Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview
Abstract
1. Introduction
Biosynthetic Pathways
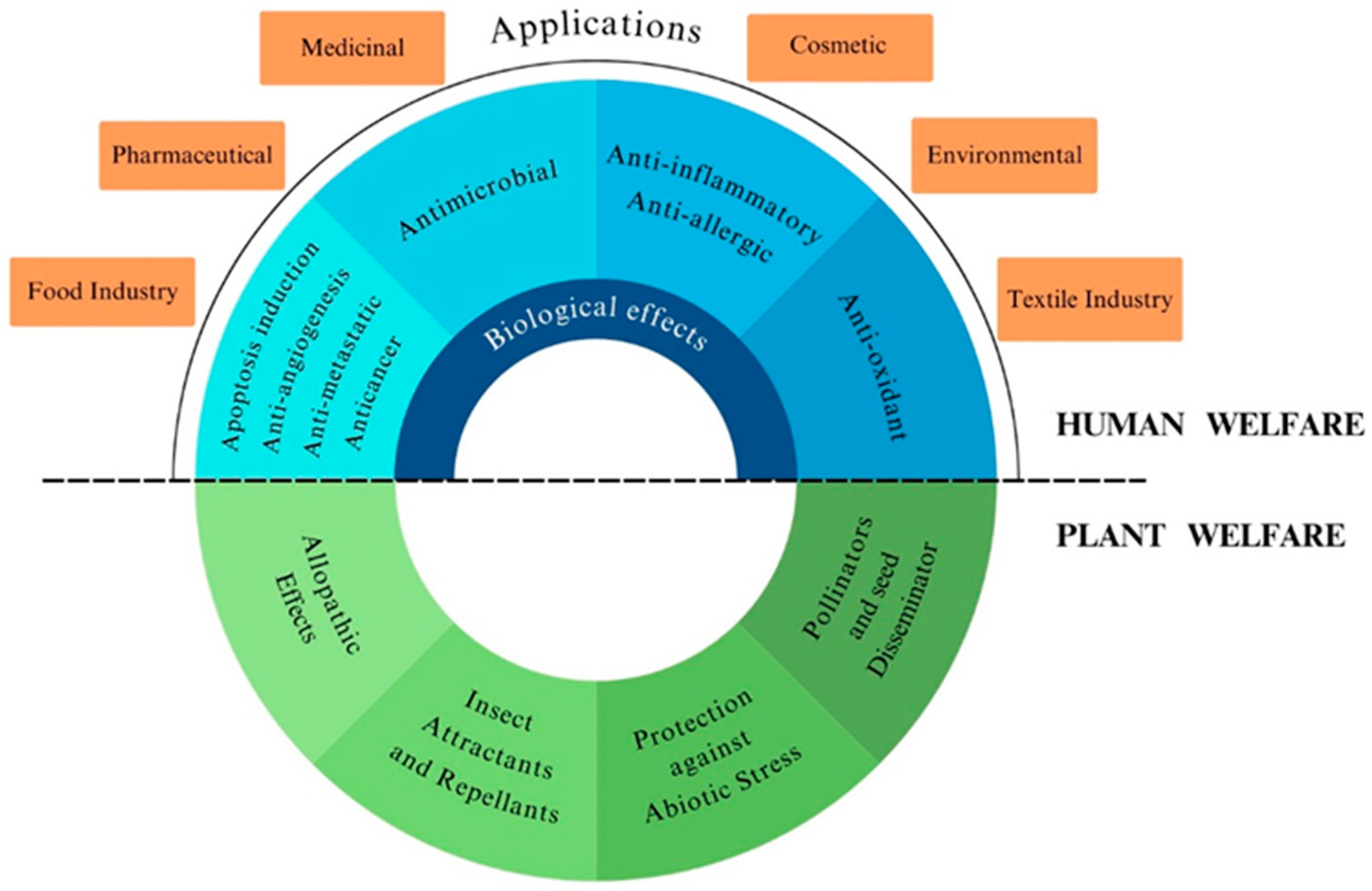
2. Terpenoids as Semiochemicals
2.1. Insect–Plant Activity
2.1.1. Terpenoids with Toxic or Repellent Properties
2.1.2. Terpenoids as Attractants
| Plant Species | Terpene ID and Class | Target | Mechanism and Effect | Ref. |
|---|---|---|---|---|
| Dalechampia (Euphorbiaceae) | Oxygenated terpenoid resins | Female euglossine (Aphidae), female anthidiine (Megachilidae) bees | Reward to pollinators | [25,26] |
| Fig (Ficus hispida) | Linalool; limonene and β-pinene (monoterpenes) | Wasp (Ceratosolen solmsi marchali) | Signaling pollinators | [27] |
| Sweet rocket (Hesperis matronalis) | Linalool; β-ocimene (monoterpenes) | Mainly syrphid flies (Syrphidade) | Attractant to pollinators | [28] |
| Cabbage (Brassica species) | 1,8-cineole (monoterpenes) | Parasitic wasps (Cotesia glomerata) | Attractant to parasitoids that lay eggs in herbivores larvae | [23] |
| Various plant species | (Z)-3-hexenyl acetate; (Z)-3-hexenol; (3E)-4,8-dimethyl-1,3,7-nonatriene; and linalool | Pest predator (Chrysopa phyllochroma) | Attractant to predators and promotes oviposition | [29] |
| Tomato (Solanum lycopersicum) and tobacco (Nicotiana tabacum) | Β-ocimene (monoterpenes) | Parasitoid (Aphidius ervi) Pest (Macrosiphum euphorbiae) | Attractant to parasitoids and defense against pest | [30] |
| Lavender (Lavandula angustifolia) | Β-trans-ocimene; (+)-R-limonene (monoterpenes) | Aphids (Aphidoidea family) | Pest deterrent | [31] |
| Cinnamon (Cinnamomum genus) and Clove (Syzygium aromaticum) | Eugenol; caryophyllene oxide; α-pinene; α-humulene and α-phellandrene (monoterpenes) | Wheat weevil (Sitophilus granaries) | Toxic and repellent effects on adult specimens | [32] |
| Eucalyptus (Eucalyptus grandis) | A-pinene; γ-terpinene (monoterpenes) | Eucalyptus gall wasp (Leptocybe invasa) | Attractant to pest | [24] |
| Various plant species | Geraniol (terpenoid) | Sweet potato whitefly (Bemisia tabaci) | Encapsulated geraniol shows attraction to that specimen | [33] |
2.2. Antifungal Activity
3. Terpenoids iR1n Human Health
3.1. Terpenoids on Cancer Prevention
3.2. Terpenoids in the Prevention of Cardiovascular Diseases
3.3. Neuroprotective Effects of Terpenoids
| Terpenoid | Assay | Reported Activities | Ref. |
|---|---|---|---|
| Artesunate | C57BL/6 mice (newborn) and primary neural stem/progenitor cells (NSPCs) | Ameliorated the insufficient endogenous neural stem/progenitor cell (NSPC) proliferation caused by ischemia. By stimulating the PI3K/Akt signaling pathway, ART could increase the phosphorylation level of FOXO-3a, downregulate p27kip1, and inhibit the transcription of FOXO-3a. | [124] |
| Asiatic acid | Neuronally differentiated PC12 cells | Protection against Aβ25-35-induced apoptosis and tau hyperphosphorylation by regulating PI3K/Akt/GSK-3β signaling. | [125] |
| Asiaticoside | Streptozotocin (STZ)-induced diabetic cognitive deficit rat model | Ameliorated cerebral oxidative stress, inflammation, and apoptosis. | [126] |
| Catalpol | Streptozotocin-induced hyperglycemic mice | Antioxidant and neuroprotective effects on mouse models of depression, improving their depressive behavior by upregulating the PI3K/Akt/Nrf2/HO-1 signaling pathway. | [127] |
| Stroke model and the primary neurons from the rat stroke model | Catalpol activated the PI3K/Akt/mTOR pathway, decreasing the expression of miR-124 and increasing the expression of downstream protein S6, thus enhancing in vivo axon growth and neuronal survival in stroke models. | [128] | |
| Celastrol | Acute spinal cord injury rats | Inhibited microglial pyroptosis and attenuated inflammatory reactions. | [129] |
| Geniposide | Epileptic rats model | Activated Akt, followed by increased PI3K and GSK-3β expression, thus improving pathological symptoms. | [130] |
| Hippocampal neurons | Inhibited apoptosis, resulting in antidepressant properties in the brain. | [131] | |
| Chronic constriction injury model of neuropathic pain | Inhibited the EGFR/PI3K/Akt signaling pathway, thereby alleviating pain symptoms in the sciatic nerve. | [132] | |
| Bilobalide and ginkgolides | Rat model of middle cerebral artery occlusion | Supported neuronal cell survival in patients suffering from ischemic stroke. | [133] |
| PC12 neuronal cells | Bilobalide derivatives (diAc-iso and diBrBn-iso) performed better than the original compound (proliferating cell activity, neuroprotective effects against Aβ (1–40) peptides, and neurite outgrowth effects). | [134] | |
| Ginkgolide B | Rats cerebral I/R damage model | Activation of Nrf2 and CREB through PI3K/Akt signaling. | [135] |
| Rat model of middle cerebral artery occlusion (MCAO) and OGD/R cell model | Antioxidant effects against cerebral ischemia injury by activating the Akt/Nrf2 pathway. | [136] | |
| Ginkgolide K | Primary cortical astrocytes from newborn mice exposed to oxygen–glucose deprivation | Superior therapeutic potential to ginkgolide B; easier to upregulate PI3K and p-Akt expression, affecting downstream pathways, thereby contributing to anti-inflammatory and antioxidant effects. | [137] |
| Echinocystic acid | Collagenase-induced intracerebral haemorrhage mice | Neuroprotective effect via the PI3K/Akt pathway. | [138] |
| Methyl lucidone | HT-22 cell line | Neuroprotective effects on glutamate-induced oxidative stress in HT-22 cells via Nrf-2/HO-1 signaling. | [139] |
| Limonene | Maternal separation mice | Antidepressant-like effects due to the reduction of nitrite levels in the hippocampus. | [140] |
| Lycopene | Primary mouse neurons | Protected against T-BHP-induced neuronal oxidative damage and apoptosis via activation of the PI3K/Akt pathway. | [141] |
| Platycodin D | Primary cortical neurons | Protected cortical neurons against oxygen–glucose deprivation/reperfusion in neonatal hypoxic–ischemic encephalopathy. | [142] |
| Polygalasaponin F | - | Glutamate-induced cytotoxicity cell model/protects hippocampal neurons against glutamate-induced cytotoxicity. | [143] |
| Rat adrenal pheochromocytoma cells (PC12) and primary rat cortical neurons | Inhibited neuronal apoptosis induced by oxygen–glucose deprivation and reoxygenation through the PI3K/Akt pathway. | [144] | |
| Ginsenosides | Oxygen–glucose deprived (OGD) SH-SY5Y cells | Neuroprotective effect of panax notoginseng saponins by activating the EGFR/PI3K/Akt pathway. | [145] |
| Mouse model | Protective effects of notoginsenoside R1 via regulation of the PI3K-Akt-mTOR/JNK pathway in neonatal cerebral hypoxic–ischemic brain injury. | [146] | |
| α-Pinene | Focal cerebral I/R in rats | Neuroprotective effect during ischemic stroke by attenuating neuroinflammation/ELISA. | [147] |
4. Industrial Applications of Terpenoids
4.1. Pharmaceutical and Medical Industry
4.2. Cosmetics Industry
4.3. Food Industry
5. Green Emerging Extraction Techniques
5.1. Pressurized Liquid Extraction (PLE)
| Matrix | Extraction Conditions | Main Conclusions | Ref. |
|---|---|---|---|
| Pressurized liquid extraction (PLE) | |||
| Orange juice by-products | Amount of 4 g orange powder residue + 8 g sea sand (1:2 w/w) placed into the extraction cell; 25 mL ethyl acetate; 96 °C, 30 min, 10 MPa, on static mode; 1 min of N2 purging; extracts stored in −20 °C in dark before drying; GC-q-TOF-MS analysis. | Terpenoids revealed promising neuroprotective action. Antioxidant activity: ABTSIC50 = 13.5 μg/mL; ROSIC50 = 4.4 μg/mL. Anticholinesterase activity: AChEIC50 = 137.1 vg /L; BChEIC50 = 147.0 μg/mL. Anti-inflammatory properties: against IL-6 and LOXIC50 = 76.1 μg/mL, with low cytotoxicity and protection against L-glutamic acid in cell models. | [182] |
| Microalgae Spirulina, Chlorella, and Phaeodactylum tricornutum | Microalgae and diatomaceous earth completely mixed (0.5 g: 1.5 g) in a mortar and placed into PLE extraction tank ASE-200 Accelerated Solvent Extractor (preheating for 1 min, heating time of 5 min, flush volume 60%, N2 for 60 s, 103.4 bars, 40 °C, 15 min); 100% DMSO; HPLC analysis. | The authors found that 100% DMSO allowed for the extraction of antioxidants and pigments from Chlorella (polyphenols 10.465 mg/g, chlorophyll a 6.206 mg/g, chlorophyll b 3.003 mg/g, carotenoids 0.971 mg/g) and was thus the chosen concentration for the recovery studies on Spirulina, Chlorella, and Phaeodactylum tricornutum. Fucoxanthin, β-carotene, zeaxanthin, and lutein were the main carotenoids found in P. tricornutum, Spirulina, and Chlorella, respectively. | [183] |
| Static headspace (HS) extraction | |||
| Cinnamon, thyme, cumin, fennel, clove, nutmeg, and orange | Amount of 20 mg of spice or 2 g of orange peels placed in a 20 mL headspace vial; 125 °C, 30 min, 250 rpm; Combi-pal + automatic HS injector; GC-MS analysis (1 mL); HS syringe heated at 130 °C. | Static HS extraction allowed for the recovery of extracts with higher concentrations in comparison with hydrodistillation and PLE. For example, eugenol LOD: Static HS: 0.0022 µg/g; PLE: 0.03 µg/g. | [176] |
