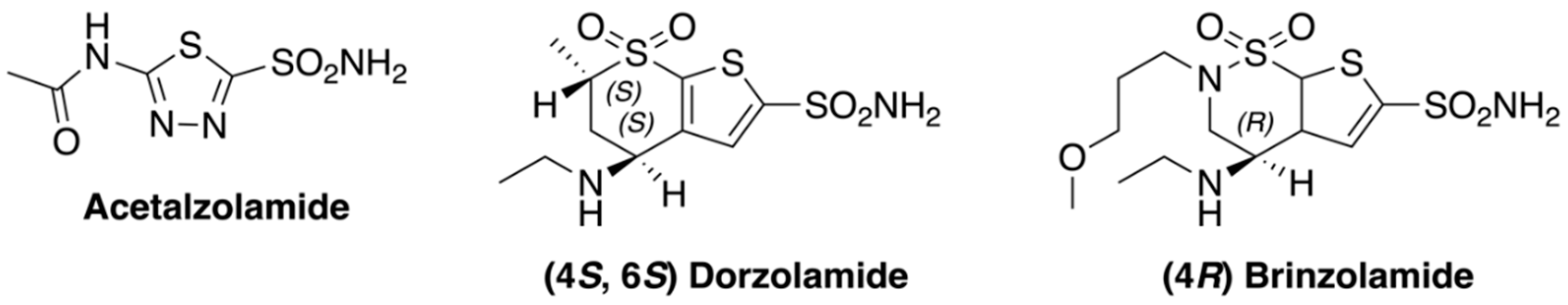
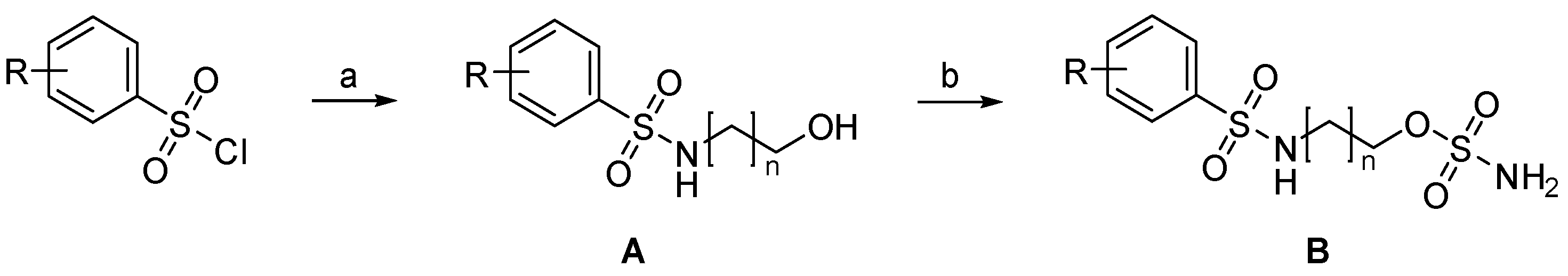
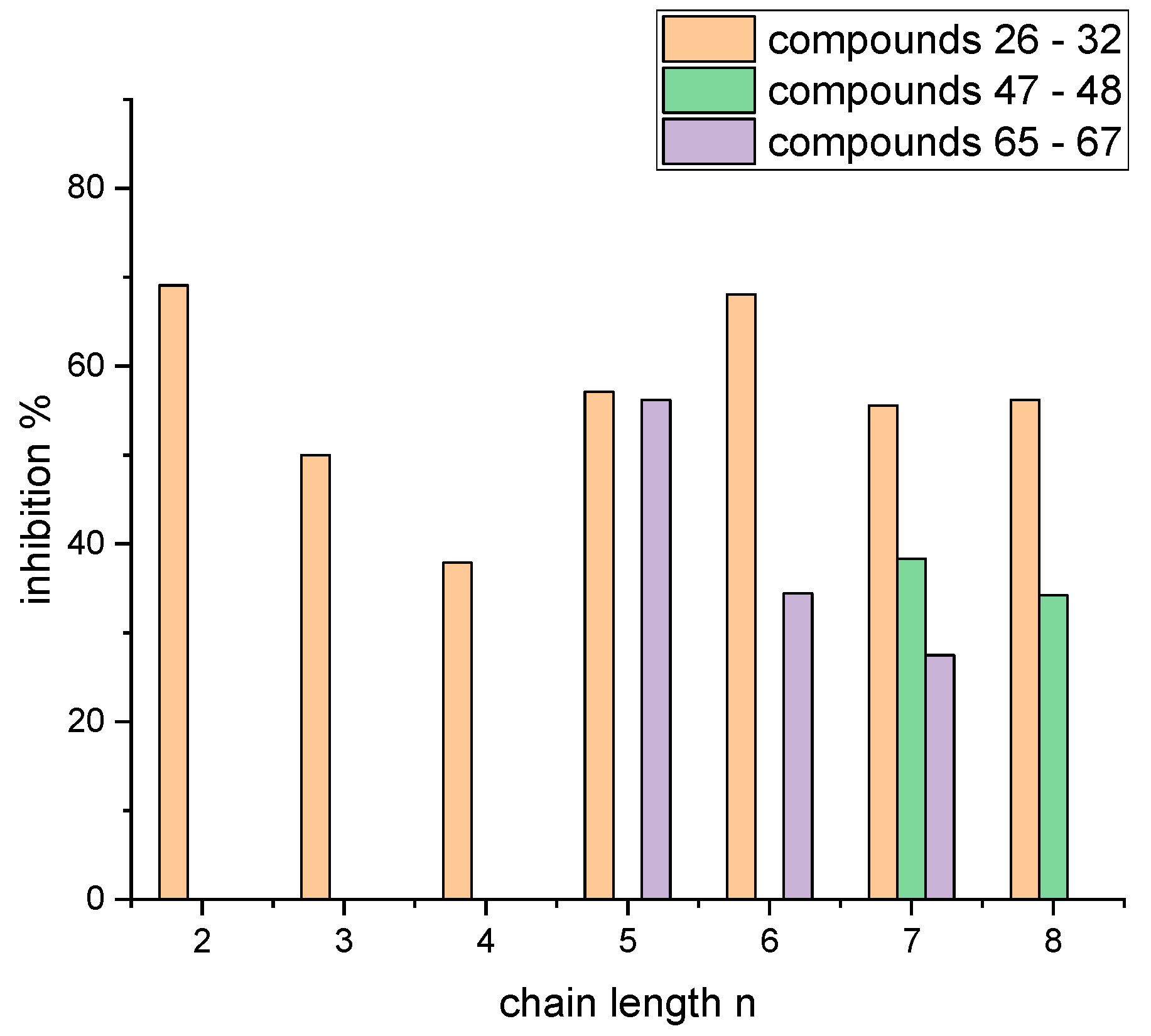
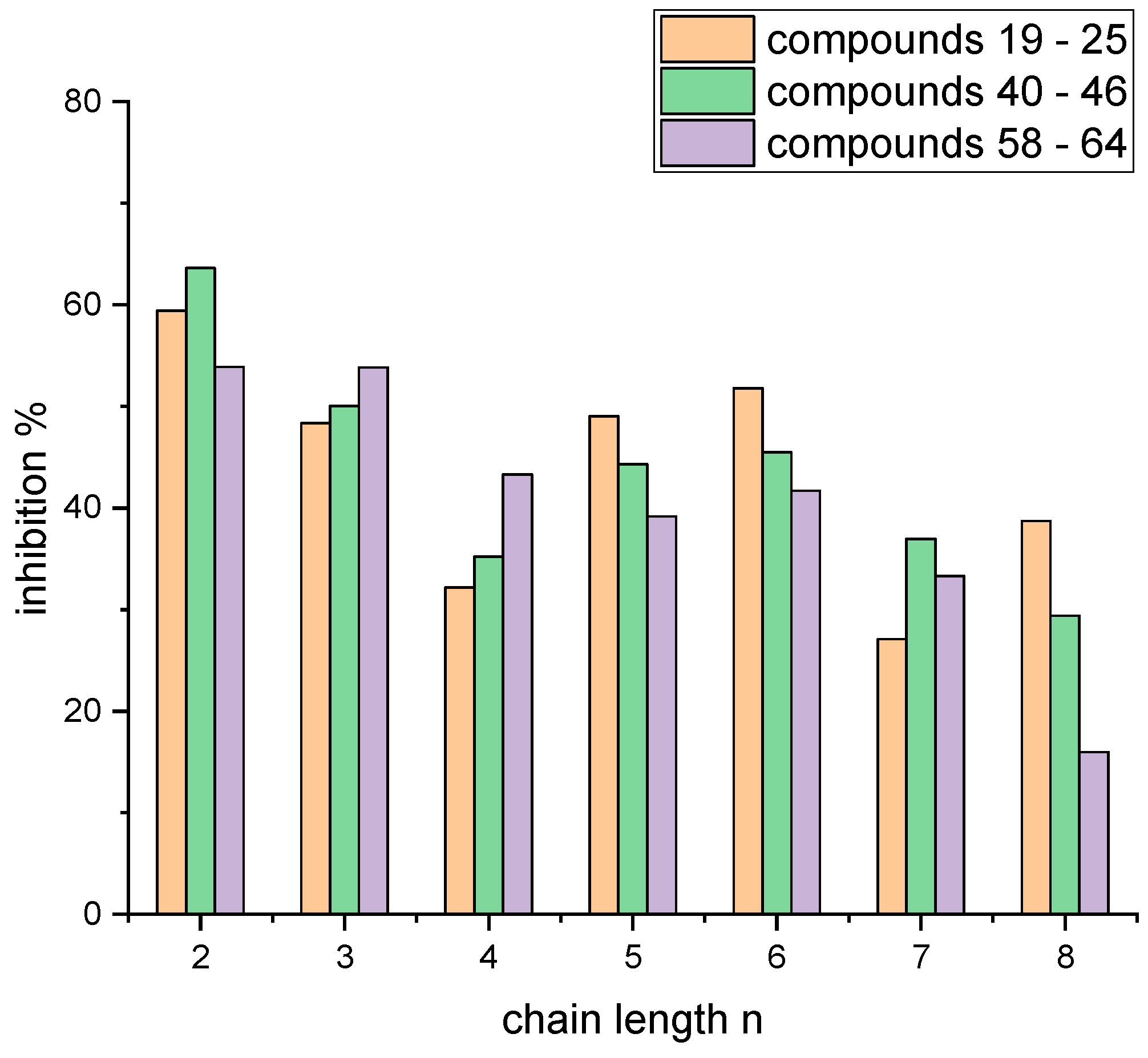
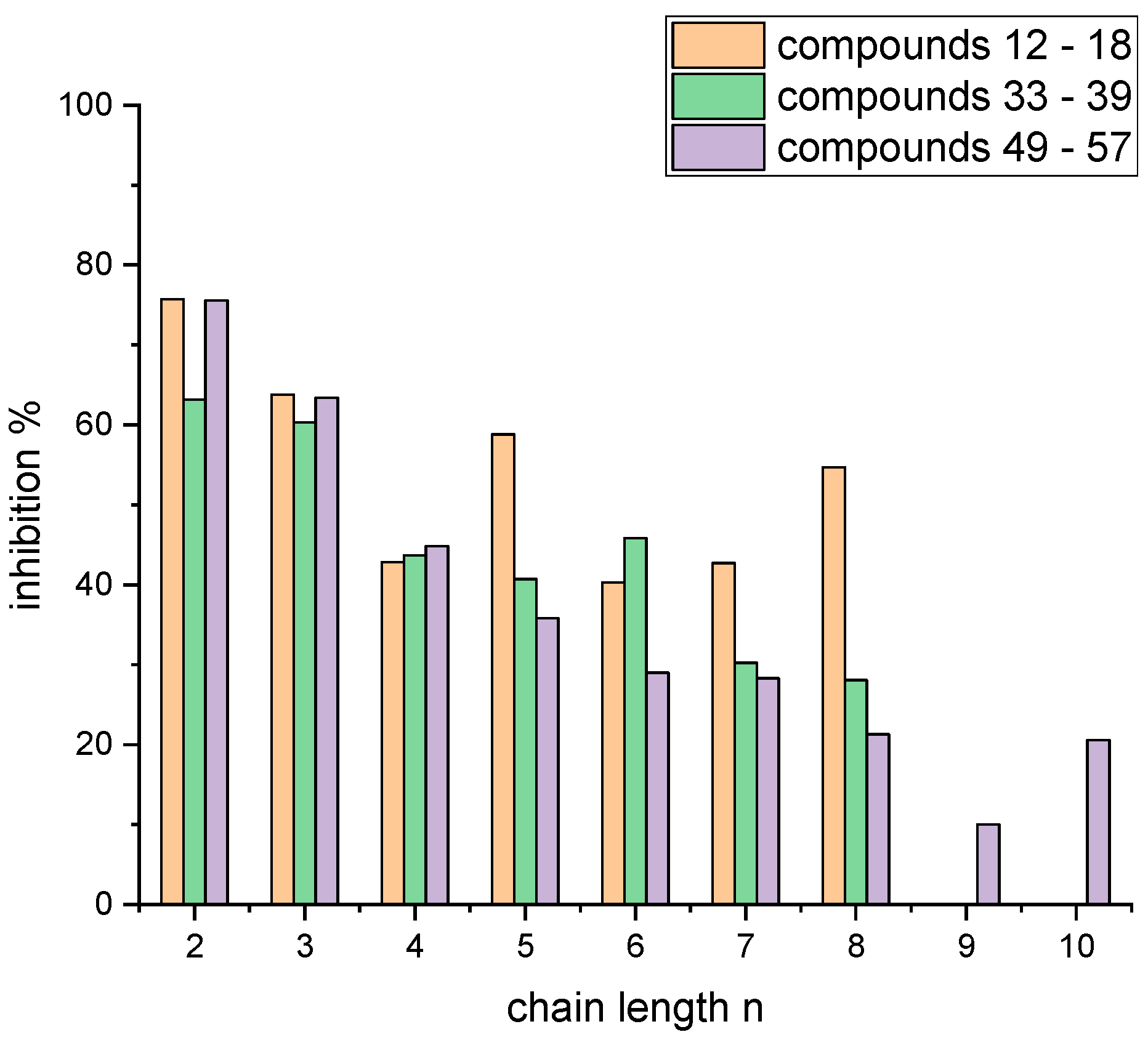
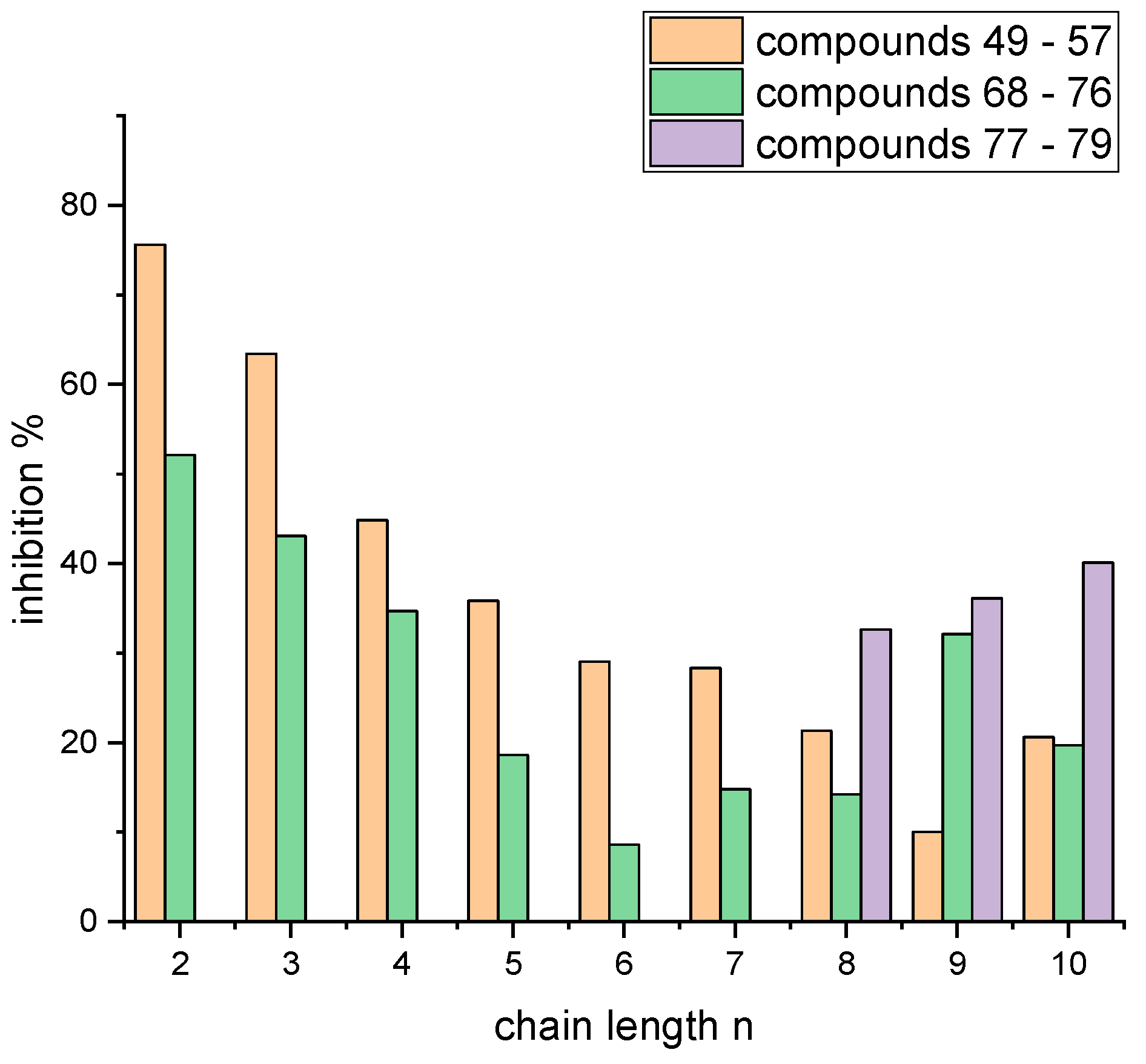
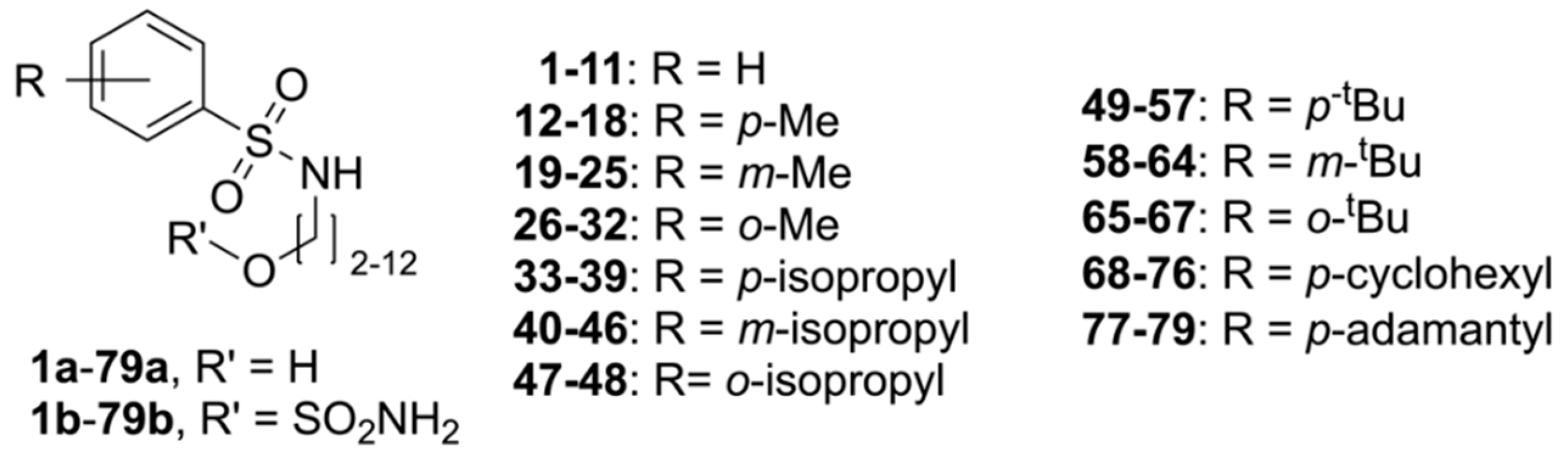4.2. Syntheses
4.2.1. Preparation of 1a–79a (General Procedure A, GPA)
Reaction of the amino-alcohol (1.5 equiv.) in dry CH2Cl2 (15 mL), with dry NEt3 (2 equiv.) and the sulfonyl chloride (1 equiv.) at 22 °C for 3 hours followed by evaporation of the solvents and chromatography gave 1a–79a. For long-chain amino-alcohols (n = 8–12), the solvent composition was modified, and a 1:1 mixture of CH2Cl2 and acetonitrile was used instead.
4.2.2. Preparation of 1b–79b (General Procedure B, GPB)
Reaction of 1a–79a (1 equiv.) in dry CH2Cl2 (6 mL), NEt3 (3 equiv.) with sulfamoyl chloride (3 equiv.) at 22 °C until completion of the reaction followed by evaporation and column chromatography gave 1b–79b.
4.2.3. N-(2-Hydroxyethyl)benzene Sulfonamide (1a) [59724-42-4]
Applying GPA: from benzenesulfonyl chloride (500 mg, 2.83 mmol) and 2-amino-ethanol (260 mg, 4.25 mmol):
1a [
73,
74,
75,
76,
77,
78,
79,
80] (542 mg, 94%); white solid; R
f = 0.07 (petrolether/EtOAc, 2:3); m.p. = 78–79 °C (lit.: [
73,
74] 79–80 °C); spectral data as previously reported [
72].
4.2.4. 2-(Phenylsulfonamido]ethyl Sulfamate (1b)
Applying GPB: from
1a (175 mg, 0.87 mmol):
1b (202 mg, 82%); white solid; R
f = 0.38 (CHCl
3/EtOAc, 2:3); m.p. = 71–73 °C; spectral data as previously reported [
72].
4.2.5. N-(3-Hydroxypropyl)benzene Sulfonamide (2a) [3351-94-8]
Applying GPA: from benzenesulfonyl chloride (500 mg, 2.83 mmol) and 3-amino-propanol (319 mg, 4.25 mmol):
2a [
81,
82,
83] (594 mg, 97%); oil; R
f = 0.09 (petrolether/EtOAc, 2:3); UV–Vis: 221 nm (4.02); IR: ν = 3500
w, 3276
m, 2943
w, 2881
w, 1478
w, 1447
m, 1309
s, 1154
vs, 1092
s, 1070
s, 1007
w, 959
w, 871
w, 754
m, 720
m, 689
s, 586
s, 469
w cm
−1;
1H NMR:δ = 7.81–7.76 (
m, 2H, 2-H, 2′-H), 7.66–7.56 (
m, 3H, 3-H, 3′-H, 4-H), 7.52 (
s, 1H, NH), 4.40 (
s, 1H, OH), 3.39–3.32 (
m, 2H, 7-H), 2.79
(t,
J = 7.3 Hz, 2H, 5-H), 1.57–1.46 (
m, 2H, 6-H);
13C NMR:δ = 140.5 (C-1), 132.3 (C-4), 129.2 (C-3), 126.4 (C-2), 58.0 (C-7), 40.0 (C-5), 32.3 (C-6) ppm; MS:
m/
z = 238.1 (100%, [M + Na]
+); anal. calcd. for C
9H
13NSO
3 (215.27): C 50.22, H 6.09, N 6.51; found: C 49.97, H 6.34, N 6.38.
