Caudiquinol: A Meroterpenoid with an Intact C20 Geranylgeranyl Chain Isolated from Garcinia caudiculata
Abstract
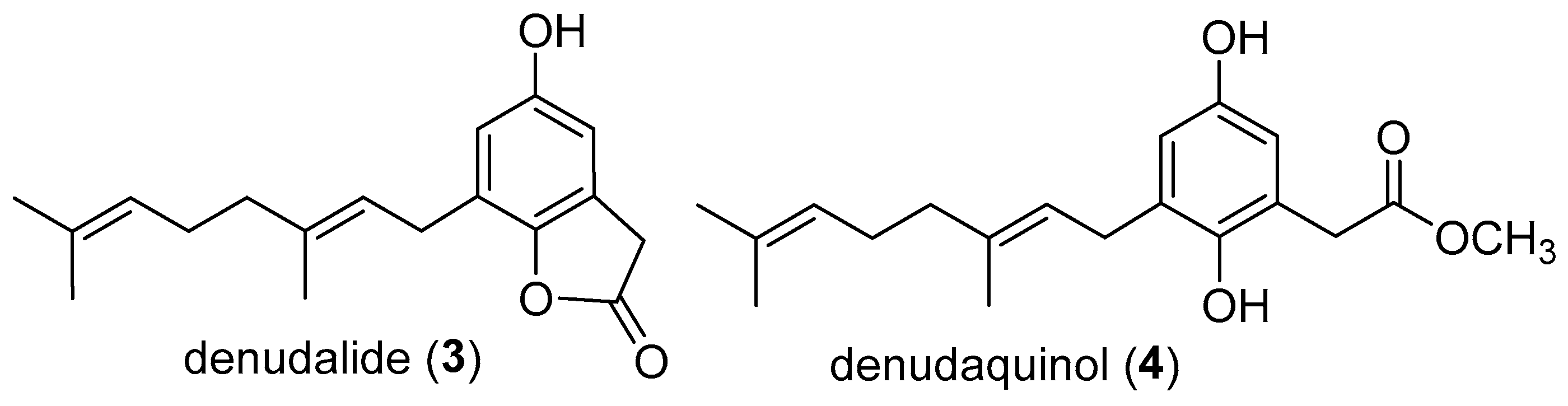
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
- Methyl 3-[(2E, 6E, 10E, 14E)-3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraen-1-yl]-2,5-dihydroxybenzeneacetate (caudiquinol 2). 6.0 mg; yellow oil; UV λmax (MeOH): 220, 232, and 294 nm; IR: 3387, 2914, 1714, and 1435 cm−1; m/z 455.316 [M + H]+ (calcd. for C29H43O4; 455.316; Δ = 0 ppm); 1H NMR (500 MHz): δ 6.50 (d, J = 3.1 Hz, 1H), 6.41 (d, J = 3.1 Hz, 1H), 5.20–5.25 (m, 1H), 5.00–5.09 (m, 3H), 3.66 (s, 3H), 3.53 (s, 2H), 3.27 (d, J = 7.0 Hz, 2H), 1.90–2.02 (m, 12H), 1.66 (s, 3H), 1.61 (s, 3H), and 1.53 (s, 9H). 13C NMR (126 MHz): δ 173.9, 149.0, 147.0, 138.0, 135.3, 135.0, 131.3, 130.6, 124.4, 124.2, 123.9, 121.5, 121.6, 115.9, 115.0, 52.5, 39.75, 39.73, 39.69, 37.2, 29.2, 26.7, 26.6, 26.5, 25.6, 17.8, 16.3, 16.20, and 16.15.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcinia L. Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:19345-1 (accessed on 24 May 2024).
