An Examination of Chemical Tools for Hydrogen Selenide Donation and Detection
Abstract
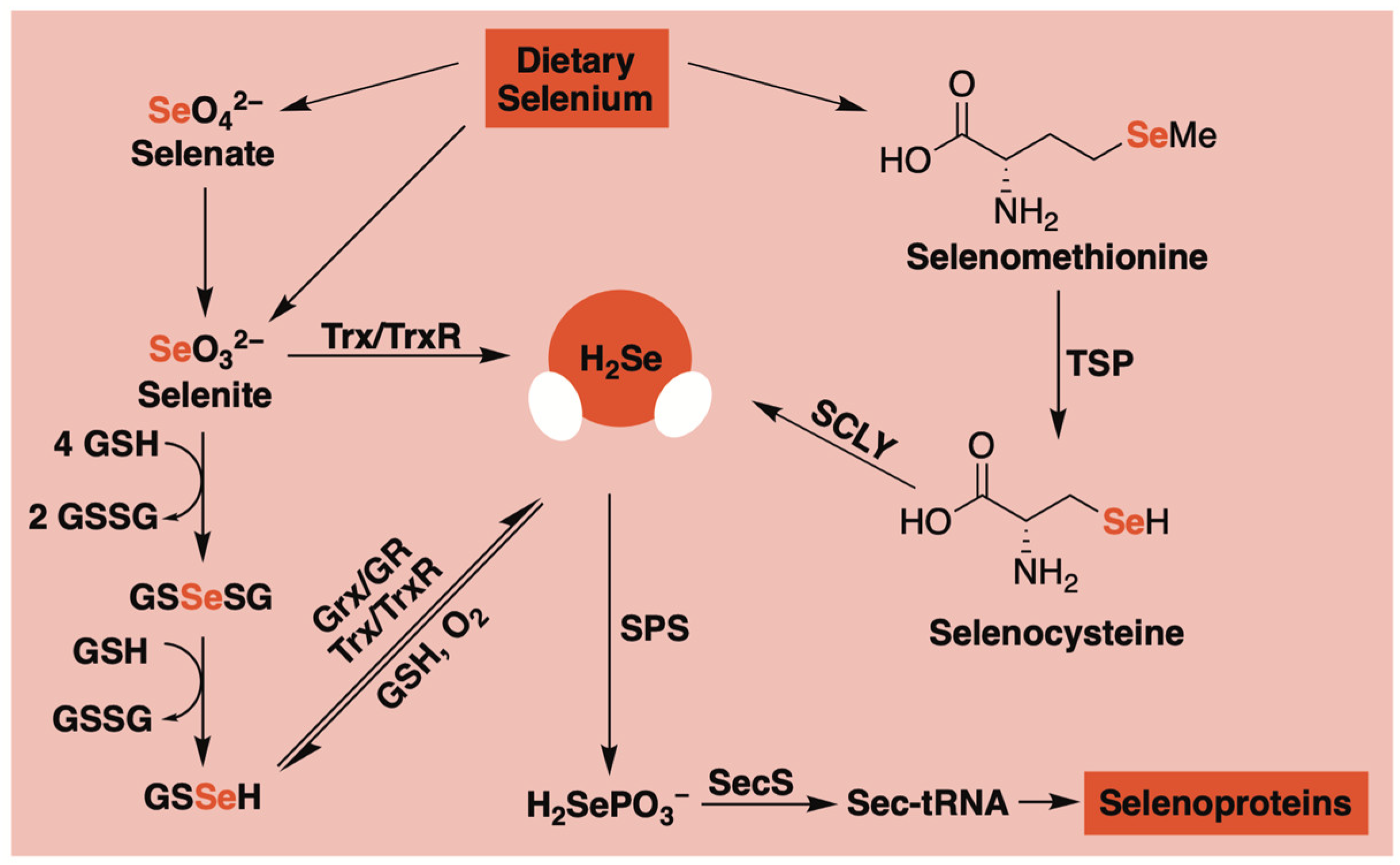
1. Introduction
2. Chemical Tools for H2Se Donation
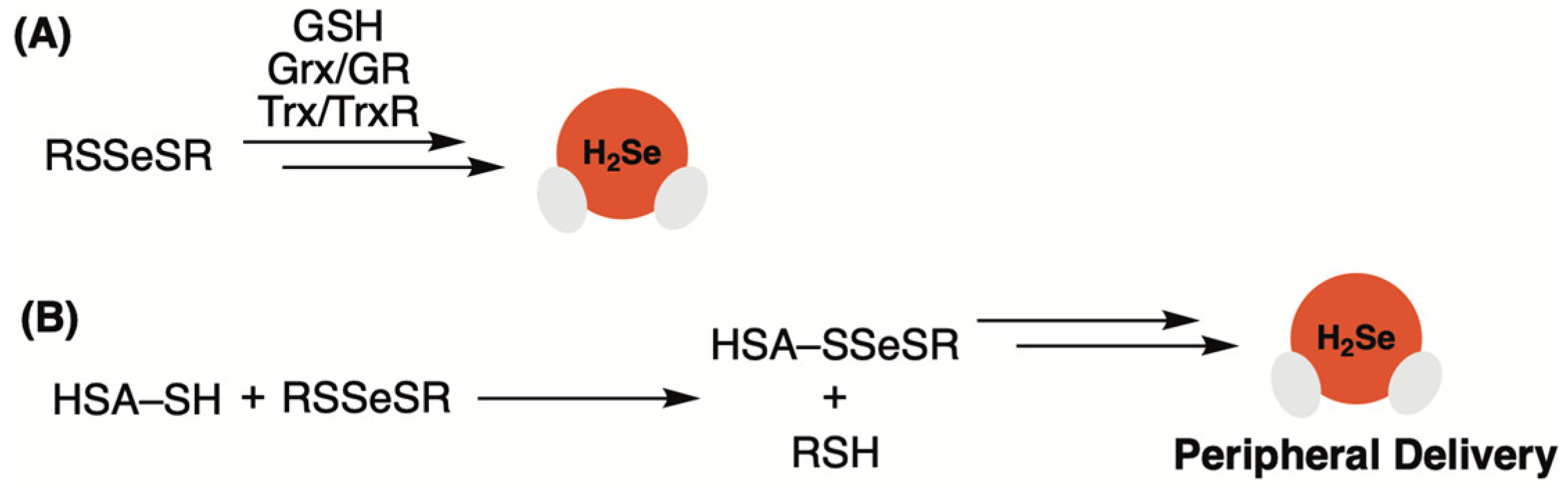
2.1. Selenotrisulfides
2.2. Selenide Salts
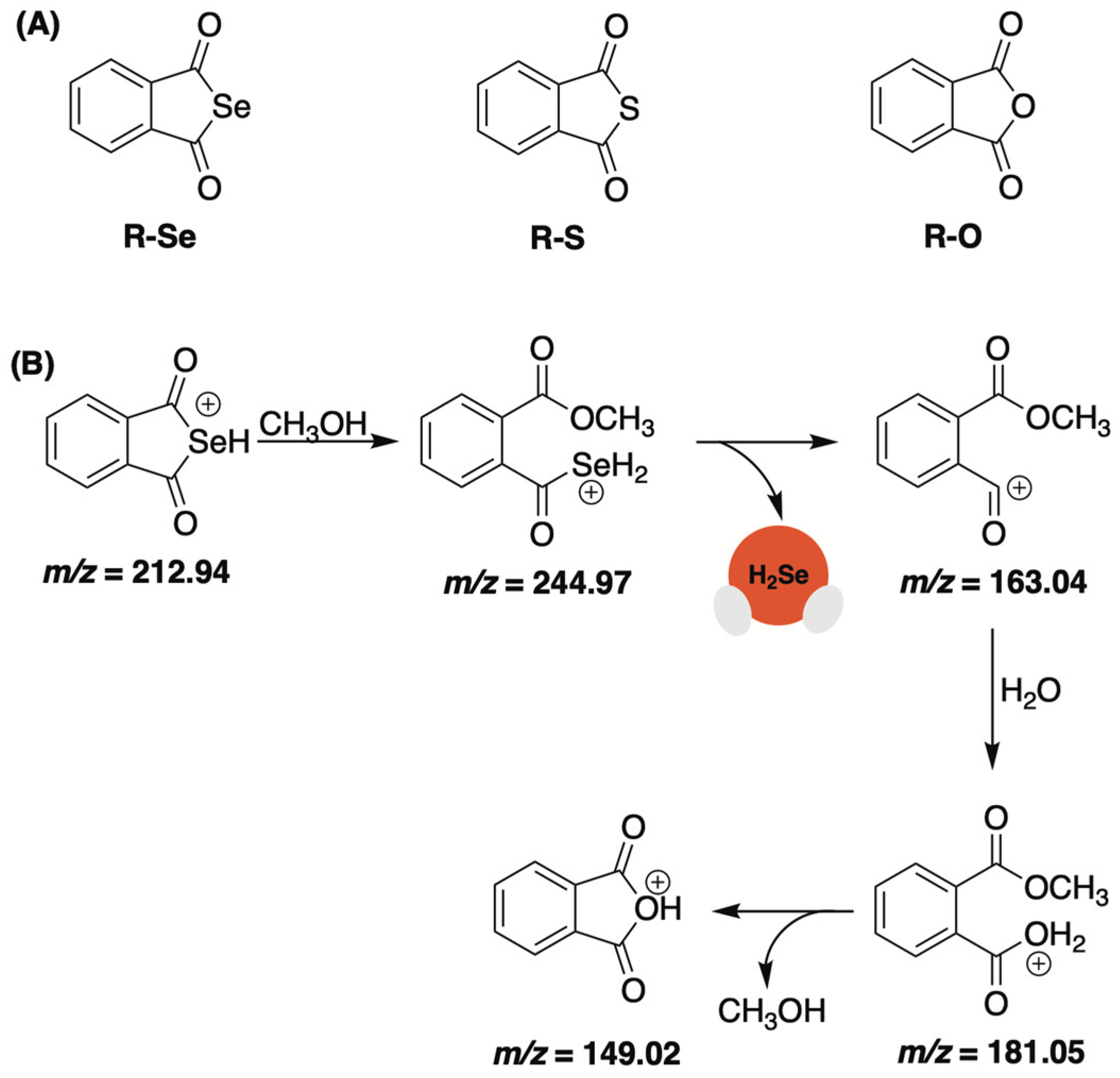
2.3. Selenoanhydrides
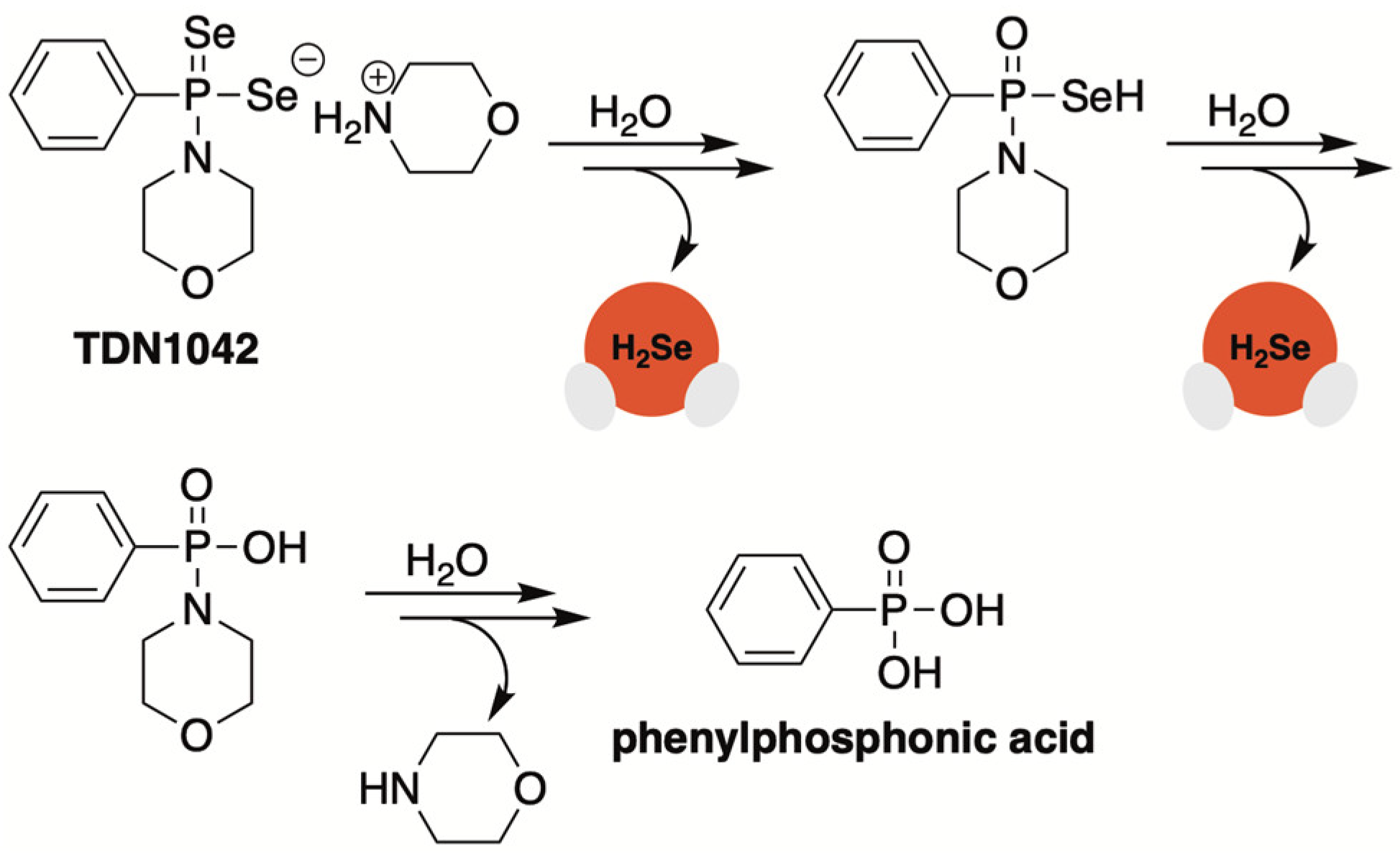
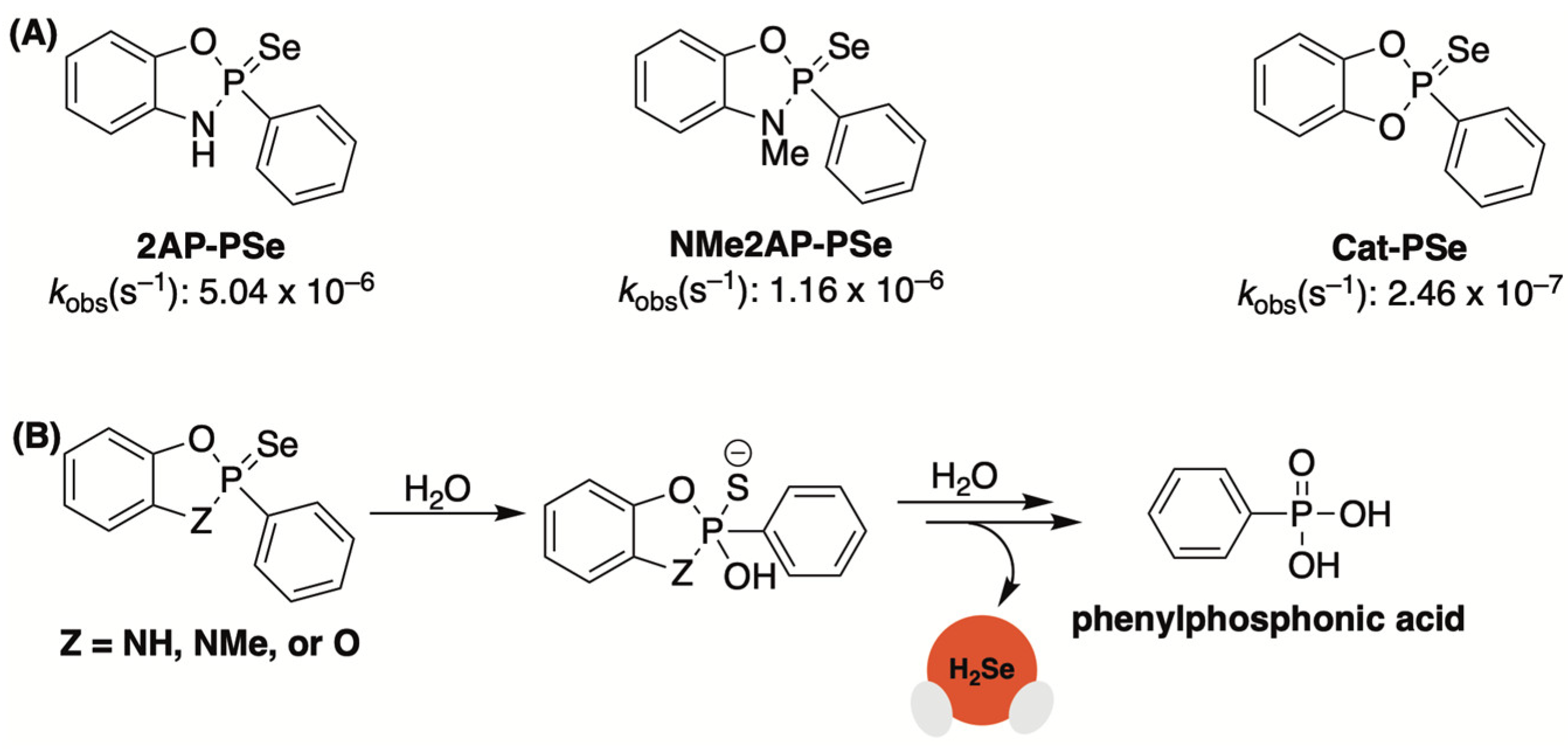
2.4. P=Se Motifs
2.5. Selenocarbonyls
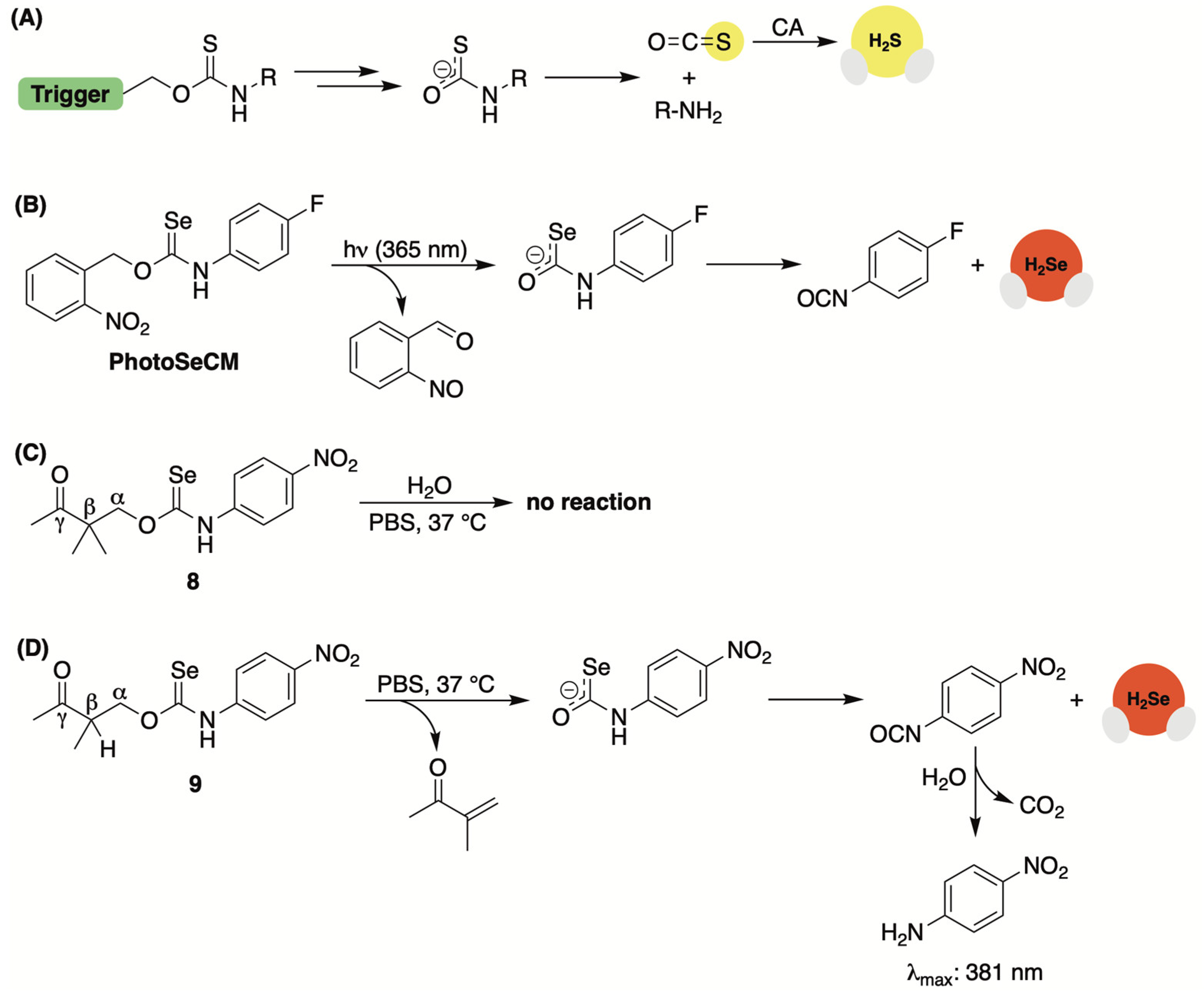
2.6. Selenocarbamates
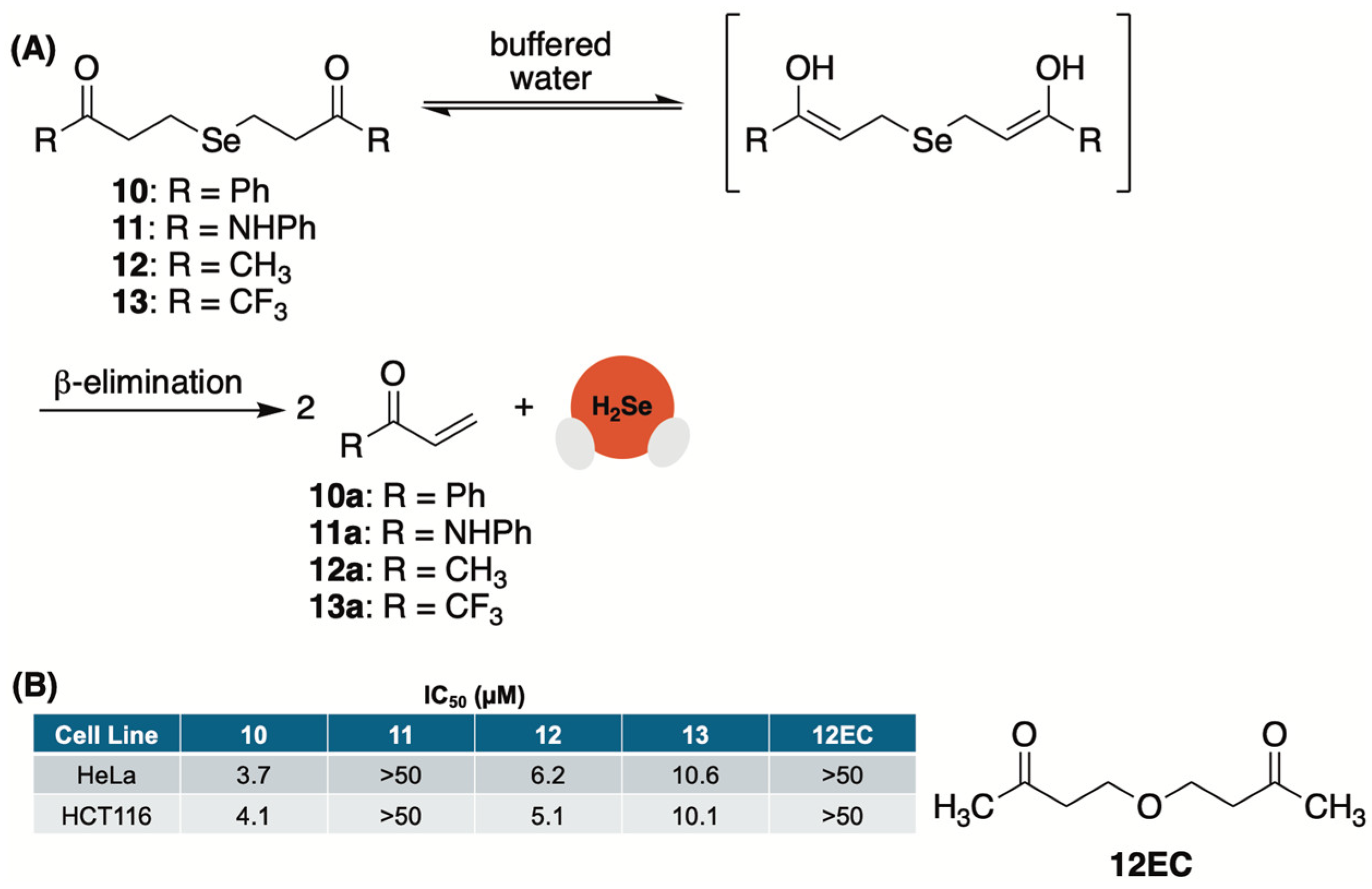
2.7. γ-Ketoselenides
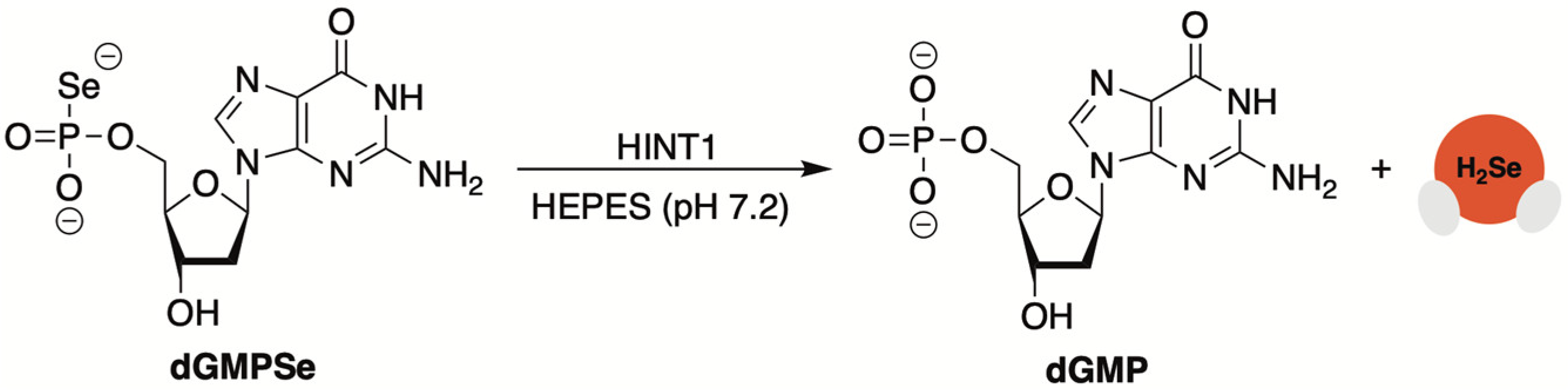
2.8. 5′-O-Selenophosphate Nucleosides
3. Chemical Tools for H2Se Detection
3.1. Nonspecific Electrophilic Traps: Dinitrofluorobenzene, Benzyl Bromide, and Iodoacetamide
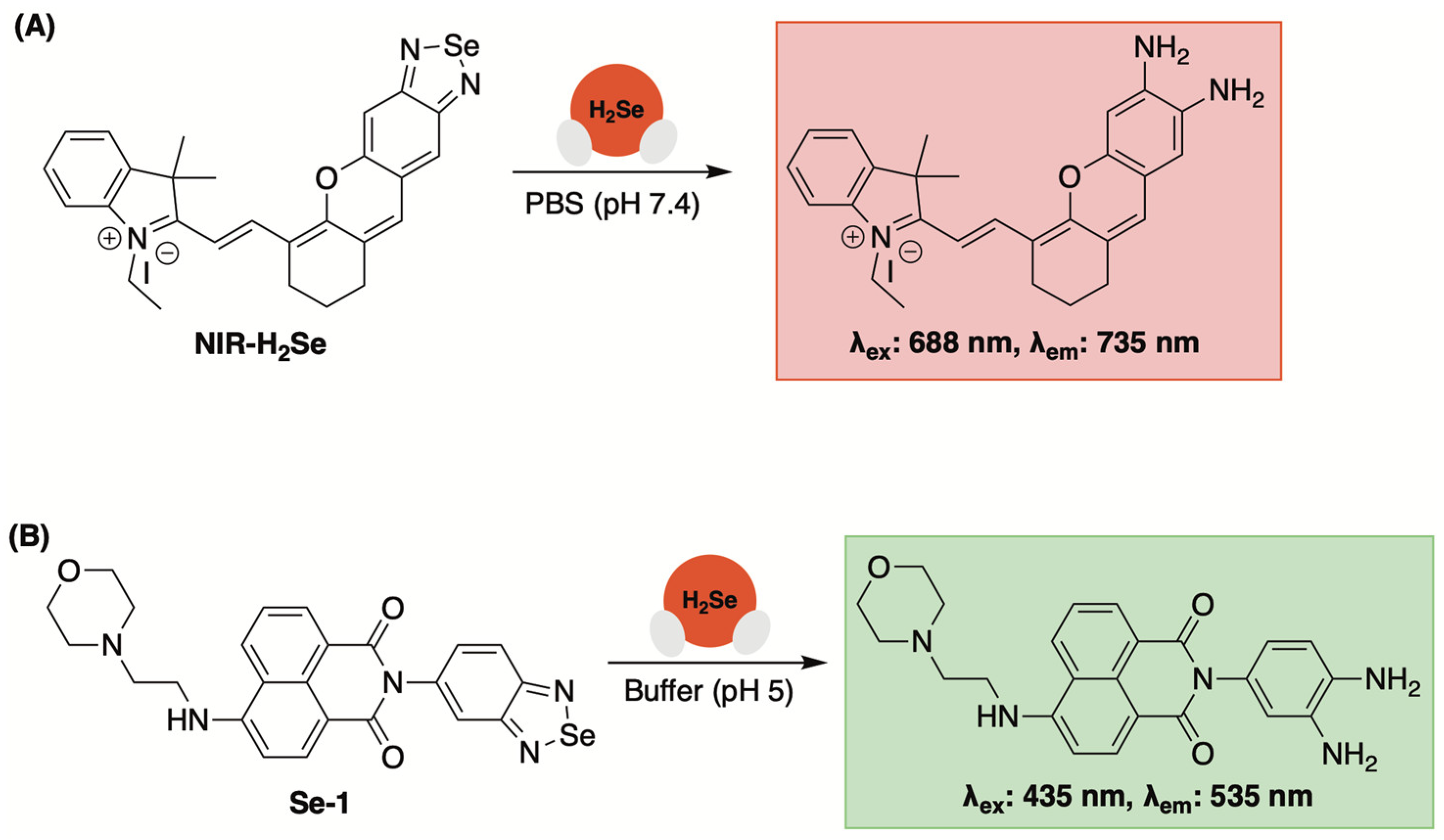
3.2. Fluorescent Sensors Based on Benzoselenadiazole Se–N Bond Cleavage
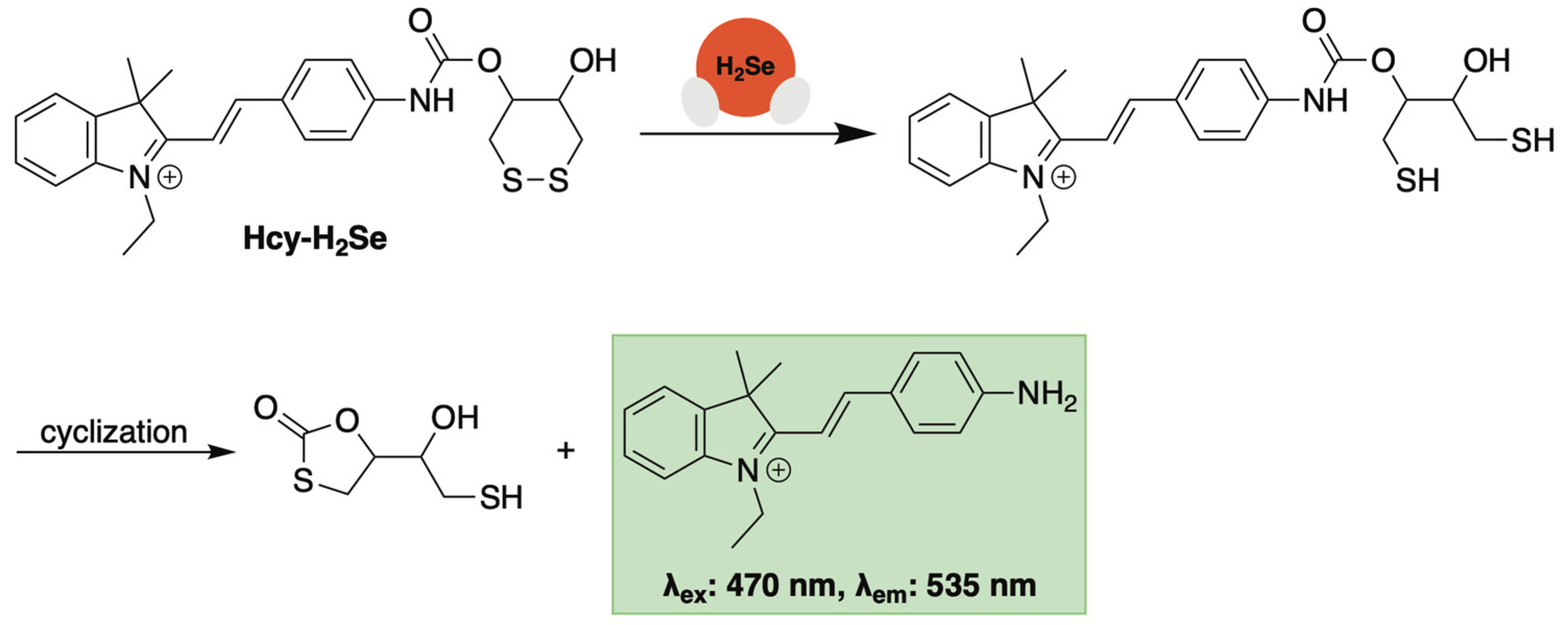
3.3. Fluorescent Sensors Based on Disulfide Bond Cleavage
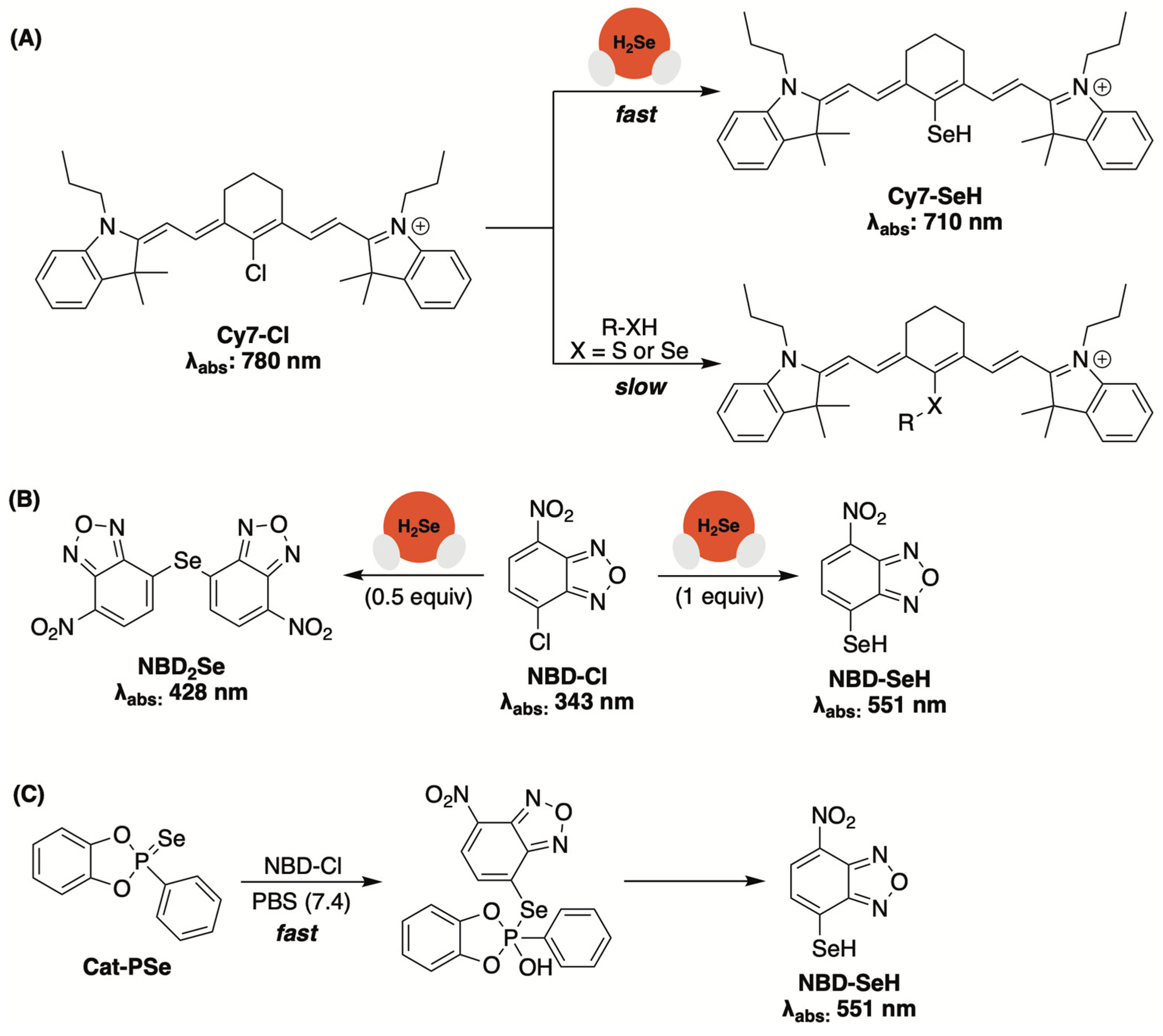
3.4. Spectroscopic Sensors Based on Nucleophilic Substitution
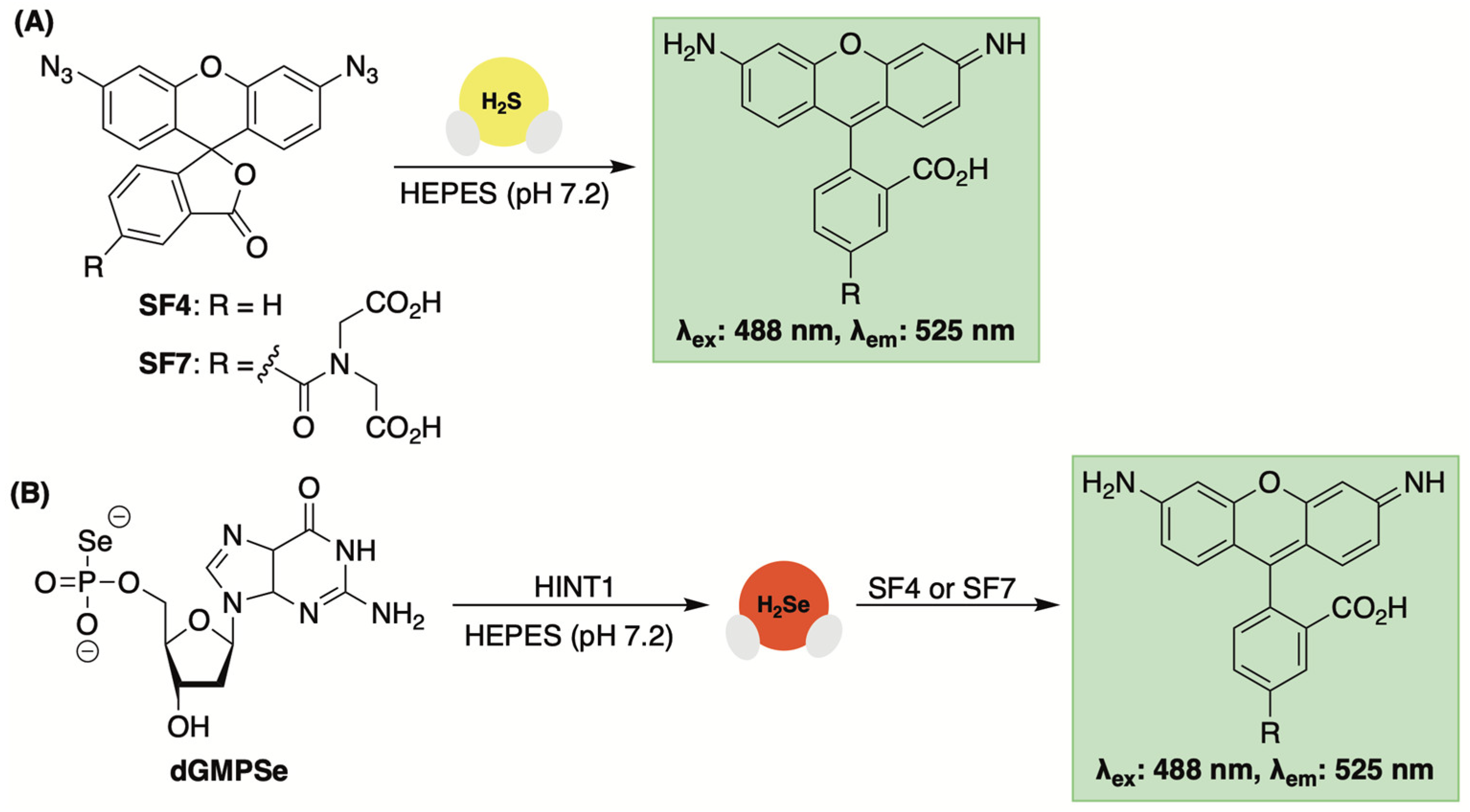
3.5. Fluorescent Sensors Based on Azide Reduction
4. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weeks, M.E. The Discovery of the Elements. VI. Tellurium and Selenium. J. Chem. Educ. 1932, 9, 474. [Google Scholar] [CrossRef]
- Levander, O.A. Scientific Rationale for the 1989 Recommended Dietary Allowance for Selenium. J. Am. Diet. Assoc. 1991, 91, 1572–1576. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Atkins, J.F.; Gesteland, R.F. The Twenty-First Amino Acid. Nature 2000, 407, 463–464. [Google Scholar] [CrossRef]
- Böck, A.; Forchhammer, K.; Heider, J.; Leinfelder, W.; Sawers, G.; Veprek, B.; Zinoni, F. Selenocysteine: The 21st Amino Acid. Mol. Microbiol. 1991, 5, 515–520. [Google Scholar] [CrossRef]
- Brown, K.M.; Arthur, J.R. Selenium, Selenoproteins and Human Health: A Review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Schomburg, L. Dietary Selenium and Human Health. Nutrients 2016, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. An Original Discovery: Selenium Deficiency and Keshan Disease (an Endemic Heart Disease). Asia Pac. J. Clin. Nutr. 2012, 21, 320–326. [Google Scholar] [PubMed]
- Shi, Y.; Yang, W.; Tang, X.; Yan, Q.; Cai, X.; Wu, F. Keshan Disease: A Potentially Fatal Endemic Cardiomyopathy in Remote Mountains of China. Front. Pediatr. 2021, 9, 576916. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, J.; Yang, B.; Qu, C.; Lei, J.; Han, J.; Guo, X. Serious Selenium Deficiency in the Serum of Patients with Kashin–Beck Disease and the Effect of Nano-Selenium on Their Chondrocytes. Biol. Trace Elem. Res. 2020, 194, 96–104. [Google Scholar] [CrossRef]