4.2.6. 3-(Phenylsulfonamido]propyl Sulfamate (2b)
Applying GPB: from 2a (200 mg, 0.93 mmol): 2b (241 mg, 88%); white solid; Rf = 0.40 (CHCl3/EtOAc, 2:3); m.p. = 64–66 °C; UV–Vis: 221 nm (3.78); IR: ν = 3344m, 3264m, 3255m, 1449w, 1373s, 1353m, 1311s, 1177s, 1155s, 1113w, 1090m, 1074w, 1052m, 1003m, 950s, 917m, 896m, 886m, 836s, 754m, 727s, 688s, 673m, 627m, 599m, 586s, 562s, 546vs, 503m, 490m, 476w, 441w cm−1; 1H NMR:δ = 7.82–7.77 (m, 2H, 2-H, 2′-H), 7.72–7.58 (m, 4H, 3-H, 3′-H, 4-H, NH), 7.41 (s, 2H, NH2), 4.02 (t, J = 6.3 Hz, 2H, 7-H), 2.85–2.78 (m, 2H, 5-H), 1.76 (dt, J = 13.2, 6.5 Hz, 2H, 6-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 140.2 (C-1), 132.4 (C-4), 129.3 (C-3), 126.4 (C-2), 66.5 (C-7), 39.2 (C-5), 28.7 (C-6) ppm; MS: m/z = 316.9 (100%, [M + Na]+); anal. calcd. for C9H14N2S2O5 (294.34): 36.73, H 4.79, N 9.52; found: C 36.50, H 4.98, N 9.36.
4.2.7. N-(4-Hydroxybutyl)benzene Sulfonamide (3a) [842146-77-4]
Applying GPA: from benzenesulfonyl chloride (500 mg, 2.83 mmol) and 4-aminobutanol (378 mg, 4.25 mmol): 3a (621 mg, 96%); oil; Rf = 0.12 (petrolether/EtOAc, 2:3); UV–Vis: 221 nm (4.07); IR: ν = 3506br, 3278br, 2940w, 2872w, 1478w, 1447m, 1318s, 1309s, 1152vs, 1092s, 1055m, 1027w, 999w, 952w, 909w, 867w, 754m, 719m, 688s, 583s, 567s, 483w cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 7.82–7.77 (m, 2H, 2-H, 2′-H), 7.65–7.53 (m, 4H, 3-H, 3′-H, 4-H, NH), 4.37 (t, J = 5.1 Hz, 1H, OH), 3.35–3.30 (m, 2H, 8-H), 2.74 (q, J = 6.0 Hz, 2H, 5-H), 1.44–1.33 (m, 4H, 6-H, 7-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 140.6 (C-1), 132.3 (C-4), 129.2 (C-3), 126.4 (C-2), 60.2 (C-8), 42.6 (C-5), 29.5 (C-7), 25.8 (C-6) ppm; MS: m/z = 243.2 (90%, [M-H]−); anal. calcd. for C10H15NSO3 (229.29): C 50.22, H 6.09, N 6.51; found: C 49.97, H 6.31, N 6.37.
4.2.8. 4-(Phenylsulfonamido]butyl Sulfamate (3b)
Applying GPB: from 3a (200 mg, 0.87 mmol): 3b (215 mg, 80%); oil; Rf = 0.43 (CHCl3/EtOAc, 2:3); UV–Vis: 221 nm (3.95); IR: ν = 3345m, 3258m, 3257m, 1442w, 1370s, 1350m, 1316s, 1187s, 1157s, 1110w, 1093m, 1072w, 1056m, 1001m, 956s, 914m, 894m, 889m, 840s, 751m, 729s, 685s, 670m, 621m, 595m, 582s, 565s, 544vs, 501m, 496m, 470w, 440w cm−1; 1H NMR:δ = 7.83–7.76 (m, 2H, 2-H, 2′-H), 7.68–7.56 (m, 4H, 3-H, 3′-H, 4-H, NH), 7.38 (s, 2H, NH2), 3.96 (t, J = 6.3 Hz, 2H, 8-H), 2.76 (q, J = 6.6 Hz, 2H, 5-H), 1.66–1.56 (m, 2H, 7-H), 1.49–1.40 (m, 2H, 6-H) ppm; 13C NMR:δ = 140.5 (C-1), 132.4 (C-4), 129.2 (C-3), 126.4 (C-2), 68.6 (C-8), 42.0 (C-5), 25.6 (C-7), 24.7 (C-6) ppm; MS: m/z = 331.1 (100%, [M + Na]+); anal. calcd. for C10H16N2S2O5 (308.37): C 38.95, H 5.23, N 9.08; found: C 38.70, H 5.51, N 8.84.
4.2.9. N-(5-Hydroxypentyl)benzene Sulfonamide (4a) [191529-31-4]
Applying GPA: from benzenesulfonyl chloride (500 mg, 2.83 mmol) and 5-amino-pentanol (438 mg, 4.25 mmol):
4a [
84,
85] (647 mg, 94%); oil; R
f = 0.12 (petrolether/EtOAc, 2:3); UV–Vis: 221 nm (3.91); IR: ν = 3503
w, 3281
m, 2937
w, 2865
w, 1478
w, 1447
m, 1320
s, 1309
s, 1153
vs, 1092
s, 1071
s, 1040
w, 999
w, 880
w, 754
s, 719
m, 689
s, 583
s, 567
s, 464
w cm
−1;
1H NMR:δ = 7.82–7.76 (
m, 2H, 2-H, 2′-H), 7.66–7.56 (
m, 3H, 3-H, 3′-H, 4-H), 7.54 (
t,
J = 5.8 Hz, 1H, NH), 4.30 (
t,
J = 5.1 Hz, 1H, OH), 3.36–3.28 (
m, 2H, 9-H), 2.71 (
td,
J = 7.0, 5.7 Hz, 2H, 5-H), 1.40–1.27 (
m, 4H, 6-H, 8-H), 1.27–1.16 (
m, 2H, 7-H) ppm;
13C NMR:δ = 140.6 (C-1), 132.2 (C-4), 129.1 (C-3), 126.4 (C-2), 60.5 (C-9), 42.6 (C-5), 32.0 (C-8), 28.8 (C-6), 22.6 (C-7) ppm; MS:
m/
z = 243.2 (90%, [M-H]
−); anal. calcd. for C
11H
17NSO
3 (243.32): C 54.30, H 7.04, N 5.76; found: C 54.11, H 7.23, N 5.43.
4.2.10. 5-(Phenylsulfonamido]pentyl Sulfamate (4b)
Applying GPB: from 4a (200 mg, 0.82 mmol): 4b (250 mg, 94%); oil; Rf = 0.46 (CHCl3/EtOAc, 2:3); UV–Vis: 221 nm (3.91); IR: ν = 3344w, 3307m, 3232w, 2953w, 2933w, 2854w, 1555w, 1469w, 1442w, 1421w, 1399vw, 1363s, 1311s, 1295w, 1276w, 1172s, 1153vs, 1092m, 1078m, 1067w, 1043w, 1025w, 993m, 945m, 922s, 911s, 808m, 766m, 750m, 722s, 693s, 599s, 570s, 553vs, 502m, 482m, 435w cm−1; 1H NMR:δ = 7.82–7.76 (m, 2H, 2-H, 2′-H), 7.67–7.55 (m, 4H, 3-H, 3′-H, 4-H, NH), 7.39–7.35 (m, 2H, NH2), 3.96 (t, J = 6.5 Hz, 2H, 9-H), 2.73 (td, J = 6.7, 5.8 Hz, 2H, 5-H), 1.55 (p, J = 6.7 Hz, 2H, 6-H), 1.44–1.34 (m, 2H, 8-H), 1.33–1.23 (m, 2H, 7-H) ppm; 13C NMR:δ = 140.6 (C-1), 132.2 (C-4), 129.1 (C-3), 126.4 (C-2), 60.5 (C-9), 42.6 (C-5), 32.0 (C-8), 28.8 (C-6), 22.6 (C-7) ppm; MS: m/z = 345.1 (100%, [M + Na]+); anal. calcd. for C11H18N2S2O5 (322.39): C 40.98, H 5.63, N 8.69; found: C 40.72, H 5.87, N 8.57.
4.2.11. N-(6-Hydroxyhexyl)benzene Sulfonamide (5a) [195003-60-2]
Applying GPA: from benzenesulfonyl chloride (500 mg, 2.83 mmol) and 6-amino-hexanol (438 g, 4.25 mmol): 5a (701 mg, 96%); white solid; Rf = 0.14 (petrolether/EtOAc, 2:3); m.p. = 40–42 °C; UV–Vis: 221 nm (3.97); IR: ν = 3506br, 3260br, 2934w, 2860w, 1447m, 1320m, 1309m, 1153vs, 1092m, 1071m, 1025w, 1000w, 754m, 719m, 689s, 584s, 568s, 475w cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 7.81–7.76 (m, 2H, 2-H, 2′-H), 7.65–7.56 (m, 3H, 3-H, 3′-H, 4-H), 7.54 (t, J = 5.8 Hz, 1H, NH), 4.30 (t, J = 5.2 Hz, 1H, OH), 3.36–3.31 (m, 2H, 10-H), 2.72 (td, J = 7.0, 5.7 Hz, 2H, 5-H), 1.38–1.30 (m, 4H, 6-H, 9-H), 1.38–1.14 (m, 4H, 7-H, 8-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 140.6 (C-1), 132.2 (C-4), 129.1 (C-3), 126.4 (C-2), 60.6 (C-10), 42.5 (C-5), 32.3 (C-9), 29.0 (C-6), 25.9 (C-8), 25.0 (C-7) ppm; MS: m/z = 280 (100%, [M + Na]+); anal. calcd. for C12H19NSO3 (257.35): C 56.01, H 7.44, N 5.44; found: C 55.76, H 7.69, N 5.21.
4.2.12. 6-(Phenylsulfonamido]hexyl Sulfamate (5b)
Applying GPB: from 5b (230 mg, 0.89 mmol): 5b (202 mg, 67%); white solid; Rf = 0.51 (CHCl3/EtOAc, 2:3); m.p. = 64–66 °C; UV–Vis: 221 nm (3.98); IR: ν = 3346w, 3306m, 3238w, 2955w, 2931w, 2858w, 1554w, 1465w, 1447w, 1421w, 1390vw, 1366s, 1314s, 1291w, 1279w, 1171s, 1155vs, 1095m, 1074m, 1063w, 1046w, 1025w, 993m, 946m, 926s, 912s, 808m, 761m, 751m, 720s, 690s, 598s, 571s, 550vs, 507m, 481m, 436w cm−1; 1H NMR:δ = 7.81–7.77 (m, 2H, 2-H, 2′-H), 7.66–7.53 (m, 4H, 3-H, 3′-H, 4-H, NH), 7.37 (s, 2H, NH2), 3.97 (t, J = 6.5 Hz, 2H, 10-H), 2.77–2.69 (m, 2H, 5-H), 1.56 (p, J = 6.7 Hz, 2H, 9-H), 1.35 (p, J = 6.9 Hz, 2H, 6-H), 1.30–1.17 (m, 4H, 7-H, 8-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 140.6 (C-1), 132.3 (C-4), 129.2 (C-3), 126.4 (C-2), 68.9 (C-10), 42.4 (C-5), 28.8 (C-9), 28.2 (C-6), 25.5 (C-8), 24.6 (C-7) ppm; MS: m/z = 359.3 (100%, [M + Na]+); anal. calcd. for C12H20N2S2O5 (336.42): C 42.84, H 5.99, N 8.33; found: C 42.57, H 6.17, N 8.04.
4.2.13. N-(7-Hydroxyheptyl)benzene Sulfonamide (6a) [2773002-10-9]
Applying GPA: from benzenesulfonyl chloride (500 mg, 2.83 mmol) and 7-amino-heptanol (558 mg, 4.25 mmol): 6a (740 mg, 96%); oil; Rf = 0.19 (petrolether/EtOAc, 2:3); UV–Vis: 221 nm (3.97); IR: ν = 3504br, 3282br, 2931m, 2858w, 1447m, 1320m, 1310m, 1153vs, 1093s, 1071m, 1058w, 1025m, 1000w, 755m, 719m, 689s, 584s, 568s, 469w cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 7.81–7.77 (m, 2H, 2-H, 2′-H), 7.65–7.56 (m, 3H, 3-H, 3′-H, 4-H), 7.54 (t, J = 5.7 Hz, 1H, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.37–3.32 (m, 3H, 11-H), 2.72 (q, J = 6.7 Hz, 2H, 5-H), 1.40–1.30 (m, 4H, 6-H, 10-H), 1.23–1.11 (m, 6H, 7-H, 8-H, 9-H) ppm; 13C NMR:δ = 140.7 (C-1), 132.2 (C-4), 129.1 (C-3), 126.4 (C-2), 60.7 (C-11), 42.5 (C-5), 32.4 (C-10), 28.9 (C-7), 28.4 (C-6), 26.0 (C-9), 25.3 (C-8) ppm; MS: m/z = 294.2 (100%, [M + Na]+); anal. calcd. for C13H21NSO3 (271.38): C 57.54, H 7.80, N 5.16; found: C 57.26, H 8.03, N 4.97.
4.2.14. 7-(Phenylsulfonamido]heptyl Sulfamate (6b)
Applying GPB: from 6a (300 mg, 1.11 mmol): 6b (315 mg, 81%); white solid; Rf = 0.60 (CHCl3/EtOAc, 2:3); m.p. = 59–61 °C; UV–Vis: 221 nm (3.99); IR: ν = 3377w, 3273m, 2959w, 2938m, 2924w, 2860w, 1545w, 1477w, 1449m, 1423m, 1397w, 1374s, 1310s, 1291m, 1229vw, 1188s, 1152vs, 1115vw, 1091s, 1063m, 1049w, 1007m, 998m, 969s, 908m, 852vw, 820s, 754m, 722s, 686s, 590s, 567s, 555vs, 524vs, 473w, 448w, 441w cm−1; 1H NMR:δ = 7.81–7.77 (m, 2H, 2-H, 2′-H), 7.66–7.52 (m, 4H, 3-H, 3′-H, 4-H, NH), 7.37 (s, 2H, NH2), 3.98 (t, J = 6.5 Hz, 2H, 11-H), 2.76–2.69 (m, 2H, 5-H), 1.62–1.53 (m, 2H, 10-H), 1.39–1.30 (m, 2H, 6-H), 1.28–1.15 (m, 6H, 7-H, 8-H, 9-H) ppm; 13C NMR:δ = 140.7 (C-1), 132.3 (C-4), 129.2 (C-3), 126.4 (C-2), 68.9 (C-11), 42.5 (C-5), 28.8 (C-10), 28.2 (C-7), 28.0 (C-6), 25.8 (C-9), 24.9 (C-8) ppm; MS: m/z = 373.3 (100%, [M + Na]+); anal. calcd. for C13H22N2S2O5 (350.45): C 44.56, H 6.33, N 7.99; found: C 44.47, H 6.50, N 7.76.
4.2.15. N-(8-Hydroxyoctyl)benzene Sulfonamide (7a) [2771470-62-1]
Applying GPA: from benzenesulfonyl chloride (500 mg, 2.83 mmol) and 8-amino-octanol (617 mg, 4.25 mmol): 7a (566 mg, 70%); white solid; Rf = 0.21 (petrolether/EtOAc, 2:3); m.p. = 59–60 °C; UV–Vis: 221 nm (3.99); IR: ν = 3280m, 2929m, 2854m, 1478w, 1447m, 1428m, 1326s, 1266w, 1158vs, 1092s, 1053m, 1031w, 982w, 905m, 755m, 720m, 687s, 596s, 563s, 530m, 500w, 481w, 455w cm−1; 1H NMR:δ = 7.81–7.76 (m, 2H, 2-H, 2′-H), 7.66–7.56 (m, 3H, 3-H, 3′-H, 4-H), 7.54 (t, J = 5.8 Hz, 1H, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.36 (td, J = 6.5, 5.1 Hz, 2H, 12-H), 2.72 (q, J = 6.7 Hz, 2H, 5-H), 1.42–1.27 (m, 4H, 6-H, 11-H), 1.27–1.09 (m, 8H, 7-H, 8-H, 9-H, 10-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 140.7 (C-1), 132.2 (C-4), 129.1 (C-3), 126.4 (C-2), 60.7 (C-12), 42.5 (C-5), 32.5 (C-11), 28.9 (C-9), 28.7 (C-6), 28.5 (C-8), 25.9 (C-7), 25.4 (C-10) ppm; MS: m/z = 308.3 (100%, [M + Na]+); anal. calcd. for C14H23NSO3 (285.40): C 58.92, H 8.12, N 4.91; found: C 58.76, H 8.37, N 4.63.
4.2.16. 8-(Phenylsulfonamido]octyl Sulfamate (7b)
Applying GPB: from 7a (300 mg, 1.05 mmol): 7b (203 mg, 53%); white solid; Rf = 0.64 (CHCl3/EtOAc, 2:3); m.p. = 77–78 °C; UV–Vis: 221 nm (3.95); IR: ν = 3338w, 3267m, 2934w, 2916w, 2856w, 1577w, 1469w, 1450w, 1427w, 1395w, 1381s, 1311m, 1179s, 1158vs, 1116vw, 1092m, 1065w, 1058m, 1039w, 1025vw, 997m, 965s, 931m, 898m, 858m, 821s, 794m, 753s, 726s, 688s, 588vs, 566s, 549s, 526s, 480w, 468w, 440w cm−1; 1H NMR:δ = 7.82–7.76 (m, 2H, 2-H, 2′-H), 7.67–7.56 (m, 3H, 3-H, 3′-H, 4-H), 7.40 (s, 3H, NH, NH2), 3.99 (t, J = 6.5 Hz, 2H, 12-H), 2.72 (t, J = 7.0 Hz, 2H, 5-H), 1.65–1.54 (m, 2H, 11-H), 1.39–1.23 (m, 4H, 6-H, 10-H), 1.23–1.14 (m, 6H, 7-H, 8-H, 9-H) ppm; 13C NMR:δ = 140.7 (C-1), 132.2 (C-4), 129.1 (C-3), 126.4 (C-2), 69.0 (C-12), 42.5 (C-5), 28.9 (C-11), 28.3 (C-9), 28.3 (C-6), 28.3 (C-8), 25.9 (C-7), 24.9 (C-10) ppm; MS: m/z = 387.3 (100%, [M + Na]+); anal. calcd. for C14H24N2S2O5 (364.48): C 46.14, H 6.64, N 7.69; found: C 45.87, H 6.84, N 7.55.
4.2.17. N-(9-Hydroxynonyl)benzene Sulfonamide (8a)
Applying GPA: from benzenesulfonyl chloride (500 mg, 2.83 mmol) and 9-amino-nonanol (676 mg, 4.25 mmol): 8a (745 mg, 88%); white solid; Rf = 0.23 (petrolether/EtOAc, 2:3); m.p. = 40–41 °C; UV–Vis: 221 nm (3.97); IR: ν = 3465br, 3263br, 2930m, 2917w, 2892w, 2849w, 1475w, 1445m, 1433w, 1313s, 1157s, 1094s, 1058s, 1051s, 1036m, 1023w, 974m, 928w, 904m, 878m, 824m, 752m, 726m, 689s, 665m, 595w, 571s, 560vs, 522s, 470w, 460w, 439w cm−1; 1H NMR:δ = 17.81–7.76 (m, 2H, 2-H, 2′-H), 7.66–7.56 (m, 3H, 3-H, 3′-H, 4-H), 7.54 (s, 1H, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.36 (td, J = 6.5, 4.7 Hz, 2H, 13-H), 2.72 (t, J = 7.0 Hz, 2H, 5-H), 1.43–1.28 (m, 4H, 6-H, 12-H), 1.28–1.11 (m, 10H, 7-H, 8-H, 9-H, 10-H, 11-H) ppm; 13C NMR:δ = 140.7 (C-1), 132.2 (C-4), 129.1 (C-3), 126.4 (C-2), 60.7 (C-13), 42.5 (C-5), 32.5 (C-12), 28.9 (C-9), 28.9 (C-10), 28.8 (C-6), 28.5 (C-8), 26.0 (C-7), 25.4 (C-11) ppm; MS: m/z = 322.3 (100%, [M + Na]+); anal. calcd. for C15H25NSO3 (299.43): C 60.17, H 8.42, N 4.68; found: C 59.87, H 8.70, N 4.39.
4.2.18. 9-(Phenylsulfonamido]nonyl Sulfamate (8b)
Applying GPB: from 8a (300 mg, 1.00 mmol): 8b (267 mg, 70%); white solid; Rf = 0.68 (CHCl3/EtOAc, 2:3); m.p. = 82–84 °C; UV–Vis: 221 nm (3.92); IR: ν = 3378w, 3273m, 2937w, 2922m, 2855w, 1545w, 1476w, 1449w, 1424w, 1397w, 1375s, 1309s, 1286w, 1275w, 1188s, 1153vs, 1118vw, 1092m, 1063m, 1033m, 994w, 969s, 910m, 883m, 820s, 776w, 753m, 723s, 686s, 589s, 568s, 555vs, 534m, 524m, 500w, 489m, 465w, 453w cm−1; 1H NMR:δ = 7.81–7.76 (m, 2H, 2-H, 2′-H), 7.66–7.55 (m, 3H, 3-H, 3′-H, 4-H), 7.41 (s, 3H, NH, NH2), 4.00 (t, J = 6.5 Hz, 2H, 13-H), 2.72 (t, J = 7.0 Hz, 2H, 5-H), 1.65–1.56 (m, 2H, 12-H), 1.37–1.27 (m, 4H, 6-H, 11-H), 1.25–1.13 (m, 8H, 7-H, 8-H, 9-H, 10-H) ppm; 13C NMR:δ = 140.7 (C-1), 132.2 (C-4), 129.1 (C-3, C-3′), 126.4 (C-2, C-2′), 69.0 (C-13), 42.5 (C-5), 28.9 (C-6), 28.7 (C-8), 28.4 (C-9), 28.4 (C-10), 28.3 (C-12), 25.9 (C-7), 25.0 (C-11) ppm; MS: m/z = 401.3 (100%, [M + Na]+); anal. calcd. for C15H26N2S2O5 (378.50): C 47.60, H 6.92, N 7.40; found: C 47.40, H 7.11, N 7.06.
4.2.19. N-(10-Hydroxydecyl)benzene Sulfonamide (9a)
Applying GPA: from benzenesulfonyl chloride (220 mg, 1.25 mmol) and 10-amino-decanol (324 mg, 4.25 mmol): 9a (350 mg, 90%); white solid; Rf = 0.28 (petrolether/EtOAc, 2:3); m.p. = 67–69 °C; UV–Vis: 221 nm (3.95); IR: ν = 3416m, 3279m, 2920w, 2889w, 2849m, 1465m, 1447m, 1426m, 1350m, 1318s, 1287w, 1153vs, 1094m, 1059m, 1033w, 1009w, 973w, 909m, 750m, 722s, 684s, 596vs, 581s, 531w, 515m, 429w, 475w cm−1; 1H NMR:δ = 7.80–7.76 (m, 2H, 2-H, 2′-H), 7.66–7.56 (m, 3H, 3-H, 3′-H, 4-H), 7.53 (t, J = 5.8 Hz, 1H, NH), 4.30 (s, 1H, OH), 3.40–3.34 (m, 2H, 15-H), 2.71 (td, J = 7.0, 5.8 Hz, 2H, 5-H), 1.43–1.28 (m, 4H, 6-H, 14-H), 1.27–1.10 (m, 12H, 7-H, 8-H, 9-H, 10-H, 11-H, 12-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 140.7 (C-1), 132.2 (C-4), 129.1 (C-3), 126.4 (C-2), 60.7 (C-15), 42.5 (C-5), 32.5 (C-14), 29.0 (C-6), 28.9 (C-8, C-11), 28.8 (C-9), 28.5 (C-10), 26.0 (C-7), 25.5 (C-12) ppm; MS: m/z = 336.4 (100%, [M + Na]+); anal. calcd. for C16H27NSO3 (313.46): C 61.31, H 8.68, N 4.47; found: C 61.07, H 8.95, N 4.24.
