Ionic Liquid-Catalyzed CO2 Conversion for Valuable Chemicals
Abstract
1. Introduction
2. Ionic Liquids
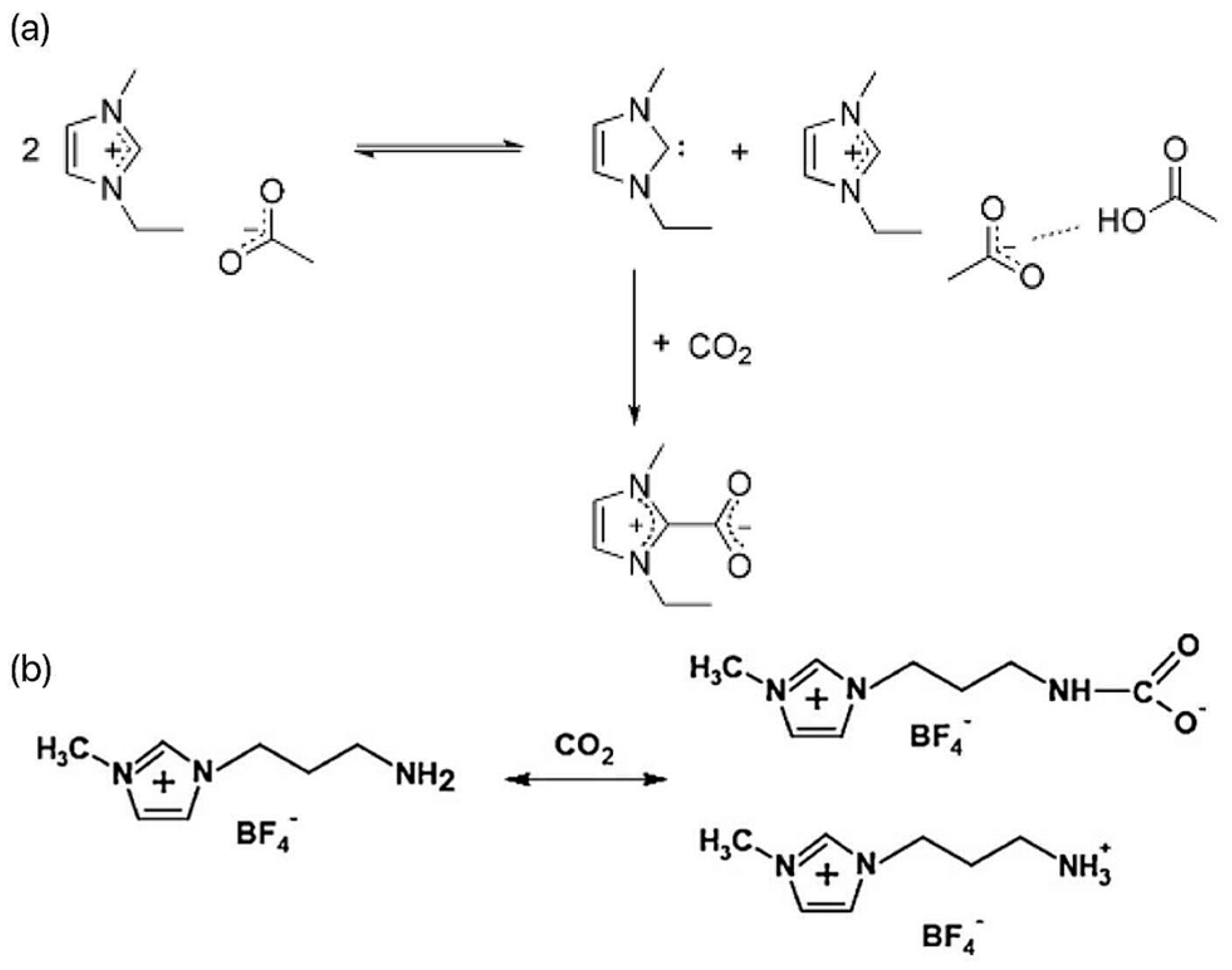
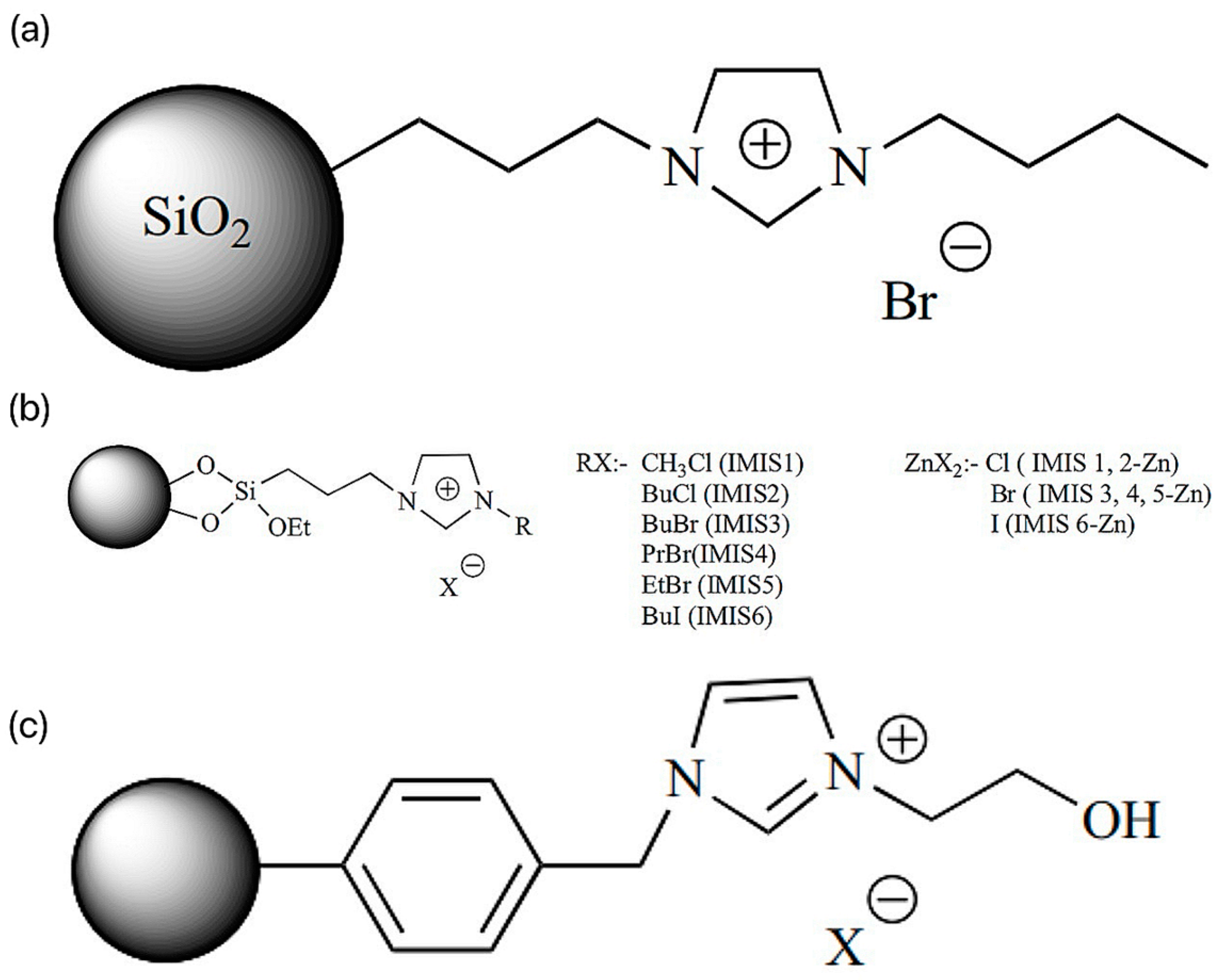
2.1. Imidazole Ionic Liquids
2.2. Pyridine Ionic Liquids
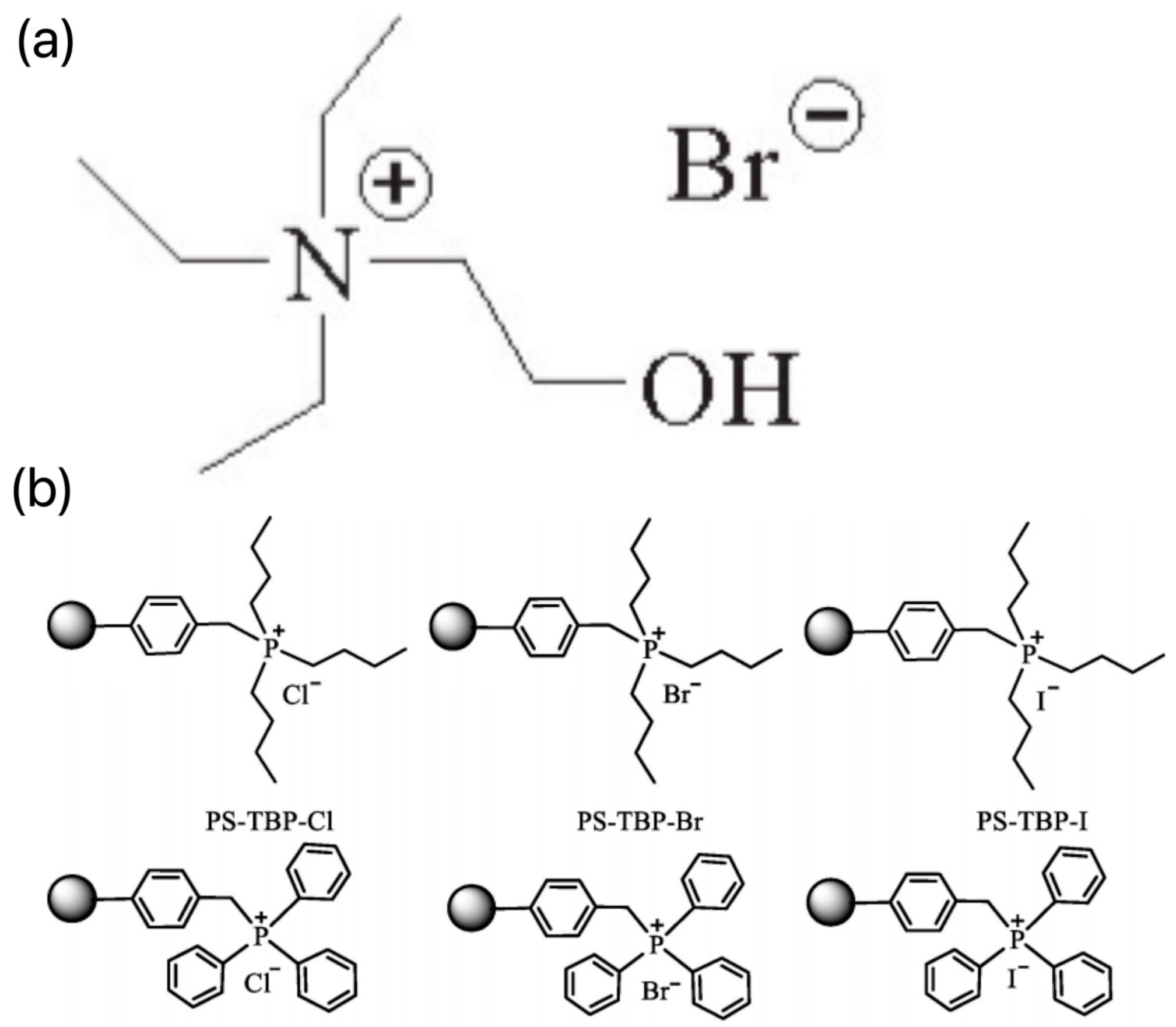
2.3. Quaternary Ammonium/Quaternary Phosphonium Salts Ionic Liquids
2.4. Loaded Ionic Liquids
2.5. Plasmonic Ionic Liquids
2.6. Non-Protonic Ionic Liquids
3. Application of Ionic Liquids in Catalytic CO2 Conversion
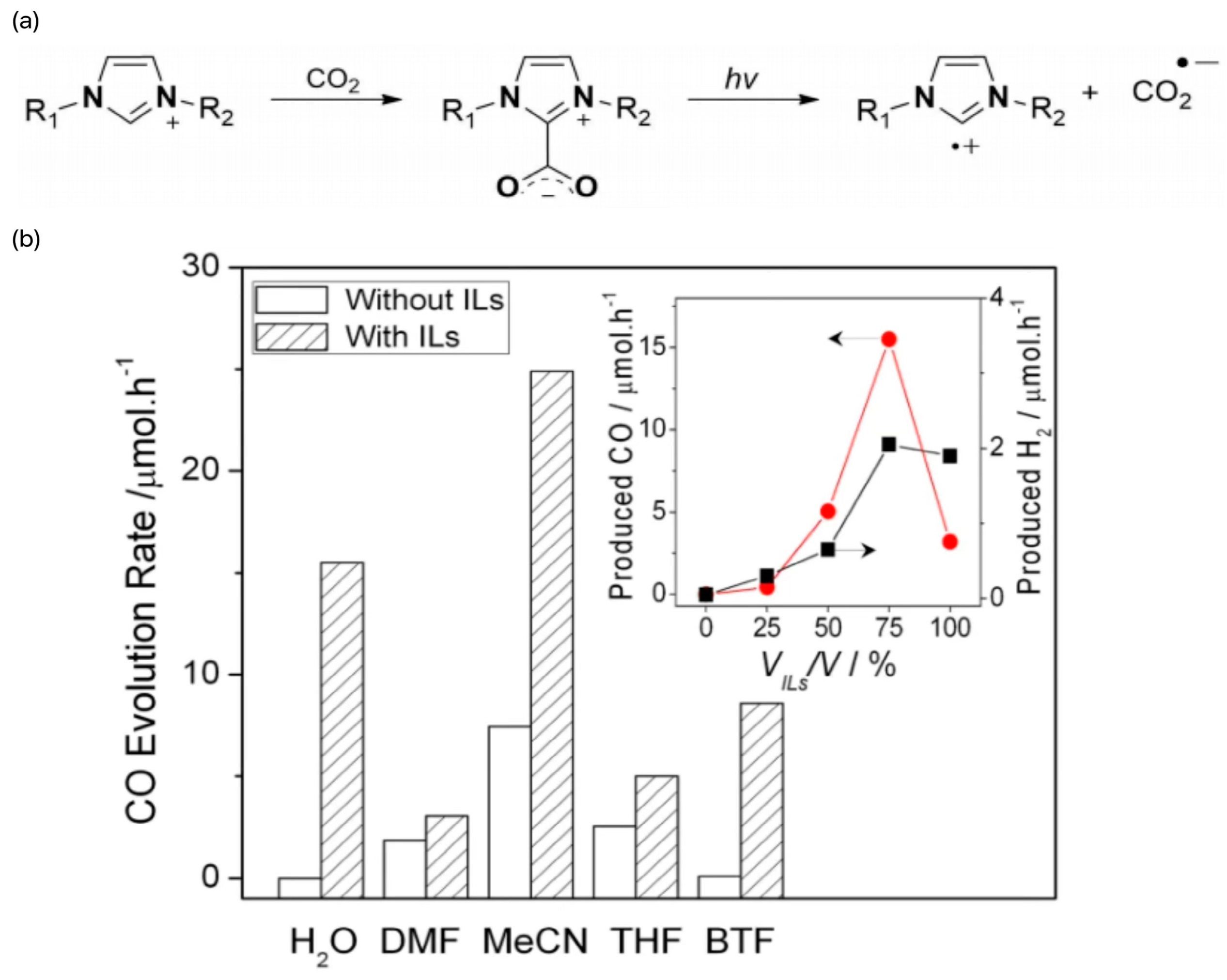
3.1. Photocatalytic Conversion of CO2 by Ionic Liquids
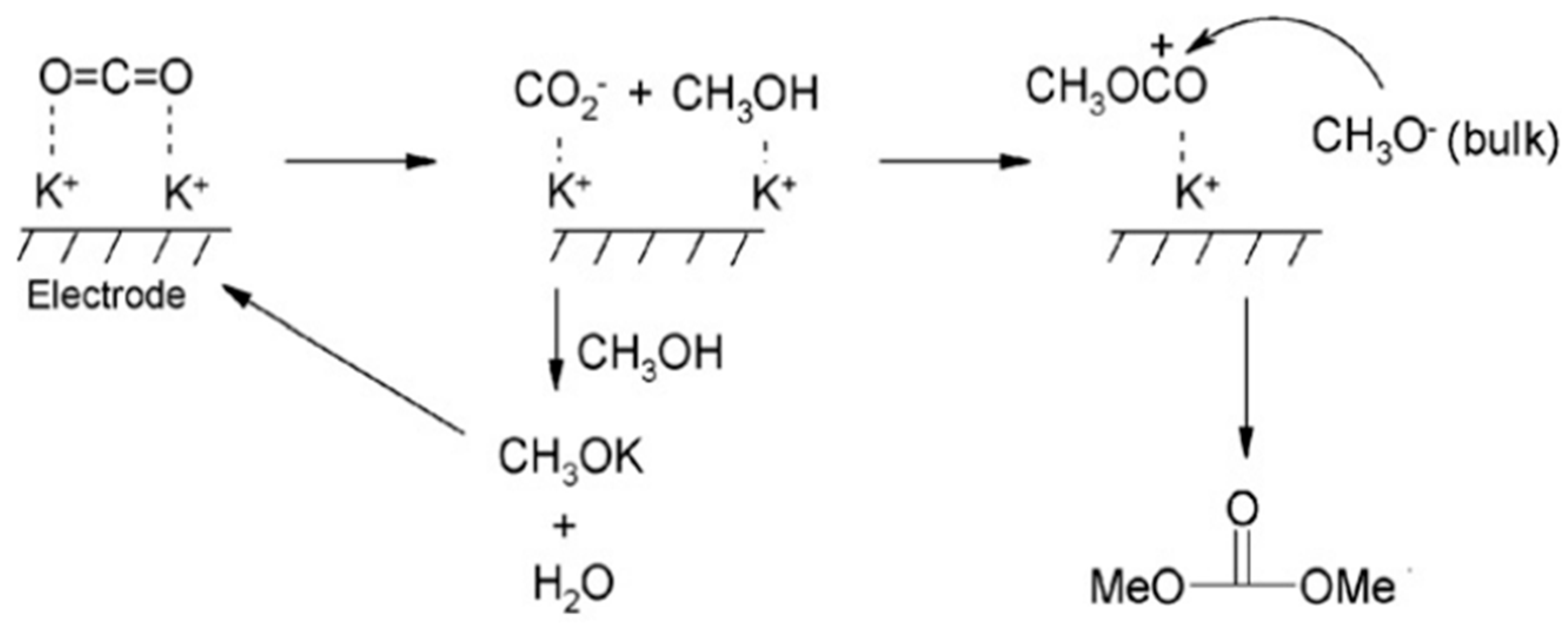
3.2. Electrocatalytic Conversion of CO2 by Ionic Liquids
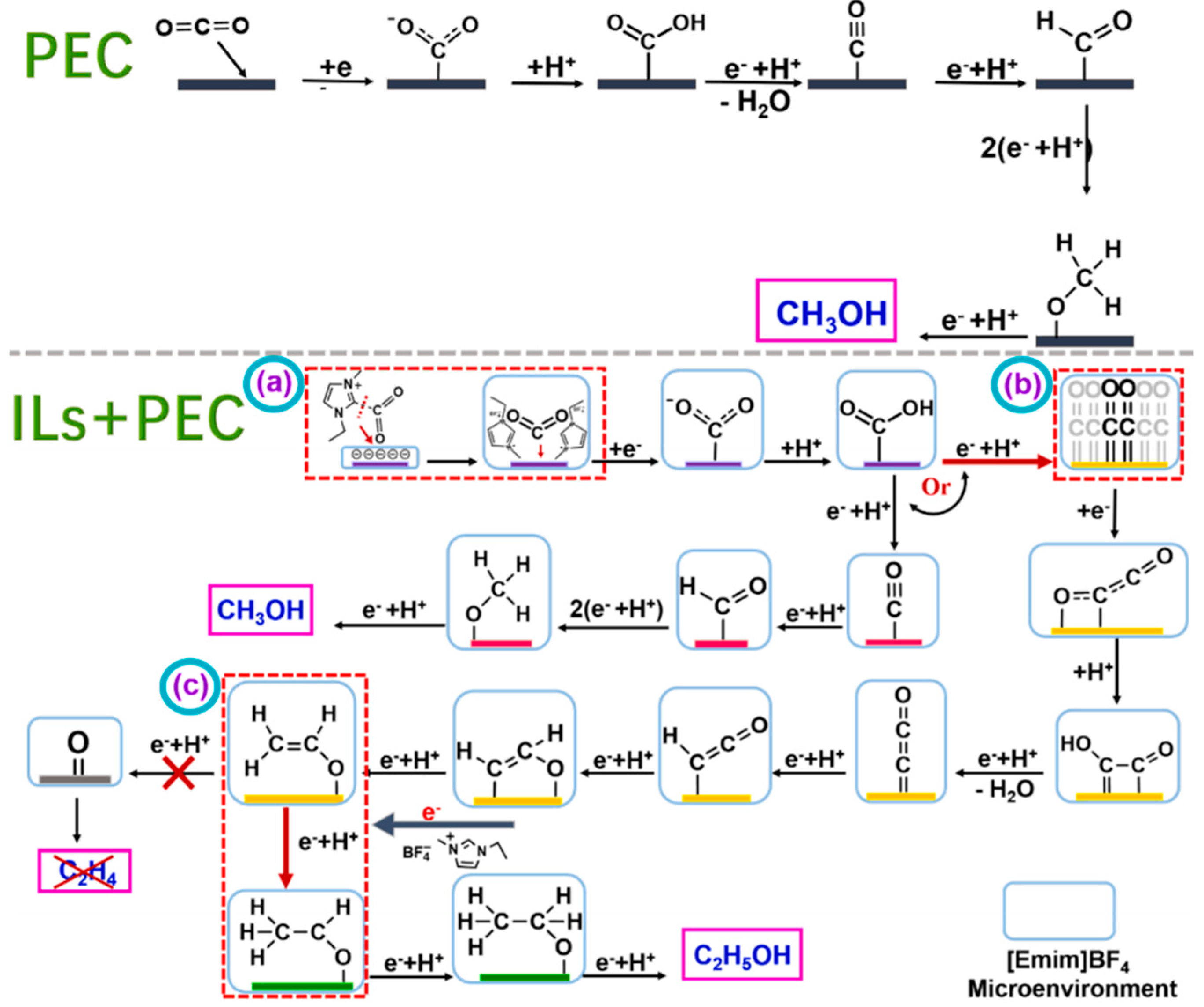
3.3. Photoelectrocatalytic Conversion of CO2 by Ionic Liquids
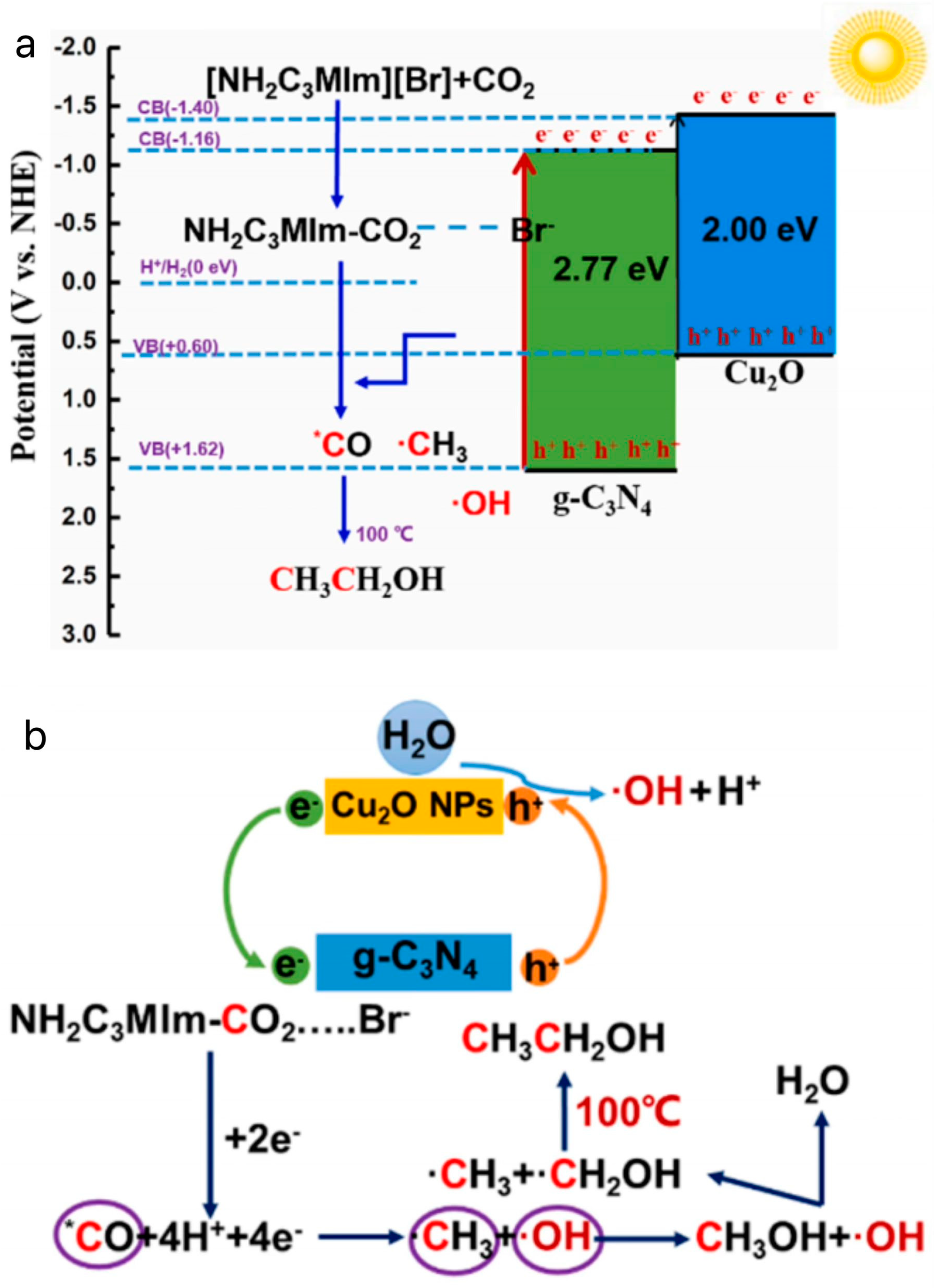
3.4. Photothermal Catalytic Conversion of CO2 by Ionic Liquids
4. Valuable Chemicals
4.1. CO2 Hydrogenation
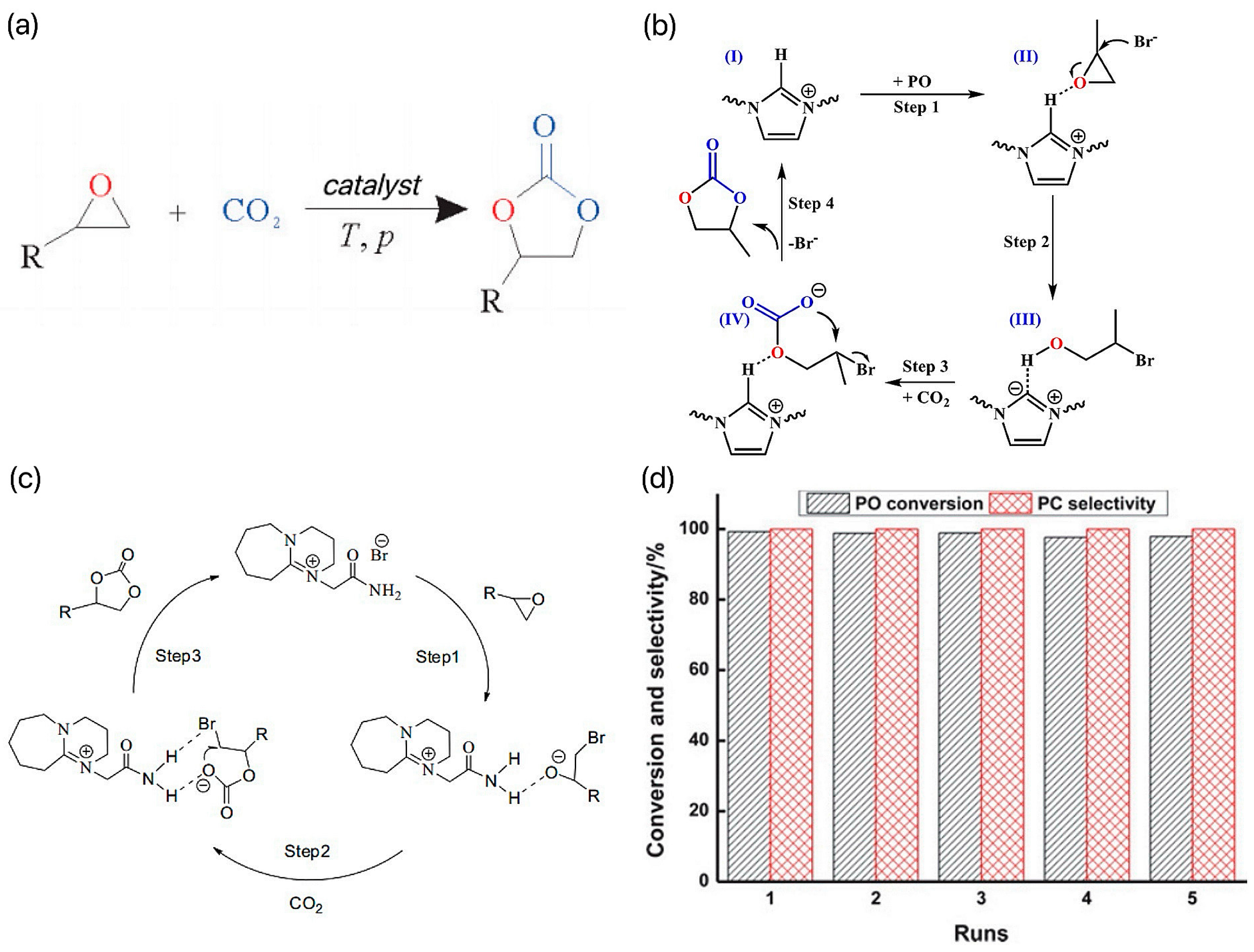
4.2. C–O Bond
4.3. C–N Bond
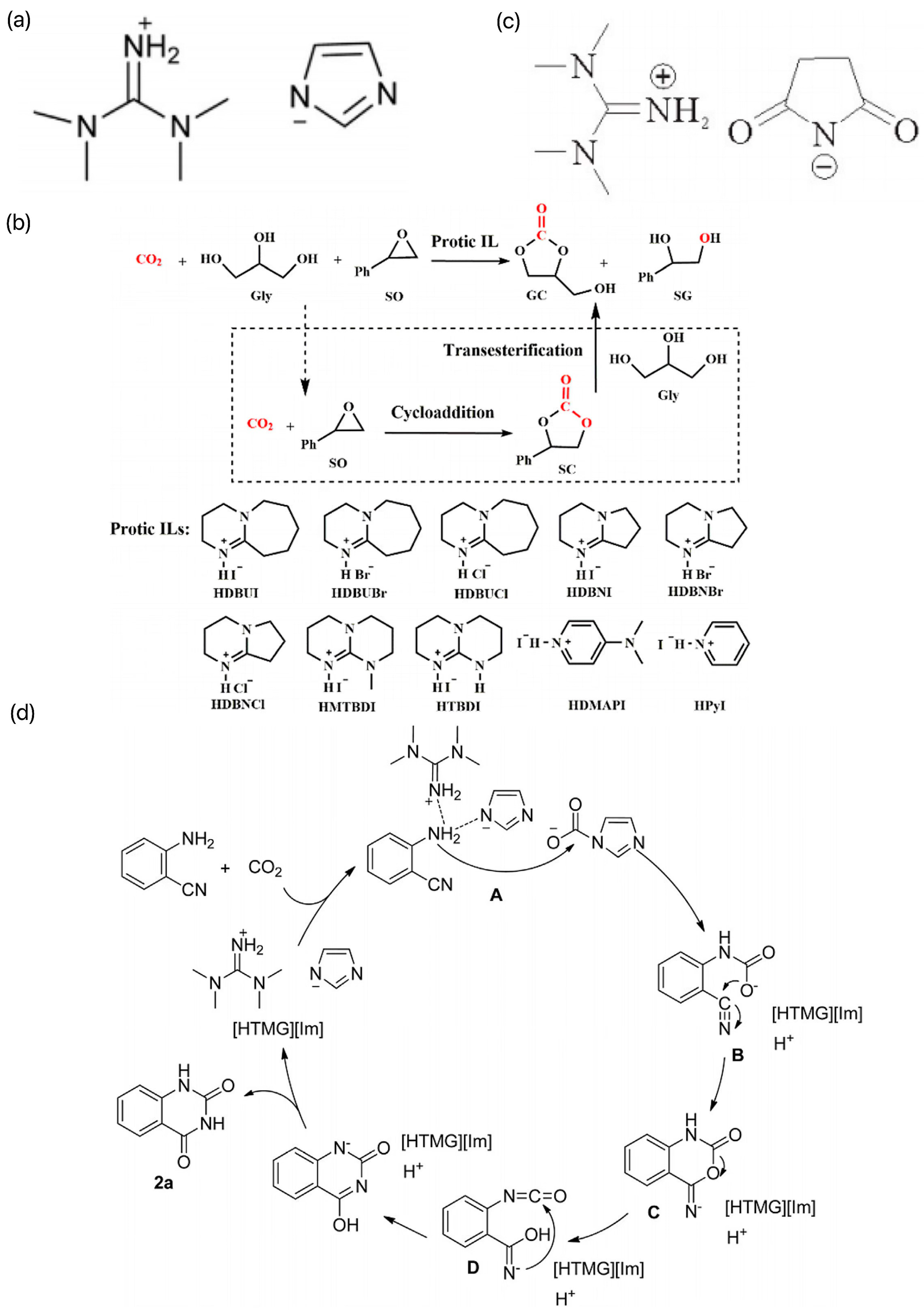
4.4. C–S Bond
4.5. C1 Products
4.6. C–C Bond
4.7. C–N Bond
5. Conclusions
- (1)
- Although functional ILs have multiple functions in CO2 conversion, problems such as high price, poor stability, and high viscosity still need to be solved. Because ILs are highly designable, they can be modified to meet a variety of requirements for industrial applications, such as reducing cost, improving stability, reducing viscosity, enhancing efficiency, increasing selectivity, and facilitating separation;
- (2)
- Combining metal-free photocatalysts with task-specific ILs to capture CO2 from the air and simulate artificial photosynthesis to produce high value-added products is a potential research direction in the future;
- (3)
- Consider the use of low-cost metal-free catalysts for thermal conversion of CO2 at room temperature and pressure to improve economy and practicality;
- (4)
- ILs should be stable during thermal, electrical, and photocatalytic processes. Many ILs may break down or react with other chemicals after prolonged exposure to the environment. In particular, the problem to be solved is how to improve the stability of functionalized task-specific ILs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, B.-H.; Wang, J.-Q.; Sun, J.; Huang, Y.; Zhang, J.-P.; Zhang, X.-P.; Zhang, S.-J. Fixation of CO2 into cyclic carbonates catalyzed by ionic liquids: A multi-scale approach. Green Chem. 2015, 17, 108–122. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, Y.; Williams, B.L.; Xiao, M.; Wang, S.; Han, D.; Sun, L.; Meng, Y. Catalytic materials for direct synthesis of dimethyl carbonate (DMC) from CO2. J. Clean. Prod. 2021, 279, 123344. [Google Scholar] [CrossRef]
- Guo, L.; Deng, L.; Hu, W.; Jin, X.; Yin, L. Ionic liquids as catalysts for conversion of CO2 to propylene carbonate. Chem. Ind. Eng. Prog. 2017, 36, 3323–3328. [Google Scholar]
- Cervantes-Reyes, A.; Farshadfar, K.; Rudolph, M.; Rominger, F.; Schaub, T.; Ariafard, A.; Hashmi, A.S.K. Copper-catalysed synthesis of α-alkylidene cyclic carbonates from propargylic alcohols and CO. Green Chem. 2021, 23, 889–897. [Google Scholar] [CrossRef]
