Recent Research Progress on the Chemical Constituents, Pharmacology, and Pharmacokinetics of Alpinae oxyphyllae Fructus
Abstract
1. Introduction
2. Active Components of AOF
2.1. Terpenes
2.2. Flavonoids
2.3. Diarylheptanoids
2.4. Other Components
3. Pharmacology
3.1. Effects of AOF on Neurological Disorders
3.1.1. Neuroprotective Effects
3.1.2. Treatment of Alzheimer’s Disease
Regulation of Metabolic Disorders
Antioxidant and Antiapoptotic Activity
Anti-Neuroinflammatory Activity
3.1.3. Treatment of Stroke
3.1.4. Others
3.2. Hypoglycemic Effects
3.2.1. Diabetes
3.2.2. Diabetic Nephropathy
3.3. Antihyperuricemia
3.4. Antiaging
3.5. Antidiuresis
3.6. Immune Regulation
3.7. Anti-Inflammatory Activity
3.8. Antitumor Activity
3.9. Renal Protection
3.10. Hepatoprotection
3.11. Antiasthma
| Pharmacology | Diseases | Model | Pathways | Effects | References |
|---|---|---|---|---|---|
| Neuroprotection | / | In vitro | / | ↓ ROS, MDA, NO | [17] |
| / | In vitro | PI3K/AKT/GSK3β, BDNF/TrkB/TLR4 | ↓ Iba-1, NO, TNF-α, iNOS ↑ TREM2, Arg-1, IL-10 | [48] | |
| Regulation of metabolic disorders | AD | In vitro | / | ↑ arginine, 2-hydroxy-2,4-pentadienoate, succinic semialdehyde, LPE, isocitrate, acetyl-l-carnitine, palmitoylcarnitine, oleoylcarnitine ↓ isoleucine, glutamine, LPC, arachidonic acid, eicosapentaenoic acid, docosapentaenoic acid, docosahexaenoic acid, adrenic acid | [32] |
| AD | In vitro | / | ↑ CA, CDCA ↓ TCA, GCA, TCDCA, GCDCA, LCA, TDCA, TLCA, GLCA | [33] | |
| AD | In vitro | / | ↓ DCA | [32,33] | |
| Antioxidant, antiapoptotic | AD | In vivo | / | ↑ BDNF, ERK, CREB, BCL-2, p-ERK1/2, p-AKT473 ↓ BAX, cleaved caspase-3 | [34] |
| AD | In vivo | / | ↓ Aβ, ACHE | [35] | |
| AD | In vivo | / | ↓ TChE, ↑ SOD | [37] | |
| AD | In vivo | / | ↓ MDA, ↑ GSH-Px | [35,37] | |
| AD | In vivo | PI3K/Akt | ↑ SOD, CAT, GSH-Px, MMP, Bcl2 ↓ ROS, caspase-3, BAX | [36] | |
| AD | In vivo | Akt-GSK3b, Nrf2-Keap1-HO-1 | ↓ APP, Aβ | [38] | |
| AD | In vitro | NF-κB, caspase9- caspase3 | ↑ IKK-α, p65 ↓ IκB-α, p53 | [39] | |
| Anti-neuroinflammatory | AD | In vitro | / | ↓ IL-1β, NFκB p65, NLRP3 | [40] |
| In vivo | / | ↓ NO | [45] | ||
| In vitro/in vivo | / | ↓ TNF-α, IL-6, | [40,45] | ||
| In vitro | PI3K/AKT/NF-κB | ↓ TNF-α, IL-6, IL-1β | [41] | ||
| In vitro | / | ↓ ROS, caspase-3 | [42] | ||
| In vivo | NF-κB | ↓ NO, TNF-α, IL-1β, NF-κBp65 | [44] | ||
| Effects on other neurological disorders | Stroke | In vivo | / | ↑ GalC, NGF, NSE, Nestin, Vimentin | [23] |
| Stroke | In vivo | p38 MAPK/ p90rsk, JNK/Cathepsin B | ↑ p-Bad, CREB, Bcl-2 (Bcl-xL)/Bax ↓ Bax, p53, cyto c, Smac/DIABLO, AIF, caspase | [55] | |
| Stroke | In vivo | TRAF3/T3JAM/JNK, JNK/NF-κ b, TLR4/T3JAM/JNK-, ASK1/JNK, TLR4/Iba1 (GFAP)/TRAF3/ T3JAM-, NF-κ b /ASK1, TLR4/JNK, ASK1/JNK | ↓ iNOS, COX-2, TNF-α, IL-6 | [56] | |
| Stroke | In vivo | BDNF/TrkB/AKT | ↑ BDNF, TrkB, p-AKT | [46] | |
| PD | In vivo | PKA/Akt/ mTOR/PSMB8 | ↓ A53T-α-syn | [60] | |
| Epilepsy | In vivo | / | ↑ SOD ↓ MDA | [61] | |
| Depression | In vitro | MyD88/NF-κ b | ↓ IL-6, TNF-α, IL-1β | [62] | |
| Reduction of blood sugar | Diabetes | In vitro | / | ↓ α-glucosidase, PTP1B ↑ GLP-1 | [20] |
| Diabetes | In vivo | / | ↑ ratio of bacteroidetes to firmicutes ↓ blood glucose | [63] | |
| DN | In vivo | / | ↓ blood glucose | [64,66] | |
| DN | In vivo | / | ↓ sphingolipin, phosphatidylcholine, lysophosphatidylcholine, phosphatidylethanolamine, urea, nitrogen, urinary creatinine, urinary albumin | [65,66] | |
| DN | In vivo | / | ↓ urinary excretion of microalbumin | [64] | |
| DN | In vivo | / | ↓ H2O2, MDA, Superoxide Anion ↑ CAT, T-GSH, SOD | [66] | |
| DN | In vivo | / | ↓ TGF-β1, UP, BUN, TG, TC, LDL-C, Scr, BUN, LDL-C, abundance of proteobacteria ↑ SCFAs, HDLC | [67] | |
| Antihyperuricemic | Hyperuricemia | In vivo | / | ↓ GOT, BUN, IL-1β, | [68] |
| Hyperuricemia | In vivo | / | ↑ OAT1 ↓ URAT1, UA | [68,69] | |
| Antiaging | / | In vitro | / | ↑ CAT, SOD, DAF-16, SOD-3 | [70] |
| / | In vitro | insulin/IGF, SKN-1 | ↑ SOD, CAT, sod-3, gst-4, daf-16, skn-1 ↓ daf-2, age-1, ROS, MDA | [71] | |
| / | In vitro/in vivo | / | ↓ P21, β-galactosidase, cyt c, caspase-3 ↑ pAKT, SIRT-1 | [72] | |
| / | In vitro | Nrf2 | ↑ Nrf2, HO-1, SOD-1, IκBα, TIMP-1 ↓ Rac-1, Nox-2, IL-6, p-NF-κB, CTGF, MMPs | [73] | |
| / | In vitro | / | ↑ DAF-16 | [74] | |
| / | In vivo | / | ↓ TGF-β1, MMP-2, MMP-9 | [75] | |
| / | In vivo | / | ↑ spermatids, testosterone ↓ 8-Hydroxydeoxyguanosine, Histone deacetylases 1, caspase-3 | [76] | |
| Antidiuretic | Overactive bladder | In vivo | TGFβ1-SMAD 3, Gq-PLCβ1 calcium | ↓ TGF-β1, SMAD 3, Collagen, Gq, PLC β1, MLCK, MLC | [77] |
| / | In vitro | / | ↓ α-smooth muscle actin; ↑ apoptosis rate of human bladder detrusor cell | [78] | |
| / | In vivo | / | ↓ urine volume | [26] | |
| / | In vivo | β3-AR-cAMP | ↑ ALD, ADH, splenic coefficients, thymus coefficients, adrenal coefficients, AC, cAMP, PKA, β3-adrenoceptor mRNA | [79] | |
| / | In vivo | / | ↓ MDA, P2X3, muscarinic M3 ↑ SOD, β3-adrenergic receptor | [80] | |
| Immune regulation | / | In vivo | / | ↑ phagocytosis rate, NK cells | [81] |
| / | In vitro | / | ↑ thymus index, spleen index | [82] | |
| / | In vitro/in vivo | / | ↑ phagocytic index | [81,82] | |
| / | In vitro | / | ↑ IL-2, IL-4, IL-6, IFN-γ, sIgG | [5] | |
| / | In vitro | / | ↑ NO, IL-10, TNF-α, iNOS, TGF-β | [83] | |
| Anti-inflammatory | / | In vitro | / | ↓ TNF-α, IL-6, iNOS, COX-2 | [16,19] |
| / | In vitro | / | ↓ PGE2 | [18] | |