4.2.20. 10-(Phenylsulfonamido]decyl Sulfamate (9b)
Applying GPB: from 9a (185 mg, 0.59 mmol): 9b (172 mg, 74%); white solid; Rf = 0.71 (CHCl3/EtOAc, 2:3); m.p. = 89–91 °C; UV–Vis: 221 nm (4.02); IR: ν = 3339w, 3266m, 2962vw, 2933w, 2917m, 2849w, 1576vw, 1470w, 1450w, 1428w, 1395w, 1381s, 1336vw, 1313m, 1308m, 1287w, 1266vw, 1235vw, 1178m, 1159vs, 1118vw, 1093m, 1078w, 1059m, 1042w, 1020w, 990m, 965s, 932m, 918m, 892m, 855w, 822s, 764w, 753m, 725s, 688s, 590s, 567s, 549s, 534s, 511w, 483w, 458w cm−1; 1H NMR:δ = 7.81–7.76 (m, 2H, 2-H, 2′-H), 7.66–7.56 (m, 3H, 3-H, 3′-H, 4-H), 7.53 (t, J = 5.8 Hz, 1H, NH), 7.37 (s, 2H, NH2), 4.00 (t, J = 6.5 Hz, 2H, 15-H), 2.72 (td, J = 7.0, 5.8 Hz, 2H, 5-H), 1.65–1.57 (m, 2H, 14-H), 1.39–1.27 (m, 4H, 6-H, 12-H), 1.27–1.12 (m, 10H, 7-H, 8-H, 9-H, 10-H, 11-H) ppm; 13C NMR:δ = 140.7 (C-1), 132.2 (C-4), 129.1 (C-3), 126.4 (C-2), 69.0 (C-15), 42.5 (C-5), 28.9 (C-6), 28.8 (C-9, C-14), 28.5 (C-8, C-11), 28.3 (C-10), 25.9 (C-7), 25.0 (C-12) ppm; MS: m/z = 415.2 (100%, [M + Na]+); anal. calcd. for C16H28N2S2O5 (392.53): C 48.96, H 7.19, N 7.14; found: C 48.75, H 7.35, N 6.87.
4.2.21. N-(11-Hydroxyundecyl)benzene Sulfonamide (10a)
Applying GPA: from benzenesulfonyl chloride (500 mg, 2.83 mmol) and 11-amino-undecanol (795 mg, 4.25 mmol): 10a (901 mg, 97%); white solid; Rf = 0.33 (petrolether/EtOAc, 2:3); m.p. = 58–59 °C; UV–Vis: 221 nm (3.97); IR: ν = 3464m, 3265m, 2918m, 2848m, 1475m, 1466m, 1445m, 1432w, 1353m, 1314s, 1287w, 1158vs, 1094s, 1052s, 1008m, 928w, 894w, 885m, 819m, 753s, 725s, 689s, 664m, 595m, 560s, 529w, 510m, 493w, 410w cm−1; 1H NMR:δ = 7.81–7.76 (m, 2H, 2-H, 2′-H), 7.66–7.56 (m, 3H, 3-H, 3′-H, 4-H), 7.53 (t, J = 5.8 Hz, 1H, NH), 4.30 (td, J = 5.2, 1.0 Hz, 1H, OH), 3.37 (td, J = 6.5, 5.1 Hz, 2H, 15-H), 2.71 (td, J = 7.0, 5.8 Hz, 2H, 5-H), 1.45–1.28 (m, 4H, 6-H, 14-H), 1.28–1.11 (m, 14H, 7-H, 8-H, 9-H, 10-H, 11-H, 12-H, 13-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 140.7 (C-1), 132.2 (C-4), 129.1 (C-3), 126.4 (C-2), 60.7 (C-15), 42.5 (C-5), 32.5 (C-14), 29.0 (C-6), 28.9 (C-12), 28.9 (C-11), 28.9 (C-10), 28.8 (C-9), 28.5 (C-8), 25.9 (C-7), 25.5 (C-13) ppm; MS: m/z = 350.2 (100%, [M + Na]+); anal. calcd. for C17H29NSO3 (327.48): C 62.35, H 8.93, N 4.28; found: C 62.17, H 9.16, N 4.01.
4.2.22. 11-(Phenylsulfonamido]undecyl Sulfamate (10b)
Applying GPB: from 10a (300 mg, 0.92 mmol): 10b (321 mg, 86%); white solid; Rf = 0.74 (CHCl3/EtOAc, 2:3); m.p. = 95–97 °C; UV–Vis: 221 nm (3.99); IR: ν = 3392w, 3269m, 2963w, 2936w, 2917m, 2852w, 1558w, 1476m, 1447w, 1429w, 1395w, 1369s, 1314s, 1258vw, 1183m, 1153s, 1120w, 1093m, 1066m, 1051m, 1028w, 991m, 970s, 928m, 903m, 869w, 824s, 755m, 719s, 690m, 639m, 590s, 565s, 555vs, 530s, 525s, 500w, 470w, 452m, 432m, 413vw, 511w, 483w, 458w cm−1; 1H NMR:δ = 7.82–7.75 (m, 2H, 2-H, 2′-H), 7.67–7.54 (m, 3H, 3-H, 3′-H, 4-H), 7.40 (s, 3H, NH, NH2), 4.00 (t, J = 6.5 Hz, 2H, 15-H), 2.72 (t, J = 6.9 Hz, 2H, 5-H), 1.67–1.57 (m, 2H, 14-H), 1.39–1.11 (m, 16H, 6-H, 7-H, 8-H, 9-H, 10-H, 11-H, 12-H, 13-H) ppm; 13C NMR:δ = 140.7 (C-1), 132.2 (C-4), 129.1 (C-3), 126.4 (C-2), 69.0 (C-15), 42.5 (C-5), 28.9 (C-6), 28.9 (C-9), 28.8 (C-10, C-11, C-13), 28.5 (C-8), 28.3 (C-14), 26.0 (C-12), 25.0 (C-7) ppm; MS: m/z = 429.3 (100%, [M + Na]+); anal. calcd. for C17H30N2S2O5 (406.56): C 50.22, H 7.44, N 6.89; found: C 49.96, H 7.69, N 6.58.
4.2.23. N-(12-Hydroxydodecyl)benzene Sulfonamide (11a)
Applying GPA: from benzenesulfonyl chloride (500 mg, 2.83 mmol) and 12-amino-dodecanol (855 mg, 4.25 mmol): 11a (944 mg, 98%); white solid; Rf = 0.37 (petrolether/EtOAc, 2:3); m.p. = 76–77 °C; UV–Vis: 221 nm (3.95); IR: ν = 3412m, 3350m, 3278s, 2920s, 2848m, 1477m, 1464m, 1447m, 1425w, 1359m, 1320s, 1266w, 1154vs, 1095s, 1064m, 1048s, 1053w, 999m, 909m, 750m, 722s, 684s, 596s, 561s, 530s, 489w, 459m cm−1; 1H NMR:δ = 7.81–7.76 (m, 2H, 2-H, 2′-H), 7.66–7.56 (m, 3H, 3-H, 3′-H, 4-H), 7.53 (t, J = 5.8 Hz, 1H, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.37 (td, J = 6.5, 5.1 Hz, 2H, 16-H), 2.71 (q, J = 6.7 Hz, 2H, 5-H), 1.44–1.28 (m, 4H, 6-H, 15-H), 1.28–1.12 (m, 16H, 7-H, 8-H, 9-H, 10-H, 11-H, 12-H, 13-H, 14-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 140.7 (C-1), 132.2 (C-4), 129.1 (C-3), 126.4 (C-2), 60.7 (C-16), 42.5 (C-5), 32.5 (C-15), 29.0 (C-12), 29.0 (C-11), 28.9 (C-9), 28.9 (C-10, C-13), 28.8 (C-6), 28.5 (C-8), 25.9 (C-7), 25.5 (C-14) ppm; MS: m/z = 364.3 (100%, [M + Na]+); anal. calcd. for C18H31NSO3 (341.51): C 63.31, H 9.15, N 4.10; found: C 63.00, H 9.43, N 3.97.
4.2.24. 12-(Phenylsulfonamido]dodecyl Sulfamate (11b)
Applying GPB: from 11a (300 mg, 0.88 mmol): 11b (229 mg, 62%); white solid; Rf = 0.77 (CHCl3/EtOAc, 2:3); m.p. = 102–103 °C; UV–Vis: λmax (log ε) =; 221 nm (3.47); IR: ν = 3338m, 3265m, 2962vw, 2917m, 2849m, 1576w, 1470w, 1450w, 1428w, 1396w, 1381s, 1313s, 1289w, 1178m, 1158vs, 1094m, 1060m, 1043m, 1024vw, 996w, 983m, 964s, 931m, 905m, 880m, 854w, 838m, 821s, 794w, 768w, 753s, 726s, 688s, 589s, 567s, 550s, 535vs, 504w, 482vw, 433m cm−1; 1H NMR:δ = 7.79–7.74 (m, 2H, 2-H, 2′-H), 7.64–7.54 (m, 3H, 3-H, 3′-H, 4-H), 7.51 (t, J = 5.8 Hz, 1H, NH), 7.35 (s, 2H, NH2), 3.98 (t, J = 6.5 Hz, 2H, 16-H), 2.69 (td, J = 7.0, 5.8 Hz, 2H, 5-H), 1.64–1.55 (m, 2H, 15-H), 1.36–1.09 (m, 18H, 6-H, 7-H, 8-H, 9-H, 10-H, 11-H, 12-H, 13-H, 14-H) ppm; 13C NMR:δ = 140.7 (C-1), 132.2 (C-4), 129.1 (C-3), 126.4 (C-2), 69.0 (C-16), 42.5 (C-5), 28.9 (C-9), 28.9 (C-6, C-11, C-12), 28.9 (C-15), 28.5 (C-8), 28.3 (C-13), 26.0 (C-14), 25.1 (C-7) ppm; MS: m/z = 443.4 (100%, [M + Na]+); anal. calcd. for C18H32N2S2O5 (420.58): C 51.40, H 7.67, N 6.66; found: C 51.15, H 7.93, N 6.42.
4.2.25. N-(2-Hydroxyethyl)-4-methylbenzene Sulfonamide (12a) [914083-49-1]
Applying GPA: from 4-methylbenzenesulfonyl chloride (300 mg, 1.57 mmol) and 2-amino-ethanol (144 mg, 2.36 mmol):
12a [
86,
87,
88,
89,
90,
91,
92] (315 mg, 93%); white solid; R
f = 0.54 (CHCl
3/EtOAc, 2:3); m.p. = 55–57 °C; UV–Vis: 227 nm (3.68); IR: ν = 3497
br, 3273
br, 2926
w, 2881
w, 1598
w, 1495
w, 1424
m, 1402
m, 1318
s, 1305
s, 1290
m, 1152
vs, 1093
s, 1055
s, 1019
w, 948
m, 880
w, 814
s, 706
w, 661
s, 550
s, 462
w cm
−1;
1H NMR:δ = 7.72–7.64 (
m, 2H, 2-H, 2′-H), 7.48 (
s, 1H, NH), 7.42–7.37 (
m, 2H, 3-H, 3′-H), 4.65 (
s, 1H, OH), 3.35 (
t,
J = 6.7 Hz, 2H, 7-H), 2.76 (
t,
J = 6.4 Hz, 2H, 6-H), 2.38 (
s, 3H, 5-H) ppm;
13C NMR:δ = 142.5 (C-1), 137.7 (C-4), 129.6 (C-3), 126.5 (C-2), 59.9 (C-7), 45.0 (C-6), 20.9 (C-5) ppm; MS:
m/
z = 238 (80%, [M + Na]
+); anal. calcd. for C
9H
13NSO
3 (215.27): C 50.22, H 6.09, N 6.51; found: C 49.87, H 6.30, N 6.33.
4.2.26. 2-[(4-Methylphenyl)sulfonamido]ethyl Sulfamate (12b) [914083-49-1]
Applying GPB: from
12a (250 mg, 1.16 mmol):
12b (330 mg, 96%) [
93]; white solid; R
f = 0.49 (CHCl
3/EtOAc, 2:3); m.p. = 79–81 °C; UV–Vis: 224 nm (3.91); IR: ν = 3358
w, 3332
w, 3265
m, 1480
w, 1448
w, 1425
w, 1377
m, 1366
s, 1322
s, 1305
m, 1238
w, 1227
w, 1178
s, 1160
s, 1148
s, 1117
w, 1098
m, 1083
s, 1033
m, 1011
s, 954
m, 931
s, 913
m, 880
w, 869
w, 818
m, 803
m, 783
s, 769
s, 702
m, 684
s, 659
m, 598
m, 589
m, 574
s, 548
vs, 529
m, 509
m, 487
m, 471
s, 448
m, 431
w, 411
w cm
−1;
1H NMR:δ = 7.82 (s, 1H, NH), 7.72–7.65 (
m, 2H, 2-H, 2′-H), 7.58–7.46 (
m, 2H, NH
2), 7.44–7.37 (
m, 2H, 3-H, 3′-H), 3.99 (
t,
J = 5.8 Hz, 2H, 7-H), 3.02 (
t,
J = 5.8 Hz, 2H, 6-H), 2.39 (
s, 3H, 5-H) ppm;
13C NMR:δ = 142.8 (C-4), 137.3 (C-1), 129.7 (C-3), 126.5 (C-2), 67.5 (C-7), 41.5 (C-6), 21.0 (C-5) ppm; MS:
m/
z = 317.1 (100%, [M + Na]
+); anal. calcd. for C
9H
14N
2S
2O
5 (294.34): C 36.73, H 4.79, N 9.52; found: C 36.41, H 4.97, N 9.35.
4.2.27. N-(3-Hydroxypropyl)-4-methylbenzene Sulfonamide (13a) [13379-98-1]
Applying GPA: from 4-methylbenzenesulfonyl chloride (200 mg, 1.05 mmol) and 3-amino-propanol (118 mg, 1.57 mmol):
13a (225 mg, 94%) [
94,
95,
96,
97,
98,
99]; white solid; R
f = 0.12 (petrolether/EtOAc, 2:3); m.p. = 55–57 °C (lit.: [
98] 55–56 °C); UV–Vis: 227 nm (4.14); IR: ν = 3492
br, 3274
br, 2945
w, 2879
w, 1598
m, 1495
w, 1423
m, 1318
s, 1305
s, 1296
m, 1185
w, 1153
vs, 1091
s, 1070
s, 1019
w, 1006
w, 959
m, 872
w, 815
s, 706
w, 662
s, 570
w, 550
s, 515
w cm
−1;
1H NMR:δ =7.69–7.64 (
m, 2H, 2-H, 2′-H), 7.46–7.35 (
m, 3H, 3-H, 3′-H, NH), 4.39 (
t,
J = 5.1 Hz, 1H, OH), 3.40–3.30 (
m, 2H, 8-H), 2.76 (
td,
J = 7.6, 3.3 Hz, 2H, 6-H), 2.38 (
s, 3H, 5-H), 1.50 (
p,
J = 6.4 Hz, 2H, 7-H) ppm;
13C NMR:δ = 142.5 (C-4), 137.6 (C-1), 129.6 (C-3), 126.5 (C-2), 58.1 (C-8), 40.0 (C-6), 32.3 (C-7), 20.9 (C-5) ppm; MS:
m/
z = 252.3 (90%, [M + Na]
+); anal. calcd. for C
10H
15NSO
3 (229.29): C 52.38, H 6.59, N 6.11; found: C 52.09, H 6.89, N 5.87.
4.2.28. 3-[(4-Methylphenyl)sulfonamido]propyl Sulfamate (13b)
Applying GPB: from 13a (300 mg, 1.31 mmol): 13b (260 mg, 64%); white solid; Rf = 0.49 (CHCl3/EtOAc, 2:3); m.p. = 86–87 °C; UV–Vis: 227 nm (3.72); IR: ν = 3352w, 3317w, 3272w, 2959vw, 2902vw, 1597vw, 1554w, 1472w, 1434vw, 1409w, 1392w, 1362m, 1321m, 1309m, 1292w, 1239vw, 1211vw, 1181m, 1160s, 1122w, 1093m, 1047m, 1020vw, 980w, 956s, 919m, 893w, 854m, 841m, 818m, 799w, 773m, 706w, 667s, 590m, 573m, 547vs, 494m, 476m, 444w, 413vw, 511w, 483w, 458w cm−1; 1H NMR:δ = 7.71–7.64 (m, 2H, 2-H, 2′-H), 7.57–7.35 (m, 5H, 3-H, 3′-H, NH, NH2), 4.02 (t, J = 6.3 Hz, 2H, 8-H), 2.79 (t, J = 7.1 Hz, 2H, 6-H), 2.39 (s, 3H, 5-H), 1.75 (p, J = 6.7 Hz, 2H, 7-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 142.7 (C-4), 137.4 (C-1), 129.7 (C-3), 126.5 (C-2), 66.5 (C-8), 39.2 (C-6), 28.7 (C-7), 20.9 (C-5) ppm; MS (ESI, MeOH) m/z = 331.2 (90%, [M + Na]+); anal. calcd. for C10H16N2S2O5 (308.37): C 38.95, H 5.23, N 9.08; found: C 38.77, H 5.54, N 8.78.
4.2.29. N-(4-Hydroxybutyl)-4-methylbenzene Sulfonamide (14a) [78521-69-4]
Applying GPA: from 4-methylbenzenesulfonyl chloride (400 mg, 2.1 mmol) and 4-amino-butanol (318 mg, 3.15 mmol):
14a (477 mg, 93%); white solid; R
f = 0.12 (petrolether/EtOAc, 2:3); m.p. = 50–51 °C (lit.: [
100] 50–52 °C); UV–Vis: 227 nm (4.18); IR: ν = 3502
br, 3279
br, 2940
w, 2871
w, 1597
m, 1475
m, 1454
m, 1433
m, 1314
s, 1305
s, 1289
m, 1150
vs, 1121
w, 1091
s, 1061
s, 1027
m, 1019
w, 941
m, 848
w, 817
s, 753
m, 721
m, 709
m, 662
s, 576
s, 551
s, 484
m, 456
w cm
−1;
1H NMR (500 MHz, DMSO-
d6): δ = 7.69–7.65 (
m, 2H, 2-H, 2′-H), 7.48–7.43 (
m, 1H, NH), 7.40–7.36 (
m, 2H, 3-H, 3′-H), 4.36 (
t,
J = 5.1 Hz, 1H, OH), 3.32 (
q,
J = 5.5 Hz, 2H, 9-H), 2.73–2.67 (
m, 2H, 6-H), 2.37 (
s, 3H, 5-H), 1.43–1.32 (
m, 4H, 7-H, 8-H) ppm;
13C NMR (126 MHz, DMSO-
d6): δ = 142.4 (C-4), 137.7 (C-1), 129.6 (C-3), 126.5 (C-2), 60.2 (C-9), 42.5 (C-6), 29.6 (C-8), 25.8 (C-7), 20.9 (C-5) ppm; MS:
m/
z = 266.1 (100%, [M + Na]
+); anal. calcd. for C
11H
17NSO
3 (243.32): C 54.30, H 7.04, N 5.76; found: C 54.11, H 7.32, N 5.55.
4.2.30. 4-[(4-Methylphenyl)sulfonamido]butyl Sulfamate (14b)
Applying GPB: from 14a (100 mg, 0.41 mmol): 14b (90 mg, 68%); white solid; Rf = 0.52 (CHCl3/EtOAc, 2:3); m.p. = 66–68 °C; UV–Vis: 227 nm (3.81); IR: ν = 3354w, 3314w, 3275w, 2955vw, 2902vw, 1597vw, 1552w, 1474w, 1434vw, 1410w, 1398w, 1362m, 1321m, 1309m, 1294w, 1235vw, 1211vw, 1180m, 1162s, 1120w, 1098m, 1046m, 1020vw, 982w, 957s, 917m, 894w, 854m, 840m, 820m, 799w, 774m, 704w, 665s, 596m, 571m, 547vs, 495m, 473m, 444w, 413vw, 511w cm−1; 1H NMR:δ = 7.70–7.65 (m, 2H, 2-H, 2′-H), 7.52 (t, J = 5.9 Hz, 1H, NH), 7.45–7.36 (m, 4H, 3-H, 3′-H, NH2), 3.96 (t, J = 6.3 Hz, 2H, 9-H), 2.73 (q, J = 6.8 Hz, 2H, 6-H), 2.38 (s, 3H, 5-H), 1.65–1.55 (m, 2H, 8-H), 1.49–1.39 (m, 2H, 7-H) ppm; 13C NMR:δ = 142.6 (C-4), 137.7 (C-1), 129.6 (C-3), 126.5 (C-2, C-2), 68.6 (C-9), 42.0 (C-6), 25.6 (C-8), 25.4 (C-7), 21.0 (C-5) ppm; MS: m/z = 345.1 (100%, [M + Na]+); anal. calcd. for C11H18N2S2O5 (322.39): C 40.98, H 5.63, N 8.69; found: C 40.78, H 5.89, N 8.43.
4.2.31. N-(5-Hydroxypentyl)-4-methylbenzene Sulfonamide (15a) [16780-44-2]
Applying GPA: from 4-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and and 5-amino-pentanol (405 mg, 3.93 mmol):
15a (522 mg, 77%) [
101,
102,
103,
104,
105,
106,
107,
108]; white solid; R
f = 0.11 (petrolether/EtOAc, 2:3); m.p. = 60–61 °C; UV–Vis: 227 nm (3.99); IR: ν = 3456
m, 3109
br, 2920
w, 2862
w, 1597
m, 1475
m, 1454
m, 1433
m, 1314
s, 1305
s, 1289
m, 1150
vs, 1121
w, 1091
s, 1061
s, 1027
m, 1019
w, 941
m, 848
w, 817
s, 753
m, 721
m, 709
m, 662
s, 576
s, 551
s, 484
m, 456
w cm
−1;
1H NMR:δ = 7.69–7.63 (
m, 2H, 2-H, 2′-H), 7.44 (
t,
J = 5.9 Hz, 1H, NH), 7.41–7.36 (
m, 2H, 3-H, 3′-H), 4.30 (
t,
J = 5.1 Hz, 1H, OH), 3.35–3.29 (
m, 2H, 10-H), 2.68 (
td,
J = 7.0, 5.9 Hz, 2H, 6-H), 2.38 (
s, 3H, 5-H), 1.39–1.27 (
m, 4H, 7-H, 9-H), 1.27–1.17 (
m, 2H, 8-H) ppm;
13C NMR:δ = 142.4 (C-4), 137.7 (C-1), 129.5 (C-3), 126.5 (C-2), 60.5 (C-10), 42.5 (C-6), 32.0 (C-9), 28.8 (C-7), 22.6 (C-8), 20.9 (C-5) ppm; MS:
m/
z = 280.0 (100%, [M + Na]
+); anal. calcd. for C
12H
19NSO
3 (257.35): C 56.01, H 7.44, N 5.44; found: C 55.76, H 7.78, N 5.16.
