Recovery of Metals from the “Black Mass” of Waste Portable Li-Ion Batteries with Choline Chloride-Based Deep Eutectic Solvents and Bi-Functional Ionic Liquids by Solvent Extraction
Abstract
1. Introduction
2. Results and Discussion
2.1. Solid BM Composition
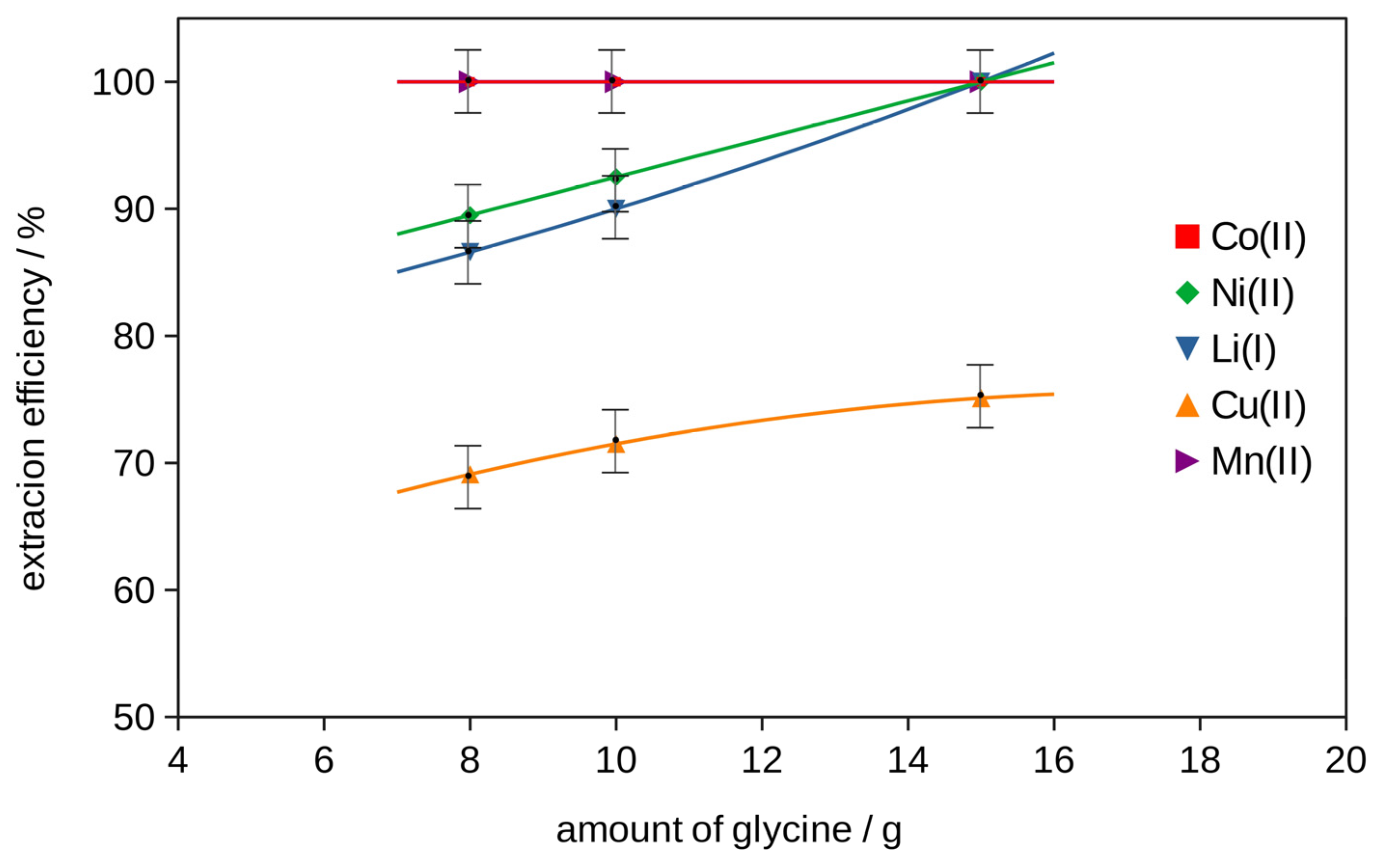
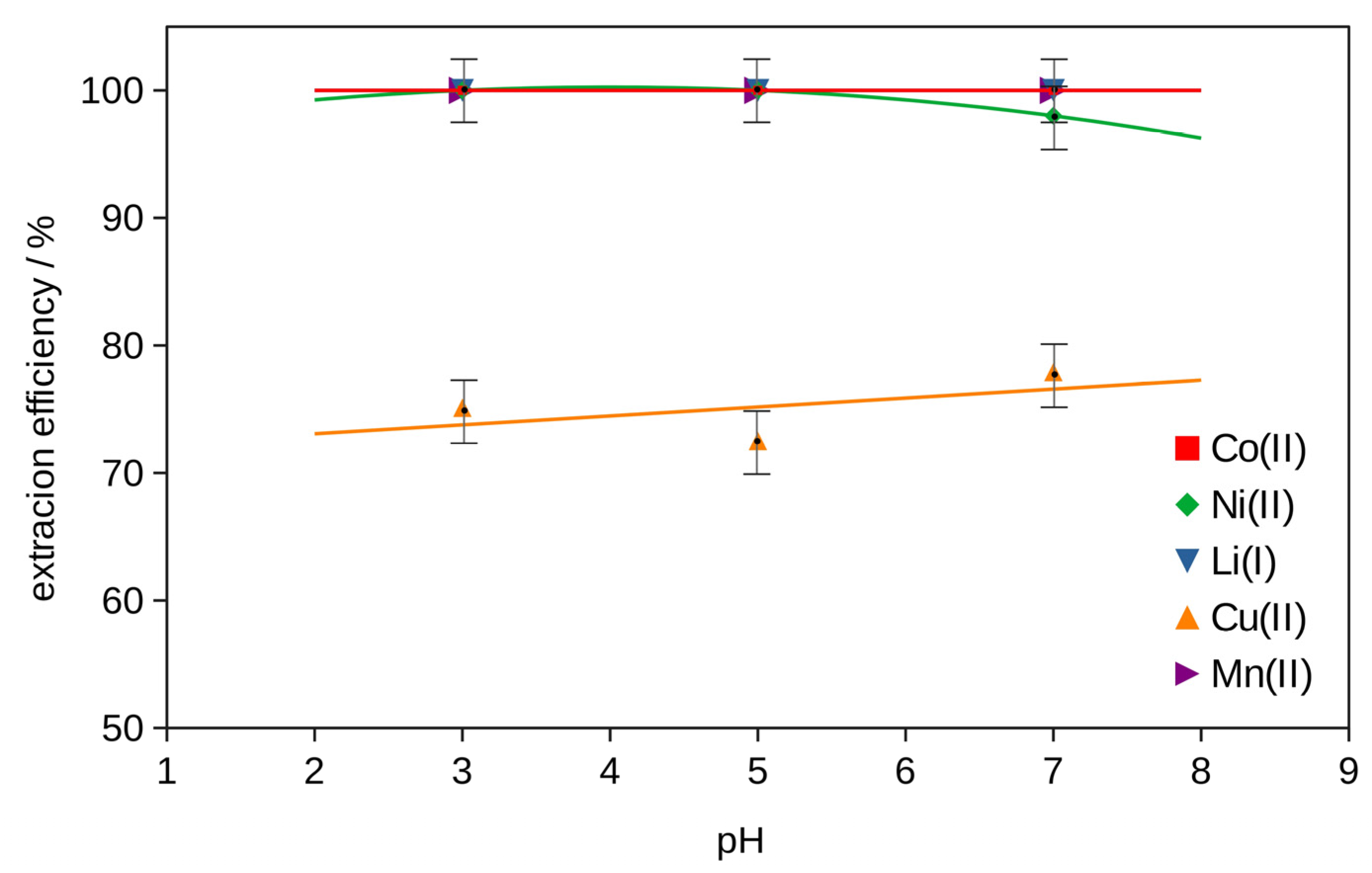
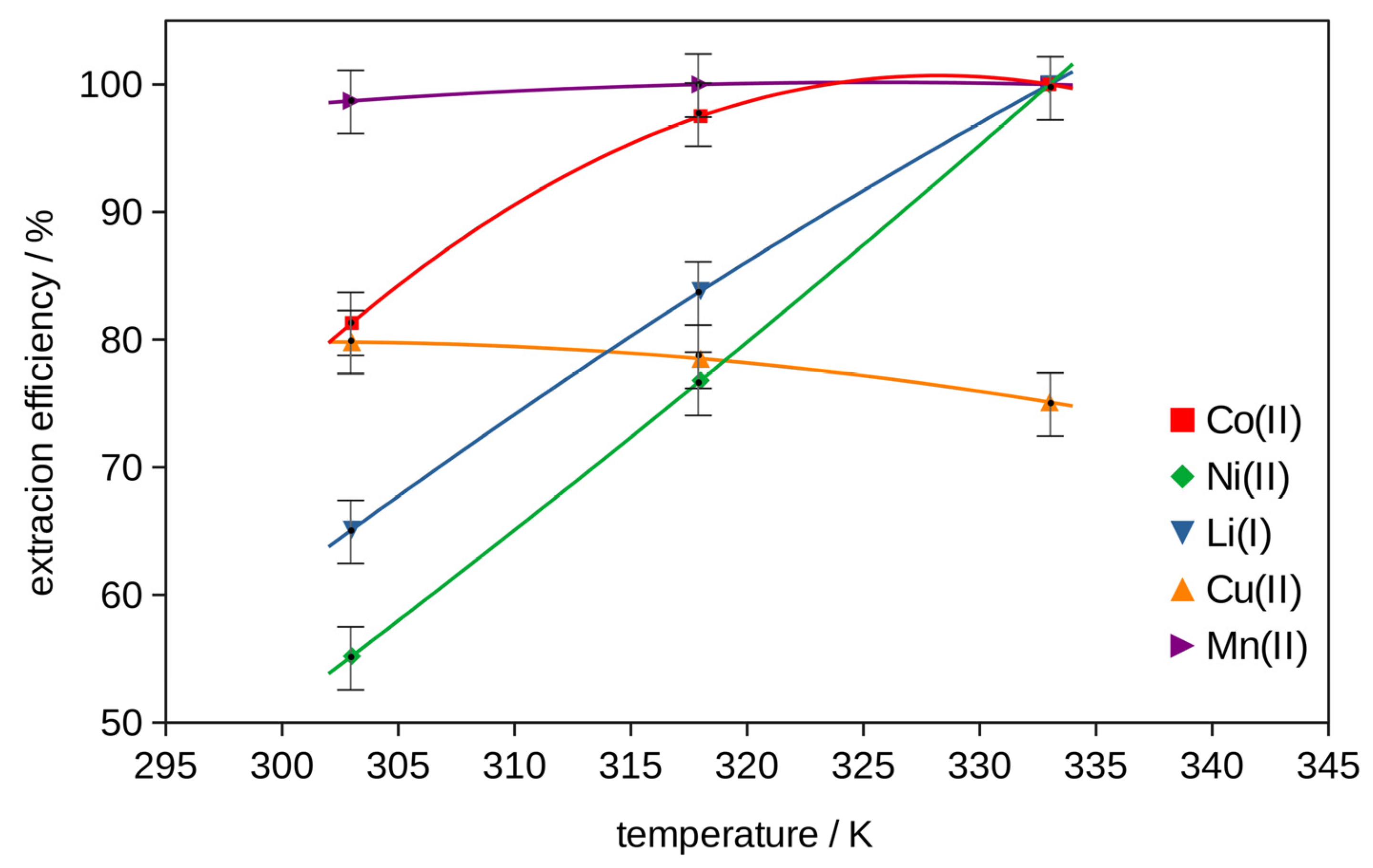
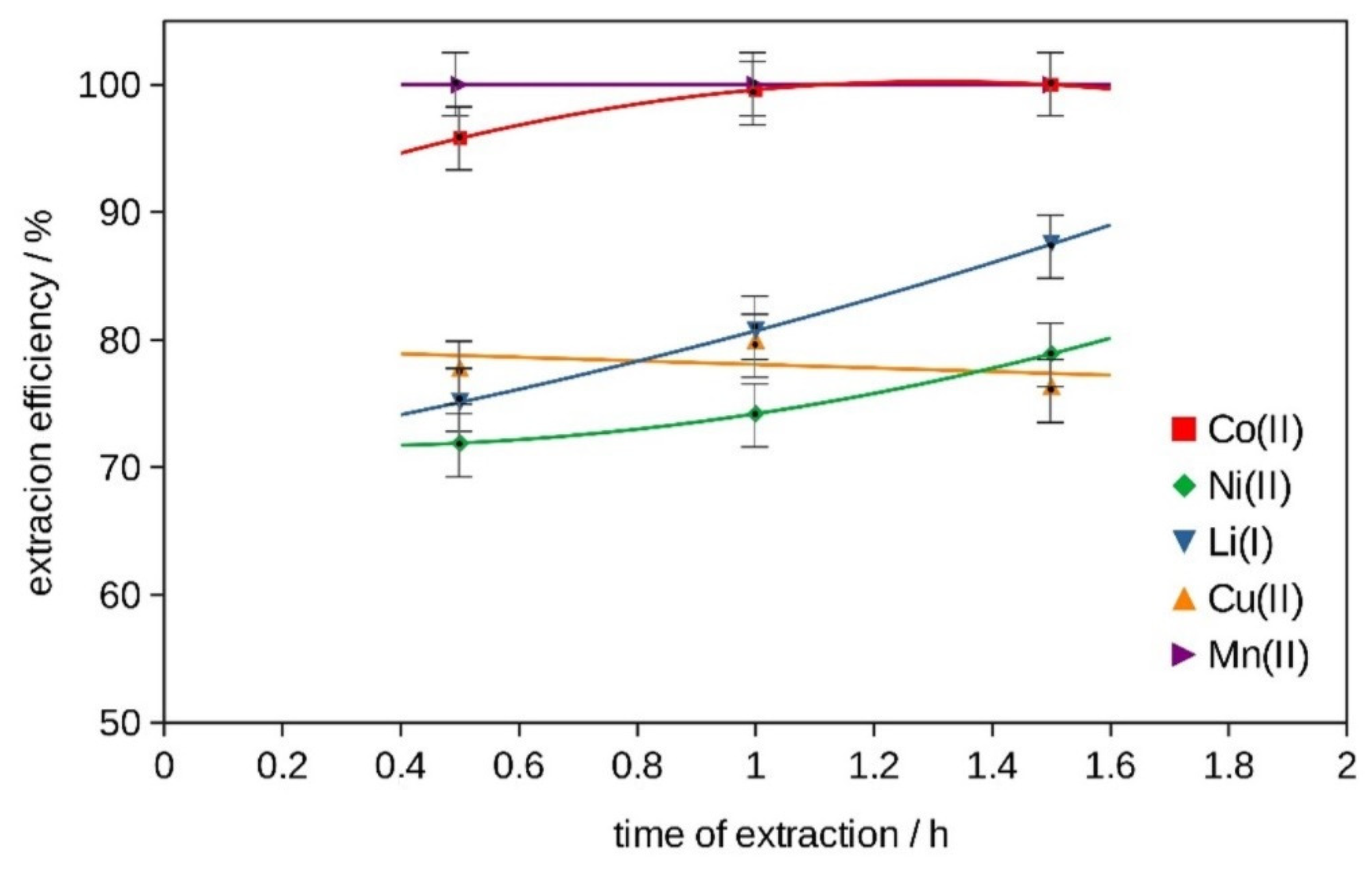
2.2. Extraction
2.2.1. Extraction with DESs
2.2.2. Extraction with Bi-Functional Ionic Liquids
3. Materials and Methods
3.1. Analysis of the Solid LiPBs’ BM Material
3.2. Chemicals
3.3. Extraction Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.-J.; Krishna, T.N.V.; Zeb, K.; Rajangam, V.; Muralee Gopi, C.V.V.; Sambasivam, S.; Raghavendra, K.V.G.; Obaidat, I.M. A Comprehensive Review of Li-Ion Battery Materials and Their Recycling Techniques. Electronics 2020, 9, 1161. [Google Scholar] [CrossRef]
- Botelho, A.B., Jr.; Stopic, S.; Friedrich, B.; Tenório, J.A.S.; Espinosa, D.C.R. Cobalt Recovery from Li-Ion Battery Recycling: A Critical Review. Metals 2021, 11, 1999. [Google Scholar] [CrossRef]
- Ma, L.; Xi, X.; Zhang, Z.; Lyu, Z. Separation and Comprehensive Recovery of Cobalt, Nickel, and Lithium from Spent Power Lithium-Ion Batterie. Minerals 2022, 12, 425. [Google Scholar] [CrossRef]
