Anti-Inflammatory, Antidiabetic, and Antioxidant Properties of Extracts Prepared from Pinot Noir Grape Marc, Free and Incorporated in Porous Silica-Based Supports
Abstract
1. Introduction
2. Results
2.1. Characterization of Extracts
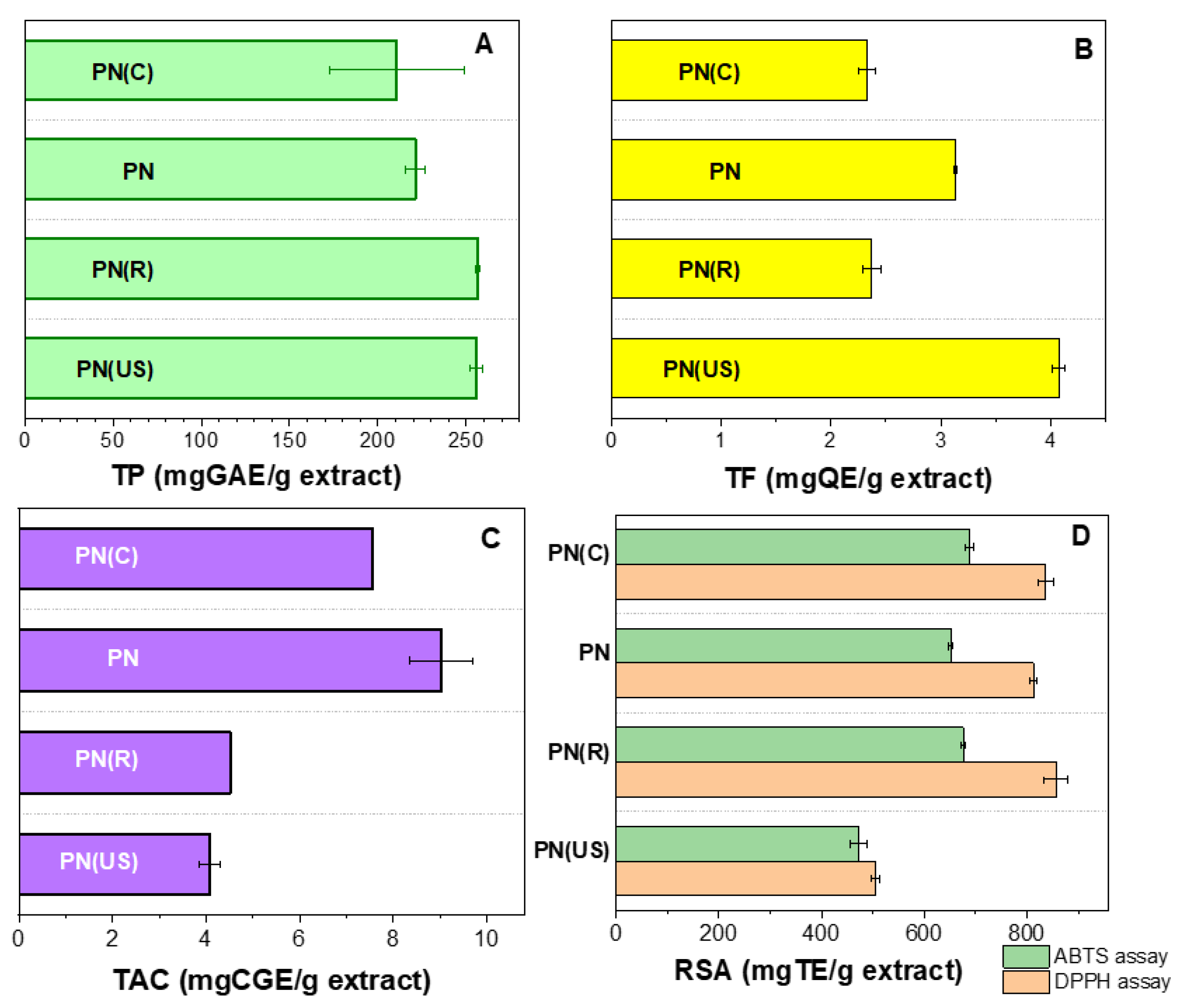
2.1.1. Spectrometric Determinations for Extracts
2.1.2. Chemical Composition of PN Grape Pomace Extracts
2.1.3. Antidiabetic Potential via α-Glucosidase Inhibitory Activity Assessment
2.1.4. Antimicrobial Activity of PN Extract
2.2. Incorporation of PN Extract in Porous Supports
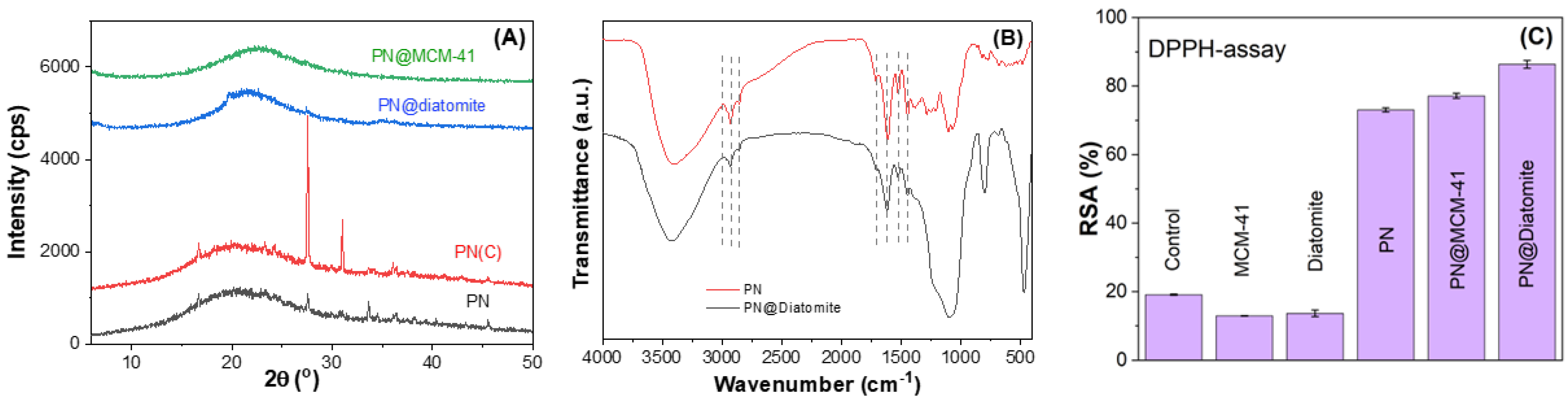
2.2.1. Supports Characterization
2.2.2. Characterization of PN-Loaded Silica-Based Supports
- Thermal analysis
- Wide-angle X-ray powder diffraction
- FTIR spectroscopy
- Radical scavenging activity
2.3. Biological Evaluation of PN Extract and PN-Loaded Supports
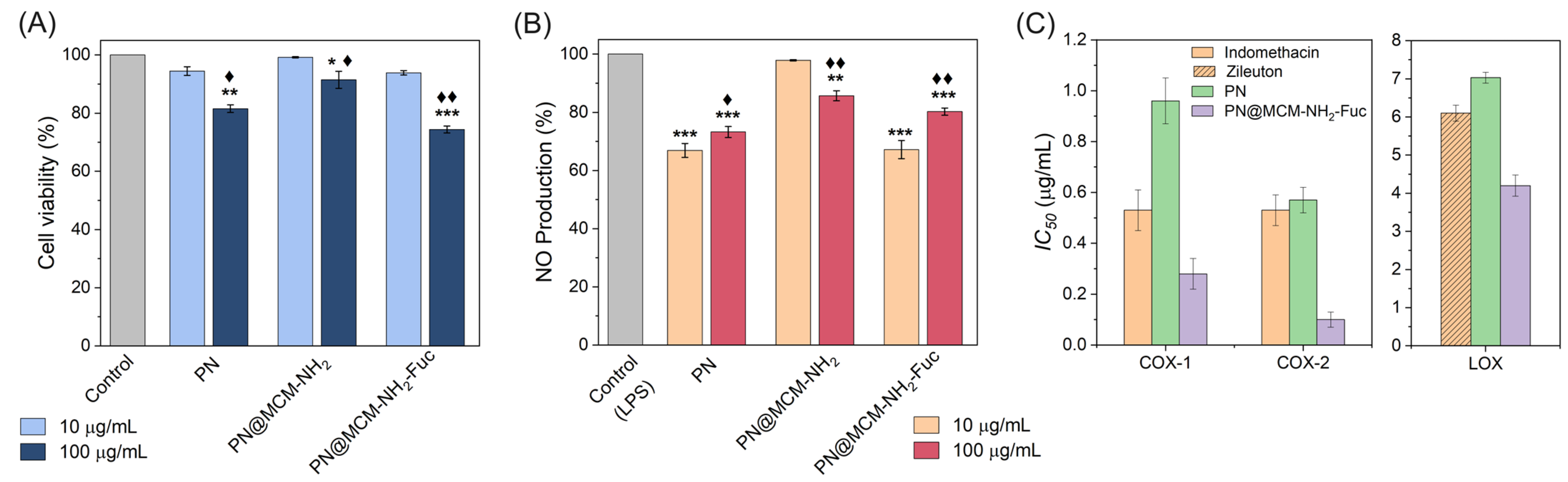
2.3.1. Cytotoxicity and NO Production Inhibitory Effects
2.3.2. Anti-Inflammatory Activity against Cyclooxygenase and Lipoxygenase Enzymes
3. Discussion
4. Materials and Methods
4.1. Preparation of Pinot Noir Extracts
4.2. Characterization of Pinot Noir Extracts
4.3. Obtaining and Characterization of Porous Supports
4.4. Biological Evaluation of PN Extract Free and Incorporated, in Porous Supports
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker Pertuzatti, P.; Cássia Mendonça, S.; Alcoléa, M.; Torres Guedes, C.; da Encarnação Amorim, F.; Simões Beckmann, A.P.; Almeida Gama, L.; Francely Américo, M. Bordo grape marc (Vitis labrusca): Evaluation of bioactive compounds in vitro and in vivo. LWT-Food Sci. Technol. 2020, 129, 109625. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Jovanović Galović, A.; Jovanović Lješković, N.; Vidović, S.; Vladić, J.; Jojić, N.; Ilić, M.; Srdić Rajić, T.; Kojić, V.; Jakimov, D. The Effects of Resveratrol-Rich Extracts of Vitis vinifera Pruning Waste on HeLa, MCF-7 and MRC-5 ells: Apoptosis, Autophagia and Necrosis Interplay. Pharmaceutics 2022, 14, 2017. [Google Scholar] [CrossRef] [PubMed]
- De Lima Cherubim, D.J.; Buzanello Martins, C.V.; Farina, L.O.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2019, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Jarma Arroyo, B.; Pinheiro Santos, A.; de Almeida de Melo, E.; Campos, A.; Lins, L.; Boyano-Orozco, L.C. Chapter 8-Bioactive Compounds and Their Potential Use as Ingredients for Food. In Bioactive Compounds. Health Benefits and Potential Applications; Segura Campos, M.R., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 143–156. [Google Scholar] [CrossRef]
