Narrow Range of Coagulation of Ion Associates of Poly(styrene sulfonate) with Alcian Blue Dye
Abstract
1. Introduction
2. Results and Discussion
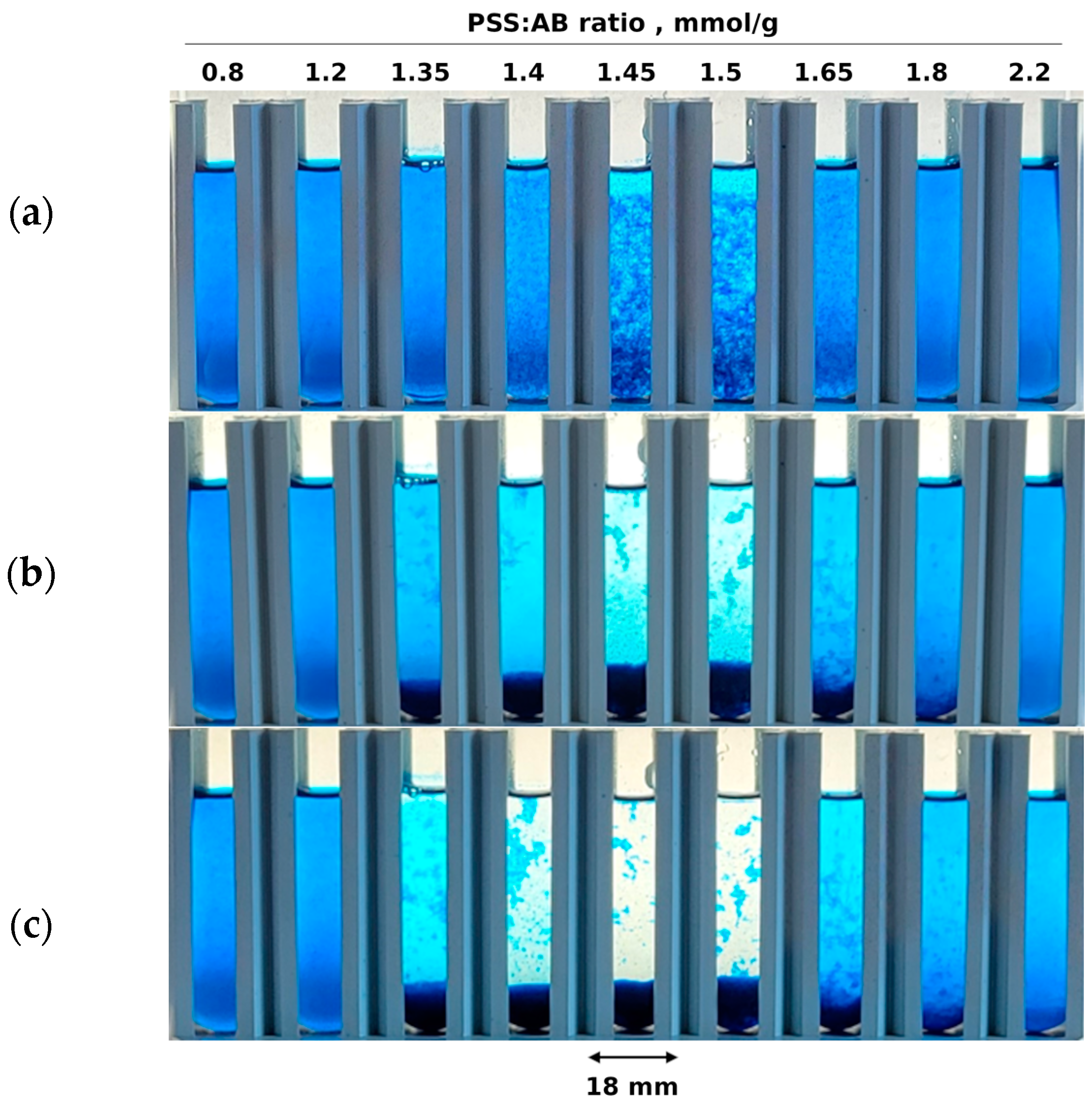
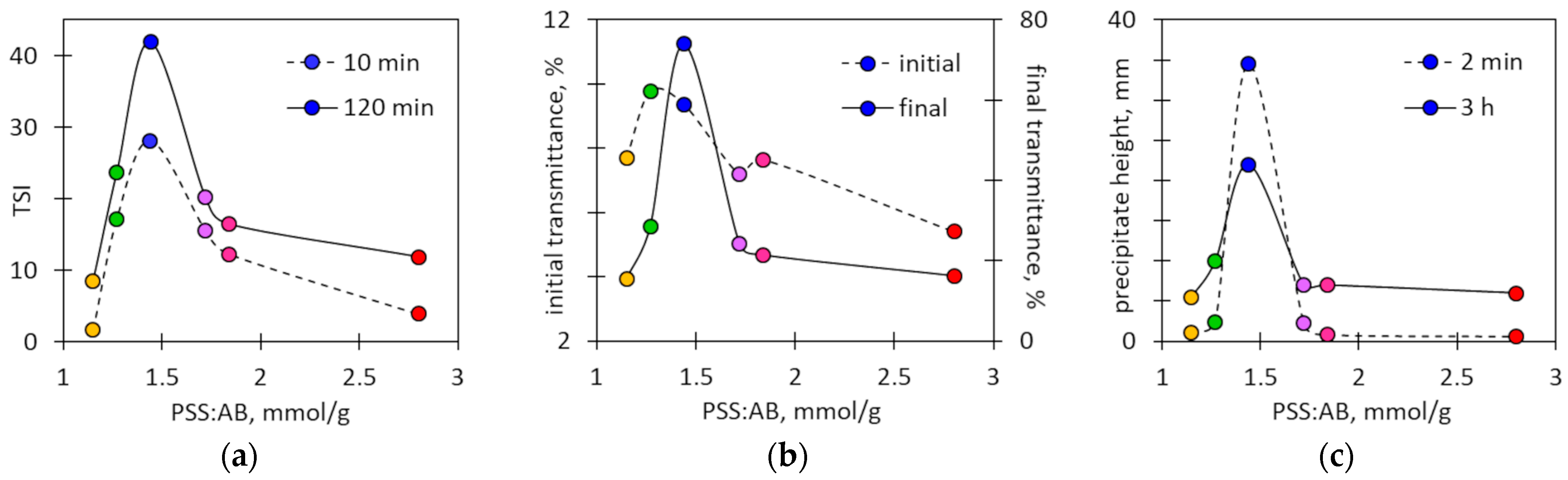
2.1. Coagulation of Ion Associates of Poly(styrene sulfonate) with Alcian Blue Dye
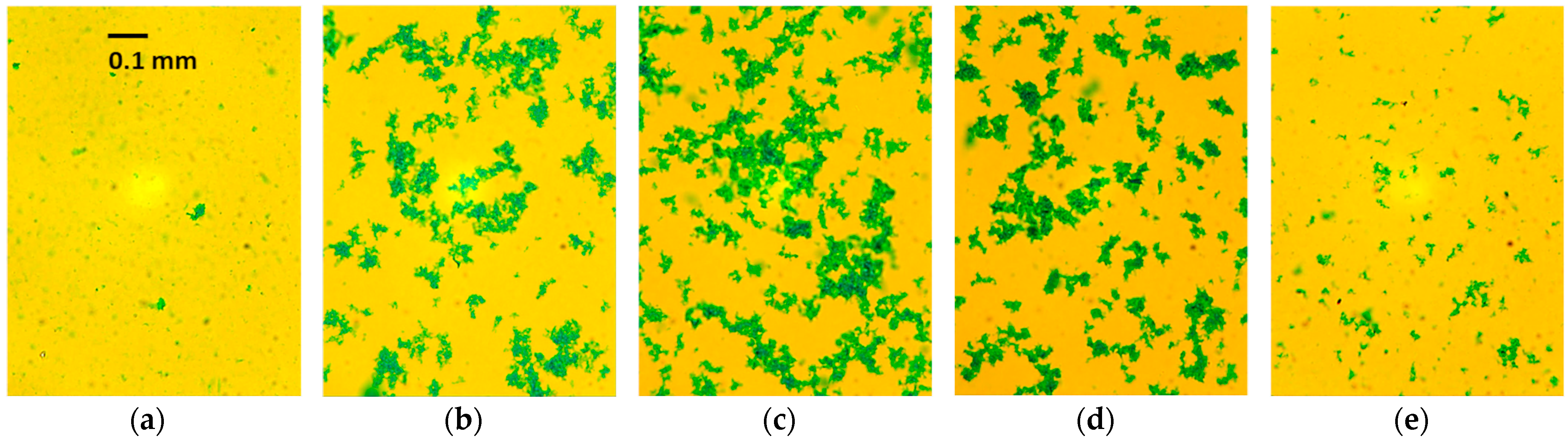
2.2. Particle Size and Charge
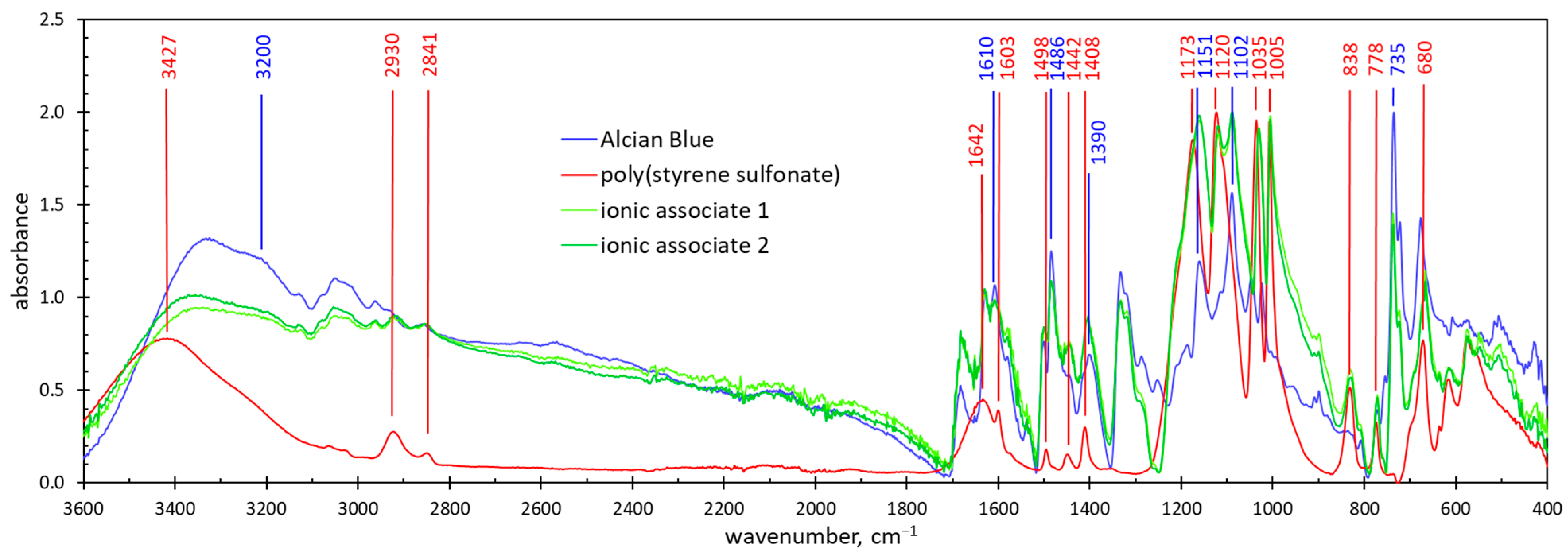
2.3. Composition of the Ionic Associates
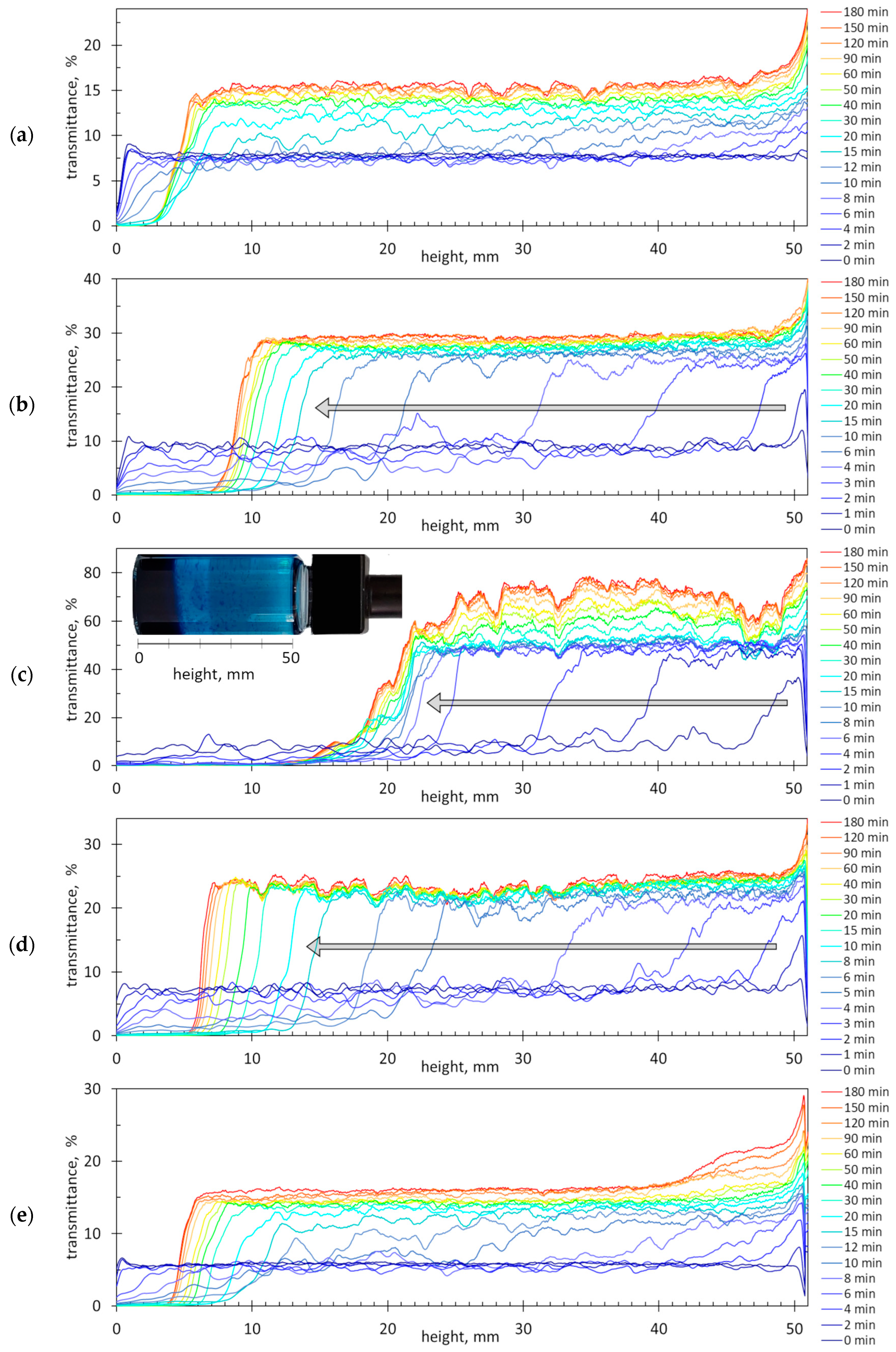
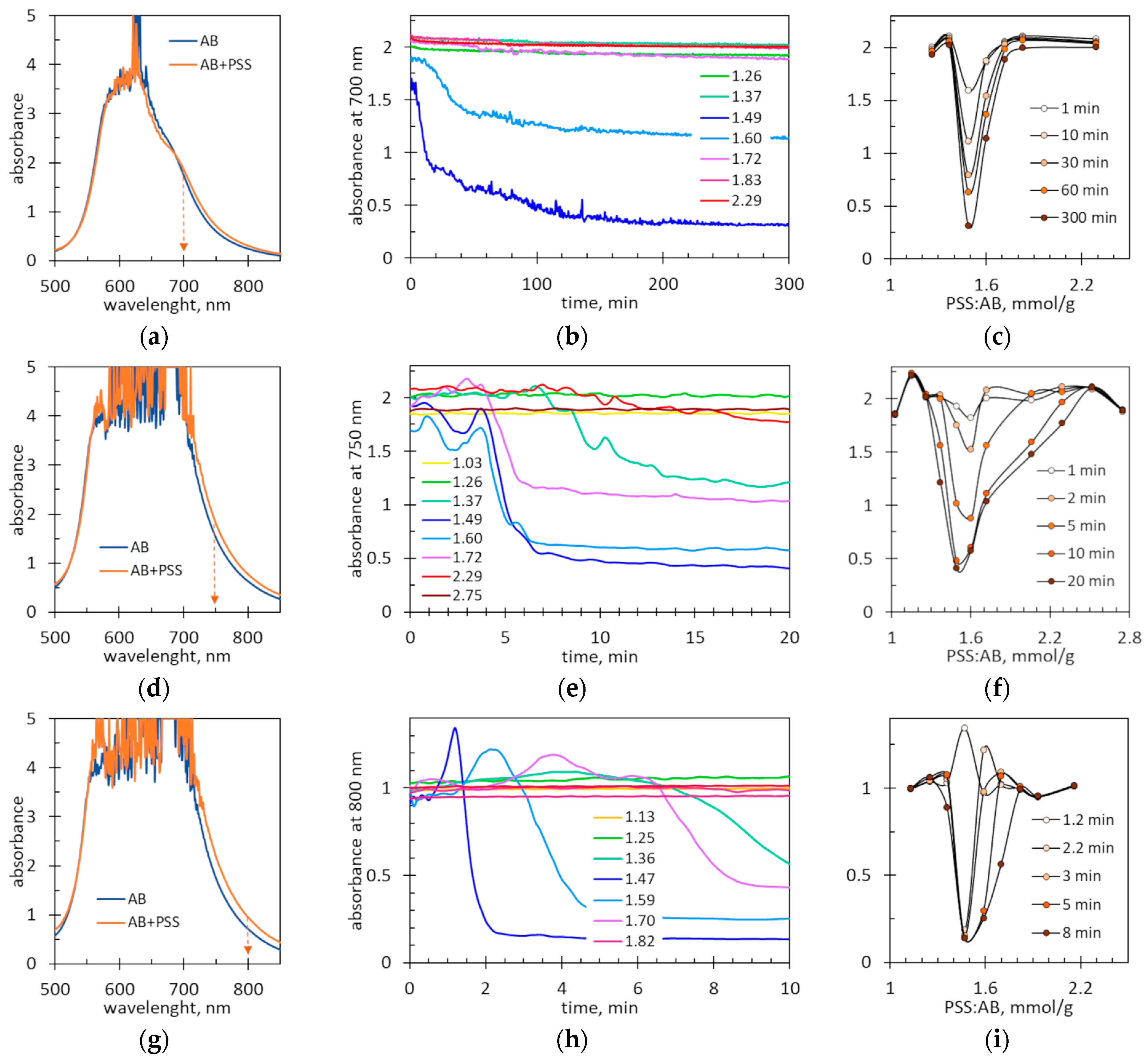
2.4. Sedimentation of Dye–Polymer Associates
2.5. Determination of Charge Equivalence Point by Photometric Measurements
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De, R.; Lee, H.; Das, B. Exploring the interactions in binary mixtures of polyelectrolytes: Influence of mixture composition, concentration, and temperature on counterion condensation. J. Mol. Liq. 2018, 251, 94–99. [Google Scholar] [CrossRef]
- Puente-Santamaría, A.; Ortega, F.; Maestro, A.; Rubio, R.G.; Guzmán, E. Non-equilibrium states in polyelectrolyte-surfactant systems at fluid interfaces: A critical review. Curr. Opin. Colloid Interface Sci. 2024, 71, 101804. [Google Scholar] [CrossRef]
