Gypensapogenin A-Liposomes Efficiently Ameliorates Hepatocellular Lipid Accumulation via Activation of FXR Receptor
Abstract
1. Introduction
2. Results and Discussion
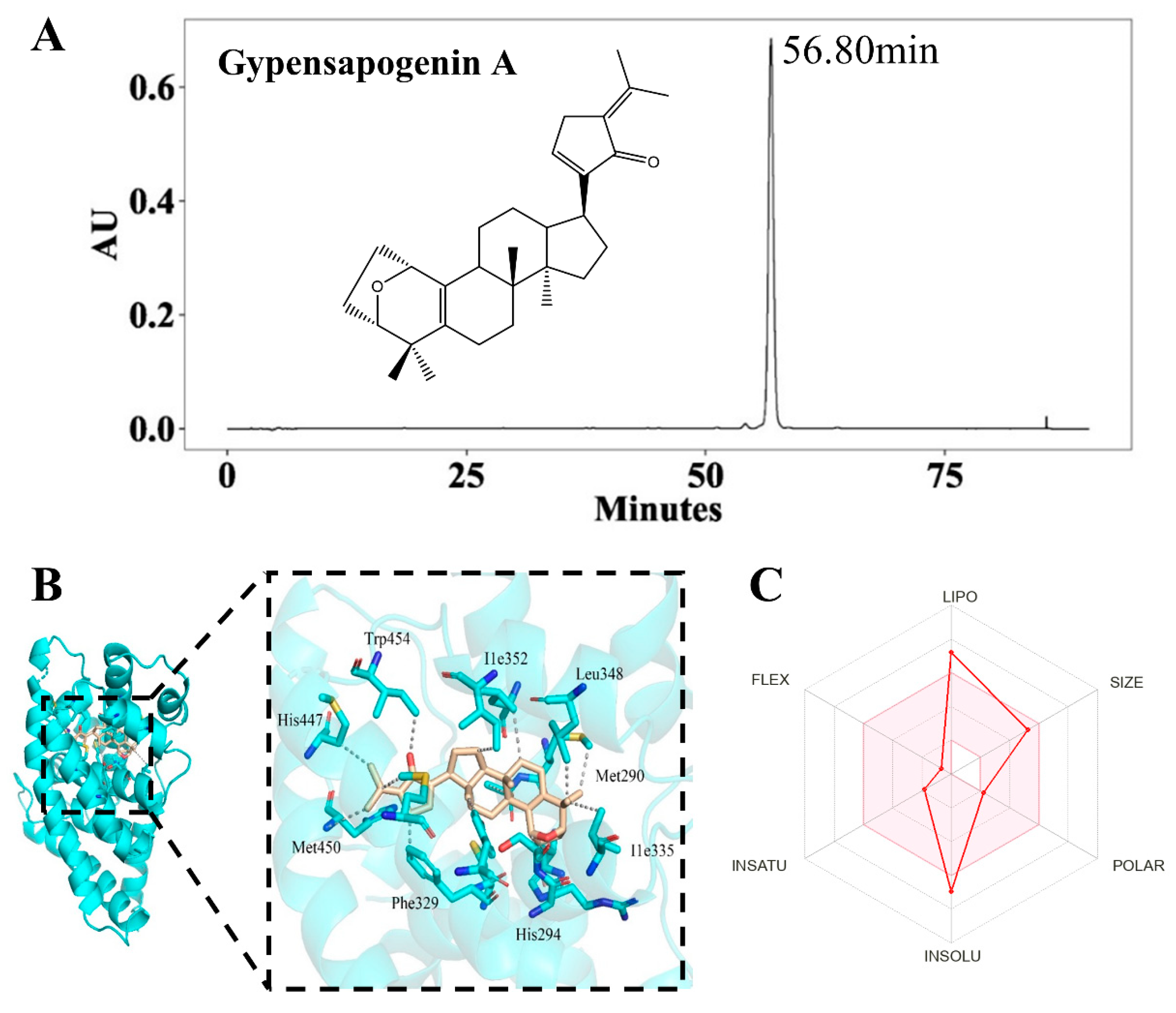
2.1. Isolation, Characterization and Druglikeness of Gypensapogenin A
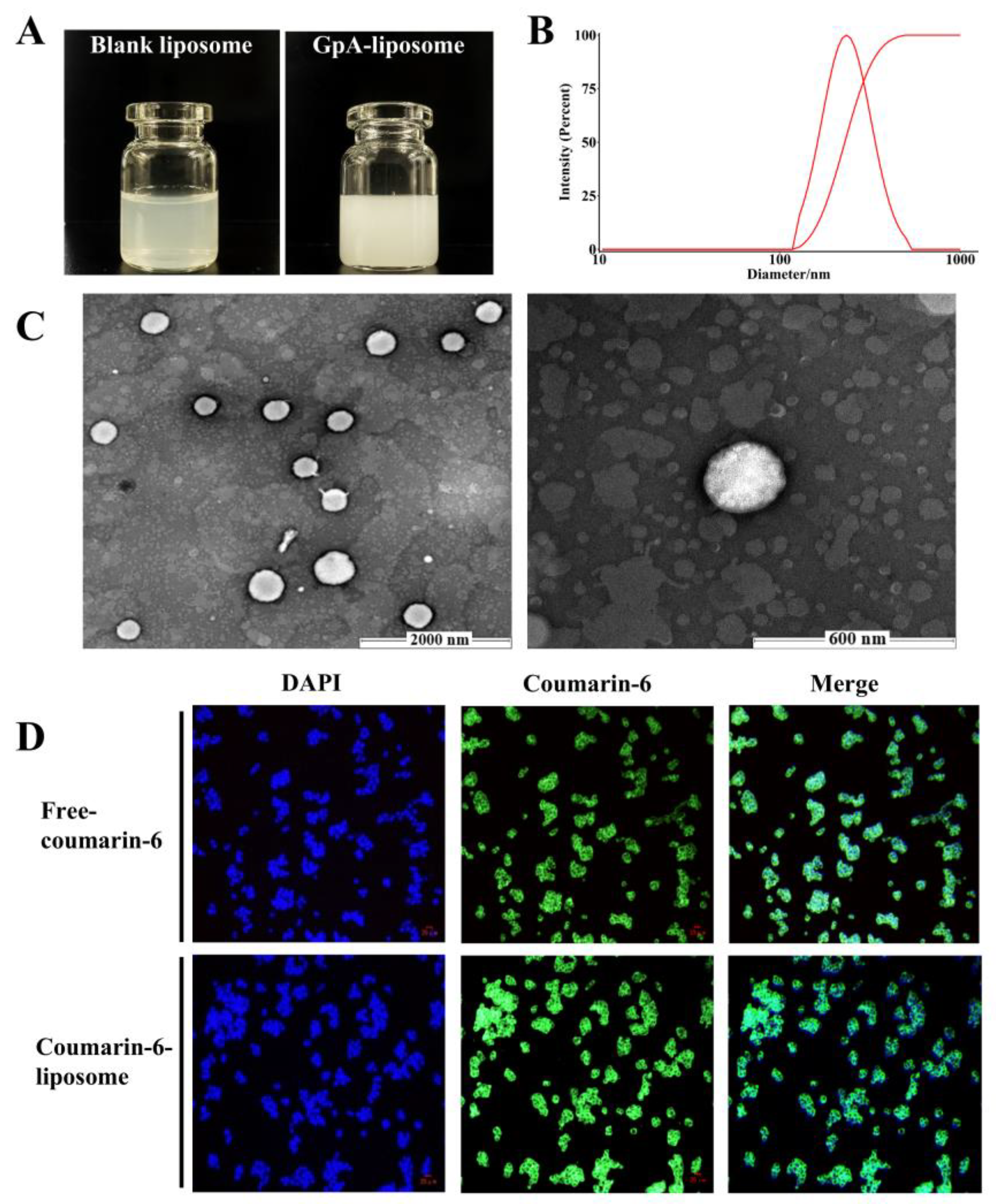
2.2. Parameters of Gypensapogenin A Liposomes
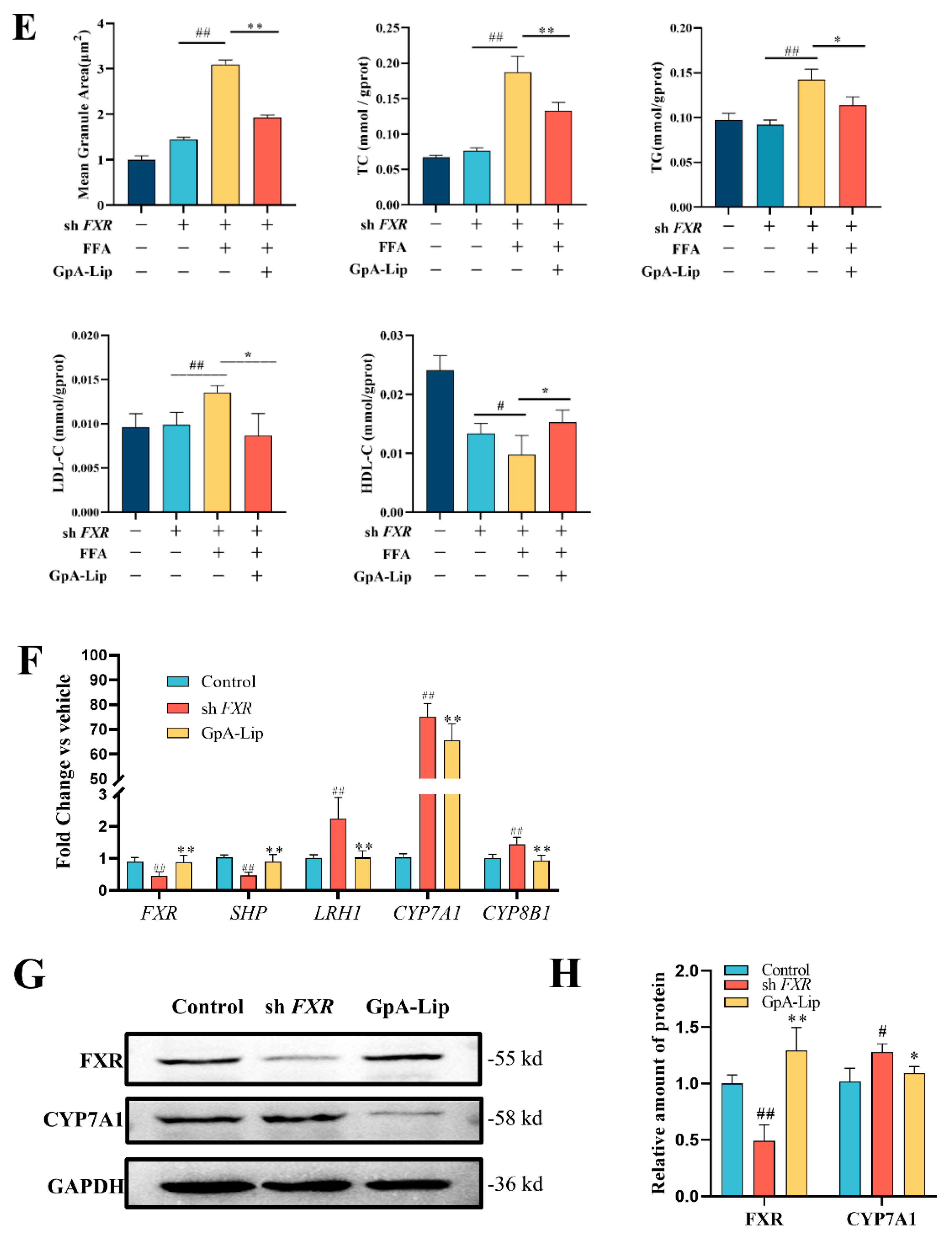
2.3. Hypolipidemic Effects of Gypensapogenin A Liposomes
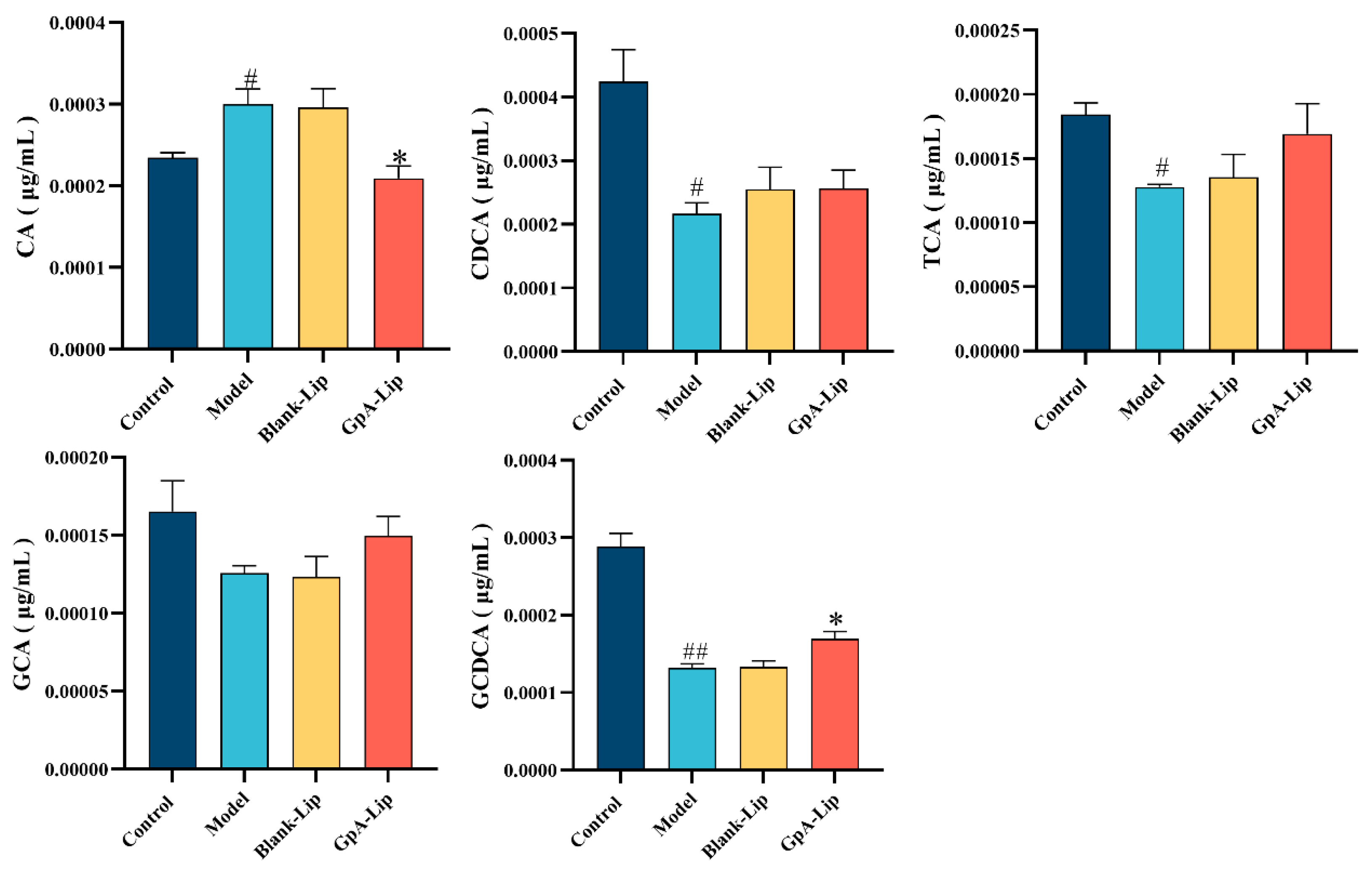
2.4. Bile Acid Analysis
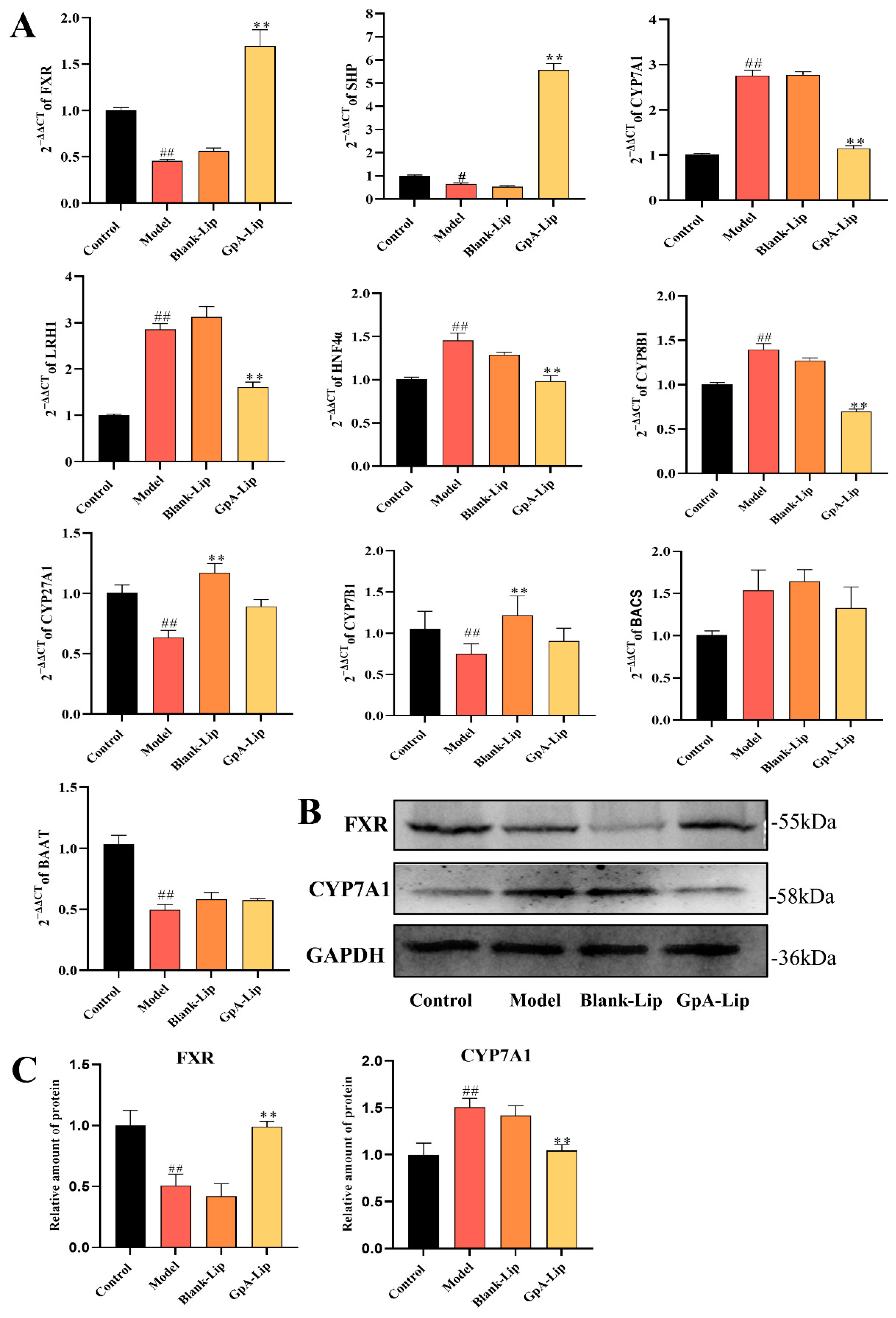
2.5. Effects of Gypensapogenin A Liposomes on Bile Acids Metabolizing Enzymes
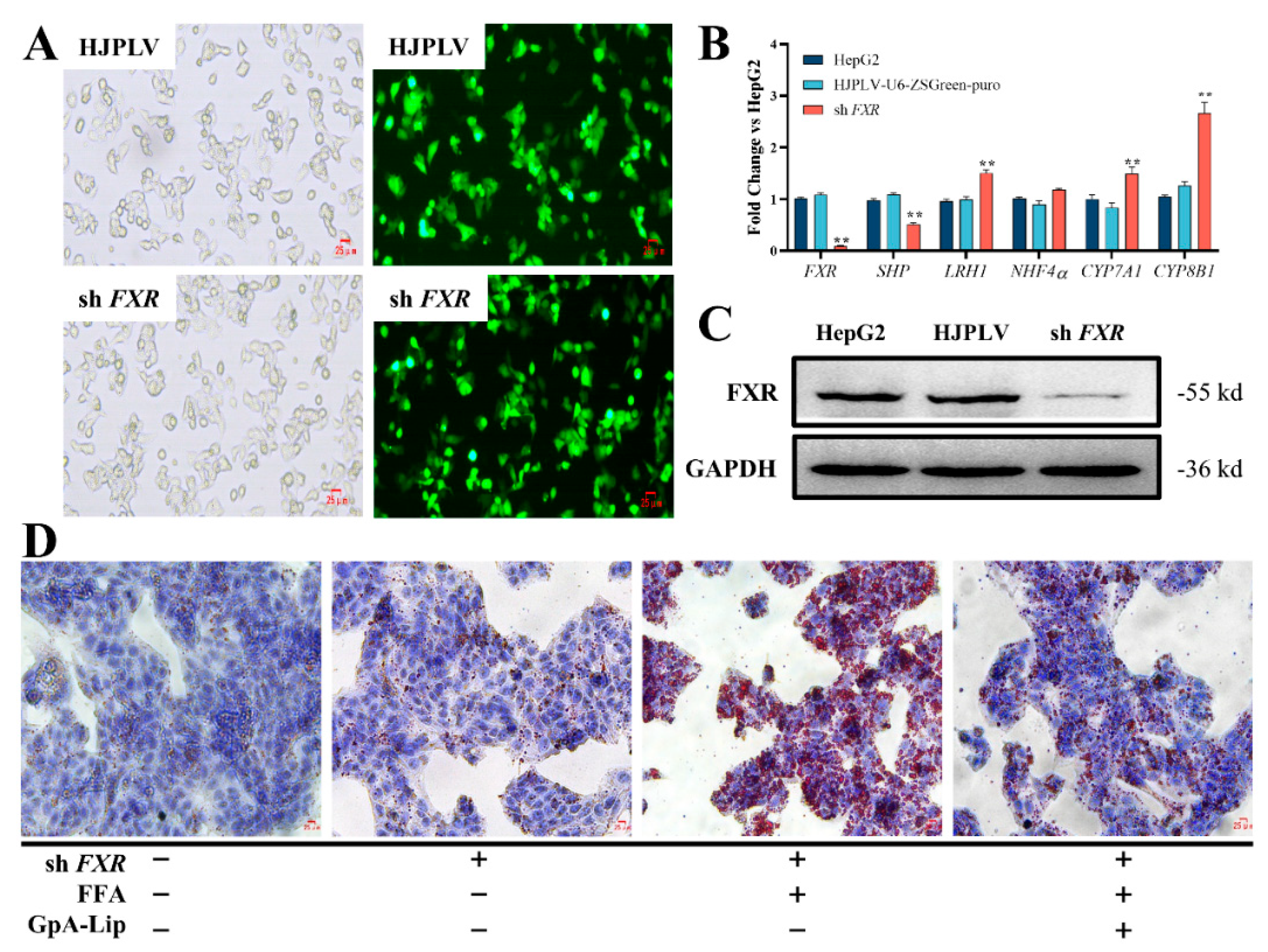
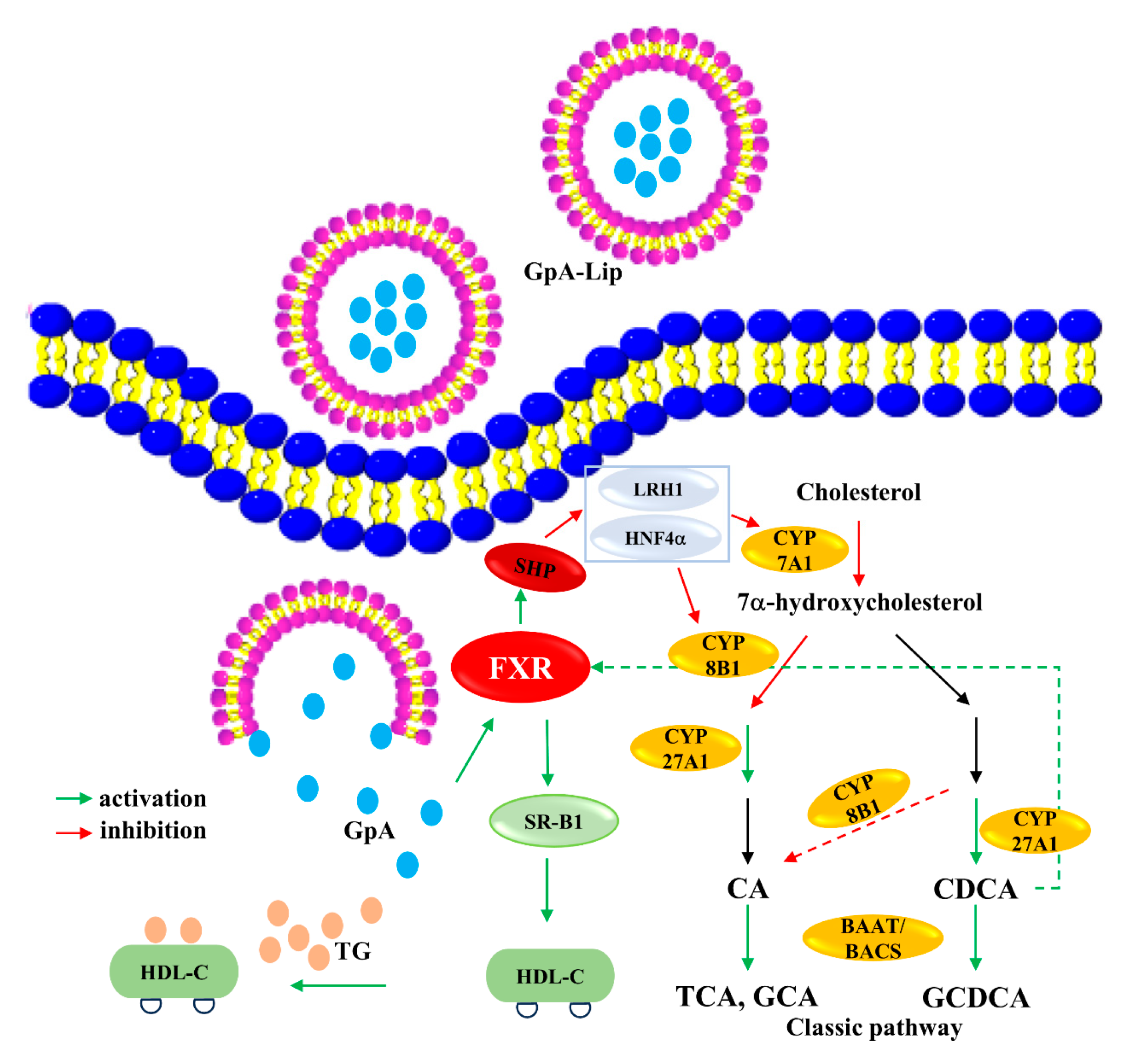
2.6. Mechanism Verification
3. Materials and Methods
3.1. Materials
3.2. Isolation and Characterization of Triterpene Aglycones
3.3. Molecular Docking
3.4. ADMET Analysis
3.5. Preparation of Gypensapogenin A Liposomes
3.6. FXR Knockdown Cells Construction
3.6.1. Lentiviral Plasmid Transfection of HEK 293T Cells
3.6.2. Infection of Target Cells with Virus Solution
3.7. Cell Culture
3.8. Oil Red O Staining
3.9. Bile Acids Analysis
3.10. Biochemical Analysis
3.11. RT-qPCR Analysis
3.12. Western Blotting Analysis
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAAT | Bile Acid-CoA: Amino Acid N-Acyltransferase |
| BACS | Bile Acid CoA Synthase |
| BCA | Bicinchoninic Acid |
| CA | Cholic Acid |
| CCK-8 | Cell Counting Kit-8 |
| CDCA | Chenodeoxycholic Acid |
| CYP7A1 | Cytochrome P450 7A1 |
