Fluorescence of Half-Twisted 10-Acyl-1-methyltetrahydrobenzoquinolines
Abstract
1. Introduction
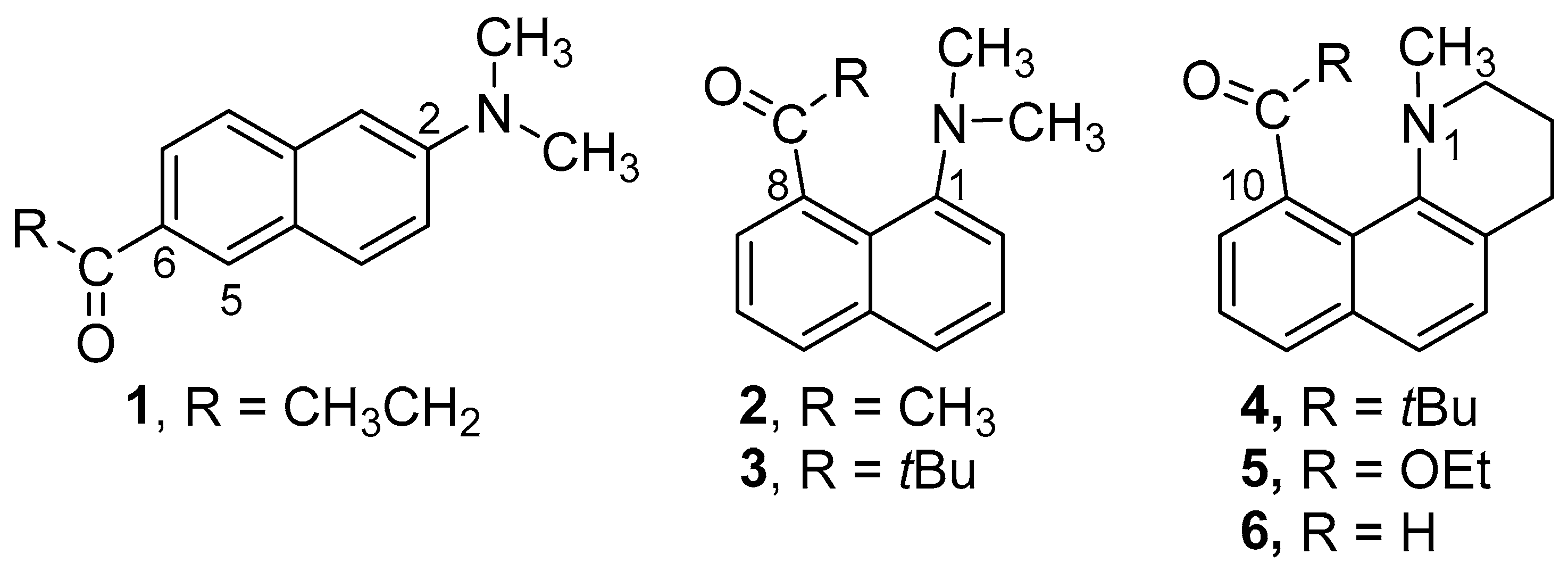
2. Results
2.1. Structural Studies
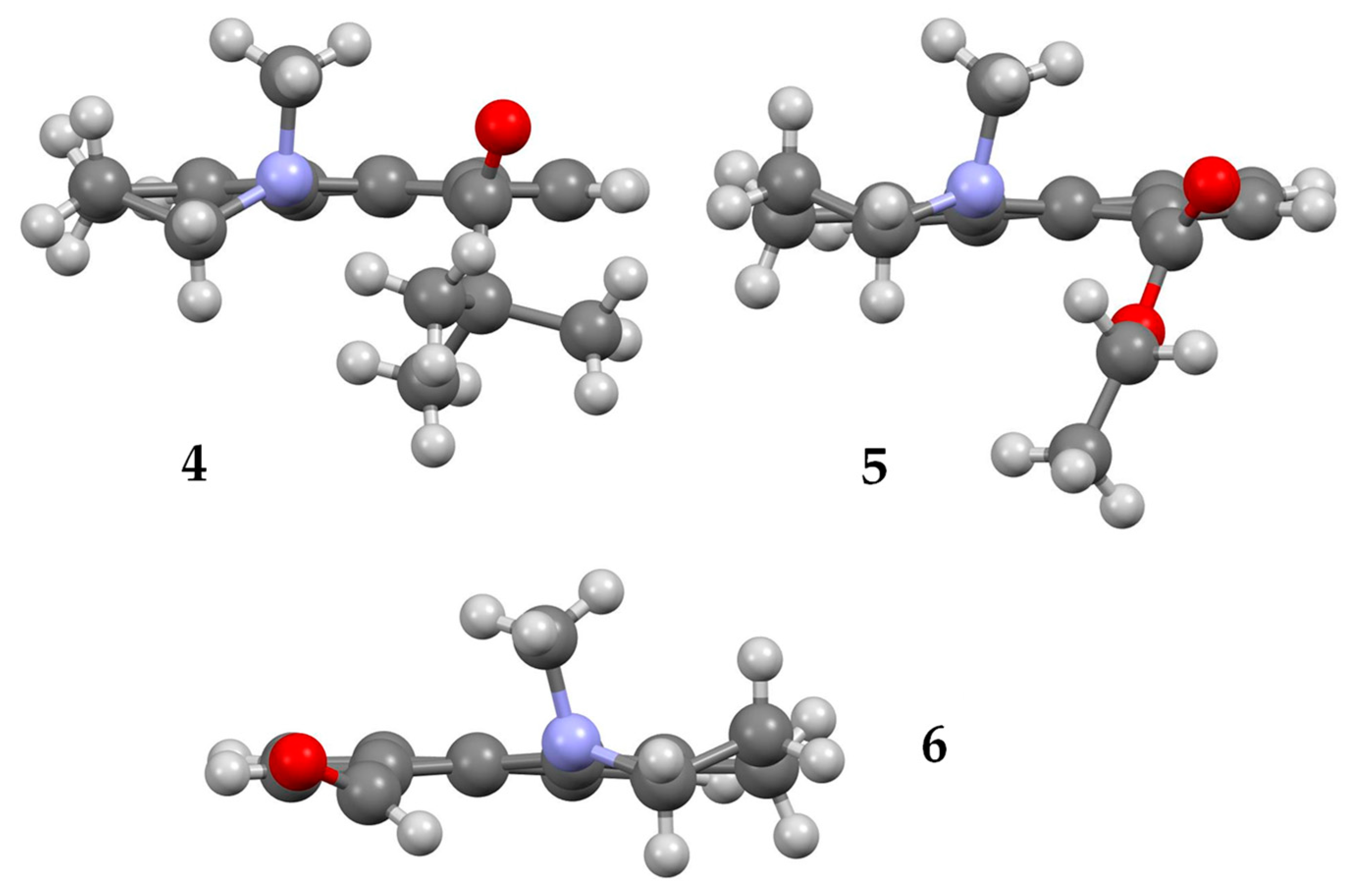
2.1.1. X-ray Structures
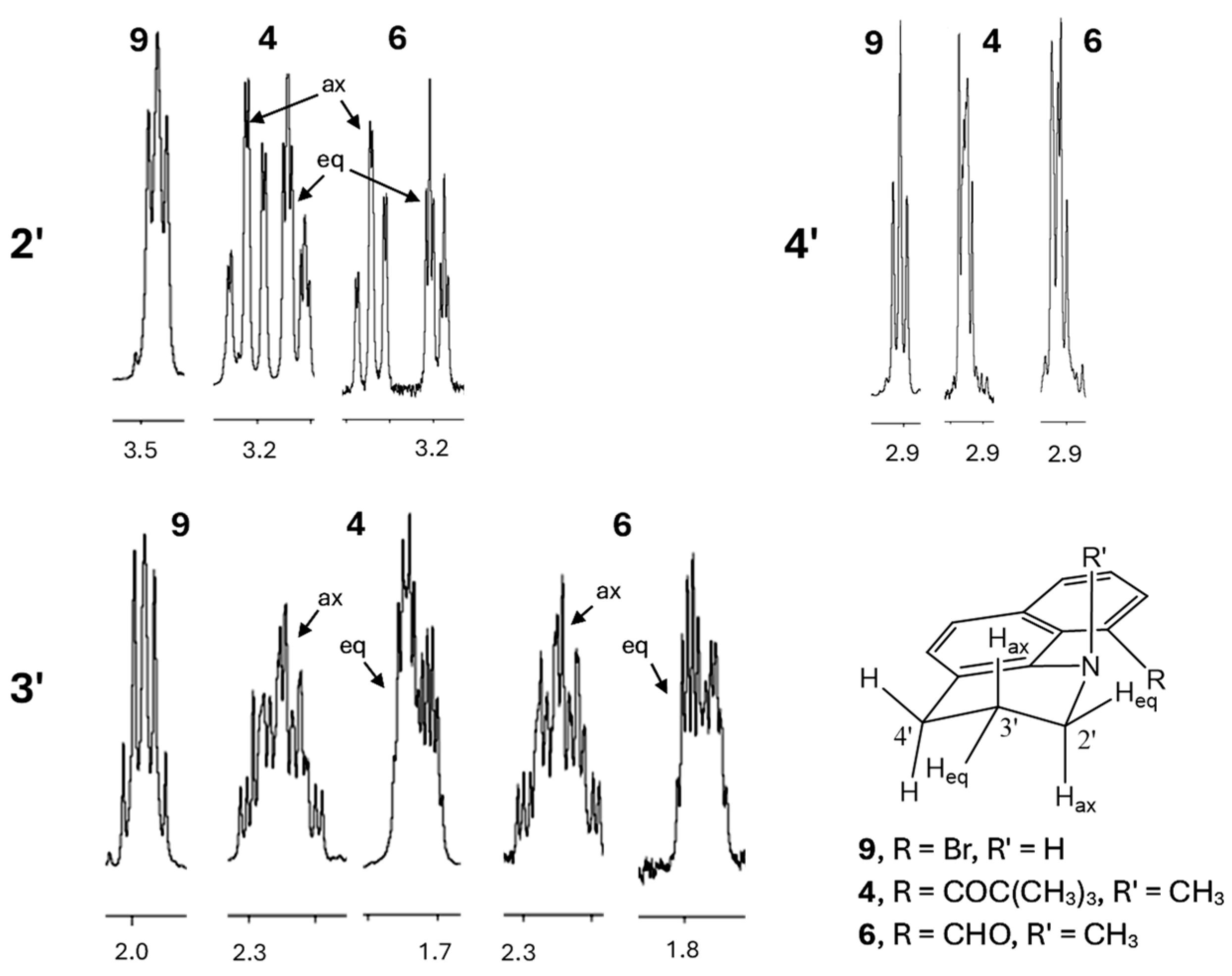
2.1.2. NMR Studies
2.2. Photophysical Studies
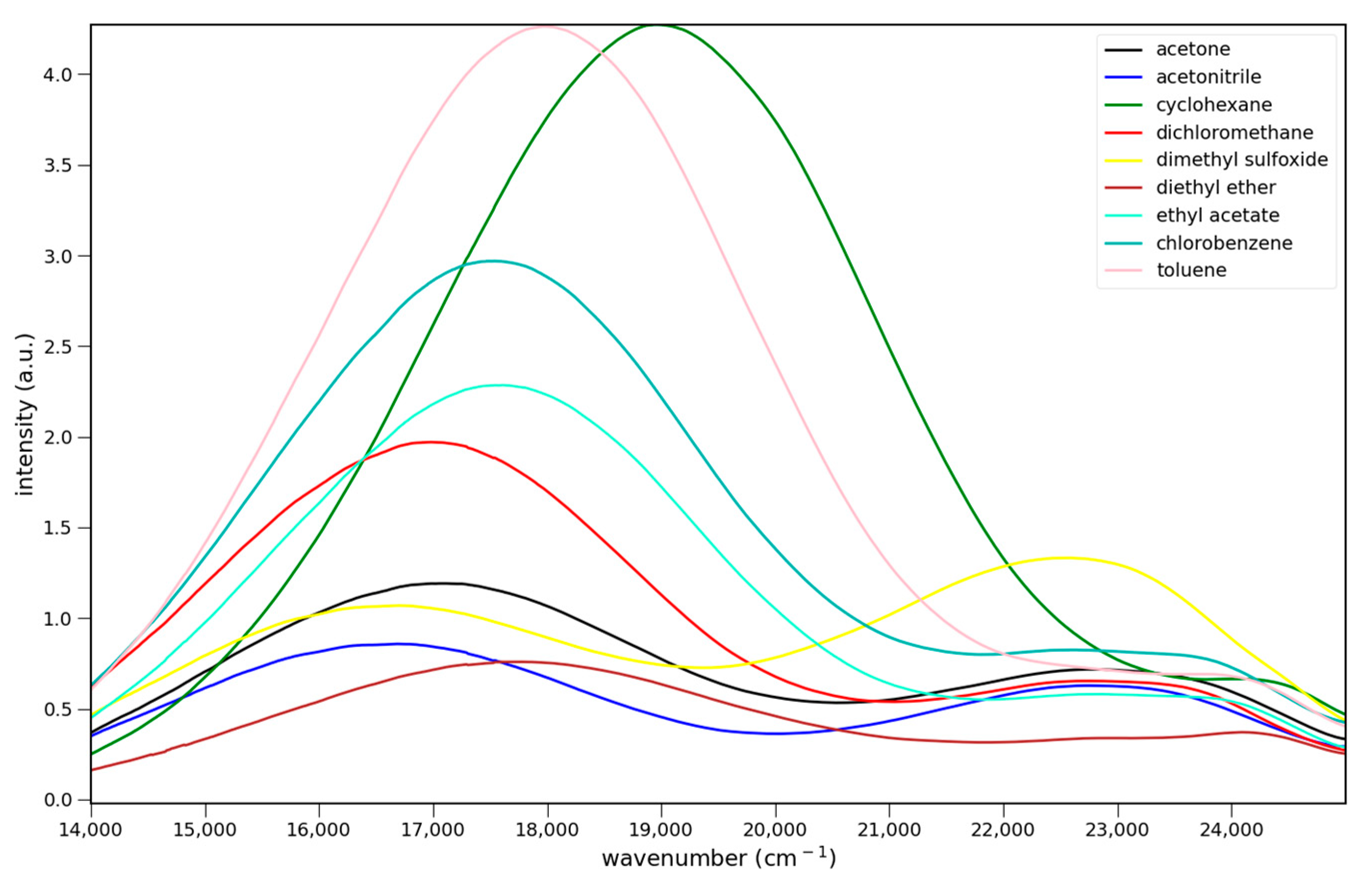
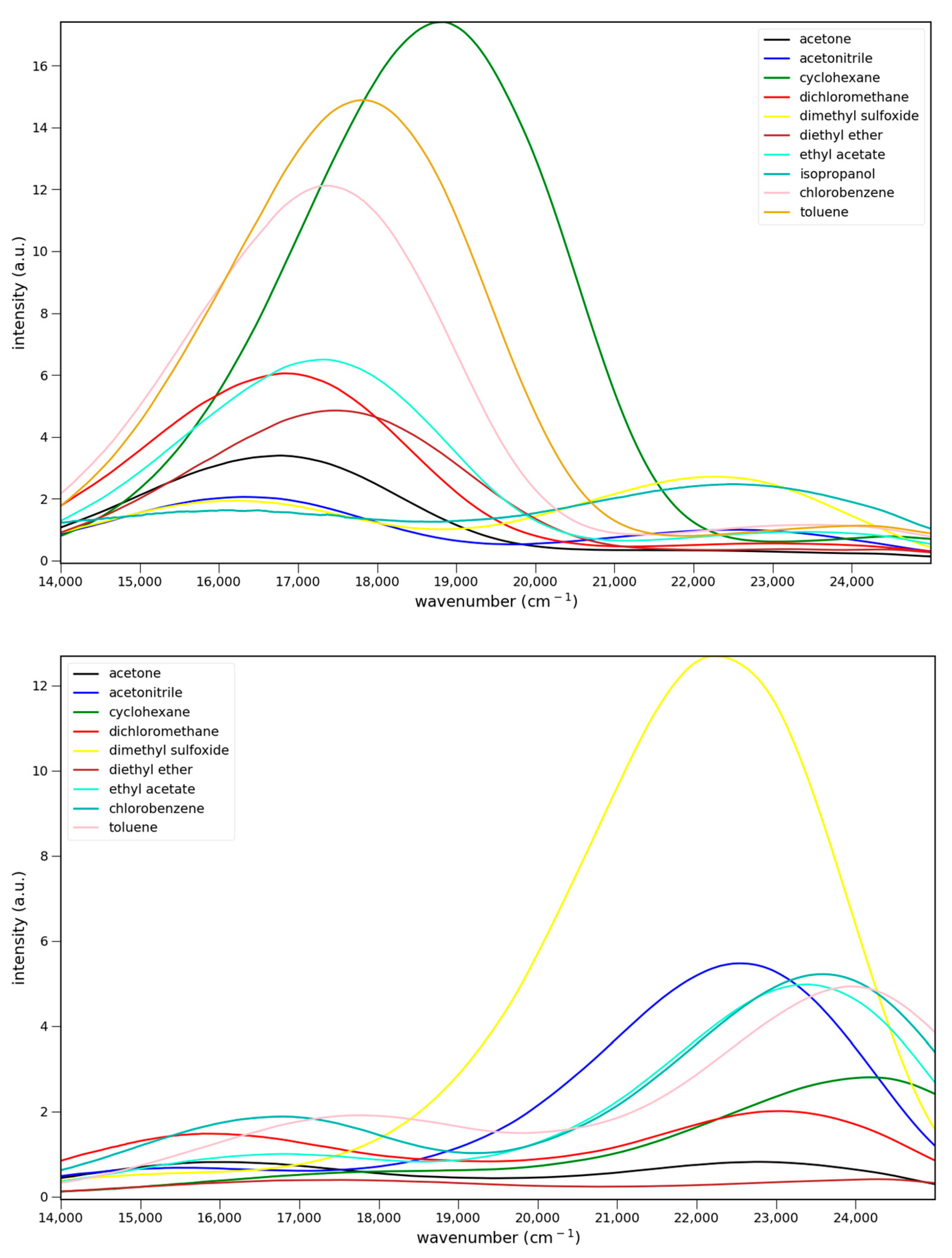
2.2.1. Absorption
2.2.2. Fluorescence
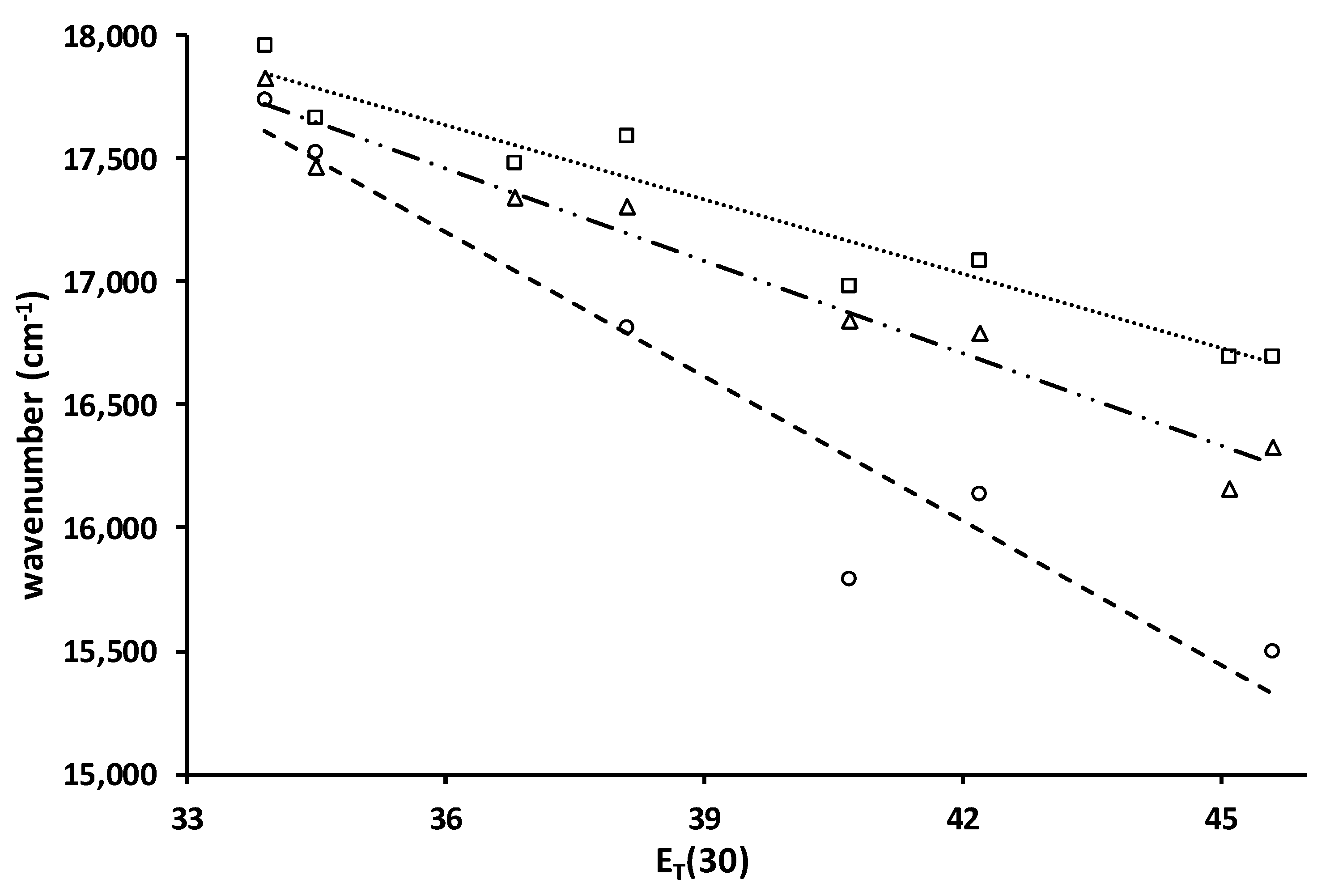
2.2.3. Solvato-Chromism
2.3. Computational Studies
3. Materials and Methods
3.1. 10-Bromobenzo[h]quinoline (8)
3.2. 10-Bromo-1,2,3,4-tetrahydrobenzo[h]quinoline (9)
3.3. 10-Bromo-1-methyl-1,2,3,4-tetrahydrobenzo[h]quinoline (10)
3.4. 2,2-Dimethyl-1-(1-methyl-1,2,3,4-tetrahydrobenzo[h]quinolin-10-yl)propan-1-one (4)
3.5. Ethyl 1-Methyl-1,2,3,4-tetrahydrobenzo[h]quinoline-10-carboxylate (5)
3.6. 1-Methyl-1,2,3,4-tetrahydrobenzo[h]quinoline-10-carbaldehyde (6)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, G.; Farris, F.J. Synthesis and Spectral Properties of a Hydrophobic Fluorescent Probe: 6-Propionyl-2-(Dimethylamino) Naphthalene. Biochemistry 1979, 18, 3075–3078. [Google Scholar] [CrossRef]