- Yao, Y.; Pei, F.; Kang, P. Selenium, Iodine, and the Relation with Kashin-Beck Disease. Nutrition 2011, 27, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.R.; McKenzie, R.C.; Beckett, G.J. Selenium in the Immune System. J. Nutr. 2003, 133, 1457S–1459S. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The Influence of Selenium on Immune Responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Al-Mubarak, A.A.; van der Meer, P.; Bomer, N. Selenium, Selenoproteins, and Heart Failure: Current Knowledge and Future Perspective. Curr. Heart Fail. Rep. 2021, 18, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Bomer, N.; Grote Beverborg, N.; Hoes, M.F.; Streng, K.W.; Vermeer, M.; Dokter, M.M.; IJmker, J.; Anker, S.D.; Cleland, J.G.F.; Hillege, H.L.; et al. Selenium and Outcome in Heart Failure. Eur. J. Heart Fail. 2020, 22, 1415–1423. [Google Scholar] [CrossRef]
- Rataan, A.O.; Geary, S.M.; Zakharia, Y.; Rustum, Y.M.; Salem, A.K. Potential Role of Selenium in the Treatment of Cancer and Viral Infections. Int. J. Mol. Sci. 2022, 23, 2215. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium in Cancer Prevention: A Review of the Evidence and Mechanism of Action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef]
- Sayehmiri, K.; Azami, M.; Mohammadi, Y.; Soleymani, A.; Tardeh, Z. The Association between Selenium and Prostate Cancer: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2018, 19, 1431–1437. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for Preventing Cancer. Cochrane Database Syst. Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef]
- Barceloux, D.G.; Barceloux, D. Selenium. J. Toxicol. Clin. Toxicol. 1999, 37, 145–172. [Google Scholar] [CrossRef]
- Hughes, M.N.; Centelles, M.N.; Moore, K.P. Making and Working with Hydrogen Sulfide. Free Radic. Biol. Med. 2009, 47, 1346–1353. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. (Eds.) CRC Handbook of Chemistry and Physics 2016–2017: A Ready-Reference Book of Chemical and Physical Data, 97th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-5429-3. [Google Scholar]
- Jiang, J.; Chan, A.; Ali, S.; Saha, A.; Haushalter, K.J.; Lam, W.-L.M.; Glasheen, M.; Parker, J.; Brenner, M.; Mahon, S.B.; et al. Hydrogen Sulfide—Mechanisms of Toxicity and Development of an Antidote. Sci. Rep. 2016, 6, 20831. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (U.S.) (Ed.) Acute Exposure Guideline Levels for Selected Airborne Chemicals; The Compass Series; National Academy Press: Washington, DC, USA, 2000; ISBN 978-0-309-07294-6. [Google Scholar]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen Sulfide and Cell Signaling. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef]