- Shyichuk, A.; Ziółkowska, D.; Szulc, J. Coagulation of Hydrophobic Ionic Associates of Cetyltrimethylammonium Bromide and Carrageenan. Molecules 2023, 28, 7584. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T. Colloidal titration: From the perspective of stability constants between oppositely charged polyelectrolytes. Bull. Chem. Soc. Jpn. 2024, 97, uoae044. [Google Scholar] [CrossRef]
- Prajapati, B.G.; Sharma, J.B.; Sharma, S.; Trivedi, N.D.; Gaur, M.; Kapoor, D.U. Harnessing polyelectrolyte complexes for precision cancer targeting: A comprehensive review. Med. Oncol. 2024, 41, 145. [Google Scholar] [CrossRef]
- Mishra, B.; Pathak, D.; Verma, D.; Gupta, M.K. Nanofibrous composite from chitosan-casein polyelectrolyte complex for rapid hemostasis in rat models in vivo. Int. J. Biol. Macromol. 2024, 269, 131882. [Google Scholar] [CrossRef]
- Yadav, D.; Dutta, J. A systematic review on recent development of chitosan/alginate-based polyelectrolyte complexes for wastewater treatment. Int. J. Environ. Sci. Technol. 2024, 21, 3381–3406. [Google Scholar] [CrossRef]
- Nguyen, A.; Jurago, A.A.; Viers, R.A.; Patten, C.; Chen, Q.; Caldona, E.B.; Advincula, R.C. 3D-printing formulated polyelectrolyte complexes (PECs) in air: Silica compositions in rheological optimization for layering. MRS Commun. 2023, 13, 1326–1334. [Google Scholar] [CrossRef]
