Host-Guest Complexes of Flavanone and 4′-Chloroflavanone with Naturals and Modified Cyclodextrin: A Calorimetric and Spectroscopy Investigations
Abstract
1. Introduction
2. Results and Discussion
2.1. Isothermal Titration Calorimetry (ITC)
2.2. Differential Scanning Calorimetry (DSC)
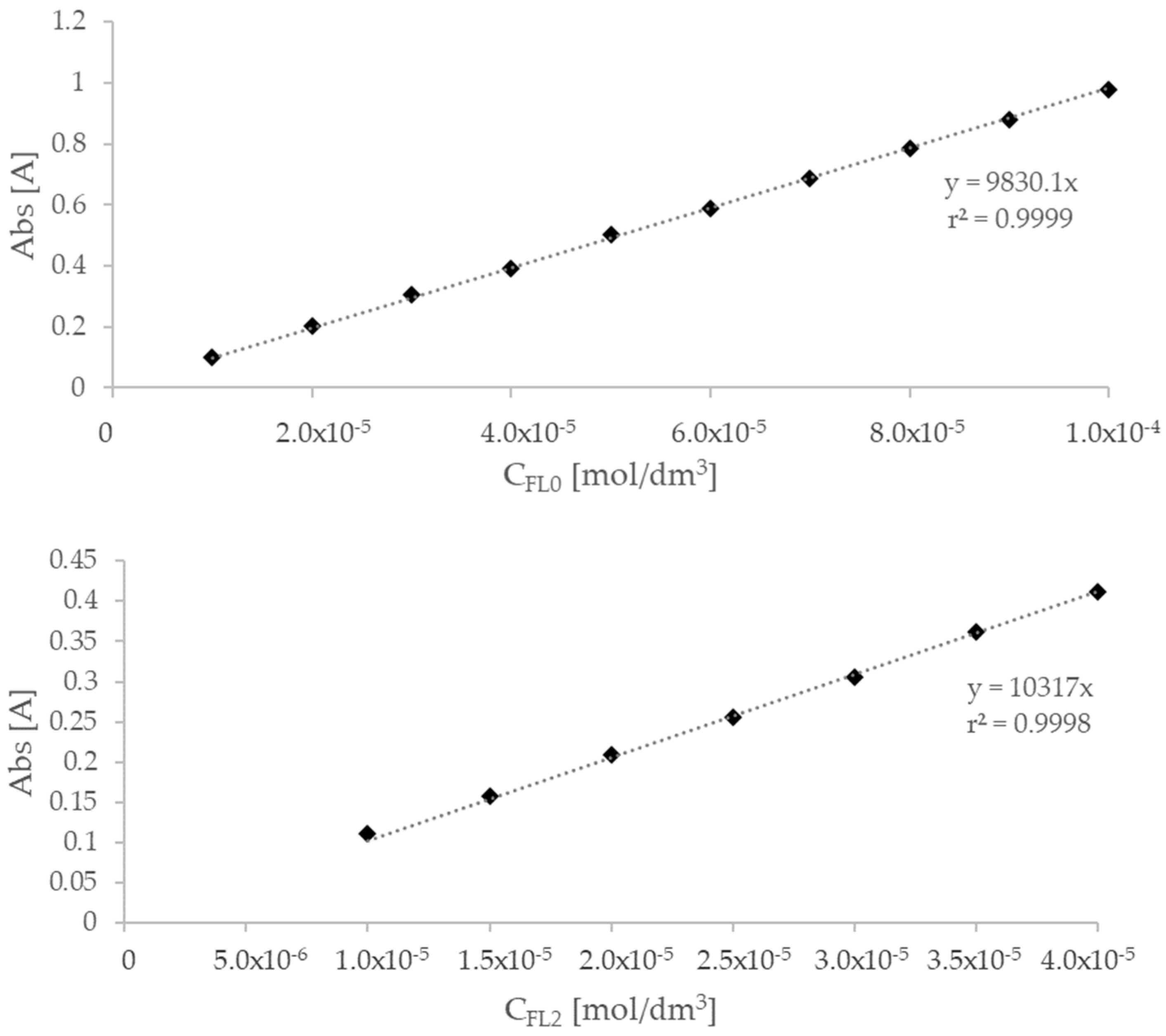
2.3. UV-Vis Spectroscopy (Phase Solubility Study)
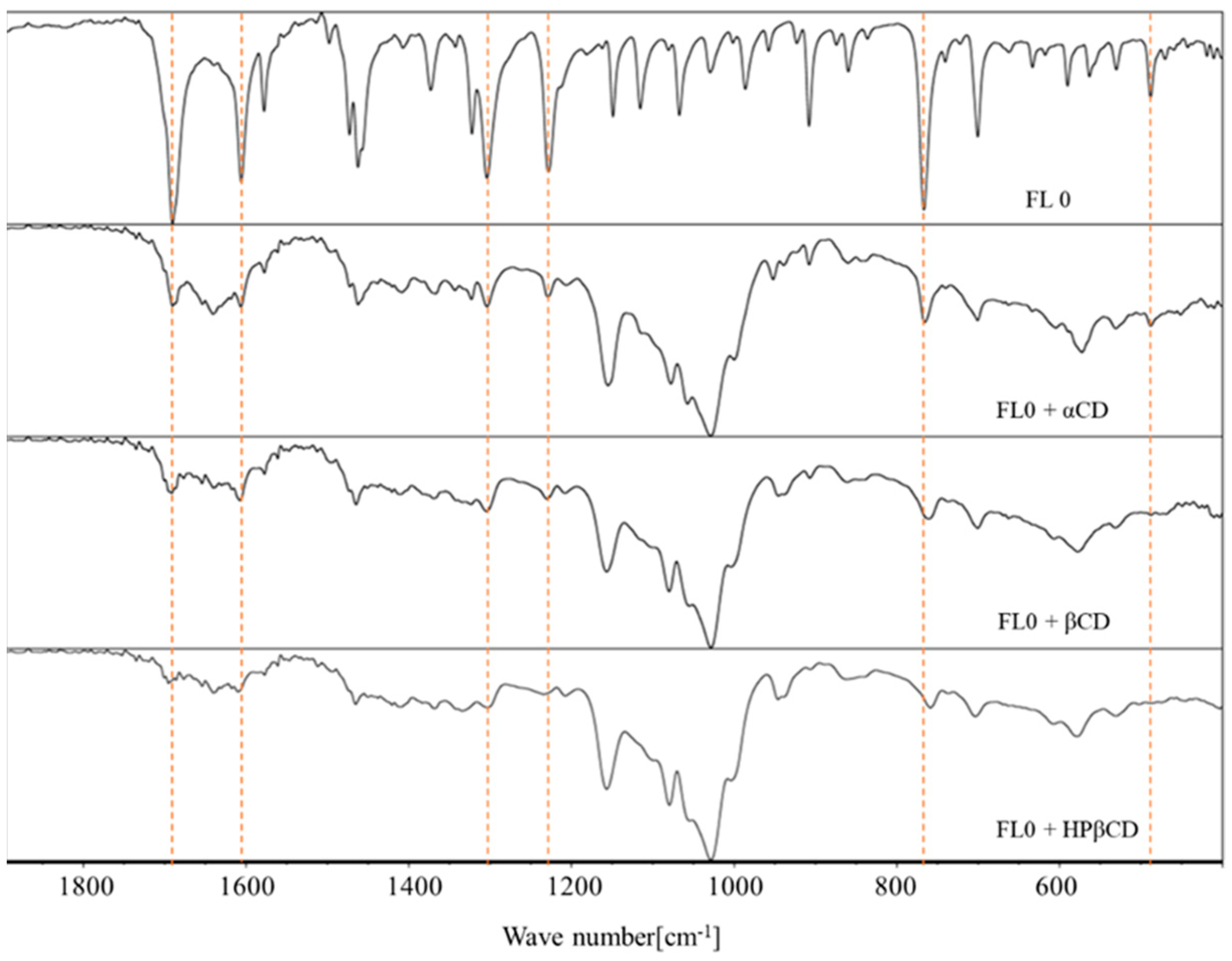
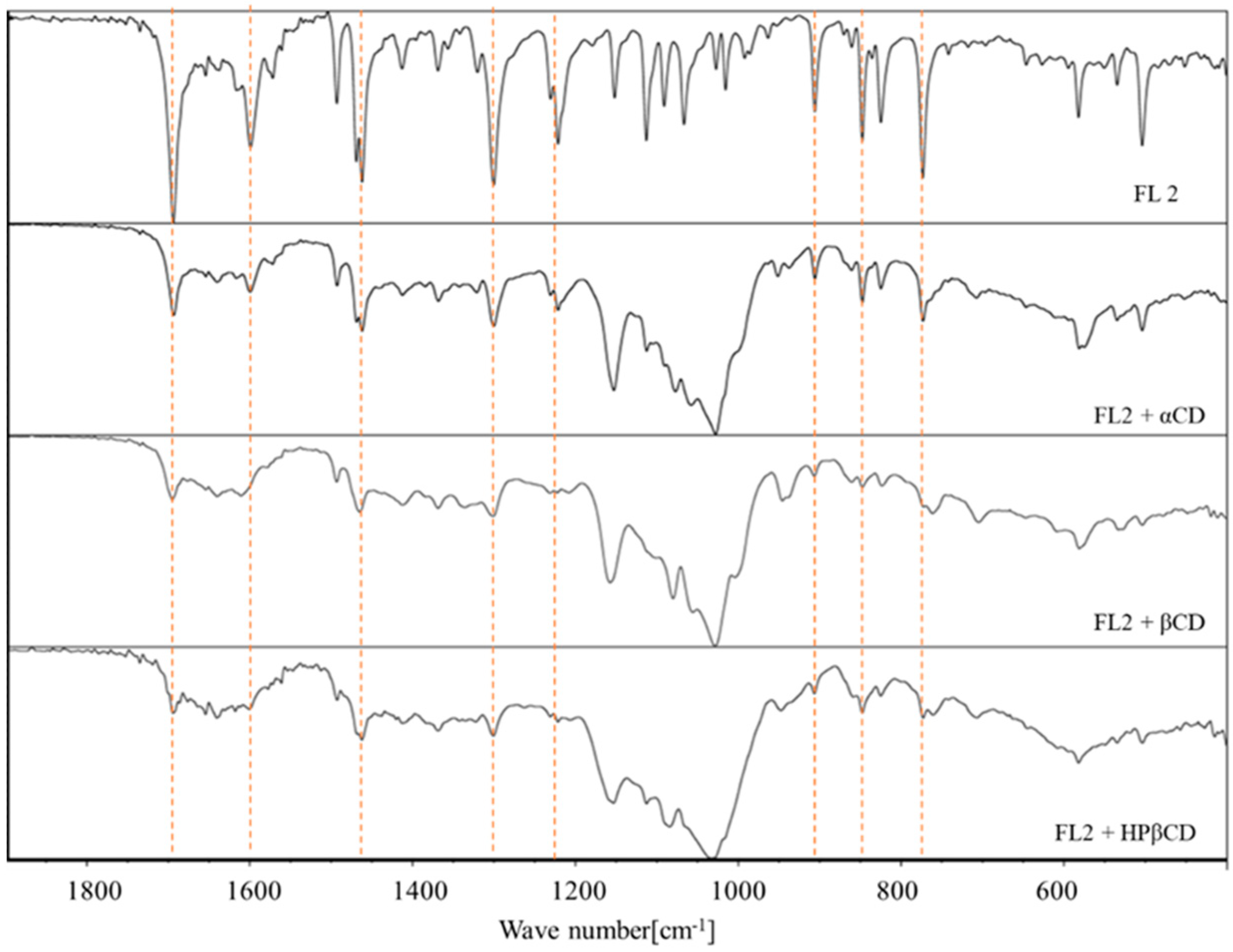
2.4. FT-IR Spectroscopy
- flavanone: 1690.2 cm−1; 1605.8 cm−1; 1462.4 cm−1; 1303.7 cm−1; 766.7 cm−1; 487.8 cm−1 [38]
- 4′-chloroflavanone: 1693.9 cm−1; 1599.2 cm−1; 1461.9 cm−1; 1300.2 cm−1; 1121.1 cm−1; 905.9 cm−1; 847.5 cm−1; 773.1 cm−1 [32]
2.5. 1H NMR Spectroscopy
3. Materials and Methods
3.1. Materials
3.2. Isothermal Titration Calorimetry (ITC)
3.3. Differential Scanning Calorimetry (DSC)
3.4. UV-Vis Spectroscopy (Phase Solubility Study)
3.5. FT-IR Spectroscopy
3.6. 1H NMR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Vazifehasl, Z.; Salatin, S.; Adibkia, K.; Javadzadeh, Y. Nanosizing of Drugs: Effect on Dissolution Rate. Res. Pharm. Sci. 2015, 10, 95–108. [Google Scholar] [PubMed]
- Elder, D.P.; Holm, R.; de Diego, H.L. Use of Pharmaceutical Salts and Cocrystals to Address the Issue of Poor Solubility. Int. J. Pharm. 2013, 453, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Berge, S.M.; Bighley, L.D.; Monkhouse, D.C. Pharmaceutical Salts. J. Pharm. Sci. 1977, 66, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Perioli, L.; Pagano, C. Inorganic Matrices: An Answer to Low Drug Solubility Problem. Expert Opin. Drug Deliv. 2012, 9, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.N.; Ahmed, M.H.; Saqib, M.; Shaikh, S.N. Chemical Modification: A Unique Solutions to Solubility Problem. J. Drug Deliv. Ther. 2019, 9, 542–546. [Google Scholar] [CrossRef]
