Abstract
Mutations of isocitrate dehydrogenase 1 (IDH1) are key biomarkers for glioma classification, but current methods for detection of mutated IDH1 (mIDH1) require invasive tissue sampling and cannot be used for longitudinal studies. Positron emission tomography (PET) imaging with mIDH1-selective radioligands is a promising alternative approach that could enable non-invasive assessment of the IDH status. In the present work, we developed efficient protocols for the preparation of four 18F-labeled derivatives of the mIDH1-selective inhibitor olutasidenib. All four probes were characterized by cellular uptake studies with U87 glioma cells harboring a heterozygous IDH1 mutation (U87-mIDH) and the corresponding wildtype cells (U87-WT). In addition, the most promising probe was evaluated by PET imaging in healthy mice and mice bearing subcutaneous U87-mIDH and U87-WT tumors. Although all four probes inhibited mIDH1 with variable potencies, only one of them ([18F]mIDH-138) showed significantly higher in vitro uptake into U87-mIDH compared to U87-WT cells. In addition, PET imaging with [18F]mIDH-138 in mice demonstrated good in vivo stability and low non-specific uptake of the probe, but also revealed significantly higher uptake into U87-WT compared to U87-mIDH tumors. Finally, application of a two-tissue compartment model (2TCM) to the PET data indicated that preferential tracer uptake into U87-WT tumors results from higher specific binding rather than from differences in tracer perfusion. In conclusion, these results corroborate recent findings that mIDH1-selective inhibition may not directly correlate with mIDH1-selective target engagement and indicate that in vivo engagement of wildtype and mutated IDH1 may be governed by factors that are not faithfully reproduced by in vitro assays, both of which could complicate development of PET probes.
1. Introduction
Members of the isocitrate dehydrogenase (IDH) family are key metabolic enzymes that catalyze the oxidative decarboxylation of isocitrate to α-ketoglutarate (α-KG) in the cytosol and peroxisomes (IDH1) or mitochondria (IDH2 and IDH3) of all eukaryotic cells [1]. Heterozygous missense mutations in the genes encoding IDH1 or, less commonly, IDH2 are frequent in diffuse gliomas and may drive malignant transformation during early stages of the disease. In the vast majority of IDH mutated gliomas (>90%), these mutations are located at codon 132 of IDH1 and result in an arginine-to-histidine (R132H) substitution at the active site, although other mutations affecting the corresponding arginine residue in IDH1 or IDH2 have been described as well [1,2,3]. All of these mutations equip the enzyme with a neomorphic activity that results in increased conversion of α-KG into the potential oncometabolite 2-hydroxyglutarate (2-HG) [4,5]. High concentrations of 2-HG competitively suppress a number of α-KG-dependent enzymes involved in epigenetic regulation, which could in turn predispose affected cells to more aggressive mutations [4,6]. Although the therapeutic benefit of targeting mutated IDH (mIDH) in gliomas remains controversial [7,8], numerous mIDH-selective inhibitors have been developed to date and in many cases shown to effectively suppress 2-HG production in patients or preclinical tumor models [3,9,10]. Interestingly, IDH-mutated gliomas have also consistently been demonstrated to be associated with a better prognosis than comparable IDH wildtype gliomas [5,11,12,13], which resulted in inclusion of the IDH status into the WHO classification of adult-type diffuse gliomas [14,15,16,17]. In particular, IDH mutations are now considered as a disease-defining feature of oligodendrogliomas and astrocytomas, while IDH wildtype gliomas (designated as glioblastomas) are recognized as a separate, more aggressive disease with distinct molecular genetics [15,16,17]. Accordingly, assessment of the IDH status by immunohistochemistry and/or genomic sequencing has become an integral part of the diagnostic algorithm for glioma classification. However, while these approaches allow for reliable detection of the most frequent IDH mutations, they require invasive tissue sampling and cannot be used for follow-up examinations or longitudinal studies. In contrast, positron emission tomography (PET) imaging enables the non-invasive visualization of molecular targets or biochemical processes with radiolabeled probes, making it a promising approach for routine assessment of the IDH status. For example, a number of established PET tracers like O-(2-[18F]fluoroethyl)tyrosine ([18F]FET) or 6-[18F]fluoro-3,4-dihydroxyphenylalanine ([18F]FDOPA) have been successfully used to infer the IDH mutation status in gliomas [18,19,20,21,22]. Nevertheless, these probes can only provide indirect evidence for the presence of IDH mutations, and their diagnostic accuracy for detection of mIDH is limited. As such, recent efforts have focused on the development of mIDH-selective PET tracers to facilitate glioma classification and possibly delineation by direct detection of mIDH expression (which is expected to be homogenous among tumor cells). Unfortunately, most candidate PET tracers evaluated in previous studies have proven to be unsuitable for glioma imaging (reviewed in [23]). Thus, radiolabeled analogs of the butyl-phenyl sulfonamide class of mIDH1 inhibitors (like [18F]I in Figure 1) showed limited mIDH selectivity in cellular uptake studies and very low brain uptake in rodents [24]. Likewise, phenylglycine-based radioligands derived from the FDA-approved mIDH1 inhibitor ivosidenib or its preclinical analogs either showed limited in vivo tumor uptake and retention ([18F]AGI-5198 & [18F]AG-135, Figure 1) or insufficient brain uptake to be useful for glioma imaging ([18F]AG-120, Figure 1) [25,26,27]. Conversely, while an 18F-labeled aminotriazine-based tracer candidate derived from the pan-mIDH1/2 inhibitor vorasidenib ([18F]II in Figure 1) showed promising in vitro and in vivo mIDH specificity, this radioligand suffered from extensive in vivo defluorination [28].
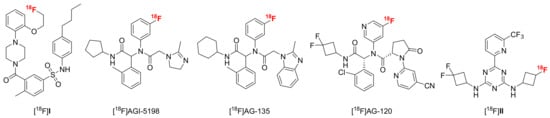
Figure 1.
Overview of 18F-labeled compounds previously evaluated as candidate PET tracers for non-invasive detection of mutated isocitrate dehydrogenase (mIDH).
Another class of potential lead structures for the development of mIDH-selective PET tracers are quinolinone-based inhibitors like the clinical candidate olutasidenib and its preclinical analogs, which have been shown to selectively inhibit several IDH1 mutations with low nanomolar IC50 values and exhibit favorable pharmacokinetic properties (e.g., high in vivo stability and effective brain penetration) [29,30]. In addition, replacement of the 6-chloro substituent in olutasidenib (1) by fluorine had no major effects on mIDH1-selective inhibition of the resulting analog (mIDH-23, 2) in functional assays (Figure 2), and its 18F-labeled isotopolog was reported to be stable during incubation in EtOH or mouse serum [31]. However, neither the cellular uptake nor the in vivo properties of this radioligand have been evaluated to date, so that the suitability of quinolinone-based tracers for mIDH-selective PET imaging remains to be established. As such, the aim of the present work was to prepare several 18F-labeled olutasidenib derivatives and to evaluate their mIDH selectivity by cellular uptake studies and (for the best candidate) in vivo PET imaging. Apart from the known 18F-labeled olutasidenib derivative (S)-[18F]mIDH-23 [(S)-[18F]2], we prepared and evaluated its (R)-methyl enantiomer (R)-[18F]mIDH-23 [(R)-[18F]2]. In addition, an early quinolinone lead structure (3) with good inhibitory potency and excellent selectivity for the most frequent mIDH1R132H mutation [29] was modified by replacement of the 6-chloro substituent with fluorine (Figure 2). The resulting analog (mIDH-138, 4), which has been shown to inhibit mIDH1R132H with similar potency as 3 [29], was either radiofluorinated without further structural modification to obtain [18F]mIDH-138 ([18F]4) or by replacement of the methoxy group on the cyanobenzene moiety with a [18F]fluoroethoxy group to obtain [18F]FE-mIDH-138 ([18]5) (Figure 2).
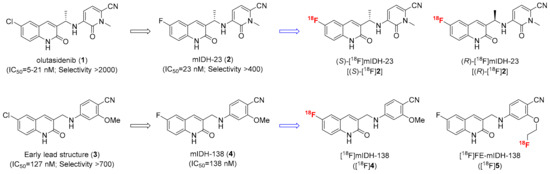
Figure 2.
mIDH1-selective quinolinone-based inhibitors selected as lead structures (left) and radiolabeled analogs prepared in the present study (right). IC50 values indicated below the lead structures correspond to the reported potency for inhibition of the most frequent mIDH1R132H mutation in biochemical assays, while the selectivity factors correspond to the ratio of the biochemical IC50 values for mIDH1R132H and the wildtype enzyme.
2. Results and Discussion
2.1. Chemistry and Radiochemistry
2.1.1. Synthesis of Reference Compound (4) and Radiolabeling Precursors (11, 18, 19) for the Preparation of [18F]mIDH-138 ([18F]4)
To prepare the reference compound and radiolabeling precursors for [18F]mIDH-138 ([18F]4), the 3-formyl-2-quinolone scaffold was first constructed according to the methodology reported by Meth-Cohn et al. [32,33]. To this end, the para-halogenated anilines 6a, and 6b were acetylated to obtain the acetanilides 7a and 7b (Scheme 1). Subsequent formylation of 7a, 7b or commercial acetamide 7c afforded mixtures of compounds 8a–c, 9a–c and 10a,c in a ratio that depended on the degree of activation of the respective starting material. The mixtures of 8a,b and 9a,b were then converted into the corresponding malondialdehydes 11a,b, which were treated with polyphosphoric acid to furnish the analytically pure 6-halogenated 3-formyl-2-quinolones 12a,b. The unsubstituted 3-formyl-2-quinolone 12c was prepared by boiling 10c with hydrochloric acid. Overall, 6-bromo-3-formyl-2-quinolone 12a and 6-fluoro-3-formyl-2-quinolone 12b were obtained in total yields of 30% and 39% over four steps, while unsubstituted 3-formyl-2-quinolone 12c was synthesized in a total yield of 40% over three steps.