| CYP7B1 | Cytochrome P450 7B1 |
| CYP8B1 | Cytochrome P450 8B1 |
| CYP27A1 | Cytochrome P450 27A1 |
| FFA | Free Fatty Acids |
| FXR | Farnesoid X Receptor |
| GCA | Glycocholic Acid |
| GCDCA | Glycochenodeoxycholic Acid |
| GpA | Gypensapogenin |
| GpA-Lip | Gypensapogenin A-liposomes |
| HDL-C | High-Density Lipoprotein Cholesterol |
| HNF4α | Hepatocyte Nuclear Factor 4 Alpha |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| LRH-1 | Liver Receptor Homolog-1 |
| NMR | Nuclear Magnetic Resonance |
| TC | Total Cholesterol |
| TCA | Taurocholate Acid |
| TG | Triglyceride |
References
- Raza, S.; Rajak, S.; Upadhyay, A.; Tewari, A.; Sinha, R.A. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front. Biosci. (Landmark Ed.) 2016, 26, 206–237. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Muthiah, M.D.; Sanyal, A.J. Current management of non-alcoholic steatohepatitis. Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62 (Suppl. S1), S47–S64. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metab. Clin. Exp. 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Wang, S.; Sheng, F.; Zou, L.; Xiao, J.; Li, P. Hyperoside attenuates non-alcoholic fatty liver disease in rats via cholesterol metabolism and bile acid metabolism. J. Adv. Res. 2021, 34, 109–122. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 432–450. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G554–G573. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Ling, L.; Qin, L.; Lu, Y.; Wu, D.; He, Y. Rosiglitazone induces hepatocyte injury by increasing DCA accumulation through OATP1A4 inhibiting in mice. Arab. J. Chem. 2023, 16, 105142. [Google Scholar] [CrossRef]