- Balter, A.; Nowak, W.; Pawekiewicz, W.; Kowalczyk, A. Some Remarks on the Interpretation of the Spectral Properties of Prodan. Chem. Phys. Lett. 1988, 143, 565–570. [Google Scholar] [CrossRef]
- Catalán, J.; Perez, P.; Laynez, J.; Blanco, F.G. Analysis of the Solvent Effect on the Photophysics Properties of 6-Propionyl-2-(Dimethylamino) Naphthalene (PRODAN). J. Fluoresc. 1991, 1, 215–223. [Google Scholar] [CrossRef]
- Kawski, A. Ground-and Excited-State Dipole Moments of 6-Propionyl-2-(Dimethylamino) Naphthalene Determined from Solvatochromic Shifts. Z. Naturforsch. A 1999, 54, 379–381. [Google Scholar] [CrossRef]
- Samanta, A.; Fessenden, R.W. Excited State Dipole Moment of PRODAN as Determined from Transient Dielectric Loss Measurements. J. Phys. Chem. A 2000, 104, 8972–8975. [Google Scholar] [CrossRef]
- Kochman, M.A.; Durbeej, B. Simulating the Nonadiabatic Relaxation Dynamics of 4-(N,N-Dimethylamino) Benzonitrile (DMABN) in Polar Solution. J. Phys. Chem. A 2020, 124, 2193–2206. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Uesugi, M.; Köhler, G.; Rechthaler, K.; Rotkiewicz, K.; Rettig, W.; Grabner, G. Time-Resolved Spectroscopy of DMABN and Its Cage Derivatives 6-Cyanobenzquinuclidine (CBQ) and Benzquinuclidine (BQ). Chem. Phys. 1999, 241, 327–337. [Google Scholar] [CrossRef]