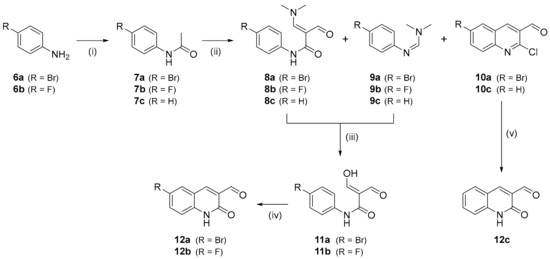
Scheme 1.
Preparation of 3-formylquinolone intermediates 12a–c. Conditions: (i) DIPEA, Ac2O, AcOEt, rt, 16 h; (ii) DMF, POCl3, 75 °C, 1.2 h; (iii) a: EtOH:20% NaOHaq (1.1:1.0), b: 0.1 m HCl, 3 h; (iv) PPA, 150 °C, 15 min; (v) 2 m HCl, reflux, 16 h.
Next, the aldehyde moiety in 12a,b was reductively aminated with 4-amino-2-methoxybenzonitrile 16 (prepared by reduction of commercial 4-nitro-2-methoxybenzonitrile 15, see Section S2.1 in Supplementary Materials) to derive bromo-substituted quinolone 13a and the fluoro-substituted reference compound 4 in 45% and 33% yield, respectively (Scheme 2) [34]. Subsequent Miyaura borylation of 13a [35] afforded the non-protected radiolabeling precursor 14 in 17% yield.
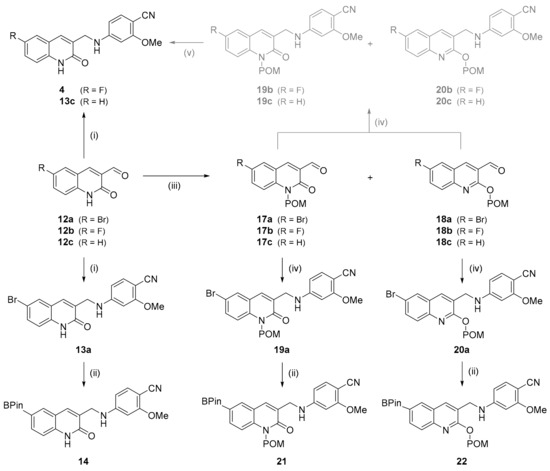
Scheme 2.
Preparation of reference compound (4) and radiolabeling precursors (14, 21, 22) for [18F]mIDH-138 ([18F]4). Conditions: (i) 16, DCE, AcOH, NaBH(OAc)3, rt, 16 h; (ii) (Bpin)2, KOAc, Pd(dppf)Cl2, dioxane, 80 °C, 22 h; (iii) POM-Cl, K2CO3, DMF, 60 °C, 2 h; (iv) 16, TMS-Cl or TMS-OTf, NaBH4, DMF, 0 °C, 75–120 min; (v) 2 N NaOH, MeOH.
Since the amide moiety of 14 interfered with Cu-mediated radiofluorination (see below), we also prepared the N- and O-protected radiolabeling precursors. To this end, a mixture of the N- and O-protected intermediates 17a and 18a was prepared by introducing a pivaloyloxymethyl (POM) protecting group into 12a (Scheme 2) [36]. After separation and isolation by column chromatography, 17a and 18a were conjugated with aniline 16, which furnished the protected quinolones 19a and 20a [37]. Finally, conversion of 19a or 20a into the corresponding boronic acid pinacol esters (as described above for preparation of 14) afforded the N- or O-POM protected radiolabeling precursors 21 or 22 in 87% or 80% yield, respectively.
Due to the higher solubility and reactivity of the POM-protected compared to the non-protected intermediates, reference compound 4 and its unsubstituted analog 13c (for identification of protodeboronated side products formed during radiofluorination) could also be prepared by conjugation of 17a or 17b and 18a or 18b with aniline 16 and subsequent de-protection of the resulting mixtures of 19b,c and 20b,c (Scheme 2).
2.1.2. Radiosynthesis of [18F]mIDH-138 ([18F]4)
The radiosynthesis of [18F]mIDH-138 ([18F]4) was initially attempted by copper-mediated radiofluorination of the non-protected boronic acid pinacol ester 14 with no carrier added [18F]fluoride ([18F]F−) (Scheme 3A). To this end, aqueous [18F]F− was trapped on a QMA cartridge and eluted with a solution of Et4NHCO3 in MeOH. After evaporation of the solvent and addition of precursor 14 and Cu(4-PhPy)4(ClO4)2 [38] (10 µmol of each) in DMA, the reaction mixture was stirred for 10 min at 110 °C. This approach afforded [18F]4, but only in low radiochemical conversions (RCCs) of 10 ± 8% (n = 3), as determined by radio-HPLC analysis. Since partial chelation of the copper mediator by the amide moiety in precursor 14 could potentially reduce the 18F-labeling efficacy, we carried out a series of spiking experiments with the bromo-substituted analog 13a to evaluate its effects on the radiofluorination of 4-acetylbenzeneboronic acid as a benchmark reaction (see Section S3.4.2 in Supplementary Materials) [39]. As suspected, addition of 13a completely prevented the formation of 4-[18F]fluoroacetophenone, indicating a need for appropriate protection groups to minimize the detrimental effects of the amide moiety in 14 on 18F-fluorination. Accordingly, the radiosynthesis of [18F]4 was instead performed with the N- or O-protected precursors 21 or 22 (Scheme 3B). After copper-mediated radiofluorination according to the same protocol as described above, the reaction mixture was cooled to 80 °C and 0.25 m NaOHaq in MeOH was added to hydrolyze the POM-protecting groups. Stirring was continued for three minutes and the mixture was acidified with 5% TFA in MeCN. Most of the solvents and the copper mediator were then removed by solid phase extraction (SPE), the product was purified by semi-preparative HPLC and the resulting eluate was loaded onto an HLB cartridge. Subsequent elution of the product with 1 mL EtOH followed by evaporation of the alcohol and formulation with PBS buffer and 1% Tween 80 afforded [18F]4 as a ready-to-inject solution with residual solvent and copper levels below the limit of detection.
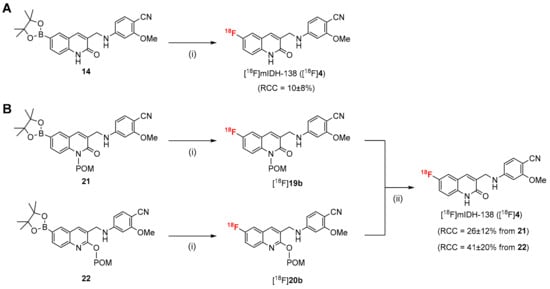
Scheme 3.
Radiosynthesis of [18F]mIDH-138 ([18F]4) by Cu-mediated radiofluorination of non-protected (A) or protected (B) precursors. Conditions: (i) elution of [18F]F− with Et4NHCO3 in MeOH followed by evaporation of MeOH, addition of precursor (14, 21 or 22) and Cu(4-PhePy)4(ClO4)2 (10 µmol of each) in DMA, 110 °C, 10 min; (ii) 0.25 m NaOHaq in MeOH, 80 °C, 3 min. Abbreviations: RCC, radiochemical conversion.
Using precursor 21, [18F]4 was obtained in RCCs of 26 ± 12% (n = 15), isolated radiochemical yields (RCYs) of 22 ± 11% (n = 11), a molar activity (Am) of 13–180 GBq/µmol (from 1–4 GBq starting activities) (n = 7) and radiochemical purities (RCPs) of >99%. Radiofluorination of precursor 22 afforded [18F]4 in RCCs of 41 ± 20% (n = 38), RCYs of 29 ± 11% (n = 30), an Am of 13–540 GBq/µmol (from 1–4 GBq starting activities) (n = 18) and RCPs of >99%.
2.1.3. Synthesis of Reference Compounds [(S)- and (R)-2] and Radiolabeling Precursors [(S)- and (R)-29, (S)- and (R)-30] for (S)- and (R)-[18F]mIDH-23 [(S)- and (R)-[18F]2]
Preparation of the reference compounds and radiolabeling precursors for (S)- and (R)-[18F]mIDH-23 [(S)- and (R)-[18F]2] started with the synthesis of formylquinolines 10a–c. Due to the poor yields of 10a,c obtained with the POCl3-based approach shown in Scheme 1, formylation of acetanilides 7a–c was instead carried out using triphosgene [40], which afforded 10a–c in about 40% yields (Scheme 4A). Subsequent condensation with the commercial chiral auxiliary (R)-(+)-2-methyl-2-propanesulfinamide (Ellman’s Sulfinamide) [41,42] followed by methylation of the resulting imines 23a–c with methyl magnesium bromide afforded (S,R)- and (R,R)-24a–c in moderate yields [43]. The chiral auxiliary was removed under acidic conditions and the resulting amine salts (S)- and (R)-25a–c were directly reacted with N-methylpyridone 31 (for preparation see Section S2.2 in Supplementary Materials) to afford bromo-substituted intermediates (S)- and (R)-26a, fluoro-substituted reference compounds (S)- and (R)-2 and their unsubstituted analogs (S)- and (R)-26c. Finally, (S)- or (R)-26a were POM-protected as described above and the resulting N- or O-protected isomers [(S)- or (R)-27 and (S)- or (R)-28] converted into the corresponding boronic acid pinacol esters to afford radiolabeling precursors (S)- or (R)-29 and (S)- or (R)-30, respectively (Scheme 4B).
