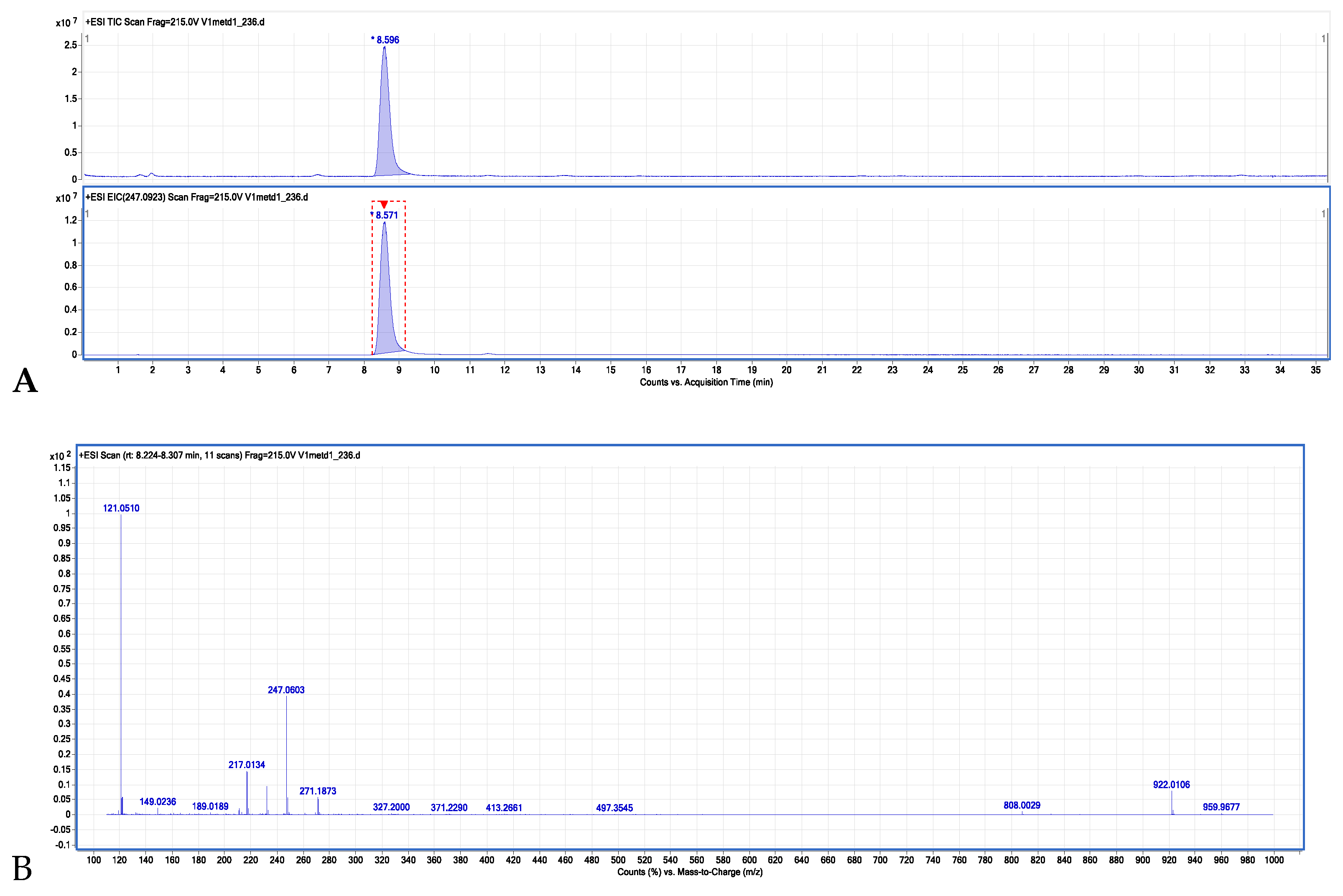
2.3. LC/CPC Isolation and Identification of Isopimpinellin
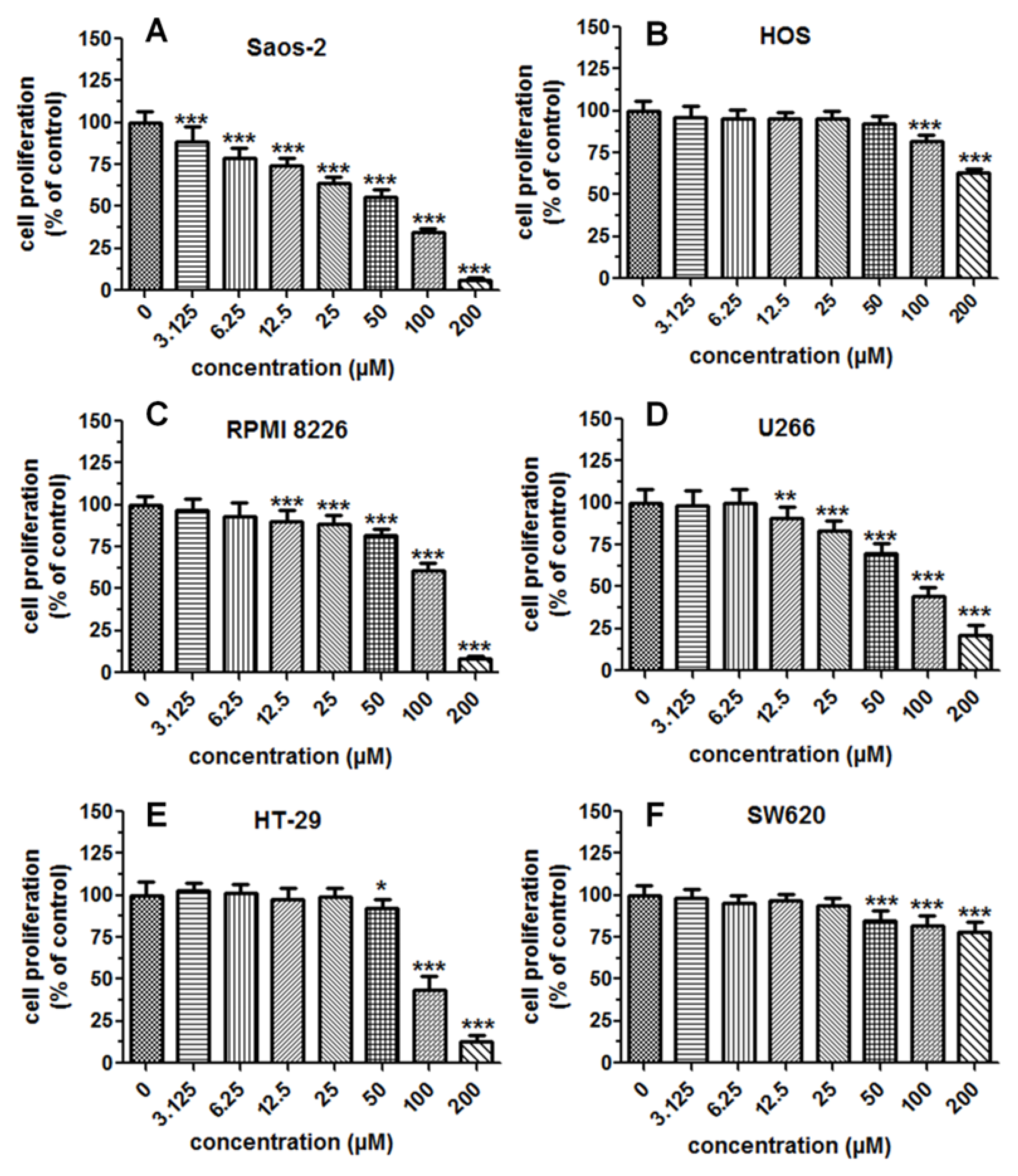
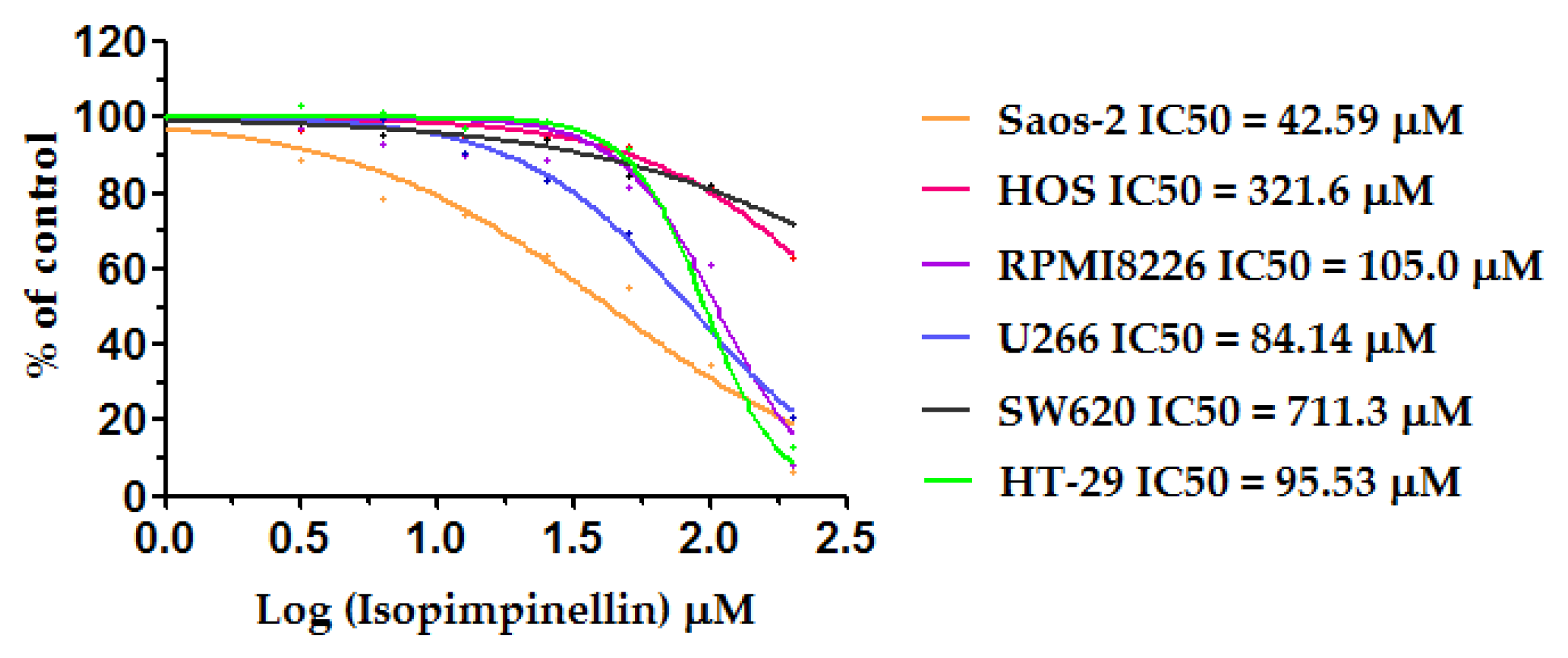
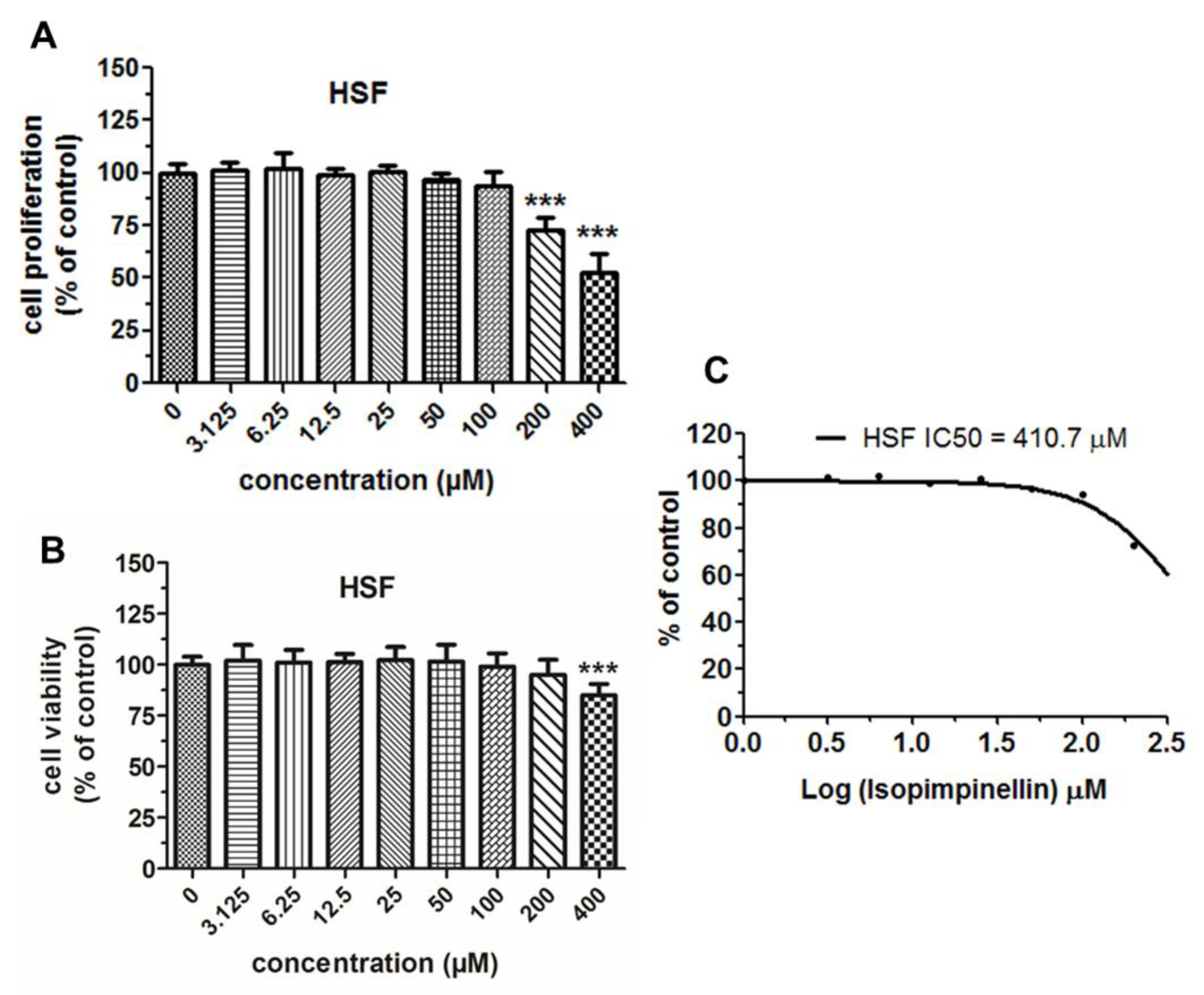
2.4. Cytostatic and Cytotoxic Activity of IsoP
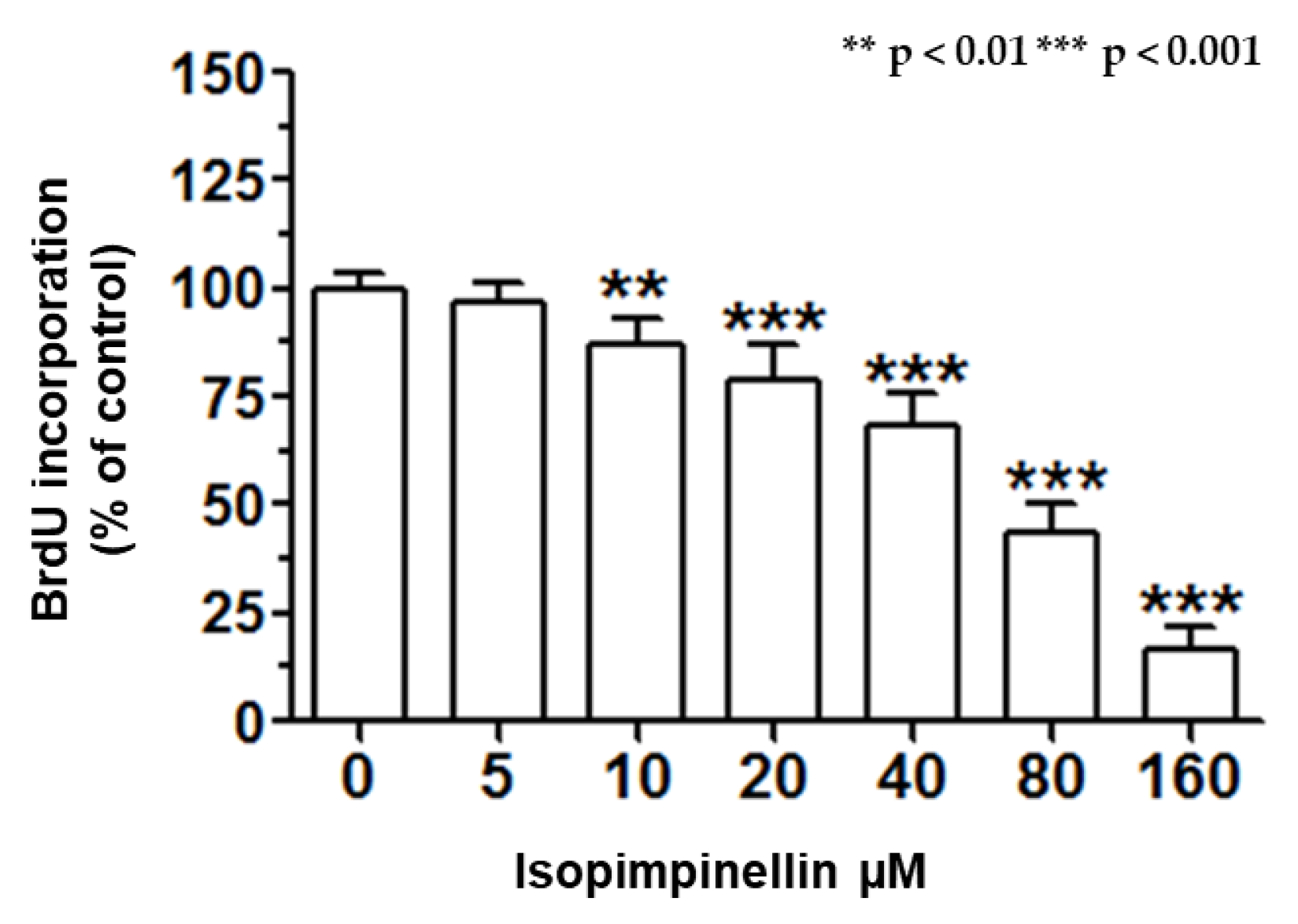
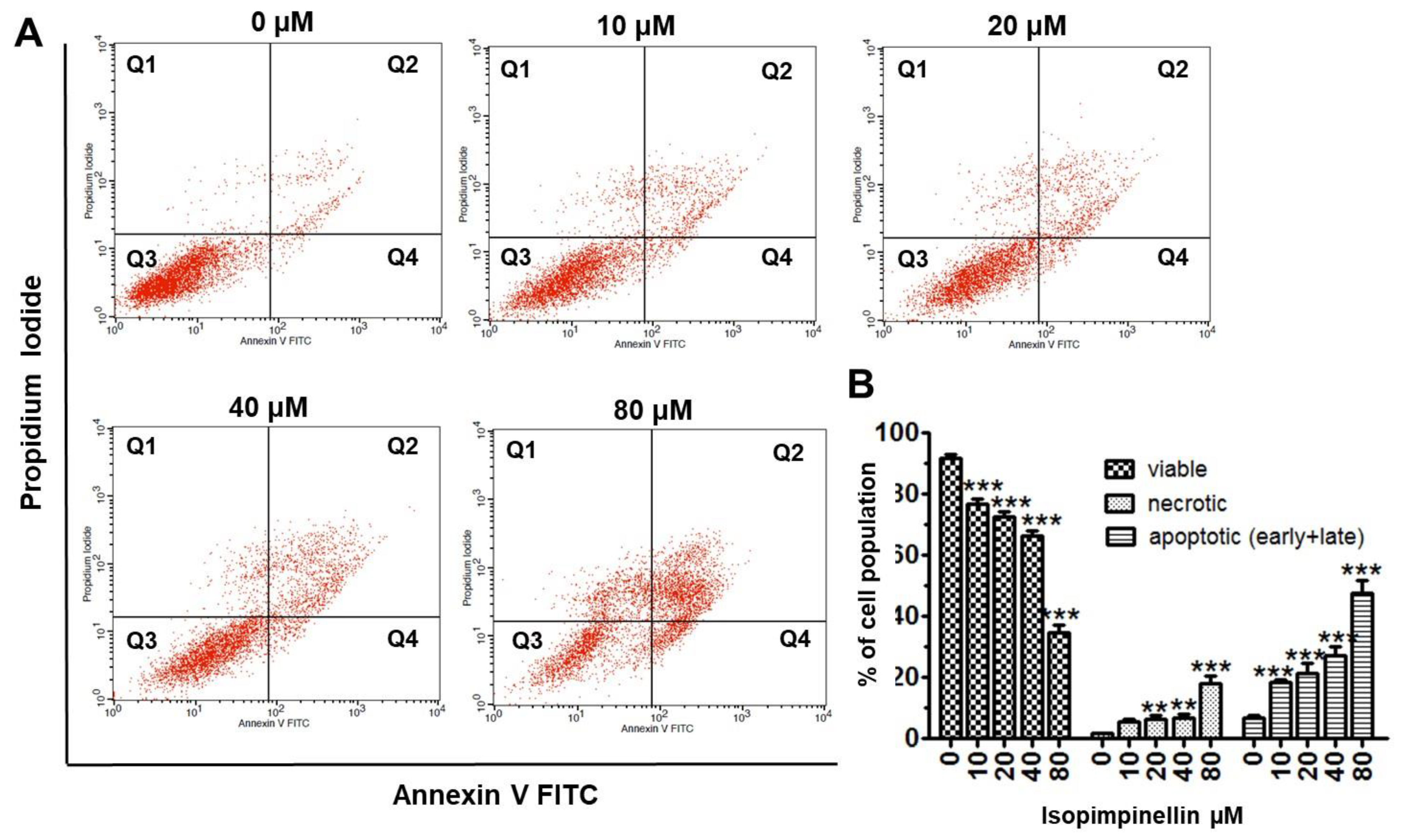
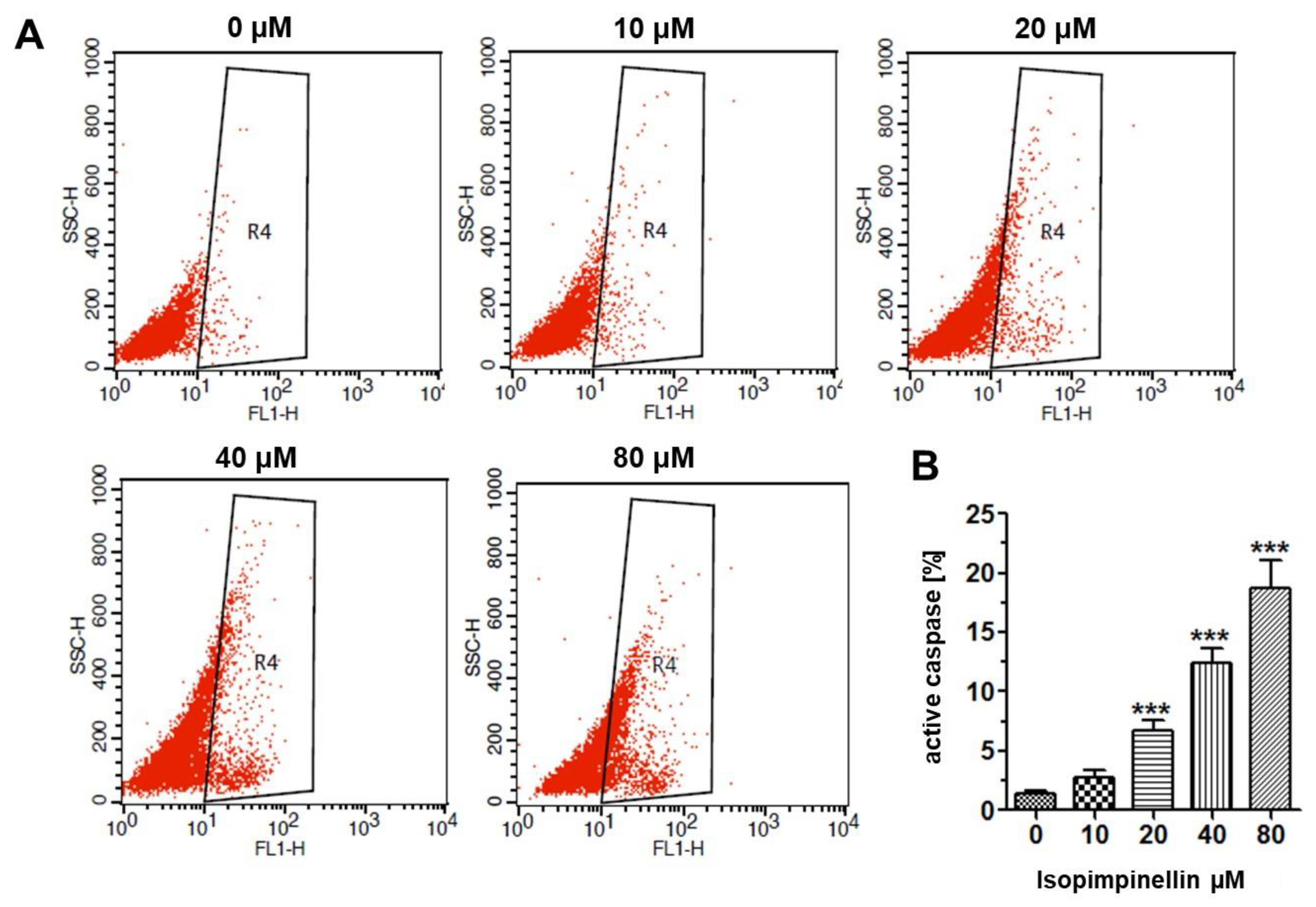
2.5. Impact of IsoP on DNA Synthesis and Apoptotic Cell Death
3. Discussion
4. Materials and Methods
4.1. Solvents and Chemicals
4.2. Plant Material
4.3. Isolation, Purification, and Identification of IsoP
4.3.1. Extraction of the Plant Material and Purification of the Samples
4.3.2. HPLC Method Validation
4.3.3. LC/CPC Isolation of Isopimpinellin
4.3.4. ESI-TOF MS Identification of Isopimpinellin
4.4. Biological Studies
4.4.1. Cell Lines
4.4.2. Proliferation MTT Assay
4.4.3. Cytotoxicity Assessment—NR Assay
4.4.4. Selectivity Index (SI)
4.4.5. BrdU ELISA Cell Proliferation Assay
4.4.6. Flow Cytometry
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, C.; Mai, Z.; Liu, C.; Yin, S.; Cai, Y.; Xia, C. Natural Products in Preventing Tumour Drug Resistance and Related Signalling Pathways. Molecules 2022, 27, 3513. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.B. Cancer treatment: Role of natural products. Time to have a serious rethink. Oral Oncol. Rep. 2023, 6, 100040. [Google Scholar] [CrossRef]
- Ali, M.; Wani, S.U.D.; Salahuddin, M.; Manjula, S.N.; Mruthunjava, K.; Dey, T.; Zargar, M.I.; Singh, J. Recent advance of herbal medicines in cancer—A molecular approach. Heliyon 2023, 9, e13684. [Google Scholar] [CrossRef] [PubMed]
- Bjoërklund, E.; Nilsson, T.; Böwadt, S. Pressurised liquid extraction of persistent organic pollutants in environmental analysis. Trends Anal. Chem. 2000, 19, 434–445. [Google Scholar] [CrossRef]
- Bucar, F.; Wube, A.; Schmid, M. Natural product isolation—How to get from biological material to pure compounds. Nat. Prod. Rep. 2013, 30, 525–545. [Google Scholar] [CrossRef] [PubMed]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Waksmundzka-Hajnos, M.; Petruczynik, A.; Dragan, A.; Wianowska, D.; Dawidowicz, A.L.; Sowa, I. Influence of the Extraction Mode on the Yield of Some Furanocoumarins from Pastinaca sativa Fruits. J. Chromatogr. A 2004, 800, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Widelski, J.; Luca, S.V.; Skiba, A.; Chinou, I.; Marcourt, L.; Wolfender, J.-L.; Skalicka-Wozniak, K. Isolation and Antimicrobial Activity of Coumarin Derivatives from Fruits of Peucedanum luxurians Tamamsch. Molecules 2018, 23, 1222. [Google Scholar] [CrossRef] [PubMed]
- Kukuła-Koch, W.; Grabarska, A.; Łuszczki, J.; Czernicka, L.; Nowosadzka, E.; Gumbarewicz, E.; Jarząb, A.; Audo, G.; Upadhyay, S.; Głowniak, K.; et al. Superior anticancer activity is demonstrated by total extract of Curcuma longa L. as opposite to individual curcuminoids separated by centrifugal partition chromatography. Phythoter. Res. 2018, 32, 933–942. [Google Scholar] [CrossRef]