- Broussely, M.; Pistoia, G. (Eds.) Industrial Applications of Batteries: From Cars to Aerospace and Energy Storage; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. OJ L 2000, 327, 1–73, as Amended. Available online: https://eur-lex.europa.eu/eli/dir/2000/60/oj (accessed on 10 May 2024).
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. OJ L 2013, 226, 1–17. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32013L0039 (accessed on 10 May 2024).
- Regulation (EU) 2023/1542 of the European Parliament and of the Council of 12 July 2023 Concerning Batteries and Waste Batteries, Amending Directive 2008/98/EC and Regulation (EU) 2019/1020 and Repealing Directive 2006/66/EC. OJ L 2023, 191, 1–117. Available online: https://eur-lex.europa.eu/eli/reg/2023/1542/oj (accessed on 10 May 2024).
- Directive 2006/66/EC of the European Parliament and of the Council of 6 September 2006 on Batteries and Accumulators and Waste Batteries and Accumulators and Repealing Directive 91/157/EEC. OJ L 2006, 266, 1–14, as Amended. Available online: https://eur-lex.europa.eu/eli/dir/2006/66/oj (accessed on 10 May 2024).
- Kolasa, D.; Lach, J.; Wróbel, K.; Samsonowska, K.; Kaszuba, A.; Stępkowska, A.; Wróbel, J. Requirements for the Content of Harmful Substances in Market Products of Plastics and Rubber. Part III. Electrical and Electronic Equipment, Batteries and Accumulators. Polimery 2023, 68, 32–47. [Google Scholar] [CrossRef]
