Pentafluorophenyl Copper–Biarylsulfoxide Complexes: Synthesis and Photoreactivity
Abstract
1. Introduction
2. Results and Discussion
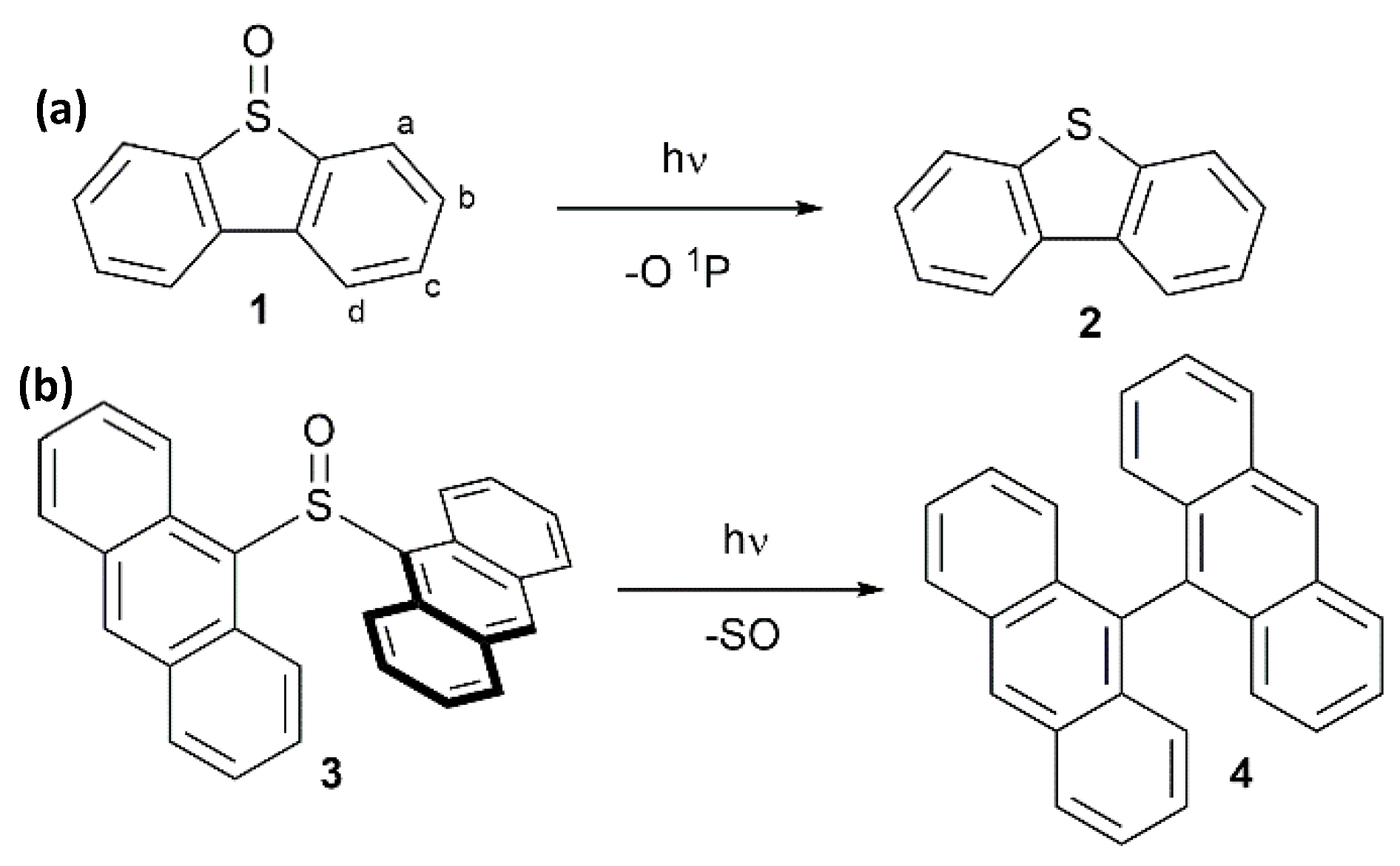
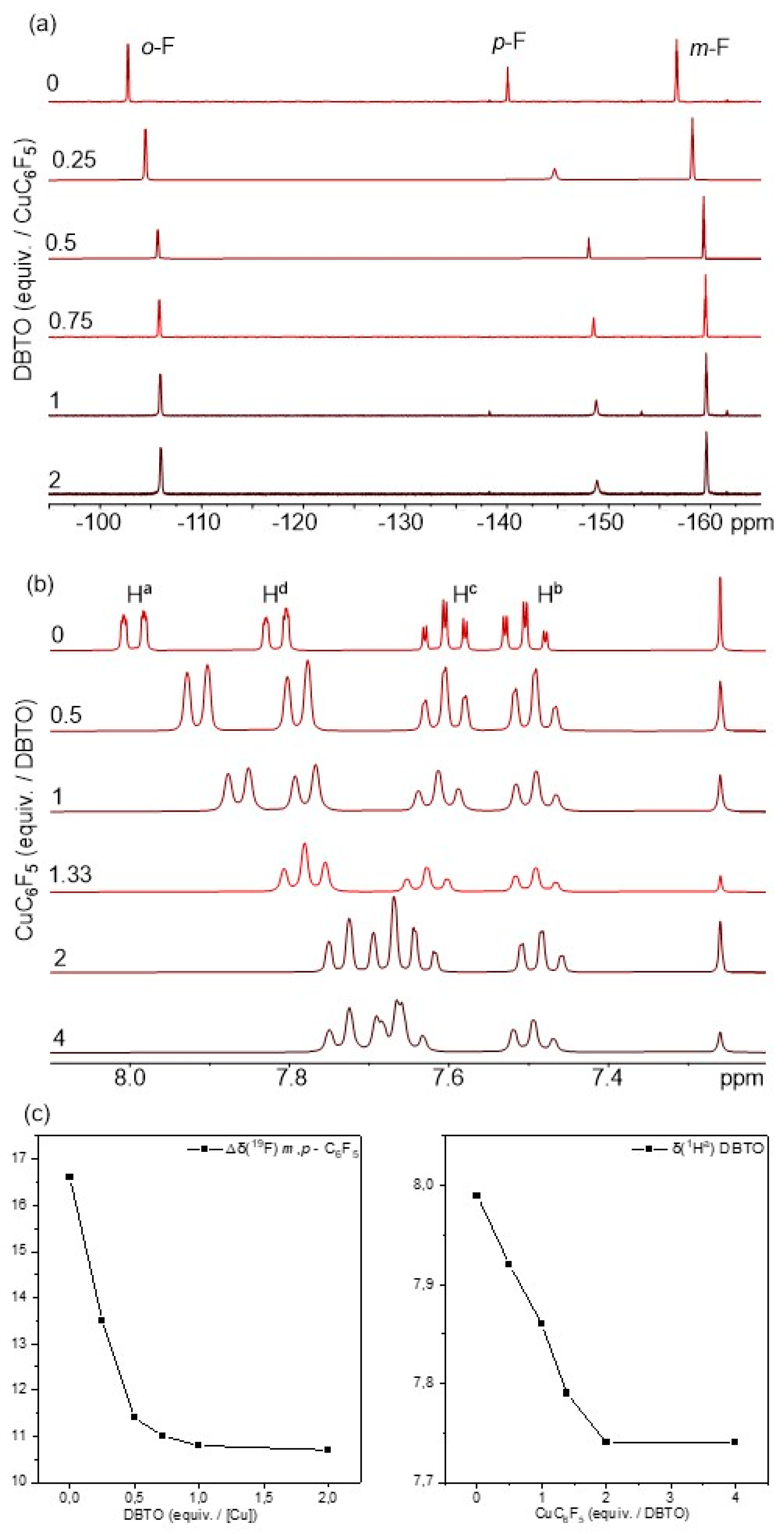
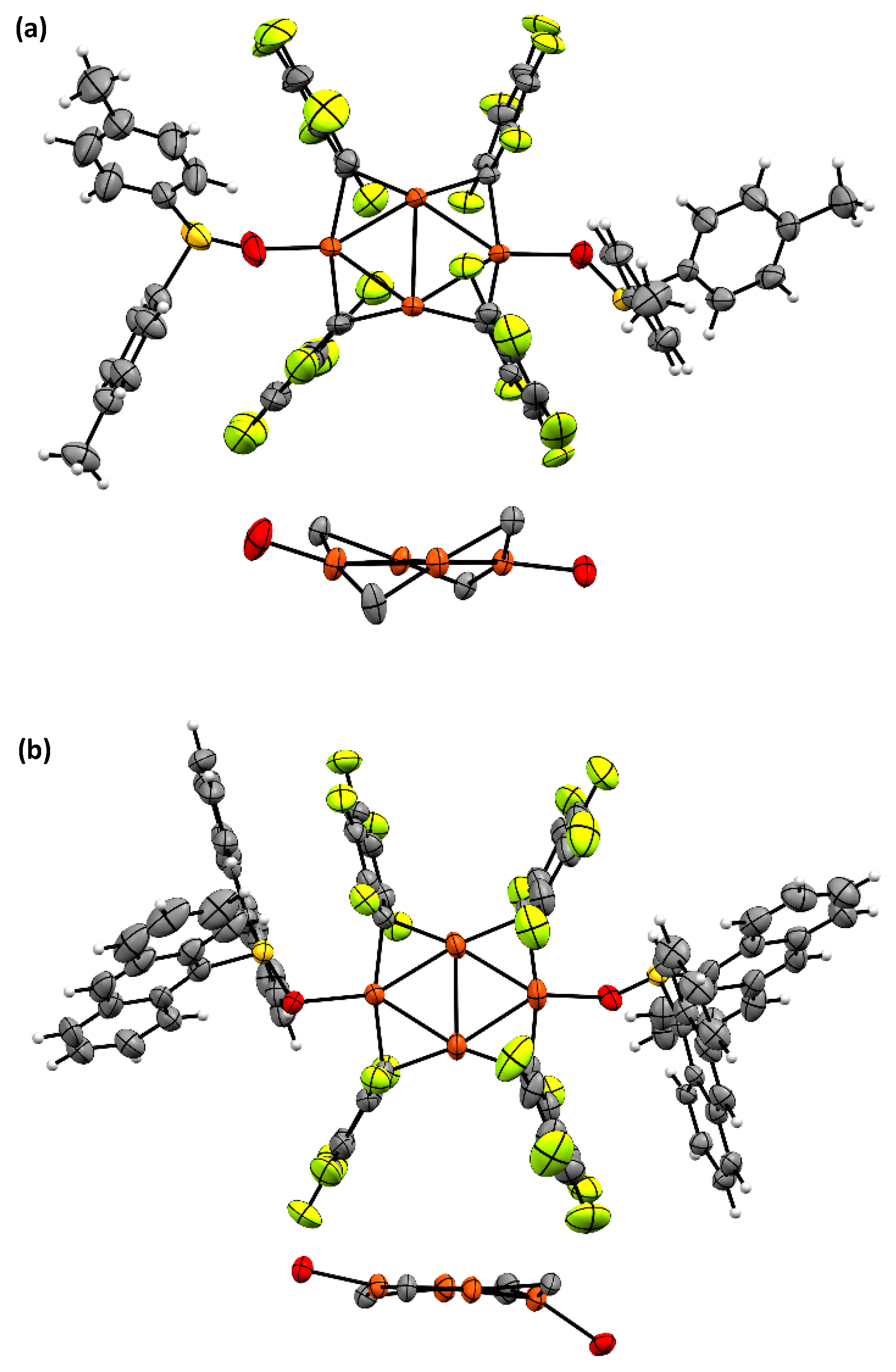
2.1. Synthesis and Photoreactivity of [Cu(C6F5)]4(DBTO)2
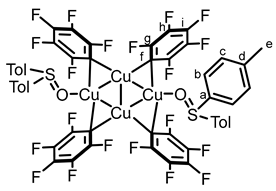
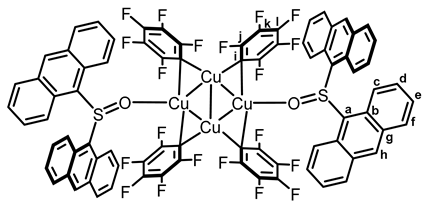
2.2. Synthesis and Photoreactivity of Complexes [Cu(C6F5)]4(Tol2SO)2 and [Cu(C6F5)]4(Anthra2SO)2
3. Materials and Methods
3.1. Reagents and Solvents
3.2. Analysis and Characterization
3.3. Synthesis of Copper–Biarylsulfoxide Complexes

3.4. Photochemistry
3.4.1. Irradiation of [Cu(C6F5)]4(DBTO)2 6
3.4.2. Irradiation of [Cu(C6F5)]4(Tol2SO)2 7
3.4.3. Irradiation of [Cu(C6F5)]4(Anthra2SO)2 8
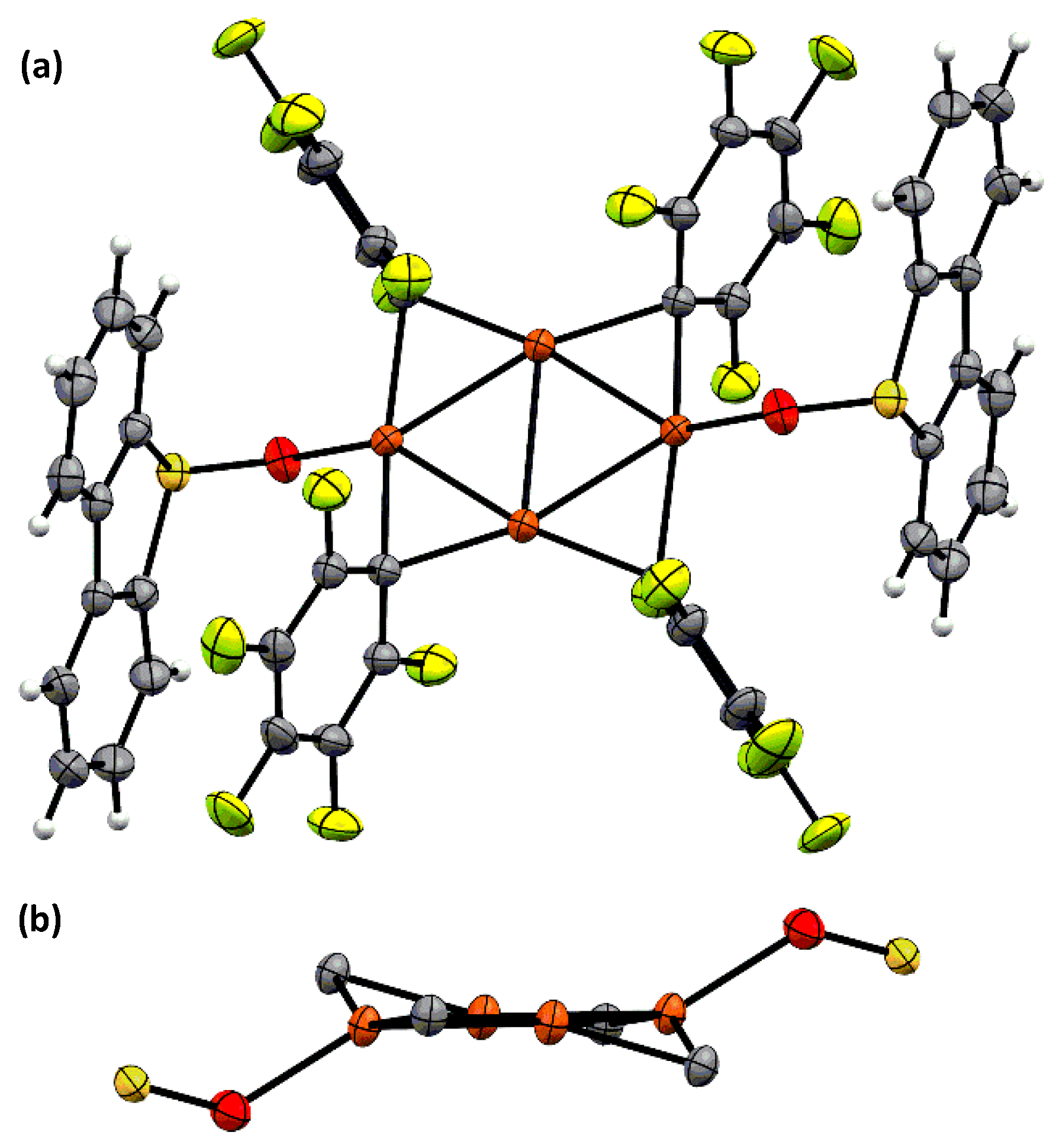
3.5. X-ray Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- David, N.A. The Pharmacology of Dimethyl Sulfoxide. Annu. Rev. Pharmacol. Toxicol. 1972, 12, 353–374. [Google Scholar] [CrossRef]