| / | In vitro | / | ↓ NO | [19,84] | |
| Antiarthritic | Osteoarthritis | In vivo | / | ↓ GAG | [85] |
| Osteoarthritis | In vivo | / | ↓ MMP-3, MMP-13, PGE2, NO ↑ TMIP-1 | [86] | |
| Osteoarthritis | In vitro/in vivo | MAPK | ↓ IL-1β, IL-6, PGE2, NO | [87] | |
| Antitumor | Bile duct cancer | In vitro | IL-6/STAT3 | ↓ IL-6, p-STAT3, MMP2, Ki-67 | [88] |
| Colorectal cancer | In vitro | / | ↑ NAG-1 ↓ cyclin D1 | [89] | |
| Liver cancer | In vitro/in vivo | / | ↑ PTEN ↓ p-AKT, PI3K | [90] | |
| Renal protection | -/ | In vivo | NOX4, NF-κB, Nrf2/HO-1 | ↑ GSH, CAT, GPX, SOD ↓ NOX4, MDA, TNF-α, IL-6, IL-1β, aspase-9, caspase-3 | [91] |
| / | In vitro | / | ↓ fibronectin | [24] | |
| Hepatoprotection | Metabolic fatty liver disease | In vivo | MAPK | ↓ IL-1β, IL-18, TNFα, IL-6, TG, HDL-c, p-ERK1/2, p-p38, p-JNK ↑ p-AMPKα | [92] |
| / | In vitro | Nrf2/HO-1 | ↑ Nrf2, Ho1 | [93] | |
| Antiasthmatic | Asthma | In vivo | / | ↓ IL-4, IL-5, IgE, CD200R ↑ CAT, GPX | [94] |
4. Research on the Herb Pairs of AOF
4.1. AOF–Schisandra Chinensis
4.2. AOF–Acorus Tatarinowii Schott
4.3. AOF–Lindera Aggregata
4.4. AOF–Other Herb Pairs
| Herb Pairs/Components | Pharmacology | Diseases | Model | Pathways | Effects | References |
|---|---|---|---|---|---|---|
| AOSC | Neuroprotection | AD | In vitro/in vivo | / | ↓ BACE1, Aβ1–42 | [96] |
| Neuroprotection | AD | In vitro | PI3K/Akt/Gsk-3β/CREB | ↓ Aβ1-42 | [98] | |
| Neuroprotection | AD | In vivo | / | ↑ ACH, M1 ↓ ACHE | [101] | |
| Anti-inflammatory | AD | In vivo | NF-κB | ↓ IKK-α, NF-κB, p53, Bad, Bax ↑ Bcl-2, Bcl-xl, i-κB-α | [97] | |
| Schisandrin and nootkatone | Neuroprotection | AD | In vivo | TLR4/NF-κB/NLRP3 | ↓ TNF-α, IL-1β, IL-6mRNA, COX-2, iNOS, GSH-Px, MDA, TAOC, NO ↑ GST, T-AOC, SOD, GSH | [99] |
| Anti-inflammatory, inhibition of apoptosis and autophagy | AD | In vitro/in vivo | PI3K/AKT/Gsk-3β/mTOR | ↓ TNF-α, IL-1β, IL-6, NF-κB, IKK, cleaved-caspase3, LC3-Ⅱ | [100] | |
| AOAT | Regulation of energy metabolism | AD | In vivo | / | ↑ ACH, GLUT-1 ↓ γ-aminobutyric acid, glutamic acid, IRS-1 | [102] |
| Protection of cerebral microvessels | AD | In vivo | / | ↑ LRP1, GLUT1, GLUT3 ↓ RAGE | [103] | |
| AOLA | Regulation of podocyte autophagy | DN | In vivo | PI3K/Akt/mTOR | ↑ Beclin-1, Atg5, LC3-Ⅱ | [105] |
| Antiproliferation of mesangial cells | DN | In vitro | PTEN/PI3K/AKT | ↓ miRNA-21, p-AKT, AKT, PI3K ↑ PTEN | [106] | |
| AOFV | Analgesia, regulation of the body’s energy metabolism and immune function | Diarrhea | In vivo | / | ↑ ATPase, cAMP, IgG, ratio of cAMP/cGMP ↓ TNF-α, NO, 5-HT | [107] |
| AOAF | Antibacterial, antitumor | / | In vivo | / | ↓ staphylococcus aureus, bacillus subtilis, pseudomonas aeruginosa, escherichia coli ↓ transplanted tumors S180 and H22 | [108] |
| Antioxidant | AD | In vivo | / | ↓ Tau, LDH | [109] | |
| AOST | Modulation of immunity, anti-inflammatory | Atopic dermatitis | In vivo | / | ↓ IL-4, IL-13, TNF-α, IgE | [110] |
5. Pharmacokinetics
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Yan, X.X.; Ren, B.L.; Wang, M.Y.; Wang, Q.L.; Yang, Q.; Tang, H.; Wang, Z.N. Present situation and development strategy of Alpinia oxyphylla. China J. Chin. Mater. Med. 2019, 44, 1960–1964. [Google Scholar]
- Pharmacopoeia of the people’s republic of China. Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Zhang, Q.; Zheng, Y.; Hu, X.; Hu, X.; Lv, W.; Lv, D.; Chen, J.; Wu, M.; Song, Q.; Shentu, J. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Alpinia oxyphylla miquel: A review. J. Ethnopharmacol. 2018, 224, 149–168. [Google Scholar] [CrossRef]
- Niu, Q.; Gao, Y.M.; Liu, P.H. Optimization of microwave-assisted extraction, antioxidant capacity, and characterization of total flavonoids from the leaves of Alpinia oxyphylla miq. Prep. Biochem. Biotechnol. 2020, 50, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Y.; Chen, H.; Xu, T.; Li, C.; Zhou, R.; Gao, L.; Han, M.; He, X.; Chen, Y. Extraction, isolation, immunoregulatory activity, and characterization of Alpiniae oxyphyllae fructus polysaccharides. Int. J. Biol. Macromol. 2020, 155, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ying, D.H.; Wu, Z.; Lin, D.; Xie, Y.Q. Study on optimization of extraction process of total flavonoids from Alpinia oxyphylllae fructus by response surface methodology. Chin. Arch. Tradit. Chin. Med. 2019, 37, 1558–1561+1795. [Google Scholar]
- Chen, Y.; Li, G.; Law, H.C.H.; Chen, H.; Lee, S.M. Determination of oxyphylla A enantiomers in the fruits of Alpinia oxyphylla by a chiral high-performance liquid chromatography-multiple reaction monitoring-mass spectrometry method and comparison of their in vivo biological activities. J. Agric. Food Chem. 2020, 68, 11170–11181. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Zuo, L.H.; Zhou, L.; Gao, Y.Q.; Guan, K.L.; Du, X.Y.; Zhang, R.; Jia, Q.Q.; Pei, J.Y.; Li, H.B.; et al. Analysis and identification of sesquiterpenes in Alpinia oxzphylla miq based on UPLC-Q-Orbitrap HRMS. Chin. Tradit. Herb. Drugs. 2020, 51, 6168–6177. [Google Scholar]
- Ying, L.; Wang, D.; Du, G. Analysis of bioactive components in the fruit, roots, and leaves of Alpinia oxyphylla by UPLC-MS/MS. Evid. Based Complement. Altern. Med. 2021, 2021, 5592518. [Google Scholar] [CrossRef]
- Duan, Z.W.; Chen, T.; Chen, L.; He, A.; Wang, S.P.; Xie, H. Optimization of different extraction process and antioxidant activity of polyphenols from Alpinia oxyphylla fructus hull. Food Sci. Technol. 2021, 46, 180–187. [Google Scholar]