- Afonso, J.; Mezzetta, A.; Marrucho, I.M.; Guazzelli, L. History repeats itself again: Will the mistakes of the past for ILs be repeated for DESs? From being considered ionic liquids to becoming their alternative: The unbalanced turn of deep eutectic solvents. Green Chem. 2023, 25, 59–105. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Mero, A.; Koutsoumpos, S.; Giannios, P.; Stavrakas, I.; Moutzouris, K.; Mezzetta, A.; Guazzelli, L. Comparison of physicochemical and thermal properties of choline chloride and betaine-based deep eutectic solvents: The influence of hydrogen bond acceptor and hydrogen bond donor nature and their molar ratios. J. Mol. Liq. 2023, 377, 121563. [Google Scholar] [CrossRef]
- Suffia, S.; Dutta, D. Applications of deep eutectic solvents in metal recovery from E-wastes in a sustainable way. J. Mol. Liq. 2024, 394, 123738. [Google Scholar] [CrossRef]
- Tran, T.T.; Moon, H.S.; Lee, M.S. Separation of Cobalt, Nickel, and Copper from synthetic metallic alloy by selective dissolution with acid solutions containing oxidizing agent. Miner. Process. Extr. Metall. Rev. 2022, 43, 313–325. [Google Scholar] [CrossRef]
- Available online: https://www.consilium.europa.eu/pl/infographics/critical-raw-materials/ (accessed on 10 May 2024).
- Pillot, C. EU Battery Demand and Supply (2019–2030) in a Global Context, Avicenne Energy, 2020. Available online: https://www.eurobat.org/wp-content/uploads/2021/05/Avicenne_EU_Market_-_summary_110321.pdf (accessed on 10 May 2024).