- Fromming, K.-H.; Szejtli, J. Cyclodextrin in Pharmacy; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, Q.X. The Driving Forces in the Inclusion Complexation of Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Gergely, V.; Sebestyén, G.; Virág, S. Toxicity Studies of Beta-Cyclodextrin. In Proceedings of the First International Symposium on Cyclodextrins, Budapest, Hungary, 30 September–2 October 1981; Springer: Dordrecht, The Netherlands, 1982; pp. 109–113. [Google Scholar]
- Gerloczy, A.; Fonagy, A.; Keresztes, P.; Perlaky, L.; Szejtli, J. Absorption, Distribution, Excretion and Metabolism of Universally Labelled 14-C-β-Cyclodextrin in Rat after per Os Administration. Arzneimittel-Forschung. Drug Res. 1985, 35, 1042–1047. [Google Scholar]
- Szente, L.; Puskás, I.; Sohajda, T.; Varga, E.; Vass, P.; Nagy, Z.K.; Farkas, A.; Várnai, B.; Béni, S.; Hazai, E. Sulfobutylether-Beta-Cyclodextrin-Enabled Antiviral Remdesivir: Characterization of Electrospun- and Lyophilized Formulations. Carbohydr. Polym. 2021, 264, 118011. [Google Scholar] [CrossRef] [PubMed]
- Donati, F. Sugammadex: A Cyclodextrin to Reverse Neuromuscular Blockade in Anaesthesia. Expert Opin. Pharmacother. 2008, 9, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Stepniak, A.; Belica-Pacha, S.; Rozalska, S.; Dlugonski, J.; Urbaniak, P.; Palecz, B. Study on a Host-Guest Interaction of β-Cyclodextrin with Tebuconazole in Water. J. Mol. Liq. 2015, 211, 288–293. [Google Scholar] [CrossRef]
- Navarro, P.; Nicolas, T.S.; Gabaldon, J.A.; Mercader-Ros, M.T.; Calín-Sánchez, Á.; Carbonell-Barrachina, Á.A.; Pérez-López, A.J. Effects of Cyclodextrin Type on Vitamin C, Antioxidant Activity, and Sensory Attributes of a Mandarin Juice Enriched with Pomegranate and Goji Berries. J. Food Sci. 2011, 76, S319–S324. [Google Scholar] [CrossRef] [PubMed]