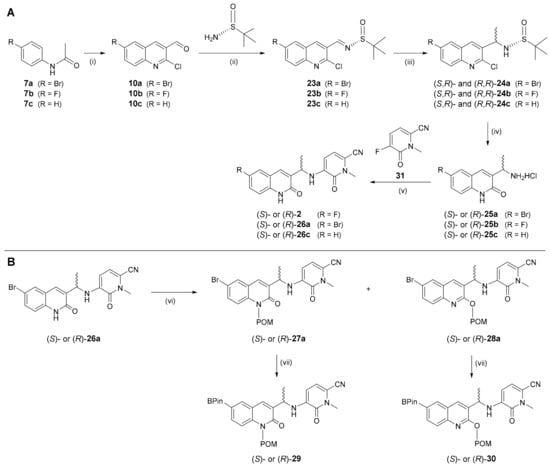
Scheme 4.
Preparation of reference compounds [(S)- and (R)-2] (A) and radiolabeling precursors [(S)- and (R)-29, (S)- and (R)-30] (B) for (S)- and (R)-[18F]mIDH-23 [(S)- and (R)-[18F]2]. Conditions: (i) triphosgene, DMF, 75 °C, 4 h; (ii) CuSO4, (R)-2-methylpropane-2-sulfinamide, DCE, 55 °C, 16 h; (iii) MeMgBr in Et2O, CH2Cl2, -60 °C to rt, 16 h; (iv) 1 N HCl:dioxane (1:1), reflux, 2 h; (v) 31, DIPEA, DMSO, 110 °C, 16 h; (vi) K2CO3, POM-Cl, DMF, rt, 16 h; (vii) (Bpin)2, KOAc, Pd(dppf)Cl2, dioxane, 80 °C, 3 h.
The relative stereochemistry of the methyl group in the prepared compounds was determined by polarimetry, while the absolute stereochemistry was assigned by comparing the NMR spectra of intermediates (S,R)- and (R,R)-24b with the ones reported by Weber et al., who also measured circular dichroism spectra of the fluorinated products [31]. This allowed for configurational assignment of the radiolabeling precursors by chiral HPLC (see Section S3.3.3 in Supplementary Materials).
2.1.4. Radiosynthesis of (S)- and (R)-[18F]mIDH-23 [(S)- and (R)-[18F]2]
The radiosynthesis of (S)- and (R)-[18F]mIDH-23 [(S)- and (R)-[18F]2] was initially performed under the same conditions as described above for [18F]4, which resulted in no 18F-incorporation in the case of the N-protected precursors [(S)- or (R)-29] and afforded the desired tracers in rather low RCCs when the O-protected precursors [(S)- or (R)-30] were used. In subsequent optimization studies with different precursor amounts and reaction solvents, temperatures or times (see Section S3.3.1 in Supplementary Materials), the highest RCCs were achieved when (S)- or (R)-30 (10 µmol) were radiofluorinated for 15 min at 100 °C in DMI, followed by de-protection of the radiolabeled intermediates with 0.25 m NaOH at 80 °C for 3 min and acidification with 5% TFA in MeCN (Scheme 5). After purification by SPE and semi-preparative HPLC (as described above for [18F]4), this protocol afforded (S)- and (R)-[18F]2 in RCCs of 61 ± 13% (n = 6), RCYs of 50 ± 10% (n = 4), an Am of 102-275 GBq/µmol (from approximately 1 GBq starting activity) (n = 2) and RCPs of >99%. The residual solvent and copper levels were well below the acceptable limit, and HPLC analyses with co-injection of the unsubstituted analogs (S)- or (R)-26c demonstrated that the protodeboronated side products formed during radiofluorination were efficiently removed by semi-preparative HPLC (see Section S3.3.1 in Supplementary Materials).
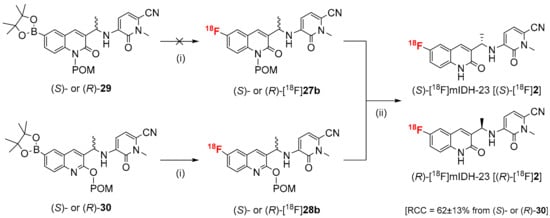
Scheme 5.
Radiosynthesis of (S)- and (R)-[18F]mIDH-23 [(S)- and (R)-[18F]2] by Cu-mediated radiofluorination. Conditions: (i) elution of [18F]F− with Et4NHCO3 in MeOH followed by evaporation of MeOH, addition of precursor [(S)- or (R)-29 or (S)- or (R)-30] and Cu(4-PhePy)4(ClO4)2 (10 µmol of each) in DMI, 100 °C, 15 min; (ii) 0.25 m NaOHaq in MeOH, 80 °C, 3 min followed by quench with 5% TFA in MeCN.
2.1.5. Synthesis of Reference Compound (5) and Radiolabeling Precursor (39) for [18F]FE-mIDH-138 ([18F]5)
The reference compound and the radiolabeling precursor for [18F]FE-mIDH-138 ([18F]5) were prepared analogous to the methodology described in Section 2.1.1 by conjugating the POM-protected 3-formylquinolone 18b with appropriate anilines. The necessary anilines 36a and 36b were obtained by O-alkylation of 2-hydroxy-4-nitrobenzonitrile 32 with 2-fluoroethyl methanesulfonate 33 (prepared by esterification of fluoroethyl alcohol with methanesulfonyl chloride [44]) or commercially available [(2-chloroethoxy)methyl]benzene 34 [36], and subsequent reduction in the resulting nitroarenes 35a,b [45] (Scheme 6A).
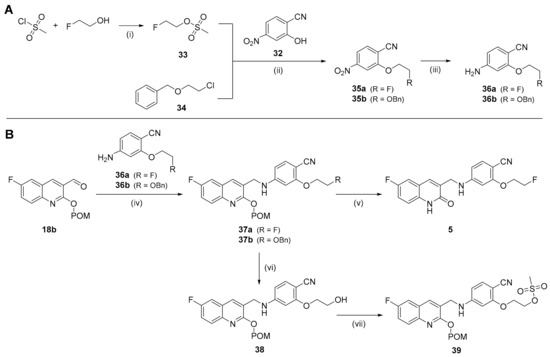
Scheme 6.
Preparation of intermediates 36a,b (A) and the reference compound (5) and radiolabeling precursor (39) (B) for [18F]FE-mIDH-138 ([18F]5). Conditions: (i) Ms-Cl, NEt3, CH2Cl2, rt, 3 h; (ii) 33 or 34, Cs2CO3, DMF, 60 °C, 2.5 h; (iii) Zn powder, NH4Cl, EtOH, rt; (iv) 36a,b, TMS-OTf, NaBH4, DMF, 0 °C, 5 h; (v) 40% NaOHaq in MeOH, 50 °C, 1 h; (vi) Pd/C, EtOH, rt, 5 h; (vii) Ms-Cl, NEt3, CH2Cl2, 0 °C to rt, 2.5 h.
Compounds 36a,b were then conjugated with 18b to afford the protected quinolones 37a,b (Scheme 6B) [37]. Subsequent de-protection of 37a with NaOHaq in MeOH furnished the reference compound 5 in 10% yield, while selective hydrogenation of the benzylic protecting group in 37b afforded POM-protected alcohol 38 in almost quantitative yield [46]. Finally, 38 was treated with methanesulfonyl chloride to obtain the O-protected radiolabeling precursor 39 [44]. Attempts to also prepare the corresponding N-protected radiolabeling precursor were unsuccessful, since Pd/C-catalyzed hydrogenation resulted in cleavage of the benzylic amine linker in the N-protected intermediate.
2.1.6. Radiosynthesis of [18F]FE-mIDH-138 ([18F]5)
Due to the base liability of the ethoxy side chain, the reaction conditions for radiofluorination of 39 and subsequent de-protection of the radiolabeled intermediate [18F]37a had to be separately optimized to identify suitably mild basic conditions (see Section S3.4.1 in Supplementary Materials). The best results were obtained when [18F]F− pre-processing was performed under ‘minimalist’ conditions by trapping aqueous [18F]F− on a QMA cartridge, removing remaining water with MeOH and air, and eluting the [18F]F− with a solution of Me4NOTf in MeOH (Scheme 7). MeOH was evaporated at 85 °C under reduced pressure for 5 min before a solution of 39 (3.5 µmol) in MeCN was added. The reaction mixture was then stirred for 15 min at 85 °C and cooled to room temperature. For de-protection of the radiolabeled intermediate, 0.1 m NaOHaq. in EtOH was added and the resulting mixture was stirred at 35 °C for 10 min (Scheme 7). Finally, the reaction was quenched by addition of H2O and the crude product was purified via semi-preparative HPLC, which afforded [18F]5 in RCYs of 26 ± 8% (n = 3), an Am of 14-47 GBq/µmol (n = 4) (from approximately 1 GBq starting activity) and RCPs of >99%.
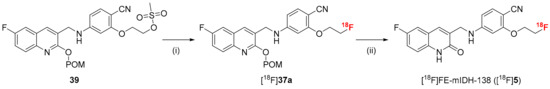
Scheme 7.
Radiosynthesis of [18F]FE-mIDH-138 ([18F]5) by ‘minimalist’ aliphatic radiofluorination. Conditions: (i) elution of [18F]F− with Me4NOTf in MeOH followed by evaporation of MeOH, addition of 39 (2 mg) in MeCN, 85 °C, 15 min; (ii) 0.1 m NaOHaq in EtOH, 35 °C, 10 min.
2.2. In Vitro Evaluation
2.2.1. Lipophilicity
As a measure for the lipophilicity of the four radiolabeled probes, their partition coefficients (logP) and distribution coefficients at pH 7.4 (logD7.4) were determined by the shake-flask method. Table 1 summarizes the results of these experiments and compares them with the predicted logD7.4 values calculated using the ADMETlab platform [47]. Overall, the experimentally determined logD7.4 values showed good agreement with the predicted values and ranged from 2.5 to 2.7, indicating a moderate lipophilicity of the compounds.

Table 1.
Summary of experimental and calculated lipophilicity metrics.