- Castro, M.L.; Ferreira, J.P.; Pintado, M.; Ramos, O.L.; Borges, S.; Baptista-Silva, S. Grape By-Products in Sustainable Cosmetics: Nanoencapsulation and Market. Trends. Appl. Sci. 2023, 13, 9168. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Cör, D.; Kotnik, P.; Knez, Ž. Extracts of White and Red Grape Skin and Rosehip Fruit: Phenolic Compounds and their Antioxidative Activity. Acta Chim. Slov. 2019, 66, 751–761. [Google Scholar] [CrossRef]
- Bolson Moro, K.I.; Beutinger Bender, A.B.; Picolli da Silva, L.; Garcia Penna, N. Green Extraction Methods and Microencapsulation Technologies of Phenolic Compounds from Grape Pomace: A Review. Food Bioprocess Technol. 2021, 14, 1407–1431. [Google Scholar] [CrossRef]
- Shouqin, Z.; Jun, X.; Changzheng, W. High hydrostatic pressure extraction of flavonoids from propolis. J. Chem. Technol. Biotechnol. 2005, 80, 50–54. [Google Scholar] [CrossRef]
- Jun, X. Caffeine extraction from green tea leaves assisted by high pressure processing. J. Food Eng. 2009, 94, 105–109. [Google Scholar] [CrossRef]
- Okur, P.S.; Okur, I. Recent Advances in the Extraction of Phenolic Compounds from Food Wastes by Emerging Technologies. Food Bioprocess Technol. 2024. [Google Scholar] [CrossRef]
- Ştefănuţa, M.N.; Căta, A.; Popa, R.; Tănasiea, C.; Pinteab, B.; David, I. Thermal stability of anthocyanins from Vaccinium myrtillus L. methanolic extract. J. Agroaliment. Process. Technol. 2010, 16, 36–40. [Google Scholar]
- Brezoiu, A.-M.; Deaconu, M.; Mitran, R.-A.; Prelipcean, A.-M.; Matei, C.; Berger, D. Optimisation of Polyphenols Extraction from Wild Bilberry Leaves—Antimicrobial Properties and Stability Studies. Molecules 2023, 28, 5795. [Google Scholar] [CrossRef] [PubMed]
- Todorović, A.; Šturm, L.; Salević-Jelić, A.; Lević, S.; Črnivec, I.G.O.; Prislan, I.; Skrt, M.; Bjeković, A.; Ulrih, N.P.; Nedović, V. Encapsulation of Bilberry Extract with Maltodextrin and Gum Arabic by Freeze-Drying: Formulation, Characterisation, and Storage Stability. Processes 2022, 10, 1991. [Google Scholar] [CrossRef]