4.2.32. 5-[(4-Methylphenyl)sulfonamido]pentyl Sulfamate (15b)
Applying GPB: from 15a (200 mg, 0.78 mmol): 15b (232 mg, 89%); white solid; Rf = 0.53 (CHCl3/EtOAc, 2:3); m.p. = 94–95 °C UV–Vis: 227 nm (3.92); IR: ν = 3351w, 3301m, 3250w, 2957vw, 2940w, 2930w, 2852w, 1554w, 1466w, 1422w, 1395vw, 1361s, 1316s, 1301m, 1294w, 1281w, 1177s, 1150vs, 1096m, 1074w, 1063w, 1044w, 1027vw, 1021w, 992m, 965vw, 947m, 915s, 870w, 815s, 810s, 749w, 722w, 708w, 671s, 630w, 598m, 574m, 550vs cm−1; 1H NMR:δ = 7.70–7.63 (m, 2H, 2-H, 2′-H), 7.47 (t, J = 5.9 Hz, 1H, NH), 7.42–7.34 (m, 4H, 3-H, 3′-H, NH2), 3.96 (t, J = 6.4 Hz, 2H, 10-H), 2.70 (q, J = 6.6 Hz, 2H, 6-H), 2.38 (s, 3H, 5-H), 1.55 (p, J = 6.7 Hz, 2H, 9-H), 1.43–1.33 (m, 2H, 7-H), 1.33–1.22 (m, 2H, 8-H) ppm; 13C NMR:δ = 142.5 (C-4), 137.7 (C-1), 129.6 (C-3), 126.5 (C-2), 68.8 (C-10), 42.3 (C-6), 28.5 (C-9), 27.8 (C-7), 22.2 (C-8), 20.9 (C-5) ppm; MS: m/z = 359.4 (100%, [M + Na]+); anal. calcd. for C12H20N2S2O5 (336.42): C 42.84, H 5.99, N 8.33; found: C 42.57, H 6.26, N 8.14.
4.2.33. N-(6-Hydroxyhexyl)-4-methylbenzene Sulfonamide (16a) [385369-83-5]
Applying GPA: from 4-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 6-amino-hexanol (461 mg, 3.93 mmol):
16a (551 mg, 77%) [
104,
109,
110,
111,
112]; white solid; R
f = 0.15 (petrolether/EtOAc, 2:3); m.p. = 49–51 °C; UV–Vis: 227 nm (4.10); IR: ν = 3423
w, 3364
w, 3290
m, 2936
m, 2891
w, 2860
w, 1589
w, 1495
w, 1476
w, 1422
m, 1385
w, 1319
m, 1303
w, 1290
w, 1154
vs, 1091
m, 1067
m, 1036
m, 983
w, 905
m, 817
s, 734
w, 707
w, 666
s, 573
s, 549
s, 523
m, 484
w, 430
w cm
−1;
1H NMR:δ = 7.70–7.63 (
m, 2H, 2-H, 2′-H), 7.47–7.41 (
m, 1H, NH), 7.41–7.35 (
m, 2H, 3-H, 3′-H), 4.30 (
t,
J = 5.1 Hz, 1H, OH), 3.38–3.30 (
m, 2H, 11-H), 2.73–2.65 (
m, 2H, 6-H), 2.37 (
s, 3H, 5-H), 1.41–1.27 (
m, 4H, 7-H, 10-H), 1.22–1.14 (
m, 4H, 8-H, 9-H) ppm;
13C NMR:δ = 142.4 (C-4), 137.8 (C-1), 129.6 (C-3, C-3′), 126.5 (C-2, C-2′), 60.6 (C-11), 42.5 (C-6), 32.4 (C-10), 29.0 (C-7), 25.9 (C-9), 25.0 (C-8), 20.9 (C-5) ppm; MS:
m/
z = 294 (100%, [M + Na]
+); anal. calcd. for C
13H
21NSO
3 (271.38): C 57.54, H 7.80, N 5.16; found: C 57.24, H 8.02, N 4.96.
4.2.34. 6-[(4-Methylphenyl)sulfonamido]hexyl Sulfamate (16b)
Applying GPB: from 16a (200 mg, 0.74 mmol): 16b (206 mg, 80%); white solid; Rf = 0.55 (CHCl3/EtOAc, 2:3); m.p. = 49–50 °C; UV–Vis: 227 nm (4.03); IR: ν = 3350w, 3300m, 3251w, 2959vw, 2939w, 2932w, 2854w, 1556w, 1464w, 1422w, 1391vw, 1363s, 1317s, 1308m, 1291w, 1280w, 1178s, 1151vs, 1094m, 1075w, 1062w, 1043w, 1027vw, 1020w, 992m, 968vw, 945m, 917s, 868w, 817s, 810s, 749w, 722w, 708w, 670s, 634w, 595m, 576m, 549vs, 513m, 487m, 473m cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 7.69–7.64 (m, 2H, 2-H, 2′-H), 7.46 (t, J = 5.8 Hz, 1H, NH), 7.41–7.35 (m, 4H, 3-H, 3′-H, NH2), 3.97 (t, J = 6.5 Hz, 2H, 11-H), 2.70 (q, J = 6.6 Hz, 2H, 6-H), 2.38 (s, 3H, 5-H), 1.59–1.52 (m, 2H, 10-H), 1.35 (p, J = 6.9 Hz, 2H, 7-H), 1.27–1.17 (m, 4H, 8-H, 9-H) ppm; 13C NMR:δ = 142.5 (C-4), 137.7 (C-1), 129.6 (C-3), 126.5 (C-2), 68.9 (C-11), 42.4 (C-6), 28.8 (C-10), 28.2 (C-7), 25.5 (C-9), 24.6 (C-8), 20.9 (C-5) ppm; MS: m/z = 373.7 (100%, [M + Na]+); anal. calcd. for C13H22N2S2O5 (350.45): C 44.56, H 6.33, N 7.99; found: C 44.34, H 6.51, N 7.65.
4.2.35. N-(7-Hydroxyheptyl)-4-methylbenzene Sulfonamide (17a) [1669425-24-4]
Applying GPA: from 4-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 7-amino-heptanol (516 mg, 3.93 mmol):
17a (557 mg, 74%) [
113] oil; R
f = 0.19 (petrolether/EtOAc, 2:3); UV–Vis: 227 nm (4.15); IR: ν = 3503
w, 3279
w, 2930
m, 2858
w, 1598
w, 1495
vw, 1429
w, 1320
m, 1305
m, 1289
m, 1152
vs, 1120
w, 1092
s, 1056
m, 1019
w, 814
m, 723
w, 707
m, 660
s, 635
w, 571
m, 549
vs, 466
w cm
−1;
1H NMR:δ = 7.68–7.65 (
m, 2H, 2-H, 2′-H), 7.44 (
t,
J = 5.8 Hz, 1H, NH), 7.40–7.36 (
m, 2H, 3-H, 3′-H), 4.30 (
t,
J = 5.1 Hz, 1H, OH), 3.37–3.34 (
m, 2H, 12-H), 2.69 (
td,
J = 7.0, 5.7 Hz, 2H, 6-H), 2.37 (
s, 3H, 5-H), 1.40–1.29 (
m, 4H, 7-H, 11-H), 1.25–1.10 (
m, 6H, 8-H, 9-H, 10-H) ppm;
13C NMR:δ = 142.4 (C-4), 137.8 (C-1), 129.5 (C-3), 126.5 (C-2), 60.7 (C-12), 42.5 (C-6), 32.4 (C-11), 28.9 (C-7), 28.4 (C-9), 26.1 (C-8), 25.3 (C-10), 20.9 (C-5) ppm; MS:
m/
z = 308.2 (100%, [M + Na]
+); anal. calcd. for C
14H
23NSO
3 (285.40): C 58.92, H 8.12, N 4.91; found: C 58.69, H 8.33, N 4.65.
4.2.36. 7-[(4-Methylphenyl)sulfonamido]heptyl Sulfamate (17b)
Applying GPB: from 17a (200 mg, 0.7 mmol): 17b (94 mg, 37%); white solid; Rf = 0.60 (CHCl3/EtOAc, 2:3); m.p. = 85–86 °C; UV–Vis: 227 nm (4.07); IR: ν = 3351w, 3298m, 3252w, 2960w, 2940w, 2921w, 2853w, 1598vw, 1558w, 1477vw, 1466w, 1457w, 1439w, 1420w, 1392w, 1362s, 1319s, 1308m, 1292w, 1281w, 1180s, 1150vs, 1137m, 1094m, 1080m, 1048w, 1020w, 1009w, 996w, 982vw, 950m, 930s, 904s, 834w, 818s, 798w, 782m, 737w, 723w, 707w, 668s, 597m, 577m, 548vs, 519m, 495m, 474m, 448w, 404vw cm−1; 1H NMR:δ = 7.69–7.64 (m, 2H, 2-H, 2′-H), 7.45 (t, J = 5.8 Hz, 1H, NH), 7.41–7.35 (m, 4H, 3-H, 3′-H, NH2), 3.98 (t, J = 6.5 Hz, 2H, 12-H), 2.69 (q, J = 6.7 Hz, 2H, 6-H), 2.38 (s, 3H, 5-H), 1.62–1.54 (m, 2H, 11-H), 1.38–1.30 (m, 2H, 7-H), 1.30–1.14 (m, 6H, 8-H, 9-H, 10-H) ppm; 13C NMR:δ = 142.4 (C-4), 137.8 (C-1), 129.5 (C-3), 126.5 (C-2), 68.9 (C-12), 42.4 (C-6), 28.8 (C-11), 28.2 (C-7), 28.0 (C-9), 25.9 (C-10), 24.9 (C-8), 20.9 (C-5) ppm; MS: m/z = 387.3 (100%, [M + Na]+); anal. calcd. for C14H24N2S2O5 (364.48): C 46.14, H 6.64, N 7.69; found: C 45.87, H 6.92, N 7.46.
4.2.37. N-(8-Hydroxyoctyl)-4-methylbenzene Sulfonamide (18a) [2772203-84-4]
Applying GPA: from 4-methylbenzenesulfonyl chloride (400 mg, 2.1 mmol) and 8-amino-octanol (457 mg, 3.15 mmol): 18a (442 mg, 70%); white solid; Rf = 0.23 (petrolether/EtOAc, 2:3); m.p. = 87–88 °C; UV–Vis: 227 nm (4.00); IR: ν = 3418w, 3278m, 2933m, 2854m, 1598w, 1478w, 1466w, 1425m, 1384w, 1364w, 1333m, 1324m, 1305m, 1290w, 1157vs, 1109w, 1092m, 1064m, 1052s, 1031w, 1020w, 992w, 982m, 905m, 817s, 733w, 707w, 668s, 571s, 551vs, 530s, 500m, 493m, 465w cm−1; 1H NMR:δ = 7.69–7.65 (m, 2H, -H, 2′-H), 7.44 (t, J = 5.8 Hz, 1H, NH), 7.41–7.36 (m, 2H, 3-H, 3′-H), 4.31 (t, J = 5.2 Hz, 1H, OH), 3.40–3.34 (m, 2H, 13-H), 2.69 (q, J = 6.8 Hz, 2H, 6-H), 2.38 (s, 3H, 5-H), 1.42–1.28 (m, 4H, 7-H, 12-H), 1.26–1.12 (m, 8H, 8-H, 9-H, 10-H, 11-H) ppm; 13C NMR:δ = 142.9 (C-4), 138.3 (C-1), 130.0 (C-3), 127.0 (C-2), 61.2 (C-13), 43.0 (C-6), 33.0 (C-12), 29.4 (C-7), 29.3 (C-9), 29.0 (C-10), 26.4 (C-8), 25.9 (C-11), 21.4 (C-5).ppm; MS: m/z = 322 (100%, [M + Na]+); anal. calcd. for C15H25NSO3 (299.43): C 60.17, H 8.42, N 4.68; found: C 59.87, H 8.69, N 4.55.
4.2.38. 8-[(4-Methylphenyl)sulfonamido]octyl Sulfamate (18b)
Applying GPB: from 18a (200 mg, 0.67 mmol): 18b (140 mg, 55%); white solid; Rf = 0.64 (CHCl3/EtOAc, 2:3); m.p. = 98–100 °C; UV–Vis: 227 nm (4.00); IR: ν = 3344m, 3311m, 3250m, 2944w, 2928w, 2857w, 2844w, 1476w, 1430m, 1362s, 1332w, 1319m, 1309m, 1183m, 1147vs, 1119w, 1095m, 1074w, 1065w, 1054m, 1040w, 963s, 927s, 902m, 818s, 724w, 707w, 666s, 591m, 561s, 550vs, 526m, 511s, 499s, 474m, 405w cm−1; 1H NMR:δ = 7.68–7.63 (m, 2H, 2-H, 2′-H), 7.44 (t, J = 5.9 Hz, 1H, NH), 7.41–7.35 (m, 4H, 3-H, 3′-H. NH2), 3.99 (t, J = 6.5 Hz, 2H, 13-H), 2.69 (td, J = 7.0, 5.8 Hz, 2H, 6-H), 2.38 (s, 3H, 5-H), 1.64–1.55 (m, 2H, 12-H), 1.38–1.31 (m, 2H, 7-H), 1.30–1.12 (m, 8H, 8-H, 9-H, 10-H, 11-H) ppm; 13C NMR:δ = 142.4 (C-4), 137.8 (C-1), 129.5 (C-3), 126.5 (C-2), 69.0 (C-13), 42.4 (C-6), 28.9 (C-12), 28.4 (C-7), 28.3 (C-10), 28.3 (C-9), 25.9 (C-8), 24.9 (C-11), 20.9 (C-5) ppm; MS: m/z = 401.7 (100%, [M + Na]+); anal. calcd. for C15H26N2S2O5 (378.50): C 47.60, H 6.92, N 7.40; found: C 47.37, H 7.19, N 7.38.
4.2.39. N-(2-Hydroxyethyl)-3-methylbenzene Sulfonamide (19a) [1082883-27-9]
Applying GPA: from 3-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 2-amino-ethanol (240 mg, 3.93 mmol): 19a (542 mg, 96%); oil; Rf = 0.1 (petrolether/EtOAc, 2:3); UV–Vis: 224 nm (3.92); IR: ν = 3493w, 3274w, 2927w, 2882w, 1601vw, 1478w, 1425w, 1321s, 1303s, 1220w, 1148vs, 1097m, 1087m, 1054s, 999w, 949m, 866w, 785m, 687s, 580vs, 524m, 492w, 459m, 435w cm−1; 1H NMR:δ = 7.63–7.61 (m, 1H, 6-H), 7.61–7.58 (m, 1H, 2-H), 7.52 (t, J = 5.8 Hz, 1H, NH), 7.50–7.42 (m, 2H, 4-H, 5-H), 4.66 (t, J = 5.6 Hz, 1H, OH), 3.37 (q, J = 6.3 Hz, 2H, 9-H), 2.79 (q, J = 6.2 Hz, 2H, 8-H), 2.39 (s, 3H, 7-H) ppm; 13C NMR:δ = 140.5 (C-1), 138.8 (C-3), 132.9 (C-4), 129.0 (C-5), 126.7 (C-6), 123.6 (C-2), 59.9 (C-9), 45.1 (C-8), 20.8 (C-7) ppm; MS: m/z = 238.4 (40%, [M + Na]+); anal. calcd. for C9H13NSO3 (215.27): C 50.22, H 6.09, N 6.51; found: C 49.97, H 6.34, N 6.27.
4.2.40. 2-[(3-Methylphenyl)sulfonamido]ethyl Sulfamate (19b)
Applying GPB: from 19a (73 mg, 0.4 mmol): 19b (72 mg, 72%); white solid; Rf = 0.52 (CHCl3/EtOAc, 2:3); m.p. = 36–38 °C; UV–Vis: 224 nm (3.65); IR: ν = 3341m, 3259m, 3100w, 2988w, 1566w, 1473w, 1444w, 1421w, 1389w, 1371vs, 1345m, 1325m, 1303s, 1226w, 1174s, 1172s, 1147vs, 1078m, 1062m, 1001m, 951s, 932s, 905m, 879m, 865w, 837s, 791m, 765m, 701s, 683s, 639s, 591s, 571s, 546vs, 504m, 452m, 429w, 544vs, 526m cm−1; 1H NMR:δ = 7.86 (t, J = 5.9 Hz, 1H, NH), 7.64–7.57 (m, 2H, 2-H, 6-H), 7.53–7.44 (m, 4H, 4-H, 5-H, NH2), 3.99 (t, J = 5.7 Hz, 2H, 9-H), 3.04 (q, J = 5.6 Hz, 2H, 8-H), 2.39 (s, 3H, 7-H) ppm; 13C NMR:δ = 140.1 (C-1), 139.0 (C-2), 133.2 (C-4), 129.1 (C-5), 126.7 (C-6), 123.6 (C-3), 67.5 (C-9), 41.6 (C-8), 20.9 (C-7) ppm; MS: m/z = 317.1 (100%, [M + Na]+); anal. calcd. for C9H14N2S2O5 (294.34): C 36.73, H 4.79, N 9.52; found: C 36.55, H 5.00, N 9.35.
4.2.41. N-(3-Hydroxypropyl)-3-methylbenzene Sulfonamide (20a) [1082805-58-0]
Applying GPA: from 3-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 3-amino-propanol (295 mg, 3.93 mmol):
20a (522 mg, 87%) [
114] oil; R
f = 0.09 (petrolether/EtOAc, 2:3); UV–Vis: 224 nm (3.91); IR: ν = 3502
w, 3276
w, 2947
w, 2882
w, 1477
w, 1423
w, 1320
m, 1303
s, 1222
w, 1148
vs, 1096
m, 1085
s, 1068
m, 1008
w, 998
w, 959
w, 879
w, 787
m, 688
s, 579
vs, 524
m, 498
w, 463
m, 434
w cm
−1;
1H NMR:δ = 7.62–7.56 (
m, 2H, 2-H, 6-H), 7.50–7.41 (
m, 3H, 4-H, 5-H, NH), 4.40 (
t,
J = 5.1 Hz, 1H, OH), 3.40–3.35 (
m, 2H, 10-H), 2.78 (
q,
J = 7.0 Hz, 2H, 8-H), 2.39 (
s, 3H, 7-H), 1.52 (
p,
J = 6.4 Hz, 2H, 9-H) ppm;
13C NMR:δ = 140.4 (C-1), 138.8 (C-3), 132.9 (C-4), 129.0 (C-5), 126.7 (C-6), 123.6 (C-2), 58.1 (C-10), 40.0 (C-8), 32.4 (C-9), 20.9 (C-7) ppm; MS:
m/
z = 252.1 (100%, [M + Na]
+); anal. calcd. for C
10H
15NSO
3 (229.29): C 52.38, H 6.59, N 6.11; found: C 52.03, H 6.88, N 5.94.
4.2.42. 3-[(3-Methylphenyl)sulfonamido]propyl Sulfamate (20b)
Applying GPB: from 20a (300 mg, 1.31 mmol): 20b (305 mg, 76%); white solid; Rf = 0.52 (CHCl3/EtOAc, 2:3); m.p. = 73–74 °C; UV–Vis: 224 nm (3.88); IR: ν = 3351m, 3256m, 3108w, 2983w, 2921vw, 1566w, 1473w, 1444w, 1421w, 1399w, 1373vs, 1352m, 1318m, 1303s, 1241w, 1226w, 1218w, 1176s, 1170s, 1147vs, 1113w, 1085m, 1056m, 1004m, 951s, 929s, 905m, 887m, 881m, 865w, 837s, 792m, 759m, 703s, 685s, 638s, 591s, 573s, 545vs, 508m, 486m, 450m, 426w, 548vs, 519m, 495m, 474m, 448w, 404vw cm−1; 1H NMR:δ = 7.67–7.57 (m, 3H, 2-H, 6-H, NH), 7.52–7.37 (m, 4H, 4-H, 5-H, NH2), 4.02 (t, J = 6.3 Hz, 2H, 10-H), 2.81 (t, J = 7.1 Hz, 2H, 8-H), 2.40 (s, 3H, 7-H), 1.76 (p, J = 6.7 Hz, 2H, 9-H) ppm; 13C NMR:δ = 140.2 (C-1), 138.9 (C-3), 133.0 (C-4), 129.1 (C-5), 126.7 (C-6), 123.6 (C-2), 66.5 (C-10), 39.2 (C-8), 28.7 (C-9), 20.8 (C-7) ppm; MS: m/z = 331.3 (100%, [M + Na]+); anal. calcd. for C10H16N2S2O5 (308.37): C 38.95, H 5.23, N 9.08; found: C 38.77, H 5.52, N 8.85.
4.2.43. N-(4-Hydroxybutyl)-3-methylbenzene Sulfonamide (21a) [1082889-69-7]
Applying GPA: from 3-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 4-amino-butanol (351 mg, 3.93 mmol): 21a (589 mg, 92%); oil; Rf = 0.56 (CHCl3/EtOAc, 2:3); UV–Vis: 224 nm (3.90); IR: ν = 3502w, 3279w, 2940w, 2871w, 1598w, 1428w, 1319m, 1305m, 1289m, 1152vs, 1120w, 1091s, 1054m, 1020m, 814m, 706w, 659s, 571m, 549vs, 491w, 469w cm−1; 1H NMR:δ = 7.62–7.56 (m, 2H, 2-H, 6-H), 7.52–7.41 (m, 3H, 4-H, 5-H, NH), 4.35 (t, J = 5.1 Hz, 1H, OH), 3.35–3.29 (m, 2H, 11-H), 2.72 (q, J = 6.7 Hz, 2H, 8-H), 2.39 (s, 3H, 7-H), 1.45–1.31 (m, 4H, 9-H, 10-H) ppm; 13C NMR:δ = 140.5 (C-1), 138.8 (C-3), 132.8 (C-4), 129.0 (C-5), 126.7 (C-6), 123.6 (C-2), 60.2 (C-11), 42.5 (C-8), 29.5 (C-10), 25.8 (C-9), 20.8 (C-7) ppm; MS: m/z = 266.2 (100%, [M + Na]+); anal. calcd. for C11H17NSO3 (243.32): C 54.30, H 7.04, N 5.76; found: C 54.00, H 7.31, N 5.52.
4.2.44. 4-[(3-Methylphenyl)sulfonamido]butyl Sulfamate (21b)
Applying GPB: from 21a (250 mg, 1.03 mmol): 21b (317 mg, 96%); white solid; Rf = 0.55 (CHCl3/EtOAc, 2:3); m.p. = 62–64 °C; UV–Vis: 225 nm (3.84); IR: ν = 3351w, 3272m, 1474w, 1428w, 1375s, 1339w, 1315m, 1303s, 1198w, 1171s, 1153vs, 1097m, 1091m, 1065m, 1014w, 970s, 930m, 897m, 888m, 823m, 793m, 746w, 701s, 697s, 688s, 609m, 594s, 582s, 553s, 525m, 491m, 436w cm−1; 1H NMR:δ = 7.59–7.50 (m, 3H, 4-H, 5-H, NH), 7.49–7.39 (m, 2H, 2-H, 6-H), 7.36 (s, 2H, NH2), 3.94 (t, J = 6.3 Hz, 2H, 11-H), 2.72 (q, J = 6.7 Hz, 2H, 8-H), 2.36 (s, 3H, 7-H), 1.63–1.54 (m, 2H, 10-H), 1.47–1.38 (m, 2H, 9-H) ppm; 13C NMR:δ = 140.5 (C-1), 138.9 (C-3), 132.9 (C-4), 129.1 (C-5), 126.7 (C-6), 123.6 (C-2), 68.6 (C-11), 42.0 (C-8), 25.6 (C-10), 24.7 (C-9), 20.9 (C-7) ppm; MS: m/z = 345.6 (90%, [M + Na]+); anal. calcd. for C11H18N2S2O5 (322.39): C 40.98, H 5.63, N 8.69; found: C 40.76, H 8.92, N 8.38.
