New 3-(Dibenzyloxyphosphoryl)isoxazolidine Conjugates of N1-Benzylated Quinazoline-2,4-diones as Potential Cytotoxic Agents against Cancer Cell Lines
Abstract
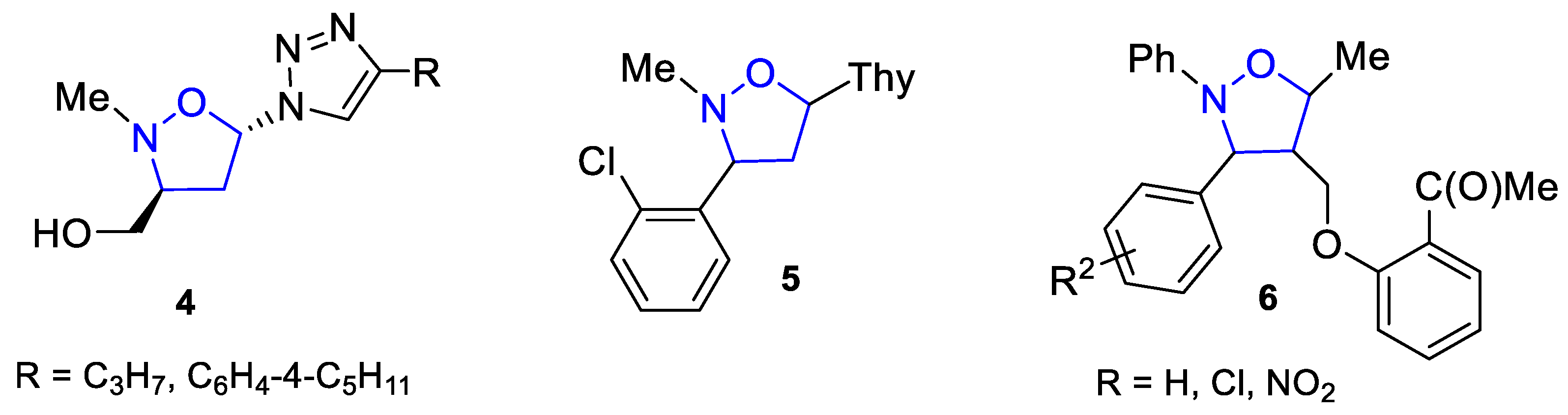
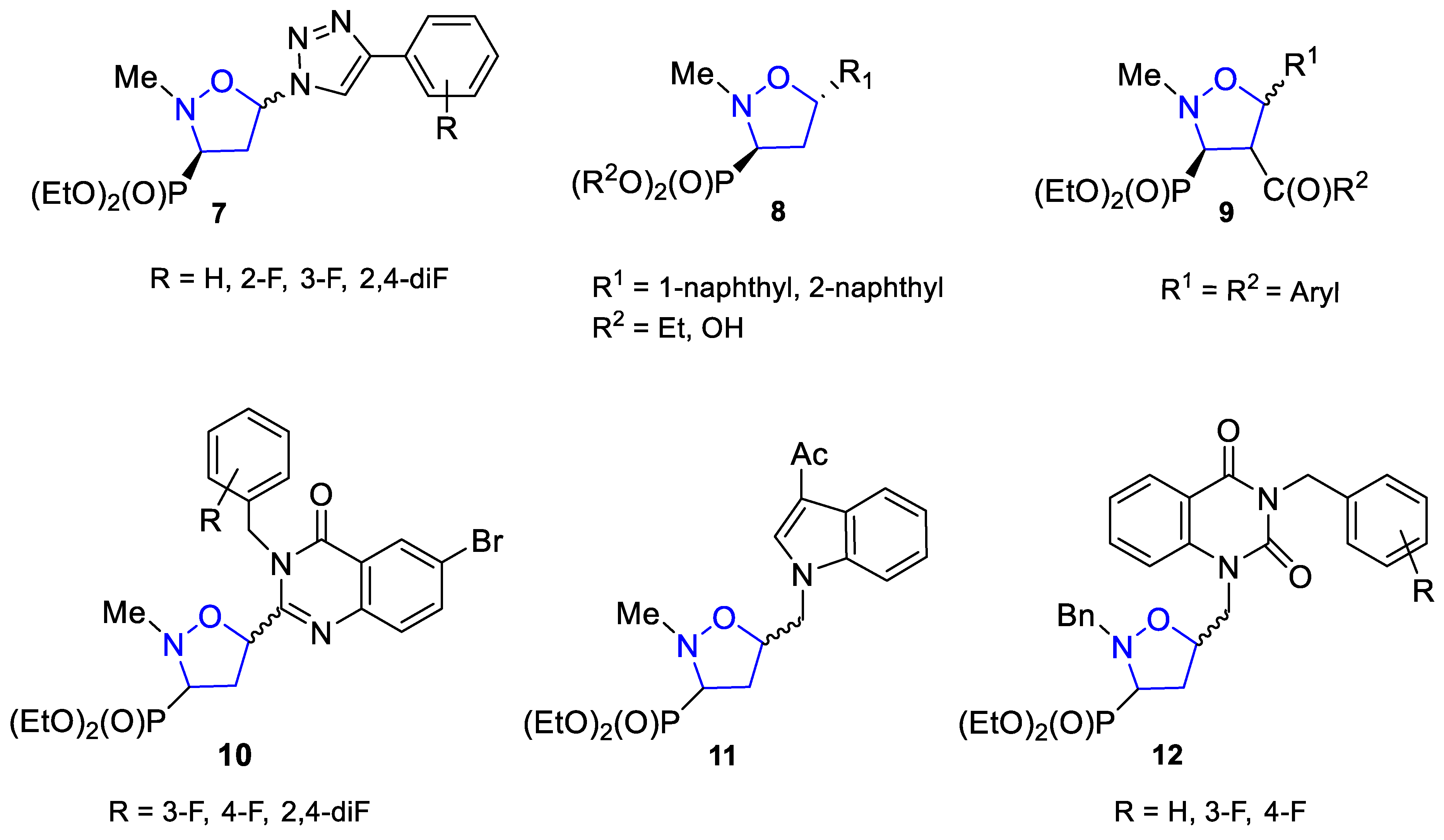
1. Introduction
2. Results and Discussion
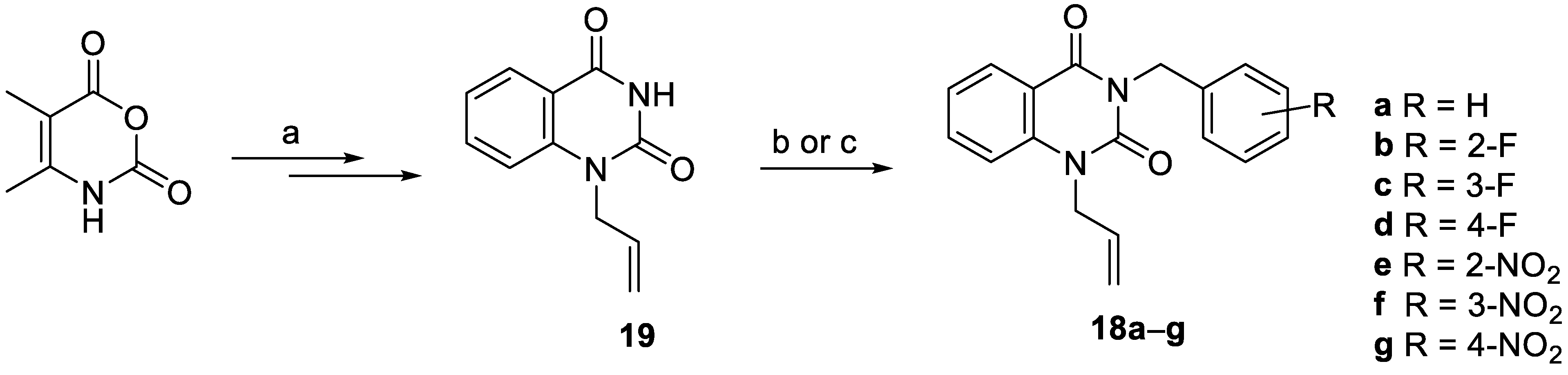
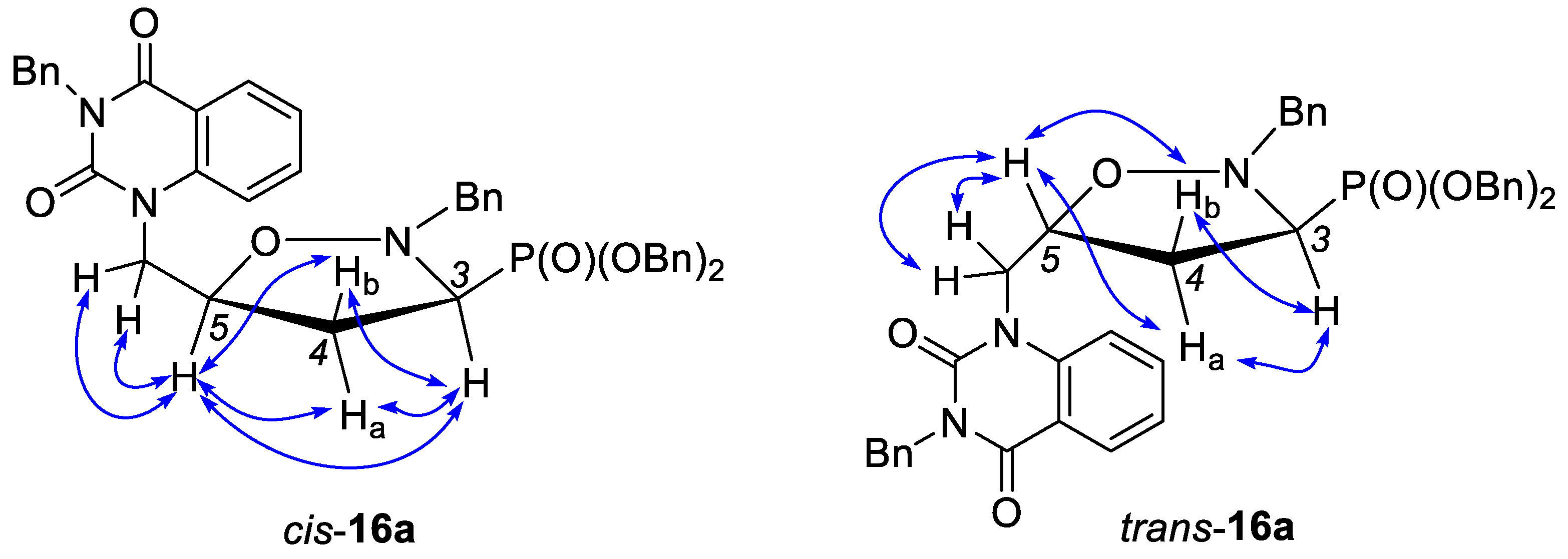
2.1. Chemistry
2.2. Pharmacology
2.2.1. Cytotoxicity towards Cancer Cell Lines
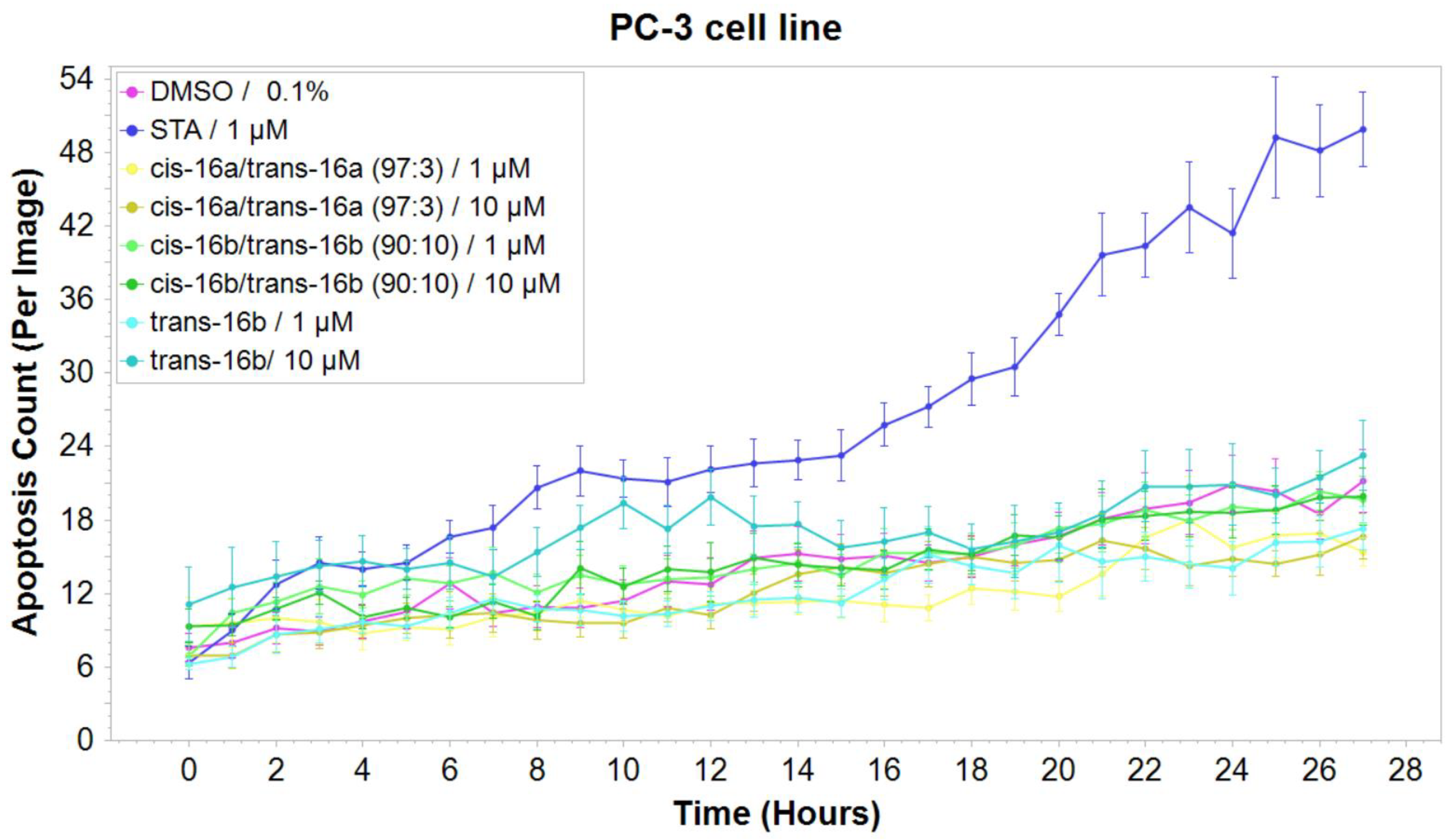
2.2.2. Mechanistic Studies: Induction of Apoptosis
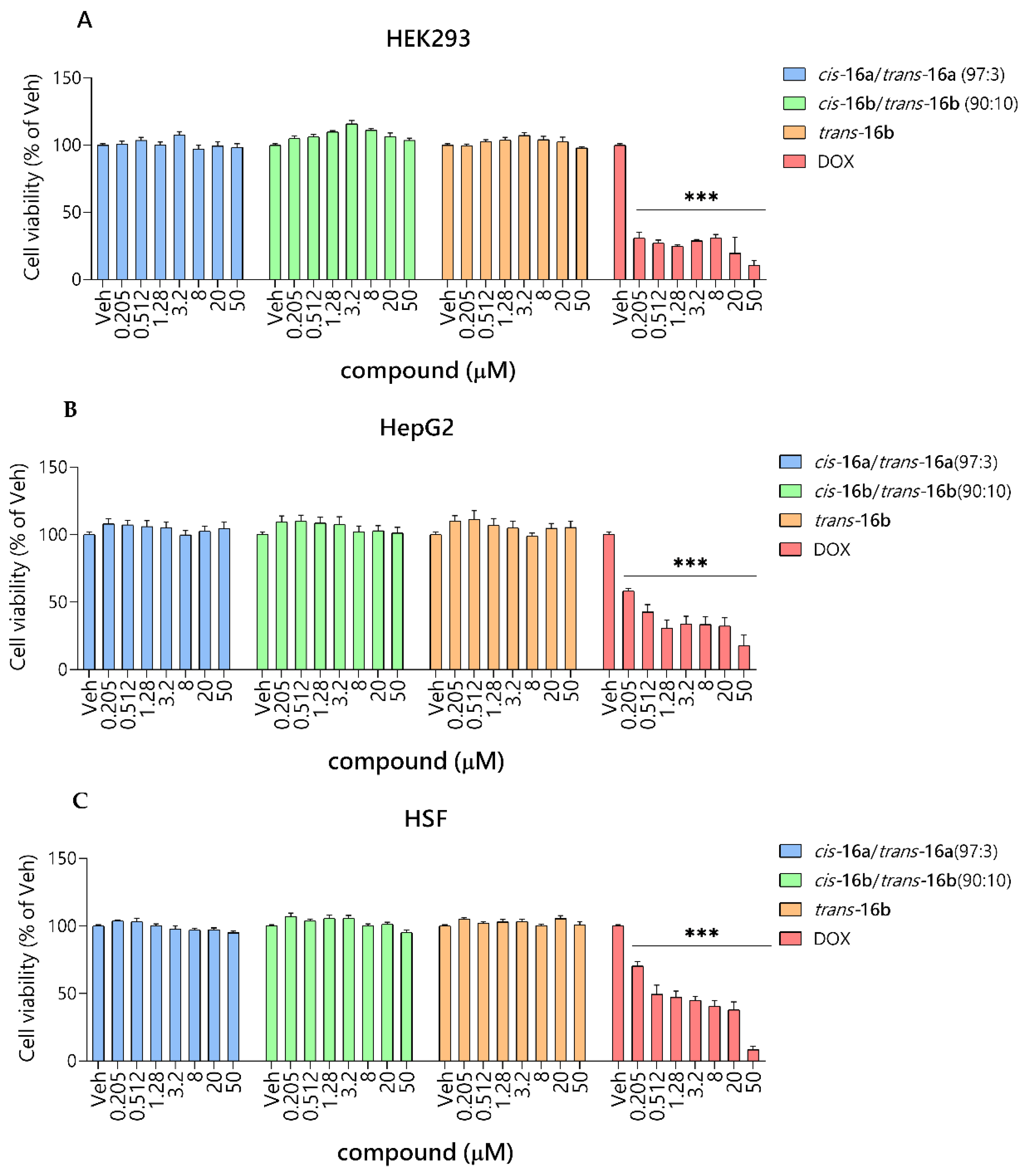
2.2.3. Safety Studies In Vitro
2.2.4. ADMET Studies In Silico
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Preparation of Quinazoline-2,4-Diones 18e–g
- N1-Allyl-N3-(2-nitrobenzyl)quinazoline-2,4(1H,3H)-dione (18e). According to the general procedure from N1-allylquinazoline-2,4-dione 19 (0.400 g, 1.98 mmol), potassium carbonate (0.328g, 2.38 mmol), and 2-nitrobenzyl bromide (0.642 g, 2.97 mmol), N1-allyl-N3-(2-nitrobenzyl)quinazoline-2,4-dione 18e (0.370 g, 55%) was obtained as a white amorphous solid, m.p. = 126–128 °C. IR (KBr, cm–1) νmax: 3081, 2851, 1708, 1652, 1526, 1483, 1412, 1336, 977, 763. 1H NMR (600 MHz, CDCl3): δ = 8.29 (dd, J = 7.9 Hz, J = 1.6 Hz, 1H), 8.10 (dd, J = 8.2 Hz, J = 1.1 Hz, 1H), 7.72 (dt, J = 7.3 Hz, J = 1.6 Hz, 1H), 7.55–7.52 (m, 1H), 7.45–7.42 (m, 1H), 7.32 (t, J = 7.9 Hz, 1H), 7.26 (t, J = 8.0 Hz, 2H), 5.95 (ddt, 3J = 17.3 Hz, 3J = 10.5 Hz, 3J = 5.2 Hz, 1H, CH2–CH=CH2), 5.72 (s, 2H, CH2Ph), 5.31 (d, 3J = 10.5 Hz, 