- Pagliaro, M.; Meneguzzo, F. Lithium battery reusing and recycling: A circular economy insight. Heliyon 2019, 5, e01866. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Luchcińska, S.; Domańska, U. Separation of cobalt, lithium and nickel from the “black mass” of waste Li-ion batteries by ionic liquids, DESs and organophosphorous-based acids extraction. J. Mol. Liq. 2021, 343, 117694. [Google Scholar] [CrossRef]
- Fischer, L.; Falta, T.; Koellensperger, G.; Stojanovic, A.; Kogelnig, D.; Galanski, M.; Krachler, R.; Keppler, B.K.; Hann, S. Ionic liquids for extraction of metals and metal containing compounds from communal and industrial waste water. Water Res. 2011, 45, 4601–4614. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Sun, Y.; Rui, J.; Yu, L.; Liu, J.; Zhang, N.; Zhao, M.; Wei, L.; Lu, C.; Zhao, J.; et al. Study of the “Oxidation-Complexation” Coordination Composite Ionic Liquid System for Dissolving Precious Metals. Appl. Sci. 2020, 10, 3625. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Domańska, U. Liquid-liquid extraction of cobalt(II) and zinc(II) from aqueous solutions using novel ionic liquids as extractants. J. Mol. Liq. 2020, 307, 112955. [Google Scholar] [CrossRef]
- Han, Y.; Yi, X.; Wang, R.; Huang, J.; Chen, M.; Sun, Z.; Sun, S.; Shu, J. Copper extraction from waste printed circuit boards by glycine. Sep. Pur. Technol. 2020, 253, 117463. [Google Scholar] [CrossRef]
- Urbańska, W. Recovery of Co, Li, and Ni from spent Li-Ion batteries by the inorganic and/or organic reducer assisted leaching method. Minerals 2020, 10, 555. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Lach, J.; Wróbel, K.; Kolasa, D.; Domańska, U. Recovery of metals from electronic waste-printed circuit boards by ionic liquids, DESs and organophosphorous-based acid extraction. Molecules 2022, 27, 4984. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, J.; Cao, H.; Zhang, H.; Sun, Z. Recovery of spent lithium-ion batteries in view of lithium recovery: A critical review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Barrueto, Y.; Hernández, H.; Jiménez, Y.P.; Morales, J. Properties and application of ionic liquids in leaching base/precious metals from e-waste. A review. Hydrometallurgy 2022, 212, 105895. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Choubey, P.K.; Dinkar, O.S.; Panda, R.; Kumari, A.; Jha, M.K.; Pathak, D.D. Selective extraction and separation of Li, Co and Mn from leach liquor of discarded lithium ion batteries (LIBs). Waste Manag. 2021, 121, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Gerold, E.; Luidold, S.; Antrekowitsch, H. Separation and efficient recovery of lithium from spent lithium-ion batteries. Metals 2021, 11, 1091. [Google Scholar] [CrossRef]
- Zheng, H.; Dong, T.; Sha, Y.; Jiang, D.; Zhang, H.; Zhang, S. Selective extraction of lithium from spent lithium batteries by functional ionic liquid. ACS Sustain. Chem. Eng. 2021, 9, 7022–7029. [Google Scholar] [CrossRef]
- Cheng, J.; Lu, T.; Wu, X.; Zhang, H.; Zhang, C.; Peng, C.-A.; Huang, S. Extraction of cobalt(II) by methyltrioctylammonium chloride in nickel(II)-containing chloride solution from spent lithium ion batteries. RSC Adv. 2019, 9, 22729–22739. [Google Scholar] [CrossRef]
- Peeters, N.; Binnemans, B.; Riaño, S. Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chem. 2020, 22, 4210–4221. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Santhosh, G.; Manjanna, J. Recovery of cobalt as cobalt oxalate from spent lithium ion batteries by using glycine as leaching agent. J. Environ. Chem. Eng. 2016, 4, 2378–2383. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7714. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Lee, M.S. Separation of Mo(VI), V(V), Ni(II), Al(III) from synthetic hydrochloric acidic leaching solution of spent catalysts by solvent extraction with ionic liquids. Sep. Pur. Technol. 2020, 247, 117005. [Google Scholar] [CrossRef]
- Tran, T.T.; Liu, Y.; Lee, M.S. Recovery of pure molybdenum and vanadium compounds from spent petroleum catalysts by treatment with ionic liquid solution in the presence of oxidizing agent. Sep. Pur. Technol. 2021, 255, 117734. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Yu, W.; Ma, X.; Lei, S.; Sun, W.; Song, S.; Hu, W. Recovering Fe, Mn and Li from LiMn1−xFexPO4 cathode material of spent lithium-ion battery by gradient precipitation. Sustain. Mater. Technol. 2023, 36, e00625. [Google Scholar] [CrossRef]