- Labib, G.S. Novel Levocetirizine HCl Tablets with Enhanced Palatability: Synergistic Effect of Combining Taste Modifiers and Effervescence Technique. Drug Des. Dev. Ther. 2015, 9, 5135–5146. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Zhang, L.; Zhang, L.; Teng, Y.; Zhang, J. Design and Evaluation of an Economic Taste-Masked Dispersible Tablet of Pyridostigmine Bromide, a Highly Soluble Drug with an Extremely Bitter Taste. Chem. Pharm. Bull. 2012, 60, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Piao, Z.Z.; Lee, M.K.; Lee, B.J. Colonic Release and Reduced Intestinal Tissue Damage of Coated Tablets Containing Naproxen Inclusion Complex. Int. J. Pharm. 2008, 350, 205–211. [Google Scholar] [CrossRef]
- Cevher, E.; Açma, A.; Sinani, G.; Aksu, B.; Zloh, M.; Mülazımoğlu, L. Bioadhesive Tablets Containing Cyclodextrin Complex of Itraconazole for the Treatment of Vaginal Candidiasis. Int. J. Biol. Macromol. 2014, 69, 124–136. [Google Scholar] [CrossRef]
- Adamkiewicz, L.; Szeleszczuk, Ł. Review of Applications of Cyclodextrins as Taste-Masking Excipients for Pharmaceutical Purposes. Molecules 2023, 28, 6964. [Google Scholar] [CrossRef]
- Bawazeer, N.A.; Choudhry, H.; Zamzami, M.A.; Abdulaal, W.H.; Middleton, B.; Moselhy, S.S. Role of Hesperetin in LDL-Receptor Expression in Hepatoma HepG2 Cells. BMC Complement. Altern. Med. 2016, 16, 182. [Google Scholar] [CrossRef]
- Choi, E.J.; Lee, J.I.; Kim, G.H. Anti-Carcinogenic Effect of a New Analogue 4′-Chloroflavanone from Flavanone in Human Breast Cancer Cells. Int. J. Mol. Med. 2010, 25, 293–298. [Google Scholar] [CrossRef]
- Safavi, M.; Esmati, N.; Ardestani, S.K.; Emami, S.; Ajdari, S.; Davoodi, J.; Shafiee, A.; Foroumadi, A. Halogenated Flavanones as Potential Apoptosis-Inducing Agents: Synthesis and Biological Activity Evaluation. Eur. J. Med. Chem. 2012, 58, 573–580. [Google Scholar] [CrossRef]
- Dimethyl Sulfoxide (DMSO) Health and Safety Information; Gaylord Chemical Company: Covington, LA, USA, 2007; Volume 106, p. 1.