- Chang, H.Y.; Cheng, T.H.; Wang, A.H. Structure, catalysis, and inhibition mechanism of prenyltransferase. IUBMB Life 2021, 73, 40–63. [Google Scholar] [CrossRef]
- Wang, Y.L. Study on Chemical Constituents of the Ethyl Acetate Part of Alpinia Oxyphylla Miq. and Their Biological Activity. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2019. [Google Scholar]
- Thapa, P.; Lee, Y.J.; Nguyen, T.T.; Piao, D.; Lee, H.; Han, S.; Lee, Y.J.; Han, A.R.; Choi, H.; Jeong, J.H.; et al. Eudesmane and eremophilane sesquiterpenes from the fruits of Alpinia oxyphylla with protective effects against oxidative stress in adipose-derived mesenchymal stem cells. Molecules. 2021, 26, 1762. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.T.; Chen, H.; Liu, X.N.; Li, K.Z.; Liu, S.J.; Feng, W.S.; Cheng, Y.X.; Wang, Y.Z. Two new terpene glycosides from the Alpiniae oxyphyllae fructus. Acta Pharm. Sin. 2023, 58, 1283–1287. [Google Scholar]
- Qiu, C.X.; Wang, J.; Mu, L.P.; Zhang, R.P.; Chen, X.L. Chemical constituents from the fruits of Alpinia oxyphylla and their neuroprotective effects. Acta Pharm. Sin. 2023, 58, 2746–2753. [Google Scholar]
- Dong, J.; Zhou, M.; Qin, Q.; Li, T.; Yao, X.; Geng, J.; Yu, Y. Structurally diverse new eudesmane sesquiterpenoids with anti-inflammatory activity from the fruits of Alpinia oxyphylla. Bioorg. Chem. 2023, 134, 106431. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Mu, L.; Wang, J.; Tang, R.; Hou, B.; Hu, W.; Zhang, R.; Chen, X. Sesquiterpenoids from the fruits of Alpinia oxyphylla miq. and their neuroprotective effect. Phytochemistry 2023, 211, 113680. [Google Scholar] [CrossRef]
- Bai, W.; Wang, T.; Yang, X.; Wang, Z.; Li, H.; Geng, J. Two new sesquiterpenoids from the fruits of Alpinia oxyphylla. Nat. Prod. Res. 2023, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhou, M.; Pan, D.B.; Qin, Q.Y.; Li, T.; Yao, X.S.; Li, H.B.; Yu, Y. Eremophilane and cadinane sesquiterpenoids from the fruits of Alpinia oxyphylla and their anti-inflammatory activities. Food Funct. 2023, 14, 9755–9766. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wu, S.L.; Chen, J.J.; Gongpan, P.; Guan, M.; Geng, C.A. Sesquiterpenoids from Alpinia oxyphylla with GLP-1 stimulative effects through Ca2+/CaMKII and PKA pathways and multiple-enzyme inhibition. J. Agric. Food Chem. 2023, 71, 16148–16159. [Google Scholar] [CrossRef]
- Lu, B.T.; Zhu, Y.T.; Liu, X.N.; Niu, H.Y.; Zhang, M.Y.; Feng, W.S.; Wang, Y.Z. Three new sesquiterpenoids from the Alpinia oxyphyllae fructus. Acta Pharm. Sin. 2024, 59, 997–1001. [Google Scholar]
- Yuan, L.; Pan, K.; Li, Y.; Yi, B.; Gao, B. Comparative transcriptome analysis of Alpinia oxyphylla miq. reveals tissue-specific expression of flavonoid biosynthesis genes. BMC Genom. Data 2021, 22, 19. [Google Scholar] [CrossRef]
- Shi, Z. Study on the Chemical Constitutions of the Ethyl Acetate Extract from Alpinia Oxyphylla and Activity of the Chrysin. Master’s Thesis, Southwest Jiaotong University, Chengdu, China, 2020. [Google Scholar]
- Zhu, Y.T.; Fang, H.B.; Liu, X.N.; Yan, Y.M.; Feng, W.S.; Cheng, Y.X.; Wang, Y.Z. Unusual acetylated flavonol glucuronides, oxyphyllvonides A-H with renoprotective activities from the fruits of Alpinae oxyphylla. Phytochemistry 2023, 215, 113849. [Google Scholar] [CrossRef] [PubMed]
- Jahng, Y.; Park, J.G. Recent studies on cyclic 1,7-diarylheptanoids: Their isolation, structures, biological activities, and chemical synthesis. Molecules 2018, 23, 3107. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.M.; Wang, Z.P.; Xu, D.P. Study on various components of Alpinia oxyphylla fructus in urine-reducing effect. Food Mach. 2019, 35, 172–175+181. [Google Scholar]
- Li, T.T. Study on the Chemical Constituents and Related Activities of Alpinia Oxyphylla miq. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2021. [Google Scholar]
- Xu, J.; Wang, F.; Guo, J.; Xu, C.; Cao, Y.; Fang, Z.; Wang, Q. Pharmacological mechanisms underlying the neuroprotective effects of Alpinia oxyphylla Miq. on alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 2071. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.H.; Li, M.; Wang, C.B.; Wang, Q.M.; Liu, Q.Q.; Shang, W.F.; Shen, Y.J.; Lin, Z.H.; Sun, T.Y.; Wu, Z.Z.; et al. Protective effects of organic extracts of Alpinia oxyphylla against hydrogen peroxide-induced cytotoxicity in PC12 cells. Neural Regen. Res. 2020, 15, 682–689. [Google Scholar] [PubMed]
- Xu, S.Y.; Ji, X.Y.; Shi, Z.; Chen, X.; Tan, R.; Jiang, H.Z. Chemical composition of Alpinia oxyphylla miq. and chrysin protective activity on neuron Cells. Pharm. Chem. J. 2023, 56, 1477–1482. [Google Scholar] [CrossRef]
- Zhang, M.Y. Pharmacodynamics and Mechanical Study of Alpiniae Oxyphyllae Fructus in the Treatment of Alzheimer’s Disease Based on Metabolomics and Network Pharmacology. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2020. [Google Scholar]
- Sun, Z.; Zhang, Y.; Zhang, M.; Zhou, S.; Cheng, W.; Xue, L.; Zhou, P.; Li, X.; Zhang, Z.; Zuo, L. Integrated brain and plasma dual-channel metabolomics to explore the treatment effects of Alpinia oxyphylla fructus on alzheimer’s disease. PLoS ONE 2023, 18, e0285401. [Google Scholar]