- Kędzierski, B.; Kukuła-Koch, W.; Głowniak, K. Application of CPC and related methods for the isolation of natural substances—A review. Acta Pol. Pharm. 2014, 71, 223–227. [Google Scholar]
- Skalicka-Woźniak, K.; Garrard, I. A comprehensive classification of solvent systems used for natural product purifications in counter current and centrifugal partition chromatography. Nat. Prod. Rep. 2012, 32, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, M.; Mazurek, A.K. Isolation of methoxyfuranocoumarins from Ammi majus by centrifugal partition chromatography. J. Chromatogr. Sci. 2016, 54, 10–16. [Google Scholar]
- Bartnik, M. Efficient Separation of the Methoxyfuranocoumarins Peucedanin, 8-Methoxypeucedanin, and Bergapten by Centrifugal Partition Chromatography (CPC). Molecules 2023, 28, 1923. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Chemical constituents and pharmacological activities of Ammi majus and Ammi visnaga: A review. Int. J. Pharm. Ind. Res. 2013, 3, 257–265. [Google Scholar]
- Usmani, Q.I.; Jahan, N.; Aleem, M.; Hasan, S.A. Aatrilal (Ammi majus L.), an important drug of Unani system of medicine: A review. J. Ethnopharmacol. 2021, 276, 114144. [Google Scholar] [CrossRef] [PubMed]
- Balkrischna, A.; Arya, V.; Sharma, I.P. Anti-cancer and anti-inflammatory potential of furanocoumarins from Ammi majus L. Anti-Cancer Agents Med. Chem. 2022, 22, 1030–1036. [Google Scholar] [CrossRef]
- Hossain, M.A.; Al Touby, S. Ammi majus an Endemic Medicinal Plant: A Review of the Medicinal Uses, Pharmacological and Phytochemicals. Annu. Toxicol. 2024, 2, 9–14. [Google Scholar] [CrossRef]
- Harsahay, M.; Hemant, K.P.; Aarti, M.; Mohd, N. Development of HPLC method for estimation of furonocumarins in Psoralea corylifolia and Ammi majus. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 290–294. [Google Scholar]
- Nazik, S.M.; Mona, S.M.; Ramzi, A.M.; Wadah, J.O.; Hassan, S.K. HPTLC fingerprint profiles and UPLC-MS identification of potential antioxidant fractions and compounds from Ambrosia maritima L. and Ammi majus L. Afr. J. Biotechnol. 2020, 19, 249–258. [Google Scholar] [CrossRef]
- Królicka, A.; Kartanowicz, R.; Wosiński, S.A.; Szpitter, A.; Kamiński, M.; Łojkowska, E. Induction of secondary metabolite production in transformed callus of Ammi majus L. grown after electromagnetic treatment of the culture medium. Enzym. Microb. Technol. 2006, 39, 1386–1391. [Google Scholar] [CrossRef]
- Song, P.-P.; Zhao, J.; Liu, Z.-L.; Duan, Y.-B.; Hou, Y.-P.; Zhao, C.-Q.; Wu, M.; Wei, M.; Wang, N.-H.; Lv, Y.; et al. Evaluation of antifungal activities and structure–activity relationships of coumarin derivatives, Pest. Menag. Sci. 2017, 73, 94–101. [Google Scholar] [CrossRef]