- Kim, J.P.; Go, C.Y.; Kang, J.; Choi, Y.; Kim, J.Y.; Kim, Y.; Kwon, O.; Kim, K.C.; Kim, D.W. Nanoporous multilayer graphene oxide membrane for forward osmosis metal ion recovery from spent Li-ion batteries. J. Membr. Sci. 2023, 676, 121590. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Nie, C.C.; Li, X.G.; Shi, S.X.; Gao, Q.; Wang, Y.S.; Zhu, X.N.; Wang, Z. Review on comprehensive recycling of spent lithium-ion batteries: A full component utilization process for green and sustainable production. Sep. Pur. Technol. 2023, 315, 123684. [Google Scholar] [CrossRef]
- Tripathy, A.; Bhuyan, A.; Padhy, R.K.; Mangla, S.K.; Roopak, R. Drivers of lithium-ion batteries recycling industry toward circular economy in industry 4.0. Comp. Ind. Eng. 2023, 179, 109157. [Google Scholar] [CrossRef]
- Yan, Z.; Sattar, A.; Li, Z. Priority Lithium recovery from spent Li-ion batteries via carbothermal reduction with water leaching. Resour. Conserv. Recycl. 2023, 192, 106937. [Google Scholar] [CrossRef]
- Mousavinezhad, S.; Kadivar, S.; Vahidi, E. Comparative life cycle analysis of critical materials recovery from spent Li-ion batteries. J. Environ. Manag. 2023, 339, 117887. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Hadinoto, K. Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review. Int. J. Mol. Sci. 2022, 23, 3381. [Google Scholar] [CrossRef]
- Pathak, A.; Vinoba, M.; Kothari, R. Emerging role of organic acids in leaching of valuable metals from refinery-spent hydroprocessing catalysts, and potential technoeconomic challenges: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1–43. [Google Scholar] [CrossRef]
- Boudesocque, S.; Mohamadou, A.; Dupont, L.; Martinez, A.; Dechamps, I. Use of dicyanamide ionic liquids for extraction of metal ions. RSC Adv. 2016, 6, 107894–107904. [Google Scholar] [CrossRef]
- Janiszewska, M.; Markiewicz, A.; Regel-Rosocka, M. Hydrometallurgical separation of Co(II) from Ni(II) from model and real solutions. J. Clean. Prod. 2019, 228, 746–754. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Chen, M.-N.; Hao, Y.; Jiang, X.; Zhou, X.-L.; Zhang, Z.-H. Choline chloride and lactic acid: A natural deep eutectic solvent for one-pot rapid construction of spiro[indoline-3,4′-pyrazolo[3,4-b]pyridines]. J. Mol. Liq. 2019, 278, 124–129. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Luchcińska, S.; Lach, J.; Wróbel, K.; Domańska, U. Recovery of zinc and manganese from “black mass” of waste Zn-MnO2 alkaline batteries by solvent extraction technique with ionic liquids, DESs and organophosphorous-based acids. J. Mol. Liq. 2021, 338, 116590. [Google Scholar] [CrossRef]

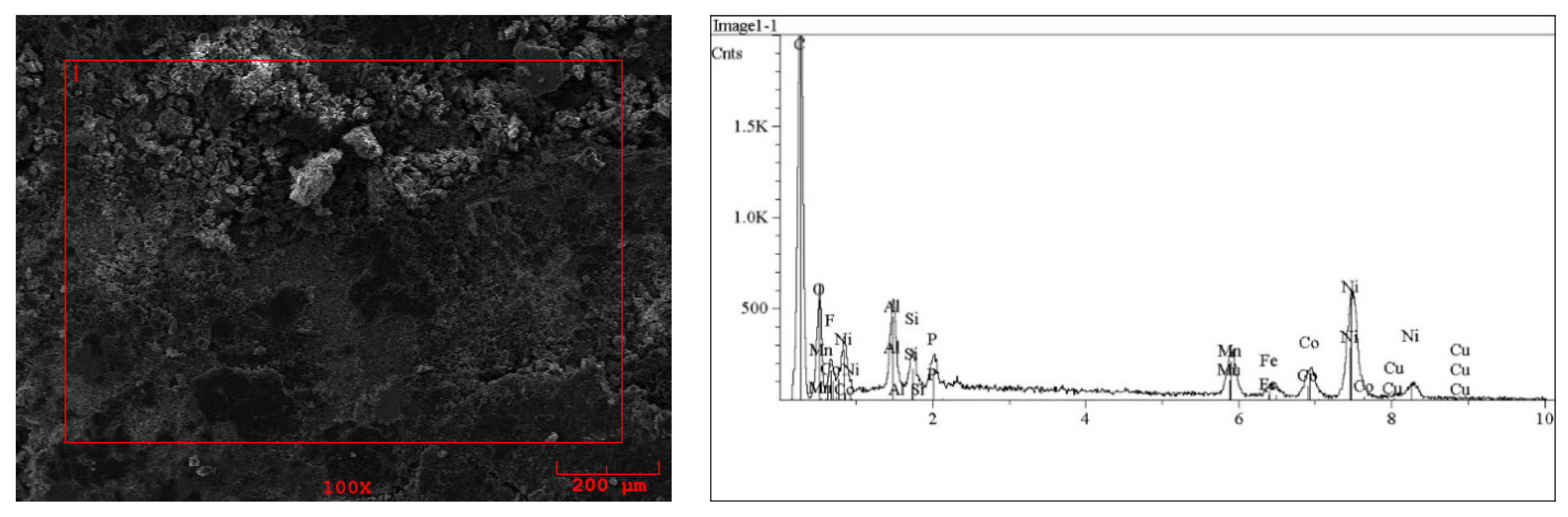


| Co wt% | Ni wt% | Cu wt% | Mn wt% | Fe wt% | Al wt% | Zn wt% | Li wt% |
|---|---|---|---|---|---|---|---|
| 2.95 | 8.6 | 4.0 | 3.4 | 1.9 | 1.65 | 0.067 | 2.2 |
| Extrahent | Ion | g0 * (mg) | gE * (mg) | E (wt%) |
|---|---|---|---|---|
| DES 1 + H2O2 | Co(II) | 295 | 82.44 | 28 |
| Ni(II) | 860 | 246.36 | 29 | |
| Li(I) | 220 | 136.42 | 62 | |
| Cu(II) | 400 | 381.19 | 95 | |
| Mn(II) | 340 | 163.00 | 48 | |
| DES 1 + TCCA | Co(II) | 295 | 141.44 | 48 |
| Ni(II) | 860 | 492.10 | 57 | |
| Li(I) | 220 | 123.61 | 56 | |
| Cu(II) | 400 | 80.83 | 20 | |
| Mn(II) | 340 | 202.87 | 60 | |
| DES 1 + NaDCC × 2H2O | Co(II) | 295 | 88.38 | 30 |
| Ni(II) | 860 | 282.45 | 33 | |
| Li(I) | 220 | 95.39 | 43 | |
| Cu(II) | 400 | 111.04 | 28 | |
| Mn(II) | 340 | 100.37 | 30 | |
| DES 1 + PHM | Co(II) | 295 | 108.61 | 37 |
| Ni(II) | 860 | 132.32 | 15 | |
| Li(I) | 220 | 167.00 | 76 | |
| Cu(II) | 400 | 388.73 | 97 | |
| Mn(II) | 340 | 231.70 | 68 | |
| DES 1 + (glycine + H2O2) | Co(II) | 295 | 153.75 | 52 |
| Ni(II) | 860 | 452.75 | 53 | |
| Li(I) | 220 | 118.02 | 54 | |
| Cu(II) | 400 | 280.82 | 70 | |
| Mn(II) | 340 | 212.27 | 62 | |
| DES 1 + (glutaric acid + H2O2) | Co(II) | 295 | 113.91 | 39 |
| Ni(II) | 860 | 371.38 | 43 | |
| Li(I) | 220 | 103.76 | 47 | |
| Cu(II) | 400 | 329.28 | 82 | |
| Mn(II) | 340 | 165.28 | 49 |
| Extrahent | Ion | g0 * (mg) | gE * (mg) | E (wt%) |
|---|---|---|---|---|
| DES 2 + H2O2 | Co(II) | 295 | 40.65 | 14 |
| Ni(II) | 860 | 104.36 | 12 | |
| Li(I) | 220 | 168.02 | 76 | |
| Cu(II) | 400 | 164.62 | 41 | |