- González-Cruz, E.M.; Calderón-Santoyo, M.; Barros-Castillo, J.C.; Ragazzo-Sánchez, J.A. Evaluation of biopolymers in the encapsulation by electrospraying of polyphenolic compounds extracted from blueberry (Vaccinium corymbosum L.) variety Biloxi. Polym. Bull. 2021, 78, 3561–3576. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Matei, C.; Deaconu, M.; Stanciuc, A.-M.; Trifan, A.; Gaspar-Pintiliescu, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Bajenaru, L.; Berger, D.; Mitran, R.-A.; Deaconu, M.; Lincu, D.; Guzun, A.S.; Matei, C.; Moisescu, M.G.; Negreanu-Pirjol, T. Effect of Nanoconfinement of Polyphenolic Extract from Grape Pomace into Functionalized Mesoporous Silica on Its Biocompatibility and Radical Scavenging Activity. Antioxidants 2020, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Murugan, B.; Sagadevan, S.; Lett, A.; Fatimah, J.I.; Fatema, K.N.; Oh, W.-C.; Mohammad, F.; Johan, M.R. Role of Mesoporous Silica Nanoparticles for the Drug Delivery Applications. Mater. Res. Express 2020, 7, 102002. [Google Scholar] [CrossRef]
- Muhamad, I.I.; Asmak Md Lazim, N.; Selvakumaran, S. Natural polysaccharide-based composites for drug delivery and biomedical applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, UK, 2019; pp. 419–440. [Google Scholar]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Alfassam, H.E.; Ashraf, M.-T.; Al Othman, S.I.; Al-Waili, M.A.; Allam, A.A.; Abukhadra, M.R. Synthesis and characterization of cellulose functionalized zeolitic diatomite as an enhanced carrier of oxaliplatin drug; loading, release, and cytotoxicity. Int. J. Biol. Macromol. 2023, 235, 123825. [Google Scholar] [CrossRef]
- Marszałek, A. Adsorption of copper and lead from rainwater using adsorbents based on diatomite and calcium alginate. Desalin Water Treat. 2022, 275, 81–91. [Google Scholar] [CrossRef]
- Zhang, P.; Cui, Y.; Zhang, K.; Wu, S.; Chen, D.; Gao, Y. Enhanced thermal storage capacity of paraffin/diatomite composite using oleophobic modification. J. Clean. Prod. 2021, 279, 123211. [Google Scholar] [CrossRef]
- Herrera-Bravo, J.; Beltrán, J.F.; Huard, N.; Saavedra, K.; Saavedra, N.; Alvear, M.; Lanas, F.; Salazar, L.A. Anthocyanins Found in Pinot Noir Waste Induce Target Genes Related to the Nrf2 Signalling in Endothelial Cells. Antioxidants 2022, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Arena, P.; Miceli, N.; Marino, A.; Davi, E.; Cavò, E.; Spadaro, V.; Maria Raimondo, F.; Cacciola, F.; Laganà Vinci, R.; Mondello, L.; et al. Comparative Study on Phenolic Profile and Biological Activities of the Aerial Parts of Sinapis pubescens L. subsp. pubescens (Brassicaceae) Wild from Sicily (Italy). Chem. Biodivers. 2023, 20, e202300309. [Google Scholar] [CrossRef]