1H, CH2–CH=CHH), 5.24 (d, 3J = 17.3 Hz, 1H, CH2–CH=CHH), 4.83–4.82 (m, 2H, CH2–CH=CH2); 13C NMR (151 MHz, CDCl3): δ = 161.77 (C=O), 150.65 (C=O), 148.78, 139.98, 135.48, 133.52, 132.48, 131.07, 129.31, 128.02, 127.65, 125.11, 123.34, 117.86, 115.35, 114.42, 46.12, 42.28. Anal. calcd. For C18H15N3O4: C, 64.09; H, 4.48; N, 12.46. Found: C, 63.85; H, 4.18; N, 12.28.
- N1-Allyl-N3-(3-nitrobenzyl)quinazoline-2,4(1H,3H)-dione (18f). According to the general procedure from N1-allylquinazoline-2,4-dione 19 (0.400 g, 1.98 mmol), potassium carbonate (0.328g, 2.38 mmol), and 3-nitrobenzyl bromide (0.642 g, 2.97 mmol), N1-allyl-N3-(3-nitrobenzyl)quinazoline-2,4-dione 18f (0.493 g, 74%) was obtained as a white amorphous solid, m.p. = 139–141 °C. IR (KBr, cm–1) νmax: 3081, 2853, 1702, 1641, 1609, 1527, 1483, 1419, 1329, 974, 739, 694. 1H NMR (600 MHz, CDCl3): δ = 8.35–8.34 (m, 1H), 8.25 (dd, J = 7.9 Hz, J = 1.6 Hz, 1H), 8.13–8.12 (m, 1H), 7.85 (d, J = 7.6 Hz, 1H), 7.67 (dt, J = 7.3 Hz, J = 1.6 Hz, 1H), 7.49 (t, J = 8.0 Hz, 1H), 7.29–7.26 (m, 1H), 7.19 (d, J = 8.5 Hz, 1H), 5.93 (ddt, 3J = 17.2 Hz, 3J = 10.2 Hz, 3J = 4.9 Hz, 1H, CH2–CH=CH2), 5.37 (s, 2H, CH2Ph), 5.28 (d, 3J = 10.2 Hz, 1H, CH2–CH=CHH), 5.21 (d, 3J = 17.2 Hz, 1H, CH2–CH=CHH), 4.79–4.78 (m, 2H, CH2–CH=CH2); 13C NMR (151 MHz, CDCl3): δ = 161.73 (C=O), 150.72 (C=O), 148.37, 139.87, 138.94, 135.43, 135.13, 131.01, 129.42, 129.22, 123.82, 123.32, 122.75, 117.84, 115.43, 114.37, 46.16, 44.37. Anal. calcd. For C18H15N3O4: C, 64.09; H, 4.48; N, 12.46. Found: C, 63.80; H, 4.18; N, 12.17.