4.2.45. N-(5-Hydroxypentyl)-3-methylbenzene Sulfonamide (22a) [1986639-01-3]
Applying GPA: from 3-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 5-amino-pentanol (406 mg, 3.93 mmol): 22a (620 mg, 92%); oil; Rf = 0.13 (petrolether/EtOAc, 2:3); UV–Vis: 224 nm (3.89); IR: ν = 3454w, 3108w, 2954w, 2919w, 2880w, 2862w, 1597vw, 1474w, 1455w, 1437w, 1313s, 1305m, 1289m, 1245w, 1150s, 1122m, 1108w, 1091s, 1061m, 1041w, 1027m, 1019w, 940m, 849w, 817m, 801w, 755m, 721m, 709m, 662s, 636w, 577s, 551vs, 484m, 458w cm−1; 1H NMR:δ = 7.62–7.60 (m, 1H, 6-H), 7.60–7.56 (m, 1H, 2-H), 7.51–7.41 (m, 3H, 4-H, 5-H, NH), 4.31 (t, J = 5.1 Hz, 1H, OH), 3.36–3.30 (m, 2H, 12-H), 2.71 (q, J = 6.5 Hz, 2H, 8-H), 2.39 (s, 3H, 7-H), 1.41–1.29 (m, 4H, 9-H, 11-H), 1.28–1.18 (m, 2H, 10-H) ppm; 13C NMR:δ = 141.0 (C-1), 139.3 (C-3), 133.3 (C-4), 129.5 (C-5), 127.1 (C-6), 124.1 (C-2), 61.0 (C-12), 43.1 (C-8), 32.5 (C-11), 29.4 (C-9), 23.1 (C-10), 21.3 (C-7) ppm; MS: m/z = 280.3 (100%, [M + Na]+); anal. calcd. for C12H19NSO3 (257.35): C 56.01, H 7.44, N 5.44; found: C 55.76, H 7.69, N 5.20.
4.2.46. 5-[(3-Methylphenyl)sulfonamido]pentyl Sulfamate (22b)
Applying GPB: from 22a (300 mg, 1.17 mmol): 22b (378 mg, 96%); white solid; Rf = 0.60 (CHCl3/EtOAc, 2:3); m.p. = 67–68 °C; UV–Vis: 224 nm (3.75); IR: ν = 3371w, 3265m, 2978vw, 2933w, 2867vw, 1536vw, 1475w, 1462w, 1439w, 1423w, 1404w, 1377m, 1358s, 1316m, 1302s, 1281w, 1227w, 1179s, 1150vs, 1138m, 1097w, 1085m, 1061m, 1049m, 1037w, 997w, 976s, 960s, 931m, 911s, 878w, 856w, 823s, 785m, 749m, 713s, 699s, 685s, 598s, 581s, 566s, 551s, 538m, 525s, 489m, 466s, 449w, 448w, 404vw cm−1; 1H NMR:δ = 7.61–7.59 (m, 1H, 6-H), 7.58–7.56 (m, 1H, 2-H), 7.54–7.42 (m, 3H, 4-H, 5-H, NH), 7.37 (s, 2H), 3.96 (t, J = 6.5 Hz, 2H, 12-H), 2.72 (q, J = 6.6 Hz, 2H, 8-H), 2.39 (s, 3H, 7-H), 1.56 (p, J = 6.7 Hz, 2H, 11-H), 1.44–1.35 (m, 2H, 9-H), 1.33–1.25 (m, 2H, 10-H) ppm; 13C NMR:δ = 140.5 (C-1), 138.8 (C-3), 132.9 (C-4), 129.0 (C-5), 126.6 (C-6), 123.6 (C-2), 68.8 (C-12), 42.3 (C-8), 28.5 (C-11), 27.8 (C-9), 22.2 (C-10), 20.8 (C-7) ppm; MS: m/z = 359.1 (100%, [M + Na]+); anal. calcd. for C12H20N2S2O5 (336.42): C 42.84, H 5.99, N 8.33; found: C 42.62, H 6.13, N 8.17.
4.2.47. N-(6-Hydroxyhexyl)-3-methylbenzene Sulfonamide (23a) [1916290-41-9]
Applying GPA: from 3-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 6-amino-hexanol (461 mg, 3.93 mmol): 23a (649 mg, 91%); oil; Rf = 0.15 (petrolether/EtOAc, 2:3); UV–Vis: 224 nm (3.90); IR: ν = 3503w, 3279w, 2933m, 2860w, 1477w, 1428w, 1321m, 1303s, 1221w, 1148vs, 1097m, 1086m, 1072m, 1054m, 882w, 787m, 688s, 591s, 581vs, 525m, 490m, 459w, 453w, 435w cm−1; 1H NMR:δ = 7.61–7.59 (m, 1H, 6-H), 7.59–7.56 (m, 1H, 2-H), 7.50–7.41 (m, 3H, 4-H, 5-H, NH), 4.30 (t, J = 5.2 Hz, 1H, OH), 3.38–3.31 (m, 2H, 13-H), 2.75–2.67 (m, 2H, 8-H), 2.39 (s, 3H, 7-H), 1.40–1.28 (m, 4H, 9-H, 12-H), 1.24–1.13 (m, 4H, 10-H, 11-H) ppm; 13C NMR:δ = 140.6 (C-1), 138.8 (C-3), 132.8 (C-4), 129.0 (C-5), 126.7 (C-6), 123.6 (C-2), 60.6 (C-13), 42.5 (C-8), 32.4 (C-12), 29.0 (C-9), 25.9 (C-10), 25.0 (C-11), 20.8 (C-7) ppm; MS: m/z = 294.4 (100%, [M + Na]+); anal. calcd. for C13H21NSO3 (271.38): C 57.54, H 7.80, N 5.16; found: C 57.26, H 8.01, N 4.97.
4.2.48. 6-[(3-Methylphenyl)sulfonamido]hexyl Sulfamate (23b)
Applying GPB: from 23a (300 mg, 1.11 mmol): 23b (364 mg, 94%); white solid; Rf = 0.64 (CHCl3/EtOAc, 2:3); m.p. = 74–75 °C; UV–Vis: 224 nm (3.84); IR: ν = 3371w, 3261m, 2975vw, 2958vw, 2943w, 2910vw, 2897vw, 2852w, 1556w, 1479w, 1472w, 1455w, 1431w, 1398w, 1369s, 1341w, 1323s, 1308m, 1282w, 1230vw, 1221vw, 1161vs, 1112vw, 1099w, 1086w, 1062w, 1053w, 1042vw, 1007m, 988vw, 963s, 940m, 912m, 881w, 861vw, 818s, 801w, 791w, 720m, 685s, 596vs, 580s, 550m, 529m, 505w, 494m, 457vw, 436w, 404vw cm−1; 1H NMR:δ = 7.62–7.55 (m, 2H, 2-H, 6-H), 7.52–7.42 (m, 3H, 4-H, 5-H), 7.37 (s, 2H, NH2), 3.97 (t, J = 6.5 Hz, 2H, 13-H), 2.72 (q, J = 6.6 Hz, 2H, 8-H), 2.39 (s, 3H, 7-H), 1.56 (p, J = 6.7 Hz, 2H, 12-H), 1.36 (p, J = 6.9 Hz, 2H, 9-H), 1.28–1.18 (m, 4H, 10-H, 11-H) ppm; 13C NMR:δ = 140.5 (C-1), 138.8 (C-3), 132.8 (C-4), 129.0 (C-5), 126.6 (C-6), 123.6 (C-2), 68.9 (C-13), 42.4 (C-8), 28.8 (C-12), 28.2 (C-9), 25.5 (C-10), 24.6 (C-11), 20.8 (C-7) ppm; MS: m/z = 373.3 (100%, [M + Na]+); anal. calcd. for C13H22N2S2O5 (350.45): C 44.56, H 6.33, N 7.99; found: C 44.26, H 6.58, N 8.19.
4.2.49. N-(7-Hydroxyheptyl)-3-methylbenzene Sulfonamide (24a)
Applying GPA: from 3-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 7-amino-heptanol (516 mg, 3.93 mmol): 24a (648 mg, 87%); oil; Rf = 0.19 (petrolether/EtOAc, 2:3); UV–Vis: 224 nm (3.78); IR: ν = 3474m, 3128m, 2930m, 2889w, 2850m, 1483w, 1465m, 1447m, 1402w, 1372w, 1317m, 1309m, 1298m, 1289m, 1222m, 1143vs, 1099s, 1083s, 1072s, 1020m, 1001m, 957m, 906m, 870w, 862w, 835w, 790s, 755w, 709s, 689s, 584vs, 555m, 545m, 524m, 483s, 472m, 439m, 408w cm−1; 1H NMR:δ = 7.61–7.56 (m, 2H, 2-H, 6-H), 7.50–7.41 (m, 3H, 4-H, 5-H, NH), 4.30 (td, J = 5.2, 1.1 Hz, 1H, OH), 3.38–3.33 (m, 2H, 14-H), 2.71 (td, J = 7.0, 5.8 Hz, 2H, 8-H), 2.39 (s, 3H, 7-H), 1.40–1.28 (m, 4H, 9-H, 13-H), 1.26–1.11 (m, 6H, 10-H, 11-H, 12-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 140.6 (C-1), 138.8 (C-3), 132.8 (C-4), 129.0 (C-5), 126.6 (C-6), 123.6 (C-2), 60.7 (C-14), 42.5 (C-8), 32.4 (C-13), 28.9 (C-9), 28.4 (C-11), 26.0 (C-12), 25.3 (C-10), 20.8 (C-7) ppm; MS: m/z = 308.1 (100%, [M + Na]+); anal. calcd. for C14H23NSO3 (285.40): C 58.92, H 8.12, N 4.91; found: C 58.78, H 8.34, N 4.65.
4.2.50. 7-[(3-Methylphenyl)sulfonamido]heptyl Sulfamate (24b)
Applying GPB: from 24a (300 mg, 1.05 mmol): 24b (279 mg, 73%) a white waxy solid; Rf = 0.77 (CHCl3/EtOAc, 2:3); m.p. = 55–57 °C; UV–Vis: 224 nm (3.80); IR: ν = 3361w, 3265m, 2961w, 2937w, 2906w, 2895w, 2856w, 1566w, 1477w, 1429m, 1396w, 1370s, 1317s, 1301m, 1226w, 1183s, 1150vs, 1114w, 1096w, 1085m, 1061m, 1049w, 1000m, 972s, 927m, 922m, 904m, 878w, 825s, 783m, 741w, 723w, 702s, 685s, 665m, 597s, 578s, 556vs, 528m, 521m, 495s, 478w, 450w, 429w, 505w, 494m, 457vw, 436w, 404vw cm−1; 1H NMR:δ = 7.62–7.56 (m, 2H, 2-H, 6-H), 7.51–7.42 (m, 3H, 4-H, 5-H, NH), 7.37 (s, 2H, NH2), 3.98 (t, J = 6.5 Hz, 2H, 14-H), 2.72 (td, J = 7.0, 5.8 Hz, 2H, 8-H), 2.39 (s, 3H, 7-H), 1.63–1.54 (m, 2H, 13-H), 1.39–1.15 (m, 8H, 9-H, 10-H, 11-H, 12-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 140.6 (C-1), 138.8 (C-3), 132.8 (C-4), 129.0 (C-5), 126.6 (C-6), 123.6 (C-2), 68.9 (C-14), 42.5 (C-8), 28.9 (C-9), 28.2 (C-13), 28.0 (C-11), 25.9 (C-10), 24.9 (C-12), 20.8 (C-7) ppm; MS: m/z = 387.2 (100%, [M + Na]+); anal. calcd. for C14H24N2S2O5 (364.48): C 46.14, H 6.64, N 7.69; found: C 45.97, H 6.90, N 7.47.
4.2.51. N-(8-Hydroxyoctyl)-3-methylbenzene Sulfonamide (25a)
Applying GPA: from 3-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 8-amino-octanol (571 mg, 3.93 mmol): 25a (702 mg, 89%); oil; Rf = 0.25 (petrolether/EtOAc, 2:3); UV–Vis: 224 nm (3.90); IR: ν = 3508w, 3282w, 2928m, 2856m, 1477w, 1458w, 1429w, 1322s, 1303s, 1221w, 1149vs, 1097m, 1086m, 999w, 883w, 786m, 689s, 592s, 581vs, 525m, 492m, 436w cm−1; 1H NMR:δ = 7.61–7.59 (m, 1H, 6-H), 7.59–7.56 (m, 1H, 2-H), 7.50–7.41 (m, 3H, 4-H, 5-H, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.39–3.32 (m, 2H, 15-H), 2.71 (td, J = 7.0, 5.8 Hz, 2H, 8-H), 2.38 (s, 3H, 7-H), 1.42–1.28 (m, 4H, 9-H, 14-H), 1.26–1.11 (m, 8H, 10-H, 11-H, 12-H, 13-H) ppm; 13C NMR:δ = 140.6 (C-1), 138.8 (C-3), 132.8 (C-4), 129.0 (C-5), 126.7 (C-6), 123.6 (C-2), 60.7 (C-15), 42.5 (C-8), 32.5 (C-14), 28.9 (C-9), 28.8 (C-11), 28.6 (C-12), 26.0 (C-10), 25.4 (C-13), 20.8 (C-7) ppm; MS: m/z = 322.1 (100%, [M + Na]+); anal. calcd. for C15H25NSO3 (299.43): C 60.17, H 8.42, N 4.68; found: C 59.87, H 8.70, N 4.45.
4.2.52. 8-[(3-Methylphenyl)sulfonamido]octyl Sulfamate (25b)
Applying GPB: from 25a (300 mg, 1.00 mmol): 25b (284 mg, 75%); white solid; Rf = 0.83 (CHCl3/EtOAc, 2:3); m.p. = 54–56 °C; UV–Vis: 224 nm (3.84); IR: ν = 3369w, 3254m, 2970w, 2938m, 2920m, 2859m, 2853w, 1562w, 1473m, 1458w, 1440m, 1431w, 1397w, 1324s, 1303s, 1217w, 1164s, 1150vs, 1116w, 1099m, 1087m, 1057m, 1053m, 1039w, 994m, 960vs, 920m, 896s, 864w, 849vw, 832m, 800w, 787m, 731w, 701s, 688vs, 602m, 578s, 569s, 524m, 517m, 493s, 478m, 446w, 431w, 415w, 457vw, 436w, 404vw cm−1; 1H NMR:δ = 7.61–7.58 (m, 2H, 6-H), 7.58–7.56 (m, 1H, 2-H), 7.50–7.42 (m, 3H, 4-H, 5-H, NH), 7.37 (s, 2H, NH2), 3.99 (t, J = 6.5 Hz, 2H, 15-H), 2.71 (td, J = 7.0, 5.8 Hz, 2H, 8-H), 2.39 (s, 3H, 7-H), 1.65–1.55 (m, 2H, 14-H), 1.40–1.24 (m, 2H, 9-H, 13-H), 1.23–1.14 (m, 6H, 10-H, 11-H, 12-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 140.6 (C-1), 138.8 (C-3), 132.8 (C-4), 129.0 (C-5), 126.6 (C-6), 123.6 (C-2), 69.0 (C-15), 42.5 (C-8), 28.9 (C-14), 28.4 (C-9), 28.3 (C-11), 28.3 (C-12), 25.9 (C-10), 24.9 (C-13), 20.8 (C-7) ppm; MS (ESI, MeOH) m/z = 401.3 (100%, [M + Na]+); anal. calcd. for C15H26N2S2O5 (378.50): C 47.60, H 6.92, N 7.40; found: C 47.36, H 7.17, N 7.15.
4.2.53. N-(2-Hydroxyethyl)-2-methylbenzene Sulfonamide (26a) [19829-14-2]
Applying GPA: from 2-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 2-amino-ethanol (3.93 mg, 3.93 mmol):
26a (420 mg, 74%) [
115,
116,
117]; white solid; R
f = 0.11 (petrolether/EtOAc, 2:3); m.p. = 73–74 °C (lit.: [
76,
116] 73–75 °C
(4)); UV–Vis: 222 nm (3.87); IR: ν = 3452
m, 3189
m, 3067
w, 2959
w, 2939
w, 2867
w, 2687
vw, 1592
vw, 1459
m, 1421
m, 1399
w, 1381
w, 1349
w, 1301
s, 1285
m, 1261
m, 1207
w, 1154
vs, 1133
s, 1094
s, 1070
m, 1059
s, 1037
m, 995
w, 963
s, 899
w, 880
vw, 839
m, 803
w, 760
s, 710
m, 687
s, 590
s, 580
vs, 542
s, 511
m, 491
m, 468
s, 443
w, 415
m cm
−1;
1H NMR (500 MHz, DMSO-
d6): δ = 7.81 (
dd,
J = 7.9, 1.4 Hz, 1H, 3-H), 7.63–7.58 (
m, 1H, 6-H), 7.51 (
td,
J = 7.5, 1.4 Hz, 1H, 4-H), 7.41–7.35 (
m, 2H, 5-H, NH), 4.65 (
t,
J = 5.5 Hz, 1H, OH), 3.36–3.32 (
m, 2H, 9-H), 2.81 (
t,
J = 6.5 Hz, 2H, 8-H), 2.57 (
s, 3H, 7-H) ppm;
13C NMR (126 MHz, DMSO-
d6): δ = 138.8 (C-1), 136.5 (C-2), 132.4 (C-4), 132.3 (C-3), 128.3 (C-6), 126.1 (C-5), 59.9 (C-9), 44.7 (C-8), 19.7 (C-7) ppm; MS:
m/
z = 238.2 (100%, [M + Na]
+); anal. calcd. for C
9H
13NSO
3 (215.27): C 50.22, H 6.09, N 6.51; found: C 49.98, H 6.37, N 6.39.
4.2.54. 2-[(2-Methylphenyl)sulfonamido]ethyl Sulfamate (26b)
Applying GPB: from 26a (150 mg, 0.70 mmol): 26b (185 mg, 90%); white solid; Rf = 0.52 (CHCl3/EtOAc, 2:3); m.p. = 70–72 °C; UV–Vis: 270 nm (3.08); IR: ν = 3394w, 3359w, 3311m, 3288m, 3256m, 1566vw, 1539w, 1479vw, 1455w, 1434w, 1417w, 1398w, 1370s, 1358vs, 1320m, 1309vs, 1290m, 1237w, 1190m, 1174s, 1155vs, 1127m, 1112m, 1091w, 1076m, 1026m, 962s, 933s, 909s, 872w, 845w, 803m, 759vs, 753vs, 708m, 691m, 590s, 567m, 548vs, 538s, 526s, 514m, 481m, 467m, 454m, 424m, 410w cm−1; 1H NMR:δ = 7.97 (t, J = 6.0 Hz, 1H, NH), 7.82 (dd, J = 7.8, 1.4 Hz, 1H, 3-H), 7.56–7.46 (m, 3H, 4-H, NH2), 7.44–7.35 (m, 2H, 5-H, 6-H), 3.97 (t, J = 5.8 Hz, 2H, 9-H), 3.08 (q, J = 5.7 Hz, 2H, 8-H), 2.58 (s, 3H, 7-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 138.4 (C-1), 136.6 (C-2), 132.6 (C-4), 132.5 (C-3), 128.3 (C-6), 126.2 (C-5), 67.5 (C-9), 41.2 (C-8), 19.8 (C-7) ppm; MS: m/z = 317.2 (100%, [M + Na]+); anal. calcd. for C9H14N2S2O5 (294.34): C 36.73, H 4.79, N 9.52; found: C 36.54, H 4.44, N 9.38.
4.2.55. N-(3-Hydroxypropyl)-2-methylbenzene Sulfonamide (27a) [1082811-80-0]
Applying GPA: from 2-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 3-amino-propanol (240 mg, 3.93 mmol): 27a (420 mg, 74%); oil; Rf = 0.15 (petrolether/EtOAc, 2:3); UV–Vis: 222 nm (3.84); IR: ν = 3498w, 3294w, 2939w, 2882w, 1472w, 1457w, 1311s, 1290m, 1153vs, 1131m, 1065s, 1006w, 957w, 872w, 806w, 759m, 710m, 689m, 591vs, 575s, 541m, 481m, 444w, 425w, 420w cm−1; 1H NMR:δ = 7.79 (dd, J = 7.8, 1.4 Hz, 1H, 3-H), 7.61–7.51 (m, 1H, 6-H), 7.48 (td, J = 7.5, 1.4 Hz, 1H, 4-H), 7.40–7.32 (m, 2H, 5-H, NH), 4.38 (s, 1H, OH), 3.36–3.30 (m, 2H, 10-H), 2.80 (t, J = 7.2 Hz, 2H, 8-H), 2.56 (s, 3H, 7-H), 1.55–1.44 (m, 2H, 9-H) ppm; 13C NMR:δ = 138.7 (C-1), 136.5 (C-2), 132.5 (C-4), 132.3 (C-3), 128.4 (C-6), 126.2 (C-5), 58.1 (C-10), 39.8 (C-8), 32.4 (C-9), 19.8 (C-7) ppm; MS: m/z = 252.2 (100%, [M + Na]+); anal. calcd. for C10H15NSO3 (229.29): C 52.38, H 6.59, N 6.11; found: C 52.04, H 6.80, N 5.96.
4.2.56. 3-[(2-Methylphenyl)sulfonamido]propyl Sulfamate (27b)
Applying GPB: from 27a (300 mg, 1.31 mmol): 27b (328 mg, 81%); white solid; Rf = 0.54 (CHCl3/EtOAc, 2:3); m.p. = 68–70 °C; UV–Vis: 224 nm (3.90); IR: ν = 3314m, 3238m, 3115w, 2942vw, 1570w, 1470w, 1439w, 1418w, 1396w, 1361s, 1315s, 1281w, 1216vw, 1199vw, 1171m, 1155vs, 1134m, 1111m, 1095m, 1066m, 1056w, 1005w, 940s, 886m, 843s, 805w, 763s, 711m, 691s, 643w, 589vs, 574s, 549vs, 543vs, 508m, 486m, 457m, 436w, 415w cm−1; 1H NMR:δ = 7.85–7.77 (m, 1H, 3-H), 7.71 (t, J = 5.8 Hz, 1H, 6-H), 7.56–7.47 (m, 1H, 4-H), 7.44–7.34 (m, 4H, 5-H, NH, NH2), 4.01 (t, J = 6.3 Hz, 2H, 10-H), 2.84 (td, J = 7.2, 5.8 Hz, 2H, 8-H), 2.57 (s, 3H, 7-H), 1.76 (p, J = 6.6 Hz, 2H, 9-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 138.5 (C-1), 136.5 (C-2), 132.5 (C-4), 132.4 (C-3), 128.3 (C-6), 126.2 (C-5), 66.5 (C-10), 39.0 (C-8), 28.9 (C-9), 19.8 (C-7) ppm; MS: m/z = 331.3 (100%, [M + Na]+); anal. calcd. for C10H16N2S2O3 (308.37): C 38.95, H 5.23, N 9.08; found: C 38.78, H 5.51, N 8.76.