- Dong, T.; Zheng, Y.-J.; Yang, G.-W.; Zhang, Y.-Y.; Li, B.; Wu, G.-P. Crosslinked Resin-Supported Bifunctional Organocatalyst for Conversion of CO2 into Cyclic Carbonates. Chemsuschem 2020, 13, 4121–4127. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Scientific Basis for Integrating Carbon Resource Processing, Utilization, and Recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhang, X.; Bai, L.; Zhang, X.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S. Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process. Chem. Rev. 2017, 117, 9625–9673. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, I.; Garcia-Gutierrez, P.; Elder, R.H.; Cuellar-Franca, R.M.; Azapagic, A.; Allen, R.W.K. Carbon dioxide utilisation for production of transport fuels: Process and economic analysis. Energy Environ. Sci. 2015, 8, 1775–1789. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Gao, Z.; Jin, F.; Zhang, J.; Li, C. Progress of functionalized ionic liquids for CO2 absorption. Mod. Chem. Ind. 2017, 37, 41–45,47. [Google Scholar]
- Torralba-Calleja, E.; Skinner, J.; Gutierrez-Tauste, D. CO2 Capture in Ionic Liquids: A Review of Solubilities and Experimental Methods. J. Chem. 2013, 2013, 473584. [Google Scholar] [CrossRef]
- Gurau, G.; Rodriguez, H.; Kelley, S.P.; Janiczek, P.; Kalb, R.S.; Rogers, R.D. Demonstration of Chemisorption of Carbon Dioxide in 1,3-Dialkylimidazolium Acetate Ionic Liquids. Angew. Chem.—Int. Ed. 2011, 50, 12024–12026. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.M.G.; Meindersma, G.W.; de Haan, A.B. Kinetics of absorption of CO2 in amino-functionalized ionic liquids. Chem. Eng. J. 2011, 166, 1104–1115. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, S.; Wang, D.; Yao, X. Hydrogen bonds in imidazolium ionic liquids. J. Phys. Chem. A 2006, 110, 9775–9782. [Google Scholar] [CrossRef] [PubMed]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 capture by a task-specific ionic liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pan, S.-Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ye, Y.; Xu, P.; Sun, J. Experimental and theoretical study on dicationic imidazolium derived poly(ionic liquid)s for catalytic cycloaddition of CO2-epoxide. J. CO2 Util. 2023, 67, 102325. [Google Scholar] [CrossRef]
- Li, W.; Cheng, W.; Yang, X.; Su, Q.; Dong, L.; Zhang, P.; Yi, Y.; Li, B.; Zhang, S. Synthesis of Cyclic Carbonate Catalyzed by DBU Derived Basic Ionic Liquids. Chin. J. Chem. 2018, 36, 293–298. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Huang, T.; Zhang, J.; He, H. Catalytic Activity of a Series of Synthesized and Newly Designed Pyridinium-Based Ionic Liquids on the Fixation of Carbon Dioxide: A DFT Investigation. Ind. Eng. Chem. Res. 2015, 54, 8093–8099. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, F.; Xing, H.; Yang, Q.; Bao, Z.; Ren, Q. Efficient Synthesis of Cyclic Carbonates from Atmospheric CO2 Using a Positive Charge Delocalized Ionic Liquid Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2841–2846. [Google Scholar] [CrossRef]
- Yuan, G.; Zhao, Y.; Wu, Y.; Li, R.; Chen, Y.; Xu, D.; Liu, Z. Cooperative effect from cation and anion of pyridine-containing anion-based ionic liquids for catalysing CO2 transformation at ambient conditions. Sci. China-Chem. 2017, 60, 958–963. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Jin, X.; Zhang, J.; He, H.; Zhang, S. Mechanism of fixation of CO2 in the presence of hydroxyl-functionalized quaternary ammonium salts. J. CO2 Util. 2015, 10, 113–119. [Google Scholar] [CrossRef]
- Liu, S.; Suematsu, N.; Maruoka, K.; Shirakawa, S. Design of bifunctional quaternary phosphonium salt catalysts for CO2 fixation reaction with epoxides under mild conditions. Green Chem. 2016, 18, 4611–4615. [Google Scholar] [CrossRef]