- Otterbach, A.; Lamprecht, A. Enhanced Skin Permeation of Estradiol by Dimethyl Sulfoxide Containing Transdermal Patches. Pharmaceutics 2021, 13, 320. [Google Scholar] [CrossRef]
- Bragger, J.M.; Dunn, R.V.; Daniel, R.M. Enzyme activity down to -100 degrees C. Biochim. Biophys. Acta 2000, 1480, 278. [Google Scholar] [CrossRef]
- Buvári-Barcza, A.; Barcza, L. Influence of the guests, the type and degree of substitution on inclusion complex formation of substituted betacyclodextrins. Talanta 1999, 49, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations II: Solubilization, binding constant, and complexation efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Koester, L.S.; Guterres, S.S.; Le Roch, M.; Eifler-Lima, V.L.; Zuanazzi, J.A.; Bassani, V.L. Ofloxacin/β-Cyclodextrin Complexation. Drug Dev. Ind. Pharm. 2021, 27, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Liu, B.; Yuan, E.; Ma, Y.; Zhu, Y. Preparation and Physicochemical Properties of the Complex of Naringenin with Hydroxypropyl-β-Cyclodextrin. Molecules 2010, 15, 4401–4407. [Google Scholar] [CrossRef]
- Rao, V.K.; Rao, M.S.; Kumar, A. Ytterbium(III) Triflate: An Efficient and Simple Catalyst for Isomerization of 2′-Hydroxychalcone and 2′-Aminochalcones in Ionic Liquid. J. Heterocycl. Chem. 2011, 48, 1356–1360. [Google Scholar] [CrossRef]
- Kostrzewa-Susłow, E.; Dmochowska-Gładysz, J.; Białońska, A.; Ciunik, Z. Microbial Transformations of Flavanone by Aspergillus Niger and Penicillium Chermesinum Cultures. J. Mol. Catal. B Enzym. 2008, 52–53, 34–39. [Google Scholar] [CrossRef]
- Zheng, X.; Jiang, H.; Xie, J.; Yin, Z.; Zhang, H. Highly Efficient and Green Synthesis of Flavanones and Tetrahydroquinolones. Synth. Commun. 2013, 43, 1023–1029. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Tao, Y.; Han, Y.; Dong, S.; Fan, X.; Wang, T.; Yan, X. Preparation, Solubilization and In vitro Anti-tumour Effect of Water-soluble Betulinic Acid/Oligo(polylvinylamino) Bridged bis(β-cyclodextrin)s Complexes. Chem. Sci. Int. J. 2018, 21, 1–15. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, Z.H.; Sun, E. Effect of β-cyclodextrin complexation on solubility and enzymatic conversion of naringin. Int. J. Mol. Sci. 2012, 13, 14251–14261. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Shin, S.Y.; Jung, Y.; Tran, T.A.; Lee, H.O.; Jung, K.Y.; Koh, D.; Cho, S.K.; Lim, Y. Quantitative Relationships between the Cytotoxicity of Flavonoids on the Human Breast Cancer Stem-Like Cells MCF7-SC and Their Structural Properties. Chem. Biol. Drug Des. 2015, 86, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S. Study of Flavonoid/Hydroxypropyl-β-Cyclodextrin Inclusion Complexes by UV-Vis, FT-IR, DSC, and X-ray Diffraction Analysis. Prev. Nutr. Food Sci. 2020, 25, 449–456. [Google Scholar] [CrossRef]
- Qiu, N.; Cheng, X.; Wang, G.; Wang, W.; Wen, J.; Zhang, Y. Inclusion complex of barbigerone with hydroxypropyl-β-cyclodextrin: Preparation and in vitro evaluation. Carbohydr. Polym. 2014, 101, 623–630. [Google Scholar] [CrossRef]
- Thakkar, A.L.; Demarco, P.V. Cycloheptaamylose inclusion complexes of barbiturates: Correlation between proton magnetic resonance and solubility studies. J. Pharm. Sci. 1971, 60, 652–653. [Google Scholar] [CrossRef]
- Greatbanks, D.; Pickford, R. Cyclodextrins as chiral complexing agents in water, and their application to optical purity measurements. Magn. Reson. Chem. 1987, 25, 208–215. [Google Scholar] [CrossRef]
- Silva, I.S.; Feitosa, E.L.; Santos, M.E.P.; Silva, R.M.; Rocha, M.S.; da Silva, F.I.; Lima, F.C.A.; Costa, A.M.S.; Alves, P.B.; de Sousa, S.A.A.; et al. Theoretical and Experimental Investigations on Inclusion Complex β-Cyclodextrin and Sulcatone: A Cardiovascular Activity Evaluation. J. Braz. Chem. Soc. 2020, 31, 1064–1077. [Google Scholar] [CrossRef]