| Cannabis | A total of 5 mg powder samples placed in a 20 mL amber rounded bottom HS vial; CTC autosampler used with an HS static tool in splitless mode; 40 min, 140 °C, 250 rpm; GC-MS/MS analysis (600 μL). | Ninety-three terpenoids were identified. Sample preparation methods significantly impacted the chemical fingerprint of the samples when compared to non-treated Cannabis. Static HS extraction allowed for the quantification of natural terpenoid contents of chemovars. | [184] |
| Citrus leaves | A total of 1 g powder sample placed in a 20 mL HS vial + 30 μL internal standard (0.1% n-hexanol); sealed vials mixed thoroughly before being placed on a static 7697A HS auto-sampler, awaiting injection; 15 min incubation at 100 °C; GC-MS analysis. | A total of 83 volatile metabolites were identified, including monoterpene hydrocarbons (68.23–95.08%, 21 compounds), alcohols (0.69–26.0%, 8 compounds), sesquiterpene hydrocarbons (0.47–5.04%, 26 compounds), aldehydes (0.12–11.26%, 10 compounds), monoterpenoids (0–0.36%, 7 compounds), esters (0–0.18%, 5 compounds), ketones (0–0.02%, 2 compounds), and miscellaneous compounds (0–1.11%, 4 compounds). | [185] |
| Microwave-assisted hydrodistillation (MAHD) | |||
| Hop (Humulus lupulus L.) | MAHD was carried out using ETHOS X and ETHOS XL extractors; GC-MS analysis. | The highest extraction yield was obtained for fresh hops (20.5 mLVF/kgdry matrix). When 3 kg of the sample were used, this value achieved a value of 17.3 mLVF/kgdry matrix. In a pilot reactor (30 kg capacity), high yield increases were seen for pelletized and dried samples in quadruple and double the lab-scale yields, respectively. | [186] |
| Sage herbal dust | Amount of 40 g dry plant material + 400 mL distilled water; MAHD performed in the oven (90, 180, 360, 600, and 800 W) for 2 h; water–oil mixture evaporated through glass pipe connector to be condensed in Unger apparatus; essential oils collected and stored at 4 °C until analysis; GC-MS analysis. | A total of 55 terpenoids were identified. The main compounds in the essential oils are obtained via the following methods: Hydrodistillation—viridiflorol (21%), camphor (16.54–19.05%), and α-thujone (11%); MAHD at 90W—camphor (24.88%), α-thujone (22.21%), and eucalyptol (18.37%); MAHD at 360W—viridiflorol (33.27%) and verticiol (13.71%) (in other MAHD samples, viridiflorol (17.17–23.7%) and camphor (14.46–18.82%)). | [187] |
| Withered flowers of Magnolia soulangeana Soul.-Bod. | MAHD with uniform heating (623 W, 54 min, 60 r/min); 50 g soaked raw materials + distilled water in a 500 mL distillation flask (6.4 mL/g liquid–solid ratio); withered flowers soaked for 8 h before essential oil preparation; anhydrous sodium sulfate added to remove the moisture; sample transferred to a low-temperature environment (4 °C ± 2 °C) for storage; GC-MS analysis. | The introduction of the rotation unit and soaking pretreatment unit increased the yield of essential oil by 16.67% and 20%, respectively. This method showed a lower energy consumption and environmental pressure than conventional approaches for essential oil preparation. The samples obtained were rich in terpenes (49.32%), such as eucalyptol, δ-cadinene, α-muurolene, and germacrene D. δ-cadinene was the main compound to exert hypolipidemic activity. | [188] |
| Lavenders (Lavandula x intermedia var. Super A) | Dried lavenders grinded at 6000 rpm for 10 s and subjected to soaking (1:10, w/v) for 1 h before extraction process. Essential oils of dried lavender extracted via MAHD (ETHOS X) at 750 W for 2 h; GC-MS analysis. | Lavender essential oil yield was around 5.5%. Based on the GC-MS data, major constituents of linalool L (29.0%), 1,8-cineole (13.9%), camphor (12.3%), and linalyl acetate (11.9%) were the main compounds identified. | [189] |
| Peppermint | Amount of 40 g dry plant material + 400 mL distilled water; MAHD performed in the oven (180, 360, 600, and 800 W) for 2 h; water–oil mixture evaporated through glass pipe connector to be condensed in Unger apparatus; essential oils collected and stored at −18 °C until analysis; GC-MS analysis. | Monoterpenes were the main class of compounds in all samples with menthol (33.07–37.43%), menthone (9.49–25.21%), isomenthol (4.27–10.21%), isomenthone (4.51–6.06%), and eucalyptol (1.16–4.89%). Sesquiterpenes were also predominant with trans-caryophyllene (4.58–10.56%) and germacrene D (2.65–7.65%). | [190] |
| Supercritical fluid extraction (SFE) | |||
| Flesh and peels of 15 matrices of vegetables | Amount of 5 g freeze-dried samples + 95 g inert glass beads; 15 g/min CO2; 30 min, 59 °C, 350 bar, 15.5% (v/v) ethanol as co-solvent; extracts collected and dissolved in ethanol and stored at −18 °C in dark glass containers until analysis; HPLC analysis. | TCR values higher than 90% w/w for most samples. β-carotene was the most successfully extracted compound (TCRs 88–100% w/w). More polar carotenoids, such as lutein and lycopene, exhibited lower TCRs. | [191] |
| Mango peel | Amount of 5 g mango peel + 6.7 g/min CO2; 180 min, 60 °C, 25 MPa, 15.5% (w/w) ethanol as co-solvent; after extraction, remanent ethanol evaporated under vacuum (35 °C, 100 mBar); dried extracts stored at −20 °C until analysis; RP-UHPLC-DAD analysis. | The extracts provided better protection to sunflower oil against lipid oxidation than all-trans-β-carotene when evaluated between 200–1000 ppm, which contained 6–28 ppm of all-trans-β-carotene. | [192] |
| Annatto seeds | Two-step sequential SFE extraction:1st step: 60 °C, 10 MPa, 290 kg/m3 CO2 to recover the geranylgeraniol-rich fraction; 2nd step: 40 °C, 20 MPa, 840 kg/m3 CO2 to recover the tocotrienols-rich fraction. Amount of 50 g annatto seeds packed in the extraction vessel + empty space filled with glass beads; 9.5 g/min CO2. | Different operational extraction conditions (temperature and pressure) resulted in extracts with different chemical compositions. The extract obtained at low CO2 density (290 kg/m3) produced a fraction enriched in geranylgeraniol with a low tocotrienols content. A two-step sequential SFE extraction process was employed to obtain a geranylgeraniol-rich fraction followed by a tocotrienols-rich fraction. | [193] |
| Carrot peels and flesh | Amont of 5 g dried peels + 95 g inert glass beads; 80 min, 59 °C, 349 bar; 15 g/min CO2; ethanol as co-solvent (15.5%); HPLC analysis. | β-Carotene represented 60% of the TCC in both flesh and peel, followed by α-carotene (30% of the TCC in both samples). In the peels, these two carotenoids accounted for almost 95% of TCC. Lycopene and lutein were also identified (1.9–30.2 μg/g). The optimum extraction conditions allowed for a carotenoid recovery of 86.1%. At 58.5 °C, 306 bar, and 14.3% ethanol, the processes retrieved maximum mass yield (5.31%, d.b.). | [192] |
| Leaves of Piper klotzschianum | Aount of 20 g leaves + the remaining extraction cell space filled with inert glass beads; after reaching 79.85 °C, the pump and extractor were simultaneously pressurized (220 bar); system left at rest to reach equilibrium (30 min); extraction was then performed up to 280 min; GC-MS analysis. | At optimum conditions, the highest extraction yield was 1.36%. The addition of organic co-solvents (5% of methanol) significantly improved the extraction yield to 2.18%, representing an increase of 40% compared to extraction using CO2 alone. | [194] |
| Caraway seeds | SWE: 1 g caraway + 2 g diatomaceous earth + 2 cellulose filter papers; sample cell placed in the oven; pump delivered solvent to the sample; cell heated to the set temperature under high pressure, and the extraction was performed for the designated time; after extraction, solvent purged out of the cell using N2 gas; extract collection. LLE: water extract + 20 mL n-hexane. Centrifugation (5 min); obtained extract stirred briefly and centrifuged (5 min); n-hexane transferred to an empty conical tube and stored in the freezer; GC and GC-MS analysis. | In SWE, smaller amounts of terpenes (limonene, carveol, and carvone) were found. The limonene concentration was higher for hydrodistillation (5 mg/gcaraway) than for SWE. The carvone yield was higher when using SWE (28.5 mg/gcaraway) than for solvent extraction (20.2 mg/gcaraway) and hydrodistillation (19.8 mg/gcaraway). | [195] |
5.2. Static Headspace (HS) Extraction
5.3. Microwave-Assisted Hydrodistillation (MAHD)
5.4. Supercritical Fluid Extraction (SFE)
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adefegha, S.A.; Oboh, G.; Oluokun, O.O. Food Bioactives: The Food Image behind the Curtain of Health Promotion and Prevention against Several Degenerative Diseases. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 72, pp. 391–421. [Google Scholar]
- Chatzivasileiou, A.O.; Ward, V.; Edgar, S.M.B.; Stephanopoulos, G. Two-Step Pathway for Isoprenoid Synthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 506–511. [Google Scholar] [CrossRef]
- Perreca, E.; Rohwer, J.; González-Cabanelas, D.; Loreto, F.; Schmidt, A.; Gershenzon, J.; Wright, L.P. Effect of Drought on the Methylerythritol 4-Phosphate (MEP) Pathway in the Isoprene Emitting Conifer Picea Glauca. Front. Plant Sci. 2020, 11, 546295. [Google Scholar] [CrossRef] [PubMed]
- Volke, D.C.; Rohwer, J.; Fischer, R.; Jennewein, S. Investigation of the Methylerythritol 4-Phosphate Pathway for Microbial Terpenoid Production through Metabolic Control Analysis. Microb. Cell Fact. 2019, 18, 192. [Google Scholar] [CrossRef]