4.2.57. N-(4-Hydroxybutyl)-2-methylbenzene Sulfonamide (28a) [1082772-69-7]
Applying GPA: from 2-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 4-amino-butanol (351 mg, 3.93 mmol): 28a (546 mg, 86%); oil; Rf = 0.15 (petrolether/EtOAc, 2:3); UV–Vis: 224 nm (3.85); IR: ν = 3502w, 3296w, 2939w, 2872w, 1472w, 1456w, 1311s, 1153vs, 1131m, 1065s, 1034m, 952vw, 867w, 808w, 760m, 711m, 688m, 591vs, 541m, 488m, 451w, 444w cm−1; 1H NMR:δ = 7.80 (dd, J = 7.8, 1.4 Hz, 1H, 3-H), 7.65–7.55 (m, 1H, 6-H), 7.50 (td, J = 7.5, 1.4 Hz, 1H, 4-H), 7.41–7.33 (m, 2H, 5-H, NH), 4.35 (s, 1H, OH), 3.30 (t, J = 6.0 Hz, 2H, 8-H), 2.76 (t, J = 6.7 Hz, 2H, 11-H), 2.57 (s, 3H, 7-H), 1.43–1.29 (m, 4H, 9-H, 10-H) ppm; 13C NMR:δ = 138.8 (C-1), 136.4 (C-2), 132.4 (C-4), 132.3 (C-3), 128.3 (C-6), 126.1 (C-5), 60.2 (C-11), 42.3 (C-8), 29.5 (C-10), 25.9 (C-9), 19.8 (C-7) ppm; MS: m/z = 266.3 (100%, [M + Na]+); anal. calcd. for C11H17NSO3 (243.32): C 54.30, H 7.04, N 5.76; found: C 53.99, H 7.34, N 5.31.
4.2.58. 4-[(2-Methylphenyl)sulfonamido]butyl Sulfamate (28b)
Applying GPB: from 28a (300 mg, 1.23 mmol): 28b (312 mg, 79%); oil; Rf = 0.55 (CHCl3/EtOAc, 2:3); UV–Vis: 224 nm (3.72); IR: ν = 3317m, 3240m, 3112w, 2940vw, 1574w, 1471w, 1438w, 1417w, 1393w, 1360s, 1317s, 1280w, 1214vw, 1198vw, 1170m, 1154vs, 1130m, 1110m, 1096m, 1068m, 1050w, 1001w, 945s, 884m, 845s, 801w, 760s, 711m, 690s, 646w, 590vs, 572s, 548vs, 543vs, 508m, 484m, 456m, 433w, 415w cm−1; 1H NMR:δ = 7.82–7.78 (m, 1H, 3-H), 7.66 (t, J = 5.9 Hz, 1H, 6-H), 7.51 (td, J = 7.5, 1.4 Hz, 1H, 4-H), 7.42–7.35 (m, 4H, 5-H, NH, NH2), 3.95 (t, J = 6.4 Hz, 2H, 11-H), 2.79 (q, J = 6.7 Hz, 2H, 8-H), 2.57 (s, 3H, 7-H), 1.64–1.55 (m, 2H, 10-H), 1.50–1.39 (m, 2H, 9-H) ppm; 13C NMR:δ = 139.2 (C-1), 136.9 (C-2), 132.9 (C-4), 132.8 (C-3), 128.7 (C-6), 126.6 (C-5), 69.0 (C-11), 42.2 (C-8), 26.0 (C-10), 26.0 (C-9), 20.2 (C-7) ppm; MS: m/z = 345.3 (100%, [M + Na]+); anal. calcd. for C11H18N2S2O5 (322.39): C 40.98, H 5.63, N 8.69; found: C 40.77, H 5.90, N 8.41.
4.2.59. N-(5-Hydroxypentyl)-2-methylbenzene Sulfonamide (29a) [1965509-68-5]
Applying GPA: from 2-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 5-amino-pentanol (406 mg, 3.93 mmol): 29a (396 mg, 59%); oil; Rf = 0.17 (petrolether/EtOAc, 2:3); UV–Vis: 222 nm (3.87); IR: ν = 3501w, 3295w, 2937w, 2864w, 1472w, 1457w, 1313s, 1154vs, 1131m, 1066m, 1046m, 1039m, 877w, 806w, 760m, 733w, 711m, 688m, 592vs, 541m, 488m, 423w, 418w cm−1; 1H NMR:δ = 7.80 (dd, J = 7.8, 1.4 Hz, 1H, 3-H), 7.62–7.56 (m, 1H, 6-H), 7.50 (td, J = 7.5, 1.4 Hz, 1H, 4-H), 7.41–7.33 (m, 2H, 5-H, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.30 (td, J = 6.4, 5.1 Hz, 2H, 12-H), 2.74 (td, J = 7.0, 5.5 Hz, 2H, 8-H), 2.57 (s, 3H, 7-H), 1.39–1.25 (m, 4H, 9-H, 11-H), 1.25–1.15 (m, 2H, 10-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 138.9 (C-1), 136.4 (C-2), 132.4 (C-4), 132.2 (C-3), 128.3 (C-6), 126.1 (C-5), 60.5 (C-12), 42.3 (C-8), 31.9 (C-11), 29.0 (C-9), 22.5 (C-10), 19.8 (C-7) ppm; MS: m/z = 280.2 (100%, [M + Na]+); anal. calcd. for C12H19NSO3 (257.35): C 56.01, H 7.44, N 5.44; found: C 55.76, H 7.69, N 5.16.
4.2.60. 5-[(2-Methylphenyl)sulfonamido]pentyl Sulfamate (29b)
Applying GPB: from 29a (300 mg, 1.17 mmol): 29b (282 mg, 72%); oil; Rf = 0.64 (CHCl3/EtOAc, 2:3); UV–Vis: 224 nm (4.02); IR: ν = 3277w, 2941w, 2868vw, 1567w, 1472w, 1458w, 1360m, 1312s, 1177s, 1154vs, 1130m, 1082m, 1066m, 1048w, 1034w, 919s, 813m, 761m, 729w, 710m, 689m, 592s, 551s, 542s, 494m, 480m cm−1; 1H NMR:δ = 7.83–7.78 (m, 1H, 3-H), 7.62 (t, J = 5.9 Hz, 1H, 6-H), 7.51 (td, J = 7.5, 1.4 Hz, 1H, 4-H), 7.42–7.34 (m, 4H, NH, 5-H, NH2), 3.94 (t, J = 6.5 Hz, 2H, 12-H), 2.76 (q, J = 6.8 Hz, 2H, 8-H), 2.57 (s, 3H, 7-H), 1.57–1.48 (m, 2H, 11-H), 1.42–1.33 (m, 2H, 9-H), 1.33–1.22 (m, 2H, 10-H) ppm; 13C NMR:δ = 138.8 (C-1), 136.4 (C-2), 132.5 (C-4), 132.3 (C-3), 128.3 (C-6), 126.2 (C-5), 68.8 (C-12), 42.0 (C-8), 28.6 (C-11), 27.8 (C-9), 22.2 (C-10), 19.8 (C-7) ppm; MS: m/z = 359.4 (100%, [M + Na]+); anal. calcd. for C12H20N2S2O5 (336.42): C 42.84, H 5.99, N 8.33; found: C 42.56, H 6.23, N 8.04.
4.2.61. N-(6-Hydroxyhexyl)-2-methylbenzene Sulfonamide (30a) [1914655-85-8]
Applying GPA: from 2-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 6-amino-hexanol (461 mg, 3.93 mmol): 30a (666 mg, 94%); oil; Rf = 0.19 (petrolether/EtOAc, 2:3); UV–Vis: 223 nm (3.97); IR: ν = 3503w, 3296w, 2934m, 2860w, 1471w, 1458w, 1314s, 1154vs, 1131m, 1066m, 807w, 759m, 728w, 711m, 688m, 592vs, 541m, 490m cm−1; 1H NMR:δ = 7.80 (dd, J = 7.8, 1.4 Hz, 1H, 3-H), 7.59 (t, J = 5.7 Hz, 1H, 6-H), 7.50 (td, J = 7.5, 1.4 Hz, 1H, 4-H), 7.41–7.33 (m, 2H, 5-H, NH), 4.29 (t, J = 5.1 Hz, 1H, OH), 3.36–3.29 (m, 2H, 13-H), 2.75 (td, J = 7.1, 5.8 Hz, 2H, 8-H), 2.57 (s, 3H, 7-H), 1.38–1.26 (m, 4H, 9-H, 12-H), 1.21–1.10 (m, 4H, 10-H, 11-H) ppm; 13C NMR:δ = 138.9 (C-1), 136.4 (C-2), 132.4 (C-4), 132.2 (C-3), 128.3 (C-6), 126.1 (C-5), 60.6 (C-13), 42.2 (C-8), 32.3 (C-12), 29.1 (C-9), 25.9 (C-10), 25.0 (C-11), 19.8 (C-7) ppm; MS: m/z = 294.2 (100%, [M + Na]+); anal. calcd. for C13H21NSO3 (271.38): C 57.54, H 7.80, N 5.16; found: C 76.26, H 8.03, N 4.76.
4.2.62. 6-[(2-Methylphenyl)sulfonamido]hexyl Sulfamate (30b)
Applying GPB: from 30a (300 mg, 1.11 mmol): 30b (333 mg, 86%); oil; Rf = 0.71 (CHCl3/EtOAc, 2:3); UV–Vis: 223 nm (3.05); IR: ν = 3277w, 3113vw, 2938w, 2863w, 1564w, 1471w, 1459w, 1360s, 1312s, 1177s, 1154vs, 1131m, 1082m, 1066m, 1048w, 922s, 807m, 761m, 726w, 710m, 689m, 592s, 551s, 543s, 492m cm−1; 1H NMR:δ = 7.80 (dd, J = 7.8, 1.4 Hz, 1H, 3-H), 7.60 (t, J = 5.8 Hz, 1H, 6-H), 7.50 (td, J = 7.4, 1.4 Hz, 1H, 4-H), 7.41–7.34 (m, 4H, 5-H, NH, NH2), 3.96 (t, J = 6.5 Hz, 2H, 13-H), 2.76 (q, J = 6.6 Hz, 2H, 8-H), 2.57 (s, 3H, 7-H), 1.59–1.47 (m, 2H, 12-H), 1.40–1.28 (m, 2H, 9-H), 1.26–1.15 (m, 4H, 10-H, 11-H) ppm; 13C NMR:δ = 139.3 (C-1), 136.9 (C-2), 132.9 (C-4), 132.7 (C-3), 128.8 (C-6), 126.6 (C-5), 69.3 (C-13), 42.6 (C-8), 29.4 (C-12), 28.6 (C-9), 25.9 (C-10), 25.0 (C-11), 20.2 (C-7) ppm; MS: m/z = 373.4 (100%, [M + Na]+); anal. calcd. for C13H22N2S2O5 (350.45): C 44.56, H 6.33, N 7.99; found: C 44.31, H 6.68, N 7.69.
4.2.63. N-(7-Hydroxyheptyl)-2-methylbenzene Sulfonamide (31a)
Applying GPA: from 2-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 7-amino-heptanol (516 mg, 3.93 mmol): 31a (394 mg, 53%); oil; Rf = 0.20 (petrolether/EtOAc, 2:3); UV–Vis: 223 nm (3.90); IR: ν = 3504w, 3295w, 3064vw, 2931m, 2858w, 1458w, 1314s, 1154vs, 1131m, 1066m, 871w, 806w, 760m, 725w, 711m, 688m, 592vs, 541m, 491m, 419vw cm−1; 1H NMR:δ = 7.80 (dd, J = 7.8, 1.4 Hz, 1H, 3-H), 7.62–7.56 (m, 1H, 6-H), 7.50 (td, J = 7.5, 1.4 Hz, 1H, 4-H), 7.40–7.33 (m, 2H, 5, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.37–3.31 (m, 2H, 14-H), 2.78–2.70 (m, 2H, 8-H), 2.57 (s, 3H, 7-H), 1.40–1.26 (m, 4H, 9-H, 13-H), 1.23–1.07 (m, 6H, 10-H, 11-H, 12-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 138.9 (C-1), 136.4 (C-2), 132.4 (C-4), 132.2 (C-3), 128.3 (C-6), 126.1 (C-5), 60.7 (C-14), 42.2 (C-8), 32.4 (C-13), 29.0 (C-9), 28.4 (C-11), 26.0 (C-10), 25.3 (C-12), 19.8 (C-7) ppm; MS: m/z = 308.3 (100%, [M + Na]+); anal. calcd. for C14H23NSO3 (285.40): C 58.92, H 8.12, N 4.91; found: C 58.76, H 8.34, N 4.78.
4.2.64. 7-[(2-Methylphenyl)sulfonamido]heptyl Sulfamate (31b)
Applying GPB: from 31a (300 mg, 1.05 mmol): 31b (294 mg, 77%); oil; Rf = 0.74 (SiO2 CHCl3/EtOAc, 2:3); UV–Vis: 269 nm (3.16); IR: ν = 3504w, 3295w, 3064vw, 2931m, 2858w, 1458w, 1314s, 1154vs, 1131m, 1066m, 871w, 806w, 760m, 725w, 711m, 688m, 592vs, 541m, 491m, 419vw cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 7.80 (dd, J = 7.8, 1.4 Hz, 1H, 3-H), 7.60 (t, J = 5.8 Hz, 1H, 6-H), 7.50 (td, J = 7.5, 1.4 Hz, 1H, 4-H), 7.41–7.34 (m, 4H, 5-H, NH, NH2), 3.98 (t, J = 6.5 Hz, 2H, 14-H), 2.75 (td, J = 7.0, 5.8 Hz, 2H, 8-H), 2.57 (s, 3H, 7-H), 1.60–1.53 (m, 2H, 13-H), 1.37–1.28 (m, 2H, 9-H), 1.27–1.20 (m, 2H, 12-H), 1.19–1.13 (m, 4H, 10-H, 11-H) ppm; 13C NMR:δ = 138.9 (C-1), 136.4 (C-2), 132.4 (C-4), 132.3 (C-3), 128.3 (C-6), 126.1 (C-5), 68.9 (C-14), 42.2 (C-8), 28.9 (C-13), 28.2 (C-9), 27.9 (C-11), 25.8 (C-12), 24.9 (C-10), 19.8 (C-7) ppm; MS: m/z = 387.4 (100%, [M + Na]+); anal. calcd. for C14H24N2S2O5 (364.48): C 46.14, H 6.64, N 7.69; found: C 45.96, H 6.90, N 7.36.
4.2.65. N-(8-Hydroxyoctyl)-2-methylbenzene Sulfonamide (32a)
Applying GPA: from 2-methylbenzenesulfonyl chloride (500 mg, 2.62 mmol) and 8-amino-octanol (571 mg, 3.93 mmol): 32a (766 mg, 98%); oil; Rf = 0.24 (petrolether/EtOAc, 2:3); UV–Vis: 223 nm (3.91); IR: ν = 3504w, 3295w, 2929m, 2856m, 1458m, 1315s, 1154vs, 1131m, 1066m, 876vw, 807w, 759m, 723w, 711m, 688m, 592vs, 541m, 489m cm−1; 1H NMR:δ = 7.80 (dd, J = 7.8, 1.4 Hz, 1H, 3-H), 7.62–7.55 (m, 1H, 6-H), 7.50 (td, J = 7.5, 1.4 Hz, 1H, 4-H), 7.41–7.34 (m, 2H, 5-H, NH), 4.29 (t, J = 5.1 Hz, 1H, OH), 3.35 (td, J = 6.6, 5.0 Hz, 2H, 15-H), 2.74 (t, J = 7.0 Hz, 2H, 8-H), 2.57 (s, 3H, 7-H), 1.41–1.26 (m, 4H, 9-H, 14-H), 1.25–1.07 (m, 8H, 10-H, 11-H, 12-H, 13-H) ppm; 13C NMR:δ = 139.4 (C-1), 136.9 (C-2), 132.9 (C-4), 132.7 (C-3), 128.8 (C-6), 126.6 (C-5), 61.2 (C-15), 42.7 (C-8), 32.9 (C-14), 29.4 (C-9), 29.2 (C-11), 29.0 (C-12), 26.3 (C-10), 25.8 (C-13), 20.2 (C-7) ppm; MS: m/z = 322.1 (100%, [M + Na]+); anal. calcd. for C15H25NSO3 (299.43): C 60.17, H 8.42, N 4.68; found: C 59.84, H 8.66, N 4.32.
4.2.66. 8-[(2-Methylphenyl)sulfonamido]octyl Sulfamate (32b)
Applying GPB: from 32a (300 mg, 1.00 mmol): 32b (283 mg, 75%); oil; Rf = 0.76 (CHCl3/EtOAc, 2:3); UV–Vis: 270 nm (3.16); IR: ν = 3280w, 3114vw, 2931w, 2857w, 1563w, 1460w, 1361m, 1314m, 1178s, 1154vs, 1131m, 1066m, 1048w, 924s, 807w, 761m, 723w, 711m, 689m, 593s, 551s, 542s, 491m cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 7.80 (dd, J = 7.8, 1.4 Hz, 1H, 3-H), 7.59 (t, J = 5.7 Hz, 1H, 6-H), 7.50 (td, J = 7.5, 1.4 Hz, 1H, 4-H), 7.40–7.34 (m, 4H, 5-H, NH, NH2), 3.99 (t, J = 6.5 Hz, 2H, 15-H), 2.75 (td, J = 7.0, 5.8 Hz, 2H, 8-H), 2.57 (s, 3H, 7-H), 1.62–1.54 (m, 2H, 14-H), 1.36–1.22 (m, 4H, 9-H, 13-H), 1.21–1.09 (m, 6H, 10-H, 11-H, 12-H) ppm; 13C NMR:δ = 138.9 (C-1), 136.4 (C-2), 132.4 (C-4), 132.3 (C-3), 128.3 (C-6), 126.1 (C-5), 69.0 (C-15), 42.2 (C-8), 29.0 (C-14), 28.3 (C-9), 28.3 (C-11), 28.3 (C-12), 25.8 (C-13), 24.9 (C-10), 19.8 (C-7) ppm; MS: m/z = 401.3 (100%, [M + Na]+); anal. calcd. for C15H26N2S2O5 (378.50): C 47.60, H 6.92, N 7.40; found: C 47.42, H 7.18, N 7.20.
4.2.67. N-(2-Hydroxyethyl)-4-isopropylbenzene Sulfonamide (33a) [117867-88-6]
Applying GPA: from 4-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 2-amino-ethanol (209 mg, 3.43 mmol):
33a (441 mg, 79%) [
115,
116,
117] oil; R
f = 0.16 (petrolether/EtOAc, 2:3); UV–Vis: 228 nm (4.08); IR: ν = 3516
m, 3168
w, 2955
m, 2891
w, 2870
w, 1599
w, 1494
w, 1464
w, 1453
w, 1433
w, 1411
m, 1354
vw, 1323
s, 1282
w, 1261
w, 1211
w, 1187
w, 1158
vs, 1107
w, 1090
m, 1070
m, 1051
s, 1018
w, 956
s, 905
w, 851
w, 845
w, 824
m, 777
s, 738
m, 724
m, 646
m, 632
w, 580
s, 562
s, 532
w, 496
w, 484
w, 462
m, 451
w cm
−1;
1H NMR:δ = 7.73–7.69 (
m, 2H, 2-H, 2′-H), 7.53–7.43 (
m, 3H, 3H-, 3′-H, NH), 4.66 (
s, 1H, OH), 3.37 (
t,
J = 6.4 Hz, 2H, 8-H), 2.97 (
hept,
J = 6.9 Hz, 1H, 5-H), 2.77 (
t,
J = 6.4 Hz, 2H, 7-H), 1.22 (
d,
J = 6.9 Hz, 6H, 6-H, 6′-H) ppm;
13C NMR:δ = 153.0 (C-4), 138.0 (C-1), 127.0 (C-2), 126.6 (C-3), 59.9 (C-8), 45.1 (C-7), 33.3 (C-5), 23.5 (C-6) ppm; MS:
m/
z = 266.2 (100%, [M + Na]
+); anal. calcd. for C
11H
17NSO
3 (243.32): C 54.30, H 7.04, N 5.76; found: C 54.03, H 7.28, N 5.44.
4.2.68. 2-[(4-Isopropylphenyl)sulfonamido]ethyl Sulfamate (33b)
Applying GPB: from 33a (300 mg, 1.23 mmol): 33b (241 mg, 61%); white solid; Rf = 0.59 (CHCl3/EtOAc, 2:3); m.p. = 81–82 °C; UV–Vis: 228 nm (4.03); IR: ν = 3276m, 2963w, 1598w, 1559w, 1411w, 1364s, 1319s, 1284w, 1179s, 1157vs, 1091m, 1054w, 1015m, 924s, 832m, 775m, 753m, 649s, 632w, 549s, 488w, 436w cm−1; 1H NMR:δ = 7.83 (t, J = 6.0 Hz, 1H, NH), 7.76–7.70 (m, 2H, 2-H, 2′-H), 7.53–7.44 (m, 4H, 3-H, 3′-H, NH2), 4.01 (t, J = 5.7 Hz, 2H, 8-H), 3.03 (q, J = 5.4 Hz, 2H, 7-H), 3.00–2.92 (m, 1H, 5-H), 1.22 (d, J = 6.9 Hz, 6H, 6-H, 6′-H) ppm; 13C NMR:δ = 153.3 (C-4), 137.7 (C-1), 127.2 (C-2), 126.6 (C-3), 67.6 (C-8), 41.6 (C-7), 33.4 (C-5), 23.5 (C-6) ppm; MS: m/z = 345.2 (100%, [M + Na]+); anal. calcd. for C11H18N2S2O5 (322.39): C 40.98, H 5.63, N 8.69; found: C 40.71, H 5.98, N 8.43.
4.2.69. N-(3-Hydroxypropyl)-4-isopropylbenzene Sulfonamide (34a) [920113-99-1]
Applying GPA: from 4-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 3-amino-propanol (258 mg, 3.43 mmol): 34a (558 mg, 95%); oil; Rf = 0.16 (petrolether/EtOAc, 2:3); UV–Vis: 228 nm (4.22); IR: ν = 3501w, 3278w, 2962w, 2874w, 1598w, 1464w, 1410m, 1386w, 1364w, 1317s, 1283m, 1156vs, 1091s, 1053s, 1016w, 1008w, 959w, 833m, 774m, 648s, 632m, 579s, 565s, 486w cm−1; 1H NMR:δ = 7.73–7.68 (m, 2H, 2-H, 2′-H), 7.48–7.40 (m, 3H, 3-H, 3′-H, NH), 4.40 (t, J = 5.1 Hz, 1H, OH), 3.37 (td, J = 6.2, 5.0 Hz, 2H, 9-H), 2.97 (hept, J = 7.0 Hz, 1H, 5-H), 2.77 (td, J = 7.3, 5.8 Hz, 2H, 7-H), 1.57–1.49 (m, 2H, 8-H), 1.22 (d, J = 6.9 Hz, 6H, 6-H, 6′-H) ppm; 13C NMR:δ = 153.0 (C-4), 137.9 (C-1), 127.0 (C-2), 126.6 (C-3), 58.1 (C-9), 40.0 (C-7), 33.3 (C-5), 32.4 (C-8), 23.5 (C-6) ppm; MS: m/z = 280.1 (100%, [M + Na]+); anal. calcd. for C12H19NSO3 (257.35): C 56.01, H 7.44, N 5.44; found: C 55.76, H 7.62, N 5.18.