- Li, Y.; Yao, X.; Liu, Y.; Li, M.; Su, Q.; Liu, Y.; Li, Y.; Yang, Z. Polymer-supported Quaternary Phosphonium Ionic Liquid Catalysts for Efficient Fixation of CO2 into Cyclic Carbonates. ChemistrySelect 2023, 8, e202301449. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Yu, K.; Yin, J. A novel supported ionic liquid membrane reactor for catalytic CO2 conversion. J. CO2 Util. 2022, 65, 102216. [Google Scholar] [CrossRef]
- Lee, M.-K.; Shim, H.-L.; Dharman, M.M.; Kim, K.-H.; Park, S.-W.; Park, D.-W. Synthesis of cyclic carbonate from allyl glycidyl ether and CO2 over silica-supported ionic liquid catalysts prepared by sol-gel method. Korean J. Chem. Eng. 2008, 25, 1004–1007. [Google Scholar] [CrossRef]
- Schaeffner, B.; Schaeffner, F.; Verevkin, S.P.; Boerner, A. Organic Carbonates as Solvents in Synthesis and Catalysis. Chem. Rev. 2010, 110, 4554–4581. [Google Scholar] [CrossRef]
- Parker, H.L.; Sherwood, J.; Hunt, A.J.; Clark, J.H. Cyclic Carbonates as Green Alternative Solvents for the Heck Reaction. ACS Sustain. Chem. Eng. 2014, 2, 1739–1742. [Google Scholar] [CrossRef]
- Beattie, C.; North, M.; Villuendas, P. Proline-Catalysed Amination Reactions in Cyclic Carbonate Solvents. Molecules 2011, 16, 3420–3432. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Bertrand, J.-P.; Lecocq, A.; Grugeon, S.; Laruelle, S.; Armand, M.; Marlair, G. Fire behavior of carbonates-based electrolytes used in Li-ion rechargeable batteries with a focus on the role of the LiPF6 and LiFSI salts. J. Power Sources 2014, 269, 804–811. [Google Scholar] [CrossRef]
- Seo, D.M.; Reininger, S.; Kutcher, M.; Redmond, K.; Euler, W.B.; Lucht, B.L. Role of Mixed Solvation and Ion Pairing in the Solution Structure of Lithium Ion Battery Electrolytes. J. Phys. Chem. C 2015, 119, 14038–14046. [Google Scholar] [CrossRef]
- Guo, W.; Laserna, V.; Martin, E.; Escudero-Adán, E.C.; Kleij, A.W. Stereodivergent Carbamate Synthesis by Selective in Situ Trapping of Organic Carbonate Intermediates. Chemistry 2015, 22, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Helou, M.; Miserque, O.; Brusson, J.M.; Carpentier, J.F.; Guillaume, S.M. Organocatalysts for the Controlled “Immortal” Ring-Opening Polymerization of Six-Membered-Ring Cyclic Carbonates: A Metal-Free, Green Process. Chemistry 2010, 16, 13805–13813. [Google Scholar] [CrossRef] [PubMed]
- Besse, V.; Camara, F.; Voirin, C.; Auvergne, R.; Caillol, S.; Boutevin, B. Synthesis and applications of unsaturated cyclocarbonates. Polym. Chem. 2013, 4, 4545–4561. [Google Scholar] [CrossRef]
- Suriano, F.; Pratt, R.; Tan, J.P.K.; Wiradharma, N.; Nelson, A.; Yang, Y.-Y.; Dubois, P.; Hedrick, J.L. Synthesis of a family of amphiphilic glycopolymers via controlled ring-opening polymerization of functionalized cyclic carbonates and their application in drug delivery. Biomaterials 2010, 31, 2637–2645. [Google Scholar] [CrossRef]
- Kaur, P.; Chopra, H.K. Recent Advances in Applications of Supported Ionic Liquids. Curr. Org. Chem. 2019, 23, 2881–2915. [Google Scholar] [CrossRef]
- Han, L.; Park, S.-W.; Park, D.-W. Silica grafted imidazolium-based ionic liquids: Efficient heterogeneous catalysts for chemical fixation of CO2 to a cyclic carbonate. Energy Environ. Sci. 2009, 2, 1286–1292. [Google Scholar] [CrossRef]
- Zheng, X.; Luo, S.; Zhang, L.; Cheng, J.-P. Magnetic nanoparticle supported ionic liquid catalysts for CO2 cycloaddition reactions. Green Chem. 2009, 11, 455–458. [Google Scholar] [CrossRef]