- Zhou, S.; Liu, L.; Zhang, Y.; Zhang, Z.; Li, H.; Fan, F.; He, J.; Kang, J.; Zuo, L. Integrated untargeted and targeted metabolomics to reveal therapeutic effect and mechanism of Alpiniae oxyphyllae fructus on alzheimer’s disease in APP/PS1 mice. Front. Pharmacol. 2023, 13, 1104954. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Q. The Effect and Mechanism of Oil Extract from Alpinia Oxyphylla miq. Fruit on Learning and Memory Impairment in. Master’s Thesis, Hubei University of Chinese Medicine, Wuhan, China, 2019. [Google Scholar]
- He, B.; Xu, F.; Xiao, F.; Yan, T.; Wu, B.; Bi, K.; Jia, Y. Neuroprotective effects of nootkatone from Alpiniae oxyphyllae fructus against amyloid-β-induced cognitive impairment. Metab. Brain Dis. 2018, 33, 251–259. [Google Scholar] [CrossRef]
- Li, R.; Wang, L.; Zhang, Q.; Duan, H.; Qian, D.; Yang, F.; Xia, J. Alpiniae oxyphyllae fructus possesses neuroprotective effects on H2O2 stimulated PC12 cells via regulation of the PI3K/Akt signaling pathway. Front. Pharmacol. 2022, 13, 966348. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Xu, F.; Yan, T.; Xiao, F.; Wu, B.; Wang, Y.; Bi, K.; Jia, Y. Tectochrysin from Alpinia oxyphylla miq. alleviates Aβ1-42 induced learning and memory impairments in mice. Eur. J. Pharmacol. 2019, 842, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Chen, Y.; Wang, X.; Cui, G.; Ung, C.O.L.; Lu, J.H.; Cong, W.; Tang, B.; Lee, S.M. Oxyphylla A ameliorates cognitive deficits and alleviates neuropathology via the Akt-GSK3β and Nrf2-Keap1-HO-1 pathways in vitro and in vivo murine models of alzheimer’s disease. J. Adv. Res. 2021, 34, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, K.M. The Potential Therapeutic Effect of Alpinia Oxyphylla Miquel Formula Granules on Cognitive Impairment in Triple-Transgenic Alzheimer’s Mice. Master’s Thesis, Dali University, Dali, China, 2023. [Google Scholar]
- Wang, Y.; Wang, M.; Xu, M.; Li, T.; Fan, K.; Yan, T.; Xiao, F.; Bi, K.; Jia, Y. Nootkatone, a neuroprotective agent from Alpiniae oxyphyllae fructus, improves cognitive impairment in lipopolysaccharide-induced mouse model of alzheimer’s disease. Int. Immunopharmacol. 2018, 62, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhang, X.; Mao, Q.; Wu, B.; He, B.; Jia, Y.; Shang, L. Alpinae oxyphyllae fructus alleviated LPS-induced cognitive impairments via PI3K/AKT/NF-κB signaling pathway. Environ. Toxicol. 2022, 37, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.H.; Zhao, H.; Liu, C.; Yu, X.Y. In-vitro neuroprotective effect and mechanism of 2β-hydroxy-δ-cadinol against amyloid β-induced neuronal apoptosis. Neuroreport 2020, 31, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, T.T.; Zhang, H.; Shi, H.S.; Zhao, S.J.; Piao, J.H.; Tian, J.N. Exploration on the action mechanism of sharpleaf galangal fruit on alzheimer’s disease based on network pharmacology. Chin. Med. Mod. Distance Edu. Chin. 2021, 19, 96–99. [Google Scholar]
- Yin, Y.; Zhang, Y.; Wu, X.R.; Huo, M.K.; Jiang, L.Z. Study on the protective effect of serum containing Yizhiren on lipopolysaccharide induced microglial cell injury in mice. Mod. J. Integr. Tradit. Chin. West. Med. 2021, 30, 2636–2640. [Google Scholar]
- Shi, W.; Zhong, J.; Zhang, Q.; Yan, C. Structural characterization and antineuroinflammatory activity of a novel heteropolysaccharide obtained from the fruits of Alpinia oxyphylla. Carbohydr. Polym. 2020, 229, 115405. [Google Scholar] [CrossRef]
- He, Y.; Chen, S.; Tsoi, B.; Qi, S.; Gu, B.; Wang, Z.; Peng, C.; Shen, J. Alpinia oxyphylla miq. and its active compound p-coumaric acid promote brain-derived neurotrophic factor signaling for inducing hippocampal neurogenesis and improving post-cerebral ischemic spatial cognitive functions. Front. Cell Dev. Biol. 2021, 8, 577790. [Google Scholar] [CrossRef]
- Xiao, T.; Pan, M.; Wang, Y.; Huang, Y.; Tsunoda, M.; Zhang, Y.; Wang, R.; Hu, W.; Yang, H.; Li, L.S.; et al. In vitro bloodbrain barrier permeability study of four main active ingredients from Alpiniae oxyphyllae fructus. J. Pharm. Biomed. Anal. 2023, 235, 115637. [Google Scholar] [CrossRef]
- Xu, M.; Yang, Y.; Peng, J.; Zhang, Y.; Wu, B.; He, B.; Jia, Y.; Yan, T. Effects of Alpinae oxyphyllae fructus on microglial polarization in a LPS-induced BV2 cells model of neuroinflammation via TREM2. J. Ethnopharmacol. 2023, 302, 115914. [Google Scholar] [CrossRef]
- Zeng, P.; Liu, Y.C.; Wang, X.M.; Ye, C.Y.; Sun, Y.W.; Su, H.F.; Qiu, S.W.; Li, Y.N.; Wang, Y.; Wang, Y.C.; et al. Targets and mechanisms of Alpinia oxyphylla miquel fruits in treating neurodegenerative dementia. Front. Aging Neurosci. 2022, 14, 1013891. [Google Scholar] [CrossRef]
- Morofuji, Y.; Nakagawa, S. Drug development for central nervous system diseases using in vitro blood-brain barrier models and drug repositioning. Curr. Pharm. Des. 2020, 26, 1466–1485. [Google Scholar] [CrossRef] [PubMed]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of early alzheimer’s disease: Clinical practice in 2021. J. Prev. Alzheimers Dis. 2021, 8, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Xiao, S.; Zheng, Q.; Zhu, L.Y.; Zhang, M.X.; Yang, M.; Yan, Y.; Wang, Z.Y. Mechanism of volatile oil from Alpinia oxyphylla in treating alzheimer’s disease based on GC-MS and network pharmacology. China J. Chin. Mater. Med. 2021, 46, 3052–3057. [Google Scholar]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Ruscu, M.; Glavan, D.; Surugiu, R.; Doeppner, T.R.; Hermann, D.M.; Gresita, A.; Capitanescu, B.; Popa-Wagner, A. Pharmacological and stem cell therapy of stroke in animal models: Do they accurately reflect the response of humans? Exp. Neurol. 2024, 376, 114753. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Huang, H.C.; Kao, S.T.; Chang, T.T.; Cheng, C.Y. Neuroprotective effects of Alpinia oxyphylla miq against mitochondria-related apoptosis by the interactions between upregulated p38 MAPK Signaling and downregulated JNK signaling in the subacute phase of cerebral ischemia-reperfusion in rats. Am. J. Chin. Med. 2022, 50, 2057–2083. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Chiang, S.Y.; Kao, S.T.; Huang, S.C. Alpinia oxyphylla miq extract reduces cerebral infarction by downregulating JNK-mediated TLR4/T3JAM- and ASK1-related inflammatory signaling in the acute phase of transient focal cerebral ischemia in rats. Chin. Med. 2021, 16, 82. [Google Scholar] [CrossRef]