- N1-Allyl-N3-(4-nitrobenzyl)quinazoline-2,4(1H,3H)-dione (18g). According to the general procedure from N1-allylquinazoline-2,4-dione 19 (0.400 g, 1.98 mmol), potassium carbonate (0.328g, 2.38 mmol), and 4-nitrobenzyl bromide (0.642 g, 2.97 mmol), N1-allyl-N3-(4-nitrobenzyl)quinazoline-2,4-dione 18g (0.443g, 66%) was obtained as a white amorphous solid, m.p. = 139–141 °C. IR (KBr, cm–1) νmax: 3108, 3008, 1701, 1665, 1481, 1397, 1345, 1213, 959, 836 1H NMR (600 MHz, CDCl3): δ = 8.24 (dd, J = 9.4 Hz, J = 1.6 Hz, 1H), 8.17–8.15 (m, 2H), 7.68–7.65 (m, 3H), 7.28 (t, J = 7.7 Hz, 1H), 7.19 (d, J = 8.4 Hz, 1H), 5.77 (ddt, 3J = 17.2 Hz, 3J = 10.2 Hz, 3J = 5.0 Hz, 1H, CH2–CH=CH2), 5.36 (s, 2H, CH2Ph), 5.28 (d, 3J = 10.2 Hz, 1H, CH2–CH=CHH), 5.20 (d, 3J = 17.2 Hz, 1H, CH2–CH=CHH), 4.78–4.77 (m, 2H, CH2–CH=CH2); 13C NMR (151 MHz, CDCl3): δ = 161.74 (C=O), 150.69 (C=O), 147.42, 144.20, 139.84, 135.47, 130.97, 129.72, 129.20, 123.74, 123.37, 117.87, 115.40, 114.38, 46.17, 44.44. Anal. calcd. For C18H15N3O4: C, 64.09; H, 4.48; N, 12.46. Found: C, 63.79; H, 4.24; N, 12.31.
3.3. General Procedure for the Preparation of Isoxsazolidines 16a–g
- Compound cis-16a. Data noted below correspond to a 97:3 mixture of cis-16a and trans-16a. A colorless oil. IR (film, cm–1) νmax: 3453, 3061, 2954, 1951, 1892, 1817, 1698, 1485, 1304, 1233, 1008. NMR signals of cis-16a were extracted from the spectrum of a 97:3 mixture of cis-16a and trans-16a. 1H NMR (600 MHz, CDCl3): δ = 8.12 (dd, J = 7.8 Hz, J = 1.5 Hz, 1H), 7.52 (d, J = 7.3 Hz, 2H), 7.42–7.37 (m, 7H), 7.36–7.30 (m, 5H), 7.29–7.23 (m, 6H), 7.15 (d, J = 8.5 Hz, 1H), 7.07 (t, J = 7.4 Hz, 1H), 7.01–6.98 (m, 1H), 5.28 (AB, JAB = 13.8 Hz, 1H, HCHN), 5.21 (AB, JAB = 13.8 Hz, 1H, HCHN), 5.21–5.18 (m, 2H, CH2OP), 5.15–5.09 (m, 2H, CH2OP), 4.56 (dddd, 3J(H5–H4α) = 9.6 Hz, 3J(H5–CH) = 7.8 Hz, 3J(H5–H4β) = 3.8 Hz, 3J(H5–CH) = 3.8 Hz, 1H, HC5), 4.39 (d, 2J = 13.7 Hz, 1H, HCHPh), 4.05 (dAB, JAB = 15.0 Hz, 3J(H5–CH) = 3.8 Hz, 1H, HCHN), 4.03 (dAB, JAB = 15.0 Hz, 3J(H5–CH) = 7.8 Hz, 1H, HCHN), 3.85 (d, 2J = 13.7 Hz, 1H, HCHPh), 3.23 (ddd, 3J(H3–H4α) = 9.6 Hz, 3J(H3–H4β) = 7.2 Hz, 2J(H3–P) = 2.6 Hz, 1H, HC3), 2.75 (dddd, 2J(H4α–H4β) = 13.2 Hz, 3J(H4α–H3) = 9.6 Hz, 3J(H4α–H5) = 9.6 Hz, 3J(H4α–P) = 9.4 Hz, 1H, HαC4), 2.38 (dddd, 3J(H4β–P) = 19.6 Hz, 2J(H4β–H4α) = 13.2 Hz, 3J(H4β–H3) = 7.2 Hz, 3J(H4β–H5) = 3.8 Hz, 1H, HβC4); 13C NMR (151 MHz, CDCl3): δ = 161.98 (C=O), 151.22 (C=O), 140.37, 137.03, 136.42, 136.02 (d, 3J(CCOP) = 6.0 Hz), 135.82 (d, 3J(CCOP) = 5.4 Hz), 134.82, 129.99, 129.08, 128.76, 128.74, 128.72, 128.43, 128.28, 128.22, 128.17, 128.13, 127.59, 127.56, 122.65, 115.39, 115.02, 75.84 (d, 3J(CCCP) = 6.6 Hz, C5), 68.37 (d, 2J(COP) = 6.4 Hz, CH2OP), 68.15 (d, 2J(COP) = 6.7 Hz, CH2OP), 62.24 (d, 3J(CNCP) = 5.1 Hz, CH2Ph), 60.75 (d, 1J(CP) = 170.2 Hz, C3), 47.62 (CH2N), 44.87 (CH2Ph), 34.97 (C4); 31P NMR (243 MHz, CDCl3): δ = 23.70. Anal. calcd. for C40H38N3O6P × 0.25 H2O: C, 69.41; H, 5.61; N, 6.07. Found: C, 69.57; H, 5.81; N, 5.89 (obtained on 97:3 mixture of cis-16a and trans-16a).
- Compound trans-16a. A colorless oil. IR (film, cm–1) νmax: 3454, 3061, 2955, 1952, 1892, 1817, 1698, 1485, 1304, 1233, 1008. 1H NMR (600 MHz, CDCl3): δ = 8.26 (dd, J = 7.9 Hz, J = 1.4 Hz, 1H), 7.63–7.61 (m, 1H), 7.52 (d, J = 7.3 Hz, 2H), 7.33–7.31 (m, 12H), 7.30–7.25 (m, 8H), 5.31 (AB, JAB = 13.9 Hz, 1H, HCHN), 5.25 (AB, JAB = 13.9 Hz, 1H, HCHN), 5.16–5.06 (m, 4H, 2 × CH2OP), 4.45 (dd, 2J = 15.1 Hz, 3J(HC–H5) = 4.2 Hz, 1H, HCHN), 4.41 (d, 2J = 13.8 Hz, 1H, HCHPh), 4.31 (dddd, 3J(H4β–H5) = 8.4 Hz, 3J(H4α–H5) = 6.6 Hz, 3J(HC–H5) = 5.9 Hz, 3J(HC–H5) = 4.2 Hz, 1H, HC5), 4.13 (dd, 2J = 15.1 Hz, 3J(HC–H5) = 5.9 Hz, 1H, HCHN), 3.90 (d, 2J = 13.8 Hz, 1H, HCHPh), 3.34 (ddd, 3J(H3–H4β) = 10.2 Hz, 3J(H3–H4α) = 6.6 Hz, 2J(H3–P) = 1.8 Hz, 1H, HC3), 2.67 (dddd, 3J(H4α–P) = 18.6 Hz, 2J(H4α–H4β) = 12.8 Hz, 3J(H4α–H3) = 6.6 Hz, 3J(H4α–H5) = 6.6 Hz, 1H, HαC4), 2.33 (dddd, 3J(H4β–P) = 16.8 Hz, 2J(H4β–H4α) = 12.8 Hz, 3J(H4β–H3) = 10.2 Hz, 3J(H4β–H5) = 8.4 Hz, 1H, HβC4); 13C NMR (151 MHz, CDCl3): δ = 161.63 (C=O), 151.26 (C=O), 140.13, 136.92, 136.38, 136.17 (d, 3J(CCOP) = 5.7 Hz), 136.04 (d, 3J(CCOP) = 5.8 Hz), 134.85, 129.70, 128.99, 128.95, 128.65, 128.62, 128.59, 128.53, 128.48, 128.17, 128.15, 127.66, 127.17, 123.54, 115.59, 114.90, 75.81 (d, 3J(CCCP) = 6.1 Hz, C5), 68.75 (d, 2J(COP) = 6.4 Hz, CH2OP), 67.97 (d, 2J(COP) = 6.7 Hz, CH2OP), 62.74 (d, 3J(CNCP) = 4.7 Hz, CH2Ph), 60.97 (d, 1J(CP) = 170.4 Hz, C3), 45.63 (CH2N), 45.08 (CH2Ph), 35.04 (C4); 31P NMR (243 MHz, CDCl3): δ = 22.81. Anal. calcd. for C40H38N3O6P × 0.25 H2O: C, 69.41; H, 5.61; N, 6.07. Found: C, 69.57; H, 5.83; N, 5.87.
- Compound cis-16b. Data noted below correspond to a 90:10 mixture of cis-16b and trans-16b. A colorless oil. IR (film, cm–1) νmax: 3453, 3063, 2956, 1956, 1885, 1817, 1662, 1483, 1318, 1230, 1008, 734. NMR signals of cis-16b were extracted from the spectrum of a 90:10 mixture of cis-16b and trans-16b. 1H NMR (600 MHz, CDCl3): δ = 8.14 (dd, J = 7.8 Hz, J = 1.4 Hz, 1H), 7.42–7.39 (m, 7H), 7.37–7.31 (m, 3H), 7.30–7.22 (m, 7H), 7.20 (d, J = 8.5 Hz, 1H), 7.10–7.05 (m, 3H), 7.03–7.01 (m, 1H), 5.38 (AB, JAB = 14.6 Hz, 1H, HCHN), 5.32 (AB, JAB = 14.6 Hz, 1H, HCHN), 5.23–5.18 (m, 2H, CH2OP), 5.15–5.08 (m, 2H, CH2OP), 4.59–4.55 (m, 1H, HC5), 4.40 (d, 2J = 13.7 Hz, 1H, HCHPh), 4.07 (d, 3J = 5.7 Hz, 2H, HCHN), 3.86 (d, 2J = 13.7 Hz, 1H, HCHPh), 3.23 (ddd, 3J(H3–H4α) = 10.1 Hz, 3J(H3–H4β) = 7.2 Hz, 2J(H3–P) = 2.6 Hz, 1H, HC3), 2.74 (dddd, 2J(H4α–H4β) = 13.2 Hz, 3J(H4α–H3) = 10.1 Hz, 3J(H4α–H5) = 9.6 Hz, 3J(H4α–P) = 9.0 Hz,1H, HαC4), 2.38 (dddd, 3J(H4β–P) = 19.8 Hz, 2J(H4β–H4α) = 13.2 Hz, 3J(H4β–H3) = 7.2 Hz, 3J(H4β–H5) = 4.2 Hz, 1H, HβC4); 13C NMR (151 MHz, CDCl3): δ = 161.94 (C=O), 160.74 (d, 1J(CF) = 247.3 Hz), 151.04 (C=O), 140.45, 136.47, 136.49 (d, 3J(CCOP) = 5.4 Hz), 136.05 (d, 3J(CCOP) = 5.6 Hz), 134.96, 129.96, 129.49 (d, 3J(CCCF) = 8.4 Hz), 129.39 (d, 4J(CCCCF) = 3.6 Hz), 128.76, 128.74, 128.72, 128.30, 128.23, 128.17, 127.58, 124.09 (d, 3J(CCCF) = 3.5 Hz), 123.94 (d, 2J(CCF) = 14.3 Hz), 122.74, 115.47 (d, 2J(CCF) = 21.7 Hz), 115.51, 114.92, 75.38 (d, 3J(CCCP) = 6.1 Hz, C5), 68.37 (d, 2J(COP) = 6.6 Hz, CH2OP), 68.18 (d, 2J(COP) = 6.9 Hz, CH2OP), 62.28 (d, 3J(CNCP) = 5.3 Hz, CH2Ph), 60.77 (d, 1J(CP) = 169.9 Hz, C3), 47.63 (CH2N), 38.59 (d, 3J(CCCF) = 4.5 Hz, CH2Ph), 34.97 (C4); 31P NMR (243 MHz, CDCl3): δ = 23.73. Anal. calcd. for C40H37FN3O6P × 3.25 H2O: C, 62.87; H, 5.74; N, 5.50. Found: C, 62.65; H, 5.88; N, 5.21 (obtained on 90:10 mixture of cis-16b and trans-16b).