2.2.2. In Vitro Stability
The in vitro stability of the radiolabeled probes was evaluated by incubation studies with phosphate-buffered saline (PBS) and rat serum. The results of these experiments showed no evidence for decomposition after incubation of the four compounds in PBS at room temperature for up to three hours (see Figure S18 in Section S4.1.2 of Supplementary Materials). In addition, all four radiotracers proved to be stable for at least one hour when incubated in rat serum at 37 °C (see Figure S19 in Section S4.1.3 of Supplementary Materials). Finally, the stability of the non-labeled reference compounds in organic solution (DMSO) was investigated, which revealed no decomposition of (S)-2 and less than 10% decomposition of 4 and 5 for at least 28 h (see Figure S17 in Section S4.1.1 of Supplementary Materials).
2.2.3. Inhibitory Potency
The inhibitory activity of the fluorinated olutasidenib derivatives against the most frequent IDH1 mutation in gliomas (mIDH1R132H) was evaluated by enzymatic assays with serially diluted solutions on the non-labeled compounds. Figure 3 shows dose-response curves derived from these experiments and the corresponding half-maximal inhibitory concentrations (IC50) for the different compounds. While all four compounds inhibited the activity of the mutant enzyme, their inhibitory potency differed markedly, with IC50 values ranging from nanomolar to micromolar concentrations. As expected based on the results of previous studies [30,31], replacement of the 6-chloro substituent in olutasidenib by a fluorine atom resulted in good retention of inhibitory potency (IC50 = 102 nM for (S)-2), while the corresponding (R)-methyl enantiomer was substantially less potent (IC50 = 1.4 µM for (R)-2). Replacement of the 6-chloro substituent in the early lead structure by fluorine resulted in a compound with intermediate inhibitory potency (IC50 = 713 nM for 4), while additional replacement of the methoxy group at the cyanobenzene moiety by a fluoroethoxy group strongly increased the IC50 value (IC50 = 8.3 µM for 5).
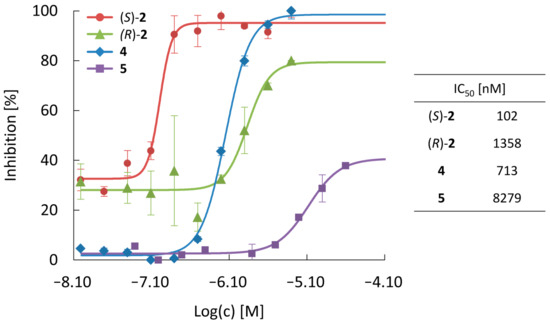
Figure 3.
Dose-response curves and half-maximal inhibitory concentrations (IC50) for suppression of mIDH1R132H by the fluorinated olutasidenib derivatives. Data are shown as mean ± standard deviation (n = 3). For additional fit parameters and 95% confidence intervals, see Table S12 in Section S4.1.4 of Supplementary Materials.
2.2.4. Cellular Uptake and Localization
Next, cellular uptake of the 18F-labeled olutasidenib derivatives by U87 glioma cells with a heterozygous IDH1R132H mutation (U87-mIDH) and the corresponding wildtype cells (U87-WT) was evaluated. Unexpectedly, the results revealed a very low cellular uptake of (S)-[18F]2 after incubation for 1 h, without clear differences between IDH-mutated (0.9 ± 0.2% per 100 µg protein) and wildtype (1.2 ± 0.7% per 100 µg protein) cells (Figure 4). Moreover, despite the >10-fold higher IC50 value of the (R)-methyl enantiomer, cellular uptake of (S)-[18F]2 by U87-mIDH cells was only approx. 2-fold higher than that of (R)-[18F]2 (0.5 ± 0.1% per 100 µg protein), suggesting that inhibitor potency and cellular uptake were not directly related. Consistent with this assumption, uptake of [18F]4 and, to a lesser extent, the 18F-fluoroethoxylated analog [18F]5 by both cell lines was considerably higher than that of (S)-[18F]2. In addition, [18F]4 showed a moderately but significantly higher uptake into U87-mIDH (6.0 ± 2.0% per 100 µg protein) compared to U87-WT (4.7 ± 1.4% per 100 µg protein, p = 0.03) cells. Conversely, 18F-fluoroethoxylated [18F]5 preferentially accumulated in the wildtype cell line (3.7 ± 0.6% per 100 µg protein) and showed significantly lower uptake into U87-mIDH cells (3.2 ± 0.6% per 100 µg protein, p = 0.03).
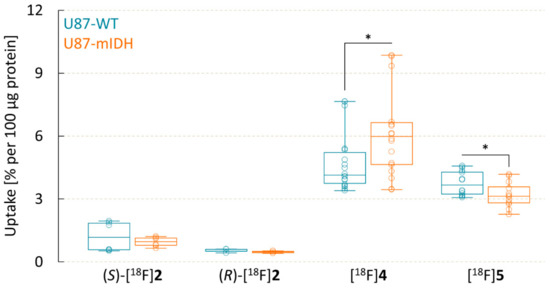
Figure 4.
Uptake of 18F-labeled olutasidenib derivatives into wildtype (U87-WT) and IDH-mutated (U87-mIDH) U87 glioma cells after 60 min. Boxplots indicate median, 25th and 75th percentile (box), minimum and maximum values (whiskers) and individual data points (circles). Statistically significant differences (p < 0.05) in cellular uptake of the compounds between U87-WT and U87-mIDH cells were identified by a two-tailed t-test and are indicated by asterisks.
To assess whether the apparent contrast between inhibitory potency and cellular uptake could be related to differential membrane partitioning or subcellular distribution of the probes, their cellular localization was also analyzed by cell fractionation experiments after incubation with the different compounds. However, the results showed that all four compounds mainly accumulated in the cytoplasm, while the fraction of radioactivity associated with the cellular membranes and organelles was always below 5%.
Taken together, these results are in line with recent findings that mIDH-selective inhibitors may not necessarily exhibit mIDH-selective binding [48] and that their cellular uptake may be governed by factors not related to inhibitor affinity or potency [27]. Thus, while (S)-2 demonstrated promising potency and selectivity in functional assays [31], cellular uptake of (S)-[18F]2 was low and showed no difference between U87-mIDH and U87-WT cells. Conversely, 4 exhibited roughly 7-fold lower potency than (S)-2 in functional assays (Figure 3), but cellular uptake of [18F]4 was 5−8-fold higher than that of (S)-[18F]2 (Figure 4). In addition, [18F]4 was the only probe that showed preferential uptake into U87-mIDH cells, so that it was selected for further in vivo evaluation.
2.3. In Vivo Evaluation of [18F]mIDH-138 ([18F]4)
2.3.1. Biodistribution Studies in Healthy C57BL/6 Mice
The in vivo stability, whole-body biodistribution and excretory pathways of [18F]4 were investigated by small animal PET imaging in healthy C57BL/6 mice (Figure 5). The results demonstrated almost exclusive hepatobiliary excretion of the radiotracer, with high uptake in liver, gall bladder and intestine. Liver uptake showed an early peak (max. SUVbw 304 ± 54 after 3 min) followed by a fast washout, while intestinal radioactivity increased steadily and reached maximum values at the end of the measurements (max. SUVbw 5344 ± 1693 after 120 min). In contrast, renal excretion was negligible, with very low radioactivity accumulation in the urinary bladder. Background uptake into muscles and other tissues was also low, with maximum SUVbw values of 52 ± 6 after 16 min followed by a decline to 18 ± 2 after 120 min. Bone uptake of radioactivity was relatively low as well but increased slightly during the measurements and reached SUVbw values of 67 ± 8 after 120 min (Figure 5), indicating that the radiotracer underwent moderate defluorination. Finally, brain uptake of radioactivity throughout all measurements remained very low, suggesting limited transfer of [18F]4 across the intact blood–brain barrier (BBB). Nevertheless, while limited BBB penetrance might be an issue in patients with an intact BBB, brain tumors like gliomas are often associated with partial BBB disruption, which could facilitate tumor delivery of the probe. In addition, mutations of IDH1 have also been detected in certain solid tumors outside of the brain [49,50,51,52,53], suggesting that mIDH1-selective PET tracers could also be used for peripheral tumor imaging. With this in mind and based on the reasonable in vivo stability and low background uptake, the tumor accumulation and mIDH-selectivity of [18F]4 were further investigated in a subcutaneous tumor model.
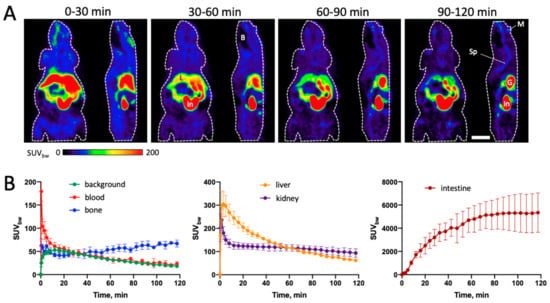
Figure 5.
Biodistribution of [18F]4 in healthy C57BL/6 mice. (A) Representative horizontal (left) and sagittal (right) summed PET images for the indicated 30 min timeframes after tracer injection. The body contour is indicated by a white dashed line. Scale bar: 10 mm. (B) Time activity curves for volumes of interest (VOIs) placed in the indicated organs, expressed as mean SUVbw values ± standard deviation (n = 4). Abbreviations: B—brain, G—gall bladder, In—intestines, L—liver, M—mandible (lower jaw), Sp—spine.