- He, Y.-H.; Shang, X.-F.; Li, H.-X.; Li, A.-P.; Tang, C.; Zhang, B.-Q.; Zhang, Z.-J.; Wang, R.; Ma, Y.; Du, S.-S.; et al. Antifungal activity and action mechanism study of coumarins from Cnidium monnieri fruit and structurally related compounds. Chem. Biodiv. 2021, 18, e2100633. [Google Scholar] [CrossRef] [PubMed]
- Łuszczki, J.J.; Bojar, H.; Jankiewicz, K.; Florek-Łuszczki, M.; Chmielewski, J.; Skalicka-Woźniak, K. Anticonvulsant effects of isopimpinellin and its interactions with classic antiseizure medications and borneol in the mouse tonic-clonic seizure model: An isobolographic transformation. Pharmacol. Rep. 2023, 75, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Borgonovo, G.; Scaglioni, L.; Bassoli, A. Phytochemicals from Ruta graveolens Activate TAS2R Bitter Taste Receptors and TRP Channels Involved in Gustation and Nociception. Molecules 2015, 20, 18907–18922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Sun, W.; Shen, X.; Gong, X.; Wang, C.; Liang, Y.; Zhou, W. CF–PK–PD analysis of natural haemostatic compounds from Toddalia asiatica (Linn.) Lam. root bark in rats. Acta Chromatogr. 2021, 33, 261–269. [Google Scholar] [CrossRef]
- Lee, Y.; Hyun, C.-G. Mechanistic Insights into the Ameliorating Effect of Melanogenesis of Psoralen Derivatives in B16F10 Melanoma Cells. Molecules 2022, 27, 2613. [Google Scholar] [CrossRef] [PubMed]
- Takomthong, P.; Waiwut, P.; Yenjai, C.; Sripanidkulchai, B.; Reubroycharoen, P.; Lai, R.; Kamau, P.; Boonyarat, C. Structure–Activity Analysis and Molecular Docking Studies of Coumarins from Toddalia asiatica as Multifunctional Agents for Alzheimer’s Disease. Biomedicines 2020, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Sonawane, V.R.; Williams, I.S.; McCann, G.J.P.; Gatchie, L.; Sharma, R.; Satti, N.; Chaudhuri, B.; Bharate, S.B. Identification of karanjin isolatd from Indian beech tree a potent CYP1 enzyme inhibitor with cellular efficacy via screening of a natural products repository. Med. Chem. Commun. 2018, 9, 371. [Google Scholar] [CrossRef]
- Robertson, A.L.; Ogryzko, N.V.; Henry, K.M.; Loynes, C.A.; Foulkes, M.J.; Meloni, M.M.; Wang, X.; Ford, C.; Jackson, M.; Ingham, P.W.; et al. Identification of benzopyrone as a common structural feature in compounds with anti-inflammatory activity in a zebrafish phenotypic screen. Dis. Model Mech. 2016, 9, 621–632. [Google Scholar] [CrossRef]
- Bartnik, M.; Sławińska-Brych, A.; Żurek, A.; Kandefer-Szerszeń, M.; Zdzisińska, B. 8-methoxypsoralen reduces AKT phosphorylation, induces intrinsic and extrinsic apoptotic pathways, and suppresses cell growth of SK-N-AS neuroblastoma and SW620 metastatic colon cancer cells. J. Ethnopharmacol. 2017, 207, 19–29. [Google Scholar] [CrossRef]