- Han, L.; Choi, H.-J.; Kim, D.-K.; Park, S.-W.; Liu, B.; Park, D.-W. Porous polymer bead-supported ionic liquids for the synthesis of cyclic carbonate from CO2 and epoxide. J. Mol. Catal. A Chem. 2011, 338, 58–64. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, W.; Fan, W.; Wang, Y.; Meng, Z.; Zhang, S. Reusable and efficient polymer-supported task-specific ionic liquid catalyst for cycloaddition of epoxide with CO2. Catal. Today 2009, 148, 361–367. [Google Scholar] [CrossRef]
- Dai, W.-L.; Chen, L.; Yin, S.-F.; Li, W.-H.; Zhang, Y.-Y.; Luo, S.-L.; Au, C.-T. High-Efficiency Synthesis of Cyclic Carbonates from Epoxides and CO2 over Hydroxyl Ionic Liquid Catalyst Grafted onto Cross-Linked Polymer. Catal. Lett. 2010, 137, 74–80. [Google Scholar] [CrossRef]
- Lang, X.-D.; Yu, Y.-C.; Li, Z.-M.; He, L.-N. Protic ionic liquids-promoted efficient synthesis of quinazolines from 2-aminobenzonitriles and CO2 at ambient conditions. J. CO2 Util. 2016, 15, 115–122. [Google Scholar] [CrossRef]
- Liu, F.; Ping, R.; Zhao, P.; Gu, Y.; Gao, J.; Liu, M. Succinimide-Based Ionic Liquids: An Efficient and Versatile Platform for Transformation of CO2 into Quinazoline-2,4(1H,3H)-diones under Mild and Solvent-Free Conditions. ACS Sustain. Chem. Eng. 2019, 7, 13517–13522. [Google Scholar] [CrossRef]
- Luo, C.; Wang, J.; Lu, H.; Wu, K.; Liu, Y.; Zhu, Y.; Wang, B.; Liang, B. Atmospheric-pressure synthesis of glycerol carbonate from CO2 and glycerol catalyzed by protic ionic liquids. Green Chem. 2022, 24, 8292–8301. [Google Scholar] [CrossRef]
- Shi, G.; Chen, K.; Wang, Y.; Li, H.; Wang, C. Highly Efficient Synthesis of Quinazoline-2,4(1H,3H)-diones from CO2 by Hydroxyl Functionalized Aprotic Ionic Liquids. ACS Sustain. Chem. Eng. 2018, 6, 5760–5765. [Google Scholar] [CrossRef]
- Zhu, A.; Tang, M.; Lv, Q.; Li, L.; Bai, S.; Li, Q.; Feng, W.; Li, Q.; Wang, J. Fixation of CO2 in structurally diverse quinazoline-2,4(1H,3H)-diones under ambient conditions. J. CO2 Util. 2019, 34, 500–506. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Yuan, G.; Hao, L.; Gao, X.; Yang, Z.; Yu, B.; Zhang, H.; Liu, Z. Azole-Anion-Based Aprotic Ionic Liquids: Functional Solvents for Atmospheric CO2 Transformation into Various Heterocyclic Compounds. Chem.—Asian J. 2016, 11, 2735–2740. [Google Scholar] [CrossRef]
- Qadir, M.I.; Dupont, J. Thermo- and Photocatalytic Activation of CO2 in Ionic Liquids Nanodomains. Angew. Chem. Int. Ed. 2023, 62, e202301497. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Zhao, Y.; Liu, Z. Recent Progress on Ionic Liquid-Mediated CO2 Conversion. Acta Phys. Chim. Sin. 2020, 37, 2010022. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Yu, B.; Wu, Y.; Yu, X.; Guo, S.; Han, B.; Liu, Z. Visible Light-Driven Photoreduction of CO2 to CH4 over TiO2 Using a Multiple-Site Ionic Liquid as an Absorbent and Photosensitizer. ACS Sustain. Chem. Eng. 2020, 8, 9088–9094. [Google Scholar] [CrossRef]
- Lin, J.; Ding, Z.; Hou, Y.; Wang, X. Ionic Liquid Co-catalyzed Artificial Photosynthesis of CO. Sci. Rep. 2013, 3, 1056. [Google Scholar] [CrossRef]
- Lu, B.; Wang, X.; Li, Y.; Sun, J.; Zhao, J.; Cai, Q. Electrochemical conversion of CO2 into dimethyl carbonate in a functionalized ionic liquid. J. CO2 Util. 2013, 3–4, 98–101. [Google Scholar] [CrossRef]