- Sekhon-Loodu, S.; Rupasinghe, H.P.V. Evaluation of antioxidant, antidiabetic and antiobesity potential of selected traditional medicinal plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Deaconu, M.; Brezoiu, A.-M.; Mitran, R.-A.; Nicu, I.; Manolescu, B.; Matei, C.; Berger, D. Exploiting the zwitterionic properties of lomefloxacin to tailor its delivery from functionalized MCM-41 silica. Micropor. Mesopor. Mater. 2020, 305, 110323. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kim, E.-A.; Gunasekara, U.K.D.S.S.; Abeytunga, D.T.U.; Nanayakkara, C.; de Silva, E.D.; et al. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. Algae 2017, 32, 75–86. [Google Scholar] [CrossRef]
- Aggrey, P.; Nartey, M.; Kan, Y.; Cvjetinovic, J.; Andrews, A.; Salimon, A.I.; Dragnevski, K.I.; Korsunsky, A.M. On the diatomite-based nanostructure-preserving material synthesis for energy applications. RSC Adv. 2021, 11, 31884. [Google Scholar] [CrossRef]
- Xia, J.; Wang, D.; Liang, P.; Zhang, D.; Du, X.; Ni, D.; Yu, Z. Vibrational (FT-IR, Raman) analysis of tea catechins based on both theoretical calculations and experiments. Biophys. Chem. 2020, 256, 106282. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies; John Wiley & Sons, Ltd.: Chichester, UK, 2001; pp. 97–98. [Google Scholar]
- Stiller, C.-O.; Hjemdahl, P. Lessons from 20 years with COX-2 inhibitors: Importance of dose–response considerations and fair play in comparative trials. J. Intern. Med. 2022, 292, 557–574. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Kashtiban, A.E.; Okpala, C.O.R.; Karimidastjerd, A.; Zahedinia, S. Recent advances in nano-related natural antioxidants, their extraction methods and applications in the food industry. Explor. Foods Foodomics 2024, 2, 125–154. [Google Scholar] [CrossRef]
- Constantin, O.E.; Stoica, F.; Rat, R.N.; Stanciuc, N.; Bahrim, G.E.; Râpeanu, G. Bioactive Components, Applications, Extractions, and Health Benefits of Winery By-Products from a Circular Bioeconomy Perspective: A Review. Antioxidants 2024, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Tsali, A.; Goula, A.M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Balea, S.S.; Pârvu, A.E.; Pop, N.; Zamora Marin, F.; Andreicuț, A.; Pârvu, M. Phytochemical profiling, antioxidant and cardio-protective properties of pinot noir cultivar pomace extracts. Farmacia 2018, 66, 431–441. [Google Scholar] [CrossRef]