- Xu, H.; Dickschat, J.S. Germacrene A–A Central Intermediate in Sesquiterpene Biosynthesis. Chem. Eur. J. 2020, 26, 17318–17341. [Google Scholar] [CrossRef]
- Lanier, E.R.; Andersen, T.B.; Hamberger, B. Plant Terpene Specialized Metabolism: Complex Networks or Simple Linear Pathways? Plant J. 2023, 114, 1178–1201. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Ma, L.T.; Lee, Y.R.; Shaw, J.F.; Wang, S.Y.; Chu, F.H. Differential Gene Expression Network in Terpenoid Synthesis of Antrodia Cinnamomea in Mycelia and Fruiting Bodies. J. Agric. Food Chem. 2017, 65, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Yazaki, K.; Arimura, G.I.; Ohnishi, T. “Hidden” Terpenoids in Plants: Their Biosynthesis, Localization and Ecological Roles. Plant Cell Physiol. 2017, 58, 1615–1621. [Google Scholar] [CrossRef]
- Georgiev, B.; Nikolova, M.; Aneva, I.; Dzhurmanski, A.; Sidjimova, B.; Berkov, S. Plant Products with Acetylcholinesterase Inhibitory Activity for Insect Control. BioRisk 2022, 2022, 309–315. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. CRC Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Tisgratog, R.; Sanguanpong, U.; Grieco, J.P.; Ngoen-Kluan, R.; Chareonviriyaphap, T. Plants Traditionally Used as Mosquito Repellents and the Implication for Their Use in Vector Control. Acta Trop. 2016, 157, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Acute, Synergistic and Antagonistic Effects of Some Aromatic Compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) Larvae. Ind. Crops Prod. 2014, 60, 247–258. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of Inhibition of Acetylcholinesterase by Monoterpenoids and Implications for Pest Control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Anderson, J.A.; Coats, J.R. Acetylcholinesterase Inhibition by Nootkatone and Carvacrol in Arthropods. Pestic. Biochem. Physiol. 2012, 102, 124–128. [Google Scholar] [CrossRef]
- Sigel, E.; Steinmann, M.E. Structure, Function, and Modulation of GABAA Receptors. J Biol. Chem. 2012, 287, 40224–40231. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, K.d.S.; e Silva, S.L.d.C.; de Souza, I.A.; Gualberto, S.A.; da Cruz, R.C.D.; dos Santos, F.R.; de Carvalho, M.G. Toxicological Evaluation of Essential Oil from the Leaves of Croton Tetradenius (Euphorbiaceae) on Aedes Aegypti and Mus Musculus. Parasitol. Res. 2016, 115, 3441–3448. [Google Scholar] [CrossRef]
- Farooqui, T. Review of Octopamine in Insect Nervous Systems. Open Access Insect Physiol. 2012, 4, 1–17. [Google Scholar] [CrossRef]
- Fongang, F.; Yannick, S.; Kezetas, B.; Jules, J. Terpenoids as Important Bioactive Constituents of Essential Oils. In Essential Oils—Bioactive Compounds, New Perspectives and Applications; Oliveria, M., Silva, S., da Costa, W., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Sampaio, L.A.; Pina, L.T.S.; Serafini, M.R.; Tavares, D.d.S.; Guimarães, A.G. Antitumor Effects of Carvacrol and Thymol: A Systematic Review. Front. Pharmacol. 2021, 12, 702487. [Google Scholar] [CrossRef]
- Chen, C.; Song, Q. Responses of the Pollinating Wasp Ceratosolen Solmsi Marchali to Odor Variation between Two Floral Stages of Ficus Hispida. J. Chem. Ecol. 2008, 34, 1536–1544. [Google Scholar] [CrossRef]
- Ahuja, I.; Rohloff, J.; Bones, A.M. Defence Mechanisms of Brassicaceae: Implications for Plant-Insect Interactions and Potential for Integrated Pest Management. Sustain. Agric. 2009, 2, 623–670. [Google Scholar] [CrossRef]
- Naidoo, S.; Christie, N.; Acosta, J.J.; Mphahlele, M.M.; Payn, K.G.; Myburg, A.A.; Külheim, C. Terpenes Associated with Resistance against the Gall Wasp, Leptocybe invasa, in Eucalyptus Grandis. Plant Cell Environ. 2018, 41, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, W.S. Reproductive Interactions Between Sympatric Dalechampia Species: Are Natural Assemblages “Random” or Organized? Ecology 1986, 67, 522–533. [Google Scholar] [CrossRef]
- Arnold, S.E.; Forbes, S.J.; Hall, D.R.; Farman, D.I.; Bridgemohan, P.; Spinelli, G.R.; Bray, D.P.; Perry, G.B.; Grey, L.; Belmain, S.R.; et al. Floral Odors and the Interaction between Pollinating Ceratopogonid Midges and Cacao. J. Chem. Ecol. 2019, 45, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Majetic, C.J.; Raguso, R.A.; Ashman, T. The Sweet Smell of Success: Floral Scent Affects Pollinator Attraction and Seed Fitness in Hesperis matronalis. Funct. Ecol. 2009, 23, 480–487. [Google Scholar] [CrossRef]
- Xu, X.; Cai, X.; Bian, L.; Luo, Z.; Xin, Z.; Chen, Z. Electrophysiological and Behavioral Responses of Chrysopa phyllochroma (Neuroptera: Chrysopidae) to Plant Volatiles. Environ. Entomol. 2015, 44, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Cascone, P.; Iodice, L.; Maffei, M.E.; Bossi, S.; Arimura, G.I.; Guerrieri, E. Tobacco Overexpressing β-Ocimene Induces Direct and Indirect Responses against Aphids in Receiver Tomato Plants. J. Plant Physiol. 2015, 173, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Dong, Y.; Hao, H.; Ling, Z.; Bai, H.; Wang, H.; Cui, H.; Shi, L. Time-Series Transcriptome Provides Insights into the Gene Regulation Network Involved in the Volatile Terpenoid Metabolism during the Flower Development of Lavender. BMC Plant Biol. 2019, 19, 313. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Campos, J.M.; da Silva Rolim, G.; Martínez, L.C.; Dos Santos, M.H.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Terpenoid Constituents of Cinnamon and Clove Essential Oils Cause Toxic Effects and Behavior Repellency Response on Granary Weevil, Sitophilus Granarius. Ecotoxicol. Environ. Saf. 2018, 156, 263–270. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.V.R.; Pereira, A.E.S.; Nunes, L.E.S.; Da Silva, C.C.L.; Pasquoto, T.; Lima, R.; Smaniotto, G.; Polanczyk, R.A.; Fraceto, L.F. Geraniol Encapsulated in Chitosan/Gum Arabic Nanoparticles: A Promising System for Pest Management in Sustainable Agriculture. J. Agric. Food Chem. 2018, 66, 5325–5334. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Freiesleben, S.H.; Jager, A.K. Correlation between Plant Secondary Metabolites and Their Antifungal Mechanisms—A Review. Med. Aromat. Plants 2014, 3, 2167-0412. [Google Scholar] [CrossRef]
- Işcan, G.; Işcan, A.; Demirci, F. Anticandidal Effects of Thymoquinone: Mode of Action Determined by Transmission Electron Microscopy (TEM). Nat. Prod. Commun. 2016, 11, 977–978. [Google Scholar] [CrossRef]
- Haque, E.; Irfan, S.; Kamil, M.; Sheikh, S.; Hasan, A.; Ahmad, A.; Lakshmi, V.; Nazir, A.; Mir, S.S. Terpenoids with Antifungal Activity Trigger Mitochondrial Dysfunction in Saccharomyces Cerevisiae. Microbiology 2016, 85, 436–443. [Google Scholar] [CrossRef]
- Seto-Young, D.; Monk, B.; Mason, A.B.; Perlin, D.S. Exploring an Antifungal Target in the Plasma Membrane H+-ATPase of Fungi. Biochim. Biophys. Acta Biomembr. 1997, 1326, 249–256. [Google Scholar] [CrossRef]
- Xu, J.; Liu, R.; Sun, F.; An, L.; Shang, Z.; Kong, L.; Yang, M. Eucalyptal D Enhances the Antifungal Effect of Fluconazole on Fluconazole-Resistant Candida Albicans by Competitively Inhibiting Efflux Pump. Front. Cell Infect. Microbiol. 2019, 9, 211. [Google Scholar] [CrossRef]
- Cotoras, M.; Castro, P.; Vivanco, H.; Melo, R.; Mendoza, L. Farnesol Induces Apoptosis-like Phenotype in the Phytopathogenic Fungus Botrytis Cinerea. Mycologia 2013, 105, 28–33. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Pitangui, N.S.; de Oliveira, H.C.; Scorzoni, L.; Galeane, M.C.; Medina-Alarcón, K.P.; Melo, W.C.M.A.; Marcelino, M.Y.; Braz, J.D.; et al. Fungal Biofilms and Polymicrobial Diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef]
- He, M.; Du, M.; Fan, M.; Bian, Z. In Vitro Activity of Eugenol against Candida Albicans Biofilms. Mycopathologia 2007, 163, 137–143. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Ahmad, I. Biofilm Inhibition by Cymbopogon Citratus and Syzygium Aromaticum Essential Oils in the Strains of Candida Albicans. J. Ethnopharmacol. 2012, 140, 416–423. [Google Scholar] [CrossRef]
- Cho, K.S.; Lim, Y.R.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.S. Terpenes from Forests and Human Health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Proshkina, E.; Plyusnin, S.; Babak, T.; Lashmanova, E.; Maganova, F.; Koval, L.; Platonova, E.; Shaposhnikov, M.; Moskalev, A. Terpenoids as Potential Geroprotectors. Antioxidants 2020, 9, 529. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Bai, L.; Li, J.; Li, H.; Song, J.; Zhou, Y.; Lu, R.; Liu, B.; Pang, Y.; Zhang, P.; Chen, J.; et al. Renoprotective Effects of Artemisinin and Hydroxychloroquine Combination Therapy on IgA Nephropathy via Suppressing NF-κB Signaling and NLRP3 Inflammasome Activation by Exosomes in Rats. Biochem. Pharmacol. 2019, 169, 113619. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Kłos, P.; Chlubek, D. Plant-Derived Terpenoids: A Promising Tool in the Fight against Melanoma. Cancers 2022, 14, 502. [Google Scholar] [CrossRef]
- Reguengo, L.M.; Nascimento, R.d.P.d.; Machado, A.P.d.F.; Marostica Junior, M.R. Signaling Pathways and the Potential Anticarcinogenic Effect of Native Brazilian Fruits on Breast Cancer. Food Res. Int. 2022, 155, 111117. [Google Scholar] [CrossRef]