- Wang, B.; Fang, T.; Chen, H. Zinc and central nervous system disorders. Nutrients 2023, 15, 2140. [Google Scholar] [CrossRef]
- Ratcliffe, C.; Pradeep, V.; Marson, A.; Keller, S.S.; Bonnett, L.J. Clinical prediction models for treatment outcomes in newly diagnosed epilepsy: A systematic review. Epilepsia 2024, 65, 1811–1846. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, B.; Cheng, Y.; Song, Y.; Li, Q.; Luo, C. Targeting molecular mediators of ferroptosis and oxidative stress for neurological disorders. Oxid. Med. Cell. Longev. 2022, 2022, 3999083. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, S.; Li, C.; Yang, X.; Li, H.; Zhong, H.; Lu, J.H.; Lee, S.M. Oxyphylla A promotes degradation of α-synuclein for neuroprotection via activation of immunoproteasome. Aging Dis. 2020, 11, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Y.T.; Liu, H.Q.; Yang, L.Q.; Wang, Z.Y.; Zhang, C.C. Study on the treatment of epileptic rats with ethanol extract of Alpinia oxyphylla fructus. World Latest Med. Inf. 2019, 19, 223–224. [Google Scholar]
- Wu, B.; Gan, A.; Wang, R.; Lin, F.; Yan, T.; Jia, Y. Alpinia oxyphylla miq. volatile oil ameliorates depressive behaviors and inhibits neuroinflammation in CUMS-exposed mice by inhibiting the TLR4-medicated MyD88/NF-κB signaling pathway. J. Chem. Neuroanat. 2023, 130, 102270. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xiao, M.; Ni, Y.; Jiang, S.; Feng, G.; Sang, S.; Du, G. Alpinia oxyphylla miq. extract prevents diabetes in mice by modulating gut microbiota. J. Diabetes Res. 2018, 2018, 4230590. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.H.; Yang, K.Y.; Yue, X.W.; Chen, Q.J.; Sun, M.; Nie, Y.X.; Ni, Y.L.; Xie, Y.Q. The effect of Yizhiren decoction on diabetic nephropathy mice. Chin. Med. Mod. Distance Edu. Chin. 2020, 18, 100–102. [Google Scholar]
- Ni, Y.L.; Yao, Y.J.; Wu, S.; Xie, Y.Q. Study on the protective mechanism of Yizhiren regulating lipid metabolism in mice with diabetic nephropathy. J. Hainan Med. Univ. 2023, 29, 801–807. [Google Scholar]
- Ni, Y.L. Studies on the Mechanism of Yizhiren in the Treatment of Diabetic Nephropathy Based on Gut Microbiota and Metabonomics Analysis. Master’s Thesis, Hainan University, Haikou, China, 2019. [Google Scholar]
- Jia, A.; Huang, X.Q.; Liu, S.H.; Chang, Z.G.; Wang, D.L.; Tan, Y.F.; Li, Y.H. Regulation of intestinal micro ecology between raw and salt-processed Alpinia oxyphylla on renal injury rats. Pak. J. Pharm. Sci. 2023, 36, 557–564. [Google Scholar]
- Sung, Y.Y.; Kim, D.S. Synergistic impacts of Alpinia oxyphylla seed extract and allopurinol against experimental hyperuricemia. BioMed Res. Int. 2022, 2022, 2824535. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Sung, Y.Y.; Yuk, H.J.; Son, E.; Lee, S.; Kim, J.S.; Kim, D.S. Anti-hyperuricemic effect of Alpinia oxyphylla seed extract by enhancing uric acid excretion in the kidney. Phytomedicine 2019, 62, 152975. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, M.; Chen, B.C.; Niu, K.; Xie, Y.Q. Mechanism study of Yizhiren (Alpinia oxyphylla) extract on senescence of caenorhabditis elegans. Chin. Arch. Tradit. Chin. Med. 2022, 40, 43–46+265. [Google Scholar]
- Xiao, M.; Chen, B.; Niu, K.; Long, Z.; Yang, F.; Xie, Y. Alpiniae oxyphylla fructus extract promotes longevity and stress resistance of C. elegans via DAF-16 and SKN-1. Front. Pharmacol. 2022, 13, 1034515. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.M.; Shibu, M.A.; Chen, C.S.; Tamilselvi, S.; Tsai, C.T.; Tsai, C.C.; Kumar, K.A.; Lin, H.J.; Mahalakshmi, B.; Kuo, W.W.; et al. Adipose derived mesenchymal stem cells along with Alpinia oxyphylla extract alleviate mitochondria-mediated cardiac apoptosis in aging models and cardiac function in aging rats. J. Ethnopharmacol. 2021, 264, 113297. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Ramesh, S.; Chang, Y.M.; Tsai, C.T.; Tsai, C.C.; Shibu, M.A.; Tamilselvi, S.; Mahalakshmi, B.; Kuo, W.W.; Huang, C.Y. D-galactose-induced toxicity associated senescence mitigated by Alpinate oxyphyllae fructus fortified adipose-derived mesenchymal stem cells. Environ. Toxicol. 2021, 36, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Tan, L.; Zhou, X.G.; Yang, Z.L.; Zhu, Q.; Chen, J.N.; Luo, H.R.; Wu, G.S. Tectochrysin increases stress resistance and extends the lifespan of caenorhabditis elegans via FOXO/DAF-16. Biogerontology 2020, 21, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.M.; Tamilselvi, S.; Lin, H.J.; Tsai, C.C.; Lin, Y.M.; Day, C.H.; Viswanadha, V.P.; Chang, H.N.; Kuo, W.W.; Huang, C.Y. Alpinia oxyphylla miq extract ameliorates cardiac fibrosis associated with D-galactose induced aging in rats. Environ. Toxicol. 2019, 34, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Park, Y.S.; Kim, B.K.; Ahn, H.S. Beneficial Effects of Alpiniae oxyphyllae fructus (AOF)on male reproductive function. Indian J Public Health Res. Dev. 2019, 10, 4696–4700. [Google Scholar] [CrossRef]