- Jurago, A.A.; Viers, R.A.; Nguyen, A.T.; Ribeiro, E.L.; Espera, A.H.; Caldona, E.B.; Advincula, R.C. On the 3D printing of polyelectrolyte complexes: A novel approach to overcome rheology constraints. MRS Commun. 2023, 13, 862–870. [Google Scholar] [CrossRef]
- Jin, Z.; Seong, H.; Srivastava, S.; McGlasson, A.; Emrick, T.; Muthukumar, M.; Russell, T.P. 3D Printing of Aqueous Two-Phase Systems with Linear and Bottlebrush Polyelectrolytes. Angew. Chem. 2024, 136, e202404382. [Google Scholar] [CrossRef]
- Che, J.; Zakri, C.; Ly, I.; Neri, W.; Laurichesse, E.; Chapel, J.; Poulin, P.; Yuan, J. High-Energy-Density Waterborne Dielectrics from Polyelectrolyte-Colloid Complexes. Adv. Funct. Mater. 2023, 33, 2213804. [Google Scholar] [CrossRef]
- Che, J.; Zakri, C.; Bronchy, M.; Neri, W.; Ly, I.; Poulin, P.; Yuan, J. Inkjet Printing of All Aqueous Inks to Flexible Microcapacitors for High-Energy Storage. Adv. Funct. Mater. 2023, 33, 2301544. [Google Scholar] [CrossRef]
- Iverson, E.T.; Legendre, H.; Chavan, S.V.; Aryal, A.; Singh, M.; Chakravarty, S.; Schmieg, K.; Chiang, H.-C.; Shamberger, P.J.; Karim, A.; et al. Nanobrick Wall Multilayer Thin Films with High Dielectric Breakdown Strength. ACS Appl. Eng. Mat. 2023, 1, 2429–2439. [Google Scholar] [CrossRef]