- Köhler, G.; Rechthaler, K.; Grabner, G.; Luboradzki, R.; Suwińska, K.; Rotkiewicz, K. Structure of Cage Amines as Models for Twisted Intramolecular Charge-Transfer States. J. Phys. Chem. A 1997, 101, 8518–8525. [Google Scholar] [CrossRef]
- Wermuth, G.; Rettig, W. The Interaction of Close-Lying Excited States: Solvent Influence on Fluorescence Rate and Polarization in Substituted Indolines. J. Phys. Chem. 1984, 88, 2729–2735. [Google Scholar] [CrossRef]
- Rotkiewicz, K.; Rubaszewska, W. Intramolecular Charge Transfer State and Unusual Fluorescence from an Upper Excited Singlet of a Nonplanar Derivative of P-Cyano-N,N-Dimethylaniline. J. Lumin. 1982, 27, 221–230. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures. Chem. Rev. 2003, 103, 3899–4031. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chi, W.; Qiao, Q.; Tan, D.; Xu, Z.; Liu, X. Twisted Intramolecular Charge Transfer (TICT) and Twists beyond TICT: From Mechanisms to Rational Designs of Bright and Sensitive Fluorophores. Chem. Soc. Rev. 2021, 50, 12656–12678. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fang, C. Fluorescence Modulation by Amines: Mechanistic Insights into Twisted Intramolecular Charge Transfer (TICT) and Beyond. Chemosensors 2023, 11, 87. [Google Scholar] [CrossRef]
- Chen, T.; Lee, S.W.; Abelt, C.J. 1,5-Prodan Emits from a Planar Intramolecular Charge-Transfer Excited State. ACS Omega 2018, 3, 4816–4823. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.K.; Nguyen, H.A.A.; Abelt, C.J. Does PRODAN Possess an O-TICT Excited State? Synthesis and Properties of Two Constrained Derivatives. J. Phys. Chem. A 2010, 114, 4946–4950. [Google Scholar] [CrossRef] [PubMed]
- Lobo, B.C.; Abelt, C.J. Does PRODAN Possess a Planar or Twisted Charge-Transfer Excited State? Photophysical Properties of Two PRODAN Derivatives. J. Phys. Chem. A 2003, 107, 10938–10943. [Google Scholar] [CrossRef]
- Abelt, C.; Sweigart, K. Twisted 8-Acyl-1-Dialkyl-Amino-Naphthalenes Emit from a Planar Intramolecular Charge Transfer Excited State. Photochem 2024, 4, 1–13. [Google Scholar] [CrossRef]
- Kiefl, C. Correlated Rotations and Unusual Fluorescence Properties of peri-Substituted, Axially Chiral Naphthyl Ketones. Eur. J. Org. Chem. 2000, 2000, 3279–3286. [Google Scholar] [CrossRef]