- Aranha, M.M.; Cortez-Pinto, H.; Costa, A.; da Silva, I.B.; Camilo, M.E.; de Moura, M.C.; Rodrigues, C.M. Bile acid levels are increased in the liver of patients with steatohepatitis. Eur. J. Gastroenterol. Hepatol. 2008, 20, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J.; et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Muthiah, M.D.; Narayan, N.; Siddiqui, M.S.; Puri, P.; Luketic, V.A.; Contos, M.J.; Idowu, M.; Chuang, J.-C.; Billin, A.N.; et al. Metabolic reprogramming of the intestinal microbiome with functional bile acid changes underlie the development of NAFLD. Hepatology 2022, 76, 1811–1824. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Chiang, J.Y.; Ferrell, J.M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol. Cell Endocrinol. 2022, 548, 111618. [Google Scholar] [CrossRef]
- Lu, T.T.; Makishima, M.; Repa, J.J.; Schoonjans, K.; Kerr, T.A.; Auwerx, J.; Mangelsdorf, D.J. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 2000, 6, 507–515. [Google Scholar] [CrossRef]
- Goodwin, B.; Jones, S.A.; Price, R.R.; Watson, M.A.; McKee, D.D.; Moore, L.B.; Galardi, C.; Wilson, J.G.; Lewis, M.C.; Roth, M.E.; et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 2000, 6, 517–526. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Dell, H.; Dowhan, D.H.; Hadzopoulou-Cladaras, M.; Moore, D.D. The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: Two mechanisms for repression. Mol. Cell Biol. 2000, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Pellicciari, R.; Pruzanski, M.; Auwerx, J.; Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008, 7, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Hart, S.N.; Kong, B.; Fang, J.; Zhong, X.B.; Guo, G.L. Genome-wide tissue-specific farnesoid X receptor binding in mouse liver and intestine. Hepatology 2010, 51, 1410–1419. [Google Scholar] [CrossRef]
- Zhang, M.; Chiang, J.Y. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): Roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J. Biol. Chem. 2001, 276, 41690–41699. [Google Scholar] [CrossRef]
- Miao, J.; Choi, S.-E.; Seok, S.M.; Yang, L.; Zuercher, W.J.; Xu, Y.; Willson, T.M.; Xu, H.E.; Kemper, J.K. Ligand-dependent regulation of the activity of the orphan nuclear receptor, small heterodimer partner (SHP), in the repression of bile acid biosynthetic CYP7A1 and CYP8B1 genes. Mol. Endocrinol. 2011, 25, 1159–1169. [Google Scholar] [CrossRef]
- Kong, B.; Wang, L.; Chiang, J.Y.; Zhang, Y.; Klaassen, C.D.; Guo, G.L. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology 2012, 56, 1034–1043. [Google Scholar] [CrossRef]
- Clifford, B.L.; Sedgeman, L.R.; Williams, K.J.; Morand, P.; Cheng, A.; Jarrett, K.E.; Chan, A.P.; Brearley-Sholto, M.C.; Wahlström, A.; Ashby, J.W.; et al. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021, 33, 1671–1684. [Google Scholar] [CrossRef]
- Yin, F.; Hu, L.; Lou, F.; Pan, R. Dammarane-type glycosides from Gynostemma pentaphyllum. J. Nat. Prod. 2004, 67, 942–952. [Google Scholar] [CrossRef]