- Bartnik, M.; Sławińska-Brych, A.; Mizerska-Kowalska, M.; Zdzisińska, B. Evaluation of the Biological Effect of Non-UV-Activated Bergapten on Selected Human Tumour Cells and the Insight into the Molecular Mechanism of Its Action. Int. J. Mol. Sci. 2023, 24, 15555. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, B.; Zhang, Q.; Yang, X.; Sun, J.; Tang, B.; Cui, G.; Yao, D.; Liu, L.; Gu, G.; et al. Simultaneous determination of osthole, bergapten and isopimpinellin in rat plasma and tissues by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 970, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Rahman, J.K.; Jaff, D.M.; Ozdemir, M.; Ahamad, J.; Hussain, F.H.S. Phytochemical Screening of Different Parts of Prangos Platychlaena Boiss by Liquid-Chromatography Tandem Mass Spectrometry (LC-MS/MS). Asian J. Sci. Eng. 2021, 7, 30–38. [Google Scholar] [CrossRef]
- Boik, J. Natural Compounds in Cancer Therapy; Oregon Medical Press: Princeton, MN, USA, 2001; p. 521. [Google Scholar]
- Suffiness, M.; Pezzuto, J.M. Assays related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity; Hostettmann, K., Ed.; Academic Press: London, UK, 1990; pp. 71–133. [Google Scholar]
- Chakravarty, M.; Ganguli, P.; Murahari, M.; Sarkar, R.R.; Peters, G.J.; Mayur, Y.C. Study of combinatorial drug synergy of novel acridone derivatives with temozolomide using in-silico and in-vitro methods in the treatment of drug-resistant glioma. Front. Oncol. 2021, 11, 625899. [Google Scholar] [CrossRef] [PubMed]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, Post-Treatment Recovery, and Selectivity Analysis of Naturally Occurring Podophyllotoxins from Bursera fagaroides var. fagaroides on Breast Cancer Cell Lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef] [PubMed]
- Noor, Z.M.; Ahmad, H.; Ain, Q.; Anjum, T.; Malik, Z.S.; Hussain, Z.; Naseer, F. Caspase 3 and Its Role in the Pathogenesis of Cancer. Clin. Oncol. 2022, 7, 1941. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Waksmundzka-Hajnos, M.; Petruczynik, A.; Dragan, A.; Wianowska, D.; Dawidowicz, A.L. Effect of extraction method on the yield of furanocumarins from fruits of Archangelica officinalis Hoffm. Phytochem. Anal. 2004, 15, 313–319. [Google Scholar] [CrossRef]
- Bartnik, M.; Głowniak, K. Furanocoumarins from Peucedanum tauricum Bieb. and their variability in the aerial parts of the plant during development. Acta Chromatogr. 2007, 18, 5–14. [Google Scholar]
- Skalicka-Woźniak, K.; Glowniak, K. Pressurized liquid extraction on coumarins from fruits of Heracleum leskowii with application of solvents with different polarity under increasing temperature. Molecules 2012, 14, 4133–4141. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2024, 10, 1367–1401, Erratum in Genes Dis. 2024, 11, 101211. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Deasy, J.O. A literature mining-based approach for identification of cellular pathways associated with chemo resistance in cancer. Brief Bioinform. 