- Machado, T.Q.; Da Fonseca, A.C.C.; Duarte, A.B.S.; Robbs, B.K.; De Sousa, D.P. A Narrative Review of the Antitumor Activity of Monoterpenes from Essential Oils: An Update. BioMed Res. Int. 2022, 2022, 6317201. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R.R. Sesquiterpenes and Their Derivatives-Natural Anticancer Compounds: An Update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef]
- Liu, G.; Chu, H. Andrographolide Inhibits Proliferation and Induces Cell Cycle Arrest and Apoptosis in Human Melanoma Cells. Oncol. Lett. 2018, 15, 5301–5305. [Google Scholar] [CrossRef]
- Coricovac, D.; Dehelean, C.A.; Pinzaru, I.; Mioc, A.; Aburel, O.M.; Macasoi, I.; Draghici, G.A.; Petean, C.; Soica, C.; Boruga, M.; et al. Assessment of Betulinic Acid Cytotoxicity and Mitochondrial Metabolism Impairment in a Human Melanoma Cell Line. Int. J. Mol. Sci. 2021, 22, 4870. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Q.; Xia, L.; Li, X.; Sun, C.; Wang, Q.; Cai, X.; Yang, G. Borneol Promotes Apoptosis of Human Glioma Cells through Regulating HIF-1a Expression via MTORC1/EIF4E Pathway. J. Cancer 2020, 11, 4810–4822. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Dong, X.; Wang, W.; Yang, L.; Zhang, X.; Li, Y.; Chen, T.; Ma, H.; Qi, D.; Su, J. Natural Borneol Enhances Paclitaxel-Induced Apoptosis of ESCC Cells by Inactivation of the PI3K/AKT. J. Food Sci. 2018, 83, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Wu, Y.J.; Chang, C.I.; Chiu, C.C.; Wu, M.L. The Effect of Bornyl Cis-4-Hydroxycinnamate on Melanoma Cell Apoptosis Is Associated with Mitochondrial Dysfunction and Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2018, 19, 1370. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Su, T.R.; Chang, C.I.; Chen, C.R.; Hung, K.F.; Liu, C. (+)-Bornyl p-Coumarate Extracted from Stem of Piper betle Induced Apoptosis and Autophagy in Melanoma Cells. Int. J. Mol. Sci. 2020, 21, 3737. [Google Scholar] [CrossRef]
- Abbas, M.M.; Kandil, Y.İ.; Abbas, M.A. R-(−)-Carvone Attenuated Doxorubicin Induced Cardiotoxicity in Vivo and Potentiated Its Anticancer Toxicity in Vitro. Balk. Med. J. 2020, 37, 98–103. [Google Scholar] [CrossRef]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Abu, N.; Mohamad, N.E.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. In Vitro Cytotoxicity and Anticancer Effects of Citral Nanostructured Lipid Carrier on MDA MBA-231 Human Breast Cancer Cells. Sci. Rep. 2019, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Estévez-Sarmiento, F.; Saavedra, E.; Ruiz-Estévez, M.; León, F.; Quintana, J.; Brouard, I.; Estévez, F. Chlorinated Guaiane-Type Sesquiterpene Lactones as Cytotoxic Agents against Human Tumor Cells. Int. J. Mol. Sci. 2020, 21, 9767. [Google Scholar] [CrossRef] [PubMed]
- Abbasifarid, E.; Bolhassani, A.; Irani, S.; Sotoodehnejadnematalahi, F. Synergistic Effects of Exosomal Crocin or Curcumin Compounds and HPV L1-E7 Polypeptide Vaccine Construct on Tumor Eradication in C57BL/6 Mouse Model. PLoS ONE 2021, 16, e0258599. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Z.; Lin, M.; Shang, Y.; Wang, F.; Zhou, J.Y.; Wang, F.; Zhang, X.; Luo, X.; Huang, W. In Vitro and in Vivo Antitumor Activity of Cucurbitacin C, a Novel Natural Product from Cucumber. Front. Pharmacol. 2019, 10, 1287. [Google Scholar] [CrossRef]
- Si, W.; Lyu, J.; Liu, Z.; Wang, C.; Huang, J.; Jiang, L.; Ma, T. Cucurbitacin E Inhibits Cellular Proliferation and Enhances the Chemo-Response in Gastric Cancer by Suppressing AKt Activation. J. Cancer 2019, 10, 5843–5851. [Google Scholar] [CrossRef] [PubMed]
- Mun, H.; Townley, H.E. Mechanism of Action of the Sesquiterpene Compound Helenalin in Rhabdomyosarcoma Cells. Pharmaceuticals 2021, 14, 1258. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Liu, C.H.; Wu, G.Y.; Lee, T.Y.; Manubolu, M.; Hsieh, C.Y.; Yang, C.H.; Sheu, J.R. Hinokitiol Inhibits Migration of A549 Lung Cancer Cells via Suppression of MMPs and Induction of Antioxidant Enzymes and Apoptosis. Int. J. Mol. Sci. 2018, 19, 939. [Google Scholar] [CrossRef] [PubMed]
- Alipanahpour Dil, E.; Asfaram, A.; Goudarzi, A.; Zabihi, E.; Javadian, H. Biocompatible Chitosan-Zinc Oxide Nanocomposite Based Dispersive Micro-Solid Phase Extraction Coupled with HPLC-UV for the Determination of Rosmarinic Acid in the Extracts of Medical Plants and Water Sample. Int. J. Biol. Macromol. 2020, 154, 528–537. [Google Scholar] [CrossRef]
- Bai, X.; Tang, J. Myrcene Exhibits Antitumor Activity Against Lung Cancer Cells by Inducing Oxidative Stress and Apoptosis Mechanisms. Nat. Prod. Commun. 2020, 15, 1934578X20961189. [Google Scholar] [CrossRef]
- Martins, B.X.; Arruda, R.F.; Costa, G.A.; Jerdy, H.; de Souza, S.B.; Santos, J.M.; de Freitas, W.R.; Kanashiro, M.M.; de Carvalho, E.C.Q.; Sant’Anna, N.F.; et al. Myrtenal-Induced V-ATPase Inhibition—A Toxicity Mechanism behind Tumor Cell Death and Suppressed Migration and Invasion in Melanoma. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1–12. [Google Scholar] [CrossRef]
- Khusnutdinova, E.; Petrova, A.; Zileeva, Z.; Kuzmina, U.; Zainullina, L.; Vakhitova, Y.; Babkov, D.; Kazakova, O. Novel A-Ring Chalcone Derivatives of Oleanolic and Ursolic Amides with Anti-Proliferative Effect Mediated through Ros-Triggered Apoptosis. Int. J. Mol. Sci. 2021, 22, 9796. [Google Scholar] [CrossRef] [PubMed]
- Hatiboglu, M.A.; Kocyigit, A.; Guler, E.M.; Akdur, K.; Nalli, A.; Karatas, E.; Tuzgen, S. Thymoquinone Induces Apoptosis in B16-F10 Melanoma Cell Through Inhibition of p-STAT3 and Inhibits Tumor Growth in a Murine Intracerebral Melanoma Model. World Neurosurg. 2018, 114, e182–e190. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, F.; He, P.; Yang, Y.; Yang, J.; Zhang, Y.; Liu, J.; Tong, Y.; Li, Q.; Mei, X.; et al. Triptolide Sensitizes Breast Cancer Cells to Doxorubicin through the DNA Damage Response Inhibition. Mol. Carcinog. 2018, 57, 807–814. [Google Scholar] [CrossRef]
- Pinheiro-Neto, F.R.; Lopes, E.M.; Acha, B.T.; Gomes, L.d.S.; Dias, W.A.; Reis Filho, A.C.d.; Leal, B.d.S.; Rodrigues, D.C.d.N.; Silva, J.d.N.; Dittz, D.; et al. α-Phellandrene Exhibits Antinociceptive and Tumor-Reducing Effects in a Mouse Model of Oncologic Pain. Toxicol. Appl. Pharmacol. 2021, 418, 115497. [Google Scholar] [CrossRef]
- Xu, Q.; Li, M.; Yang, M.; Yang, J.; Xie, J.; Lu, X.; Wang, F.; Chen, W. α-Pinene Regulates MiR-221 and Induces G2/M Phase Cell Cycle Arrest in Human Hepatocellular Carcinoma Cells. Biosci. Rep. 2018, 38, BSR20180980. [Google Scholar] [CrossRef]
- Negreiros, H.A.; de Moura, K.G.; Barreto do Nascimento, M.L.L.; do Nascimento Rodrigues, D.C.; Ferreir, P.M.P.; Braz, D.C.; de Farias, M.G.; de Sousa Corrêia, L.; Pereira, A.R.S.; Santos, L.K.B.; et al. Alpha-Terpineol as Antitumor Candidate in Pre-Clinical Studies. Anticancer Agents Med. Chem. 2021, 21, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Pudełek, M.; Catapano, J.; Kochanowski, P.; Mrowiec, K.; Janik-Olchawa, N.; Czyż, J.; Ryszawy, D. Therapeutic Potential of Monoterpene α-Thujone, the Main Compound of Thuja occidentalis L. Essential Oil, against Malignant Glioblastoma Multiforme Cells in Vitro. Fitoterapia 2019, 134, 172–181. [Google Scholar] [CrossRef]
- Bai, Z.; Yao, C.; Zhu, J.; Xie, Y.; Ye, X.Y.; Bai, R.; Xie, T. Anti-Tumor Drug Discovery Based on Natural Product β-Elemene: Anti-Tumor Mechanisms and Structural Modification. Molecules 2021, 26, 1499. [Google Scholar] [CrossRef]
- Balavandi, Z.; Neshasteh-Riz, A.; Koosha, F.; Eynali, S.; Hoormand, M.; Shahidi, M. The Use of SS-Elemene to Enhance Radio Sensitization of A375 Human Melanoma Cells. Cell J. 2020, 21, 419–425. [Google Scholar] [CrossRef]
- Dhyani, P.; Sati, P.; Sharma, E.; Attri, D.C.; Bahukhandi, A.; Tynybekov, B.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Suleria, H.A.R.; et al. Sesquiterpenoid Lactones as Potential Anti-Cancer Agents: An Update on Molecular Mechanisms and Recent Studies. Cancer Cell Int. 2022, 22, 305. [Google Scholar] [CrossRef]
- Silva, B.I.M.; Nascimento, E.A.; Silva, C.J.; Silva, T.G.; Aguiar, J.S. Anticancer Activity of Monoterpenes: A Systematic Review. Mol. Biol. Rep. 2021, 48, 5775–5785. [Google Scholar] [CrossRef]
- Alipanah, H.; Farjam, M.; Zarenezhad, E.; Roozitalab, G.; Osanloo, M. Chitosan Nanoparticles Containing Limonene and Limonene-Rich Essential Oils: Potential Phytotherapy Agents for the Treatment of Melanoma and Breast Cancers. BMC Complement. Med. Ther. 2021, 21, 186. [Google Scholar] [CrossRef]
- Abdul Ghani, M.A.; Ugusman, A.; Latip, J.; Zainalabidin, S. Role of Terpenophenolics in Modulating Inflammation and Apoptosis in Cardiovascular Diseases: A Review. Int. J. Mol. Sci. 2023, 24, 5339. [Google Scholar] [CrossRef]