- Yang, F.; Shi, H.; Zhang, X.; Yu, L. Two novel anti-inflammatory 21-nordammarane saponins from tetraploid Jiaogulan (Gynostemma pentaphyllum). J. Agric. Food Chem. 2013, 61, 12646–12652. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, L.; Du, Y.; Qin, L.; Lu, Y.; Zhang, Q.; He, Y.; Tan, D. Gypenosides ameliorate high-fat diet-induced nonalcoholic fatty liver disease in mice by regulating lipid metabolism. PeerJ 2023, 11, 15225. [Google Scholar] [CrossRef] [PubMed]
- Razmovski-Naumovski, V.; Huang, T.H.-W.; Tran, V.H.; Li, G.Q.; Duke, C.C.; Roufogalis, B.D. Chemistry and Pharmacology of Gynostemma pentaphyllum. Phytochem. Rev. 2005, 4, 197–219. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, G.; Sun, Y.; Wu, X.; Zhao, Y. Triterpenes derived from hydrolyzate of total Gynostemma pentaphyllum saponins with anti-hepatic fibrosis and protective activity against H2O2-induced injury. Phytochemistry 2017, 144, 226–232. [Google Scholar] [CrossRef]
- Lee, C.; Lee, J.W.; Jin, Q.; Jang, H.; Jang, H.J.; Rho, M.C.; Lee, M.K.; Lee, C.K.; Lee, M.K.; Hwang, B.Y. Isolation and Characterization of Dammarane-Type Saponins from Gynostemma pentaphyllum and Their Inhibitory Effects on IL-6-Induced STAT3 Activation. J. Nat. Prod. 2015, 78, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, J.-L.; Zhou, P.-P.; Meng, X.-H.; Shi, Y.-P. Further New Gypenosides from Jiaogulan (Gynostemma pentaphyllum). J. Agric. Food Chem. 2017, 65, 5926–5934. [Google Scholar] [CrossRef]