2.3.2. Biodistribution Studies in CB17-SCID Mice with Subcutaneous U87-WT and U87-mIDH Tumors
The in vivo tumor uptake and mIDH selectivity of [18F]4 were investigated by small animal PET imaging in CB17-SCID mice bearing subcutaneous U87-WT and U87-mIDH tumors, which were implanted in the right and left shoulder region, respectively (Figure 6). Due to premature death of three mice during the 120 min measurements (after 65, 80 and 95 min), full time activity curves (TACs) could only be calculated for the remaining three animals, while data from a total of five mice could be used for kinetic modelling (see below). Unexpectedly, analysis of the PET measurements revealed more pronounced uptake of [18F]4 into U87-WT compared to U87-mIDH tumors. Thus, while initial tracer uptake by both tumors was similar, accumulation of radioactivity in the U87-mIDH tumors already peaked after 8 mins (max. SUVbw 35 ± 8), which was followed by a quick washout (Figure 6B). In contrast, the U87-WT tumors showed a sustained accumulation phase (max. SUVbw 45 ± 8 after 38 min) and better retention of radioactivity (Figure 6B), which resulted in almost two-fold higher SUVbw values during the second half of the measurements (Table 2). Interestingly, very similar differences in the time course of tracer uptake (i.e., early peak followed by fast washout in IDH1 mutated tumors versus sustained tracer accumulation in IDH1 wildtype tumors) have previously been observed in a preclinical study with the TSPO-radioligand [18F]DPA-714 [22], suggesting that they could be related to factors like, e.g., differences in tracer perfusion between the tumors. Indeed, considering that expression of mIDH is well known to have profound effects on numerous aspects of tumor biology and growth [54,55,56,57,58], it seemed conceivable that differences in the composition and/or structure of the tumors (percentage and distribution of hypoxic, necrotic, etc., cells) could result in genotype-dependent differences in the wash-in/wash-out phases, although visual inspection of the dissected tumors did not substantiate this hypothesis.
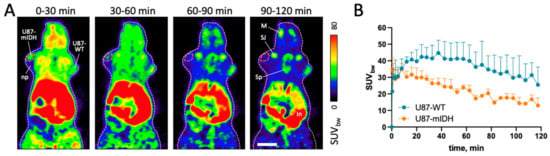
Figure 6.
Biodistribution of [18F]4 in CB17-SCID mice with subcutaneous U87-mIDH and U87-WT tumors. (A) Representative horizontal summed PET images for the indicated 30 min timeframes after tracer injection. The body contour is indicated by a white dashed line. The U87-mIDH tumor was located in the left shoulder region (indicated by the orange dashed line), while the U87-WT tumor was located in the right shoulder region (indicated by the blue dashed line). Scale bar: 10 mm. (B) Time activity curves for volumes of interest (VOIs) placed in the non-necrotic parts of the tumors, expressed as mean SUVbw values ± standard deviation (n = 3). Abbreviations: In—intestines, L—liver, M—mandible (lower jaw), np—necrotic part of the tumor, SJ—shoulder joint, Sp—spine.

Table 2.
Comparison of [18F]4 uptake between U87-WT and U87-mIDH tumors.
To obtain further insight into the potential mechanisms underlying these unexpected findings, tumoral tracer uptake was analyzed with a two-tissue compartment model (2TCM), using radioactivity in the heart as image-derived input function. Although the use of an image-derived input function instead of an invasive arterial input function could introduce some degree of systematic bias (due to, e.g., a lack of correction for spill-over and metabolism), this should affect the parameter estimation for both tumors in a given animal in the same manner and therefore have little impact on inter-tumor comparisons. The results of the kinetic modelling showed that both specific distribution volume (VS) and non-displaceable binding potential (BPND) were significantly higher for U87-WT compared to U87-mIDH tumors, whereas the influx and efflux rate constants (K1, k2) for the two tumor types were comparable (Table 3). These results indicate that the higher tracer uptake in U87-WT tumors resulted from higher specific binding rather than from differences in tracer perfusion or wash-in/wash-out phases. In particular, VS and BPND depend on the density of receptors available for radioligand binding (Bavail), the affinity of the radioligand (KD) and its free fraction (fND) [59], suggesting that the differences in tracer uptake were related to differences in one or more of these parameters between the two tumor types. Assuming that nonspecific binding of the radioligand in both tumors was similar, differences in fND can most likely be excluded. Likewise, although recent findings indicate that most mIDH1-selective inhibitors can indeed bind to both wildtype and mutant enzymes [27,48], a significantly lower KD of the radiotracer for the wildtype enzyme seems unlikely and should have been evident in the in vitro studies. Thus, while our observation that inhibitor potency and cellular uptake are not directly related clearly supports the assumption that mIDH1-selective inhibition may not correlate with mIDH1-selective binding, the preferential in vitro uptake of [18F]4 into mIDH1-expressing cells argues against a higher affinity of this radioligand for the wildtype enzyme (but see below). On the other hand, the expression and function of mIDH1 have been shown to be affected by various intracellular and extracellular factors [58], which means that differences in Bavail between the tumors could potentially explain the apparent contrast between our in vitro and in vivo findings. For example, while expression of the mutated enzyme in the isogenic U87-mIDH cell line under well-defined in vitro conditions should be comparable to that of the wildtype enzyme in the parental U87-WT cell line, it seems conceivable that environmental stimuli in the tumor microenvironment could differentially affect the expression levels of the two enzymes in vivo. In addition, olutasidenib and most other mIDH1-selective inhibitors are thought to preferentially or exclusively bind to resting enzyme conformations [23] so that differences in the equilibrium between active and inactive states could affect the number of enzymes available for binding. Indeed, a recent study with the mIDH1-selective radioligand [18F]AG-120 reported significant differences in the apparent number of binding sites between cells expressing mutated and wildtype IDH1, despite similar expression levels of both enzymes [27]. In particular, while [18F]AG-120 showed comparable affinities for both enzymes, the number of binding sites and cellular tracer accumulation in mIDH1-expressing cells were much higher, suggesting that a larger fraction of enzymes in these cells was in the binding-permissive resting conformation. Based on these findings and considering that AG-120 and olutasidenib have been shown to target the same induced-fit pocket [23], the preferential in vitro uptake of [18F]4 by U87-mIDH cells observed in the present study could be explained not only by a higher affinity of the radioligand for the mutated enzyme, but also by a higher fraction of resting enzymes in the U87-mIDH cells. Since the equilibrium between active and inactive states is well known to be highly dynamic and sensitive to many factors like the concentration of substrates, co-factors and catalytic metal ions [60,61,62], the latter could in turn provide an attractive explanation for the opposite results observed in vivo. Thus, preferential uptake into the wildtype tumors could result if the exact conformational ensemble in vivo is different from that in the in vitro assays and, e.g., a larger fraction of the enzymes in these tumors resides in the inactive conformation. In any case, while further studies are required to firmly establish the underlying mechanisms, our findings demonstrate that in vivo engagement of wildtype and mutated IDH1 is governed by factors that are not faithfully reproduced by in vitro assay.

Table 3.
Micro- and macro-parameters obtained via kinetic modeling of the PET data (2TCM) 1.
3. Materials and Methods
3.1. Chemistry and Radiochemistry
A detailed description of all chemical and radiochemical procedures is provided in Supplementary Materials.
3.2. Cell Culture and In Vitro Studies
3.2.1. Cell Culture and Reagents
Human glioblastoma cells with a c.395G>A knock-in mutation encoding mIDH1R132H (HTB-14IG™) and the corresponding wildtype cells (HTB-14™) were purchased from the American Type Culture Collection (ATCC). The cells were grown under standard culture conditions (5% CO2 and 95% air at 37 °C) in Eagle’s Minimum Essential Medium (MEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics (penicillin–streptomycin mixed solution, Gibco, Thermo Fisher Scientific, Oberhausen, Germany). The identity of the cell lines and functional expression of the mutated enzyme were confirmed by measurement of 2-HG production (for details see below), which demonstrated 3.6-fold higher levels in the HTB-14IG™ (7.56 ± 1.25 nmol per 106 cells) compared to the parental HTB-14™ (2.08 ± 0.83 nmol per 106 cells) cell line.
3.2.2. 2-Hydroxyglutarate (2-HG) Assays
Cellular 2-HG production was measured with a commercially available assay kit (Abcam, Cambridge, UK, ab211070). To this end, the cells were cultured in 9 cm dishes as described above until they had reached about 90% confluency. The cells were then detached with trypsin, suspended in 1.6 mL MEM, counted, and centrifuged for 5 min (1000× g) at ambient temperature. The resulting cell pellet was resuspended in 100 µL assay buffer and the cell membranes were disrupted by ultrasonication for 1 min on ice. After centrifugation (50,000× g) for 5 min at 4 °C, the supernatant was collected and used for determination of the 2-HG concentration according to the manufacturer specifications.
3.2.3. Determination of Lipophilicity
A solution of the corresponding radiotracer in DMSO (1 µL) was added to a mixture of n-octanol and PBS (pH = 7.4) or water (750 µL of each) in a 2 mL Eppendorf vial and the vial was vortexed for 2 min and centrifuged for another 2 min to separate the two phases. A total of 300 µL of the upper n-octanol phase was then carefully removed from the top, while 300 µL of the aqueous phase was removed by piercing the bottom of the vial with a syringe and cannula to avoid contact with the organic phase. Both fractions were transferred into fresh vials and counted in a gamma counter to determine the ratio between the activity in the organic and the aqueous phase. All measurements were performed in triplicate and the results are expressed as mean ± standard deviation of the logarithm of the partition coefficient between n-octanol and water (logP) or the logarithm of the distribution coefficient between n-octanol and PBS (logD7.4), respectively.
3.2.4. Determination of Stability in DMSO
A solution of the corresponding reference compound in DMSO (1 mg/mL) was incubated at room temperature for 28–30 h, during which aliquots were removed every few hours and analyzed by analytical HPLC. The fraction of intact reference compound at each time point was calculated by dividing the peak area of the reference compound by the total area of all peaks in the chromatogram and plotted as a function of time.