2016, 17, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz-Gil, J.; Paduch, R.; Ulz, Z.; Bądziul, D.; Głowniak, K.; Gawron, A. Cell death in HeLa cells upon imperatorin and cisplatin treatment. Folia Histochem. Cytobiol. 2012, 50, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Küpeli Akkol, E.; Capasso, R. Anticancer Potential of Furanocoumarins: Mechanistic and Therapeutic Aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, X.; Cheng, K.; Zhao, W.; Hua, Y.; Xu, C.; Yang, Z. Psoralen reverses the P-glycoprotein-mediated multidrug resistance in human breast cancer MCF-7/ADR cells. Mol. Med. Rep. 2016, 13, 4745–4750. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, C.; Cheng, B.; Jin, L.; Li, J.; Gong, Y.; Lin, W.; Pan, Z.; Pan, C.-W. Imperatorin acts as a cisplatin sensitizer via downregulating Mcl-1 expression in HCC chemotherapy. Tumor Biol. 2015, 37, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, S.; Ogmundsdottir, H.M.; Gudbjarnason, S. Antiproliferative effect of Angelica archangelica fruits. Z. Naturforsch. C 2004, 59, 523–527. [Google Scholar] [CrossRef]
- Li, G.; Song, X.; He, Y.; Yao, J.; Huang, C.; Deng, Y.; Xie, S.; Ren, J.; Jin, M.; Liu, H. Angelicin inhibits human lung carcinoma A549 cell growth and migration through regulating JNK and ERK pathways. Oncol. Rep. 2016, 36, 3504–3512. [Google Scholar] [CrossRef] [PubMed]
- Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical Sources, Chemistry, Analysis, and Biological Activity of Furanocoumarins of Pharmaceutical Interest. Molecules 2019, 24, 2163. [Google Scholar] [CrossRef]
- Kubrak, T.; Bogucka-Kocka, A.; Komsta, Ł.; Załuski, D.; Bogucki, J.; Galkowski, D.; Kaczmarczyk, R.; Feldo, M.; Adamczyk-Cioch, M.; Kocki, J. Modulation of multidrug resistance gene expression by coumarin derivatives in human leukemic cells. Oxid. Med. Cell. Longev. 2017, 2017, 5647281. [Google Scholar] [CrossRef]
- Mirzaei, S.A.; Dehkordi, N.G.; Ghamghami, M.; Amiri, A.; Abdolahinia, E.D.; Elahian, F. ABC-transporter blockage mediated by xanthotoxin and bergapten is the major pathway for chemo sensitization of multidrug-resistant cancer cells. Toxicol. Appl. Pharmacol. 2017, 337, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, A.; Raho, E.; Marconi, B.; Nicoli, S.; Santini, M.; Allegra, F.; Colombo, P.; Bettini, R.; Santi, P. Plasma and skin concentration of 5-methoxypsoralen in psoriatic patients after oral administration. J. Investig. Dermatol. 2001, 117, 379–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, A.-H.; Zhang, Y.-B.; Yang, X.-W. Simultaneous determination, and pharmacokinetics of sixteen Angelicae dahuricae coumarins in vivo by LC–ESI-MS/MS following oral delivery in rats. Phytomedicine 2016, 23, 1029–1036. [Google Scholar] [CrossRef]