- Tan, D.; Wang, J.; Wang, X.; Qin, L.; Du, Y.; Zhao, C.; Liu, P.; Zhang, Q.; Ma, F.; Xie, J.; et al. New dammarane-type triterpenoids from hydrolyzate of total Gynostemma pentaphyllum saponins with protein tyrosine phosphatase 1B inhibitory activity. J. Enzym. Inhib. Med. Chem. 2023, 38, 2281263. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xi, Y.; Liu, H.; Xin, X. Gypenosides ameliorate high-fat diet-induced non-alcoholic steatohepatitis via farnesoid X receptor activation. Front. Nutr. 2022, 9, 914079. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Du, Y.; Qin, L.; Wu, D.; Wang, W.; Ling, L.; Ma, F.; Ling, H.; Yang, L.; Wang, C.; et al. Gypenosides Altered Hepatic Bile Acids Homeostasis in Mice Treated with High Fat Diet. Evid.-Based Complement. Altern. Med. 2018, 2018, 8098059. [Google Scholar] [CrossRef]
- Li, N.; Wu, C.-F.; Xu, X.-Y.; Liu, Z.-Y.; Li, X.; Zhao, Y.-Q. Triterpenes possessing an unprecedented skeleton isolated from hydrolyzate of total saponins from Gynostemma pentaphyllum. Eur. J. Med. Chem. 2012, 50, 173–178. [Google Scholar] [CrossRef]
- Azman, M.; Sabri, A.H.; Anjani, Q.K.; Mustaffa, M.F.; Hamid, K.A. Intestinal Absorption Study: Challenges and Absorption Enhancement Strategies in Improving Oral Drug Delivery. Pharmaceuticals 2022, 15, 975. [Google Scholar] [CrossRef]
- Ye, J.; Wu, D.; Wu, X.; Qin, L.; Yang, M.; Lu, Y.-L.; Tan, D.-P.; He, Y.-Q. Dendrobium officinale aqueous extract regulates bile acid synthesis to improve acute alcoholic liver injury in mice. Food Biosci. 2023, 55, 103087. [Google Scholar] [CrossRef]