- Li, H.; Fauquignon, M.; Haddou, M.; Schatz, C.; Chapel, J.-P. Interfacial Behavior of Solid- and Liquid-like Polyelectrolyte Complexes as a Function of Charge Stoichiometry. Polymers 2021, 13, 3848. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, D.; Lamkiewicz, J.; Shyichuk, A. Structure and Flocculation of Ion Associates of Carrageenan and Poly(diallyldimethylammonium chloride) Depending on the Component Ratio. Molecules 2022, 27, 8075. [Google Scholar] [CrossRef]
- Fehér, B.; Wacha, A.; Jezsó, B.; Bóta, A.; Pedersen, J.S.; Varga, I. The evolution of equilibrium poly(styrene sulfonate) and dodecyl trimethylammonium bromide supramolecular structure in dilute aqueous solution with increasing surfactant binding. J. Colloid Interface Sci. 2023, 651, 992–1007. [Google Scholar] [CrossRef]
- Jemili, N.; Fauquignon, M.; Grau, E.; Fatin-Rouge, N.; Dole, F.; Chapel, J.-P.; Essafi, W.; Schatz, C. Complexation in Aqueous Solution of a Hydrophobic Polyanion (PSSNa) Bearing Different Charge Densities with a Hydrophilic Polycation (PDADMAC). Polymers 2022, 14, 2404. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, H.; Han, X.; Han, L.; Xu, Z.; Wang, P. Rapid, Highly-Efficient and Selective Removal of Anionic and Cationic Dyes from Wastewater Using Hollow Polyelectrolyte Microcapsules. Molecules 2023, 28, 3010. [Google Scholar] [CrossRef]
- Scott, J.E. Histochemistry of Alcian blue. Histochemistry 1972, 32, 191–212. [Google Scholar] [CrossRef]
- Bhattarai, A. Micellization behavior of cetyltrimethylammonium bromide in the absence and presence of sodium polystyrene sulfonate in water and methanol-water mixture: A conductivity approach. J. Mol. Liq. 2019, 292, 111352. [Google Scholar] [CrossRef]
- Lopez, C.G.; Matsumoto, A.; Shen, A.Q. Dilute polyelectrolyte solutions: Recent progress and open questions. Soft Matter 2024, 20, 2635–2687. [Google Scholar] [CrossRef] [PubMed]
- Grzeczkowicz, A.; Lipko, A.; Kwiatkowska, A.; Strawski, M.; Bącal, P.; Więckowska, A.; Granicka, L.H. Polyelectrolyte Membrane Nanocoatings Aimed at Personal Protective and Medical Equipment Surfaces to Reduce Coronavirus Spreading. Membranes 2022, 12, 946. [Google Scholar] [CrossRef]