- Compound trans-16b. A colorless oil. IR (film, cm–1) νmax: 3453, 3063, 2956, 1956, 1886, 1817, 1663, 1482, 1347, 1231, 1008, 735. 1H NMR (600 MHz, CDCl3): δ = 8.27 (dd, J = 7.9 Hz, J = 1.2 Hz, 1H), 7.64 (t, J = 8.2 Hz, 1H), 7.35–7.30 (m, 11H), 7.29–7.23 (m, 7H), 7.22–7.20 (m, 1H), 7.07–7.02 (m, 2H), 5.40 (AB, JAB = 14.8 Hz, 1H, HCHN), 5.36 (AB, JAB = 14.8 Hz, 1H, HCHN), 5.14–5.05 (m, 4H, 2 × CH2OP), 4.46 (dd, 2J = 15.1 Hz, 3J(HC–H5) = 4.1 Hz, 1H, HCHN), 4.40 (d, 2J = 13.7 Hz, 1H, HCHPh), 4.35–4.31 (m, 1H, HC5), 4.16 (dd, 2J = 15.1 Hz, 3J(HC–H5) = 5.9 Hz, 1H, HCHN), 3.90 (d, 2J = 13.7 Hz, 1H, HCHPh), 3.33 (ddd, 3J(H4β–H3) = 10.1 Hz, 3J(H4α–H3) = 6.3 Hz, 2J(H3–P) = 1.8 Hz, 1H, HC3), 2.66 (dddd, 3J(H4α –P) = 18.6 Hz, 2J(H4α–H4β) = 12.7 Hz, 3J(H4α–H3) = 6.3 Hz, 3J(H4α–H5) = 6.3 Hz, 1H, HαC4), 2.32 (dddd, 3J(H4β–P) = 16.8 Hz, 2J(H4β–H4α) = 12.7 Hz, 3J(H4β–H3) = 10.1 Hz, 3J(H4β–H5) = 9.2 Hz, 1H, HβC4); 13C NMR (151 MHz, CDCl3): δ = 161.64 (C=O), 160.74 (d, 1J(CF) = 247.4 Hz), 151.09 (C=O), 140.20, 136.35, 136.16 (d, 3J(CCOP) = 5.6 Hz), 136.03 (d, 3J(CCOP) = 5.7 Hz), 134.97, 129.69, 129.28 (d, 4J(CCCCF) = 3.8 Hz), 129.04 (d, 3J(CCCF) = 6.7 Hz), 128.64, 128.61, 128.58, 128.51, 128.16, 128.13, 128.11, 127.53, 124.11 (d, 3J(CCCF) = 3.8 Hz), 123.78 (d, 2J(CCF) = 14.3 Hz), 123.25, 115.50 (d, 2J(CCF) = 21.4 Hz), 115.47, 114.98, 75.76 (d, 3J(CCCP) = 6.1 Hz, C5), 68.72 (d, 2J(COP) = 6.5 Hz, CH2OP), 67.95 (d, 2J(COP) = 6.7 Hz, CH2OP), 62.71 (d, 3J(CNCP) = 5.1 Hz, CH2Ph), 60.93 (d, 1J(CP) = 170.1 Hz, C3), 45.61 (CH2N), 38.94 (d, 3J(CCCF) = 4.5 Hz, CH2Ph), 34.97 (C4); 31P NMR (243 MHz, CDCl3): δ = 22.80. Anal. calcd. for C40H37FN3O6P × 2.5 H2O: C, 64.00; H, 5.64; N, 5.60. Found: C, 63.74; H, 5.96; N, 5.34.
- Compound cis-16c. Data noted below correspond to a 90:10 mixture of cis-16c and trans-16c. A colorless oil. IR (film, cm–1) νmax: 3442, 3063, 2954, 1960, 1893, 1820, 1657, 1485, 1346, 1233, 1010, 698. NMR signals of cis-16c were extracted from the spectrum of a 90:10 mixture of cis-16c and trans-16c. 1H NMR (600 MHz, CDCl3): δ = 8.12 (dd, J = 7.8 Hz, J = 1.0 Hz, 1H), 7.42–7.37 (m, 6H), 7.36–7.30 (m, 4H), 7.29–7.24 (m, 7H), 7.20–7.18 (m, 1H), 7.17 (d, J = 8.5 Hz, 1H), 7.08 (t, J = 7.4 Hz, 1H), 7.01–6.95 (m, 2H), 5.26 (AB, JAB = 14.2 Hz, 1H, HCHN), 5.20 (AB, JAB = 14.2 Hz, 1H, HCHN), 5.15–5.08 (m, 2H, CH2OP), 5.23–5.18 (m, 2H, CH2OP), 4.58–4.54 (m, 1H, HC5), 4.40 (d, 2J = 13.6 Hz, 1H, HCHPh), 4.05 (d, 3J = 5.6 Hz, 2H, HCHN), 3.85 (d, 2J = 13.6 Hz, 1H, HCHPh), 3.23 (ddd, 3J(H3–H4α) = 10.1 Hz, 3J(H3–H4β) = 7.4 Hz, 2J(H3–P) = 2.4 Hz, 1H, HC3), 2.76 (dddd, 2J(H4α–H4β) = 12.6 Hz, 3J(H4α–P) = 10.1 Hz, 3J(H4α–H3) = 10.1 Hz, 3J(H4α–H5) = 9.0 Hz, 1H, HαC4), 2.38 (dddd, 3J(H4β–P) = 19.2 Hz, 2J(H4β–H4α) = 12.6 Hz, 3J(H4β–H3) = 7.4 Hz, 3J(H4β–H5) = 4.1 Hz, 1H, HβC4); 13C NMR (151 MHz, CDCl3): δ = 162.78 (d, 1J(CF) = 245.7 Hz), 161.89 (C=O), 151.15 (C=O), 140.38, 139.37 (d, 3J(CCCF) = 7.1 Hz), 136.43, 136.03 (d, 3J(CCOP) = 5.7 Hz), 135.97 (d, 3J(CCOP) = 5.6 Hz), 134.97, 129.98, 129.89 (d, 3J(CCCF) = 8.4 Hz), 128.77, 128.75, 128.73, 128.29, 128.23, 128.18, 128.15, 128.13, 127.57, 124.60 (d, 4J(CCCCF) = 2.3 Hz), 122.76, 115.96 (d, 2J(CCF) = 21.9 Hz), 115.49, 114.91, 114.55 (d, 2J(CCF) = 21.0 Hz), 75.82 (d, 3J(CCCP) = 6.7 Hz, C5), 68.38 (d, 2J(COP) = 6.6 Hz, CH2OP), 68.18 (d, 2J(COP) = 7.2 Hz, CH2OP), 62.27 (d, 3J(CNCP) = 5.2 Hz, CH2Ph), 60.77 (d, 1J(CP) = 169.8 Hz, C3), 47.66 (CH2N), 44.41 (CH2Ph), 34.98 (C4); 31P NMR (243 MHz, CDCl3): δ = 23.70. Anal. calcd. for C40H37FN3O6P × 0.25 H2O: C, 67.65; H, 5.32; N, 5.92. Found: C, 67.76; H, 5.25; N, 5.90 (obtained on 90:10 mixture of cis-16c and trans-16c).
- Compound trans-16c. A colorless oil. IR (film, cm–1) νmax: 3441, 3063, 2954, 1960, 1893, 1820, 1657, 1485, 1346, 1233, 1010, 698. 1H NMR (600 MHz, CDCl3): δ = 8.26 (dd, J = 7.8 Hz, J = 1.0 Hz, 1H), 7.63 (t, J = 7.4 Hz, 1H), 7.35–7.31 (m, 11H), 7.29–7.21 (m, 9H), 6.97–6.95 (m, 1H), 5.28 (AB, JAB = 14.0 Hz, 1H, HCHN), 5.23 (AB, JAB = 14.0 Hz, 1H, HCHN), 5.15–5.05 (m, 4H, 2 × CH2OP), 4.45 (dd, 2J = 15.1 Hz, 3J(HC–H5) = 4.1 Hz, 1H, HCHN), 4.40 (d, 2J = 13.8 Hz, 1H, HCHPh), 4.34–4.29 (m, 1H, HC5), 4.14 (dd, 2J = 15.1 Hz, 3J(HC–H5) = 6.1 Hz, 1H, HCHN), 3.90 (d, 2J = 13.8 Hz, 1H, HCHPh), 3.34 (ddd, 3J(H4β–H3) = 10.2 Hz, 3J(H4α–H3) = 6.3 Hz, 2J(H3–P) = 1.2 Hz, 1H, HC3), 2.67 (dddd, 3J(H4α –P) = 18.5 Hz, 2J(H4α–H4β) = 12.6 Hz, 3J(H4α–H3) = 6.3 Hz, 3J(H4α–H5) = 6.3 Hz, 1H, HαC4), 2.32 (dddd, 3J(H4β–P) = 15.0 Hz, 2J(H4β–H4α) = 12.6 Hz, 3J(H4β–H3) = 10.2 Hz, 3J(H4β–H5) = 10.2 Hz, 1H, HβC4); 13C NMR (150 MHz, CDCl3): δ = 162.79 (d, 1J(CF) = 246.1 Hz), 161.63 (C=O), 151.18 (C=O), 140.13, 139.23 (d, 3J(CCCF) = 7.1 Hz), 136.34, 136.15 (d, 3J(CCOP) = 5.6 Hz), 136.03 (d, 3J(CCOP) = 5.6 Hz), 134.99, 129.95 (d, 3J(CCCF) = 8.5 Hz), 129.70, 128.97, 128.64, 128.61, 128.59, 128.53, 128.15, 128.13, 127.54, 124.54 (d, 4J(CCCCF) = 3.0 Hz), 123.28, 115.83 (d, 2J(CCF) = 21.7 Hz), 115.47, 114.97 114.62 (d, 2J(CCF) = 21.1 Hz), 75.74 (d, 3J(CCCP) = 5.8 Hz, C5), 68.74 (d, 2J(COP) = 6.4 Hz, CH2OP), 67.98 (d, 2J(COP) = 6.8 Hz, CH2OP), 62.73 (d, 3J(CNCP) = 4.7 Hz, CH2Ph), 60.95 (d, 1J(CP) = 170.3 Hz, C3), 45.71 (CH2N), 44.59 (CH2Ph), 35.05 (d, 2J(CCP) = 1.8 Hz, C4); 31P NMR (243 MHz, CDCl3): δ = 22.78. Anal. calcd. for C40H37FN3O6P × H2O: C, 66.39; H, 5.43; N, 5.81. Found: C, 66.20; H, 5.34; N, 5.72.