- Haines, D.D.; Cowan, F.M.; Tosaki, A. Evolving Strategies for Use of Phytochemicals in Prevention and Long-Term Management of Cardiovascular Diseases (CVD). Int. J. Mol. Sci. 2024, 25, 6176. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, İ.; Özmen, O.; Tutun, H.; Yalçın, A.; Türk, A. Artemisinin Attenuates Doxorubicin Induced Cardiotoxicity and Hepatotoxicity in Rats. Biotech. Histochem. 2020, 95, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Choe, J.Y.; Park, K.Y. Anti-Inflammatory Effect of Artemisinin on Uric Acid-Induced NLRP3 Inflammasome Activation through Blocking Interaction between NLRP3 and NEK7. Biochem. Biophys. Res. Commun. 2019, 517, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, Q.; Yang, J.; Wang, C.; Cao, J.; Sun, J.; Fan, Z.; Fu, L. Artemisinin Suppresses Myocardial Ischemia–Reperfusion Injury via NLRP3 Inflammasome Mechanism. Mol. Cell Biochem. 2020, 474, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Guo, W.; Shang, F.; Li, Y.; Li, W.; Liu, J.; Ma, C.; Teng, J. Bakuchiol Alleviates Hyperglycemia-Induced Diabetic Cardiomyopathy by Reducing Myocardial Oxidative Stress via Activating the SIRT1/Nrf2 Signaling Pathway. Oxid. Med. Cell Longev. 2020, 2020, 3732718. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, L.; Xiao, L.; Kong, L.; Shi, H.; Tian, X.; Zhao, L. Bakuchiol Protects against Pathological Cardiac Hypertrophy by Blocking NF-ΚB Signaling Pathway. Biosci. Rep. 2018, 38, BSR20181043. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Geng, L.; Zhou, L.; Pei, X.; Yang, Z.; Ding, Z. Betulin Alleviates on Myocardial Inflammation in Diabetes Mice via Regulating Siti1/NLRP3/NF-ΚB Pathway. Int. Immunopharmacol. 2020, 85, 106653. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Cai, X.; Liu, X.; Liu, J.; Zhu, N. Betulin Alleviates Myocardial Ischemia–Reperfusion Injury in Rats via Regulating the Siti1/NLRP3/NF-ΚB Signaling Pathway. Inflammation 2021, 44, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lim, W.; Sung, M.K. Carnosic Acid Modulates Increased Hepatic Lipogenesis and Adipocytes Differentiation in Ovariectomized Mice Fed Normal or High-Fat Diets. Nutrients 2018, 10, 1984. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xu, H.; Chen, J.; Yang, X.; Xiong, J.; Wang, J.; Cheng, F. Carnosic Acid Protects against Pressure Overload-induced Cardiac Remodelling by Inhibiting the AKT/GSK3β/NOX4 Signalling Pathway. Exp. Ther. Med. 2020, 20, 3709–3719. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Yang, J.J.; Zhang, H.S. Carvedilol (CAR) Combined with Carnosic Acid (CAA) Attenuates Doxorubicin-Induced Cardiotoxicity by Suppressing Excessive Oxidative Stress, Inflammation, Apoptosis and Autophagy. Biomed. Pharmacother. 2019, 109, 71–83. [Google Scholar] [CrossRef]
- Xu, D.; Ye, B.; Lin, L.; Jin, Y.; Jiang, Y.; Zheng, Z.; Chen, Y.; Han, X.; Wang, W.; Wu, G.; et al. Carnosol attenuates angiotensin II-induced cardiac remodeling and inflammation via directly binding to p38 and inhibiting p38 activation. Int. Immunopharm. 2024, 134, 112143. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Lv, J.; Peng, L.; Zhao, J.; Chi, L. Carnosol Promotes Endothelial Differentiation under H2O2-Induced Oxidative Stress. Arch. Biol. Sci. 2016, 69, 101. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.; Hejazian, S.; Jamhiri, M.; Hafizibarjin, Z.; Sadeghzadeh, S.; Safari, F. The Effect of Carvacrol on Transcription Levels of Bcl-2 Family Proteins in Hypertrophied Heart of Rats. Physiol. Pharmacol. 2018, 22, 54–62. [Google Scholar]
- Ye, S.; Luo, W.; Khan, Z.A.; Wu, G.; Xuan, L.; Shan, P.; Lin, K.; Chen, T.; Wang, J.; Hu, X.; et al. Celastrol Attenuates Angiotensin II-Induced Cardiac Remodeling by Targeting STAT3. Circ. Res. 2020, 126, 1007–1023. [Google Scholar] [CrossRef]
- Shih, M.F.; Pan, K.H.; Liu, C.C.; Shen, C.R.; Cherng, J.Y. Treatment of β-Thujaplicin Counteracts Di(2-Ethylhexyl)Phthalate (DEHP)-Exposed Vascular Smooth Muscle Activation, Inflammation and Atherosclerosis Progression. Regul. Toxicol. Pharmacol. 2018, 92, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Liang, S.; Cai, Q.; Liu, J.; Jin, L.; Yang, Z.; Chen, X. Hinokitiol Protects Cardiomyocyte from Oxidative Damage by Inhibiting GSK3 β-Mediated Autophagy. Oxid. Med. Cell Longev. 2022, 2022, 2700000. [Google Scholar] [CrossRef]
- Li, W.; Cao, J.; Wang, X.; Zhang, Y.; Sun, Q.; Jiang, Y.; Yao, J.; Li, C.; Wang, Y.; Wang, W. Ferruginol Restores SIRT1-PGC-1α-Mediated Mitochondrial Biogenesis and Fatty Acid Oxidation for the Treatment of DOX-Induced Cardiotoxicity. Front. Pharmacol. 2021, 12, 773834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Wang, C.; Li, H.; Wang, L.; Chen, Y.; Feng, J.; Ali Alharbi, S.; Deng, Y. Ameliorative Effect of Ferruginol on Isoprenaline Hydrochloride-Induced Myocardial Infarction in Rats. Environ. Toxicol. 2021, 36, 249–256. [Google Scholar] [CrossRef]
- Fu, Y.; Yuan, P.; Cao, Y.; Ke, Y.; Zhang, Q.; Hou, Y.; Zhang, Y.; Feng, W.; Zheng, X. Geniposide in Gardenia Jasminoides Var. Radicans Makino Modulates Blood Pressure via Inhibiting WNK Pathway Mediated by the Estrogen Receptors. J. Pharm. Pharmacol. 2020, 72, 1956–1969. [Google Scholar] [CrossRef]
- Luo, X.; Wu, S.; Jiang, Y.; Wang, L.; Li, G.; Qing, Y.; Liu, J.; Zhang, D. Inhibition of Autophagy by Geniposide Protects against Myocardial Ischemia/Reperfusion Injury. Int. Immunopharmacol. 2020, 85, 106609. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, X.; Lu, Y.; Wang, F.; Xu, Z.; Liu, S.; Zheng, H.; Liu, X. Geniposide Inhibits NLRP3 Inflammasome Activation via Autophagy in BV-2 Microglial Cells Exposed to Oxygen–Glucose Deprivation/Reoxygenation. Int. Immunopharmacol. 2020, 84, 106547. [Google Scholar] [CrossRef]
- Song, P.; Shen, D.F.; Meng, Y.Y.; Kong, C.Y.; Zhang, X.; Yuan, Y.P.; Yan, L.; Tang, Q.Z.; Ma, Z.G. Geniposide Protects against Sepsis-Induced Myocardial Dysfunction through AMPKα-Dependent Pathway. Free Radic. Biol. Med. 2020, 152, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Huang, M.; Ouyang, H.; Peng, J.; Liang, J. Oridonin Inhibits Vascular Inflammation by Blocking NF-κB and MAPK Activation. Eur. J. Pharmacol. 2018, 826, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Lan, C.C.; Hsu, Y.T.; Tsai, C.L.; Tzeng, I.S.; Wang, P.; Kuo, C.Y.; Hsieh, P.C. Oridonin Attenuates Lipopolysaccharide-Induced ROS Accumulation and Inflammation in HK-2 Cells. Evid.-Based Complement. Altern. Med. 2020, 2020, 9724520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, Y.; Sun, Y.; Yan, H.; Han, W.; Wang, X.; Wang, K.; Wei, B.; Xu, X. Beneficial Effects of Oridonin on Myocardial Ischemia/Reperfusion Injury: Insight Gained by Metabolomic Approaches. Eur. J. Pharmacol. 2019, 861, 172587. [Google Scholar] [CrossRef]
- Lu, C.; Chen, C.; Chen, A.; Wu, Y.; Wen, J.; Huang, F.; Zeng, Z. Oridonin Attenuates Myocardial Ischemia/Reperfusion Injury via Downregulating Oxidative Stress and NLRP3 Inflammasome Pathway in Mice. Evid.-Based Complement. Altern. Med. 2020, 2020, 7395187. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Jiang, H.; Chen, Y.; Ye, J.; Wang, A.; Wang, C.; Liu, Q.; Liang, G.; Deng, X.; Jiang, W.; et al. Oridonin Is a Covalent NLRP3 Inhibitor with Strong Anti-Inflammasome Activity. Nat. Commun. 2018, 9, 2550. [Google Scholar] [CrossRef]
- Yang, H.; Lv, H.; Li, H.; Ci, X.; Peng, L. Oridonin Protects LPS-Induced Acute Lung Injury by Modulating Nrf2-Mediated Oxidative Stress and Nrf2-Independent NLRP3 and NF-ΚB Pathways. Cell Commun. Signal. 2019, 17, 62. [Google Scholar] [CrossRef]
- Wang, C.; Sun, H.; Song, Y.; Ma, Z.; Zhang, G.; Gu, X.; Zhao, L. Retraction Pterostilbene Attenuates Inflammation in Rat Heart Subjected to Ischemia-Reperfusion: Role of TLR4/NF-κB Signaling Pathway. Int. J. Clin. Exp. Med. 2015, 8, 1737. [Google Scholar]
- Li, J.; Zhao, C.; Zhu, Q.; Wang, Y.; Li, G.; Li, X.; Li, Y.; Wu, N.; Ma, C. Sweroside Protects Against Myocardial Ischemia–Reperfusion Injury by Inhibiting Oxidative Stress and Pyroptosis Partially via Modulation of the Keap1/Nrf2 Axis. Front. Cardiovasc. Med. 2021, 8, 650368. [Google Scholar] [CrossRef]
- Ma, L.Q.; Yu, Y.; Chen, H.; Li, M.; Ihsan, A.; Tong, H.Y.; Huang, X.J.; Gao, Y. Sweroside Alleviated Aconitine-Induced Cardiac Toxicity in H9c2 Cardiomyoblast Cell Line. Front. Pharmacol. 2018, 9, 1138. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Jang, J.H.; Kim, S.W.; Han, S.H.; Ma, K.H.; Jang, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Sweroside Prevents Non-Alcoholic Steatohepatitis by Suppressing Activation of the NLRP3 Inflammasome. Int. J. Mol. Sci. 2020, 21, 2790. [Google Scholar] [CrossRef] [PubMed]
- El-Marasy, S.A.; El Awdan, S.A.; Hassan, A.; Abdallah, H.M.I. Cardioprotective Effect of Thymol against Adrenaline-Induced Myocardial Injury in Rats. Heliyon 2020, 6, e04431. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.C.; Liu, Y.; Cen, Y.Y.; Xiong, Y.L.; Li, J.M.; Ding, Y.Y.; Tong, Y.F.; Liu, T.; Chen, X.H.; Zhang, H.G. Dual Role of Triptolide in Interrupting the NLRP3 Inflammasome Pathway to Attenuate Cardiac Fibrosis. Int. J. Mol. Sci. 2019, 20, 360. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Shi, L.; Zhou, P. Phenols and Terpenoids: Natural Products as Inhibitors of NLRP3 Inflammasome in Cardiovascular Diseases. Inflammopharmacology 2022, 30, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Shi, H.; Yu, Y.; Yu, Y.; Li, M.; Chen, R. NLRP3 Inflammasome, an Immune-inflammatory Target in Pathogenesis and Treatment of Cardiovascular Diseases. Clin. Transl. Med. 2020, 10, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, Z.J.S.; Álvarez-Rivera, G.; Sánchez-Martínez, J.D.; Gallego, R.; Valdés, A.; Bueno, M.; Cifuentes, A.; Ibáñez, E. Neuroprotective Effect of Terpenoids Recovered from Olive Oil By-Products. Foods 2021, 10, 1507. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Bai, L.; Chen, L.; Tong, R.; Feng, Y.; Shi, J. Terpenoid Natural Products Exert Neuroprotection via the PI3K/Akt Pathway. Front. Pharmacol. 2022, 13, 1. [Google Scholar] [CrossRef]