- Hu, Y.L.; Liu, X.B.; Rong, Q. Novel and highly efficient electro-catalytic cycloaddition of CO2 and epoxides to cyclic carbonates over reusable ionic liquid-based cooperative catalytic system. Green Chem. Lett. Rev. 2023, 16, 2163192. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Guo, H.; Liu, L.; Wang, H.; Cui, W. Ionic liquids enhanced highly efficient photoelectrochemical reduction of CO2 to ethanol over Cu2O/TiO2 nanoarrays. Mol. Catal. 2023, 543, 113161. [Google Scholar] [CrossRef]
- Chaugule, A.A.; Bandhal, H.A.; Tamboli, A.H.; Chung, W.-J.; Kim, H. Highly efficient synthesis of dimethyl carbonate from methanol and carbon dioxide using IL/DBU/SmOCl as a novel ternary catalytic system. Catal. Commun. 2016, 75, 87–91. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, B.; Li, X.; Zhang, W.; Zhao, J.; Cai, Q. Hydroxyl-functionalized ionic liquid for activation and conversion of CO2 and methanol into dimethyl carbonate. J. CO2 Util. 2015, 12, 49–53. [Google Scholar] [CrossRef]
- Strehmel, V. Radicals in Ionic Liquids. ChemPhysChem 2012, 13, 1649–1663. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Imidazolium Ionic Liquids, Imidazolylidene Heterocyclic Carbenes, and Zeolitic Imidazolate Frameworks for CO2 Capture and Photochemical Reduction. Angew. Chem.—Int. Ed. 2016, 55, 2308–2320. [Google Scholar] [CrossRef]
- Wu, F.; Xu, C.; Margulis, C.J. Dynamics of an excess hole in the 1-methyl-1-butyl-pyrrolidinium dicyanamide ionic-liquid. J. Chem. Phys. 2018, 148, 193831. [Google Scholar] [CrossRef]
- Yilmaz, P.; Lacerda, A.M.; Larrosa, I.; Dunn, S. Photoelectrocatalysis of Rhodamine B and Solar Hydrogen Production by TiO2 and Pd/TiO2 Catalyst Systems. Electrochim. Acta 2017, 231, 641–649. [Google Scholar] [CrossRef]
- Wei, Z.; Liang, F.; Liu, Y.; Luo, W.; Wang, J.; Yao, W.; Zhu, Y. Photoelectrocatalytic degradation of phenol-containing wastewater by TiO2/g-C3N4 hybrid heterostructure thin film. Appl. Catal. B-Environ. 2017, 201, 600–606. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 2013, 6, 3007–3014. [Google Scholar] [CrossRef]
- Peng, Y.; Szeto, K.C.; Santini, C.C.; Daniele, S. Ionic Liquids as homogeneous photocatalyst for CO2 reduction in protic solvents. Chem. Eng. J. Adv. 2022, 12, 100379. [Google Scholar] [CrossRef]
- Rosen, B.A.; Salehi-Khojin, A.; Thorson, M.R.; Zhu, W.; Whipple, D.T.; Kenis, P.J.A.; Masel, R.I. Ionic Liquid-Mediated Selective Conversion of CO2 to CO at Low Overpotentials. Science 2011, 334, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Hu, X. Ionic liquids enhance the electrochemical CO2 reduction catalyzed by MoO2. Chem. Commun. 2015, 51, 13698–13701. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, L.; Wang, Q.; Wang, X.; Li, Q.; Shi, J. Catalytic Mechanism of Ionic Liquid for CO2 Electrochemical Reduction. Chem. J. Chin. Univ.—Chin. 2016, 37, 94–99. [Google Scholar]
- Yuan, D.; Yan, C.; Lu, B.; Wang, H.; Zhong, C.; Cai, Q. Electrochemical activation of carbon dioxide for synthesis of dimethyl carbonate in an ionic liquid. Electrochim. Acta 2009, 54, 2912–2915. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Q.; Kang, X.; Liu, H.; Qian, Q.; Zhang, Z.; Han, B. Molybdenum–Bismuth Bimetallic Chalcogenide Nanosheets for Highly Efficient Electrocatalytic Reduction of Carbon Dioxide to Methanol. Angew. Chem. Int. Ed. 2016, 55, 6771–6775. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, S.; Wang, H.; Wu, Z.; Wang, L. Recent Progress on Photo-Electrocatalytic Reduction of Carbon Dioxide. Part. Part. Syst. Charact. 2018, 35, 1700371. [Google Scholar]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazabal, G.O.; Perez-Ramirez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef]