- Semedo, M.C.; Karmali, A.; Fonseca, L. A novel colorimetric assay of β-D-glucans in basidiomycete strains by alcian blue dye in a 96-well microtiter plate. Biotechnol. Prog. 2015, 31, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Cao, L. Sulfonated Hydrogel Formed via CO2-in-Water Emulsion: Potential in Antibiotic Removal. Gels 2023, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Farouq, R. Functionalized graphene/polystyrene composite, green synthesis and characterization. Sci. Rep. 2022, 12, 21757. [Google Scholar] [CrossRef] [PubMed]
- Balding, P.; Borrelli, R.; Volkovinsky, R.; Russo, P.S. Physical Properties of Sodium Poly(styrene sulfonate): Comparison to Incompletely Sulfonated Polystyrene. Macromolecules 2022, 55, 1747–1762. [Google Scholar] [CrossRef]
- Suhail, M.; Chiu, I.-H.; Hung, M.-C.; Vu, Q.L.; Lin, I.-L.; Wu, P.-C. In Vitro Evaluation of Smart and pH-Sensitive Chondroitin Sulfate/Sodium Polystyrene Sulfonate Hydrogels for Controlled Drug Delivery. Gels 2022, 8, 406. [Google Scholar] [CrossRef]
- Szafran, M.; Dega-Szafran, Z.; Katrusiak, A.; Komasa, A. Molecular structure and spectral properties of 4-(1-pyridinium)-butyrate dihydrate and its hydrobromide. Vib. Spectrosc. 2017, 88, 40–48. [Google Scholar] [CrossRef]
- Fagan, C.; Dapson, R.W.; Horobin, R.W.; Kiernan, J.A. Revised tests and standards for Biological Stain Commission certification of alcian blue dyes. Biotech. Histochem. 2020, 95, 333–340. [Google Scholar] [CrossRef]