- Gallucci, J.C.; Hart, D.J.; Young, D.G.J. Nucleophile–Electrophile Interactions in 1,8-Disubstituted Naphthalenes: Structures of Three 1-Naphthaldehydes and a 1-Naphthyl Methyl Ketone. Acta Crystallog. B Struct. Sci. 1998, 54, 73–81. [Google Scholar] [CrossRef]
- Schweizer, W.B.; Procter, G.; Kaftory, M.; Dunitz, J.D. Structural Studies of 1,8-Disubstituted Naphthalenes as Probes for nucleophile-electrophile interactions. Helv. Chim. Acta 1978, 61, 2783–2808. [Google Scholar] [CrossRef]
- Bulgarevich, S.B.; Ivanova, N.A.; Movshovich, D.Y.; Mannschreck, A.; Kiefl, C. Conformational Investigation of 1,8-Disubstituted Naphthalenes as Solutes by Kerr Effect and Dipole Moment Methods. J. Mol. Struct. 1994, 326, 17–24. [Google Scholar] [CrossRef]
- Hodgson, D.R.W.; Kirby, A.J.; Feeder, N. The Case of the Missing Acetylene. The Mechanism of an Intramolecular SN(V) Reaction and a New Route to 1-Methylbenzo[de]Quinolines. J. Chem. Soc. Perkin Trans. 1 1999, 949–954. [Google Scholar] [CrossRef]