4.2.70. 3-[(4-Isopropylphenyl)sulfonamido]propyl Sulfamate (34b)
Applying GPB: from 34a (200 mg, 0.78 mmol): 34b (226 mg, 86%); white solid; Rf = 0.60 (CHCl3/EtOAc, 2:3); m.p. = 83–84 °C; UV–Vis: 228 nm (4.11); IR: ν = 3359m, 3285m, 3262m, 2963w, 1599w, 1565w, 1474w, 1468w, 1435m, 1402m, 1372vs, 1337w, 1311s, 1283m, 1255w, 1176s, 1157vs, 1111w, 1091m, 1068m, 1054m, 1039m, 1017w, 943s, 920s, 887m, 835s, 827s, 774m, 756m, 733w, 676s, 635m, 596m, 579s, 561s, 546vs, 520s, 486m, 426m cm−1; 1H NMR:δ = 7.73–7.68 (m, 2H, 2-H, 2′-H), 7.60 (t, J = 5.9 Hz, 1H, NH), 7.49–7.44 (m, 2H, 3-H, 3′-H), 7.41 (s, 2H, NH2), 4.03 (t, J = 6.3 Hz, 2H, 9-H), 2.98 (hept, J = 7.0 Hz, 1H, 5-H), 2.81 (td, J = 7.1, 5.8 Hz, 2H, 7-H), 1.77 (p, J = 6.7 Hz, 2H, 8-H), 1.22 (d, J = 6.9 Hz, 6H, 6-H, 6′-H) ppm; 13C NMR:δ = 153.2 (C-4), 137.7 (C-1), 127.3 (C-2), 126.6 (C-3), 66.5 (C-9), 39.2 (C-7), 33.3 (C-5), 28.8 (C-8), 23.5 (C-6) ppm; MS: m/z = 359.3 (100%, [M + Na]+); anal. calcd. for C12H20N2S2O5 (336.42): C 42.84, H 5.99, N 8.33; found: C 42.67, H 6.20, N 8.02.
4.2.71. N-(4-Hydroxybutyl)-4-isopropylbenzene Sulfonamide (35a) [1082772-50-6]
Applying GPA: from 4-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 4-amino-butanol (306 mg, 3.43 mmol): 35a (540 mg, 87%); oil; Rf = 0.14 (petrolether/EtOAc, 2:3); UV–Vis: 228 nm (4.32); IR: ν = 3501w, 3281w, 2961m, 2871w, 1598w, 1463w, 1410m, 1386w, 1364w, 1318s, 1283w, 1156vs, 1091s, 1053s, 1017w, 991vw, 833m, 773w, 735w, 648s, 632m, 581s, 565s, 488w, 471w cm−1; 1H NMR:δ = 7.72–7.68 (m, 2H, 2-H, 2′-H), 7.48–7.43 (m, 3H, 3-H, 3′-H, NH), 4.39–4.31 (m, 1H, OH), 3.37–3.27 (m, 2H, 10-H), 2.97 (hept, J = 6.9 Hz, 1H, 5-H), 2.75–2.67 (m, 2H, 7-H), 1.44–1.31 (m, 4H, 8-H, 9-H), 1.22 (d, J = 6.9 Hz, 6H, 6-H, 6′-H) ppm; 13C NMR:δ = 152.9 (C-4), 138.1 (C-1), 127.0 (C-2), 126.6 (C-3), 60.2 (C-10), 42.5 (C-7), 33.3 (C-5), 29.5 (C-9), 25.8 (C-8), 23.5 (C-6) ppm; MS: m/z = 294.3 (100%, [M + Na]+); anal. calcd. for C13H21NSO3 (271.38): C 57.54, H 7.80, N 5.16; found: C 57.21, H 8.04, N 4.87.
4.2.72. 4-[(4-Isopropylphenyl)sulfonamido]butyl Sulfamate (35b)
Applying GPB: from 35a (200 mg, 0.74 mmol): 35b (233 mg, 90%); white solid; Rf = 0.61 (CHCl3/EtOAc, 2:3); m.p. = 95–97 °C; UV–Vis: 228 nm (4.16); IR: ν = 3356m, 3286m, 3262m, 2965w, 2879w, 1558w, 1481w, 1476w, 1431w, 1409w, 1398w, 1384w, 1366s, 1337m, 1320s, 1284w, 1203w, 1175s, 1157vs, 1143m, 1093m, 1063m, 1047w, 969s, 942w, 922s, 901s, 840m, 824s, 773m, 750w, 732w, 666s, 632m, 588s, 580s, 552vs, 513m, 505m, 415w cm−1; 1H NMR:δ = 7.73–7.68 (m, 2H, 2-H, 2′-H), 7.53 (t, J = 5.9 Hz, 1H, NH), 7.48–7.43 (m, 2H, 3-H, 3′-H), 7.38 (s, 2H, NH2), 3.96 (t, J = 6.3 Hz, 2H, 10-H), 2.98 (hept, J = 6.9 Hz, 1H, 5-H), 2.75 (td, J = 6.9, 6.0 Hz, 2H, 7-H), 1.66–1.56 (m, 2H, 9-H), 1.50–1.41 (m, 2H, 8-H), 1.22 (d, J = 6.9 Hz, 6H, 6-H, 6′-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 153.0 (C-4), 138.0 (C-1), 127.1 (C-2), 126.6 (C-3), 68.5 (C-10), 42.0 (C-7), 33.3 (C-5), 25.6 (C-8), 25.4 (C-9), 23.5 (6) ppm; MS: m/z = 373.3 (100%, [M + Na]+); anal. calcd. for C13H22N2S2O5 (350.45): C 44.56, H 6.33, N 7.99; found: C 44.27, H 6.68, N 7.65.
4.2.73. N-(5-Hydroxypentyl)-4-isopropylbenzene Sulfonamide (36a) [1925596-35-5]
Applying GPA: from 4-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 5-amino-pentanol (354 mg, 3.43 mmol): 36a (604 mg, 93%); oil; Rf = 0.18 (petrolether/EtOAc, 2:3); UV–Vis: 228 nm (4.42); IR: ν = 3503w, 3278w, 2960w, 2936m, 2869w, 1598w, 1460w, 1410m, 1386w, 1364w, 1318s, 1283w, 1156vs, 1091s, 1053m, 1016w, 833m, 774w, 733w, 648s, 632m, 581s, 565s cm−1; 1H NMR:δ = 7.72–7.67 (m, 2H, 2-H, 2′-H), 7.48–7.42 (m, 3H, 3-H, 3′-H, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.35–3.29 (m, 2H, 11-H), 2.97 (hept, J = 7.0 Hz, 1H, 5-H), 2.70 (td, J = 6.8, 6.0 Hz, 2H, 7-H), 1.40–1.27 (m, 4H, 8-H, 10-H), 1.27–1.16 (m, 8H, 6-H, 6′-H, 9-H) ppm; 13C NMR:δ = 152.9 (C-4), 138.1 (C-1), 127.0 (C-2), 126.6 (C-3), 60.5 (C-11), 42.6 (C-7), 33.3 (C-5), 32.0 (C-10), 28.9 (C-8), 23.5 (C-6), 22.6 (C-9) ppm; MS: m/z = 308.1 (100%, [M + Na]+); anal. calcd. for C14H23NSO3 (285.40): C 58.92, H 8.12, N 4.91; found: C 58.76, H 8.47, N 4.59.
4.2.74. 5-[(4-Isopropylphenyl)sulfonamido]pentyl Sulfamate (36b)
Applying GPB: from 36a (200 mg, 0.7 mmol): 36b (227 mg, 89%); white solid; Rf = 0.66 (CHCl3/EtOAc, 2:3); m.p. = 95–97 °C; UV–Vis: 228 nm (4.14); IR: ν = 3350w, 3280m, 2963w, 2946w, 2869w, 2859vw, 1475vw, 1432w, 1407w, 1400w, 1386w, 1366s, 1328s, 1310m, 1286w, 1191w, 1174m, 1157vs, 1142m, 1112w, 1094m, 1068w, 1058w, 1038m, 963s, 922m, 900m, 843w, 825s, 771w, 737w, 733w, 661s, 632m, 584s, 552vs, 525m, 517m, 501w, 444w cm−1; 1H NMR:δ = 7.72–7.68 (m, 2H, 2-H, 2′-H), 7.51–7.43 (m, 3H, 3-H, 3′-H, NH), 7.37 (s, 2H, NH2), 3.96 (t, J = 6.5 Hz, 2H, 11-H), 2.97 (hept, J = 7.0 Hz, 1H, 5-H), 2.72 (q, J = 6.5 Hz, 2H, 7-H), 1.55 (p, J = 6.7 Hz, 2H, 10-H), 1.43–1.34 (m, 2H, 8-H), 1.34–1.26 (m, 2H, 9-H), 1.22 (d, J = 6.9 Hz, 6H, 6-H, 6′-H) ppm; 13C NMR:δ = 153.0 (C-4), 138.0 (C-1), 127.1 (C-2), 126.6 (C-3), 68.8 (C-11), 42.3 (C-7), 28.5 (C-10), 27.8 (C-8), 23.5 (C-6), 22.2 (C-9) ppm; MS: m/z = 387.3 (100%, [M + Na]+); anal. calcd. for C14H24N2S2O5 (364.48): C 46.14, H 6.64, N 7.69; found: C 45.84, H 6.99, N 7.44.
4.2.75. N-(6-Hydroxyhexyl)-4-isopropylbenzene Sulfonamide (37a) [1912844-40-6]
Applying GPA: from 4-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 6-amino-hexanol (402 mg, 3.43 mmol): 37a (612 mg, 89%); oil; Rf = 0.20 (petrolether/EtOAc, 2:3); UV–Vis: 228 nm (4.08); IR: ν = 3505w, 3282w, 2960w, 2933m, 2864w, 1598w, 1462w, 1410m, 1386w, 1364w, 1319s, 1283w, 1157vs, 1092s, 1073m, 1053m, 1016w, 891vw, 833m, 773w, 727w, 649s, 633m, 581s, 565s, 493w, 456w cm−1; 1H NMR:δ = 7.73–7.68 (m, 2H, 2-H, 2′-H), 7.48–7.43 (m, 3H, 3-H, 3′-H, NH), 4.32–4.27 (m, 1H, OH), 3.34 (td, J = 6.3, 2.9 Hz, 2H, 12-H), 2.97 (hept, J = 6.9 Hz, 1H, 5-H), 2.71 (td, J = 7.0, 5.9 Hz, 2H, 7-H), 1.38–1.29 (m, 4H, 8-H, 11-H), 1.22 (d, J = 6.9 Hz, 6H, 6-H, 6′-H), 1.20–1.15 (m, 4H, 9-H, 10-H) ppm; 13C NMR:δ = 152.9 (C-4), 138.2 (C-1), 127.0 (C-2), 126.6 (C-3), 60.6 (C-12), 42.5 (C-7), 33.3 (C-5), 32.3 (C-11), 29.0 (C-8), 25.9 (C-10), 25.0 (C-9), 23.5 (C-6) ppm; MS: m/z = 322.1 (100%, [M + Na]+); anal. calcd. for C15H25NSO3 (299.43): C 60.17, H 8.42, N 4.68; found: C 59.76, H 8.72, N 4.43.
4.2.76. 6-[(4-Isopropylphenyl)sulfonamido]hexyl Sulfamate (37b)
Applying GPB: from 37a (300 mg, 1.00 mmol): 37b (260 mg, 69%); white solid; Rf = 0.72 (CHCl3/EtOAc, 2:3); m.p. = 102–103 °C; UV–Vis: 228 nm (4.19); IR: ν = 3384w, 3278m, 2962m, 2933w, 2860w, 1600w, 1544w, 1477w, 1469w, 1420m, 1396w, 1374s, 1320s, 1312s, 1283w, 1179s, 1157vs, 1146s, 1112w, 1092m, 1065m, 1054m, 1003s, 977s, 951w, 924m, 907m, 873m, 845w, 830m, 814s, 802s, 774m, 757w, 697s, 647m, 633m, 579s, 564s, 551vs, 537s, 505m, 484w, 456w cm−1; 1H NMR:δ = 7.72–7.67 (m, 2H, 2-H, 2′-H), 7.49–7.43 (m, 3H, 3-H, 3′-H, NH), 7.37 (s, 2H, NH2), 3.97 (t, J = 6.5 Hz, 2H, 12-H), 2.97 (hept, J = 6.9 Hz, 1H, 5-H), 2.71 (q, J = 6.7 Hz, 2H, 7-H), 1.56 (p, J = 6.6 Hz, 2H, 11-H), 1.35 (p, J = 6.8 Hz, 2H, 8-H), 1.28–1.19 (m, 10H, 6-H, 6′-H, 9-H, 10-H) ppm; 13C NMR:δ = 153.0 (C-4), 138.1 (C-1), 127.0 (C-2), 126.6 (C-3), 68.9 (C-12), 42.4 (C-7), 33.3 (C-5), 28.8 (C-11), 28.2 (C-8), 25.5 (C-10), 24.6 (C-9), 23.5 (C-6) ppm; MS: m/z = 401.2 (100%, [M + Na]+); anal. calcd. for C15H26N2S2O5 (378.50): C 47.60, H 6.92, N 7.40; found: C 47.36, H 7.20, N 7.05.
4.2.77. N-(7-Hydroxyheptyl)-4-isopropylbenzene Sulfonamide (38a)
Applying GPA: from 4-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 7-amino-heptanol (450 mg, 3.43 mmol): 38a (658 mg, 92%); oil; Rf = 0.26 (petrolether/EtOAc, 2:3); UV–Vis: 228 nm (4.11); IR: ν = 3503w, 3281w, 2960w, 2931m, 2859m, 1598w, 1495vw, 1463w, 1410m, 1386w, 1364w, 1319s, 1283w, 1157vs, 1092s, 1053m, 1016w, 891vw, 833m, 773w, 724w, 648s, 633m, 581s, 565s, 493w, 485w cm−1; 1H NMR:δ = 7.70–7.66 (m, 2H, 2-H, 2′-H), 7.45–7.40 (m, 3H, 3-H, 3′-H, NH), 3.97 (s, 1H, OH), 3.33 (t, J = 6.6 Hz, 2H, 13-H), 2.94 (hept, J = 6.9 Hz, 1H, 5-H), 2.69 (td, J = 7.0, 5.9 Hz, 2H, 7-H), 1.39–1.27 (m, 4H, 8-H, 12-H), 1.19 (d, J = 6.9 Hz, 6H, 6-H, 6′-H), 1.17–1.10 (m, 6H, 9-H, 10-H, 11-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 152.9 (C-4), 138.2 (C-1), 127.0 (C-2), 126.6 (C-3), 60.7 (C-13), 42.5 (C-7), 33.3 (C-5), 32.4 (C-12), 28.9 (C-8), 28.4 (C-10), 26.1 (C-9), 25.3 (C-11), 23.5 (C-6) ppm; MS: m/z = 336.2 (100%, [M + Na]+); anal. calcd. for C16H27NSO3 (313.46): C 61.31, H 8.68, N 4.47; found: C 61.00, H 8.95, N 4.18.
4.2.78. 7-[(4-Isopropylphenyl)sulfonamido]heptyl Sulfamate (38b)
Applying GPB: from 38a (200 mg, 0.67 mmol): 38b (230 mg, 90%); white solid; Rf = 0.75 (CHCl3/EtOAc, 2:3); m.p. = 70–72 °C; UV–Vis: 228 nm (4.10); IR: ν = 3382w, 3274m, 2966w, 2921m, 2911w, 2852w, 1605w, 1543w, 1475w, 1467w, 1420m, 1392w, 1376vs, 1320s, 1281w, 1181s, 1159s, 1110w, 1090m, 1065w, 1056m, 1040m, 1018w, 1000m, 978s, 945w, 926m, 892m, 852w, 843w, 831m, 815s, 776m, 762w, 733m, 698s, 645m, 639w, 580s, 566s, 551vs, 530s, 510m, 486w, 477w, 445w cm−1; 1H NMR:δ = 7.72–7.68 (m, 2H, 2-H, 2′-H), 7.48–7.43 (m, 3H, 3-H, 3′-H, NH), 7.37 (s, 2H, NH2), 3.98 (t, J = 6.5 Hz, 2H, 13-H), 2.97 (hept, J = 6.9 Hz, 1H, 5-H), 2.71 (q, J = 6.8 Hz, 2H, 7-H), 1.63–1.53 (m, 2H, 12-H), 1.38–1.31 (m, 2H, 8-H), 1.22 (d, J = 6.9 Hz, 6H, 6-H, 6′-H), 1.30–1.14 (m, 6H, 9-H, 10-H, 11-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 153.0 (C-4), 138.1 (C-1), 127.0 (C-2), 126.6 (C-3), 68.9 (C-13), 42.5 (C-7), 33.3 (C-5), 28.9 (C-12), 28.2 (C-8), 28.0 (C-10), 25.8 (C-11), 24.9 (C-9), 23.5 (C-6) ppm; MS: m/z = 415.3 (100%, [M + Na]+); anal. calcd. for C16H28N2S2O5 (392.53): C 48.96, H 7.19, N 7.14; found: C 48.73, H 7.41, N 6.87.
4.2.79. N-(8-Hydroxyoctyl)-4-isopropylbenzene Sulfonamide (39a)
Applying GPA: from 4-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 8-amino-octanol (498 mg, 3.43 mmol): 39a (721 mg, 96%); oil; Rf = 0.28 (petrolether/EtOAc, 2:3); UV–Vis: 228 nm (4.23); IR: ν = 3500w, 3281w, 2929m, 2857m, 1599w, 1463w, 1410w, 1364w, 1320m, 1283m, 1158s, 1092m, 1053m, 1017w, 924w, 892w, 833m, 809w, 773w, 722m, 649m, 582w, 566m, 514w, 488w cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 7.71–7.68 (m, 2H, 2-H, 2′-H), 7.47–7.43 (m, 3H, 3-H, 3′-H, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.36 (td, J = 6.5, 5.1 Hz, 2H, 14-H), 2.97 (hept, J = 6.9 Hz, 1H, 5-H), 2.71 (td, J = 7.0, 5.8 Hz, 2H, 7-H), 1.41–1.29 (m, 4H, 8-H, 13-H), 1.21 (d, J = 6.9 Hz, 6H, 6-H, 6′-H), 1.20–1.11 (m, 8H, 9-H, 10-H, 11-H, 12-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 152.9 (C-4), 138.2 (C-1), 127.0 (C-2), 126.6 (C-3), 60.7 (C-14), 42.5 (C-7), 33.3 (C-5), 32.5 (C-13), 28.9 (C-8), 28.8 (C-12), 28.6 (C-9), 25.9 (C-11), 25.4 (C-10), 23.5 (C-6) ppm; MS: m/z = 350.2 (100%, [M + Na]+); anal. calcd. for C17H29NSO3 (327.48): C 62.35, H 8.93, N 4.28; found: C 62.03, H 9.22, N 3.93.
4.2.80. 8-[(4-Isopropylphenyl)sulfonamido]octyl Sulfamate (39b)
Applying GPB: from 39a (200 mg, 0.61 mmol): 39b (220 mg, 89%); white solid; Rf = 0.80 (CHCl3/EtOAc, 2:3); m.p. = 69–70 °C; UV–Vis: 229 nm (4.08); IR: ν = 3385w, 3278m, 2961w, 2929m, 2915w, 2853w, 1600w, 1543w, 1477w, 1468w, 1419m, 1396w, 1376vs, 1319s, 1283w, 1182s, 1158s, 1112w, 1093m, 1069w, 1055m, 1038m, 1016w, 1001m, 977s, 945w, 925m, 894m, 858w, 845w, 830m, 814s, 775m, 762w, 731m, 699s, 648m, 633w, 582s, 564s, 550vs, 532s, 513m, 485w, 477w, 443w cm−1; 1H NMR:δ = 7.72–7.66 (m, 2H, 2-H, 2′-H), 7.47–7.43 (m, 3H, 3-H, 3′-H, NH), 7.37 (s, 2H, NH2), 3.99 (t, J = 6.5 Hz, 2H, 14-H), 2.97 (hept, J = 6.9 Hz, 1H, 5-H), 2.75–2.66 (m, 2H, 7-H), 1.64–1.54 (m, 2H, 13-H), 1.40–1.11 (m, 10H, 8-H, 9-H, 10-H, 11-H, 12-H), 1.22 (d, J = 6.9 Hz, 6H, 6-H, 6′-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 153.0 (C-4), 138.2 (C-1), 127.0 (C-2), 126.6 (C-3), 69.0 (C-14), 42.5 (C-7), 33.3 (C-5), 28.9 (C-13), 28.4 (C-11), 28.3 (C-8), 28.3 (C-10), 25.9 (C-9), 24.9 (C-12), 23.5 (C-6) ppm; MS: m/z = 429.4 (100%, [M + Na]+); anal. calcd. for C17H30N2S2O5 (406.56): C 50.22, H 7.44, N 6.89; found: C 49.93, H 7.71, N 6.65.
4.2.81. N-(2-Hydroxyethyl)-3-isopropylbenzene Sulfonamide (40a)
Applying GPA: from 3-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 2-amino-ethanol (209 mg, 3.43 mmol): 40a (548 mg, 98%); oil; Rf = 0.16 (petrolether/EtOAc, 2:3); UV–Vis: 222 nm (3.94); IR: ν = 3492w, 3275w, 2962w, 2874w, 1478w, 1461w, 1428m, 1386w, 1365w, 1323s, 1306s, 1217w, 1156vs, 1144s, 1090m, 1052m, 998vw, 949m, 902w, 798m, 695s, 623m, 586vs, 564m, 542m, 517m, 470m, 460w cm−1; 1H NMR:δ = 7.69–7.66 (m, 1H, 5-H), 7.64–7.60 (m, 1H, 6-H), 7.55 (t, J = 5.9 Hz, 1H, NH), 7.53–7.48 (m, 2H, 2-H, 4-H), 4.67 (t, J = 5.6 Hz, 1H, OH), 3.38 (td, J = 6.3, 5.5 Hz, 2H, 10-H), 2.98 (hept, J = 6.9 Hz, 1H, 7-H), 2.80 (q, J = 6.2 Hz, 2H, 9-H), 1.22 (d, J = 6.9 Hz, 6H, 8-H, 8′-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 149.6 (C-3), 140.7 (C-1), 130.4 (C-4), 129.1 (C-5), 124.1 (C-2), 124.0 (C-6), 59.9 (C-10), 45.1 (C-9), 33.3 (C-7), 23.6 (C-8) ppm; MS: m/z = 266.2 (100%, [M + Na]+); anal. calcd. for C11H17NSO3 (243.32): C 54.30, H 7.04, N 5.76; found: C 53.99, H 7.36, N 5.41.
4.2.82. 2-[(3-Isopropylphenyl)sulfonamido]ethyl Sulfamate (40b)
Applying GPB: from 40a (200 mg, 0.82 mmol): 40b (238 mg, 90%); oil; Rf = 0.64 (CHCl3/EtOAc, 2:3); UV–Vis: 269 nm (2.95); IR: ν = 3275m, 2963w, 2873vw, 1558w, 1479w, 1461w, 1430w, 1364s, 1325s, 1307s, 1179s, 1157vs, 1101m, 1091m, 1070w, 1021m, 998w, 924s, 798m, 757m, 694s, 624m, 585vs, 549s, 491w, 445w, 432w cm−1; 1H NMR:δ = 7.88 (t, J = 6.0 Hz, 1H, NH), 7.69–7.66 (m, 1H, 5-H), 7.63 (dt, J = 6.7, 2.0 Hz, 1H, 6-H), 7.56–7.47 (m, 4H, 2-H, 4-H, NH2), 4.01 (t, J = 5.7 Hz, 2H, 10-H), 3.05 (q, J = 5.0, 4.3 Hz, 2H, 9-H), 3.03–2.94 (m, 1H, 7-H), 1.23 (d, J = 6.9 Hz, 6H, 8-H, 8′-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 149.7 (C-3), 140.3 (C-1), 130.6 (C-4), 129.3 (C-5), 124.1 (C-2), 124.0 (C-6), 67.6 (C-10), 41.6 (C-9), 33.3 (C-7), 23.6 (C-8) ppm; MS: m/z = 345.3 (100%, [M + Na]+); anal. calcd. for C11H18N2S2O5 (322.39): C 40.98, H 5.63, N 8.69; found: C 40.66, H 6.01, N 8.40.