- Mony, T.J.; Elahi, F.; Choi, J.W.; Park, S.J. Neuropharmacological Effects of Terpenoids on Preclinical Animal Models of Psychiatric Disorders: A Review. Antioxidants 2022, 11, 1834. [Google Scholar] [CrossRef]
- González-Cofrade, L.; De Las Heras, B.; Apaza Ticona, L.; Palomino, O.M. Molecular Targets Involved in the Neuroprotection Mediated by Terpenoids. Planta Med. 2019, 85, 1304–1315. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, Y.; Ge, H.; Wang, J.; Chen, X.; Lei, X.; Zhong, J.; Zhang, C.; Xian, J.; Lu, Y.; et al. Artesunate Promotes the Proliferation of Neural Stem/Progenitor cells and Alleviates Ischemia-Reperfusion Injury through PI3K/Akt/FOXO3a/P27kip1 Signaling Pathway. Aging 2020, 12, 8029–8048. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, W.; Wang, P.; Chu, J. Asiatic Acid Protects Differentiated PC12 Cells from Aβ25–35-Induced Apoptosis and Tau Hyperphosphorylation via Regulating PI3K/Akt/GSK-3β Signaling. Life Sci. 2018, 208, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Giribabu, N.; Karim, K.; Kilari, E.K.; Nelli, S.R.; Salleh, N. Oral Administration of Centella asiatica (L.) Urb Leave Aqueous Extract Ameliorates Cerebral Oxidative Stress, Inflammation, and Apoptosis in Male Rats with Type-2 Diabetes. Inflammopharmacology 2020, 28, 1599–1622. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Song, L.; Guan, Y.; Cao, C.; Cui, Y.; Zhang, Y.; Liu, C. Catalpol Weakens Depressive-like Behavior in Mice with Streptozotocin-Induced Hyperglycemia via PI3K/AKT/Nrf2/HO-1 Signaling Pathway. Neuroscience 2021, 473, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, J.; Shao, Y.; Wan, D. Catalpol May Improve Axonal Growth via Regulating MiR-124 Regulated PI3K/AKT/MTOR Pathway in Neurons after Ischemia. Ann. Transl. Med. 2019, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, X.; Teng, H.; Li, C.; Wang, B.; Wang, J. Celastrol Inhibits Microglial Pyroptosis and Attenuates Inflammatory Reaction in Acute Spinal Cord Injury Rats. Int. Immunopharmacol. 2019, 66, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Duan, G.; He, J.; Meng, Q.; Liu, Y.; Chen, W.; Meng, Y. Geniposide Attenuates Epilepsy Symptoms in a Mouse Model through the PI3K/Akt/GSK-3β Signaling Pathway. Exp. Ther. Med. 2018, 15, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, L.; Chen, Z.; Dai, L.; Xi, C.; Wu, X.; Wu, G.; Wang, Y.; Hu, J. Geniposide Ameliorates Chronic Unpredictable Mild Stress Induced Depression-like Behavior through Inhibition of Ceramide-PP2A Signaling via the PI3K/Akt/GSK3β Axis. Psychopharmacology 2021, 238, 2789–2800. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Q.; Yao, L. Geniposide Alleviates Neuropathic Pain in CCI Rats by Inhibiting the EGFR/PI3K/AKT Pathway and Ca2+ Channels. Neurotox. Res. 2022, 40, 1057–1069. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, Z.; Yi, F.; Orange, M.; Yao, M.; Yang, B.; Liu, J.; Zhu, H. By Activating Akt/ENOS Bilobalide B Inhibits Autophagy and Promotes Angiogenesis Following Focal Cerebral Ischemia Reperfusion. Cell Physiol. Biochem. 2018, 47, 604–616. [Google Scholar] [CrossRef]
- Usuki, T.; Yoshimoto, Y.; Sato, M.; Takenaka, T.; Takezawa, R.; Yoshida, Y.; Satake, M.; Suzuki, N.; Hashizume, D.; Dzyuba, S.V. Bilobalide and PC12 Cells: A Structure Activity Relationship Study. Bioorg. Med. Chem. 2020, 28, 115251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, J.; Yan, R.; Li, L.; Xiao, Z.; Zhou, W.; Wang, Z.; Xiao, W.; Du, G. Diterpene Ginkgolides Protect against Cerebral Ischemia/Reperfusion Damage in Rats by Activating Nrf2 and CREB through PI3K/Akt Signaling. Acta Pharmacol. Sin. 2018, 39, 1259–1272. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, Z.; Xu, Z.; Yang, H.; Li, L.; Li, G.; Li, F.; Gu, S.; Zong, S.; Zhou, J.; et al. Antioxidant Effects of Ginkgolides and Bilobalide against Cerebral Ischemia Injury by Activating the Akt/Nrf2 Pathway in Vitro and in Vivo. Cell Stress Chaperones 2019, 24, 441–452. [Google Scholar] [CrossRef]
- Yu, W.B.; Cao, L.; Zhao, Y.Y.; Xiao, W.; Xiao, B.G. Comparing the Role of Ginkgolide B and Ginkgolide K on Cultured Astrocytes Exposed to Oxygen-glucose Deprivation. Mol. Med. Rep. 2018, 18, 4417–4427. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, Y.; Li, W.; Hang, J.; Yin, M.; Yu, H. Echinocystic Acid Provides a Neuroprotective Effect via the PI3K/AKT Pathway in Intracerebral Haemorrhage Mice. Ann. Transl. Med. 2020, 8, 6. [Google Scholar] [CrossRef]
- Park, J.Y.; Amarsanaa, K.; Cui, Y.; Lee, J.H.; Wu, J.; Yang, Y.S.; Eun, S.Y.; Jung, S.C. Methyl Lucidone Exhibits Neuroprotective Effects on Glutamate-Induced Oxidative Stress in HT-22 Cells via Nrf-2/HO-1 Signaling. Appl. Biol. Chem. 2019, 62, 67. [Google Scholar] [CrossRef]
- Lorigooini, Z.; Boroujeni, S.N.; Sayyadi-Shahraki, M.; Rahimi-Madiseh, M.; Bijad, E.; Amini-Khoei, H. Limonene through Attenuation of Neuroinflammation and Nitrite Level Exerts Antidepressant-like Effect on Mouse Model of Maternal Separation Stress. Behav. Neurol. 2021, 2021, 8817309. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wen, C.; Yang, M.; Gan, D.; Fan, C.; Li, A.; Li, Q.; Zhao, J.; Zhu, L.; Lu, D. Lycopene Protects against T-BHP-Induced Neuronal Oxidative Damage and Apoptosis via Activation of the PI3K/Akt Pathway. Mol. Biol. Rep. 2019, 46, 3387–3397. [Google Scholar] [CrossRef]
- Wang, G.; Guo, H.; Wang, X. Platycodin D Protects Cortical Neurons against Oxygen-Glucose Deprivation/Reperfusion in Neonatal Hypoxic-Ischemic Encephalopathy. J. Cell Biochem. 2019, 120, 14028–14034. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Cao, X.C.; Liu, Z.Y.; Ma, C.L.; Li, B.M. Polygalasaponin F Protects Hippocampal Neurons against Glutamate-Induced Cytotoxicity. Neural. Regen. Res. 2022, 17, 178–184. [Google Scholar] [CrossRef]
- Xie, W.; Wulin, H.; Shao, G.; Wei, L.; Qi, R.; Ma, B.; Chen, N.; Shi, R. Polygalasaponin F Inhibits Neuronal Apoptosis Induced by Oxygen-Glucose Deprivation and Reoxygenation through the PI3K/Akt Pathway. Basic. Clin. Pharmacol. Toxicol. 2020, 127, 196–204. [Google Scholar] [CrossRef]
- Wu, S.; Yang, T.; Cen, K.; Zou, Y.; Shi, X.; Zhou, D.; Gao, Y.; Chai, L.; Zhao, Y.; Sun, Y.; et al. In Vitro Evaluation of the Neuroprotective Effect of Panax Notoginseng Saponins by Activating the EGFR/PI3K/AKT Pathway. Evid. Based Complement. Altern. Med. 2020, 2020, 1403572. [Google Scholar] [CrossRef]
- Tu, L.; Wang, Y.; Chen, D.; Xiang, P.; Shen, J.; Li, Y.; Wang, S. Protective Effects of Notoginsenoside R1 via Regulation of the PI3K-Akt-MTOR/JNK Pathway in Neonatal Cerebral Hypoxic-Ischemic Brain Injury. Neurochem. Res. 2018, 43, 1210–1226. [Google Scholar] [CrossRef]
- Khoshnazar, M.; Parvardeh, S.; Bigdeli, M.R. Alpha-Pinene Exerts Neuroprotective Effects via Anti-Inflammatory and Anti-Apoptotic Mechanisms in a Rat Model of Focal Cerebral Ischemia-Reperfusion. J. Stroke Cerebrovasc. Dis. 2020, 29, 104977. [Google Scholar] [CrossRef] [PubMed]
- Mehri, S.; Meshki, M.A.; Hosseinzadeh, H. Linalool as a Neuroprotective Agent against Acrylamide-Induced Neurotoxicity in Wistar Rats. Drug Chem Toxicol 2015, 38, 162–166. [Google Scholar] [CrossRef]
- Weston-Green, K.; Clunas, H.; Jimenez Naranjo, C. A Review of the Potential Use of Pinene and Linalool as Terpene-Based Medicines for Brain Health: Discovering Novel Therapeutics in the Flavours and Fragrances of Cannabis. Front. Psychiatry 2021, 12, 583211. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Bueno, M.; Alvarez-Rivera, G.; Tudela, J.; Ibañez, E.; Cifuentes, A. In Vitro Neuroprotective Potential of Terpenes from Industrial Orange Juice by-Products. Food Funct. 2021, 12, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Maitra, U.; Stephen, C.; Ciesla, L.M. Drug Discovery from Natural Products—Old Problems and Novel Solutions for the Treatment of Neurodegenerative Diseases. J. Pharm. Biomed. Anal. 2022, 210, 114553. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural Monoterpenes: Much More than Only a Scent. Chem. Biodivers. 2019, 16, e1900434. [Google Scholar] [CrossRef]
- Akhtar, A.; Andleeb, A.; Waris, T.S.; Bazzar, M.; Moradi, A.R.; Awan, N.R.; Yar, M. Neurodegenerative Diseases and Effective Drug Delivery: A Review of Challenges and Novel Therapeutics. J. Control. Release 2021, 330, 1152–1167. [Google Scholar] [CrossRef]
- Yoo, K.Y.; Park, S.Y. Terpenoids as Potential Anti-Alzheimer’s Disease Therapeutics. Molecules 2012, 17, 3524–3538. [Google Scholar] [CrossRef]
- Marino, A.; Battaglini, M.; Moles, N.; Ciofani, G. Natural Antioxidant Compounds as Potential Pharmaceutical Tools against Neurodegenerative Diseases. ACS Omega 2022, 7, 25974–25990. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L. Progress in Research on Paclitaxel and Tumor Immunotherapy. Cell Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef]
- Jiang, Z.; Jacob, J.A.; Loganathachetti, D.S.; Nainangu, P.; Chen, B. β-Elemene: Mechanistic Studies on Cancer Cell Interaction and Its Chemosensitization Effect. Front. Pharmacol. 2017, 8, 105. [Google Scholar] [CrossRef]
- Nijst, P.; Tang, W.H.W. Managing Cancer Patients and Survivors with Advanced Heart Failure. Curr. Treat Options Cardiovasc. Med. 2021, 23, 73. [Google Scholar] [CrossRef]
- Gallaher, J.R.; Charles, A. Acute Cholecystitis A Review. JAMA Netw. 2022, 327, 965–975. [Google Scholar] [CrossRef]