- Clayden, J.; McCarthy, C.; Helliwell, M. Bonded Peri-Interactions Govern the Rate of Racemisation of Atropisomeric 8-Substituted 1-Naphthamides. Chem. Commun. 1999, 2059–2060. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 1999; ISBN 978-1-4757-3063-0. [Google Scholar]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH Verlag GmbH: Hoboken, NJ, USA, 2011. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Version 1.1; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Shuai, Z.; Peng, Q. Organic light-emitting diodes: Theoretical understanding of highly efficient materials and development of computational methodology. Natl. Sci. Rev. 2017, 4, 224–239. [Google Scholar] [CrossRef]
- Weimar, M.; Correa Da Costa, R.; Lee, F.-H.; Fuchter, M.J. A Scalable and Expedient Route to 1-Aza[6]Helicene Derivatives and Its Subsequent Application to a Chiral-Relay Asymmetric Strategy. Org. Lett. 2013, 15, 1706–1709. [Google Scholar] [CrossRef]
- Wang, C.; Qiao, Q.; Chi, W.; Chen, J.; Liu, W.; Tan, D.; McKechnie, S.; Lyu, D.; Jiang, X.; Zhou, W.; et al. Quantitative Design of Bright Fluorophores and AIEgens by the Accurate Prediction of Twisted Intramolecular Charge Transfer (TICT). Angew. Chem. Int. Ed. 2020, 59, 10160–10172. [Google Scholar] [CrossRef]