- Espirito Santo, B.L.S.D.; Santana, L.F.; Kato Junior, W.H.; de Araújo, F.O.; Bogo, D.; Freitas, K.C.; Guimarães, R.C.A.; Hiane, P.A.; Pott, A.; Filiú, W.F.O.; et al. Medicinal Potential of Garcinia Species and Their Compounds. Molecules 2020, 25, 4513. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.C.; Marques, A.M.; da Camillo, F.C.; Figueiredo, M.R. Garcinia Spp: Products and by-products with potential pharmacological application in cancer. Food Biosci. 2022, 50, 102110. [Google Scholar] [CrossRef]
- Nchiozem-Ngnitedem, V.-A.; Mukavi, J.; Omosa, L.K.; Kuete, V. Phytochemistry and antibacterial potential of the genus Garcinia. In Advances in Botanical Research; Kuete, V., Ed.; Academic Press: London, UK, 2023; Volume 107, pp. 105–175. [Google Scholar]
- Ridley, H.N. Additions to the Flora of Borneo and Other Malay Islands: VI. Bull. Misc. Inf. (Royal Gardens Kew) 1938, 1938, 110–123. [Google Scholar] [CrossRef]
- Vieira, P.C.; Gottlieb, O.R.; Gottlieb, H.E. Tocotrienols from Iryanthera grandis. Phytochemistry 1983, 22, 2281–2286. [Google Scholar] [CrossRef]
- Gu, Q.; Wang, R.R.; Zhang, X.M.; Wang, Y.H.; Zheng, Y.T.; Zhou, J.; Chen, J.J. A New Benzofuranone and Anti-HIV Constituents from the Stems of Rhus chinensis. Planta Med. 2007, 73, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Z.; Geng, C.A.; Chen, J.J. First total synthesis of rhuscholide A, glabralide B and denudalide. Tetrahedron Lett. 2019, 60, 151059. [Google Scholar] [CrossRef]
- Noshita, T.; Kiyota, H.; Kidachi, Y.; Ryoyama, K.; Funayama, S.; Hanada, K.; Murayama, T. New Cytotoxic Phenolic Derivatives from Matured Fruits of Magnolia denudata. Biosci. Biotechnol. Biochem. 2009, 73, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, G.W.; Rodriguez, E. Prenylated hydroquinones: Contact allergens from trichomes of Phacelia minor and P. parryi. Phytochemistry 1981, 20, 1365–1366. [Google Scholar] [CrossRef]
- Voutquenne, L.; Lavaud, C.; Massiot, G.; Sevenet, T.; Hadi, H.A. Cytotoxic polyisoprenes and glycosides of long-chain fatty alcohols from Dimocarpus fumatus. Phytochemistry 1999, 50, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, V.; Kamkaew, M.; Sukavisit, D.; Phongpaichit, S.; Sawangchote, P.; Taylor, W.C. Antibacterial Xanthones from the Leaves of Garcinia nigrolineata. J. Nat. Prod. 2003, 66, 1531–1535. [Google Scholar] [CrossRef] [PubMed]



| Position | δC, Type | δH, Type |
|---|---|---|
| 1 | 147.0, C | |
| 2 | 121.5, C | |
| 3 | 115.0, CH | 6.50, d |
| 4 | 149.0, C | |
| 5 | 115.9, CH | 6.41, d |
| 6 | 131.3, C | |
| 7 | 29.2, CH2 | 3.27, d |
| 8 | 121.6, CH | 5.20–5.25, m |
| 9 | 138.0, C | |
| 10 | 37.2, CH2 | 1.90–2.02, m |
| 11 | 26.5, CH2 | 1.90–2.02, m |
| 12 | 123.9, CH | 5.00–5.09, m |
| 13 | 135.3, C | |
| 14 | 39.69, CH2 | 1.90–2.02, m |
| 15 | 26.6, CH2 | 1.90–2.02, m |
| 16 | 124.2, CH | 5.00–5.09, m |
| 17 | 135.0, C | |
| 18 | 39.73, CH2 | 1.90–2.02, m |
| 19 | 26.7, CH2 | 1.90–2.02, m |
| 20 | 124.4, CH | 5.00–5.09, m |
| 21 | 130.6, C | |
| 22 | 25.6, CH3 | 1.54, s |
| 1′ | 39.75, CH2 | 3.53, s |
| 2′ | 173.9, C | |
| 3′ | 52.5, CH3 | 3.66, s |
| 1″ | 16.3, CH3 | 1.66, s |
| 2″ | 16.2, CH3 | 1.61, s |
| 3‴ | 16.2, CH3 | 1.54, s |
| 4″ | 17.8, CH3 | 1.54, s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valmiki, M.; Teo, S.P.; de Resende, P.E.; Gibbons, S.; Ganesan, A. Caudiquinol: A Meroterpenoid with an Intact C20 Geranylgeranyl Chain Isolated from Garcinia caudiculata. Molecules 2024, 29, 3613. https://doi.org/10.3390/molecules29153613
Valmiki M, Teo SP, de Resende PE, Gibbons S, Ganesan A. Caudiquinol: A Meroterpenoid with an Intact C20 Geranylgeranyl Chain Isolated from Garcinia caudiculata. Molecules. 2024; 29(15):3613. https://doi.org/10.3390/molecules29153613
Chicago/Turabian StyleValmiki, Maya, Stephen Ping Teo, Pedro Ernesto de Resende, Simon Gibbons, and A. Ganesan. 2024. "Caudiquinol: A Meroterpenoid with an Intact C20 Geranylgeranyl Chain Isolated from Garcinia caudiculata" Molecules 29, no. 15: 3613. https://doi.org/10.3390/molecules29153613
APA StyleValmiki, M., Teo, S. P., de Resende, P. E., Gibbons, S., & Ganesan, A. (2024). Caudiquinol: A Meroterpenoid with an Intact C20 Geranylgeranyl Chain Isolated from Garcinia caudiculata. Molecules, 29(15), 3613. https://doi.org/10.3390/molecules29153613