- Wang, R. Hydrogen Sulfide: The Third Gasotransmitter in Biology and Medicine. Antioxid. Redox Signal. 2010, 12, 1061–1064. [Google Scholar] [CrossRef]
- Wang, R. The Gasotransmitter Role of Hydrogen Sulfide. Antioxid. Redox Signal. 2003, 5, 493–501. [Google Scholar] [CrossRef]
- Miles, E.W.; Kraus, J.P. Cystathionine β-Synthase: Structure, Function, Regulation, and Location of Homocystinuria-Causing Mutations. J. Biol. Chem. 2004, 279, 29871–29874. [Google Scholar] [CrossRef]
- Pan, L.L.; Liu, X.H.; Gong, Q.H.; Yang, H.B.; Zhu, Y.Z. Role of Cystathionine γ-Lyase/Hydrogen Sulfide Pathway in Cardiovascular Disease: A Novel Therapeutic Strategy? Antioxid. Redox Signal. 2012, 17, 106–118. [Google Scholar] [CrossRef]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bian, J.-S. Hydrogen Sulfide: A Neuromodulator and Neuroprotectant in the Central Nervous System. ACS Chem. Neurosci. 2014, 5, 876–883. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The Possible Role of Hydrogen Sulfide as an Endogenous Neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Guo, W.; Kan, J.; Cheng, Z.; Chen, J.; Shen, Y.; Xu, J.; Wu, D.; Zhu, Y. Hydrogen Sulfide as an Endogenous Modulator in Mitochondria and Mitochondria Dysfunction. Oxidative Med. Cell. Longev. 2012, 2012, 878052. [Google Scholar] [CrossRef][Green Version]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen Sulfide Signaling in Mitochondria and Disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A Review of Hydrogen Sulfide (H2S) Donors: Chemistry and Potential Therapeutic Applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef]
- Xu, S.; Hamsath, A.; Neill, D.L.; Wang, Y.; Yang, C.; Xian, M. Strategies for the Design of Donors and Precursors of Reactive Sulfur Species. Chem. A Eur. J. 2019, 25, 4005–4016. [Google Scholar] [CrossRef] [PubMed]
- Levinn, C.M.; Cerda, M.M.; Pluth, M.D. Activatable Small-Molecule Hydrogen Sulfide Donors. Antioxid. Redox Signal. 2020, 32, 96–109. [Google Scholar] [CrossRef]
- Hu, Q.; Lukesh, J.C. H2S Donors with Cytoprotective Effects in Models of MI/R Injury and Chemotherapy-Induced Cardiotoxicity. Antioxidants 2023, 12, 650. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, A.; Foresti, R.; Ferdinandy, P. Pharmacology of the ‘Gasotransmitters’ NO, CO and H2S: Translational Opportunities. Br. J. Pharmacol. 2015, 172, 1395–1396. [Google Scholar] [CrossRef]