- Tie, Y.; Sun, Z.; Tong, X.; Cheng, M.; Wu, Y.; Shi, Z.; Xu, P.; Xue, M.; Xu, L.; Zhou, X. Multi-omic analysis revealed the therapeutic mechanisms of Alpinia oxyphylla fructus water extract against bladder overactivity in spontaneously hypertensive rats. Phytomedicine 2024, 123, 155154. [Google Scholar] [CrossRef]
- Su, M.S.; Xue, S.C.; Xu, L.; Ren, X.K.; Huang, N.; Tang, Z.Q.; Xu, M.H. Effect of intermittent hypoxia on bladder detrusor cell apoptosis and regulatory mechanism of Alpiniae oxyphyllae fructus. Chin. J. Pathophysiol. 2020, 36, 2212–2219. [Google Scholar]
- Han, Y.; Wu, J.; Liu, Y.; Qi, J.; Wang, C.; Yu, T.; Xia, Y.; Li, H. Therapeutic effect and mechanism of polysaccharide from Alpiniae oxyphyllae fructus on urinary incontinence. Int. J. Biol. Macromol. 2019, 128, 804–813. [Google Scholar] [CrossRef]
- Su, M.S.; Xu, L.; Gu, S.G.; Huang, N.; Ren, X.K.; Cai, X.H.; Li, C.C. Therapeutic effects and modulatory mechanism of Alpiniae oxyphyllae fructus in chronic intermittent hypoxia induced enuresis in rats. Sleep Breath. 2020, 24, 329–337. [Google Scholar] [CrossRef]
- Liu, T.H. Immunomodulatory effects of Alpiniae oxyphyllae fructus in rats. Clin. Lab J. (Electron. Ed.). 2019, 8, 98–99. [Google Scholar]
- Tian, J.X.; Zhong, J.W. Studies on the immunomodulatory effects of Alpiniae oxyphyllae fructus on mice. World Latest Med. Inf. 2018, 18, 87. [Google Scholar]
- Yang, X.; Zhou, S.; Li, H.; An, J.; Li, C.; Zhou, R.; Teng, L.; Zhu, Y.; Liao, S.; Yang, Y.; et al. Structural characterization of Alpiniae oxyphyllae fructus polysaccharide 2 and its activation effects on RAW264.7 macrophages. Int. Immunopharmacol. 2021, 97, 107708. [Google Scholar] [CrossRef]
- Busayo, F.K.; Yang, J.L.; Ding, X.P.; Wang, Y.L.; Gai, C.J.; Wu, F.; Dai, H.F.; Mei, W.L.; Chen, H.Q. Identification of volatile compounds and their bioactivities from unpolar fraction of Alpinia oxyphylla miq. and mining key genes of nootkatone biosynthesis. Nat. Prod. Res. 2024, 38, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Kim, H.J.; Jeon, S.Y.; Kim, M.R.; Lee, B.S.; Lee, J.J.; Kim, D.S.; Lee, Y.C. Anti-inflammatory and anti-nociceptive activities of Alpinia oxyphylla miquel extracts in animal models. J. Ethnopharmacol. 2020, 260, 112985. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.Y.; Yu, S.H.; Lee, B.S.; Kim, H.J.; Kim, C.G.; Jang, E.J.; Lee, J.J.; Kim, D.S.; Kim, M.R. Chondroprotective effect of Alpinia oxyphylla extract in experimentally induced cartilage degradation in rabbit articular cartilage explants. J. Food Biochem. 2021, 45, e13713. [Google Scholar] [CrossRef]
- Lee, Y.M.; Son, E.; Kim, S.H.; Kim, D.S. Effect of Alpinia oxyphylla extract in vitro and in a monosodium iodoacetate-induced osteoarthritis rat model. Phytomedicine 2019, 65, 153095. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chen, Y.; He, Z.T.; Wu, H.Z. Experimental study of ethyl acetate extract of Alpinia oxyphylla on TFK-1 cell line of cholangiocarcinoma. Chin. J. Clin. Pharmacol. 2020, 36, 3670–3673. [Google Scholar]
- Yoo, E.; Lee, J.; Lertpatipanpong, P.; Ryu, J.; Kim, C.T.; Park, E.Y.; Baek, S.J. Anti-proliferative activity of A. Oxyphylla and its bioactive constituent nootkatone in colorectal cancer cells. BMC Cancer 2020, 20, 881. [Google Scholar] [CrossRef]
- Hui, F.; Qin, X.; Zhang, Q.; Li, R.; Liu, M.; Ren, T.; Zhao, M.; Zhao, Q. Alpinia oxyphylla oil induces apoptosis of hepatocellular carcinoma cells via PI3K/Akt pathway in vitro and in vivo. Biomed. Pharmacother. 2019, 109, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Liu, M.; Zhang, Q.; Das Gupta, S.; Tang, S.; Shen, J. Nootkatone supplementation attenuates carbon tetrachloride exposure-induced nephrotoxicity in mice. Antioxidants 2023, 12, 370. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Z.B.; Zhou, Z.; Ma, N.; Wang, R.Q.; Chen, M.M.; He, X.W.; Dong, L.; Xia, Z.X.; Liu, Q.; et al. Nootkatone, a sesquiterpene ketone from Alpiniae oxyphyllae fructus, ameliorates metabolic-associated fatty liver by regulating AMPK and MAPK signaling. Front. Pharmacol. 2022, 13, 909280. [Google Scholar]
- Park, C.L.; Kim, J.H.; Jeon, J.S.; Lee, J.H.; Zhang, K.; Guo, S.; Lee, D.H.; Gao, E.M.; Son, R.H.; Kim, Y.M.; et al. Protective effect of Alpinia oxyphylla fruit against tert-butyl hydroperoxide-induced toxicity in HepG2 cells via Nrf2 activation and free radical scavenging and its active molecules. Antioxidants 2022, 11, 1032. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Yan, Y.; Xu, Z.; He, Z.; Zhou, S.; Jiang, X.; Wu, F.; Yuan, X.; Zhang, T.; Yu, D. Tectochrysin ameliorates murine allergic airway inflammation by suppressing Th2 response and oxidative stress. Eur. J. Pharmacol. 2021, 902, 174100. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, Y.; Tan, W.; Wu, X.; Chen, R.; Cao, J.; Chen, M.; Wang, Y. Compatibility art of traditional Chinese medicine: From the perspective of herb pairs. J. Ethnopharmacol. 2012, 143, 412–423. [Google Scholar] [CrossRef]
- Wang, M.; Lin, F.; Zhang, X.; Zhang, M.; Yan, T.; Wu, B.; Du, Y.; He, B.; Jia, Y. Combination of Alpinia oxyphylla fructus and Schisandra chinensis fructus ameliorates aluminum-induced alzheimer’s disease via reducing BACE1 expression. J. Chem. Neuroanat. 2022, 126, 102180. [Google Scholar] [CrossRef]