| Mn(II) | 340 | 178.52 | 53 | |
| DES 2 + TCCA | Co(II) | 295 | 175.07 | 59 |
| Ni(II) | 860 | 429.03 | 50 | |
| Li(I) | 220 | 143.09 | 65 | |
| Cu(II) | 400 | 344.28 | 86 | |
| Mn(II) | 340 | 236.02 | 69 | |
| DES 2 + NaDCC × 2H2O | Co(II) | 295 | 166.65 | 57 |
| Ni(II) | 860 | 481.23 | 56 | |
| Li(I) | 220 | 144.06 | 66 | |
| Cu(II) | 400 | 146.64 | 37 | |
| Mn(II) | 340 | 210.80 | 62 | |
| DES 2 + PHM | Co(II) | 295 | 58.60 | 20 |
| Ni(II) | 860 | 19.55 | 2 | |
| Li(I) | 220 | 179.27 | 82 | |
| Cu(II) | 400 | 311.42 | 78 | |
| Mn(II) | 340 | 208.91 | 61 | |
| DES 2 + (glycine + H2O2) | Co(II) | 295 | 177.22 | 60 |
| Ni(II) | 860 | 568.86 | 66 | |
| Li(I) | 220 | 147.06 | 67 | |
| Cu(II) | 400 | 300.80 | 75 | |
| Mn(II) | 340 | 221.00 | 65 | |
| DES 2 + (glutaric acid + H2O2) | Co(II) | 295 | 53.14 | 18 |
| Ni(II) | 860 | 234.61 | 27 | |
| Li(I) | 220 | 157.42 | 72 | |
| Cu(II) | 400 | 82.98 | 21 | |
| Mn(II) | 340 | 152.34 | 45 |
| Extrahent | Ion | g0 * (mg) | gE * (mg) | E (wt%) |
|---|---|---|---|---|
| DES 3 + H2O2 | Co(II) | 295 | 103.37 | 35 |
| Ni(II) | 860 | 284.53 | 33 | |
| Li(I) | 220 | 106.13 | 48 | |
| Cu(II) | 400 | 44.26 | 12 | |
| Mn(II) | 340 | 144.32 | 42 | |
| DES 3 + TCCA (T = 318 K) | Co(II) | 295 | 157.82 | 53 |
| Ni(II) | 860 | 381.36 | 44 | |
| Li(I) | 220 | 161.45 | 73 | |
| Cu(II) | 400 | 376.52 | 94 | |
| Mn(II) | 340 | 249.45 | 73 | |
| DES 3 + NaDCC × 2H2O | Co(II) | 295 | 34.92 | 12 |
| Ni(II) | 860 | 164.55 | 19 | |
| Li(I) | 220 | 131.92 | 60 | |
| Cu(II) | 400 | 191.64 | 48 | |
| Mn(II) | 340 | 28.21 | 8 | |
| DES 3 + PHM | Co(II) | 295 | 60.03 | 20 |
| Ni(II) | 860 | 13.87 | 2 | |
| Li(I) | 220 | 141.54 | 64 | |
| Cu(II) | 400 | 87.14 | 22 | |
| Mn(II) | 340 | 186.00 | 55 | |
| DES 3 + (glycine + H2O2) | Co(II) | 295 | 116.19 | 39 |
| Ni(II) | 860 | 378.38 | 44 | |
| Li(I) | 220 | 118.93 | 54 | |
| Cu(II) | 400 | 147.84 | 37 | |
| Mn(II) | 340 | 153.19 | 45 | |
| DES 3 + (glutaric acid + H2O2) | Co(II) | 295 | 120.76 | 41 |
| Ni(II) | 860 | 418.61 | 49 | |
| Li(I) | 220 | 115.26 | 52 | |
| Cu(II) | 400 | 109.57 | 27 | |
| Mn(II) | 340 | 146.68 | 43 |
| Extrahent | Ion | g0 * (mg) | gE * (mg) | E (wt%) |
|---|---|---|---|---|
| DES 4 + H2O2 | Co(II) | 295 | 133.41 | 45 |
| Ni(II) | 860 | 360.16 | 42 | |
| Li(I) | 220 | 108.70 | 49 | |
| Cu(II) | 400 | 66.99 | 17 | |
| Mn(II) | 340 | 178.68 | 53 | |
| DES 4 + TCCA (T = 318 K) | Co(II) | 295 | 151.76 | 51 |
| Ni(II) | 860 | 426.40 | 50 | |
| Li(I) | 220 | 131.68 | 60 | |
| Cu(II) | 400 | 348.66 | 87 | |
| Mn(II) | 340 | 221.27 | 65 | |
| DES 4 + NaDCC × 2H2O | Co(II) | 295 | 36.56 | 12 |
| Ni(II) | 860 | 174.50 | 20 | |
| Li(I) | 220 | 112.19 | 51 | |
| Cu(II) | 400 | 130.56 | 33 | |
| Mn(II) | 340 | 36.31 | 11 | |
| DES 4 + PHM | Co(II) | 295 | 55.46 | 19 |
| Ni(II) | 860 | 12.67 | 2 | |
| Li(I) | 220 | 143.40 | 65 | |
| Cu(II) | 400 | 108.95 | 27 | |
| Mn(II) | 340 | 206.00 | 61 | |
| DES 4 + (glycine + H2O2) | Co(II) | 295 | 109.32 | 37 |
| Ni(II) | 860 | 333.66 | 39 | |
| Li(I) | 220 | 108.69 | 49 | |
| Cu(II) | 400 | 303.02 | 76 | |
| Mn(II) | 340 | 164.63 | 48 | |
| DES 4 + (glutaric acid + H2O2) | Co(II) | 295 | 122.85 | 42 |
| Ni(II) | 860 | 371.96 | 43 | |
| Li(I) | 220 | 106.67 | 49 | |
| Cu(II) | 400 | 81.87 | 20 | |
| Mn(II) | 340 | 167.52 | 49 |
| Extrahent | Ion | g0 * (mg) | gE * (mg) | E (wt%) |
|---|---|---|---|---|
| DES 5 + H2O2 | Co(II) | 295 | 147.47 | 50 |
| Ni(II) | 860 | 439.56 | 51 | |
| Li(I) | 220 | 136.47 | 62 | |
| Cu(II) | 400 | 335.74 | 84 | |
| Mn(II) | 340 | 206.09 | 61 | |
| DES 5 + TCCA (T = 318 K) | Co(II) | 295 | 147.12 | 50 |
| Ni(II) | 860 | 381.17 | 44 | |
| Li(I) | 220 | 132.92 | 60 | |
| Cu(II) | 400 | 350.78 | 88 | |
| Mn(II) | 340 | 206.69 | 61 | |
| DES 5 + NaDCC × 2H2O | Co(II) | 295 | 142.85 | 48 |
| Ni(II) | 860 | 442.10 | 51 | |
| Li(I) | 220 | 145.17 | 66 | |
| Cu(II) | 400 | 374.00 | 94 | |
| Mn(II) | 340 | 210.90 | 62 | |
| DES 5 + PHM | Co(II) | 295 | 115,16 | 39 |
| Ni(II) | 860 | 208.16 | 24 | |
| Li(I) | 220 | 193.01 | 88 | |
| Cu(II) | 400 | 347.42 | 87 | |
| Mn(II) | 340 | 250.52 | 74 | |
| DES 5 + (glycine + H2O2) | Co(II) | 295 | 125.91 | 43 |
| Ni(II) | 860 | 388.85 | 45 | |
| Li(I) | 220 | 134.53 | 62 | |
| Cu(II) | 400 | 341.59 | 85 | |
| Mn(II) | 340 | 178.51 | 53 | |
| DES 5 + (glutaric acid + H2O2) | Co(II) | 295 | 116.05 | 39 |