- Kumar, L.; Tian, B.; Harrison, R. Interactions of Vitis vinifera L. cv. Pinot Noir grape anthocyanins with seed proanthocyanidins and their effect on wine color and phenolic composition. LWT-Food Sci. Technol. 2022, 162, 113428. [Google Scholar] [CrossRef]
- Xu, L.; Li, W.; Chen, Z.; Guo, Q.; Wang, C.; Santhanam, R.K.; Chen, H. Inhibitory effect of epigallocatechin-3-O-gallate on α-glucosidase and its hypoglycemic effect via targeting PI3K/AKT signaling pathway in L6 skeletal muscle cells. Int. J. Biol. Macromol. 2019, 125, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Yupanqui, M.; Lante, A.; Mihaylova, D.; Krastanov, A.I.; Rizzi, C. The α-Amylase and α-Glucosidase Inhibition Capacity of Grape Pomace: A Review. Food Bioprocess. Technol. 2023, 16, 691–703. [Google Scholar] [CrossRef]
- Liang, J.H.; Lin, H.R.; Yang, C.S.; Liaw, C.C.; Wang, I.C.; Chen, J.J. Bioactive Components from Ampelopsis japonica with Antioxidant, Anti-α-Glucosidase, and Antiacetylcholinesterase Activities. Antioxidants 2022, 11, 1228. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Iriondo-DeHond, A.; Dellacassa, E.; Medrano-Fernandez, A.; del Castillo, M.D. Assessment of antioxidant, antidiabetic, antiobesity, and anti-inflammatory properties of a Tannat winemaking by-product. Eur. Food Res. Technol. 2019, 245, 1539–1551. [Google Scholar] [CrossRef]
- Kong, F.; Su, Z.; Zhang, L.; Qin, Y.; Zhang, K. Inclusion complex of grape seeds extracts with sulfobutyl ether β-cyclodextrin: Preparation, characterization, stability and evaluation of α-glucosidase and α-amylase inhibitory effects in vitro. LWT 2019, 101, 819–826. [Google Scholar] [CrossRef]
- Deaconu, M.; Prelipcean, A.-M.; Brezoiu, A.-M.; Mitran, R.-A.; Seciu, A.M.; Matei, C.; Berger, D. Design of scaffolds based on zinc-modified marine collagen and bilberry leaves extract-loaded silica nanoparticles as wound dressings. Int. J. Nanomed. 2024; submitted. [Google Scholar]
- Takács, I.; Szekeres, A.; Takács, Á.; Rakk, D.; Mézes, M.; Polyák, A.; Lakatos, L.; Gyémánt, G.; Csupor, D.; Kovács, C.J.; et al. Wild Strawberry, Blackberry, and Blueberry Leaf Extracts Alleviate Starch-Induced Hyperglycemia in Prediabetic and Diabetic Mice. Planta Med. 2020, 86, 790–799. [Google Scholar] [CrossRef]