- Rubin, L.F. Toxicity of Dimethyl Sulfoxide, alone and in combination. Ann. N. Y. Acad. Sci. 1975, 243, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Brayton, C.F. Dimethyl Sulfoxide (DMSO): A Review. Cornell Vet. 1986, 76, 61–90. [Google Scholar] [PubMed]
- Dimethylsulfoxide. In Meyler’s Side Effects of Drugs, 16th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 992–993. [CrossRef]
- Gurria, G.M.; Posner, G.H. Photochemical Deoxygenation of Aryl Sulfoxides. J. Org. Chem. 1973, 38, 2419–2420. [Google Scholar] [CrossRef]
- Gregory, D.D.; Wan, Z.; Jenks, W.S. Photodeoxygenation of Dibenzothiophene Sulfoxide: Evidence for a Unimolecular S-O Cleavage Mechanism. J. Am. Chem. Soc. 1997, 119, 94–102. [Google Scholar] [CrossRef]
- Omlid, S.M.; Dergunov, S.A.; Isor, A.; Sulkowski, K.L.; Petroff, J.T.; Pinkhassik, E.; McCulla, R.D. Evidence for Diffusing Atomic Oxygen Uncovered by Separating Reactants with a Semi-Permeable Nanocapsule Barrier. Chem. Commun. 2019, 55, 1706–1709. [Google Scholar] [CrossRef]
- Christensen, P.R.; Patrick, B.O.; Caron, É.; Wolf, M.O. Oxidation-State-Dependent Photochemistry of Sulfur-Bridged Anthracenes. Angew. Chem. Int. Ed. 2013, 52, 12946–12950. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, M.; Carugo, O. Structure and Bonding in Metal Sulfoxide Complexes. Coord. Chem. Rev. 1996, 153, 83–154. [Google Scholar] [CrossRef]