- Wang, R. Shared Signaling Pathways among Gasotransmitters. Proc. Natl. Acad. Sci. USA 2012, 109, 8801–8802. [Google Scholar] [CrossRef]
- Yang, G.; Sener, A.; Ji, Y.; Pei, Y.; Pluth, M.D. Gasotransmitters in Biology and Medicine: Molecular Mechanisms and Drug Targets. Oxidative Med. Cell. Longev. 2016, 2016, 4627308. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Two’s Company, Three’s a Crowd: Can H2S Be the Third Endogenous Gaseous Transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef]
- Kuganesan, M.; Samra, K.; Evans, E.; Singer, M.; Dyson, A. Selenium and Hydrogen Selenide: Essential Micronutrient and the Fourth Gasotransmitter? Intensive Care Med. Exp. 2019, 7, 71. [Google Scholar] [CrossRef]
- Samra, K.; Kuganesan, M.; Smith, W.; Kleyman, A.; Tidswell, R.; Arulkumaran, N.; Singer, M.; Dyson, A. The Pharmacology and Therapeutic Utility of Sodium Hydroselenide. Int. J. Mol. Sci. 2021, 22, 3258. [Google Scholar] [CrossRef]
- Tarze, A.; Dauplais, M.; Grigoras, I.; Lazard, M.; Ha-Duong, N.-T.; Barbier, F.; Blanquet, S.; Plateau, P. Extracellular Production of Hydrogen Selenide Accounts for Thiol-Assisted Toxicity of Selenite against Saccharomyces Cerevisiae. J. Biol. Chem. 2007, 282, 8759–8767. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Song, X.; Wang, C.; Cheng, T.; Luan, D.; Xu, K.; Tang, B. H2Se Induces Reductive Stress in HepG2 Cells and Activates Cell Autophagy by Regulating the Redox of HMGB1 Protein under Hypoxia. Theranostics 2019, 9, 1794–1808. [Google Scholar] [CrossRef]
- Iwata, A.; Morrison, M.L.; Blackwood, J.E.; Roth, M.B. Selenide Targets to Reperfusing Tissue and Protects It from Injury. Crit. Care Med. 2015, 43, 1361–1367. [Google Scholar] [CrossRef]
- Painter, E.P. The Chemistry and Toxicity of Selenium Compounds, with Special Reference to the Selenium Problem. Chem. Rev. 1941, 28, 179–213. [Google Scholar] [CrossRef]
- Ganther, H.E. Selenotrisulfides. Formation by the Reaction of Thiols with Selenious Acid. Biochemistry 1968, 7, 2898–2905. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Aoyama, E.; Kobayashi, N.; Tanaka, H.; Chikuma, M.; Sakurai, H.; Nakayama, M. Thiol Exchange Reactions Involving Selenotrisulfides. Biochem. Biophys. Res. Commun. 1988, 150, 1149–1154. [Google Scholar] [CrossRef]
- Haratake, M.; Ono, M.; Nakayama, M. Penicillamine Selenotrisulfide as a Selenium-Source in Mice. J. Health Sci. 2004, 50, 366–371. [Google Scholar] [CrossRef]
- Haratake, M.; Hongoh, M.; Miyauchi, M.; Hirakawa, R.; Ono, M.; Nakayama, M. Albumin-Mediated Selenium Transfer by a Selenotrisulfide Relay Mechanism. Inorg. Chem. 2008, 47, 6273–6280. [Google Scholar] [CrossRef]