- Compound cis-16d. Data noted below correspond to a 96:4 mixture of cis-16d and trans-16d. A colorless oil. IR (film, cm–1) νmax: 3457, 3063, 2957, 1956, 1893, 1816, 1658, 1496, 1347, 1220, 1007, 825. NMR signals of cis-16d were extracted from the spectrum of a 96:4 mixture of cis-16d and trans-16d. 1H NMR (600 MHz, CDCl3): δ = 8.12 (dd, J = 7.9 Hz, J = 1.4 Hz, 1H), 7.54–7.51 (m, 2H), 7.41–7.35 (m, 7H), 7.34–7.30 (m, 3H), 7.29–7.22 (m, 5H), 7.17 (d, J = 8.5 Hz, 1H), 7.08 (t, J = 7.5 Hz, 1H), 7.01–6.98 (m, 3H), 5.24–5.15 (m, 4H, HCHN, CH2OP), 5.14–5.09 (m, 2H, CH2OP), 4.59–4.55 (m, 1H, HC5), 4.40 (d, 2J = 13.7 Hz, 1H, HCHPh), 4.06 (d, 3J = 5.7 Hz, 2H, HCHN), 3.85 (d, 2J = 13.7 Hz, 1H, HCHPh), 3.23 (ddd, 3J(H3–H4α) = 9.8 Hz, 3J(H3–H4β) = 7.3 Hz, 2J(H3–P) = 2.5 Hz, 1H, HC3), 2.76 (dddd, 2J(H4α–H4β) = 12.8 Hz, 3J(H4α–P) = 11.4 Hz, 3J(H4α–H3) = 9.8 Hz, 3J(H4α–H5) = 9.8 Hz, 1H, HαC4), 2.39 (dddd, 3J(H4β–P) = 19.0 Hz, 2J(H4β–H4α) = 12.8 Hz, 3J(H4β–H3) = 7.3 Hz, 3J(H4β–H5) = 4.3 Hz, 1H, HβC4); 13C NMR (151 MHz, CDCl3): δ = 162.27 (d, 1J(CF) = 245.9 Hz), 161.90 (C=O), 151.18 (C=O), 140.37, 136.44, 136.04 (d, 3J(CCOP) = 5.5 Hz), 135.98 (d, 3J(CCOP) = 5.5 Hz), 134.90, 132.69 (d, 4J(CCCCF) = 3.2 Hz), 131.00 (d, 3J(CCCF) = 8.2 Hz), 129.96, 128.77, 128.75, 128.73, 128.29, 128.23, 128.17, 127.57, 122.71, 115.46, 115.22 (d, 2J(CCF) = 21.2 Hz), 114.98, 75.82 (d, 3J(CCCP) = 6.7 Hz, C5), 68.38 (d, 2J(COP) = 6.6 Hz, CH2OP), 68.18 (d, 2J(COP) = 7.1 Hz, CH2OP), 62.27 (d, 3J(CNCP) = 5.1 Hz, CH2Ph), 60.79 (d, 1J(CP) = 170.2 Hz, C3), 47.63 (CH2N), 44.16 (CH2Ph), 34.98 (C4); 31P NMR (243 MHz, CDCl3): δ = 23.70. Anal. calcd. for C40H37FN3O6P × 0.75 H2O: C, 66.80; H, 5.40; N, 5.84. Found: C, 66.78; H, 5.56; N, 5.91 (obtained on 96:4 mixture of cis-16d and trans-16d).
- Compound trans-16d. A colorless oil. IR (film, cm–1) νmax: 3455, 3063, 2957, 1956, 1896, 1817, 1659 1497, 1347, 1220, 993, 855. 1H NMR (600 MHz, CDCl3): δ = 8.24 (dd, J = 7.9 Hz, J = 1.5 Hz, 1H), 7.64–7.61 (m, 1H), 7.54–7.50 (m, 2H), 7.36–7.31 (m, 10H), 7.30–7.23 (m, 7H), 6.99–6.95 (m, 2H), 5.25 (AB, JAB = 13.8 Hz, 1H, HCHN), 5.20 (AB, JAB = 13.8 Hz, 1H, HCHN), 5.12–5.05 (m, 4H, 2 × CH2OP), 4.45 (dd, 2J = 15.1 Hz, 3J(HC–H5) = 4.1 Hz, 1H, HCHN), 4.39 (d, 2J = 13.8 Hz, 1H, HCHPh), 4.32–4.28 (m, 1H, HC5), 4.13 (dd, 2J = 15.1 Hz, 3J(HC–H5) = 6.1 Hz, 1H, HCHN), 3.89 (d, 2J = 13.8 Hz, 1H, HCHPh), 3.33 (ddd, 3J(H3–H4β) = 9.9 Hz, 3J(H3–H4α) = 6.3 Hz, 2J(H3–P) = 1.7 Hz, 1H, HC3), 2.67 (dddd, 3J(H4α–P) = 18.5 Hz, 2J(H4α–H4β) = 12.6 Hz, 3J(H4α–H3) = 6.3 Hz, 3J(H4α–H5) = 6.3 Hz, 1H, HαC4), 2.32 (dddd, 3J(H4β–P) = 16.7 Hz, 2J(H4β–H4α) = 12.6 Hz, 3J(H4β–H3) = 9.9 Hz, 3J(H4β–H5) = 8.8 Hz, 1H, HβC4); 13C NMR (151 MHz, CDCl3): δ = 162.30 (d, 1J(CF) = 246.5 Hz), 161.66 (C=O), 151.20 (C=O), 140.09, 136.33, 136.59 (d, 3J(CCOP) = 6.0 Hz), 136.13 (d, 3J(CCOP) = 5.5 Hz), 134.92, 132.69 (d, 4J(CCCCF) = 3.3 Hz), 131.05 (d, 3J(CCCF) = 8.0 Hz), 129.68, 128.92, 128.64, 128.61, 128.53, 128.15, 128.13, 128.12, 127.54, 123.23, 115.53, 115.26 (d, 2J(CCF) = 21.1 Hz), 114.92, 75.74 (d, 3J(CCCP) = 5.7 Hz, C5), 68.73 (d, 2J(COP) = 6.5 Hz, CH2OP), 67.97 (d, 2J(COP) = 6.8 Hz, CH2OP), 62.73 (CH2Ph), 60.95 (d, 1J(CP) = 170.2 Hz, C3), 45.67 (CH2N), 44.33 (CH2Ph), 35.05 (d, 2J(CCP) = 2.2 Hz, C4); 31P NMR (243 MHz, CDCl3): δ = 22.76. Anal. calcd. for C40H37FN3O6P × 0.5 H2O: C, 67.22; H, 5.36; N, 5.88. Found: C, 67.38; H, 5.37; N, 5.63.
- Compound cis-16e. Data noted below correspond to a 70:30 mixture of cis-16e and trans-16e. A colorless oil. IR (film, cm–1) νmax: 3442, 3062, 2956, 1957, 1896, 1658, 1482, 1338, 1246, 1019, 760. NMR signals of cis-16e were extracted from the spectrum of a 70:30 mixture of cis-16e and trans-16e. 1H NMR (600 MHz, CDCl3): δ = 8.13 (dd, J = 7.8 Hz, J = 1.4 Hz, 1H), 8.07 (d, J = 8.2 Hz, 1H), 7.51–7.50 (m, 1H), 7.42–7.39 (m, 5H), 7.35–7.32 (m, 5H), 7.29–7.22 (m, 8H), 7.11 (t, J = 7.5 Hz, 1H), 7.07–7.04 (m, 1H), 5.67–5.64 (m, 2H, HCHN), 5.22–5.07 (m, 4H, 2 CH2OP), 4.57–4.53 (m 1H, HC5), 4.40 (d, 2J = 13.7 Hz, 1H, HCHPh), 4.12 (dd, 2J = 14.9 Hz, 3J(HC–H5) = 9.1 Hz, 1H, HCHN), 4.05 (dd, 2J = 14.9 Hz, 3J(HC–H5) = 2.2 Hz, 1H, HCHN), 3.86 (d, 2J = 13.7 Hz, 1H, HCHPh), 3.23 (ddd, 3J(H3–H4α) = 10.0 Hz, 3J(H3–H4β) = 7.3 Hz, 2J(H3–P) = 2.7 Hz, 1H, HC3), 2.74 (dddd, 2J(H4α–H4β) = 12.0 Hz, 3J(H4α–P) = 10.0 Hz, 3J(H4α–H3) = 10.0 Hz, 3J(H4α–H5) = 10.0 Hz, 1H, HαC4), 2.37 (dddd, 3J(H4β–P) = 19.7 Hz, 2J(H4β–H4α) = 12.0 Hz, 3J(H4β–H3) = 7.3 Hz, 3J(H4β–H5) = 4.0 Hz, 1H, HβC4); 13C NMR (151 MHz, CDCl3): δ = 161.94 (C=O), 151.00 (C=O), 148.77, 140.51, 136.04, 135.97 (d, 3J(CCOP) = 5.7 Hz), 135.95 (d, 3J(CCOP) = 5.4 Hz), 135.23, 135.22, 133.50, 129.92, 128.77, 128.74, 128.72, 128.65, 128.62, 128.56, 128.23, 128.17, 128.14, 125.01, 122.94, 115.68, 114.70, 75.79 (d, 3J(CCCP) = 6.6 Hz, C5), 68.38 (d, 2J(COP) = 6.6 Hz, CH2OP), 68.22 (d, 2J(COP) = 7.0 Hz, CH2OP), 62.27 (d, 3J(CNCP) = 5.3 Hz, CH2Ph), 61.81 (d, 1J(CP) = 145.3 Hz, C3), 47.61 (CH2N), 42.00 (CH2Ph), 34.91 (C4); 31P NMR (243 MHz, CDCl3): δ = 23.62. Anal. calcd. for C40H37N4O8P × 1.5 H2O: C, 63.24; H, 5.31; N, 7.38. Found: C, 63.50; H, 5.09; N, 7.55 (obtained on 70:30 mixture of cis-16e and trans-16e).
- Compound trans-16e. A colorless oil. IR (film, cm–1) νmax: 3441, 3063, 2957, 1957, 1884, 1660, 1482, 1338, 1259, 1020, 760. 1H NMR (600 MHz, CDCl3): δ = 8.26 (dd, J = 7.9 Hz, J = 1.3 Hz, 1H), 8.07 (dd, J = 8.2 Hz, J = 1.0 Hz, 1H), 7.67 (t, J = 7.5 Hz, 1H), 7.46–7.43 (m, 1H), 7.39–7.31 (m, 12H), 7.27–7.23 (m, 6H), 7.19 (d, J = 7.8 Hz, 1H), 5.68 (AB, JAB = 16.3 Hz, 1H, HCHN), 5.65 (AB, JAB = 16.3 Hz, 1H, HCHN), 5.14–5.03 (m, 4H, 2 × CH2OP), 4.43 (dd, 2J = 15.0 Hz, 3J(HC–H5) = 4.0 Hz, 1H, HCHN), 4.40 (d, 2J = 13.7 Hz, 1H, HCHPh), 4.36–4.30 (m 1H, HC5), 4.18 (dd, 2J = 15.0 Hz, 3J(HC–H5) = 6.1 Hz, 1H, HCHN), 3.91 (d, 2J = 13.7 Hz, 1H, HCHPh), 3.37–3.30 (br m, 1H, HC3), 2.64 (dddd, 3J(H4α–P) = 18.5 Hz, 2J(H4α–H4β) = 12.7 Hz, 3J(H4α–H3) = 6.3 Hz, 3J(H4α–H5) = 6.3 Hz, 1H, HαC4), 2.32–2.25 (m, 1H, HβC4); 13C NMR (151 MHz, CDCl3): δ = 161.65 (C=O), 151.04 (C=O), 148.73, 140.21, 136.10, 136.08 (d, 3J(CCOP) = 5.4 Hz), 135.95 (d, 3J(CCOP) = 5.5 Hz), 135.26, 133.54, 132.33, 129.74, 129.11, 128.66, 128.62, 128.57, 128.17, 128.14, 128.02, 127.68, 127.61, 125.07, 123.46, 115.25, 115.08, 75.71 (d, 3J(CCCP) = 5.4 Hz, C5), 68.75 (d, 2J(COP) = 6.5 Hz, CH2OP), 67.86 (d, 2J(COP) = 6.9 Hz, CH2OP), 62.59 (CH2Ph), 60.83 (d, 1J(CP) = 170.3 Hz, C3), 45.68 (CH2N), 42.27 (CH2Ph), 34.96 (d, 2J(CCP) = 1.4 Hz, C4); 31P NMR (243 MHz, CDCl3): δ = 22.47. Anal. calcd. for C40H37N4O8P × 1.75 H2O: C, 62.87; H, 5.34; N, 7.33. Found: C, 62.71; H, 5.03; N, 7.03.