- Ali, S.; Dapson, R.; Horobin, R.; Kiernan, J.; Kazlauciunas, A. At least four distinct blue cationic phthalocyanine dyes sold as “alcian blue” raises the question: What is alcian blue? Biotech. Histochem. 2022, 97, 11–20. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziółkowska, D.; Shyichuk, A.; Shyychuk, I. Narrow Range of Coagulation of Ion Associates of Poly(styrene sulfonate) with Alcian Blue Dye. Molecules 2024, 29, 4017. https://doi.org/10.3390/molecules29174017
Ziółkowska D, Shyichuk A, Shyychuk I. Narrow Range of Coagulation of Ion Associates of Poly(styrene sulfonate) with Alcian Blue Dye. Molecules. 2024; 29(17):4017. https://doi.org/10.3390/molecules29174017
Chicago/Turabian StyleZiółkowska, Dorota, Alexander Shyichuk, and Iryna Shyychuk. 2024. "Narrow Range of Coagulation of Ion Associates of Poly(styrene sulfonate) with Alcian Blue Dye" Molecules 29, no. 17: 4017. https://doi.org/10.3390/molecules29174017
APA StyleZiółkowska, D., Shyichuk, A., & Shyychuk, I. (2024). Narrow Range of Coagulation of Ion Associates of Poly(styrene sulfonate) with Alcian Blue Dye. Molecules, 29(17), 4017. https://doi.org/10.3390/molecules29174017