- Calligaris, M. Structure and Bonding in Metal Sulfoxide Complexes: An Update. Coord. Chem. Rev. 2004, 248, 351–375. [Google Scholar] [CrossRef]
- Mellah, M.; Voituriez, A.; Schulz, E. Chiral Sulfur Ligands for Asymmetric Catalysis. Chem. Rev. 2007, 107, 5133–5209. [Google Scholar] [CrossRef]
- Sipos, G.; Drinkel, E.E.; Dorta, R. The Emergence of Sulfoxides as Efficient Ligands in Transition Metal Catalysis. Chem. Soc. Rev. 2015, 44, 3834–3860. [Google Scholar] [CrossRef]
- Jastrzebski, J.T.B.H.; van Koten, G. Structures and Reactivities of Organocopper Compounds. In Modern Organocopper Chemistry; Wiley: Hoboken, NJ, USA, 2002; Volume 34, pp. 1–44. [Google Scholar] [CrossRef]
- Liu, L.; Chen, H.; Yang, Z.; Wei, J.; Xi, Z. C,C- and C,N-Chelated Organocopper Compounds. Molecules 2021, 26, 5806. [Google Scholar] [CrossRef] [PubMed]
- Guss, J.M.; Mason, R.; Søtofte, I.; Van Koten, G.; Noltes, J.G. A Tetranuclear Cluster Complex of Copper(I) with Bridging Aryl Ligands: The Crystal Structure of (4-Methyl-2-Cupriobenzyl)Dimethylamine. J. Chem. Soc. Chem. Commun. 1972, 8, 446–447. [Google Scholar] [CrossRef]