- Qi, Y.; Cheng, X.; Jing, H.; Yan, T.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Effect of Alpinia oxyphylla-Schisandra chinensis herb pair on inflammation and apoptosis in alzheimer’s disease mice model. J. Ethnopharmacol. 2019, 237, 28–38. [Google Scholar] [CrossRef]
- Qi, Y.; Jing, H.; Cheng, X.; Yan, T.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Alpinia oxyphylla-Schisandra chinensis herb pair alleviates amyloid-β induced cognitive deficits via PI3K/Akt/Gsk-3β/CREB pathway. Neuromolecular Med. 2020, 22, 370–383. [Google Scholar] [CrossRef]
- Qi, Y.; Cheng, X.; Jing, H.; Yan, T.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Combination of schisandrin and nootkatone exerts neuroprotective effect in alzheimer’s disease mice model. Metab. Brain Dis. 2019, 34, 1689–1703. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Cheng, X.; Gong, G.; Yan, T.; Du, Y.; Wu, B.; Bi, K.; Jia, Y. Synergistic neuroprotective effect of schisandrin and nootkatone on regulating inflammation, apoptosis and autophagy via the PI3K/AKT pathway. Food Funct. 2020, 11, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Bi, W.; Fan, K.; Li, T.; Yan, T.; Xiao, F.; He, B.; Bi, K.; Jia, Y. Ameliorating effect of Alpinia oxyphylla-Schisandra chinensis herb pair on cognitive impairment in a mouse model of alzheimer’s disease. Biomed. Pharmacother. 2018, 97, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. Comparison of Extraction Process of Acorus Tatarinowii-Alpinia Oxyphylla miq Essential Oil and Study on the Effect of Inhalation Administration on AD. Master’s Thesis, Jiangxi University of Traditional Chinese Medicine, Nanchang, China, 2019. [Google Scholar]
- Li, W.J. Effect of Volatile Oil from Acorus Tatarinowii and Alpinia Oxyphylla on hCMEC/D3 Cells Injured by Aβ25-35 and Preparation of Microemulsion. Master’s Thesis, Jiangxi University of Traditional Chinese Medicine, Nanchang, China, 2021. [Google Scholar]
- Yin, D.H.; Song, L.; Xie, Y.Q.; Zhu, Y.; Wu, Z. Protective effects of a combination of Alpinia oxyphylla fructus and Lindera aggregata on podocytes of mice with diabetic nephropathy through modulation of cellular autophagy. Lishizhen Med. Mater. Med. Res. 2021, 32, 2088–2090. [Google Scholar]
- Yin, D.H.; Tang, S.Y.; Wu, Z.; Chen, Y.Q.; Zhu, Y. Study on mechanism of Yizhiren(Alpiniae oxyphyllae fructus)-Wuyao(Linderae Radix) drug pair in regulating PI3K/Akt/mTOR pathway-mediated cellular autophagy to protect podocytes. Chin. Arch. Tradit. Chin. Med. 2024, 42, 30–34+262–264. [Google Scholar]
- Mao, L.Q.; Huang, L.; Xiao, M.; Du, G.K.; Yao, Y.J.; Li, X.; Tang, C.; Xie, Y.Q.; Ni, Y.L. Inhibitory effect of Yizhiren combined with Obtusiloba on proliferation of high glucose-cultured mesangial cells. Shandong Med. J. 2018, 58, 32–35. [Google Scholar]
- Xu, R.Y.; Dou, S.R.; Cao, Y.G.; Tian, L.Q.; Zhu, J.G.; Li, H.W.; Li, K.; Feng, W.S. The effect of salt-processing on the antidiarrheal efficacy of Alpiniae oxyphyllae fructus-Foeniculi fructus medicines. Chin. J. Hosp. Pharm. 2023, 43, 351–355. [Google Scholar]
- Gao, L.L.; Wang, Q.; Zhang, J.W.; Huang, R.Q.; Zhang, X.W. Study on purification and antibacterial and antitumor activity of flavonoids from Amomum villosum lour and Alpinia oxyphylla miq. J. Food Saf. Qual. 2019, 10, 4659–4666. [Google Scholar]
- Lin, W.X.; Huang, L.P.; Deng, M.Z.; Wang, N.B.; Lin, M.Q.; Ma, R.X. Effect of Component of Fructus Alpiniae oxyphyllae and Amomum Villosum on alzheimer’s disease cell model induced by okadaic acid. Chin. J. Chin. Med. 2018, 33, 106–110. [Google Scholar]
- Zhang, T.; Qiu, J.; Wu, X.; Huang, S.; Yuan, H.; Park, S. Schizonepeta tenuifolia with Alpinia oxyphylla alleviates atopic dermatitis and improves the gut microbiome in Nc/Nga mice. Pharmaceutics 2020, 12, 722. [Google Scholar] [CrossRef]
- Zuo, L.; Li, J.; Xue, L.; Jia, Q.; Li, Z.; Zhang, M.; Zhao, M.; Wang, M.; Kang, J.; Du, S.; et al. Integrated UPLC-MS/MS and UHPLC-Q-orbitrap HRMS analysis to reveal pharmacokinetics and metabolism of five terpenoids from Alpiniae oxyphyllae fructus in rats. Curr. Drug Metab. 2021, 22, 70–82. [Google Scholar] [PubMed]
- Zhao, M.F. Pharmacokinetics of Nootkatone Alpinia Oxyphylla Fructus in Rats. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2022. [Google Scholar]
- Wen, Q.; Li, H.L.; Mai, S.Y.; Tan, Y.F.; Chen, F. Tissue distribution of active principles from Alpiniae oxyphyllae fructus extract: An experimental study in rats. Curr. Pharm. Anal. 2019, 15, 286–293. [Google Scholar] [CrossRef]
- Qi, Y.; Cheng, X.; Jing, H.; Yan, T.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Comparative pharmacokinetic study of the components in Alpinia oxyphylla miq.-Schisandra chinensis (Turcz.) Baill. herb pair and its single herb between normal and alzheimer’s disease rats by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2020, 177, 112874. [Google Scholar] [CrossRef] [PubMed]
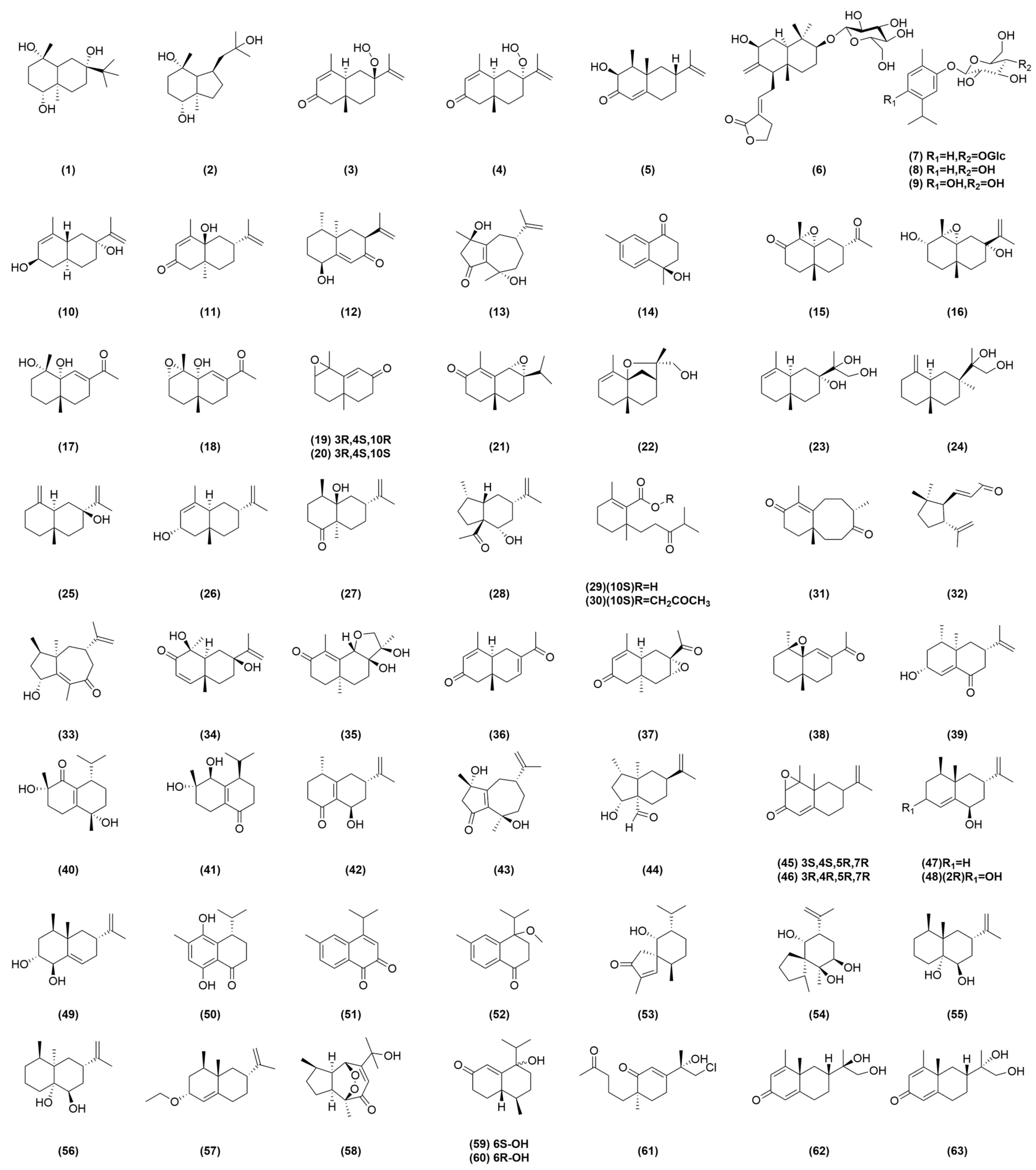
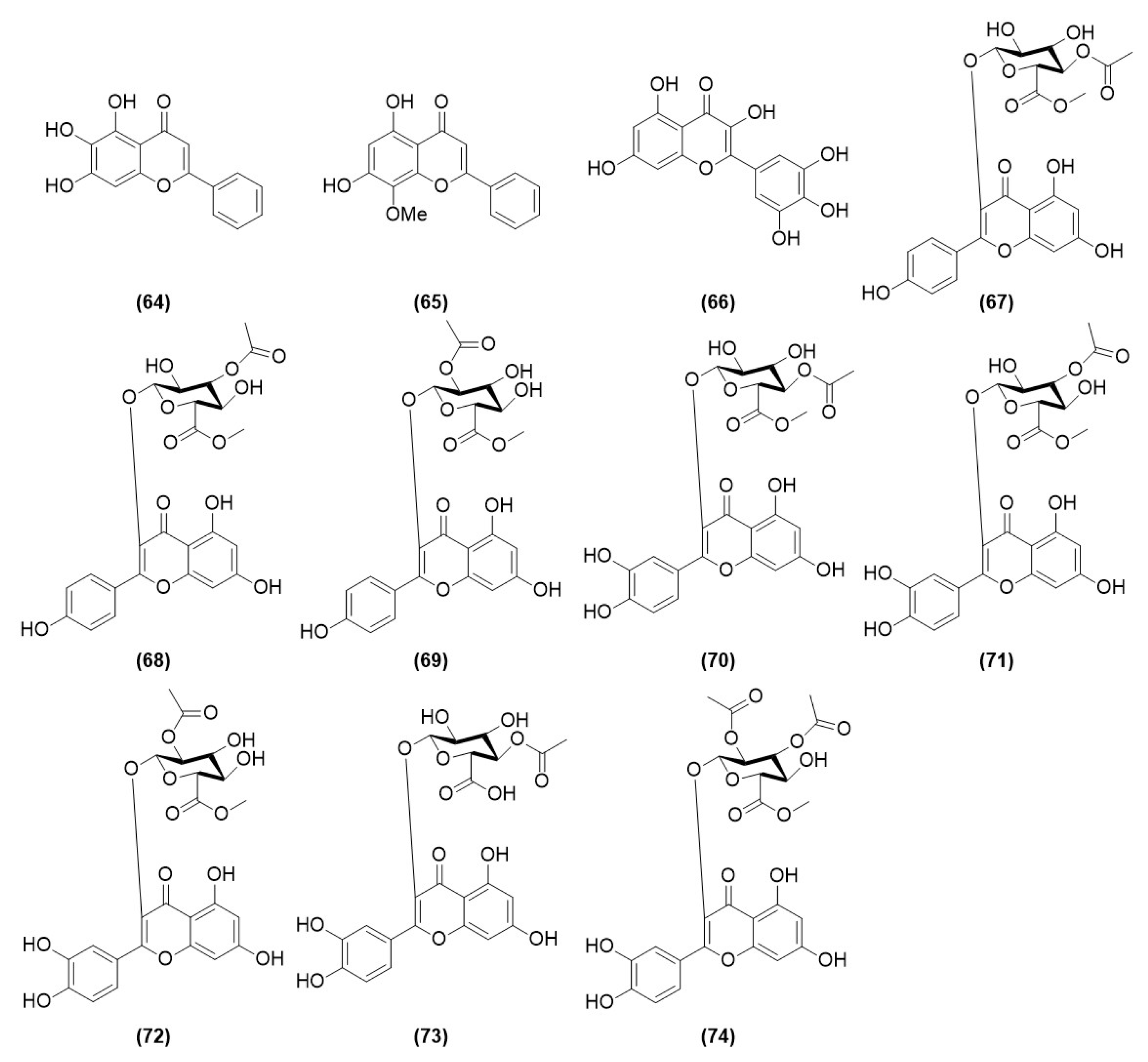
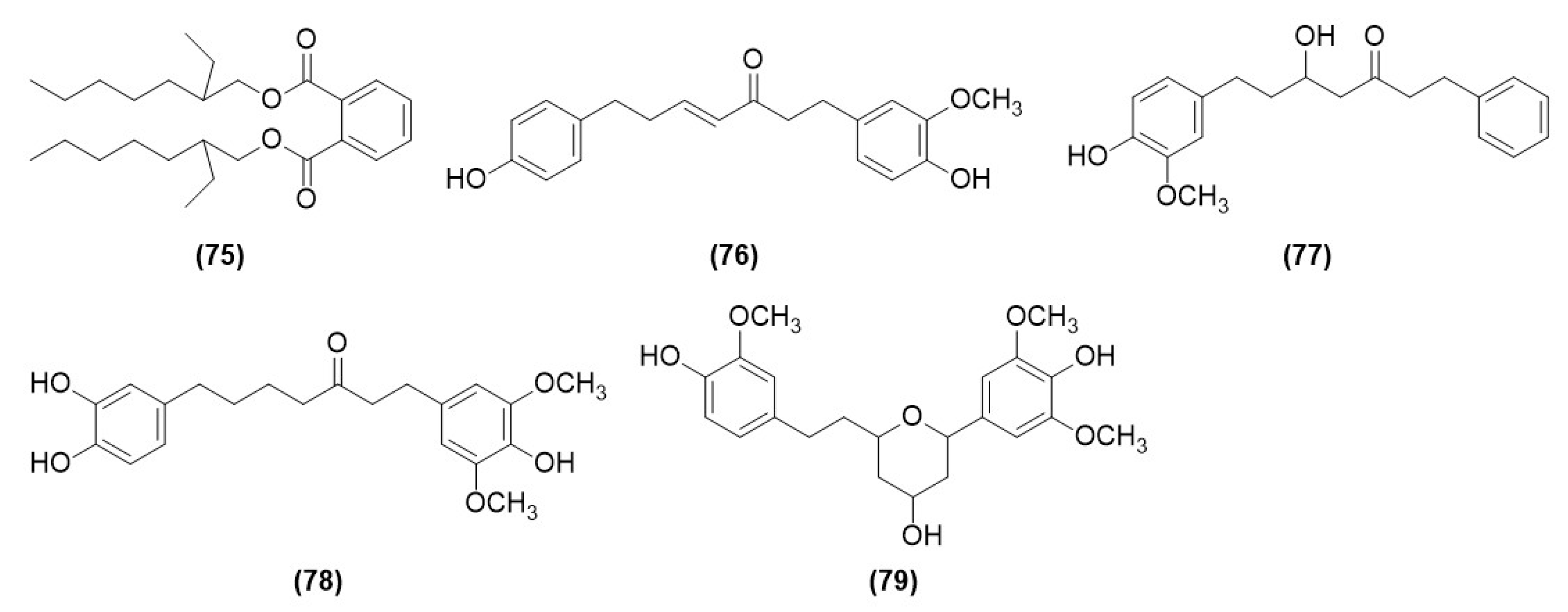
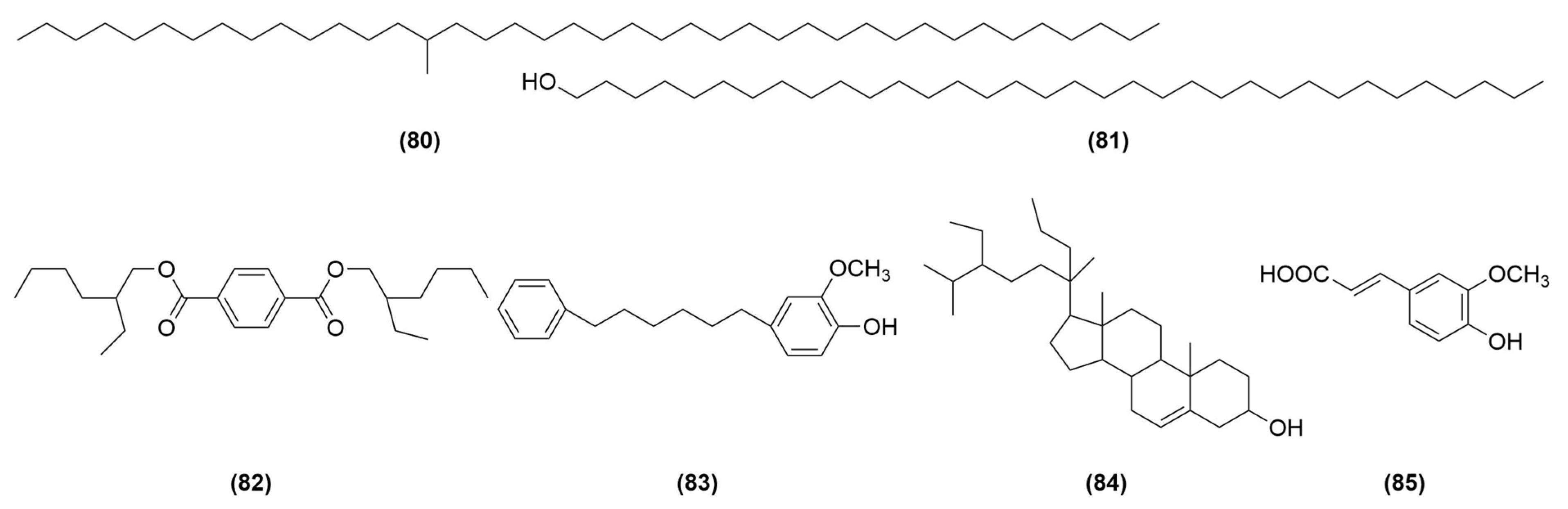

| Compound No. | Components | Molecular Formula | Ref. |
|---|---|---|---|
| 1 | 1β,4β,7β-trihydroxyeudesmane | C15H28O3 | [12] |
| 2 | bullatantriol | C15H28O3 | [12] |
| 3 | 7α-hydroperoxy eudesma-3,11-diene-2-one | C15H22O3 | [13] |
| 4 | 7β-hydroperoxy eudesma-3,11-diene-2-one | C15H22O3 | [13] |
| 5 | 3α-hydroxynootkatone | C15H22O2 | [13] |
| 6 | oxyphylloneside A | C26H40O9 | [14] |
| 7 | oxyphylloneside B | C22H34O11 | [14] |