- Bljajić, K.; Petlevski, R.; Vujić, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; de Carvalho, I.S.; Zovko Končić, M. Chemical composition, antioxidant and α-glucosidase-inhibiting activities of the aqueous and hydroethanolic extracts of Vaccinium myrtillus leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial Potential, Antioxidant Activity, and Phenolic Content of Grape Seed Extracts from Four Grape Varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four Virginia-grown grape varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Sateriale, D.; Forgione, G.; Di Rosario, M.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Vine-Winery Byproducts as Precious Resource of Natural Antimicrobials: In Vitro Antibacterial and Antibiofilm Activity of Grape Pomace Extracts against Foodborne Pathogens. Microorganisms 2024, 12, 437. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Deaconu, M.; Mitran, R.-A.; Sedky, N.K.; Schiets, F.; Marote, P.; Voicu, I.-S.; Matei, C.; Ziko, L.; Berger, D. The Antioxidant and Anti-Inflammatory Properties of Wild Bilberry Fruit Extracts Embedded in Mesoporous Silica-Type Supports: A Stability Study. Antioxidants 2024, 13, 250. [Google Scholar] [CrossRef]
- Deaconu, M.; Prelipcean, A.-M.; Brezoiu, A.-M.; Mitran, R.-A.; Isopencu, G.; Matei, C.; Berger, D. Novel Collagen-Polyphenols-Loaded Silica Composites for Topical Application. Pharmaceutics 2023, 15, 312. [Google Scholar] [CrossRef]
- Mukerabigwi, J.F.; Wang, Q.; Ma, X.; Liu, M.; Lei, S.; Wei, H.; Huang, X.; Cao, Y. Urea fertilizer coated with biodegradable polymers and diatomite for slow release and water retention. J. Coat. Technol. Res. 2015, 12, 1085–1094. [Google Scholar] [CrossRef]
- Kulikova, A.K.; Yashin, E.A.; Kozlov, A.V.; Toigildina, I.A.; Zakharov, N.G.; Hairtdinova, N.A.; Karpov, A.V.; Toigildin, A.L. Influence of Diatomite on Crop Productivity. Res. J. Pharm. Biolog. Chem. Sci. 2016, 7, 1037–1041. [Google Scholar]
- Artem, V.; Negreanu–Pirjol, T.; Ranca, A.; Ciobanu, C.; Abduraman, A.; Coroiu, V.; Negreanu-Pijol, B.-S. Experimental studies on the residual marine and viticultural bioresources valorization for new organic fertilizers. UPB Sci. Bull. Ser. B 2021, 83, 65–76. [Google Scholar]