- Liu, L.; Geng, W.; Yang, Q.; Zhang, W.X.; Xi, Z. Well-Defined Butadienyl Organocopper(I) Aggregates from Zirconacyclopentadienes and CuCl: Synthesis and Structural Characterization. Organometallics 2015, 34, 4198–4201. [Google Scholar] [CrossRef]
- Doshi, A.; Sundararaman, A.; Venkatasubbaiah, K.; Zakharov, L.N.; Rheingold, A.L.; Myahkostupov, M.; Piotrowiak, P.; Jäkle, F. Pentafluorophenyl Copper-Pyridine Complexes: Synthesis, Supramolecular Structures via Cuprophilic and π-Stacking Interactions, and Solid-State Luminescence. Organometallics 2012, 31, 1546–1558. [Google Scholar] [CrossRef]
- Gambarotta, S.; Strologo, S.; Floriani, C.; Chlesl-Vllla, A.; Guastini, C. Synthesis and Structure of a Mononuclear Copper(I) Complex Containing the Copper(I) σ-Phenyl Functionality. Organometallics 1984, 3, 1444–1445. [Google Scholar] [CrossRef]
- Cairncross, A.; Omura, H.; Sheppard, W.A. Organocopper Cluster Compounds. II. Pentafluorophenylcopper and o-(Trifluoromethyl)Phenylcopper Tetramers. J. Am. Chem. Soc. 1971, 93, 248–249. [Google Scholar] [CrossRef]