| Ni(II) | 860 | 367.34 | 43 | |
| Li(I) | 220 | 116.92 | 53 | |
| Cu(II) | 400 | 277.37 | 69 | |
| Mn(II) | 340 | 165.34 | 49 |
| Extrahent | Ion | g0 * (mg) | gE * (mg) | E (wt%) |
|---|---|---|---|---|
| DES 6 + H2O2 | Co(II) | 295 | 151.95 | 52 |
| Ni(II) | 860 | 510.33 | 59 | |
| Li(I) | 220 | 111.67 | 51 | |
| Cu(II) | 400 | 271.66 | 68 | |
| Mn(II) | 340 | 210.02 | 62 | |
| DES 6 + TCCA (T = 318 K) | Co(II) | 295 | 178.91 | 61 |
| Ni(II) | 860 | 173.15 | 20 | |
| Li(I) | 220 | 132.22 | 60 | |
| Cu(II) | 400 | 298.25 | 75 | |
| Mn(II) | 340 | 327.5 | 96 | |
| DES 6 + NaDCC × 2H2O | Co(II) | 295 | 103.75 | 35 |
| Ni(II) | 860 | 98.36 | 11 | |
| Li(I) | 220 | 92.80 | 42 | |
| Cu(II) | 400 | 259.72 | 65 | |
| Mn(II) | 340 | 229.67 | 68 | |
| DES 6 + PHM | Co(II) | 295 | 94.19 | 32 |
| Ni(II) | 860 | 311.5 | 36 | |
| Li(I) | 220 | 137.06 | 62 | |
| Cu(II) | 400 | 263.81 | 66 | |
| Mn(II) | 340 | 281.11 | 83 | |
| DES 6 + (glycine + H2O2) | Co(II) | 295 | 168.76 | 57 |
| Ni(II) | 860 | 169.91 | 20 | |
| Li(I) | 220 | 114.94 | 52 | |
| Cu(II) | 400 | 282.63 | 71 | |
| Mn(II) | 340 | 286.07 | 84 | |
| DES 6 + (glutaric acid + H2O2) | Co(II) | 295 | 147.55 | 50 |
| Ni(II) | 860 | 487.22 | 57 | |
| Li(I) | 220 | 108.68 | 49 | |
| Cu(II) | 400 | 272.95 | 68 | |
| Mn(II) | 340 | 271.84 | 80 |
| Extrahent | Ion | g0 * (mg) | gEAq * (mg) | gEOrg * (mg) | E (wt%) |
|---|---|---|---|---|---|
| [N10,10,1,1][Cyanex272] + H2O2 | Co(II) | 295 | 14.15 | 45.99 | 20 |
| Ni(II) | 860 | 50.74 | 75.32 | 15 | |
| Li(I) | 220 | 33.37 | 32.63 | 30 | |
| Cu(II) | 400 | 1.32 | 133.60 | 34 | |
| Mn(II) | 340 | 10.79 | 74.10 | 25 | |
| [N10,10,1,1][Cyanex272] + TCCA | Co(II) | 295 | 12.62 | 69.93 | 28 |
| Ni(II) | 860 | 99.03 | 141.69 | 28 | |
| Li(I) | 220 | 46.23 | 75.38 | 55 | |
| Cu(II) | 400 | 0.74 | 134.34 | 34 | |
| Mn(II) | 340 | 15.49 | 124.67 | 41 | |
| [N10,10,1,1][Cyanex272] + PHM | Co(II) | 295 | 34.60 | 18.06 | 18 |
| Ni(II) | 860 | 26.07 | 16.24 | 5 | |
| Li(I) | 220 | 80.33 | 20.82 | 46 | |
| Cu(II) | 400 | 7.25 | 106.76 | 29 | |
| Mn(II) | 340 | 26.07 | 15.75 | 12 | |
| [N10,10,1,1][Cyanex272] + (glycine + H2O2) | Co(II) | 295 | 73.06 | 14.77 | 30 |
| Ni(II) | 860 | 171.00 | 31.56 | 24 | |
| Li(I) | 220 | 66.57 | 14.87 | 37 | |
| Cu(II) | 400 | 114.62 | 45.77 | 40 | |
| Mn(II) | 340 | 122.86 | 131.56 | 75 |
| Extrahent | Ion | g0 * (mg) | gEAq * (mg) | gEOrg * (mg) | E (wt%) |
|---|---|---|---|---|---|
| [N10,10,1,1][D2EHPA] + H2O2 | Co(II) | 295 | 1.47 | 5.79 | 3 |
| Ni(II) | 860 | 1.55 | 10.48 | 1 | |
| Li(I) | 220 | 21.61 | 15.37 | 17 | |
| Cu(II) | 400 | 2.31 | 97.83 | 25 | |
| Mn(II) | 340 | 3.02 | 12.61 | 5 | |
| [N10,10,1,1][D2EHPA] + TCCA | Co(II) | 295 | 20.23 | 117.74 | 47 |
| Ni(II) | 860 | 82.59 | 112.63 | 23 | |
| Li(I) | 220 | 57.03 | 51.27 | 49 | |
| Cu(II) | 400 | 1.63 | 119.37 | 30 | |
| Mn(II) | 340 | 39.82 | 100.58 | 41 | |
| [N10,10,1,1][D2EHPA] + PHM | Co(II) | 295 | 35.87 | 6.12 | 14 |
| Ni(II) | 860 | 19.22 | 6.01 | 3 | |
| Li(I) | 220 | 91.74 | 11.09 | 47 | |
| Cu(II) | 400 | 63.62 | 55.32 | 30 | |
| Mn(II) | 340 | 100.73 | 12.92 | 33 | |
| [N10,10,1,1][D2EHPA] + (glycine + H2O2) | Co(II) | 295 | 73.97 | 6.49 | 27 |
| Ni(II) | 860 | 168.63 | 12.63 | 21 | |
| Li(I) | 220 | 62.74 | 8.95 | 33 | |
| Cu(II) | 400 | 126.44 | 25.58 | 38 | |
| Mn(II) | 340 | 123.22 | 9.26 | 39 |
| Extrahent | Ion | g0 * (mg) | gEAq * (mg) | gEOrg * (mg) | E (wt%) |
|---|---|---|---|---|---|
| [P6,6,6,14][Cyanex272] + H2O2 | Co(II) | 295 | 41.89 | 34.69 | 26 |
| Ni(II) | 860 | 115.04 | 43.50 | 18 | |
| Li(I) | 220 | 65.67 | 14.88 | 37 | |
| Cu(II) | 400 | 1.81 | 39.59 | 10 | |
| Mn(II) | 340 | 36.13 | 63.85 | 29 | |
| [P6,6,6,14][Cyanex272] + TCCA | Co(II) | 295 | 14.96 | 82.97 | 33 |
| Ni(II) | 860 | 221.54 | 25.84 | 29 | |
| Li(I) | 220 | 96.37 | 14.71 | 50 | |
| Cu(II) | 400 | 0.27 | 76.36 | 19 | |
| Mn(II) | 340 | 33.98 | 120.69 | 45 | |
| [P6,6,6,14][Cyanex272] + PHM | Co(II) | 295 | 46.51 | 1.54 | 16 |
| Ni(II) | 860 | 22.31 | 2.25 | 3 | |
| Li(I) | 220 | 98.73 | 1.34 | 46 | |
| Cu(II) | 400 | 69.86 | 16.09 | 22 | |
| Mn(II) | 340 | 109.97 | 2.66 | 33 | |
| [P6,6,6,14][Cyanex272] + (glycine + H2O2) | Co(II) | 295 | 106.34 | 1.79 | 37 |
| Ni(II) | 860 | 257.16 | 3.02 | 30 | |
| Li(I) | 220 | 90.73 | 0.85 | 42 | |
| Cu(II) | 400 | 142.89 | 4.03 | 37 | |
| Mn(II) | 340 | 200.30 | 2.73 | 60 |