- Kir, S.; Zhang, Y.; Gerard, R.D.; Kliewer, S.A.; Mangelsdorf, D.J. Nuclear receptors HNF4α and LRH-1 cooperate in regulating Cyp7a1 in vivo. J. Biol. Chem. 2012, 287, 41334–41341. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Zheng, C.; Yi, K.; Mintz, R.L.; Lv, S.; Tao, Y.; Li, M. Structural and componential design: New strategies regulating the behavior of lipid-based nanoparticles In Vivo. Biomaterias Sci. 2023, 11, 4774–4788. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, K.; Hu, B.; Li, C.; Zhang, M.; Hussain, A.; Wang, X.; Cheng, Q.; Yang, F.; Ge, K.; et al. Membrane-Destabilizing ionizable lipid empowered imaging-guided siRNA delivery and cancer treatment. Exploration 2021, 1, 35–49. [Google Scholar] [CrossRef]
- Lee, M.R.; Yang, H.J.; Park, K.I.; Ma, J.Y. Lycopus lucidus Turcz. ex Benth. Attenuates free fatty acid-induced steatosis in HepG2 cells and non-alcoholic fatty liver disease in high-fat diet-induced obese mice. Phytomedicine Int. J. Phytother. Phytopharm. 2019, 55, 14–22. [Google Scholar] [CrossRef]
- Lv, W.-J.; Huang, J.-Y.; Lin, J.; Ma, Y.-M.; He, S.-Q.; Zhang, Y.-W.; Wang, T.-Z.; Cheng, K.; Xiong, Y.; Sun, F.-G.; et al. Phytosterols Alleviate Hyperlipidemia by Regulating Gut Microbiota and Cholesterol Metabolism in Mice. Oxidative Med. Cell Longev. 2023, 2023, 6409385. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| FXR | GCGACAAGTGACCTCGACA | TGGTTGCCATTTCCGTCAAAA |
| CYP7A1 | CATGCTGTTGTCTATGGCTTATTC | ACAGCCCAGGTATGGAATTAATC |