- Mukhtar, Y.M.; Adu-Frimpong, M.; Xu, X.; Yu, J. Biochemical Significance of Limonene and Its Metabolites: Future Prospects for Designing and Developing Highly Potent Anticancer Drugs. Biosci. Rep. 2018, 38, BSR20181253. [Google Scholar] [CrossRef]
- Weston-Green, K. The United Chemicals of Cannabis: Beneficial effects of Cannabis phytochemicals on the brain and cognition. In Recent Advances in Cannabinoid Research; Costain, W., Laprairie, R.B., Eds.; InTechOpen: London, UK, 2018; pp. 83–100. [Google Scholar] [CrossRef]
- Xiong, Y.; Huang, J. Anti-Malarial Drug: The Emerging Role of Artemisinin and Its Derivatives in Liver Disease Treatment. Chin. Med. 2021, 16, 80. [Google Scholar] [CrossRef]
- Bridgford, J.L.; Xie, S.C.; Cobbold, S.A.; Pasaje, C.F.A.; Herrmann, S.; Yang, T.; Gillett, D.L.; Dick, L.R.; Ralph, S.A.; Dogovski, C.; et al. Artemisinin Kills Malaria Parasites by Damaging Proteins and Inhibiting the Proteasome. Nat. Commun. 2018, 9, 3801. [Google Scholar] [CrossRef]
- Fan, M.; Yuan, S.; Li, L.; Zheng, J.; Zhao, D.; Wang, C.; Wang, H.; Liu, X.; Liu, J. Application of Terpenoid Compounds in Food and Pharmaceutical Products. Fermentation 2023, 9, 119. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and Isoprenoids: A Wealth of Compounds for Global Use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef]
- Shahidi, F.; De Camargo, A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular Evidence That Oral Supplementation with Lycopene or Lutein Protects Human Skin against Ultraviolet Radiation: Results from a Double-Blinded, Placebo-Controlled, Crossover Study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, M.J.; Chung, B.Y.; Bang, D.Y.; Lim, S.K.; Choi, S.M.; Lim, D.S.; Cho, M.C.; Yoon, K.; Kim, H.S.; et al. Safety Evaluation and Risk Assessment of D-Limonene. J. Toxicol. Environ. Health B Crit. Rev. 2013, 16, 17–38. [Google Scholar] [CrossRef]
- Liang, Z.; Zhi, H.; Fang, Z.; Zhang, P. Genetic Engineering of Yeast, Filamentous Fungi and Bacteria for Terpene Production and Applications in Food Industry. Food Res. Int. 2021, 147, 110487. [Google Scholar] [CrossRef]
- Gutiérrez-Del-río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczyńska, E. Comparison of the Oxidative Stability of Soybean and Sunflower Oils Enriched with Herbal Plant Extracts. Chem. Pap. 2018, 72, 2607–2615. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, I.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Moctezuma, E.; Gutierrez-Pacheco, M.M.; Tapia-Rodriguez, M.R.; Ortega-Ramirez, L.A.; Ayala-Zavala, J.F. Oregano (Lippia Graveolens) Essential Oil Added within Pectin Edible Coatings Prevents Fungal Decay and Increases the Antioxidant Capacity of Treated Tomatoes. J. Sci. Food Agric. 2016, 96, 3772–3778. [Google Scholar] [CrossRef]
- Kutyna, D.R.; Borneman, A.R. Heterologous Production of Flavour and Aroma Compounds in Saccharomyces Cerevisiae. Genes 2018, 9, 326. [Google Scholar] [CrossRef]
- Triaux, Z.; Petitjean, H.; Marchioni, E.; Steyer, D.; Marcic, C. Comparison of Headspace, Hydrodistillation and Pressurized Liquid Extraction of Terpenes and Terpenoids from Food Matrices—Qualitative and Quantitative Analysis. J. Anal. Chem. 2021, 76, 284–295. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, K. Production of Terpenoids by Synthetic Biology Approaches. Front. Bioeng. Biotechnol. 2020, 8, 1. [Google Scholar] [CrossRef]
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of Phenolic Compounds and Terpenes from Cannabis Sativa l. By-Products: From Conventional to Intensified Processes. Antioxidants 2021, 10, 942. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A Review of Ultrasound-Assisted Extraction for Plant Bioactive Compounds: Phenolics, Flavonoids, Thymols, Saponins and Proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Llorente, D.; Cañada-Barcala, A.; Álvarez-Torrellas, S.; Águeda, V.I.; García, J.; Larriba, M. A Review of the Use of Eutectic Solvents, Terpenes and Terpenoids in Liquid–Liquid Extraction Processes. Processes 2020, 8, 1220. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Alvarez-Rivera, G.; Gallego, R.; Fagundes, M.B.; Valdés, A.; Mendiola, J.A.; Ibañez, E.; Cifuentes, A. Neuroprotective Potential of Terpenoid-Rich Extracts from Orange Juice by-Products Obtained by Pressurized Liquid Extraction. Food Chem. X 2022, 13, 100242. [Google Scholar] [CrossRef]
- Wang, M.; Morón-Ortiz, Á.; Zhou, J.; Benítez-González, A.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Barba, F.J. Effects of Pressurized Liquid Extraction with Dimethyl Sulfoxide on the Recovery of Carotenoids and Other Dietary Valuable Compounds from the Microalgae Spirulina, Chlorella and Phaeodactylum tricornutum. Food Chem. 2023, 405, 134885. [Google Scholar] [CrossRef]
- Shapira, A.; Berman, P.; Futoran, K.; Guberman, O.; Meiri, D. Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections. Anal. Chem. 2019, 91, 11425–11432. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; He, R.; Huang, R.; Pang, C.; Ma, Y.; Xia, H.; Liang, D.; Liao, L.; Xiong, B.; Wang, X.; et al. Optimization of a Static Headspace GC-MS Method and Its Application in Metabolic Fingerprinting of the Leaf Volatiles of 42 Citrus Cultivars. Front. Plant Sci. 2022, 13, 1050289. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, L.; Grillo, G.; Gallina, L.; Carnaroglio, D.; Chemat, F.; Cravotto, G. Microwave-Assisted Hydrodistillation of Hop (Humulus lupulus L.) Terpenes: A Pilot-Scale Study. Foods 2021, 10, 2726. [Google Scholar] [CrossRef] [PubMed]
- Radivojac, A.; Bera, O.; Micić, D.; Đurović, S.; Zeković, Z.; Blagojević, S.; Pavlić, B. Conventional versus Microwave-Assisted Hydrodistillation of Sage Herbal Dust: Kinetics Modeling and Physico-Chemical Properties of Essential Oil. Food Bioprod. Process. 2020, 123, 90–101. [Google Scholar] [CrossRef]
- Peng, X.; Feng, C.; Jia, A.; Gao, J.; Zhang, R. Recovery of Essential Oils from the Withered Flowers of Magnolia Soulangeana Soul.-Bod. by Microwave-Assisted Hydrodistillation with Uniform Heating and Its New Application in the Hypolipidemic Field. Ind. Crops Prod. 2023, 204, 117355. [Google Scholar] [CrossRef]
- Çelebi Uzkuç, N.M.; Uzkuç, H.; Berber, M.M.; Tarhan Kuzu, K.; Özmen Toğay, S.; İşleten Hoşoğlu, M.; Kırca Toklucu, A.; Kurt, S.B.; Sahiner, N.; Karagül Yüceer, Y. Stabilisation of Lavender Essential Oil Extracted by Microwave-Assisted Hydrodistillation: Characteristics of Starch and Soy Protein-Based Microemulsions. Ind. Crops Prod. 2021, 172, 114034. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.K.; Zeković, Z. Antioxidant and Enzyme-Inhibitory Activity of Peppermint Extracts and Essential Oils Obtained by Conventional and Emerging Extraction Techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.d.P.; Gutiérrez, L.F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.E. Valorisation of Mango Peel: Proximate Composition, Supercritical Fluid Extraction of Carotenoids, and Application as an Antioxidant Additive for an Edible Oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Vardanega, R.; Nogueira, G.C.; Nascimento, C.D.O.; Faria-Machado, A.F.; Meireles, M.A.A. Selective Extraction of Bioactive Compounds from Annatto Seeds by Sequential Supercritical CO2 Process. J. Supercrit. Fluids 2019, 150, 122–127. [Google Scholar] [CrossRef]
- Lima, R.N.; Ribeiro, A.S.; Cardozo-Filho, L.; Vedoy, D.; Alves, P.B. Extraction from Leaves of Piper Klotzschianum Using Supercritical Carbon Dioxide and Co-Solvents. J. Supercrit. Fluids 2019, 147, 205–212. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, H.J.; Chung, M.S.; Ko, M.J. Selective Extraction of Oxygenated Terpene in Caraway (Carum carvi L.) Using Subcritical Water Extraction (SWE) Technique. Food Chem. 2022, 381, 132192. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, E.; Ferrati, M.; Lo Giudice, D.; Felicioni, E.; Petrelli, R.; Benelli, G.; Maggi, F.; Cespi, M. Microwave-Assisted Hydrodistillation of the Insecticidal Essential Oil from Carlina Acaulis: A Fractional Factorial Design Optimization Study. Plants 2023, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Verma, D.K.; Thakur, M.; Tripathy, S.; Patel, A.R.; Shah, N.; Utama, G.L.; Srivastav, P.P.; Benavente-Valdés, J.R.; Chávez-González, M.L.; et al. Supercritical Fluid Extraction (SCFE) as Green Extraction Technology for High-Value Metabolites of Algae, Its Potential Trends in Food and Human Health. Food Res. Internat. 2021, 150, 110746. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef]
- Essien, S.O.; Young, B.; Baroutian, S. Recent Advances in Subcritical Water and Supercritical Carbon Dioxide Extraction of Bioactive Compounds from Plant Materials. Trends Food Sci. Technol. 2020, 97, 156–169. [Google Scholar] [CrossRef]




| Terpenoid (Source) | Cancer Type Assay/Reported Activities | Ref. |
|---|---|---|
| Andrographolide (Andrographis paniculate) | C8161 and A375 human malignant melanoma cells/cell cycle arrest and apoptosis | [53] |
| Betulinic acid | A375 melanoma cells/dose-dependent inhibitory effect in both mitochondrial respiration and glycolysis; induced mitochondrial dysfunction (10 μM) | [54] |