- Compound cis-16f. Data noted below correspond to a 96:4 mixture of cis-16f and trans-16f. A colorless oil. IR (film, cm–1) νmax: 3441, 3063, 2924, 1960, 1885, 1658, 1482, 1347, 1235, 1018, 696. NMR signals of cis-16f were extracted from the spectrum of a 96:4 mixture of cis-16f and trans-16f. 1H NMR (600 MHz, CDCl3): δ = 8.36 (s, 1H), 8.15–8.11 (m, 2H), 7.84 (d, J = 7.6 Hz, 1H), 7.49 (t, J = 7.9 Hz, 1H), 7.42–7.37 (m, 7H), 7.36–7.32 (m, 3H), 7.30–7.23 (m, 5H), 7.20 (d, J = 8.5 Hz, 1H), 7.10 (t, J = 7.4 Hz, 1H), 7.03–7.01 (m, 1H), 5.34 (AB, JAB = 14.0 Hz, 1H, HCHN), 5.29 (AB, JAB = 14.0 Hz, 1H, HCHN), 5.23–5.18 (m, 2H, CH2OP), 5.14–5.09 (m, 2H, CH2OP), 4.59–4.55 (m 1H, HC5), 4.40 (d, 2J = 13.7 Hz, 1H, HCHPh), 4.09 (dd, 2J = 14.9 Hz, 3J(HC–H5) = 8.8 Hz, 1H, HCHN), 4.04 (dd, 2J = 14.9 Hz, 3J(HC–H5) = 2.5 Hz, 1H, HCHN), 3.85 (d, 2J = 13.7 Hz, 1H, HCHPh), 3.23 (dd, 3J(H3–H4α) = 9.9 Hz, 3J(H3–H4β) = 7.3 Hz, 2J(H3–P) = 2.5 Hz, 1H, HC3), 2.77 (dddd, 2J(H4α–H4β) = 12.0 Hz, 3J(H4α–P) = 10.0 Hz, 3J(H4α–H3) = 9.9 Hz, 3J(H4α–H5) = 9.9 Hz, 1H, HαC4), 2.39 (dddd, 3J(H4β–P) = 19.9 Hz, 2J(H4β–H4α) = 12.0 Hz, 3J(H4β–H3) = 7.3 Hz, 3J(H4β–H5) = 4.0 Hz, 1H, HβC4); 13C NMR (151 MHz, CDCl3): δ = 161.87 (C=O), 151.10 (C=O), 148.33, 140.39, 138.95, 136.33, 136.02 (d, 3J(CCOP) = 5.7 Hz), 135.95 (d, 3J(CCOP) = 5.4 Hz), 135.22, 135.16, 129.97, 129.38, 128.78, 128.77, 128.74, 128.28, 128.24, 128.18, 128.16, 127.60, 124.00, 122.91, 122.72, 115.62, 114.77, 75.79 (d, 3J(CCCP) = 6.6 Hz, C5), 68.40 (d, 2J(COP) = 6.6 Hz, CH2OP), 68.21 (d, 2J(COP) = 6.7 Hz, CH2OP), 62.23 (d, J = 4.9 Hz, CH2Ph), 60.76 (d, 1J(CP) = 169.8 Hz, C3), 47.69 (CH2N), 44.22 (CH2Ph), 34.92 (C4); 31P NMR (243 MHz, CDCl3): δ = 23.57. Anal. calcd. for C40H37N4O8P × 1.5 H2O: C, 63.24; H, 5.31; N, 7.38. Found: C, 63.50; H, 5.09; N, 7.55 (obtained on 96:4 mixture of cis-16f and trans-16f).
- Compound trans-16f. A colorless oil. IR (film, cm–1) νmax: 3441, 3063, 2955, 1959, 1815, 1658, 1482, 1347, 1235, 993, 696. 1H NMR (600 MHz, CDCl3): δ = 8.37 (s, 1H), 8.25 (d, J = 7.9 Hz, 1H), 8.12 (d, J = 8.2 Hz, 1H), 7.84 (d, J = 7.7 Hz, 1H), 7.65 (t, J = 7.9 Hz, 1H), 7.46 (t, J = 7.9 Hz, 1H), 7.37–7.29 (m, 12H), 7.27–7.23 (m, 5H), 5.36 (AB, JAB = 14.2 Hz, 1H, HCHN), 5.32 (AB, JAB = 14.2 Hz, 1H, HCHN), 5.14–5.05 (m, 4H, 2 × CH2OP), 4.45 (dd, 2J = 15.1 Hz, 3J(HC–H5) = 3.8 Hz, 1H, HCHN), 4.40 (d, 2J = 13.8 Hz, 1H, HCHPh), 4.35– 4.31 (m, 1H, HC5), 4.17 (dd, 2J = 15.1 Hz, 3J(HC–H5) = 6.2 Hz, 1H, HCHN), 3.90 (d, 2J = 13.8 Hz, 1H, HCHPh), 3.36–3.34 (m, 1H, HC3), 2.69 (dddd, 3J(H4α–P) = 19.1 Hz, 2J(H4α–H4β) = 13.2 Hz, 3J(H4α–H3) = 6.8 Hz, 3J(H4α–H5) = 6.8 Hz, 1H, HαC4), 2.36–2.28 (m, 1H, HβC4); 13C NMR (151 MHz, CDCl3): δ = 161.60 (C=O), 151.14 (C=O), 148.34, 140.13, 138.81, 136.20, 136.13 (d, 3J(CCOP) = 5.7 Hz), 136.01 (d, 3J(CCOP) = 5.5 Hz), 135.18, 135.13, 129.71, 129.43, 129.00, 128.65, 128.61, 128.55, 128.15, 127.57, 123.93, 123.43, 122.77, 115.34, 115.07, 75.75 (d, 3J(CCCP) = 5.7 Hz, C5), 68.74 (d, 2J(COP) = 6.5 Hz, CH2OP), 68.03 (d, 2J(COP) = 6.7 Hz, CH2OP), 62.69 (d, J = 2.5 Hz, CH2Ph), 60.95 (d, 1J(CP) = 170.3 Hz, C3), 45.79 (CH2N), 44.38 (CH2Ph), 35.03 (C4); 31P NMR (243 MHz, CDCl3): δ = 22.69 Anal. calcd. for C40H37N4O8P × 0.75 H2O: C, 64.39; H, 5.20; N, 7.51. Found: C, 64.69; H, 5.15; N, 7.22.
- Compound cis-16g. Data noted below correspond to an 88:12 mixture of cis-16g and trans-16g. A colorless oil. IR (film, cm–1) νmax: 3442, 3062, 2956, 1954, 1657, 1609, 1482, 1343, 1214, 1023, 803. NMR signals of cis-16g were extracted from the spectrum of a 88:12 mixture of cis-16g and trans-16g. 1H NMR (600 MHz, CDCl3): δ = 8.18–8.16 (m, 2H), 8.12 (d, J = 7.8 Hz, J = 1.6 Hz, 1H), 7.64 (t, J = 8.8 Hz, 2H), 7.41–7.37 (m, 6H), 7.36–7.32 (m, 4H), 7.30–7.25 (m, 2H), 7.24–7.21 (m, 4H), 7.10 (t, J = 7.4 Hz, 1H), 7.05–7.02 (m, 1H), 5.33 (AB, JAB = 14.2 Hz, 1H, HCHN), 5.29 (AB, JAB = 14.2 Hz, 1H, HCHN), 5.23–5.17 (m, 2H, CH2OP), 5.14–5.08 (m, 2H, CH2OP), 4.58–4.55 (m 1H, HC5), 4.40 (d, 2J = 13.7 Hz, 1H, HCHPh), 4.11 (dd, 2J = 14.9 Hz, 3J(HC–H5) = 9.1 Hz, 1H, HCHN), 4.03 (dd, 2J = 14.9 Hz, 3J(HC–H5) = 2.3 Hz, 1H, HCHN), 3.86 (d, 2J = 13.7 Hz, 1H, HCHPh), 3.23 (ddd, 3J(H3–H4α) = 10.0 Hz, 3J(H3–H4β) = 7.4 Hz, 2J(H3–P) = 2.7 Hz, 1H, HC3), 2.76 (dddd, 2J(H4α–H4β) = 12.7 Hz, 3J(H4α–H3) = 10.0 Hz, 3J(H4α–H5) = 10.0 Hz, 3J(H4α–P) = 9.3 Hz, 1H, HαC4), 2.39 (dddd, 3J(H4β–P) = 19.1 Hz, 2J(H4β–H4α) = 12.7 Hz, 3J(H4β–H3) = 7.4 Hz, 3J(H4β–H5) = 4.0 Hz, 1H, HβC4); 13C NMR (151 MHz, CDCl3): δ = 161.88 (C=O), 151.07 (C=O), 147.37, 144.23, 140.38, 136.35, 136.01 (d, 3J(CCOP) = 5.6 Hz), 135.94 (d, 3J(CCOP) = 5.4 Hz), 135.21, 129.94, 129.70, 128.78, 128.75, 128.60, 128.29, 128.23, 128.17, 128.14, 127.61, 123.70, 122.97, 115.65 114.75, 75.76 (d, 3J(CCCP) = 5.9 Hz, C5), 68.40 (d, 2J(COP) = 6.6 Hz, CH2OP), 68.22 (d, 2J(COP) = 7.1 Hz, CH2OP), 62.25 (d, J = 4.6 Hz, CH2Ph), 60.76 (d, 1J(CP) = 170.0 Hz, C3), 47.68 (CH2N), 44.28 (CH2Ph), 34.92 (C4); 31P NMR (243 MHz, CDCl3): δ = 23.50. Anal. calcd. for C40H37N4O8P × 3 H2O: C, 61.07; H, 5.51; N, 7.12. Found: C, 60.81; H, 5.45; N, 6.82 (obtained on 88:12 mixture of cis-16g and trans-16g).
- Compound trans-16g. A colorless oil. IR (film, cm–1) νmax: 3454, 3062, 2926, 1954, 1812, 1660, 1612, 1485, 1346, 1216, 1043, 806. 1H NMR (600 MHz, CDCl3): δ = 8.25 (dd, J = 7.9 Hz, J = 1.4 Hz, 1H), 8.15 (d, J = 10.9 Hz, 2H), 7.67–7.63 (m, 3H), 7.39–7.29 (m, 12H), 7.27–7.23 (m, 5H), 5.33 (AB, JAB = 14.3 Hz, 1H, HCHN), 5.31 (AB, JAB = 14.3 Hz, 1H, HCHN), 5.14–5.05 (m, 4H, 2 × CH2OP), 4.44 (dd, 2J = 15.1 Hz, 3J(HC–H5) = 3.8 Hz, 1H, HCHN), 4.40 (d, 2J = 13.8 Hz, 1H, HCHPh), 4.35–4.31 (m, 1H, HC5), 4.15 (dd, 2J = 15.1 Hz, 3J(HC–H5) = 6.5 Hz, 1H, HCHN), 3.91 (d, 2J = 13.8 Hz, 1H, HCHPh), 3.36–3.34 (m, 1H, HC3), 2.69 (dddd, 3J(H4α–P) = 18.5 Hz, 2J(H4α–H4β) = 12.7 Hz, 3J(H4α–H3) = 6.4 Hz, 3J(H4α–H5) = 6.4 Hz, 1H, HαC4), 2.35–2.28 (m, 1H, HβC4); 13C NMR (151 MHz, CDCl3): δ = 161.60 (C=O), 151.09 (C=O), 147.41, 144.06, 140.10, 136.25, 136.49 (d, 3J(CCOP) = 5.6 Hz), 136.10 (d, 3J(CCOP) = 5.3 Hz), 135.22, 129.67, 128.99, 128.65, 128.62, 128.56, 128.24, 128.16, 128.13, 127.57, 123.73, 123.47, 115.31, 115.07, 75.71 (d, 3J(CCCP) = 5.7 Hz, C5), 68.73 (d, 2J(COP) = 6.5 Hz, CH2OP), 68.04 (d, 2J(COP) = 6.7 Hz, CH2OP), 62.69 (d, J = 3.5 Hz, CH2Ph), 60.95 (d, 1J(CP) = 170.2 Hz, C3), 45.88 (CH2N), 44.45 (CH2Ph), 35.11 (C4); 31P NMR (243 MHz, CDCl3): δ = 22.63. Anal. calcd. for C40H37N4O8P × 1.5 H2O: C, 63.24; H, 5.31; N, 7.38. Found: C, 63.54; H, 5.30; N, 7.18.