- Biernacka, M.; Ilyich, T.; Zavodnik, I.; Palecz, B.; Stepniak, A. Studies of the Formation and Stability of Ezetimibe-Cyclodextrin Inclusion Complexes. Int. J. Mol. Sci. 2022, 23, 455. [Google Scholar] [CrossRef] [PubMed]
- Buczkowski, A.; Urbaniak, P.; Palecz, B. Thermochemical and spectroscopic studies on the supramolecular complex of PAMAM-NH2 G4 dendrimer and 5-fluorouracil in aqueous solution. Int. J. Pharm. 2012, 428, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Buczkowski, A.; Urbaniak, P.; Piekarski, H.; Palecz, B. Spectroscopic and calorimetric studies on the interaction between PAMAM G4-OH and 5-fluorouracil in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Rajewski, R.A. Cyclodextrins: Their future in drug formulation and delivery. Pharm. Res. 1997, 14, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Fourmentin, S.; Ciobanu, A.; Landy, D.; Wenz, G. Space filling of β-cyclodextrin and β-cyclodextrin derivatives by volatile hydrophobic guests. Beilstein J. Org. Chem. 2013, 9, 1185–1191. [Google Scholar] [CrossRef]
- Dhruve, P.; Tripathi, A.; Gidwani, B.; Vyas, A. Investigating the phase complexed with -solubility and compatibility study of Anticancer drug β-cyclodextrin and hp–β-cyclodextrin. Int. J. Adv. Pharm. Sci. 2017, 9, 69–74. [Google Scholar] [CrossRef][Green Version]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Soe, H.M.S.H.; Kerdpol, K.; Rungrotmongkol, T.; Pruksakorn, P.; Autthateinchai, R.; Wet-osot, S.; Loftsson, T.; Jansook, P. Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether β-Cyclodextrin/Polyvinyl Alcohol Complexes. Int. J. Mol. Sci. 2023, 24, 2343. [Google Scholar] [CrossRef]
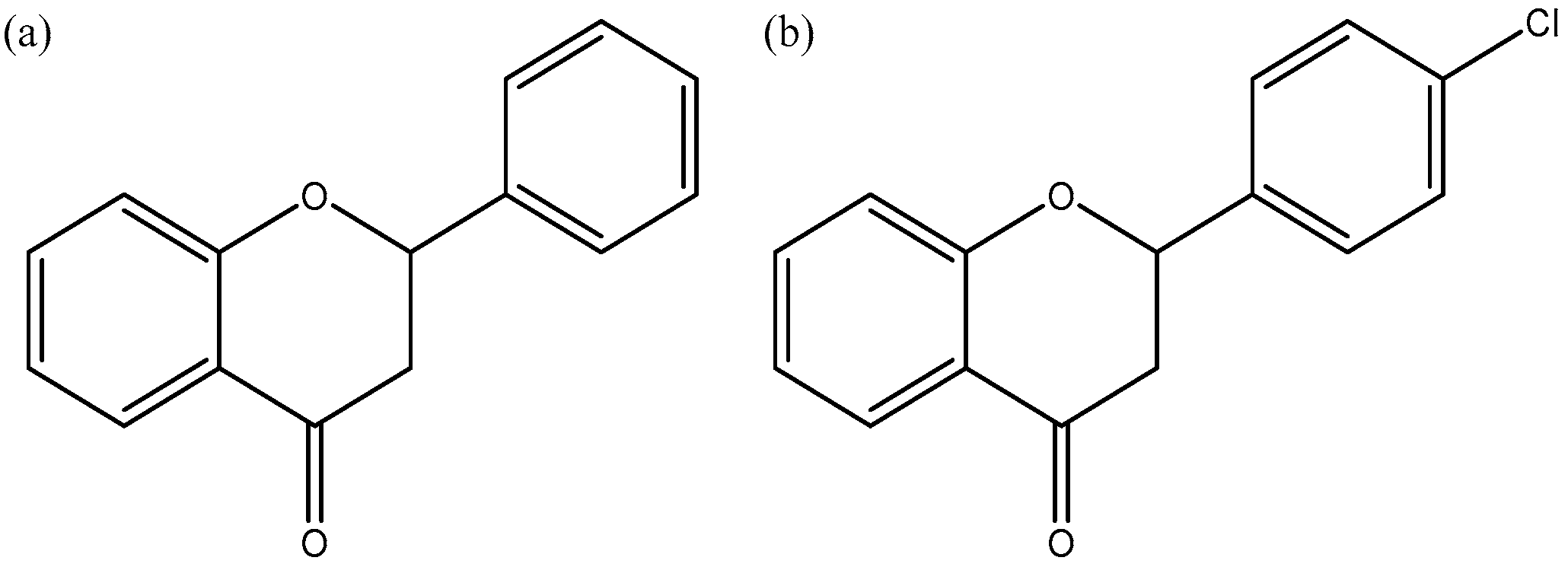
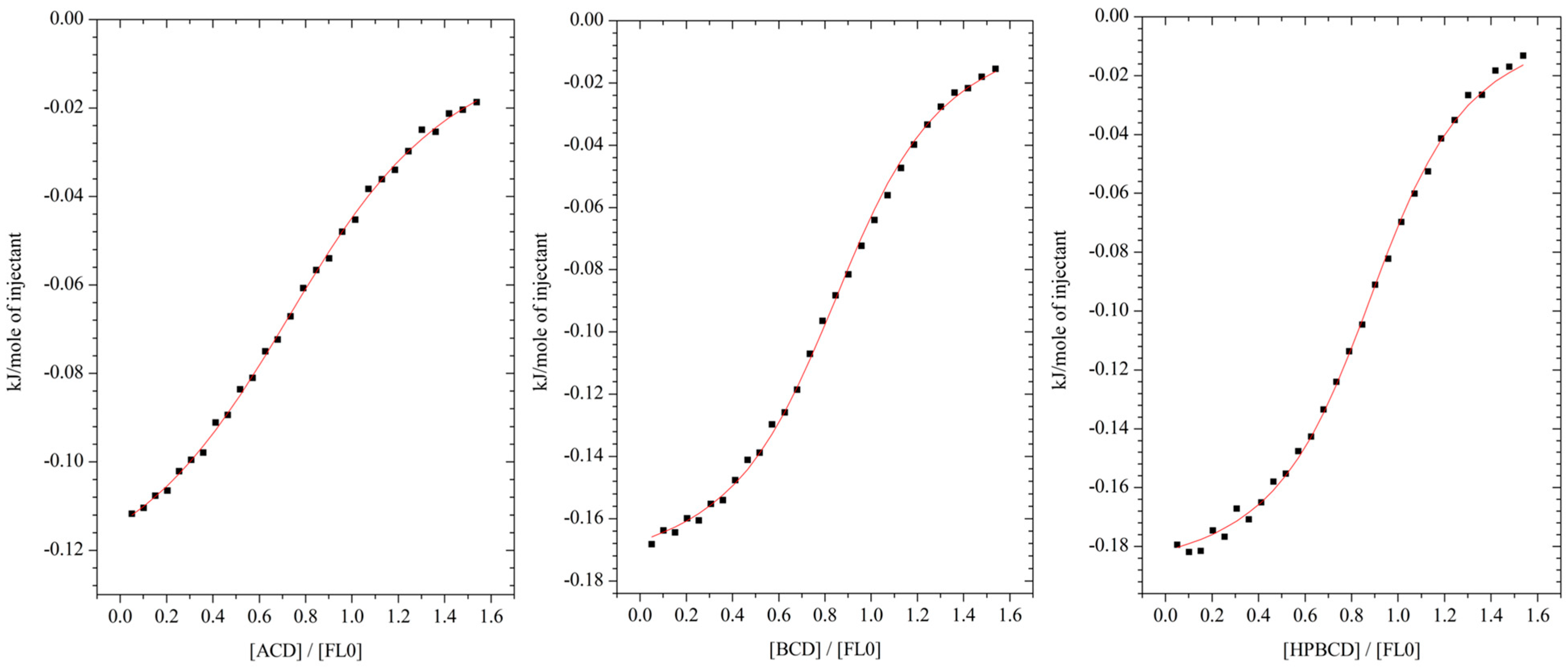
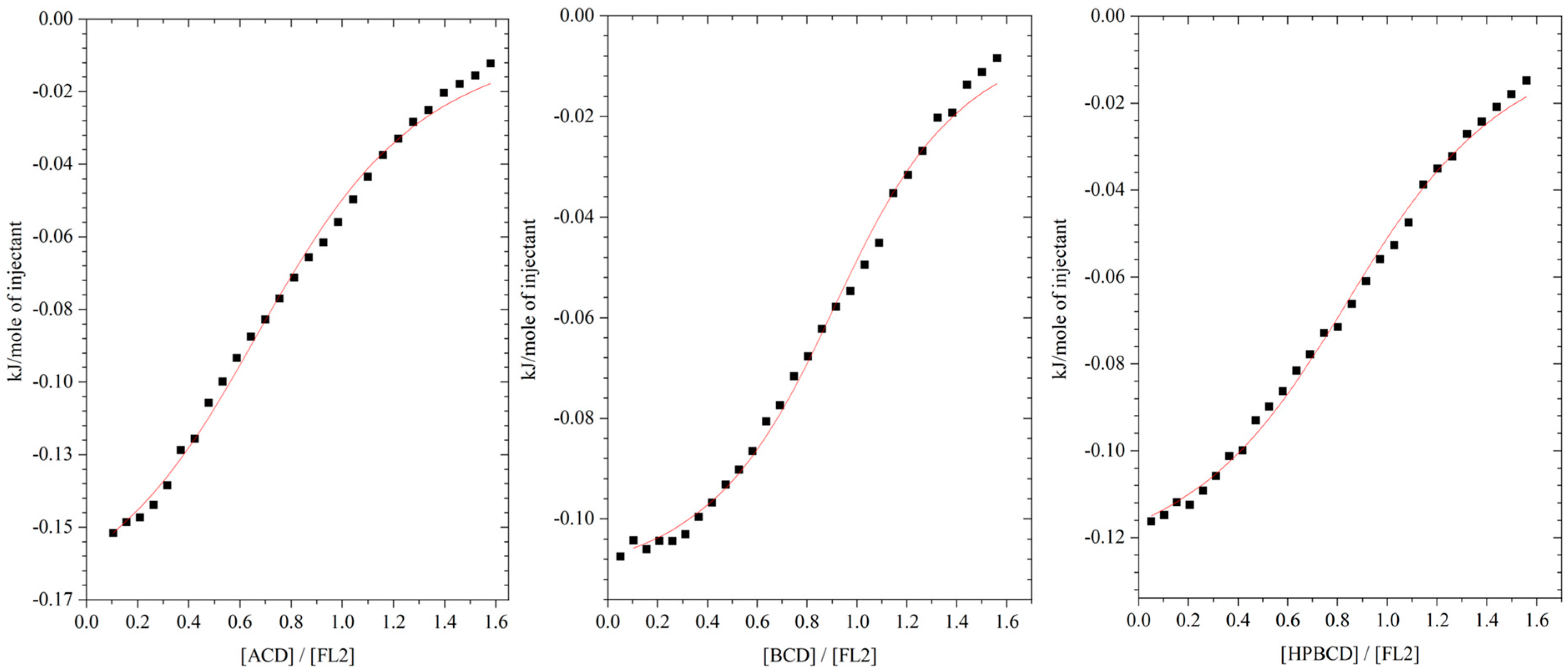
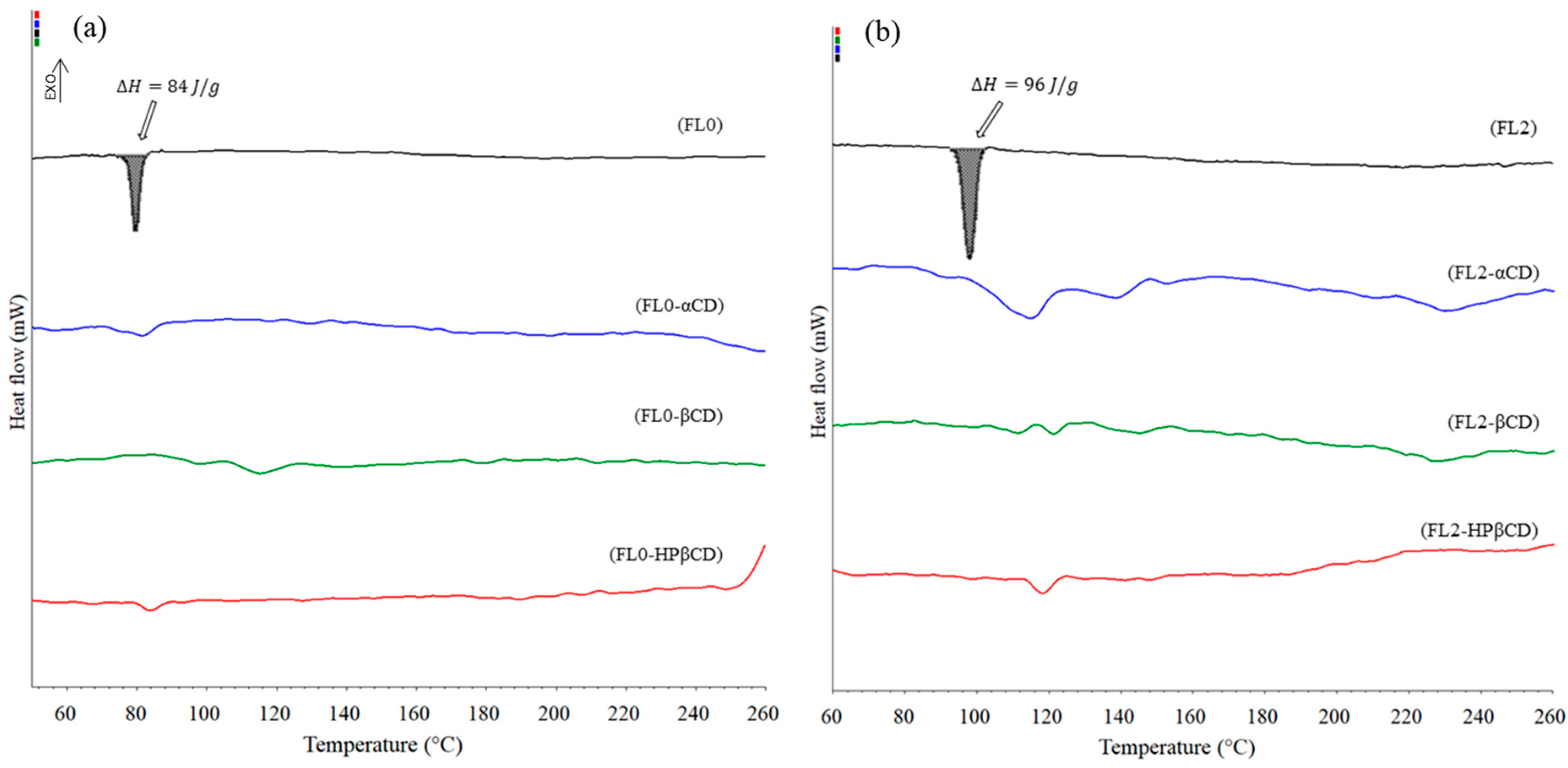
 HP-βcyclodextrin).
HP-βcyclodextrin).
 HP-βcyclodextrin).
HP-βcyclodextrin).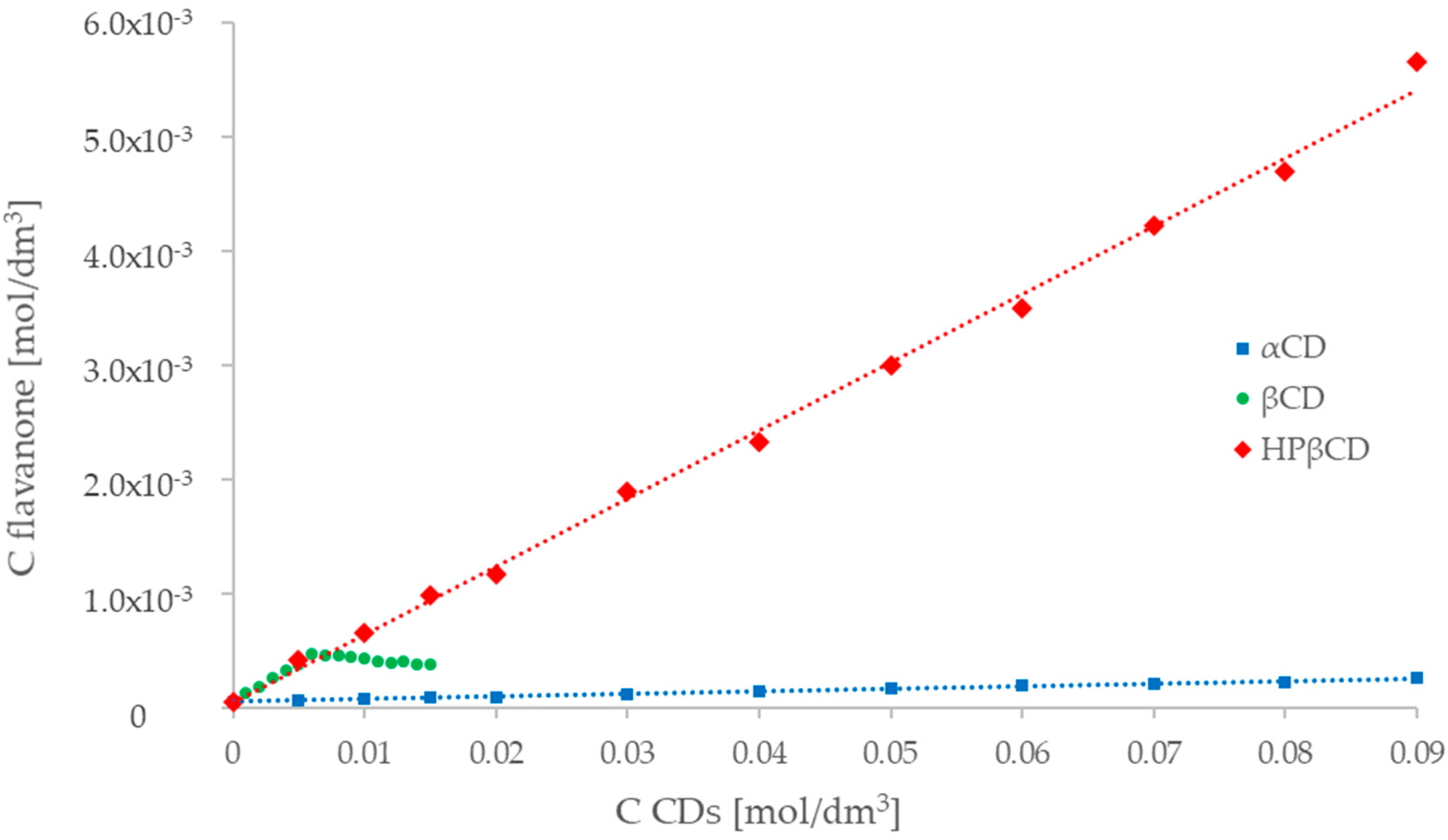
 HP-βcyclodextrin).