- Vania Sáez, V.; Pastene, E.; Vergara, C.; Mardones, C.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Gómez, M.V.; Theoduloz, C.; Riquelme, S.; von Baer, D. Oligostilbenoids in Vitis vinifera L. Pinot Noir grape cane extract: Isolation, characterization, in vitro antioxidant capacity and anti-proliferative effect on cancer cells. Food Chem. 2018, 265, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Macovei, S.O.; Bocsan, I.C.; Măgureanu, D.C.; Levai, A.M.; Buzoianu, A.D.; Pop, R.M. Grape Pomace Polyphenols as a Source of Compounds for Management of Oxidative Stress and Inflammation—A Possible Alternative for Non-Steroidal Anti-Inflammatory Drugs? Molecules 2022, 27, 6826. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.; Bogdan, C.; Fizesan, I.; Iurian, S.; Carpa, R.; Bacali, C.; Vlase, L.; Benedec, D.; Moldovan, M.L. In Vitro Evaluation of Biological Activities of Canes and Pomace Extracts from Several Varieties of Vitis vinifera L. for Inclusion in Freeze-Drying Mouthwashes. Antioxidants 2022, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Fariña, E.; Daghero, H.; Bollati-Fogolín, M.; Boido, E.; Cantero, J.; Moncada-Basualto, M.; Olea-Azar, C.; Polticelli, F.; Paulino, M. Antioxidant Capacity and NF-kB-Mediated Anti-Inflammatory Activity of Six Red Uruguayan Grape Pomaces. Molecules 2023, 28, 3909. [Google Scholar] [CrossRef] [PubMed]
- Balea, S.S.; Pârvu, A.E.; Pârvu, M.; Vlase, L.; Dehelean, C.A.; Pop, T.I. Antioxidant, Anti-Inflammatory and Antiproliferative Effects of the Vitis vinifera L. var. Fetească Neagră and Pinot Noir Pomace Extracts. Front. Pharmacol. Sec. Ethnopharmacol. 2020, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Kutil, Z.; Temml, V.; Maghradze, D.; Pribylova, M.; Dvorakova, M.; Schuster, D.; Vanek, T.; Landa, P. Impact of Wines and Wine Constituents on Cyclooxygenase-1, Cyclooxygenase-2, and 5-Lipoxygenase Catalytic Activity. Mediat. Inflamm. 2014, 178931. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Balaha, M.; Acquaviva, A.; Ferrante, C.; Cataldi, A.; Menghini, L.; Rapino, M.; Orlando, G.; Brunetti, L.; Leone, S.; et al. Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts. Molecules 2021, 26, 6216. [Google Scholar] [CrossRef]
- Mehrabian, M.; Allayee, H. 5-Lipoxygenase and atherosclerosis. Curr. Opin. Lipidol. 2003, 14, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Een Kim, C.; Lee, M. Serratane triterpenoids isolated from Lycopodium clavatum by bioactivity-guided fractionation attenuate the production of inflammatory mediators. Bioorg. Chem. 2020, 96, 103632. [Google Scholar] [CrossRef] [PubMed]