- Lu, W.; Jia, B.; Cui, B.; Zhang, Y.; Yao, K.; Zhao, Y.; Wang, J. Efficient Photoelectrochemical Reduction of Carbon Dioxide to Formic Acid: A Functionalized Ionic Liquid as an Absorbent and Electrolyte. Angew. Chem. Int. Ed. 2017, 56, 11851–11854. [Google Scholar] [CrossRef]
- Zeng, G.; Qiu, J.; Hou, B.; Shi, H.; Lin, Y.; Hettick, M.; Javey, A.; Cronin, S.B. Enhanced Photocatalytic Reduction of CO2 to CO through TiO2 Passivation of InP in Ionic Liquids. Chemistry 2015, 21, 13502–13507. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Zheng, G. Designing Copper-Based Catalysts for Efficient Carbon Dioxide Electroreduction. Adv. Mater. 2021, 33, 2005798. [Google Scholar] [CrossRef]
- Zheng, Y.; Vasileff, A.; Zhou, X.; Jiao, Y.; Jaroniec, M.; Qiao, S.-Z. Understanding the Roadmap for Electrochemical Reduction of CO2 to Multi-Carbon Oxygenates and Hydrocarbons on Copper-Based Catalysts. J. Am. Chem. Soc. 2019, 141, 7646–7659. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [PubMed]
- Feng, J.; Zeng, S.; Liu, H.; Feng, J.; Gao, H.; Bai, L.; Dong, H.; Zhang, S.; Zhang, X. Insights into Carbon Dioxide Electroreduction in Ionic Liquids: Carbon Dioxide Activation and Selectivity Tailored by Ionic Microhabitat. Chemsuschem 2018, 11, 3191–3197. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, W.; Feng, L.; Fan, Y.; Liang, J.; Wang, X.; Zhang, X. Greenery-inspired nanoengineering of bamboo-like hierarchical porous nanotubes with spatially organized bifunctionalities for synergistic photothermal catalytic CO2 fixation. J. Mater. Chem. A 2022, 10, 12418–12428. [Google Scholar] [CrossRef]
- Bai, L.; Shang, D.; Li, M.; Dai, Z.; Deng, L.; Zhang, X. CO2 absorption with ionic liquids at elevated temperatures. J. Energy Chem. 2017, 26, 1001–1006. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; An, W.; Wang, H.; Cui, W. Efficient photothermal catalytic CO2 reduction to CH3CH2OH over Cu2O/g-C3N4 assisted by ionic liquids. Appl. Surf. Sci. 2021, 565, 150448. [Google Scholar] [CrossRef]
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R.; et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016, 353, 467–470. [Google Scholar] [CrossRef]
- Rosen, B.A.; Haan, J.L.; Mukherjee, P.; Braunschweig, B.; Zhu, W.; Salehi-Khojin, A.; Dlott, D.D.; Masel, R.I. In Situ Spectroscopic Examination of a Low Overpotential Pathway for Carbon Dioxide Conversion to Carbon Monoxide. J. Phys. Chem. C 2012, 116, 15307–15312. [Google Scholar] [CrossRef]
- Sun, L.; Ramesha, G.K.; Kamat, P.V.; Brennecke, J.F. Switching the Reaction Course of Electrochemical CO2 Reduction with Ionic Liquids. Langmuir 2014, 30, 6302–6308. [Google Scholar] [CrossRef]
- Qadir, M.I.; Weilhard, A.; Fernandes, J.A.; de Pedro, I.; Vieira, B.J.C.; Waerenborgh, J.C.; Dupont, J. Selective Carbon Dioxide Hydrogenation Driven by Ferromagnetic RuFe Nanoparticles in Ionic Liquids. ACS Catal. 2018, 8, 1621–1627. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, E.; Li, W.; Hu, S.; Song, J.; Jiang, T.; Han, B. Hydrogenation of carbon dioxide is promoted by a task-specific ionic liquid. Angew. Chem.