HP-βcyclodextrin).
 HP-βcyclodextrin).
HP-βcyclodextrin).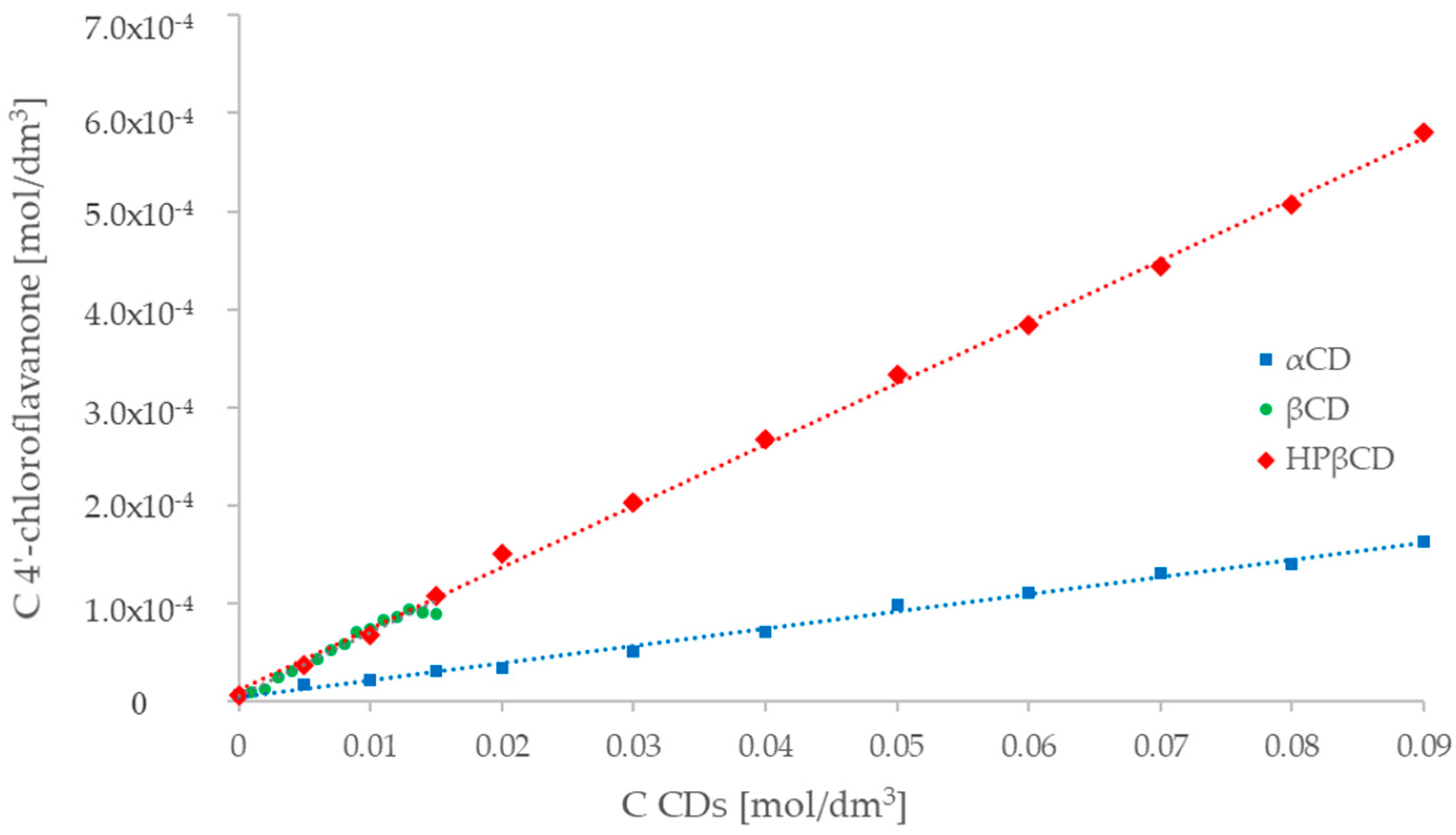
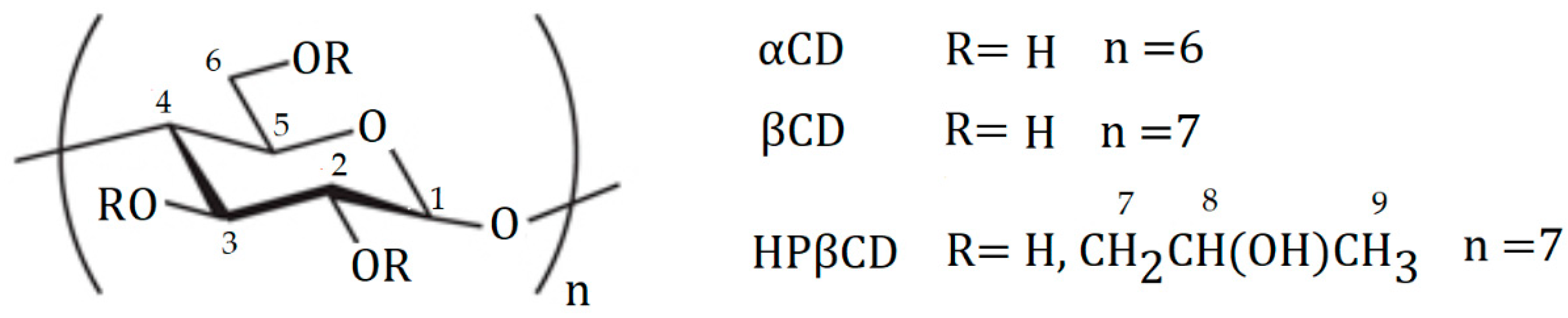
| αCD | βCD | HPβCD (Mw Approximately 1380) | |
|---|---|---|---|
| n | 0.83 ± 0.12 | 0.93 ± 0.09 | 0.95 ± 0.10 |
| K [dm3 mol−1] | 1350 ± 130 | 3830 ± 490 | 1940 ± 190 |
| ∆H [J mol−1] | −179 ± 5 | −109 ± 2 | −120 ± 2 |
| ∆S [J mol−1 K−1] | 59.5 ± 0.2 | 68.3 ± 0.3 | 62.4 ± 0.1 |
| ∆G [kJ mol−1] | −17.9 ± 0.1 | −20.5 ± 0.2 | −18.9 ± 0.2 |
| αCD | βCD | HPβCD (Mw Approximately 1380) | |
|---|---|---|---|
| n | 0.91 ± 0.09 | 0.92 ± 0.12 | 0.98 ± 0.11 |
| K [dm3 mol−1] | 1130 ± 180 | 3160 ± 220 | 1640 ± 350 |
| ∆H [J mol−1] | −103 ± 2 | −158 ± 5 | − 150 ± 3 |
| ∆S [J mol−1 K−1] | 58.0 ± 0.3 | 66.5 ± 0.2 | 61.1 ± 0.3 |
| ∆G [kJ mol−1] | −17.4 ± 0.2 | −19.9 ± 0.2 | −18.4 ± 0.1 |
| αCD | βCD | HPβCD | |
|---|---|---|---|
| Solubility increase 0–15 mM | 1.6 ± 0.2 | 8.3 ± 0.6 | 17.3 ± 0.6 |
| Solubility increase 0–90 mM | 4.6 ± 0.4 | - | 98.7 ± 8.8 |
| K [dm3 mol−1] | 39.5 ± 5 | 1286.3 ± 84.9 | 1106.8 ± 84.5 |
| CE | 0.002 | 0.07 | 0.06 |
| αCD | βCD | HPβCD | |
|---|---|---|---|
| Solubility increase 0–15 mM | 5.1 ± 1.0 | 17.1 ± 1.1 | 19.6 ± 1.0 |
| Solubility increase 0–90 mM | 27.4 ± 1.1 | - | 109.1 ± 6.9 |
| K [dm3 mol−1] | 299.4 ± 18.5 | 1252.0 ± 158.9 | 1185.9 ± 93.1 |
| CE | 0.0016 | 0.0067 | 0.0065 |
| αCD | βCD | HPβCD | |
|---|---|---|---|
| flavanone (FL0) | 39.5 ± 5.0 | 1286.3 ± 84.9 | 1106.8 ± 84.5 |
| 4′-chloroflavanone (FL2) | 299.4 ± 18.5 | 1252.0 ± 158.9 | 1185.9 ± 93.1 |
| 1H Assignment | H1 | H2 | H3 | H4 | H5 | H6 | H 4,7,8 | H9 |
|---|---|---|---|---|---|---|---|---|
| δαCD | 4.806 | 3.393 | 4.476 | 3.284 | 3.779 | 3.595 | - | - |
| δcomplex (αCD+FL0) | 4.806 | 3.393 | 4.480 | 3.286 | 3.779 | 3.596 | - | - |
| ∆δ | 0.000 | 0.000 | −0.004 | −0.002 | 0.000 | −0.001 | - | - |
| δβCD | 4.839 | 3.359 | 4.427 | * | 3.631 | 3.580 | - | - |
| δcomplex (βCD+FL0) | 4.835 | 3.355 | 4.472 | * | 3.637 | 3.575 | - | - |
| ∆δ | 0.004 | 0.004 | −0.045 | * | −0.006 | 0.005 | - | - |