- Liu, M.; Bu, F.; Li, G.; Xie, W.; Xu, H.; Wang, X. S-Se-S Type Molecule: A Bactericidal Promoter against H2S-Induced Antibiotic Resistance. Innov. Life 2024, 2, 100076. [Google Scholar] [CrossRef]
- Asfar, P.; Radermacher, P. Drug-Induced “Suspended Animation”: Can a Dream Become True? Crit. Care Med. 2015, 43, 1528–1530. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Gaseotransmitters: New Frontiers for Translational Science. Sci. Transl. Med. 2010, 2, 59ps54. [Google Scholar] [CrossRef]
- Klayman, D.L.; Griffin, T.S. Reaction of Selenium with Sodium Borohydride in Protic Solvents. A Facile Method for the Introduction of Selenium into Organic Molecules. J. Am. Chem. Soc. 1973, 95, 197–199. [Google Scholar] [CrossRef]
- Kharma, A.; Misak, A.; Grman, M.; Brezova, V.; Kurakova, L.; Baráth, P.; Jacob, C.; Chovanec, M.; Ondrias, K.; Domínguez-Álvarez, E. Release of Reactive Selenium Species from Phthalic Selenoanhydride in the Presence of Hydrogen Sulfide and Glutathione with Implications for Cancer Research. New J. Chem. 2019, 43, 11771–11783. [Google Scholar] [CrossRef]
- Domínguez-Álvarez, E.; Gajdács, M.; Spengler, G.; Palop, J.A.; Marć, M.A.; Kieć-Kononowicz, K.; Amaral, L.; Molnár, J.; Jacob, C.; Handzlik, J.; et al. Identification of Selenocompounds with Promising Properties to Reverse Cancer Multidrug Resistance. Bioorg. Med. Chem. Lett. 2016, 26, 2821–2824. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Álvarez, E.; Plano, D.; Font, M.; Calvo, A.; Prior, C.; Jacob, C.; Palop, J.A.; Sanmartín, C. Synthesis and Antiproliferative Activity of Novel Selenoester Derivatives. Eur. J. Med. Chem. 2014, 73, 153–166. [Google Scholar] [CrossRef]
- Gajdács, M.; Spengler, G.; Sanmartín, C.; Marć, M.A.; Handzlik, J.; Domínguez-Álvarez, E. Selenoesters and Selenoanhydrides as Novel Multidrug Resistance Reversing Agents: A Confirmation Study in a Colon Cancer MDR Cell Line. Bioorg. Med. Chem. Lett. 2017, 27, 797–802. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.-H.; Moore, P.K. Characterization of a Novel, Water-Soluble Hydrogen Sulfide–Releasing Molecule (GYY4137): New Insights into the Biology of Hydrogen Sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Newton, T.D.; Pluth, M.D. Development of a Hydrolysis-Based Small-Molecule Hydrogen Selenide (H2Se) Donor. Chem. Sci. 2019, 10, 10723–10727. [Google Scholar] [CrossRef]
- Newton, T.D.; Bolton, S.G.; Garcia, A.C.; Chouinard, J.E.; Golledge, S.L.; Zakharov, L.N.; Pluth, M.D. Hydrolysis-Based Small-Molecule Hydrogen Selenide (H2Se) Donors for Intracellular H2Se Delivery. J. Am. Chem. Soc. 2021, 143, 19542–19550. [Google Scholar] [CrossRef]