- Li, H.; Zeng, H.; He, D.; Wang, M.; Liu, L.; Liang, W.; Shu, Y.; Zhao, S.; Sun, G.; Lv, C.; et al. A new approach to examining the extracton process of Zhishi and Zhiqiao considering the synergistic effect of complex mixtures by PAMPA. J. Chrom. B 2018, 1099, 10–17. [Google Scholar] [CrossRef]
- Liao, M.; Song, G.; Cheng, X.; Diao, X.; Sun, Y.; Zhang, L. Simultaneous determination of six coumarins in rat plasma and metabolites identification of bergapten in vitro and in vivo. J. Agric. Food Chem. 2018, 66, 4602–4613. [Google Scholar] [CrossRef]
- Melough, M.M.; Chun, O.K. Dietary furocoumarins and skin cancer: A review of current biological evidence. Food Chem. Toxicol. 2018, 122, 163–171. [Google Scholar] [CrossRef]
- Sumorek-Wiadro, J.; Zając, A.; Maciejczyk, A.; Jakubowicz-Gil, J. Furanocoumarins in anticancer therapy—For and against. Fitoterapia 2020, 142, 104492. [Google Scholar] [CrossRef]
- Grabarska, A.; Skalicka-Woźniak, K.; Kiełbus, M.; Dmoszyńska-Graniczka, M.; Miziak, P.; Szumiło, J.; Nowosadzka, E.; Kowalczuk, K.; Khalifa, S.; Smok-Kalwat, J.; et al. Imperatorin as a Promising Chemotherapeutic Agent against Human Larynx Cancer and Rhabdomyosarcoma Cells. Molecules 2020, 25, 2046. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, M. Methoxyfuranocoumarins of Natural Origin-Updating Biological Activity Research and Searching for New Directions—A Review. Curr. Issues Mol. Biol. 2024, 46, 856–883. [Google Scholar] [CrossRef]
- Kleiner, H.E.; Vulimiri, S.V.; Miller, L.; Johnson, J.W.H.; Whitman, C.P. Oral administration of naturally occurring coumarins leads to altered phase I and II enzyme activities and reduced DNA adduct formation by polycyclic aromatic hydrocarbons in various tissues of SENCAR mice. Carcinogenesis 2001, 22, 73–82. [Google Scholar] [CrossRef][Green Version]
- Kleiner, H.E.; Vulimiri, S.V.; Starost, M.F.; Reed, M.J.; Di Giovanni, J. Oral administration of the citrus coumarin, isopimpinellin, blocks DNA adduct formation and skin tumour initiation by 7,12-dimethylbenz[α]anthracene in SENCAR mice. Carcinogenesis 2002, 23, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, H.E.; Reed, M.J.; Di Giovanni, J. Naturally Occurring Coumarins Inhibit Human Cytochromes P450 and Block Benzo[α]pyrene and 7,12-Dimethylbenz[α]anthracene DNA Adduct Formation in MCF-7 Cells. Chem. Res. Toxicol. 2003, 16, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Bhagavatheeswaran, S.; Ramachandran, V.; Shanmugam, S.; Balakrishnan, A. Isopimpinellin extends antiangiogenic effect through overexpression of miR-15b-5p and downregulating angiogenic stimulators. Mol. Biol. Rep. 2022, 49, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Łuczka, P.; Grabarska, A.; Florek-Łuszczki, M.; Plewa, Z.; Łuszczki, J.J. Synergy, Additivity, and Antagonism between Cisplatin and Selected Coumarins in Human Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 537. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.; Jayaprakasha, G.K.; Kim, J.; Murthy, K.; Chetti, M.; Nam, S.-Y.; Patil, B.S. 5-Geranyloxy-7-Methoxycoumarin inhibits colon cancer (SW480) cells growth by inducing apoptosis. Planta Med. 2013, 79, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Li, J.; Wu, S.; Wu, X.; Chen, M.; Zhong, X.; Liu, K. Comparative profiling between primary colorectal carcinomas and metastases identifies heterogeneity on drug resistance. Oncotarget 2016, 7, 63937–63949. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, W.; Xiong, J.; Gui, H.Y.; Feng, X.M.; Chen, R.N.; Hu, G.; Yang, J. Down regulation of differentiated embryonic chondrocytes 1 (DEC1) is involved in 8-methoxypsoralen-induced apoptosis in HepG2 cells. Toxicology 2012, 301, 58–65. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, J.; Luo, W.; Yang, J.; Xi, T. 8-Methoxypsoralen Induces Intrinsic Apoptosis in HepG2 Cells: Involvement of Reactive Oxygen Species Generation and ERK1/2 Pathway Inhibition. Cell Physiol. Biochem. 2015, 37, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Europaean Pharmacopoeia, 10th ed.; European Directoriate for the Quality of Medicines and Healthcare—EDQM, Council of Europe: Strasbourg, France, 2020.
- Zgórka, G.; Głowniak, K. Simultaneous Determination of Phenolic Acids and Linear Furanocoumarins in Fruits of Libanotis dolichostyla by Solid-Phase Extraction and High Performance Liquid Chromatography. Phytochem. Anal. 1999, 10, 268–271. [Google Scholar] [CrossRef]
- Piechowska, K.; Mizerska-Kowalska, M.; Zdzisińska, B.; Cytarska, J.; Baranowska-Łączkowska, A.; Jaroch, K.; Łuczykowski, K.; Płaziński, W.; Bojko, B.; Kruszewski, S.; et al. Tropinone-Derived Alkaloids as Potent Anticancer Agents: Synthesis, Tyrosinase Inhibition, Mechanism of Action, DFT Calculation, and Molecular Docking Studies. Int. J. Mol. Sci. 2020, 21, 9050. [Google Scholar] [CrossRef]
- Lauvrak, S.U.; Munthe, E.; Kresse, S.H.; Stratford, E.W.; Namløs, H.M.; Meza-Zepeda, L.A.; Myklebost, O. Functional characterisation of osteosarcoma cell lines and identification of mRNAs and miRNAs associated with aggressive cancer phenotypes. Br. J. Cancer. 2013, 109, 2228–2236. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.L.; Liang, L.; Ding, Y.Q. Expression of FMNL2 and its relation to the metastatic potential of human colorectal cancer cells. Nan Fang Yi Ke Da Xue Xue Bao 2008, 28, 1775–1778. [Google Scholar] [PubMed]
- Jiang, S.; Zhou, F.; Zhang, Y.; Zhou, W.; Zhu, L.; Zhang, M.; Luo, J.; Ma, R.; Xu, X.; Zhu, J.; Dong, X.; Zhang, S.; Fang, J.; Sun, J.; Yang, X. Identification of tumorigenicity-associated genes in osteosarcoma cell lines based on bioinformatic analysis and experimental validation. J. Cancer. 2020, 11, 3623–3633. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://cancer.sanger.ac.uk/cosmic (accessed on 6 May 2024).