—Int. Ed. 2008, 47, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Melo, C.I.; Szczepanska, A.; Bogel-Lukasik, E.; da Ponte, M.N.; Branco, L.C. Hydrogenation of Carbon Dioxide to Methane by Ruthenium Nanoparticles in Ionic Liquid. Chemsuschem 2016, 9, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhao, Y.; Li, Z.; Wang, H.; Fan, M.; Wang, J. Efficient Ionic-Liquid-Promoted Chemical Fixation of CO2 into α-Alkylidene Cyclic Carbonates. Chemsuschem 2017, 10, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Tak, R.K.; Patel, P.; Subramanian, S.; Kureshy, R.I.; Khan, N.-u.H. Cycloaddition Reaction of Spiro-Epoxy Oxindole with CO2 at Atmospheric Pressure Using Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2018, 6, 11200–11205. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, R.; Xu, Q.; Jiang, J.; Zhou, X.; Ji, H. Metalloporphyrin Polymers with Intercalated Ionic Liquids for Synergistic CO2 Fixation via Cyclic Carbonate Production. ACS Sustain. Chem. Eng. 2018, 6, 1074–1082. [Google Scholar] [CrossRef]
- Zhao, T.; Hu, X.; Wu, D.; Li, R.; Yang, G.; Wu, Y. Direct Synthesis of Dimethyl Carbonate from Carbon Dioxide and Methanol at Room Temperature Using Imidazolium Hydrogen Carbonate Ionic Liquid as a Recyclable Catalyst and Dehydrant. Chemsuschem 2017, 10, 2046–2052. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, H.-Y.; Fukaya, N.; Yasuda, H.; Choi, J.-C. Direct synthesis of carbamate from CO2 using a task-specific ionic liquid catalyst. Green Chem. 2017, 19, 5614–5624. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; He, L.-N.; Peng, S.-Y.; Liu, A.-H. Lewis basic ionic liquids-catalyzed synthesis of 5-aryl-2-oxazolidinones from aziridines and CO2 under solvent-free conditions. Green Chem. 2010, 12, 1850–1854. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Li, Y.-N.; Wei, Y.-Y.; He, L.-N. Protic onium salts-catalyzed synthesis of 5-aryl-2-oxazolidinones from aziridines and CO2 under mild conditions. Green Chem. 2011, 13, 2351–2353. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Zhang, Z.; Zhu, Q.; Zhou, H.; Lu, W.; Han, B. A route to convert CO2: Synthesis of 3,4,5-trisubstituted oxazolones. Green Chem. 2015, 17, 1219–1225. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Zhu, Q.; Zhang, Z.; Wu, C.; Han, B. Transformation of Atmospheric CO2 Catalyzed by Protic Ionic Liquids: Efficient Synthesis of 2-Oxazolidinones. Angew. Chem.—Int. Ed. 2015, 54, 5399–5403. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yu, B.; Yang, Z.; Zhao, Y.; Zhang, H.; Hao, L.; Han, B.; Liu, Z. Ionic Liquid-Catalyzed C–S Bond Construction using CO2 as a C1 Building Block under Mild Conditions: A Metal-Free Route to Synthesis of Benzothiazoles. ACS Catal. 2015, 5, 6648–6652. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Jiang, T.; Han, B.X.; Li, Z.H.; Zhang, J.M.; Liu, Z.M.; He, J.; Wu, W.Z. Electrochemical reduction of supercritical carbon dioxide in ionic liquid 1-n-butyl-3-methylimidazolium hexafluorophosphate. J. Supercrit. Fluids 2004, 32, 287–291. [Google Scholar] [CrossRef]
- Atifi, A.; Boyce, D.W.; DiMeglio, J.L.; Rosenthal, J. Directing the Outcome of CO2 Reduction at Bismuth Cathodes Using Varied Ionic Liquid Promoters. ACS Catal. 2018, 8, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Ma, J.; Kang, X.; Sun, X.; Hu, J.; Yang, G.; Han, B. Electrochemical reduction of CO2 to CO using graphene oxide/carbon nanotube electrode in ionic liquid/acetonitrile system. Sci. China-Chem. 2016, 59, 551–556. [Google Scholar] [CrossRef]
- Kang, X.; Zhu, Q.; Sun, X.; Hu, J.; Zhang, J.; Liu, Z.; Han, B. Highly efficient electrochemical reduction of CO2 to CH4 in an ionic liquid using a metal-organic framework cathode. Chem. Sci. 2016, 7, 266–273. [Google Scholar] [CrossRef]