| Chemical Structure | Name, Abbreviation, Supplier, CAS Number | Molar Mass M (g mol−1) | Purity * in Mass Percent (%) |
|---|---|---|---|
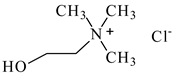 | Choline chloride, [N2OH,1,1,1][Cl], Sigma-Aldrich, Darmstadt, Germany, CAS: 67-48-1 | 139.62 | >98 |
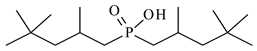 | Bis(2,4,4-trimethylpentyl)phosphinic acid, Cyanex 272, Chem Scene LLC (Glenside, (PA)/ USA), CAS: 83411-71-6 | 290.42 | 90 |
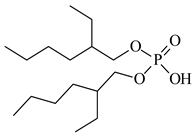 | Bis(2-ethylhexyl) phosphate, D2EHPA, Heavy Water, Darmstadt, Germany, CAS: 298-07-7 | 322.40 | >95 |
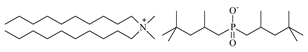 | Didecyldimethylammonium bis(2,4,4-trimethylpentyl)phosphinate [N10,10,1,1][Cyanex272], Synthesized, Ł-IChP | 616.12 | >95 |
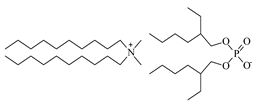 | Didecyldimethylammonium bis(2-ethylhexyl)phosphate [N10,10,1,1][D2EHPA], C38H82NO4P, Synthesized, Ł-IChP | 648.13 | >95 |
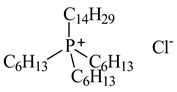 | Trihexyltetradecylphosphonium chloride, Cyphos IL 101, [P6,6,6,14][Cl], IoLiTec, Heilbronn, Germany, CAS: 258864-54-9 | 519.42 | >95 |
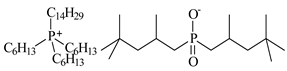 | Trihexyltetradecylphosphonium bis(2,4,4-trimethylopentyl)phosphinate [P6,6,6,14][Cyanex272], ([P6,6,6,14][BTMPP]), IoLiTec, Heilbronn, Germany, CAS: 465527-59-7 | 773.27 | >90 |
 | Didecyldimethylammonium chloride, [N10,10,1,1][Cl], DDACl, Alpinus Sp. z o.o. (Miszewko, Poland), CAS: 7173-51-5 | 362.16 | 50 wt% aq. solution |
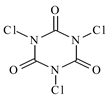 | Trichloroizocyjanuric acid, C3Cl3N3O3, TCCA, Sigma-Aldrich, Darmstadt, Germany, CAS: 87-90-1, | 232.40 | 95 |
 | Sodium dichloroizocyjanurian dihydrat, C3Cl2N3NaO2 × 2H2O, NaDCC × 2H2O, Sigma-Aldrich, Darmstadt, Germany, CAS: 51580-86-0 | 239.99 | ≥98 |
 | Glycine, C2H5NO2, Sigma-Aldrich, Darmstadt, Germany, CAS: 56-40-6 | 75.07 | 95 |
| (2KHSO5·KHSO4·K2SO4) | Pentapotassium bis(peroxymonosulphate) bis(sulphate), PHM, Sigma-Aldrich, Darmstadt, Germany, CAS: 70693-62-8 | 614.76 | 98.0 |
 | Lactic acid, C3H6O3, Sigma-Aldrich, Darmstadt, Germany, CAS: 50-21-5 | 90.08 | 98.0 |
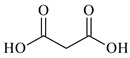 | Malonic acid, C3H4O4, Reachim, Darmstadt, Germany, CAS: 141-82-2 | 104.06 | 99.0 |
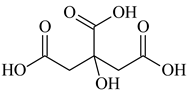 | Citric Acid, C6H8O7, Riedel-de Haën, Seelze, Germany, CAS: 77-92-2 | 192.13 | 99.8 |
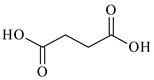 | Succinic acid, C4H6O4, Avantor (POCh), Gliwice, Poland, CAS: 110-15-6 | 118.09 | >99 |
 | Glutaric acid, C5H8O4, Sigma-Aldrich, Darmstadt, Germany, CAS: 110-94-1 | 132.12 | 99 |
| Kerosene | Kerosene, Dragon Poland Sp.z.o.o., Skawina, Poland | - | - |
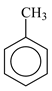 | Toluene, C6H5CH3, Chempur, Karlsruhe, Germany, CAS: 108-88-3 | 92.14 | 98.8 |
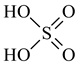 | Sulphuric acid, H2SO4, Riedel-de Haën, Seelze, Germany, CAS 7664-93-9 | 98.08 | 96.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domańska, U.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Wróbel, K.; Lach, J. Recovery of Metals from the “Black Mass” of Waste Portable Li-Ion Batteries with Choline Chloride-Based Deep Eutectic Solvents and Bi-Functional Ionic Liquids by Solvent Extraction. Molecules 2024, 29, 3142. https://doi.org/10.3390/molecules29133142
Domańska U, Wiśniewska A, Dąbrowski Z, Kolasa D, Wróbel K, Lach J. Recovery of Metals from the “Black Mass” of Waste Portable Li-Ion Batteries with Choline Chloride-Based Deep Eutectic Solvents and Bi-Functional Ionic Liquids by Solvent Extraction. Molecules. 2024; 29(13):3142. https://doi.org/10.3390/molecules29133142
Chicago/Turabian StyleDomańska, Urszula, Anna Wiśniewska, Zbigniew Dąbrowski, Dorota Kolasa, Kamil Wróbel, and Jakub Lach. 2024. "Recovery of Metals from the “Black Mass” of Waste Portable Li-Ion Batteries with Choline Chloride-Based Deep Eutectic Solvents and Bi-Functional Ionic Liquids by Solvent Extraction" Molecules 29, no. 13: 3142. https://doi.org/10.3390/molecules29133142
APA StyleDomańska, U., Wiśniewska, A., Dąbrowski, Z., Kolasa, D., Wróbel, K., & Lach, J. (2024). Recovery of Metals from the “Black Mass” of Waste Portable Li-Ion Batteries with Choline Chloride-Based Deep Eutectic Solvents and Bi-Functional Ionic Liquids by Solvent Extraction. Molecules, 29(13), 3142. https://doi.org/10.3390/molecules29133142