| δHPβCD | 5.703 | 3.395 | 4.530 | - | 3.754 | 3.619 | * | 1.030 |
| δcomplex (HPβCD+FL0) | * | 3.412 | 4.544 | - | 3.752 | 3.620 | * | 1.028 |
| ∆δ | * | −0.017 | −0.014 | - | 0.002 | −0.001 | * | 0.002 |
| δαCD | 4.806 | 3.393 | 4.476 | 3.284 | 3.779 | 3.595 | - | - |
| δcomplex (αCD+FL2) | 4.805 | * | 4.489 | 3.286 | 3.775 | 3.593 | - | - |
| ∆δ | 0.001 | * | −0.013 | -0.002 | 0.004 | 0.002 | - | - |
| δβCD | 4.839 | 3.359 | 4.427 | * | 3.631 | 3.580 | - | - |
| δcomplex (βCD+FL2) | 4.806 | 3.392 | 4.480 | * | 3.643 | 3.595 | - | - |
| ∆δ | 0.033 | −0.033 | −0.053 | * | −0.012 | −0.015 | - | - |
| δHPβCD | 5.703 | 3.395 | 4.530 | - | 3.754 | 3.619 | * | 1.030 |
| δcomplex (HPβCD+FL2) | 5.699 | 3.402 | 4.555 | - | * | 3.619 | * | 1.027 |
| ∆δ | 0.004 | −0.007 | −0.025 | - | * | 0.000 | * | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepniak, A.; Biernacka, M.; Malecka, M.; Palecz, B. Host-Guest Complexes of Flavanone and 4′-Chloroflavanone with Naturals and Modified Cyclodextrin: A Calorimetric and Spectroscopy Investigations. Molecules 2024, 29, 3123. https://doi.org/10.3390/molecules29133123
Stepniak A, Biernacka M, Malecka M, Palecz B. Host-Guest Complexes of Flavanone and 4′-Chloroflavanone with Naturals and Modified Cyclodextrin: A Calorimetric and Spectroscopy Investigations. Molecules. 2024; 29(13):3123. https://doi.org/10.3390/molecules29133123
Chicago/Turabian StyleStepniak, Artur, Marta Biernacka, Magdalena Malecka, and Bartlomiej Palecz. 2024. "Host-Guest Complexes of Flavanone and 4′-Chloroflavanone with Naturals and Modified Cyclodextrin: A Calorimetric and Spectroscopy Investigations" Molecules 29, no. 13: 3123. https://doi.org/10.3390/molecules29133123
APA StyleStepniak, A., Biernacka, M., Malecka, M., & Palecz, B. (2024). Host-Guest Complexes of Flavanone and 4′-Chloroflavanone with Naturals and Modified Cyclodextrin: A Calorimetric and Spectroscopy Investigations. Molecules, 29(13), 3123. https://doi.org/10.3390/molecules29133123