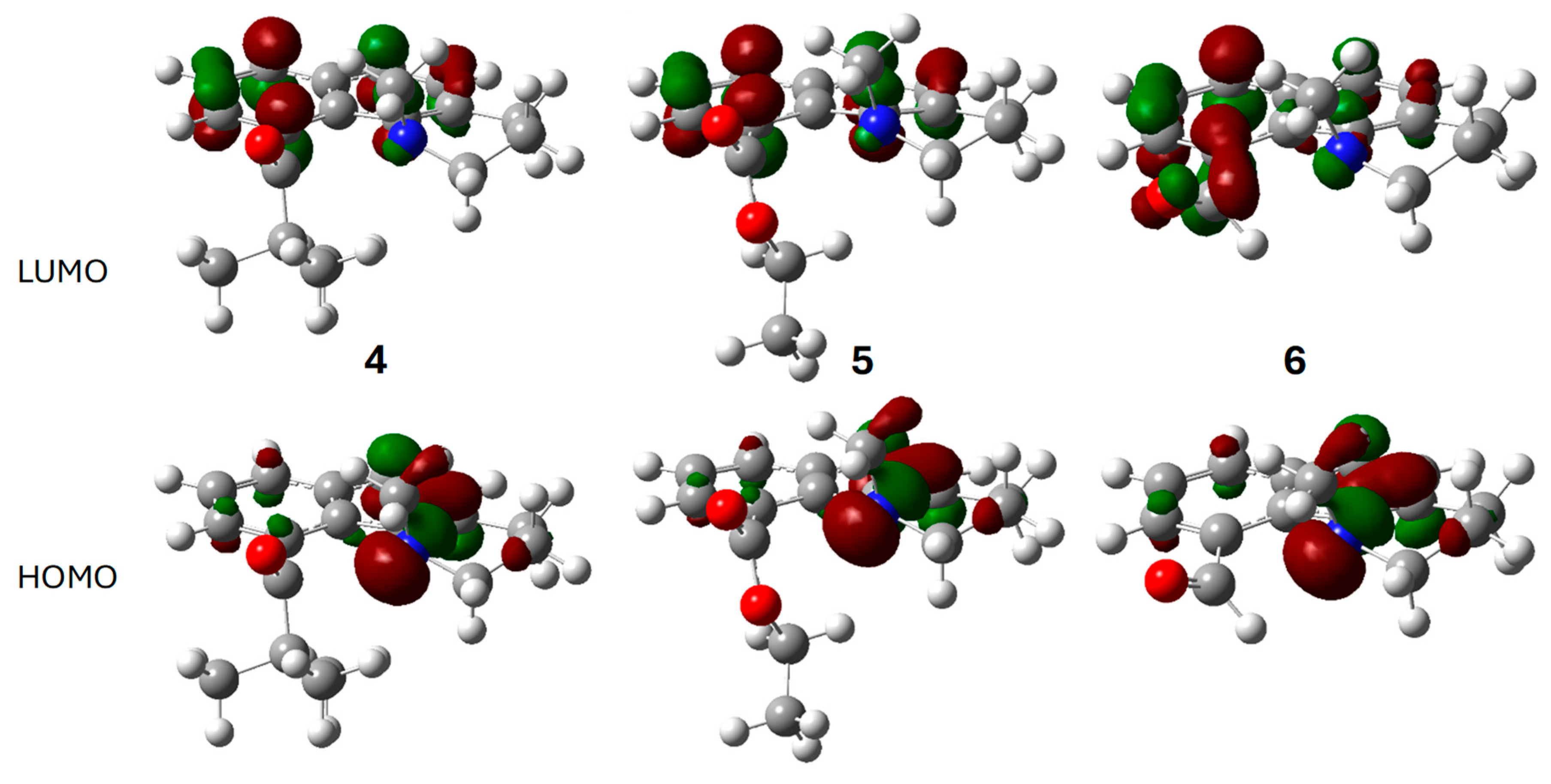

| 4 | 5 | 6 | ||||
|---|---|---|---|---|---|---|
| Φf | λem | Φf | λem | Φf | λem c | |
| Solvent a | ×10−2 | (nm) | ×10−2 | (nm) | ×10−2 | (nm) |
| Cyc | 2.6 | 528 | 7.6 | 531 | 0.5 | sh b |
| Tol | 2.4 | 557 | 6.0 | 561 | 1.9 | 564 |
| PhCl | 1.5 | 572 | 4.5 | 576 | 0.1 | sh |
| CH2Cl2 | 0.9 | 589 | 2.2 | 594 | 1.0 | 633 |
| EtOAc | 1.2 | 568 | 2.4 | 578 | 0.9 | 595 |
| Et2O | 0.4 | 566 | 1.8 | 572 | 0.3 | 571 |
| Me2CO | 0.5 | 585 | 1.2 | 596 | 0.1 | 620 |
| MeCN | 0.3 | 599 | 0.7 | 612 | 2.4 | 645 |
| DMSO | 0.4 | 599 | 0.5 | 619 | 1.0 | sh |
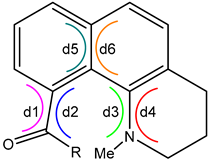 | d1 | d2 | twist | d3 | d4 | twist | d5 | d6 | twist | ||
| 4 | S1 | −35 | −46 | 40 | −27 | −21 | 24 | 13 | 18 | 16 | |
| S0 | −72 | −81 | 76 | −76 | −25 | 50 | 6 | 6 | 6 | ||
| 5 | S1 | −7 | 2 | 2 | −19 | −25 | 22 | 11 | 11 | 11 | |
| S0 | −71 | −77 | 74 | −77 | −25 | 51 | 6 | 6 | 6 | ||
| 6 | S1 | −9 | −5 | 7 | −29 | −23 | 26 | 12 | 13 | 12 | |
| S0 | −45 | −43 | 44 | −74 | −25 | 49 | 6 | 8 | 7 |
| 4 | 5 | 6 | |||||
|---|---|---|---|---|---|---|---|
| em | abs | em | abs | em | abs | ||
| calculated | λmax (nm) | 488 | 294 | 518 | 300 | 511 | 317 |