| CYP8B1 | GAGGACAGCCTCTTTCGCTT | TGTAGCCGAACAAGCTCAGG |
| LRH1 | AGCAGGCTAACCGAAGCAAG | TGGAATAGTCCACTTGTTGCC |
| HNF4a | AAGAGGAACCAGTGCCGCTACT | GCTTGACCTTCGAGTGCTGATCC |
| CYP27A1 | GCAACGGAGCTTAGAGGAGA | CAGGTTCACGTGCATCTGAG |
| CYP7B1 | AGTGCGTGACGAAATTGACC | CAAGTCTCCCTTTCGCACAC |
| BACS | CTGAGAACATCCGCTGCTTC | ATAGATGAAGAGGGCAGGGC |
| BAAT | CCCCGCAAACCAGAAGTAAC | GAAGGGGCTGATGGATCTGA |
| GAPDH | CAGCCTCAAGATCATCAGCA | ATGATGTTCTGGAGAGCCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Wang, J.; Wu, D.; Qin, L.; He, Y.; Tan, D. Gypensapogenin A-Liposomes Efficiently Ameliorates Hepatocellular Lipid Accumulation via Activation of FXR Receptor. Molecules 2024, 29, 4080. https://doi.org/10.3390/molecules29174080
Deng Y, Wang J, Wu D, Qin L, He Y, Tan D. Gypensapogenin A-Liposomes Efficiently Ameliorates Hepatocellular Lipid Accumulation via Activation of FXR Receptor. Molecules. 2024; 29(17):4080. https://doi.org/10.3390/molecules29174080
Chicago/Turabian StyleDeng, Yidan, Jianmei Wang, Di Wu, Lin Qin, Yuqi He, and Daopeng Tan. 2024. "Gypensapogenin A-Liposomes Efficiently Ameliorates Hepatocellular Lipid Accumulation via Activation of FXR Receptor" Molecules 29, no. 17: 4080. https://doi.org/10.3390/molecules29174080
APA StyleDeng, Y., Wang, J., Wu, D., Qin, L., He, Y., & Tan, D. (2024). Gypensapogenin A-Liposomes Efficiently Ameliorates Hepatocellular Lipid Accumulation via Activation of FXR Receptor. Molecules, 29(17), 4080. https://doi.org/10.3390/molecules29174080