| Borneol | Glioma cells/promote apoptosis through the regulation of HIF-1a expression via theMTORC1/EIF4E pathway; esophageal squamous cell carcinoma cells/enhances paclitaxel-induced apoptosis via the inactivation of the PI3K/AKT pathway | [55,56] |
| Bornyl cis-4-hydroxycinnamate | Melanoma cell/apoptosis via mitochondrial dysfunction and endoplasmic reticulum stress | [57] |
| (+)-Bornyl p-coumarate | Melanoma cells/induced apoptosis and autophagy | [58] |
| Carvone | MCF-7 breast ductal carcinoma/Protective effect against tumor (IC50 14.22 μM) | [59] |
| Citral (nanostructured lipid carrier) | MBA-231 breast cancer cells/in vitro cytotoxicity and anticancer activity | [60] |
| Chlorinated guaiane-type sesquiterpene lactones (Centaurea plants) | U-937 leukemia HL-60 cells/cytotoxicity | [61] |
| Curcumin and crocin | Cervical cancer cells/protective and therapeutic effects against tumor cells | [62] |
| Cucurbitacin C (Cucumber) | Cancer-cell-derived xenograft tumors in athymic nude mice/dose-dependent inhibited proliferation and clonogenic potential: cell cycle arrest at G1 or G2/M stage at low-dose; apoptosis at high-dose treatment | [63] |
| Cucurbitacin E | NCI-N87 gastric cancer cells/enhanced doxorubicin cytotoxicity | [64] |
| Helenalin | Embryonal rhabdomyosarcoma cells/increase ROS levels, decrease mitochondrial membrane potential, trigger endoplasmic reticulum stress, and deactivate the NF-κB pathway | [65] |
| Hinokitiol (>90%) | A549 lung adenocarcinoma/reduced cell migration and chemoprevention | [66] |
| Limonene (chitosan nanoparticles containing limonene and limonene-rich essential oils) | Melanoma and breast cancers/potential phytotherapy agents for cancer treatment | [67] |
| Myrcene | A549 lung adenocarcinoma/increased apoptosis via caspase induction (IC50 0.5 μg/mL), MTT assay | [68] |
| Myrtenal | B16F0, B16F10, and SkMel-5 melanoma cells/decreased tumor cells migration and invasion | [69] |
| Oleanolic and ursolic acid derivatives | NCI-60 cancer cells/antiproliferative and cytotoxic effects | [70] |
| Thymoquinone | B16-F10 melanoma cells model/inhibition of melanogenesis | [71] |
| Triptolide | Breast cancer cells/enhanced sensitization to doxorubicin (DNA damage response inhibition) | [72] |
| α-Phellandrene | Melanoma (B-16/F-10), and murine (S-180) cells/antinociceptive and tumor-reducing effect (CI50 436.0 and 217.9 μg/mL); MTT assay | [73] |
| α-Pinene | HepG2 liver cancer/reduced cell growth (IR 39.3%), MTT assay | [74] |
| α-Terpineol | Murine sarcoma 180 cell line/antitumor activity against different tumor cell lines (lung, breast, leukemias, and colorectal); blockage of NF-kB expression | [75] |
| α-Thujone (Thuja occidentalis L.) | T98G and U-87 MG glioblastoma cells/induction of cell death, reduced proliferation and invasives; TB exclusion | [76] |
| β-elemene (Curcuma wenyujin) | β-elemene-derived antitumor drug/antitumor mechanisms and structural modification A375 melanoma cells/β-elemene enhances radio sensitization | [77,78] |
| Terpenoid | Assay/Reported Activities | Ref. |
|---|---|---|
| Artemisinin | Rats/attenuates doxorubicin-induced cardiotoxicity and hepatotoxicity | [84] |
| Rats/renoprotective effects on IgA nephropathy by suppressing NF-κB signaling and NLRP3 inflammasome activation by exosomes | [47] | |
| Human macrophage U937 cells/anti-inflammatory effect on uric acid-induced NLRP3 inflammasome activation through blocking the interaction between NLRP3 and NEK7 | [85] | |
| I/R model rats/suppresses myocardial ischemia–reperfusion injury via NLRP3 inflammasome mechanism | [86] | |
| Bakuchiol | C57BL6 male mice/protective effect limiting the synthesis of fibrosis, preventing oxidative damage and cell death in diabetic myocardium | [87] |
| C57BL/6J mice and NRCM cells/antihypertrophy effects by modulating the synthesis of fibrosis and inflammatory responses | [88] | |
| Betulin | Diabetic mice and glucose-stimulated H9c2 cells/protective impact on injured myocardium; significant reduction in cardiac inflammation (anticardiac inflammatory factor via the SIRT1/NLRP3/NF-κB pathway) | [89] |
| I/R model rats/significantly improved the abnormal electrocardiograms; decreased myocardial infarction area and expression of myocardial enzymes and inflammatory cytokines and SITI1; decreased protein expression levels of NLRP3 and NF-κB (anti-inflammatory mechanism is associated with the NLRP3/NF-κB signaling pathway) | [90] | |
| Carnosic acid | C57BL/6 mice/antiobesity effect by improving hormone homeostasis and reduced gene expression of liver lipogenesis possibly via the PPAR-γ pathway | [91] |
| C57BL/6 mice/cardioprotective effect against myocardial remodeling by modulation oxidative stress and apoptosis via the AKT/GSK3β/NOX 4 signaling pathway | [92] | |
| C57BL/mice and H9c2 cells/protects the heart against toxicity via the suppression of oxidative damage, inflammation, apoptosis, and autophagy | [93] | |
| Carnosol | H9c2 cells/protective effect against inflammation in the cardiomyoblasts may be via the NF-κB signaling pathway | [94] |
| MAPC cells/promote vascular health by regulating redox status and downregulating inflammation and apoptosis | [95] | |
| Carvacrol | Wistar rats/protective effect against myocardial hypertrophy by improving blood pressure and inhibiting apoptosis via regulation of the Bcl-2 family protein | [96] |
| Celastrol | Rat primary cardiomyocytes and H9C2 cells/prevents myocardium fibrosis and hypertrophy produced by angiotensin II | [97] |
| Hinokitiol | SEVC4-10 and A7r5 cells/protective effect against atherosclerosis by modulating cell adhesion molecules and members of the matrix metalloproteinase (MMP) family | [98] |
| AC16 cells/protects cardiomyocytes from oxidative damage by regulating apoptosis and autophagy, probably through the GSK3β signaling pathway | [99] | |
| Ferruginol | C57BL/mice and H9c2 cells/cardioprotective action by preserving the mitochondrial from the production of ROS, limiting damage to heart function, and attenuating the apoptotic process, possibly via the SIRT1 pathway that mediates mitochondrial biogenesis and fatty acid oxidation | [100] |
| Wistar rats/cardioprotective effect against myocardial infarction via modulation of inflammatory response and upregulation of antioxidant enzymes | [101] | |
| Geniposide | Spontaneous hypertensive rat/modulates blood pressure by inhibiting the WNK pathway mediated by the estrogen receptors | [102] |
| I/R model rats and H9C2 cells/inhibition of autophagy via geniposide protects against myocardial I/R injury | [103] | |
| Neurons and PC-12 cells/inhibits NLRP3 inflammasome activation via autophagy in BV-2 microglial cells exposed to oxygen–glucose deprivation/reoxygenation | [104] | |
| Mice/protects against sepsis-induced myocardial dysfunction through AMPKα-dependent pathway | [105] | |
| Oridonin | Renal proximal tubular epithelial cells and acute lung injury mice model/suppressed NF-κB or MAPK activation and release of TNF-α and IL-6 | [106,107] |
| Rats/drastically diminish the extent of myocardial infarction and the blood cardiac enzymes | [108] | |
| Mice/attenuates myocardial I/R injury by downregulating oxidative stress and the NLRP3 inflammasome pathway | [109] | |
| Mouse models of peritonitis, gouty arthritis, and type 2 diabetes/specific covalent inhibitor of NLRP3 inflammasomes, inhibiting the assembly and activation of NLRP3 inflammasomes | [110] | |
| RAW264.7 cells and mouse model/protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways | [111] | |
| Pterostilbene | Rat heart subjected to ischemia–reperfusion/attenuates inflammation via the TLR4/NF-kB signaling pathway | [112] |
| Sweroside | H9c2 cells/protects against myocardial ischemia–reperfusion injury by inhibiting oxidative stress and pyroptosis partially via modulation of the keap1/Nrf2 axis | [113] |
| H9c2 cells/ameliorate the cardiotoxicity of aconitine and the incidence of arrhythmias generated by aconitine | [114] | |
| H9c2 cells/protect against myocardial ischemia–reperfusion injury by inhibiting oxidative stress and pyroptosis partially via modulation of the keap1/Nrf2 axis. | [113] | |
| NASH mouse model/prevents non-alcoholic steatohepatitis by suppressing activation of the NLRP3 inflammasome | [115] | |
| Thymol | Albino Wistar rats/cardioprotective effect against myocardial infarction by modulating oxidative stress, inflammation, and apoptosis | [116] |
| Triptolide | Mice and mouse cardiac fibroblasts/significantly inhibit the activation of NLRP3 inflammasome and show an antifibrosis effect | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules 2024, 29, 3861. https://doi.org/10.3390/molecules29163861
Câmara JS, Perestrelo R, Ferreira R, Berenguer CV, Pereira JAM, Castilho PC. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules. 2024; 29(16):3861. https://doi.org/10.3390/molecules29163861
Chicago/Turabian StyleCâmara, José S., Rosa Perestrelo, Rui Ferreira, Cristina V. Berenguer, Jorge A. M. Pereira, and Paula C. Castilho. 2024. "Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview" Molecules 29, no. 16: 3861. https://doi.org/10.3390/molecules29163861
APA StyleCâmara, J. S., Perestrelo, R., Ferreira, R., Berenguer, C. V., Pereira, J. A. M., & Castilho, P. C. (2024). Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules, 29(16), 3861. https://doi.org/10.3390/molecules29163861