3.4. Biological Study In Vitro
3.4.1. Cytotoxicity Assay
3.4.2. Safety Studies
3.4.3. Apoptosis Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berthet, M.; Cheviett, T.; Dujardin, G.; Parrot, I.; Martinez, J. Isoxazolidine: A privileged scaffold for organic and medicinal chemistry. Chem. Rev. 2016, 116, 15235–15283. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, D.G.; Andrei, G.; Schols, D.; Snoeck, R.; Lysakowska, M. Synthesis, anti-varicella-zoster virus and anti-cytomegalovirus activity of quinazoline-2,4-diones containing isoxazolidine and phosphonate substructures. Eur. J. Med. Chem. 2017, 126, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Lysakowska, M.; Balzarini, J.; Piotrowska, D.G. Design, Synthesis, Antiviral, and Cytostatic Evaluation of Novel Isoxazolidine Analogs of Homonucleotides. Arch. Pharm. 2014, 347, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Romeo, R.; Giofre, S.V.; Carnovale, C.; Campisi, A.; Parenti, R.; Bandini, L.; Chiacchio, M.A. Synthesis and biological evaluation of 3-hydroxymethyl-5-(1H-1,2,3-triazol) isoxazolidines. Bioorg. Med. Chem. 2013, 21, 7929–7937. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, O.; De Nino, A.; Eliseo, T.; Gavioli, R.; Maiuolo, L.; Russo, B.; Sforza, F. Synthesis and biological evaluation of diastereoisomerically pure N,O-nucleosides. Bioorg. Med. Chem. 2010, 18, 6970–6976. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bhella, S.S.; Sexana, A.K.; Shanmugavel, M.; Faruk, A.; Ishar, M.P.S. Investigations of regio- and stereoselectivities in the synthesis of cytotoxic isoxazolidines through 1,3-dipolar cycloadditions of nitrones to dipolarophiles bearing an allylic oxygen. Tetrahedron 2007, 63, 2283–2291. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Balzarini, J.; Glowacka, I.E. Design, synthesis, antiviral and cytostatic evaluation of novel isoxazolidine nucleotide analogues with a 1,2,3-triazole linker. Eur. J. Med. Chem. 2012, 47, 501–509. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Cieslak, M.; Krolewska, K.; Wroblewski, A.E. Design, Synthesis and Cytotoxicity of a New Series of Isoxazolidine Based Nucleoside Analogues. Arch. Pharm. 2011, 344, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, D.G.; Cieslak, M.; Krolewska, K.; Wroblewski, A.E. Design, synthesis and cytotoxicity of a new series of isoxazolidines derived from substituted chalcones. Eur. J. Med. Chem. 2011, 46, 1382–1389. [Google Scholar] [CrossRef]
- Grabkowska-Druzyc, M.; Andrei, G.; Schols, D.; Snoeck, R.; Piotrowska, D.G. Isoxazolidine Conjugates of N3-Substituted 6-Bromoquinazolinones-Synthesis, Anti-Varizella-Zoster Virus, and Anti-Cytomegalovirus Activity. Molecules 2018, 23, 1889. [Google Scholar] [CrossRef]
- Eneama, W.A.; Salman, H.H.; Mousa, M.N. Synthesis of a New Isoxazolidine and Evaluation Anticancer Activity against MCF 7 Breast Cancer Cell Line. Radiother. Oncol. 2023, 17, 1–8. [Google Scholar]
- Wang, Q.; He, X.; Li, R.; Le, Y.; Liu, L. New isoxazolidine derivatives: Synthesis, spectroscopic analysis, X-ray, DFT calculation, biological activity studies. J. Mol. Struct. 2024, 1312, 138547. [Google Scholar] [CrossRef]
- Mellaoui, M.D.; Zaki, K.; Abbiche, K.; Imjjad, A.; Rachid Boutiddar, R.; Sbai, A.; Jmiai, A.; Issami, S.E.; Lamsabhi, A.M.; Zejli, H. In silico anticancer activity of isoxazolidine and isoxazolines derivatives: DFT study, ADMET prediction, and molecular docking. J. Mol. Struct. 2024, 1308, 138330. [Google Scholar] [CrossRef]
- Alminderej, F.; Ghannay, S.; Elsamani, M.O.; Alhawday, F.; Albadri, A.; Elbehairi, S.E.I.; Alfaifi, M.Y.; Kadri, A.; Aouadi, K. In Vitro and In Silico Evaluation of Antiproliferative Activity of New Isoxazolidine Derivatives Targeting EGFR: Design, Synthesis, Cell Cycle Analysis, and Apoptotic Inducers. Pharmaceuticals 2023, 16, 1025. [Google Scholar] [CrossRef] [PubMed]
- Stadániová, R.; Sahulcík, M.; Dohánosová, J.; Moncol, J.; Janotka, L.; Simonicová, K.; Messingerová, L.; Fischer, R. Synthesis of 1,2,3-Triazoles Bearing a 4-Hydroxyisoxazolidine Moiety from 4,5-Unsubstituted 2,3-Dihydroisoxazoles. Eur. J. Org. Chem. 2020, 2020, 4775–4786. [Google Scholar] [CrossRef]
- Al-Adhreai, A.; Alsaeedy, M.; Alrabie, A.; Al-Qadsy, I.; Dawbaa, S.; Alaizeri, Z.M.; Alhadlaq, H.A.; Al-Kubati, A.; Ahamed, M.; Farooqui, M. Design and synthesis of novel enantiopure Bis(5-Isoxazolidine) derivatives: Insights into their antioxidant and antimicrobial potential via in silico drug-likeness, pharmacokinetic, medicinal chemistry properties, and molecular docking studies. Heliyon 2022, 8, e09746. [Google Scholar] [CrossRef] [PubMed]
- Arwa Al Adhreai, Mohammed Alsaeedy, Mazahar Farooqui and Usama Al-Timari, Regio-and stereoselectivity of 1,3-dipolar cycloaddition reaction of cinnarizine drug with chiral nitrones, and their antimicrobial activity. Rasayan J. Chem. 2021, 4, 2728–2738. [CrossRef]
- Al-Adhreai, A.; Alsaeedy, M.; Alrabie, A.; Al-Horaibi, S.A.; Al-Qadsy, I.; Alezzy, A.A.; Al-Odayni, A.B.; Saeed, W.S.; Farooqui, M. Enhanced synthesis of novel multisubstituted isoxazolidines as potential antimicrobial and antioxidant agents using zinc (II) catalyst, and in silico studies. J. Mol. Struct. 2023, 1292, 136146. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, A.; Kaur, H.; Ishar, M.P.S. Chromanyl-isoxazolidines as Antibacterial agents: Synthesis, Biological Evaluation, Quantitative Structure Activity Relationship, and Molecular Docking Studies. Chem. Biol. Drug Des. 2016, 87, 213–223. [Google Scholar] [CrossRef]
- Hussam Hamza Salman, H.H. Synthesis and Antimicrobial Evaluation of Some Isoxazolidine Derivatives. J. Educ. Pure Sci. 2019, 9, 217–225. [Google Scholar] [CrossRef]
- Yanmaz, V.; Disli, A.; Yavuz, S.; Ogutcu, H.; Dilek, G. Synthesis of Some Novel Isoxazolidine Derivatives via 1,3-Dipolar Cycloaddition and Their Biological Evaluation. GU J. Sci. 2019, 32, 78–89. [Google Scholar]
- Lysakowska, M.; Glowacka, I.E.; Andrei, G.; Schols, D.; Snoeck, R.; Lisiecki, P.; Szemraj, M.; Piotrowska, D.G. Design, Synthesis, Anti-Varicella-Zoster and Antimicrobial Activity of (Isoxazolidin-3-yl)Phosphonate Conjugates of N1-Functionalised Quinazoline-2,4-Diones. Molecules 2022, 27, 6526. [Google Scholar] [CrossRef] [PubMed]
- Leggio, A.; Liguori, A.; Procopio, A.; Siciliano, C.; Sindona, G. A novel class of 4′-aza analogues of 2′,3′-dideoxynucleosides as potential anti-HIV drugs. Nucleosides Nucleotides 1997, 16, 1515–1518. [Google Scholar] [CrossRef]
- Romeo, R.; Iannazzo, D.; Veltri, L.; Gabriele, B.; Macchi, B.; Frezza, C.; Marino-Merlo, F.; Giofre, S.V. Pyrimidine 2,4-Diones in the Design of New HIV RT Inhibitors. Molecules 2019, 24, 1718. [Google Scholar] [CrossRef]
- Ghannay, S.; Bakari, S.; Msaddek, M.; Vidal, S.; Kadri, A.; Aouadi, K. Design, synthesis, molecular properties and in vitro antioxidant and antibacterial potential of novel enantiopure isoxazolidine derivatives. Arab. J. Chem. 2020, 13, 2121–2131. [Google Scholar] [CrossRef]
- Mosbah, H.; Chahdoura, H.; Mannai, A.; Snoussi, M.; Aouadi, K.; Abreu, R.M.V.; Bouslama, A.; Achour, L.; Selmi, B. Biological activities evaluation of enantiopure isoxazolidine derivatives: In vitro, in vivo and in silico studies. Appl. Biochem. Biotechnol. 2019, 187, 1113–1130. [Google Scholar] [CrossRef]
- Sadashiva, M.P.; Nataraju, A.; Mallesha, H.; Rajesh, R.; Vishwanath, B.S.; Rangappa, K.S. Synthesis and evaluation of trimethoxyphenyl isoxazolidines as inhibitors of secretory phospholipase A(2) with anti-inflammatory activity. Int. J. Mol. Med. 2005, 16, 895–904. [Google Scholar] [CrossRef]