3.2.5. Determination of Stability in PBS
A solution of the corresponding radiotracer (approximately 0.5–1.0 MBq) in 0.5 µL DMSO was added to 0.5 mL PBS and the mixture was incubated at room temperature for three hours. After 0, 1, 2 and 3 h, 2.5 µL aliquots of the solution were removed and spotted on a radio TLC plate, which was air-dried and developed with an appropriate eluent for the respective tracer. For determination of the amount of intact tracer, the TLC plate was scanned with a phosphor imager and all peaks were integrated and baseline subtracted. The radiotracers were identified by comparison of the migration distances with the non-radioactive reference compounds. If the TLC plates were developed after collection of all samples, partial or complete defluorination was detected for the samples collected during early time-points but not for the last sample (see Figure S18A,B in Section S4.1.2 of Supplementary Materials), indicating silica-induced degradation of the tracers. If the aliquots for each time-point were spotted on separate TLC plates that were immediately developed to avoid silica-induced tracer degradation, no evidence for defluorination was observed for any of the time-points examined (see Figure S18C,D in Section S4.1.2 of Supplementary Materials).
3.2.6. Determination of Stability in Rat Serum
A vial with 0.5 mL of rat serum was prewarmed in a thermoshaker at 37 °C for 5 min before a solution of the corresponding radiotracer (approximately 0.5–1.0 MBq) in 0.5 µL DMSO was added. The mixture was shaken for 1 h at 37 °C, during which 60 µL aliquots were removed after 5, 15, 30 and 60 min (n = 3 per time-point). Each aliquot was added to a vial with 120 µL MeCN and the vials were vortexed for 2 min and centrifuged for another 2 min. Then, 2.5 µL of the supernatants and the respective reference compound were spotted on separate lanes of a radio TLC plate and the plate was developed with a suitable eluent for the respective tracer. Baseline, frontline and the reference compound peak were then detected with a UV lamp and spotted with activity to be visualized with a phosphor imager. The TLC plate was scanned at the phosphor imager, all peaks were integrated and the background was subtracted. The radiotracers were identified by comparison of the migration distance of the radioactive spots with the non-radioactive reference compounds.
3.2.7. Determination of Inhibitory Potency
The inhibitory activity of the candidate probes against mIDH1 was evaluated by enzymatic assays with serially diluted solutions of the non-labeled compounds using a commercially available assay kit (IDH1 (R132H) from BPS Bioscience, San Diego, CA, USA). IC50 values were determined with GraphPad Prism 4.00 by fitting the resulting dose-response curves with the Hill function.
3.2.8. Cellular Uptake Studies
For the cellular uptake studies, 1.5 × 105 cells were seeded into gelatin-coated 12-well transwell plates and incubated in 1 mL MEM (without FBS and antibiotics) containing 18.5 kBq (0.5 µCi) of the corresponding radiotracer. After incubation at 21 °C for 1 h, the supernatant was collected, the cells were lysed with 40 mM NaOH and the radioactivity of both fractions was measured with a gamma counter. The total protein concentration of the cell lysate was measured by a bicinchoninic acid (BCA) assay. The radiotracer uptake was calculated as the ratio of radioactivity in the cell fraction and the supernatant and normalized by protein content (% uptake/100 µg protein). Statistical analysis of the results was performed with Originlab Pro 2023b using two-tailed t-tests with a significance level of 0.05 to identify significant differences between groups.
To assess the ability of the radiotracers to cross the cell membrane and enter the intracellular compartment, separate cell fractionation experiments were conducted after incubation with the corresponding radiotracer as described above. To this end, the cells were detached with trypsin and dispersed with an ultra-turrax (1 min at maximum speed on ice). After centrifugation (50,000× g) for 5 min at 4 °C, the supernatant containing the cell plasma was separated from the pellet containing the cell membranes, organelles, and nuclei. The radioactivity of both fractions was measured with a gamma counter and the percentage of radiotracer in the cell plasma was calculated from the activity ratio.
3.3. In Vivo Studies
3.3.1. Animals
All experiments were carried out in accordance with the EU directive 2010/63/EU for animal experiments and the German Animal Welfare Act (TierSchG, 2006) and were approved by the regional authorities (LANUV NRW; 81-02.04.2022.A442). Six male CB17-SCID mice and four male C57BL/6 mice (Janvier Labs, Le Genest-Saint-Isle, France) were housed in groups in individually ventilated cages (Allentown LLC, Allentown, NJ, USA) in a temperature- and humidity-controlled room (20 ± 2 °C, 50–60% humidity) on a 12:12 h light–dark cycle. Throughout the experiments, the animals had ad libitum access to water and food.
3.3.2. Subcutaneous Tumor Model
Six male CB17-SCID mice (24–26 g) were used for the subcutaneous tumor model. Twenty-four hours before tumor implantation, the animals were injected (i.p.) with 20 µL anti-asialo GM1 rabbit (Fujifilm Wako Chemicals Europe GmbH, Neuss, Germany) in 0.9% NaCl (total volume: 100 µL) to promote tumor cell survival and growth through a temporary reduction in natural killer cell activity. For tumor inoculation, suspensions of 5 × 106 tumor cells in 75 µL culture medium and 75 µL Corning Matrigel (Merck, Darmstadt, Germany) were injected subcutaneously into the right (U87-WT cells) and left (U87-mIDH cells) shoulder region. Due to faster growth of the U87-mIDH tumors, U87-WT cells were injected five days before the U87-mIDH cells.
3.3.3. PET Measurements
The mice were anesthetized with isoflurane (5% for induction, 2% for maintenance) in O2/air (3:7), and a catheter for tracer injection was inserted into the lateral tail vein. They were placed on an animal holder (Medres, Cologne, Germany) and fixed with a tooth bar in a respiratory mask. Body temperature was maintained at 37 °C by warming the animal bed. Eyes were protected from drying with Bepanthen eye and nose ointment (Bayer, Leverkusen, Germany). Respiratory rate was monitored and maintained at around 40–60 breaths per minute by adjusting the isoflurane concentration. A PET scan in list mode was conducted using a Focus 220 micro PET scanner (CTI-Siemens, Erlangen, Germany) with a resolution at the center of the field of view of 1.4 mm. Data acquisition started with intravenous tracer injection and ended after 120 min. This was followed by a 10 min transmission scan using a 57Co point source for attenuation correction. After the scan was completed, the catheter was removed and the animals were allowed to wake up in their home cage. Data were histogrammed in three ways: (1) 4 × 30 min frames for visual display, (2) 28 frames (2 × 1 min, 2 × 2 min, 6 × 4 min, 18 × 5 min) for determination of time–activity curves, (3) 53 frames (12 × 10 s, 6 × 30 s, 15 × 1 min, 20 × 5 min) for kinetic modelling. Full 3D rebinning was followed by an iterative OSEM3D/MAP reconstruction algorithm with attenuation and decay correction. The resulting voxel sizes were 0.47 mm × 0.47 mm × 0.80 mm. All further analysis was performed with the software VINCI 5.21 (Max Planck Institute for Metabolism Research, Cologne, Germany). Standardized uptake values based on body weight (SUVbw) were determined according to the following equation: SUVbw = radioactivity [Bq/g] × body weight [g] × 100/injected dose [Bq]. Statistical analysis of the results was performed with a 2-way analysis of variance (ANOVA) followed by Sidak’s multiple comparison test to identify significant differences between the two tumor types.
3.3.4. Kinetic Modelling
Kinetic modeling of the PET data was performed with PMOD V.4.403 (PMOD Technologies GmbH, Zürich, Switzerland). Time–activity curves (TACs) were generated from volumes of interest (VOIs) drawn around the tumors and the heart. The heart TAC served as image-derived input function for kinetic modeling. Using a two-tissue compartment model (2TCM) with the blood volume fraction (vB) constrained to 0.05, the kinetic rate constants K1 to k4 as well as VT, VS and BPND (k3/k4) were estimated for both tumors. The choice of the specific model was based on both goodness of fit (AIC) and fitting robustness (standard deviation, parameter stability). Statistical analysis of the results was performed with Originlab Pro 2023b, using two-tailed t-tests with a significance level of 0.05 to identify significant differences between the two tumor types.
4. Conclusions
In conclusion, our in vitro studies with four 18F-labeled derivatives of olutasidenib corroborate recent findings that mIDH1-selective inhibition may not directly correlate with mIDH1-selective target engagement. In addition, biodistribution studies with [18F]4 demonstrated that in vivo engagement of wildtype and mutated IDH1 by this radioligand is governed by factors that are not faithfully reproduced by in vitro assays. Taken together, these findings highlight several obstacles that could complicate preclinical development of mIDH1-selective PET tracers. For example, given the discrepancies between mIDH1-selective inhibition, in vitro uptake and in vivo engagement observed in the present and previous studies, it remains to be firmly established whether structural modification of existing mIDH1-selective ligands through medicinal chemistry approaches can be used to achieve selective target engagement in vivo. Furthermore, as target engagement could be influenced by environmental factors, more sophisticated animal models, such as patient-derived xenografts that closely mimic human glioma pathology, may be required to reliably assess a tracer’s in vivo performance. Future research utilizing such models will be crucial to determine the suitability of existing mIDH1-selective inhibitors as leads for development of PET probes as well as to identify potential structural modifications that could be used to improve in vivo target engagement.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29163939/s1, Detailed description of all chemical and radiochemical procedures including HPLC chromatograms of the radiolabeled compounds (Sections S1–S3); additional results of the preclinical in vitro evaluation (Section S4); 1H-, 13C and 19F-NMR spectra of all prepared compounds (Section S5).
Author Contributions
Conceptualization, R.C., M.H., D.S., D.B., H.E., J.E., F.N. and B.N.; formal analysis, R.C., D.S., D.B., H.E. and F.N.; investigation, R.C., M.H., D.S., D.B., A.S., C.S., H.E. and F.N.; resources, B.N.; writing—original draft preparation, R.C., M.H., D.S., D.B., H.E. and F.N.; writing—review and editing, R.C., M.H., D.S., D.B., A.S., C.S., H.E., J.E., F.N. and B.N.; visualization, R.C., D.S., D.B., H.E. and F.N.; supervision, B.N. and J.E.; project administration, B.N.; funding acquisition, B.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deutsche Forschungsgemeinschaft (DFG), grant number NE 890/9-1, and by the Helmholtz European Partnering program (‘Innovative high-performance computing approaches for molecular neuromedicine’).