- Miao, Y.; Siri-Nguan, N.; Sornchamni, T.; Jovanovic, G.N.; Yokochi, A.F. CO2 reduction in wet ionic liquid solution in microscale-based electrochemical reactor. Chem. Eng. J. 2018, 333, 300–309. [Google Scholar] [CrossRef]
- Tateno, H.; Nakabayashi, K.; Kashiwagi, T.; Senboku, H.; Atobe, M. Electrochemical fixation of CO2 to organohalides in room-temperature ionic liquids under supercritical CO2. Electrochim. Acta 2015, 161, 212–218. [Google Scholar] [CrossRef]
- Dai, Y.; Niu, L.; Liu, H.; Zou, J.; Yu, L.; Feng, Q. Cu-Ni Alloy Catalyzed Electrochemical Carboxylation of Benzyl Bromide with Carbon Dioxide in Ionic Liquid 1-Butyl-3-methylimidazolium tetrafluoroborate. Int. J. Electrochem. Sci. 2018, 13, 1084–1095. [Google Scholar]
- Feng, Q.; Huang, K.; Liu, S.; Yu, J.; Liu, F. Electrocatalytic carboxylation of aromatic ketones with carbon dioxide in ionic liquid 1-butyl-3-methylimidazoliumtetrafluoborate to α-hydroxy-carboxylic acid methyl ester. Electrochim. Acta 2011, 56, 5137–5141. [Google Scholar] [CrossRef]
- Hiejima, Y.; Hayashi, M.; Uda, A.; Oya, S.; Kondo, H.; Senboku, H.; Takahashi, K. Electrochemical carboxylation of α-chloroethylbenzene in ionic liquids compressed with carbon dioxide. Phys. Chem. Chem. Phys. 2010, 12, 1953–1957. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Huang, K.; Liu, S.; Wang, X. Electrocatalytic carboxylation of 2-amino-5-bromopyridine with CO2 in ionic liquid 1-butyl-3-methyllimidazoliumtetrafluoborate to 6-aminonicotinic acid. Electrochim. Acta 2010, 55, 5741–5745. [Google Scholar] [CrossRef]
- Feroci, M.; Chiarotto, I.; Orsini, M.; Sotgiu, G.; Inesi, A. Carbon dioxide as carbon source: Activation via electrogenerated O2 − in ionic liquids. Electrochim. Acta 2011, 56, 5823–5827. [Google Scholar] [CrossRef]
- Feroci, M.; Orsini, M.; Rossi, L.; Sotgiu, G.; Inesi, A. Electrochemically promoted C–N bond formation from amines and CO2 in ionic liquid BMIm-BF4: Synthesis of carbamates. J. Org. Chem. 2007, 72, 200–203. [Google Scholar] [CrossRef]

| ILs | Catalytic Mode | Catalytic Condition | Catalyst | Sel% | Ref. |
|---|---|---|---|---|---|
| [P4444]3[p-2,6-O-4-COO] | Photocatalytic conversion | visible light > 420 nm | CH4 | 96.2% | [51] |
| [Ru(bpy)3]Cl2 | Photocatalytic conversion | 1 atm, visible light > 420 nm | CO | 96.3% | [52] |
| ApmimBr | Electrocatalytic conversion | 20 °C, voltage 3.5 V, | C3H6O3 | 94.5% | [53] |
| [APMIm]DCA | Electrocatalytic conversion | 50 °C | 4-(hydroxymethyl)-1,3-dioxolan-2-one | 96.8% | [54] |
| [Emim]BF4 | Photoelectrocatalytic conversion | visible light, −0.9 vs. NHE | CH3CH2OH | 82.7% | [55] |
| [EmimOH]/[NTF2]/DBU/SmOCl | Thermocatalytic conversion | 140 °C | C3H6O3 | 99.1% | [56] |
| BzMDH | Thermocatalytic conversion | 140 °C | C3H6O3 | 99.7% | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Wang, R. Ionic Liquid-Catalyzed CO2 Conversion for Valuable Chemicals. Molecules 2024, 29, 3805. https://doi.org/10.3390/molecules29163805
Wang P, Wang R. Ionic Liquid-Catalyzed CO2 Conversion for Valuable Chemicals. Molecules. 2024; 29(16):3805. https://doi.org/10.3390/molecules29163805
Chicago/Turabian StyleWang, Peng, and Rui Wang. 2024. "Ionic Liquid-Catalyzed CO2 Conversion for Valuable Chemicals" Molecules 29, no. 16: 3805. https://doi.org/10.3390/molecules29163805
APA StyleWang, P., & Wang, R. (2024). Ionic Liquid-Catalyzed CO2 Conversion for Valuable Chemicals. Molecules, 29(16), 3805. https://doi.org/10.3390/molecules29163805