- Available online: https://maayanlab.cloud/Harmonizome/dataset/COSMIC+Cell+Line+Gene+Mutation+Profiles (accessed on 6 May 2024).
| Solvent | Temp. (°C) | ASE Extraction Yield (% ± SD) * | RSD (%) |
|---|---|---|---|
| dichloromethane | Dex/50 | 13.44 ± 0.32 | 2.38 |
| Dex/70 | 13.87 ± 0.17 | 1.23 | |
| Dex/90 | 16.69 ± 0.26 | 1.56 | |
| Dex/110 | 17.47 ± 0.31 | 1.77 | |
| Dex/130 | 18.43 ± 0.67 | 3.64 | |
| methanol | Mex/50 | 18.01 ± 0.41 | 2.28 |
| Mex/70 | 21.60 ± 0.66 | 3.06 | |
| Mex/90 | 24.79 ± 0.73 | 2.94 | |
| Mex/110 | 27.09 ± 0.58 | 2.14 | |
| Mex/130 | 28.81 ± 0.49 | 1.70 |
| Compound | tR (min ± SD) * | Calibration Eq. & | R2 | Range (µg/mL) | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|---|
| UM | 6.301 ± 0.01 | y = 43.468x − 1.8877 | 0.9999 | 1–100 | 0.02 | 0.07 |
| 8 MOP | 12.929 ± 0.04 | y = 19.618x + 59.806 | 0.9999 | 7–220 | 0.20 | 0.61 |
| IsoP | 14.419 ± 0.06 | y = 23.379x + 19.515 | 0.9999 | 5–100 | 0.06 | 0.19 |
| 5 MOP | 14.860 ± 0.10 | y = 31.995x + 63.463 | 0.9996 | 1–220 | 0.42 | 1.26 |
| IMP | 19.189 ± 0.09 | y = 14.949x + 27.773 | 0.9996 | 1–220 | 0.15 | 0.45 |
| No | Compound | mg/g (Mean ± SD) | mg/100 g | RSD (%) & | (%) * | tR (min) |
|---|---|---|---|---|---|---|
| 1 | UM | 0.084671 ± 0.0008 | 8.4671 | 0.98 | 0.52 | 6.301 |
| 2 | u | 1.005768 ± 0.0739 | 100.5768 | 7.35 | 6.11 | 8.668 |
| 3 | u | 0.152076 ± 0.0076 | 15.2076 | 5.01 | 0.92 | 9.845 |
| 4 | u | 0.155196 ± 0.0025 | 15.5196 | 1.60 | 0.94 | 12.000 |
| 5 | 8 MOP | 3.680345 ± 0.0451 | 368.0345 | 1.23 | 22.36 | 12.929 |
| 6 | x | 0.828362 ± 0.0170 | 82.8363 | 2.06 | 5.03 | 11.795 |
| 7 | x | 1.156771 ± 0.0437 | 115.6771 | 3.76 | 7.03 | 14.699 |
| 8 | IsoP | 4.041358 ± 0.0354 | 404.1358 | 0.88 | 24.56 | 14.419 |
| 9 | 5 MOP | 2.530536 ± 0.0356 | 253.0536 | 1.41 | 15.38 | 14.860 |
| 10 | y | 0.584874 ± 0.0145 | 58.4874 | 2.48 | 3.55 | 13.282 |
| 11 | y | 0.130425 ± 0.0069 | 13.0425 | 5.30 | 0.79 | 16.676 |
| 12 | IMP | 0.416027 ± 0.0263 | 41.6027 | 6.32 | 2.52 | 19.189 |
| 13 | z | 1.677398 ± 0.0442 | 167.7398 | 2.64 | 10.19 | 16.319 |
| 14 | z | 0.012632 ± 0.0010 | 1.2632 | 7.77 | 0.08 | 21.967 |
| Total | 16.456440 ± 0.0265 | 1645.6440 | 0.16 | 100.0 | ||
| 5 MOP + 8 MOP + IsoP | 10.252239 ± 0.0875 | 1025.2239 | 0.85 | 62.3 | ||
| Temp. (°C) Solvent | mg/100 g Dry Weight of Plant Substance (mean ± SD) */(% = g/100 g DW) & | ||
|---|---|---|---|
| 8 MOP | IsoP | 5 MOP | |
| dichloromethane | |||
| Dex/50 | 187.91 ± 1.75/0.19 | 303.94 ± 2.11/0.30 | 129.20 ± 2.34/0.13 |
| Dex/70 | 205.28 ± 2.06/0.21 | 311.04 ± 3.01/0.31 | 141.15 ± 1.53/0.14 |
| Dex/90 | 227.36 ± 1.23/0.23 | 327.73 ± 3.70/0.33 | 156.33 ± 2.78/0.16 |
| Dex/110 | 238.79 ± 3.02/0.24 | 333.53 ± 1.65/0.33 | 168.19 ± 1.54/0.17 |
| Dex/130 | 259.53 ± 3.41/0.26 | 346.53 ± 2.76/0.35 | 178.45 ± 3.02/0.18 |
| methanol | |||
| Mex/50 | 296.05 ± 1.24/0.30 | 358.76 ± 2.01/0.36 | 203.56 ± 1.40/0.20 |
| Mex/70 | 330.16 ± 1.41/0.33 | 374.56 ± 2.09/0.38 | 227.01 ± 2.54/0.23 |
| Mex/90 | 351.37 ± 2.34/0.35 | 397.25 ± 1.98/0.40 | 241.60 ± 2.03/0.24 |
| Mex/110 | 354.25 ± 2.60/0.35 | 403.59 ± 2.62/0.40 | 243.58 ± 1.78/0.24 |
| Mex/130 | 368.03 ± 4.51/0.37 | 404.14 ± 3.54/0.40 | 253.05 ± 3.55/0.25 |
| Normal Cells / Tumor Cells (IC50) | HSF / Saos-2 | HSF / HOS | HSF / RPMI8226 | HSF / U266 | HSF / SW620 | HSF / HT-29 |
|---|---|---|---|---|---|---|
| SI * | 9.62 | 1.27 | 3.91 | 4.88 | 0.57 | 4.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartnik, M.; Sławińska-Brych, A.; Mizerska-Kowalska, M.; Kania, A.K.; Zdzisińska, B. Quantitative Analysis of Isopimpinellin from Ammi majus L. Fruits and Evaluation of Its Biological Effect on Selected Human Tumor Cells. Molecules 2024, 29, 2874. https://doi.org/10.3390/molecules29122874
Bartnik M, Sławińska-Brych A, Mizerska-Kowalska M, Kania AK, Zdzisińska B. Quantitative Analysis of Isopimpinellin from Ammi majus L. Fruits and Evaluation of Its Biological Effect on Selected Human Tumor Cells. Molecules. 2024; 29(12):2874. https://doi.org/10.3390/molecules29122874
Chicago/Turabian StyleBartnik, Magdalena, Adrianna Sławińska-Brych, Magdalena Mizerska-Kowalska, Anna Karolina Kania, and Barbara Zdzisińska. 2024. "Quantitative Analysis of Isopimpinellin from Ammi majus L. Fruits and Evaluation of Its Biological Effect on Selected Human Tumor Cells" Molecules 29, no. 12: 2874. https://doi.org/10.3390/molecules29122874
APA StyleBartnik, M., Sławińska-Brych, A., Mizerska-Kowalska, M., Kania, A. K., & Zdzisińska, B. (2024). Quantitative Analysis of Isopimpinellin from Ammi majus L. Fruits and Evaluation of Its Biological Effect on Selected Human Tumor Cells. Molecules, 29(12), 2874. https://doi.org/10.3390/molecules29122874