- Jäkle, F. Pentafluorophenyl Copper: Aggregation and Complexation Phenomena, Photoluminescence Properties, and Applications as Reagent in Organometallic Synthesis. Dalton Trans. 2007, 27, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Sundararaman, A.; Lalancette, R.A.; Zakharov, L.N.; Rheingold, A.L.; Jäkle, F. Structural Diversity of Pentafluorophenylcopper Complexes. First Evidence of π-Coordination of Unsupported Arenes to Organocopper Aggregates. Organometallics 2003, 22, 3526–3532. [Google Scholar] [CrossRef]
- Akihiko, I.; Norio, N.; Yasunaka, T. 5,9b-dihydro-5,9b-banzonaphtho[1,2-b] Chalcogenophene Derivative, and Production Method of the Same. JP2012041295A, 1 March 2013. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Nevado, C. Cross-coupling of arene–gold(III) complexes. Tetrahedron 2013, 69, 5751–5757. [Google Scholar] [CrossRef]
- Meyer, B.K.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Kramm, B.; Klar, P.J.; Sander, T.; Reindl, C.; Heiliger, C.; et al. The Physics of Copper Oxide (Cu2O). Semicond. Semimet. 2013, 88, 201–226. [Google Scholar] [CrossRef]
- Zoolfakar, A.S.; Rani, R.A.; Morfa, A.J.; O’Mullane, A.P.; Kalantar-Zadeh, K. Nanostructured Copper Oxide Semiconductors: A Perspective on Materials, Synthesis Methods and Applications. J. Mater. Chem. C 2014, 2, 5247–5270. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magné, V.; Lenk, R.; Mallet-Ladeira, S.; Maerten, E.; Madec, D. Pentafluorophenyl Copper–Biarylsulfoxide Complexes: Synthesis and Photoreactivity. Molecules 2024, 29, 3332. https://doi.org/10.3390/molecules29143332
Magné V, Lenk R, Mallet-Ladeira S, Maerten E, Madec D. Pentafluorophenyl Copper–Biarylsulfoxide Complexes: Synthesis and Photoreactivity. Molecules. 2024; 29(14):3332. https://doi.org/10.3390/molecules29143332
Chicago/Turabian StyleMagné, Valentin, Romaric Lenk, Sonia Mallet-Ladeira, Eddy Maerten, and David Madec. 2024. "Pentafluorophenyl Copper–Biarylsulfoxide Complexes: Synthesis and Photoreactivity" Molecules 29, no. 14: 3332. https://doi.org/10.3390/molecules29143332
APA StyleMagné, V., Lenk, R., Mallet-Ladeira, S., Maerten, E., & Madec, D. (2024). Pentafluorophenyl Copper–Biarylsulfoxide Complexes: Synthesis and Photoreactivity. Molecules, 29(14), 3332. https://doi.org/10.3390/molecules29143332