Institutional Review Board Statement
The animal study protocol was approved by the regional authorities (LANUV NRW; 81-02.04.2022.A442).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Reitman, Z.J.; Yan, H. Isocitrate Dehydrogenase 1 and 2 Mutations in Cancer: Alterations at a Crossroads of Cellular Metabolism. JNCI J. Natl. Cancer Inst. 2010, 102, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Horbinski, C. What Do We Know about IDH1/2 Mutations so Far, and How Do We Use It? Acta Neuropathol. 2013, 125, 621–636. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH Mutation in Glioma: Molecular Mechanisms and Potential Therapeutic Targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-Associated IDH1 Mutations Produce 2-Hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 Mutations in Gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.-H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.-T.; et al. Oncometabolite 2-Hydroxyglutarate Is a Competitive Inhibitor of α-Ketoglutarate-Dependent Dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef]
- Johannessen, T.-C.A.; Mukherjee, J.; Viswanath, P.; Ohba, S.; Ronen, S.M.; Bjerkvig, R.; Pieper, R.O. Rapid Conversion of Mutant IDH1 from Driver to Passenger in a Model of Human Gliomagenesis. Mol. Cancer Res. 2016, 14, 976–983. [Google Scholar] [CrossRef]
- Kayabolen, A.; Yilmaz, E.; Bagci-Onder, T. IDH Mutations in Glioma: Double-Edged Sword in Clinical Applications? Biomedicines 2021, 9, 799. [Google Scholar] [CrossRef]
- Golub, D.; Iyengar, N.; Dogra, S.; Wong, T.; Bready, D.; Tang, K.; Modrek, A.S.; Placantonakis, D.G. Mutant Isocitrate Dehydrogenase Inhibitors as Targeted Cancer Therapeutics. Front. Oncol. 2019, 9, 417. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, W.; Wang, Y.; Jin, R.; Wang, Y.; Guo, H.; Tang, Y.; Yao, X. Recent Advances of IDH1 Mutant Inhibitor in Cancer Therapy. Front. Pharmacol. 2022, 13, 982424. [Google Scholar] [CrossRef]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.-H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.-M.; Gallia, G.L.; et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. The Cancer Genome Atlas Research Network Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [CrossRef]
- Sanson, M.; Marie, Y.; Paris, S.; Idbaih, A.; Laffaire, J.; Ducray, F.; El Hallani, S.; Boisselier, B.; Mokhtari, K.; Hoang-Xuan, K.; et al. Isocitrate Dehydrogenase 1 Codon 132 Mutation Is an Important Prognostic Biomarker in Gliomas. J. Clin. Oncol. 2009, 27, 4150–4154. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Osborn, A.G.; Louis, D.N.; Poussaint, T.Y.; Linscott, L.L.; Salzman, K.L. The 2021 World Health Organization Classification of Tumors of the Central Nervous System: What Neuroradiologists Need to Know. Am. J. Neuroradiol. 2022, 43, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.R.; Wen, P.Y.; Lang-Orsini, M.; Chukwueke, U.N. World Health Organization 2021 Classification of Central Nervous System Tumors and Implications for Therapy for Adult-Type Gliomas: A Review. JAMA Oncol. 2022, 8, 1493–1501. [Google Scholar] [CrossRef]
- Whitfield, B.T.; Huse, J.T. Classification of Adult-type Diffuse Gliomas: Impact of the World Health Organization 2021 Update. Brain Pathol. 2022, 32, e13062. [Google Scholar] [CrossRef]
- Lohmann, P.; Lerche, C.; Bauer, E.K.; Steger, J.; Stoffels, G.; Blau, T.; Dunkl, V.; Kocher, M.; Viswanathan, S.; Filss, C.P.; et al. Predicting IDH Genotype in Gliomas Using FET PET Radiomics. Sci. Rep. 2018, 8, 13328. [Google Scholar] [CrossRef]
- Unterrainer, M.; Winkelmann, I.; Suchorska, B.; Giese, A.; Wenter, V.; Kreth, F.W.; Herms, J.; Bartenstein, P.; Tonn, J.C.; Albert, N.L. Biological Tumour Volumes of Gliomas in Early and Standard 20–40 Min 18F-FET PET Images Differ According to IDH Mutation Status. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Verger, A.; Stoffels, G.; Bauer, E.K.; Lohmann, P.; Blau, T.; Fink, G.R.; Neumaier, B.; Shah, N.J.; Langen, K.-J.; Galldiks, N. Static and Dynamic 18F–FET PET for the Characterization of Gliomas Defined by IDH and 1p/19q Status. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Vettermann, F.; Suchorska, B.; Unterrainer, M.; Nelwan, D.; Forbrig, R.; Ruf, V.; Wenter, V.; Kreth, F.-W.; Herms, J.; Bartenstein, P.; et al. Non-Invasive Prediction of IDH-Wildtype Genotype in Gliomas Using Dynamic 18F-FET PET. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2581–2589. [Google Scholar] [CrossRef]
- Clément, A.; Zaragori, T.; Filosa, R.; Ovdiichuk, O.; Beaumont, M.; Collet, C.; Roeder, E.; Martin, B.; Maskali, F.; Barberi-Heyob, M.; et al. Multi-Tracer and Multiparametric PET Imaging to Detect the IDH Mutation in Glioma: A Preclinical Translational in Vitro, in Vivo, and Ex Vivo Study. Cancer Imaging 2022, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Neumaier, F.; Zlatopolskiy, B.D.; Neumaier, B. Mutated Isocitrate Dehydrogenase (MIDH) as Target for PET Imaging in Gliomas. Molecules 2023, 28, 2890. [Google Scholar] [CrossRef]
- Chitneni, S.K.; Reitman, Z.J.; Gooden, D.M.; Yan, H.; Zalutsky, M.R. Radiolabeled Inhibitors as Probes for Imaging Mutant IDH1 Expression in Gliomas: Synthesis and Preliminary Evaluation of Labeled Butyl-Phenyl Sulfonamide Analogs. Eur. J. Med. Chem. 2016, 119, 218–230. [Google Scholar] [CrossRef]
- Chitneni, S.K.; Reitman, Z.J.; Spicehandler, R.; Gooden, D.M.; Yan, H.; Zalutsky, M.R. Synthesis and Evaluation of Radiolabeled AGI-5198 Analogues as Candidate Radiotracers for Imaging Mutant IDH1 Expression in Tumors. Bioorg. Med. Chem. Lett. 2018, 28, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, Q.; Zhang, Y.; Xu, Z.; Shi, D.; Cheng, Y.; Fu, Z.; Tan, H.; Cheng, D.; Shi, H. Synthesis and Biological Evaluation of Novel PET Tracers [18F]AG120 & [18F]AG135 for Imaging Mutant Isocitrate Dehydrogenase 1 Expression. Bioorg. Med. Chem. 2022, 53, 116525. [Google Scholar] [CrossRef]
- Lai, T.H.; Wenzel, B.; Dukić-Stefanović, S.; Teodoro, R.; Arnaud, L.; Maisonial-Besset, A.; Weber, V.; Moldovan, R.-P.; Meister, S.; Pietzsch, J.; et al. Radiosynthesis and Biological Evaluation of [18F]AG-120 for PET Imaging of the Mutant Isocitrate Dehydrogenase 1 in Glioma. Eur. J. Nucl. Med. Mol. Imaging 2023, 51, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Chitneni, S.K.; Yan, H.; Zalutsky, M.R. Synthesis and Evaluation of a 18F-Labeled Triazinediamine Analogue for Imaging Mutant IDH1 Expression in Gliomas by PET. ACS Med. Chem. Lett. 2018, 9, 606–611. [Google Scholar] [CrossRef]
- Lin, J.; Lu, W.; Caravella, J.A.; Campbell, A.M.; Diebold, R.B.; Ericsson, A.; Fritzen, E.; Gustafson, G.R.; Lancia, D.R.; Shelekhin, T.; et al. Discovery and Optimization of Quinolinone Derivatives as Potent, Selective, and Orally Bioavailable Mutant Isocitrate Dehydrogenase 1 (mIDH1) Inhibitors. J. Med. Chem. 2019, 62, 6575–6596. [Google Scholar] [CrossRef]
- Caravella, J.A.; Lin, J.; Diebold, R.B.; Campbell, A.-M.; Ericsson, A.; Gustafson, G.; Wang, Z.; Castro, J.; Clarke, A.; Gotur, D.; et al. Structure-Based Design and Identification of FT-2102 (Olutasidenib), a Potent Mutant-Selective IDH1 Inhibitor. J. Med. Chem. 2020, 63, 1612–1623. [Google Scholar] [CrossRef]
- Weber, V.; Arnaud, L.; Dukic-Stefanovic, S.; Wenzel, B.; Roux, V.; Chezal, J.-M.; Lai, T.-H.; Teodoro, R.; Kopka, K.; Miot-Noirault, E.; et al. Novel Radioiodinated and Radiofluorinated Analogues of FT-2102 for SPECT or PET Imaging of mIDH1 Mutant Tumours. Molecules 2022, 27, 3766. [Google Scholar] [CrossRef] [PubMed]
- Meth-Cohn, O.; Narine, B.; Tarnowski, B. A Versatile New Synthesis of Quinolines and Related Fused Pyridines, Part 5. The Synthesis of 2-Chloroquinoline-3-Carbaldehydes. J. Chem. Soc. Perkin Trans. 1 1981, 1520–1530. [Google Scholar] [CrossRef]