- Myhre, O.; Andersen, J.M.; Aarnes, H.; Fonnum, F. Evaluation of the Probes 2′,7′-Dichlorofluorescin Diacetate, Luminol, and Lucigenin as Indicators of Reactive Species Formation. Biochem. Pharmacol. 2003, 65, 1575–1582. [Google Scholar] [CrossRef]
- Kang, X.; Huang, H.; Jiang, C.; Cheng, L.; Sang, Y.; Cai, X.; Dong, Y.; Sun, L.; Wen, X.; Xi, Z.; et al. Cysteine-Activated Small-Molecule H2Se Donors Inspired by Synthetic H2S Donors. J. Am. Chem. Soc. 2022, 144, 3957–3967. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Pugliesi, I.; Barresi, E.; Nesi, G.; Rapposelli, S.; Taliani, S.; Da Settimo, F.; et al. Arylthioamides as H2S Donors: L-Cysteine-Activated Releasing Properties and Vascular Effects In Vitro and In Vivo. ACS Med. Chem. Lett. 2013, 4, 904–908. [Google Scholar] [CrossRef]
- Hu, Q.; Suarez, S.I.; Hankins, R.A.; Lukesh, J.C. Intramolecular Thiol- and Selenol-Assisted Delivery of Hydrogen Sulfide. Angew. Chem. Int. Ed. 2022, 61, e202210754. [Google Scholar] [CrossRef]
- Hu, Q.; Zhu, C.; Hankins, R.A.; Murmello, A.R.; Marrs, G.S.; Lukesh, J.C. An ROS-Responsive Donor That Self-Reports Its H2S Delivery by Forming a Benzoxazole-Based Fluorophore. J. Am. Chem. Soc. 2023, 145, 25486–25494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pluth, M.D. Hydrogen Sulfide Donors Activated by Reactive Oxygen Species. Angew. Chem. Int. Ed. 2016, 55, 14638–14642. [Google Scholar] [CrossRef]
- Zhao, Y.; Henthorn, H.A.; Pluth, M.D. Kinetic Insights into Hydrogen Sulfide Delivery from Caged-Carbonyl Sulfide Isomeric Donor Platforms. J. Am. Chem. Soc. 2017, 139, 16365–16376. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Jos, S.; Chakrapani, H. Reactive Oxygen Species-Triggered Tunable Hydrogen Sulfide Release. Org. Lett. 2018, 20, 3766–3770. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Fang, Y.; Shi, W.; Li, X.; Chen, W.; Xian, M.; Ma, H. Reactive Oxygen Species-Triggered off-on Fluorescence Donor for Imaging Hydrogen Sulfide Delivery in Living Cells. Chem. Sci. 2019, 10, 7690–7694. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Suarez, S.I.; Lukesh, J.C. Illuminating and Alleviating Cellular Oxidative Stress with an ROS-Activated, H2S-Donating Theranostic. Tetrahedron Lett. 2021, 69, 152944. [Google Scholar] [CrossRef]
- Zhao, Y.; Bolton, S.G.; Pluth, M.D. Light-Activated COS/H2S Donation from Photocaged Thiocarbamates. Org. Lett. 2017, 19, 2278–2281. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Nair, M.; Chauhan, P.; Gupta, K.; Saini, D.K.; Chakrapani, H. Visible-Light-Triggered Uncaging of Carbonyl Sulfide for Hydrogen Sulfide (H2S) Release. Org. Lett. 2017, 19, 4822–4825. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.K.; Zhao, Y.; Otteson, C.E.; Pluth, M.D. Development of Acid-Mediated H2S/COS Donors That Respond to a Specific pH Window. J. Org. Chem. 2019, 84, 14469–14475. [Google Scholar] [CrossRef]