| kr × 107 (s−1) b | 2.7 | 2.3 | 2.2 | ||||
| μ (D) | 3.6 | 3.5 | 3.8 | 5.4 | 4.9 | 4.7 | |
| Stokes shift (cm−1) | 13,500 | 14,100 | 12,000 | ||||
| experimental | |||||||
| λmax (nm) | 557 | 315 | 561 | 323 | 564 | 333 | |
| Φ (em), log ε (abs) | 0.024 | 4.0 | 0.060 | 3.9 | 0.019 | 3.9 | |
| Stokes shift (cm−1) | 13,800 | 13,100 | 12,300 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abelt, C.; Day, I.; Zhao, J.; Pike, R. Fluorescence of Half-Twisted 10-Acyl-1-methyltetrahydrobenzoquinolines. Molecules 2024, 29, 3016. https://doi.org/10.3390/molecules29133016
Abelt C, Day I, Zhao J, Pike R. Fluorescence of Half-Twisted 10-Acyl-1-methyltetrahydrobenzoquinolines. Molecules. 2024; 29(13):3016. https://doi.org/10.3390/molecules29133016
Chicago/Turabian StyleAbelt, Christopher, Ian Day, Junkai Zhao, and Robert Pike. 2024. "Fluorescence of Half-Twisted 10-Acyl-1-methyltetrahydrobenzoquinolines" Molecules 29, no. 13: 3016. https://doi.org/10.3390/molecules29133016
APA StyleAbelt, C., Day, I., Zhao, J., & Pike, R. (2024). Fluorescence of Half-Twisted 10-Acyl-1-methyltetrahydrobenzoquinolines. Molecules, 29(13), 3016. https://doi.org/10.3390/molecules29133016