- Vilsmeier, A.; Haack, A. Über die Einwirkung von Halogenphosphor auf Alkyl-formanilide. Eine neue Methode zur Darstellung Sekundärer und Tertiärer p-Alkylamino-benzaldehyde. Berichte Dtsch. Chem. Ges. 1927, 60, 119–122. [Google Scholar] [CrossRef]
- Abdel-Magid, A.F.; Mehrman, S.J. A Review on the Use of Sodium Triacetoxyborohydride in the Reductive Amination of Ketones and Aldehydes. Org. Process Res. Dev. 2006, 10, 971–1031. [Google Scholar] [CrossRef]
- Ishiyama, T.; Murata, M.; Miyaura, N. Palladium(0)-Catalyzed Cross-Coupling Reaction of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters. J. Org. Chem. 1995, 60, 7508–7510. [Google Scholar] [CrossRef]
- Abonia, R.; Gutiérrez, L.F.; Insuasty, B.; Quiroga, J.; Laali, K.K.; Zhao, C.; Borosky, G.L.; Horwitz, S.M.; Bunge, S.D. Catalyst-Free Assembly of Giant Tris(Heteroaryl)Methanes: Synthesis of Novel Pharmacophoric Triads and Model Sterically Crowded Tris(Heteroaryl/Aryl)Methyl Cation Salts. Beilstein J. Org. Chem. 2019, 15, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Pletz, J.; Berg, B.; Breinbauer, R. A General and Direct Reductive Amination of Aldehydes and Ketones with Electron-Deficient Anilines. Synthesis 2016, 48, 1301–1317. [Google Scholar] [CrossRef][Green Version]
- Hoffmann, C.; Kolks, N.; Smets, D.; Haseloer, A.; Gröner, B.; Urusova, E.A.; Endepols, H.; Neumaier, F.; Ruschewitz, U.; Klein, A.; et al. Next Generation Copper Mediators for the Efficient Production of 18F-Labeled Aromatics. Chem. A Eur. J. 2023, 29, e202202965. [Google Scholar] [CrossRef]
- Taylor, N.J.; Emer, E.; Preshlock, S.; Schedler, M.; Tredwell, M.; Verhoog, S.; Mercier, J.; Genicot, C.; Gouverneur, V. Derisking the Cu-Mediated 18F-Fluorination of Heterocyclic Positron Emission Tomography Radioligands. J. Am. Chem. Soc. 2017, 139, 8267–8276. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Weng, Y.; Jiang, L.; Yang, Y.; Zhao, L.; Chen, Z.; Li, Z.; Li, J. Recent Progress in the Use of Vilsmeier-Type Reagents. Org. Prep. Proced. Int. 2010, 42, 503–555. [Google Scholar] [CrossRef]
- Davis, F.A.; Reddy, R.E.; Szewczyk, J.M.; Reddy, G.V.; Portonovo, P.S.; Zhang, H.; Fanelli, D.; Reddy, R.T.; Zhou, P.; Carroll, P.J. Asymmetric Synthesis and Properties of Sulfinimines (Thiooxime S-Oxides). J. Org. Chem. 1997, 62, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cogan, D.A.; Ellman, J.A. Catalytic Asymmetric Synthesis of tert-Butanesulfinamide. Application to the Asymmetric Synthesis of Amines. J. Am. Chem. Soc. 1997, 119, 9913–9914. [Google Scholar] [CrossRef]
- Davis, F.A.; Chen, B.-C. Asymmetric Synthesis of Amino Acids Using Sulfinimines (Thiooxime S-Oxides). Chem. Soc. Rev. 1998, 27, 13–18. [Google Scholar] [CrossRef]
- Pearson, A.J.; Roush, W.R. Handbook of Reagents for Organic Synthesis Vol. 4: Activating Agents and Protecting Groups; John Wiley & Sons: Hoboken, NJ, USA; ISBN 978-0-471-97927-2.
- Saikia, B. Zinc Ammonium Chloride. Synlett 2011, 2011, 2597–2598. [Google Scholar] [CrossRef][Green Version]
- Cristau, H.-J.; Hervé, A.; Loiseau, F.; Virieux, D. Synthesis of New Arylhydroxymethylphosphinic Acids and Derivatives. Synthesis 2003, 35, 2216–2220. [Google Scholar] [CrossRef]
- Dong, J.; Wang, N.-N.; Yao, Z.-J.; Zhang, L.; Cheng, Y.; Ouyang, D.; Lu, A.-P.; Cao, D.-S. ADMETlab: A Platform for Systematic ADMET Evaluation Based on a Comprehensively Collected ADMET Database. J. Cheminformatics 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Abboud, M.; Mikhailov, V.; Liu, X.; Reinbold, R.; Schofield, C.J. Differentiating Inhibition Selectivity and Binding Affinity of Isocitrate Dehydrogenase 1 Variant Inhibitors. J. Med. Chem. 2023, 66, 5279–5288. [Google Scholar] [CrossRef] [PubMed]
- Borger, D.R.; Tanabe, K.K.; Fan, K.C.; Lopez, H.U.; Fantin, V.R.; Straley, K.S.; Schenkein, D.P.; Hezel, A.F.; Ancukiewicz, M.; Liebman, H.M.; et al. Frequent Mutation of Isocitrate Dehydrogenase (IDH)1 and IDH2 in Cholangiocarcinoma Identified Through Broad-Based Tumor Genotyping. Oncologist 2012, 17, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Amary, M.F.; Bacsi, K.; Maggiani, F.; Damato, S.; Halai, D.; Berisha, F.; Pollock, R.; O’Donnell, P.; Grigoriadis, A.; Diss, T.; et al. IDH1 and IDH2 Mutations Are Frequent Events in Central Chondrosarcoma and Central and Periosteal Chondromas but Not in Other Mesenchymal Tumours. J. Pathol. 2011, 224, 334–343. [Google Scholar] [CrossRef]
- Kato Kaneko, M.; Liu, X.; Oki, H.; Ogasawara, S.; Nakamura, T.; Saidoh, N.; Tsujimoto, Y.; Matsuyama, Y.; Uruno, A.; Sugawara, M.; et al. Isocitrate Dehydrogenase Mutation Is Frequently Observed in Giant Cell Tumor of Bone. Cancer Sci. 2014, 105, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.T.; Sadrzadeh, H.; Comander, A.H.; Higgins, M.J.; Bardia, A.; Perry, A.; Burke, M.; Silver, R.; Matulis, C.R.; Straley, K.S.; et al. Isocitrate Dehydrogenase 1 (IDH1) Mutation in Breast Adenocarcinoma Is Associated With Elevated Levels of Serum and Urine 2-Hydroxyglutarate. Oncologist 2014, 19, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Murugan, A.K.; Bojdani, E.; Xing, M. Identification and Functional Characterization of Isocitrate Dehydrogenase 1 (IDH1) Mutations in Thyroid Cancer. Biochem. Biophys. Res. Commun. 2010, 393, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Reitman, Z.J.; Jin, G.; Karoly, E.D.; Spasojevic, I.; Yang, J.; Kinzler, K.W.; He, Y.; Bigner, D.D.; Vogelstein, B.; Yan, H. Profiling the Effects of Isocitrate Dehydrogenase 1 and 2 Mutations on the Cellular Metabolome. Proc. Natl. Acad. Sci. USA 2011, 108, 3270–3275. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ye, D.; Guan, K.-L.; Xiong, Y. IDH1 and IDH2 Mutations in Tumorigenesis: Mechanistic Insights and Clinical Perspectives. Clin. Cancer Res. 2012, 18, 5562–5571. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, P.; Radoul, M.; Izquierdo-Garcia, J.L.; Ong, W.Q.; Luchman, H.A.; Cairncross, J.G.; Huang, B.; Pieper, R.O.; Phillips, J.J.; Ronen, S.M. 2-Hydroxyglutarate-Mediated Autophagy of the Endoplasmic Reticulum Leads to an Unusual Downregulation of Phospholipid Biosynthesis in Mutant IDH1 Gliomas. Cancer Res. 2018, 78, 2290–2304. [Google Scholar] [CrossRef] [PubMed]
- Lita, A.; Pliss, A.; Kuzmin, A.; Yamasaki, T.; Zhang, L.; Dowdy, T.; Burks, C.; de Val, N.; Celiku, O.; Ruiz-Rodado, V.; et al. IDH1 Mutations Induce Organelle Defects via Dysregulated Phospholipids. Nat. Commun. 2021, 12, 614. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.E. Friend or Foe—IDH1 Mutations in Glioma 10 Years On. Carcinogenesis 2019, 40, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Innis, R.B.; Cunningham, V.J.; Delforge, J.; Fujita, M.; Gjedde, A.; Gunn, R.N.; Holden, J.; Houle, S.; Huang, S.-C.; Ichise, M.; et al. Consensus Nomenclature for in Vivo Imaging of Reversibly Binding Radioligands. J. Cereb. Blood Flow Metab. 2007, 27, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, J.; Xu, Z.; Peng, B.; Huang, Q.; Arnold, E.; Ding, J. Structures of Human Cytosolic NADP-Dependent Isocitrate Dehydrogenase Reveal a Novel Self-Regulatory Mechanism of Activity. J. Biol. Chem. 2004, 279, 33946–33957. [Google Scholar] [CrossRef] [PubMed]
- Sabo, K.A.; Albekioni, E.; Caliger, D.; Coleman, N.J.; Thornberg, E.; Avellaneda Matteo, D.; Komives, E.A.; Silletti, S.; Sohl, C.D. Capturing the Dynamic Conformational Changes of Human Isocitrate Dehydrogenase 1 (IDH1) upon Ligand and Metal Binding Using Hydrogen–Deuterium Exchange Mass Spectrometry. Biochemistry 2023, 62, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Roman, J.V.; Melkonian, T.R.; Silvaggi, N.R.; Moran, G.R. Transient-State Analysis of Human Isocitrate Dehydrogenase I: Accounting for the Interconversion of Active and Non-Active Conformational States. Biochemistry 2019, 58, 5366–5380. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).