- Zhao, Y.; Steiger, A.K.; Pluth, M.D. Colorimetric Carbonyl Sulfide (COS)/Hydrogen Sulfide (H2S) Donation from γ-Ketothiocarbamate Donor Motifs. Angew. Chem. Int. Ed. 2018, 57, 13101–13105. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Bora, P.; Ravikumar, G.; Jos, S.; Chakrapani, H. Esterase Activated Carbonyl Sulfide/Hydrogen Sulfide (H2S) Donors. Org. Lett. 2017, 19, 62–65. [Google Scholar] [CrossRef]
- Steiger, A.K.; Marcatti, M.; Szabo, C.; Szczesny, B.; Pluth, M.D. Inhibition of Mitochondrial Bioenergetics by Esterase-Triggered COS/H2S Donors. ACS Chem. Biol. 2017, 12, 2117–2123. [Google Scholar] [CrossRef]
- Steiger, A.K.; Zhao, Y.; Pluth, M.D. Emerging Roles of Carbonyl Sulfide in Chemical Biology: Sulfide Transporter or Gasotransmitter? Antioxid. Redox Signal. 2018, 28, 1516–1532. [Google Scholar] [CrossRef] [PubMed]
- Newton, T.D.; Li, K.; Sharma, J.; Champagne, P.A.; Pluth, M.D. Direct Hydrogen Selenide (H2Se) Release from Activatable Selenocarbamates. Chem. Sci. 2023, 14, 7581–7588. [Google Scholar] [CrossRef] [PubMed]
- Hankins, R.A.; Carter, M.E.; Zhu, C.; Chen, C.; Lukesh, J.C. Enol-Mediated Delivery of H2Se from γ-Keto Selenides: Mechanistic Insight and Evaluation. Chem. Sci. 2022, 13, 13094–13099. [Google Scholar] [CrossRef] [PubMed]
- Ozga, M.; Dolot, R.; Janicka, M.; Kaczmarek, R.; Krakowiak, A. Histidine Triad Nucleotide-Binding Protein 1 (HINT-1) Phosphoramidase Transforms Nucleoside 5′-O-Phosphorothioates to Nucleoside 5′-O-Phosphates. J. Biol. Chem. 2010, 285, 40809–40818. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, A.; Kaczmarek, R.; Baraniak, J.; Wieczorek, M.; Stec, W.J. Stereochemistry of rHint1 Hydrolase Assisted Cleavage of P–N Bond in Nucleoside 5′-O-Phosphoramidothioates. Chem. Commun. 2007, 2163–2165. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, A.; Czernek, L.; Pichlak, M.; Kaczmarek, R. Intracellular HINT1-Assisted Hydrolysis of Nucleoside 5′-O-Selenophosphate Leads to the Release of Hydrogen Selenide That Exhibits Toxic Effects in Human Cervical Cancer Cells. Int. J. Mol. Sci. 2022, 23, 607. [Google Scholar] [CrossRef] [PubMed]
- Guga, P.; Maciaszek, A.; Stec, W.J. Oxathiaphospholane Approach to the Synthesis of Oligodeoxyribonucleotides Containing Stereodefined Internucleotide Phosphoroselenoate Function. Org. Lett. 2005, 7, 3901–3904. [Google Scholar] [CrossRef]