| Compounds | Concentration in Extract (mgcompound/gextract) | |||
|---|---|---|---|---|
| PN | PN(R) | PN(C) | PN(US) | |
| Gallic Acid | 0.791 ± 0.001 | 0.933 ± 0.001 | 0.736 ± 0.002 | 0.820 ± 0.005 |
| Protocatechuic acid | 0.337 ± 0.001 | 0.396 ± 0.002 | 0.250 ± 0.002 | 0.278 ± 0.001 |
| Catechin hydrate | 14.673 ± 0.018 | 18.722 ± 0.015 | 11.505 ± 0.016 | 12.784 ± 0.004 |
| Vanillic acid | 0.687 ± 0.001 | 0.833 ± 0.002 | 0.579 ± 0.002 | 0.640 ± 0.001 |
| Siringic acid | 0.928 ± 0.002 | 1.168 ± 0.002 | 0.745 ± 0.001 | 0.913 ± 0.005 |
| (-) Epicatechin | 10.613 ± 0.031 | 13.218 ± 0.008 | 8.772 ± 0.027 | 9.700 ± 0.037 |
| Delphinidin | 0.401 ± 0.004 | 0.402 ± 0.003 | 0.453 ± 0.001 | 0.080 ± 0.001 |
| Rutin hydrate | 0.517 ± 0.001 | 0.559 ± 0.002 | 0.523 ± 0.002 | 0.499 ±0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deaconu, M.; Abduraman, A.; Brezoiu, A.-M.; Sedky, N.K.; Ioniță, S.; Matei, C.; Ziko, L.; Berger, D. Anti-Inflammatory, Antidiabetic, and Antioxidant Properties of Extracts Prepared from Pinot Noir Grape Marc, Free and Incorporated in Porous Silica-Based Supports. Molecules 2024, 29, 3122. https://doi.org/10.3390/molecules29133122
Deaconu M, Abduraman A, Brezoiu A-M, Sedky NK, Ioniță S, Matei C, Ziko L, Berger D. Anti-Inflammatory, Antidiabetic, and Antioxidant Properties of Extracts Prepared from Pinot Noir Grape Marc, Free and Incorporated in Porous Silica-Based Supports. Molecules. 2024; 29(13):3122. https://doi.org/10.3390/molecules29133122
Chicago/Turabian StyleDeaconu, Mihaela, Anil Abduraman, Ana-Maria Brezoiu, Nada K. Sedky, Simona Ioniță, Cristian Matei, Laila Ziko, and Daniela Berger. 2024. "Anti-Inflammatory, Antidiabetic, and Antioxidant Properties of Extracts Prepared from Pinot Noir Grape Marc, Free and Incorporated in Porous Silica-Based Supports" Molecules 29, no. 13: 3122. https://doi.org/10.3390/molecules29133122
APA StyleDeaconu, M., Abduraman, A., Brezoiu, A.-M., Sedky, N. K., Ioniță, S., Matei, C., Ziko, L., & Berger, D. (2024). Anti-Inflammatory, Antidiabetic, and Antioxidant Properties of Extracts Prepared from Pinot Noir Grape Marc, Free and Incorporated in Porous Silica-Based Supports. Molecules, 29(13), 3122. https://doi.org/10.3390/molecules29133122