- Ghabi, A.; Brahmi, J.; Alminderej, F.; Messaoudi, S.; Vidal, S.; Kadri, A.; Aouadi, K. Multifunctional isoxazolidine derivatives as α-amylase and α-glucosidase inhibitors. Bioorganic Chem. 2020, 98, 103713. [Google Scholar] [CrossRef]
- Ghannay, S.; Aldhafeeri, B.S.; Ahmad, I.; Albadri, A.; Patel, H.; Kadri, A.; Aouadi, K. Identification of dual-target isoxazolidine-isatin hybrids with antidiabetic potential: Design, synthesis, in vitro and multiscale molecular modeling approaches. Heliyon 2024, 10, e25911. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Kim, Y.H.; Dudley, S.C., Jr.; Choudhary, G.; Pfahnl, A.; Oshima, Y.; Daly, J.W. The structure of zetekitoxin AB, a saxitoxin analog from the Panamanian golden frog Atelopus zeteki: A potent sodium-channel blocker. Proc. Natl. Acad. Sci. USA 2004, 101, 4346–4351. [Google Scholar] [CrossRef]
- Nishikawa, T.; Wang, C.; Akimoto, T.; Koshino, H.; Nagasawa, K. Synthesis of an Advanced Model of Zetekitoxin AB Focusing on the N-Acylisoxazolidine Amide Structure Corresponding to C13-C17. Asian J. Org. Chem. 2014, 3, 1308–1311. [Google Scholar] [CrossRef]
- Tsuda, M.; Hirano, K.; Kubota, T.; Kobayashi, J. Pyrinodemin A, a cytotoxic pyridine alkaloid with an isoxazolidine moiety from sponge Amphimedon sp. Tetrahedron Lett. 1999, 40, 4819–4820. [Google Scholar] [CrossRef]
- Serna, A.V.; Kurti, L.; Siitonen, J.H. Synthesis of (+/−)-Setigerumine I: Biosynthetic Origins of the Elusive Racemic Papaveraceae Isoxazolidine Alkaloids**. Angew. Chem. Int. Ed. Engl. 2021, 60, 27236–27240. [Google Scholar] [CrossRef] [PubMed]
- Tronchet, J.M.J.; Iznaden, M.; Barbalatrey, F.; Dhimane, H.; Ricca, A.; Balzarini, J.; Declercq, E. Isoxazolidine analogs of nucleosides. Eur. J. Med. Chem. 1992, 27, 555–560. [Google Scholar] [CrossRef]
- Gheidari, D.; Mehrdad, M.; Maleki, S. The quinazoline-2,4(1H,3H)-diones skeleton: A key intermediate in drug synthesis. Sustain. Chem. Pharm. 2022, 27, 100696. [Google Scholar] [CrossRef]
- Hassan, A.; Mosallam, A.M.; Ibrahim, A.O.A.; Badr, M.; Abdelmonsef, A.H. Novel 3-phenylquinazolin-2,4(1H,3H)-diones as dual VEGFR-2/c-Met-TK inhibitors: Design, synthesis, and biological evaluation. Sci. Rep. 2023, 13, 18567. [Google Scholar] [CrossRef]
- El-Adl, K.; El-Helby, A.G.A.; Sakr, H.; El-Hddad, S.S.A. Design, synthesis, molecular docking, and anticancer evaluations of 1-benzylquinazoline-2,4(1H,3H)-dione bearing different moieties as VEGFR-2 inhibitors. Arch. Pharm. Chem. Life Sci. 2020, 353, e2000068. [Google Scholar] [CrossRef]
- Zhou, J.; Du, T.; Wang, X.; Yao, H.P.; Deng, J.; Li, Y.; Chen, X.; Sheng, L.; Ji, M.; Xu, B. Discovery of Quinazoline-2,4(1H,3H)-dione Derivatives Containing a Piperizinone Moiety as Potent PARP-1/2 Inhibitors-Design, Synthesis, In Vivo Antitumor Activity, and X-ray Crystal Structure Analysis. J. Med. Chem. 2023, 66, 14095–14115. [Google Scholar] [CrossRef]
- Pradere, U.; Garnier-Amblard, E.C.; Coats, S.J.; Amblard, F.; Schinazi, R.F. Synthesis of Nucleoside Phosphate and Phosphonate Prodrugs. Chem. Rev. 2014, 114, 9154–9218. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Mediavilla, L.; Cuarental, L.; Glowacka, I.E.; Marco-Contelles, J.; Hadjipavlou-Litina, D.; Lopez-Munoz, F.; Oset-Gasque, M.J. Synthesis and Neuroprotective Properties of N-Substituted C-Dialkoxyphosphorylated Nitrones. Acs Omega 2019, 4, 8581–8587. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Mak, S.K.; Kültz, D.; Hammock, B.D. Determination of cytotoxicity of nephrotoxins on murine and human kidney cell lines. J. Environ. Sci. Heal. B 2008, 43, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, T.; Strigun, A.; Verlohner, A.; Huener, H.A.; Peter, E.; Herold, M.; Bordag, N.; Mellert, W.; Walk, T.; Spitzer, M.; et al. Prediction of liver toxicity and mode of action using metabolomics in vitro in HepG2 cells. Arch. Toxicol. 2018, 92, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Ayla, S.; Seckin, I.; Tanriverdi, G.; Cengiz, M.; Eser, M.; Soner, B.C.; Oktem, G. Doxorubicin Induced Nephrotoxicity: Protective Effect of Nicotinamide. Int. J. Cell Biol. 2011, 2011, 390238. [Google Scholar] [CrossRef]
- Prasanna, P.L.; Renu, K.; Gopalakrishnan, A.V. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020, 250, 117599. [Google Scholar] [CrossRef]

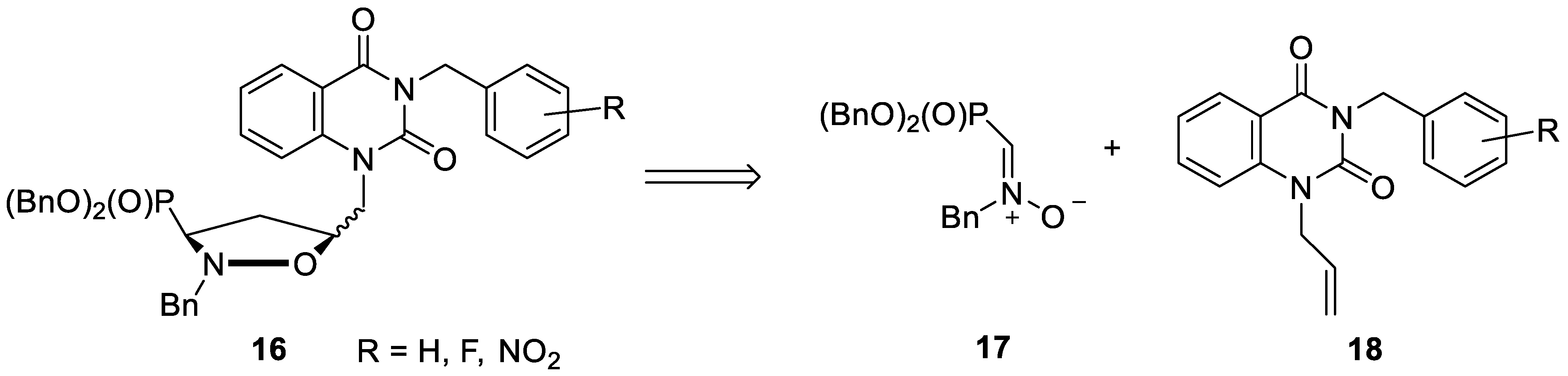
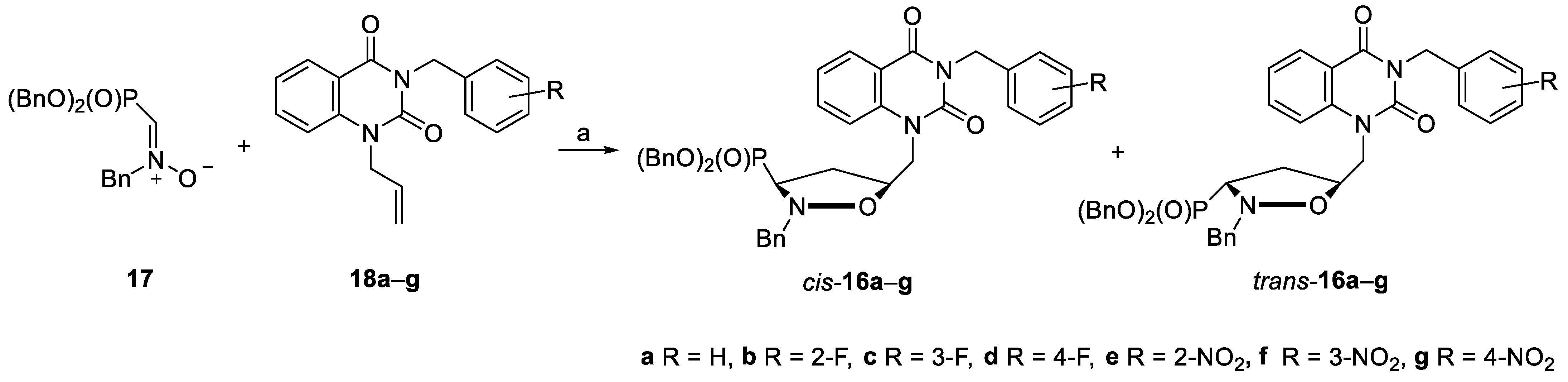
| Entry | Alkene 18 (R) | Ratio of cis-16:trans-16 | Yield (%) |
|---|---|---|---|
| a | H | 35:65 | trans-16a (27%) a + cis-16a and trans-16a (35%) b |
| b | 2-F | 33:67 | trans-16b (35%) a + cis-16b and trans-16b (33%) b |
| c | 3-F | 39:61 | trans-16c (34%) a + cis-16c and trans-16c (35%) b |
| d | 4-F | 39:61 | trans-16d (39%) a + cis-16d and trans-16d (30%) b |
| e | 2-NO2 | 39:61 | trans-16e (17%) a + cis-16e and trans-16e (44%) b |
| f | 3-NO2 | 42:58 | trans-16f (38%) a + cis-16f and trans-16f (28%) b |
| g | 4-NO2 | 40:60 | trans-16g (26%) a + cis-16g and trans-16g (39%) b |
| Compound | IC50 ± SEM [μM] a | ||
|---|---|---|---|
| MCF-7 | HT-1080 | PC-3 | |
| cis-16a/trans-16a (97:3) | 90.33 ± 4.57 | 19.94 ± 8.13 | 12.64 ± 5.56 |
| trans-16a | 96.04 ± 4.66 | 40.45 ± 5.44 | 12.67 ± 3.45 |
| cis-16b/trans-16b (90:10) | 103.69 ± 7.38 | 27.29 ± 5.43 | 11.21 ± 1.99 |
| trans-16b | 78.66 ± 2.35 | 34.56 ± 5.30 | 9.84 ± 3.69 |
| cis-16c/trans-16c (90:10) | 237.55 ± 20.72 | 20.47 ± 1.56 | 17.64 ± 6.21 |
| trans-16c | 130.35 ± 9.97 | 42.34 ± 3.48 | 16.37 ± 4.32 |
| cis-16d/trans-16d (96:4) | 116.45 ± 5.73 | 10.36 ± 2.69 | 16.43 ± 3.69 |
| trans-16d | 88.89 ± 3.86 | 35.62 ± 3.03 | 13.93 ± 2.14 |
| cis-16e/trans-16e (70:30) | 59.08 ± 3.77 | 59.60 ± 0.36 | 26.57 ± 4.69 |
| trans-16e | 57.87 ± 8.36 | 29.80 ± 4.75 | 26.58 ± 1.09 |
| cis-16f/trans-16f (96:4) | 91.68 ± 1.47 | 17.07 ± 5.73 | 24.80 ± 2.15 |
| trans-16f | 59.40 ± 0.78 | 23.08 ± 9.22 | 18.14 ± 0.98 |
| cis-16g/trans-16g (88:12) | 116.45 ± 9.69 | 16.45 ± 2.03 | 21.51 ± 4.63 |
| trans-16g | 142.49 ± 5.11 | 16.64 ± 3.11 | 16.68 ± 3.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łysakowska, M.; Głowacka, I.E.; Honkisz-Orzechowska, E.; Handzlik, J.; Piotrowska, D.G. New 3-(Dibenzyloxyphosphoryl)isoxazolidine Conjugates of N1-Benzylated Quinazoline-2,4-diones as Potential Cytotoxic Agents against Cancer Cell Lines. Molecules 2024, 29, 3050. https://doi.org/10.3390/molecules29133050
Łysakowska M, Głowacka IE, Honkisz-Orzechowska E, Handzlik J, Piotrowska DG. New 3-(Dibenzyloxyphosphoryl)isoxazolidine Conjugates of N1-Benzylated Quinazoline-2,4-diones as Potential Cytotoxic Agents against Cancer Cell Lines. Molecules. 2024; 29(13):3050. https://doi.org/10.3390/molecules29133050
Chicago/Turabian StyleŁysakowska, Magdalena, Iwona E. Głowacka, Ewelina Honkisz-Orzechowska, Jadwiga Handzlik, and Dorota G. Piotrowska. 2024. "New 3-(Dibenzyloxyphosphoryl)isoxazolidine Conjugates of N1-Benzylated Quinazoline-2,4-diones as Potential Cytotoxic Agents against Cancer Cell Lines" Molecules 29, no. 13: 3050. https://doi.org/10.3390/molecules29133050
APA StyleŁysakowska, M., Głowacka, I. E., Honkisz-Orzechowska, E., Handzlik, J., & Piotrowska, D. G. (2024). New 3-(Dibenzyloxyphosphoryl)isoxazolidine Conjugates of N1-Benzylated Quinazoline-2,4-diones as Potential Cytotoxic Agents against Cancer Cell Lines. Molecules, 29(13), 3050. https://doi.org/10.3390/molecules29133050