| 8 | carvacrol 2-O-β-glucopyranoside | C16H24O6 | [14] |
| 9 | thymoquinol 2-O-β-glucopyranoside | C16H24O7 | [14] |
| 10 | (2R,5R,7R,10S)-2,7-dihydroxyl-eudesmane-3(4),11(12)-diene | C15H24O2 | [15] |
| 11 | α-rotunol | C15H22O2 | [15] |
| 12 | (1S,4S,5R,7S)-1-hydroxyl-eremophilane-9(10),11(12)-diene-8-one | C15H22O2 | [15] |
| 13 | cyperusol A1 | C15H22O3 | [15] |
| 14 | (6R,9S,10S)-10-hydroxyl-11,12,13-trinor-cadinane-4(5)-ene-3-one | C12H14O2 | [15] |
| 15 | (4R,5R,7R,10R)-12-nor-eudesma-4,5-epoxy-3-one | C14H20O3 | [16] |
| 16 | (3S,4S,5R,7R,10S)-eudesma-4,5-epoxy-11-en-3,7-diol | C15H24O3 | [16] |
| 17 | (4S,5R,10S)-12-nor-eudesma-6-en-4,5-diol-11-one | C14H22O3 | [16] |
| 18 | (3S,4S,5R,10R)-12-nor-eudesma-3,4-epoxy-6-en-5-ol-11-one | C14H20O3 | [16] |
| 19 | (3R,4S,10R)-11,12,13-trinor-eudesma-3,4-epoxy-5-en-7-one | C12H16O2 | [16] |
| 20 | (3R,4S,10S)-11,12,13-trinor-eudesma-3,4-epoxy-5-en-7-one | C12H16O2 | [16] |
| 21 | (6S,7S,10R)-eudesma-6,7-epoxy-4-en-3-one | C15H22O2 | [16] |
| 22 | (5R,7R,10S,11S)-eudesma-5,11-epoxy-3-en-12-ol | C15H24O2 | [16] |
| 23 | (5S,7R,10S)-eudesma-3-en-7,11,12-triol | C15H26O3 | [16] |
| 24 | (5R,7R,10S)-eudesma-4(15)-en-7,11,12-triol | C15H26O3 | [16] |
| 25 | (5R,7S,10S)-eudesma-4(15),11-dien-7-ol | C15H24O | [16] |
| 26 | (2R,5R,7S,10S)-eudesma-4,11-dien-2-ol | C15H24O | [16] |
| 27 | (4R,5R,7R,10R)-eudesma-11-en-5-ol-1-one | C15H24O2 | [16] |
| 28 | epialpiniol | C15H24O2 | [16] |
| 29 | alpinoxyphllaone A | C15H24O3 | [16] |
| 30 | alpinoxyphllaone B | C18H28O4 | [16] |
| 31 | neoxyphyllanene | C15H22O2 | [16] |
| 32 | oxyphyllin A | C13H20O2 | [17] |
| 33 | oxyphyllin B | C15H22O2 | [17] |
| 34 | oxyphyllin C | C15H22O3 | [17] |
| 35 | oxyphyllin D | C15H22O4 | [17] |
| 36 | oxyphyllin E | C14H18O2 | [17] |
| 37 | oxyphyllin F | C14H18O3 | [17] |
| 38 | oxyphyllin G | C14H20O2 | [17] |
| 39 | oxyphyllin H | C15H22O2 | [17] |
| 40 | oxyphyllin I | C15H24O3 | [17] |
| 41 | oxyphyllin J | C14H22O3 | [17] |
| 42 | (4S*,7S*,9R*)-14-nor-5(10),11(12)-dien-9-ol-1-one-eudesma | C14H20O2 | [18] |
| 43 | cyperusol A4 | C15H22O3 | [18] |
| 44 | alpinoxyphyllone C | C15H24O2 | [19] |
| 45 | (3S,4S,5R,7R)-eremophila-3,4-epoxy-1(10),11-dien-2-one | C15H20O2 | [19] |
| 46 | (3R,4R,5R,7R)-eremophila-3,4-epoxy-1(10),11-dien-2-one | C15H20O2 | [19] |
| 47 | (4R,5S,7S,9R)-eremophila-1(10),11-dien-9-ol | C15H24O | [19] |
| 48 | (2R,4R,5S,7S,9R)-eremophila-1(10),11-dien-2,9-diol | C15H24O2 | [19] |
| 49 | (1R,2R,4R,5S,7R)-eremophila-9,11-dien-1,2-diol | C15H24O2 | [19] |
| 50 | oxyphyllone J | C14H18O3 | [19] |
| 51 | oxyphyllone K | C14H24O2 | [19] |
| 52 | (±)oxyphyllone I | C15H20O2 | [19] |
| 53 | oxyspirone A | C15H24O2 | [19] |
| 54 | oxyspirone B | C15H26O2 | [19] |
| 55 | alpinoxyphyllol A | C15H26O2 | [20] |
| 56 | alpinoxyphyllol B | C15H26O2 | [20] |
| 57 | 2-O-ethyl-β-nootkatol | C17H28O | [20] |
| 58 | 11-Hydroxyisohanalpinone | C15H22O4 | [20] |
| 59 | 6S-Oxyphyllenone H | C14H22O2 | [20] |
| 60 | 6R-Oxyphyllenone H | C14H22O2 | [20] |
| 61 | oxyphylleudne J | C15H23ClO3 | [21] |
| 62 | oxyphyllerene D | C15H22O3 | [21] |
| 63 | oxyphyllerene E | C15H22O3 | [21] |
| Compound No. | Components | Molecular Formula | Ref. |
|---|---|---|---|
| 64 | baicalein | C15H10O5 | [23] |
| 65 | wogonin | C16H12O5 | [23] |
| 66 | myricetin | C15H10O8 | [23] |
| 67 | Oxyphyllvonide A | C24H22O13 | [24] |
| 68 | Oxyphyllvonide B | C24H22O13 | [24] |
| 69 | Oxyphyllvonide C | C24H22O13 | [24] |
| 70 | Oxyphyllvonide D | C24H22O14 | [24] |
| 71 | Oxyphyllvonide E | C24H22O14 | [24] |
| 72 | Oxyphyllvonide F | C24H22O14 | [24] |
| 73 | Oxyphyllvonide G | C23H20O14 | [24] |
| 74 | Oxyphyllvonide H | C26H24O15 | [24] |
| Compound No. | Components | Molecular Formula | Ref. |
|---|---|---|---|
| 75 | 1, 2-benzenedicarboxylic acid | C26H42O4 | [12] |
| 76 | (E)-1-(4-hydroxy-3-methoxy-phenyl)-7-(4-hydroxy-phenyl)-hept-4en-3-one | C20H22O4 | [12] |
| 77 | 5-hydroxy-7-(4″-hydroxy-3″-methoxyphenyl)-1-phenyl-3-heptanone | C20H24O4 | [12] |
| 78 | dihydrogingerenone B | C21H26O6 | [12] |
| 79 | 1,5-epoxy-3-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane | C22H28O7 | [12] |
| Compound No. | Components | Molecular Formula | Ref. |
|---|---|---|---|
| 80 | 15-methyl-tetracontane | C41H84 | [12] |
| 81 | 1-tetratriacontanol | C34H70O | [12] |
| 82 | bis-(2-ethylhexyl) terephthalate | C24H38O4 | [15] |
| 83 | 3-methoxy-4-Hydroxy-diphenylhexane | C19H24O2 | [26] |
| 84 | 20-propyl-β-sitosterol | C32H56O | [26] |
| 85 | ferulic acid | C10H10O4 | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Zhao, X. Recent Research Progress on the Chemical Constituents, Pharmacology, and Pharmacokinetics of Alpinae oxyphyllae Fructus. Molecules 2024, 29, 3905. https://doi.org/10.3390/molecules29163905
Liao J, Zhao X. Recent Research Progress on the Chemical Constituents, Pharmacology, and Pharmacokinetics of Alpinae oxyphyllae Fructus. Molecules. 2024; 29(16):3905. https://doi.org/10.3390/molecules29163905
Chicago/Turabian StyleLiao, Junfa, and Xueying Zhao. 2024. "Recent Research Progress on the Chemical Constituents, Pharmacology, and Pharmacokinetics of Alpinae oxyphyllae Fructus" Molecules 29, no. 16: 3905. https://doi.org/10.3390/molecules29163905
APA StyleLiao, J., & Zhao, X. (2024). Recent Research Progress on the Chemical Constituents, Pharmacology, and Pharmacokinetics of Alpinae oxyphyllae Fructus. Molecules, 29(16), 3905. https://doi.org/10.3390/molecules29163905