- Lin, V.S.; Lippert, A.R.; Chang, C.J. Cell-Trappable Fluorescent Probes for Endogenous Hydrogen Sulfide Signaling and Imaging H2O2-Dependent H2S Production. Proc. Natl. Acad. Sci. USA 2013, 110, 7131–7135. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Ge, L.; Pan, X.; Xu, K.; Liu, X.; Tang, B. A Highly Selective Near-Infrared Fluorescent Probe for Imaging H2Se in Living Cells and In Vivo. Chem. Sci. 2016, 7, 1051–1056. [Google Scholar] [CrossRef]
- Tian, Y.; Xin, F.; Jing, J.; Zhang, X. Fluorescence Imaging of Lysosomal Hydrogen Selenide under Oxygen-Controlled Conditions. J. Mater. Chem. B 2019, 7, 2829–2834. [Google Scholar] [CrossRef]
- Choi, N.-E.; Lee, J.-Y.; Park, E.-C.; Lee, J.-H.; Lee, J. Recent Advances in Organelle-Targeted Fluorescent Probes. Molecules 2021, 26, 217. [Google Scholar] [CrossRef]
- Kong, F.; Zhao, Y.; Liang, Z.; Liu, X.; Pan, X.; Luan, D.; Xu, K.; Tang, B. Highly Selective Fluorescent Probe for Imaging H2Se in Living Cells and In Vivo Based on the Disulfide Bond. Anal. Chem. 2017, 89, 688–693. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, Z.; Lim, C.W.; Lee, Y.H.; Dongbang, S.; Kang, C.; Kim, J.S. Disulfide-Cleavage-Triggered Chemosensors and Their Biological Applications. Chem. Rev. 2013, 113, 5071–5109. [Google Scholar] [CrossRef] [PubMed]
- Cleland, W.W. Dithiothreitol, a New Protective Reagent for SH Groups. Biochemistry 1964, 3, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Montoya, L.A.; Pearce, T.F.; Hansen, R.J.; Zakharov, L.N.; Pluth, M.D. Development of Selective Colorimetric Probes for Hydrogen Sulfide Based on Nucleophilic Aromatic Substitution. J. Org. Chem. 2013, 78, 6550–6557. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Lippert, A.R.; Chang, C.J. Azide-Based Fluorescent Probes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 554, pp. 63–80. ISBN 978-0-12-801512-4. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hankins, R.A.; Lukesh, J.C. An Examination of Chemical Tools for Hydrogen Selenide Donation and Detection. Molecules 2024, 29, 3863. https://doi.org/10.3390/molecules29163863
Hankins RA, Lukesh JC. An Examination of Chemical Tools for Hydrogen Selenide Donation and Detection. Molecules. 2024; 29(16):3863. https://doi.org/10.3390/molecules29163863
Chicago/Turabian StyleHankins, Rynne A., and John C. Lukesh. 2024. "An Examination of Chemical Tools for Hydrogen Selenide Donation and Detection" Molecules 29, no. 16: 3863. https://doi.org/10.3390/molecules29163863
APA StyleHankins, R. A., & Lukesh, J. C. (2024). An Examination of Chemical Tools for Hydrogen Selenide Donation and Detection. Molecules, 29(16), 3863. https://doi.org/10.3390/molecules29163863