4.2.83. N-(3-Hydroxypropyl)-3-isopropylbenzene Sulfonamide (41a)
Applying GPA: from 3-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 3-amino-propanol (258 mg, 3.43 mmol): 41a (562 mg, 95%); oil; Rf = 0.16 (petrolether/EtOAc, 2:3); UV–Vis: 222 nm (3.91); IR: ν = 3496w, 3279w, 2962m, 2875w, 1478w, 1462w, 1426m, 1386w, 1365w, 1323m, 1305s, 1217w, 1156vs, 1144s, 1084m, 1068s, 1008w, 998w, 960w, 904w, 871w, 799m, 696s, 623m, 587vs, 530m, 488w cm−1; 1H NMR:δ = 7.67–7.64 (m, 1H, 5-H), 7.62–7.58 (m, 1H, 6-H), 7.54–7.45 (m, 3H, 2-H, 4-H, NH), 4.39 (t, J = 5.1 Hz, 1H, OH), 3.36 (td, J = 6.3, 5.0 Hz, 2H, 11-H), 2.99 (hept, J = 6.9 Hz, 1H, 7-H), 2.79 (td, J = 7.1, 5.2 Hz, 2H, 9-H), 1.57–1.47 (m, 2H, 10-H), 1.23 (d, J = 6.9 Hz, 6H, 8-H, 8′-H) ppm; 13C NMR:δ = 149.6 (C-3), 140.5 (C-1), 130.4 (C-4), 129.1 (C-5), 124.1 (C-2), 124.0 (C-6), 58.1 (C-11), 40.0 (C-9), 33.3 (C-7), 32.3 (C-10), 23.6 (C-8) ppm; MS: m/z = 280.2 (100%, [M + Na]+); anal. calcd. for C12H19NSO3 (257.35): C 56.01, H 7.44, N 5.44; found: C 55.71, H 7.73, N 5.18.
4.2.84. 3-[(3-Isopropylphenyl)sulfonamido]propyl Sulfamate (41b)
Applying GPB: from 41a (200 mg, 0.78 mmol): 41b (176 mg, 67%); oil; Rf = 0.64 (CHCl3/EtOAc, 2:3); UV–Vis: 269 nm (2.90); IR: ν = 3273m, 2964w, 2874w, 1560w, 1478w, 1464w, 1427w, 1364s, 1324s, 1306s, 1217w, 1177s, 1156vs, 1145s, 1090m, 1070w, 1051w, 940s, 824w, 798m, 738w, 695s, 623m, 585vs, 550s, 494m cm−1; 1H NMR:δ = 7.69–7.63 (m, 2H, 5-H, NH), 7.63–7.59 (m, 1H, 6-H), 7.56–7.49 (m, 2H, 2-H, 4-H), 7.41 (s, 2H, NH2), 4.03 (t, J = 6.3 Hz, 2H, 11-H), 3.00 (hept, J = 6.9 Hz, 1H, 7-H), 2.83 (q, J = 6.7 Hz, 2H, 9-H), 1.80–1.72 (m, 2H, 10-H), 1.23 (d, J = 6.9 Hz, 6H, 8-H, 8′-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 149.7 (C-3), 140.3 (C-1), 130.5 (C-4), 129.2 (C-5), 124.1 (C-2), 124.0 (C-6), 66.5 (C-11), 39.2 (C-9), 33.3 (C-7), 28.7 (C-10), 23.6 (C-8) ppm; MS: m/z = 359.3 (100%, [M + Na]+); anal. calcd. for C12H20N2S2O5 (336.42): C 42.84, H 5.99, N 8.33; found: C 42.57, H 6.21, N 8.02.
4.2.85. N-(4-Hydroxybutyl)-3-isopropylbenzene Sulfonamide (42a)
Applying GPA: from 3-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 4-amino-butanol (306 mg, 3.43 mmol): 42a (593 mg, 95%); oil; Rf = 0.18 (petrolether/EtOAc, 2:3); UV–Vis: 223 nm (3.91); IR: ν = 3500w, 3277w, 2961w, 2871w, 1478w, 1462w, 1427m, 1386w, 1365w, 1323m, 1305s, 1217w, 1156s, 1144s, 1087m, 1067m, 1054m, 1034w, 998w, 904w, 866vw, 798m, 737w, 696s, 623m, 587vs, 564m, 517m, 493w, 477w, 464w cm−1; 1H NMR:δ = 7.66–7.64 (m, 1H, 5-H), 7.62–7.58 (m, 1H, 6-H), 7.54–7.47 (m, 3H, 2-H, 4-H, NH), 4.35 (t, J = 5.1 Hz, 1H, OH), 3.35–3.28 (m, 2H, 12-H), 2.99 (hept, J = 6.9 Hz, 1H, 7-H), 2.78–2.69 (m, 2H, 9-H), 1.44–1.31 (m, 4H, 10-H, 11-H), 1.22 (d, J = 6.9 Hz, 6H, 8-H, 8′-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 149.6 (C-3), 140.7 (C-1), 130.3 (C-4), 129.1 (C-5), 124.0 (C-2), 124.0 (C-6), 60.2 (C-12), 42.5 (C-9), 33.3 (C-7), 29.5 (C-11), 25.8 (C-10), 23.6 (C-8) ppm; MS: m/z = 294.3 (100%, [M + Na]+); anal. calcd. for C13H21NSO3 (271.38): C 57.54, H 7.80, N 5.16; found: C 57.27, H 8.05, N 4.92.
4.2.86. 4-[(3-Isopropylphenyl)sulfonamido]butyl Sulfamate (42b)
Applying GPB: from 42a (200 mg, 0.68 mmol): 42b (240 mg, 93%); oil; Rf = 0.64 (CHCl3/EtOAc, 2:3); UV–Vis: 269 nm (3.01); IR: ν = 3274m, 2963w, 2874w, 1562w, 1478w, 1463w, 1452w, 1428w, 1364m, 1324s, 1306s, 1268w, 1217vw, 1178s, 1156vs, 1145s, 1089m, 1069m, 1051w, 997w, 922s, 800m, 735w, 696s, 626m, 587vs, 551s cm−1; 1H NMR:δ = 7.67–7.64 (m, 1H, 5-H), 7.63–7.56 (m, 2H, 6-H, NH), 7.55–7.48 (m, 2H, 2-H, 4-H), 7.38 (s, 2H, NH2), 3.95 (t, J = 6.3 Hz, 2H, 12-H), 2.99 (hept, J = 6.9 Hz, 1H, 7-H), 2.81–2.72 (m, 2H, 9-H), 1.66–1.55 (m, 2H, 11-H), 1.49–1.39 (m, 2H, 10-H), 1.23 (d, J = 7.0 Hz, 6H, 8-H, 8′-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 149.6 (C-3), 140.6 (C-1), 130.4 (C-4), 129.2 (C-5), 124.0 (C-2), 123.9 (C-6), 68.5 (C-12), 42.0 (C-9), 33.3 (C-7), 25.6 (C-10), 25.4 (C-11), 23.6 (C-8) ppm; MS: m/z = 373.3 (100%, [M + Na]+); anal. calcd. for C13H22N2S2O5 (350.45): C 44.56, H 6.33, N 7.99; found: C 44.18, H 6.67, N 7.68.
4.2.87. N-(5-Hydroxypentyl)-3-isopropylbenzene Sulfonamide (43a)
Applying GPA: from 3-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 5-amino-pentanol (354 mg, 3.43 mmol): 43a (611 mg, 94%); oil; Rf = 0.20 (petrolether/EtOAc, 2:3; UV–Vis: 223 nm (4.03); IR: ν = 3503w, 3279w, 2960w, 2936w, 2868w, 1478w, 1460w, 1427w, 1386w, 1365w, 1323m, 1305s, 1217w, 1156s, 1144s, 1087m, 1069m, 1050m, 998w, 905w, 799m, 730w, 696s, 623m, 587vs, 564m, 526m, 500m, 469w, 465w, 458w, 449w cm−1; 1H NMR:δ = 7.66–7.64 (m, 1H, 5-H), 7.62–7.58 (m, 1H, 6-H), 7.53–7.47 (m, 3H, 2-H, 4-H, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.34–3.29 (m, 2H, 13-H), 2.99 (hept, J = 6.9 Hz, 1H, 7-H), 2.77–2.67 (m, 2H, 9-H), 1.39–1.27 (m, 4H, 10-H, 12-H), 1.22 (d, J = 6.9 Hz, 8H, 8-H, 8′-H, 11-H) ppm; 13C NMR:δ = 149.6 (C-3), 140.7 (C-1), 130.3 (C-4), 129.1 (C-5), 124.0 (C-2), 124.0 (C-6), 60.5 (C-13), 42.6 (C-9), 33.3 (C-7), 32.0 (C-12), 28.8 (C-10), 23.6 (C-8), 22.6 (C-11) ppm; MS: m/z = 308.1 (100%, [M + Na]+); anal. calcd. for C14H23NSO3 (285.40): C 58.92, H 8.12, N 4.91; found: C 58.66, H 8.32, N 4.63.
4.2.88. 5-[(3-Isopropylphenyl)sulfonamido]pentyl Sulfamate (43b)
Applying GPB: from 43a (200 mg, 0.70 mmol): 43b (160 mg, 63%); oil; Rf = 0.65 (CHCl3/EtOAc, 2:3); UV–Vis: 269 nm (2.99); IR: ν = 3275m, 2962w, 2871w, 1561w, 1478w, 1463w, 1428w, 1363s, 1323s, 1305s, 1217vw, 1176s, 1156vs, 1089m, 1070m, 1032w, 998w, 919s, 816m, 799m, 769w, 728w, 696s, 624m, 587vs, 551s cm−1; 1H NMR:δ = 7.66–7.64 (m, 1H, 5-H), 7.63–7.57 (m, 1H, 6-H), 7.56–7.47 (m, 3H, 2-H, 4-H, NH), 7.37 (s, 2H, NH2), 3.95 (t, J = 6.5 Hz, 2H, 13-H), 2.99 (hept, J = 7.0 Hz, 1H, 7-H), 2.73 (q, J = 6.6 Hz, 2H, 9-H), 1.55 (p, J = 6.7 Hz, 2H, 12-H), 1.43–1.34 (m, 2H, 10-H), 1.33–1.26 (m, 2H, 11-H), 1.23 (d, J = 6.9 Hz, 6H, 8-H, 8′-H) ppm; 13C NMR:δ = 149.6 (C-3), 140.6 (C-1), 130.4 (C-4), 129.2 (C-5), 124.0 (C-2), 124.0 (C-6), 68.8 (C-13), 42.3 (C-9), 33.3 (C-7), 28.5 (C-12), 27.8 (C-10), 23.6 (C-8), 22.2 (C-11) ppm; MS: m/z = 387.4 (100%, [M + Na]+); anal. calcd. for C14H24N2S2O5 (364.48): C 46.14, H 6.64, N 7.69; found: C 45.86, H 6.98, N 7.41.
4.2.89. N-(6-Hydroxyhexyl)-3-isopropylbenzene Sulfonamide (44a)
Applying GPA: from 3-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 6-amino-hexanol (402 mg, 3.43 mmol): 44a (658 mg, 96%); oil; Rf = 0.24 (petrolether/EtOAc, 2:3); UV–Vis: 223 nm (3.88); IR: ν 3502w, 3281w, 2960w, 2933m, 2863w, 1598vw, 1478w, 1462w, 1427w, 1386w, 1365w, 1323m, 1305s, 1217vw, 1156s, 1144s, 1087m, 1069m, 1051m, 999w, 904w, 798m, 726w, 696s, 624m, 587vs, 563m, 529m, 454w cm−1; 1H NMR:δ = 7.66–7.64 (m, 1H, 5-H), 7.62–7.57 (m, 1H, 6-H), 7.53–7.46 (m, 3H, 2-H, 4-H, NH), 4.29 (t, J = 5.1 Hz, 1H, OH), 3.33 (td, J = 6.5, 5.1 Hz, 2H, 14-H), 2.99 (hept, J = 6.9 Hz, 1H, 7-H), 2.77–2.69 (m, 2H, 9-H), 1.38–1.28 (m, 4H, 10-H, 13-H), 1.22 (d, J = 6.9 Hz, 6H, 8-H, 8′-H), 1.21–1.14 (m, 4H, 11-H, 12-H) ppm; 13C NMR:δ = 149.5 (C-3), 140.7 (C-1), 130.3 (C-4), 129.1 (C-5), 124.0 (C-2), 124.0 (C-6), 60.6 (C-14), 42.5 (C-9), 33.3 (C-7), 32.3 (C-13), 29.0 (C-10), 25.9 (C-12), 25.0 (C-11), 23.6 (C-8) ppm; MS: m/z = 322.1 (100%, [M + Na]+); anal. calcd. for C15H25NSO3 (299.43): C 60.17, H 8.42, N 4.68; found: C 59.84, H 8.76, N 4.37.
4.2.90. 6-[(3-Isopropylphenyl)sulfonamido]hexyl Sulfamate (44b)
Applying GPB: from 44a (300 mg, 1.00 mmol): 44b (260 mg, 69%); oil; Rf = 0.66 (CHCl3/EtOAc, 2:3); UV–Vis: 269 nm (2.99); IR: ν = 3276m, 2961w, 2866w, 1561w, 1478w, 1463w, 1428w, 1363s, 1323s, 1306s, 1217vw, 1177s, 1156vs, 1145s, 1088m, 1069m, 1050w, 922s, 799m, 725w, 696s, 624m, 587vs, 551s cm−1; 1H NMR:δ = 7.66–7.64 (m, 1H, 5-H), 7.63–7.58 (m, 1H, 6-H), 7.54–7.47 (m, 3H, 2-H, 4-H, NH), 7.37 (s, 2H, NH2), 3.97 (t, J = 6.5 Hz, 2H, 14-H), 2.99 (hept, J = 6.9 Hz, 1H, 7-H), 2.77–2.69 (m, 2H, 9-H), 1.55 (p, J = 6.7 Hz, 2H, 13-H), 1.34 (p, J = 7.0 Hz, 2H, 10-H), 1.22 (d, J = 6.9 Hz, 10H, 8-H, 8′-H, 11-H, 12-H) ppm; 13C NMR:δ = 149.6 (C-3), 140.7 (C-1), 130.4 (C-4), 129.1 (C-5), 124.0 (C-2), 124.0 (C-6), 68.9 (C-14), 42.4 (C-9), 33.3 (C-7), 28.8 (C-13), 28.2 (C-10), 25.5 (C-12), 24.6 (C-11), 23.6 (C-8) ppm; MS: m/z = 401.4 (100%, [M + Na]+); anal. calcd. for C15H26N2S2O5 (378.50): C 47.60, H 6.92, N 7.40; found: C 47.29, H 7.18, N 7.35.
4.2.91. N-(7-Hydroxyheptyl)-3-isopropylbenzene Sulfonamide (45a)
Applying GPA: from 3-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol), and 7-amino-heptanol (450 mg, 3.43 mmol): 45a (667 mg, 93%); oil; Rf = 0.23 (petrolether/EtOAc, 2:3); UV–Vis: 223 nm (3.99); IR: ν = 3504w, 3280w, 2960w, 2930m, 2859w, 1478w, 1462w, 1427w, 1385w, 1365w, 1324m, 1306s, 1217vw, 1157s, 1144s, 1088m, 1069m, 999w, 904w, 798m, 696s, 624m, 587vs, 564m, 527m, 462w cm−1; 1H NMR:δ = 7.66–7.64 (m, 1H, 2-H), 7.63–7.57 (m, 1H, 6-H), 7.52–7.46 (m, 3H, 4-H, 5-H, NH), 4.29 (t, J = 5.1 Hz, 1H, OH), 3.37–3.32 (m, 2H, 15-H), 2.98 (hept, J = 6.9 Hz, 1H, 7-H), 2.72 (q, J = 6.9 Hz, 2H, 9-H), 1.40–1.27 (m, 4H, 10-H, 14-H), 1.22 (d, J = 6.9 Hz, 6H, 8-H, 8′-H), 1.20–1.11 (m, 6H, 11-H, 12-H, 13-H) ppm; 13C NMR:δ = 149.5 (C-3), 140.8 (C-1), 130.3 (C-4), 129.1 (C-5), 124.0 (C-2), 124.0 (C-6), 60.7 (C-15), 42.5 (C-9), 33.3 (C-7), 32.4 (C-14), 28.9 (C-10), 28.4 (C-12), 26.0 (C-11), 25.3 (C-13), 23.6 (C-8) ppm; MS: m/z = 336.3 (100%, [M + Na]+); anal. calcd. for C16H27N2S2O5 (313.46): C 61.31, H 8.68, N 4.47; found: C 61.07, H 8.97, N 4.29.
4.2.92. 7-[(3-Isopropylphenyl)sulfonamido]heptyl Sulfamate (45b)
Applying GPB: from 45a (200 mg, 0.67 mmol): 45b (227 mg, 90%); oil; Rf = 0.68 (CHCl3/EtOAc, 2:3); UV–Vis: 269 nm (2.93); IR: ν = 3276m, 2961w, 2933w, 2862w, 1561w, 1478w, 1464w, 1428w, 1363s, 1323s, 1306s, 1217vw, 1178s, 1157vs, 1145s, 1089m, 1069m, 1052w, 997w, 923s, 799m, 724w, 696s, 624m, 587vs, 551s cm−1; 1H NMR:δ = 7.66–7.64 (m, 1H, 5-H), 7.62–7.58 (m, 1H, 6-H), 7.53–7.47 (m, 3H, 2-H, 4-H, NH), 7.37 (s, 2H, NH2), 3.98 (t, J = 6.5 Hz, 2H, 15-H), 2.99 (hept, J = 6.9 Hz, 1H, 7-H), 2.76–2.69 (m, 2H, 9-H), 1.63–1.52 (m, 2H, 14-H), 1.39–1.28 (m, 2H, 10-H), 1.23 (d, J = 6.9 Hz, 6H, 8-H, 8′-H), 1.29–1.13 (m, 6H, 11-H, 12-H, 13-H) ppm; 13C NMR:δ = 149.6 (C-3), 140.7 (C-1), 130.3 (C-4), 129.1 (C-5), 124.0 (C-2), 124.0 (C-6), 68.9 (C-15), 42.5 (C-9), 33.3 (C-7), 28.8 (C-14), 28.2 (C-11), 28.0 (C-10), 25.8 (C-13), 24.9 (C-12), 23.6 (C-8) ppm; MS: m/z = 415.4 (100%, [M + Na]+); anal. calcd. for C16H28N2S2O5 (392.53): C 48.96, H 7.19, N 7.14; found: C 48.75, H 7.34, N 6.86.
4.2.93. N-(8-Hydroxyoctyl)-3-isopropylbenzene Sulfonamide (46a)
Applying GPA: from 3-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 8-amino-octanol (498 mg, 3.43 mmol): 46a (649 mg, 87%); oil; Rf = 0.30 (petrolether/EtOAc, 2:3; UV–Vis: 223 nm (3.88); IR: ν = 3504vw, 3281w, 2960w, 2929m, 2857m, 1478w, 1462w, 1428w, 1385w, 1365w, 1324m, 1306m, 1217vw, 1157s, 1144s, 1088m, 1069m, 1051m, 998vw, 904w, 798m, 722w, 696s, 624m, 587vs, 564m, 511m, 456w cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 7.66–7.64 (m, 1H, 2-H), 7.62–7.58 (m, 1H, 6-H), 7.52–7.47 (m, 3H, 4-H, 5-H, NH), 4.29 (td, J = 5.2, 0.8 Hz, 1H, OH), 3.35 (td, J = 6.6, 5.1 Hz, 2H, 16-H), 2.99 (hept, J = 6.9 Hz, 1H, 7-H), 2.72 (td, J = 7.0, 5.8 Hz, 2H, 9-H), 1.41–1.28 (m, 4H, 10-H, 15-H), 1.22 (d, J = 6.9 Hz, 6H, 8-H, 8′-H), 1.20–1.11 (m, 8H, 11-H, 12-H, 13-H, 14-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 149.5 (C-3), 140.8 (C-1), 130.3 (C-4), 129.1 (C-5), 124.0 (C-2), 124.0 (C-6), 60.7 (C-16), 42.5 (C-9), 33.3 (C-7), 32.5 (C-15), 28.9 (C-10), 28.8 (C-12), 28.6 (C-13), 25.9 (C-11), 25.4 (C-14), 23.6 (C-8) ppm; MS: m/z = 350.3 (100%, [M + Na]+); anal. calcd. for C17H29NSO3 (327.48): C 62.35, H 8.93, N 4.28; found: C 62.17, H 9.20, N 3.97.
4.2.94. 8-[(3-Isopropylphenyl)sulfonamido]octyl Sulfamate (46b)
Applying GPB: from 46a (500 mg, 2.29 mmol): 46b (196 mg, 79%); oil; Rf = 0.73 (CHCl3/EtOAc, 2:3); UV–Vis: 268 nm (3.00); IR: ν = 3276m, 2961w, 2930m, 2859w, 1561w, 1478w, 1464w, 1428w, 1363s, 1324m, 1306s, 1217vw, 1178s, 1157vs, 1145s, 1089m, 1070m, 1051w, 998w, 922s, 799m, 723w, 696s, 624m, 587vs, 552s, 461w cm−1; 1H NMR:δ = 7.66–7.64 (m, 1H, 5-H), 7.61–7.58 (m, 1H, 6-H), 7.53–7.48 (m, 3H, 2-H, 4-H, NH), 7.37 (s, 2H, NH2), 3.99 (t, J = 6.5 Hz, 2H, 17-H), 2.99 (hept, J = 6.9 Hz, 1H, 7-H), 2.73 (td, J = 7.0, 5.8 Hz, 2H, 9-H), 1.63–1.55 (m, 2H, 16-H), 1.38–1.10 (m, 10H, 10-H, 11-H, 12-H, 13-H, 14-H), 1.23 (d, J = 6.9 Hz, 6H, 8-H, 8′-H) ppm; 13C NMR:δ = 149.6 (C-3), 140.7 (C-1), 130.3 (C-4), 129.1 (C-5), 124.0 (C-2), 124.0 (C-6), 69.0 (C-17), 42.5 (C-9), 33.3 (C-7), 28.8 (C-16), 28.4 (C-13), 28.3 (C-10), 28.3 (C-12), 25.9 (C-11), 24.9 (C-14), 23.6 (C-8) ppm; MS: m/z = 429.5 (100%, [M + Na]+); anal. calcd. for C17H30N2S2O5 (406.56): C 50.22, H 7.44, N 6.89; found: C 49.97, H 7.70, N 6.54.
4.2.95. N-(7-Hydroxyheptyl)-2-isopropylbenzene Sulfonamide (47a)
Applying GPA: from 2-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 7-amino-heptanol (450 mg, 3.43 mmol): 47a (634 mg, 88%); oil; Rf = 0.32 (petrolether/EtOAc, 2:3); UV–Vis: 224 nm (4.02); IR: ν = 3502w, 3292w, 2931m, 2859m, 1474m, 1461w, 1444m, 1385w, 1363w, 1316s, 1204w, 1148vs, 1115m, 1056s, 1031m, 878w, 763s, 725w, 686m, 595vs, 565s, 545s, 462w, 448w cm−1; 1H NMR:δ = 7.80–7.76 (m, 1H, 3-H), 7.66 (t, J = 5.7 Hz, 1H, NH), 7.60–7.53 (m, 2H, 6-H, 4-H), 7.33 (ddd, J = 8.0, 5.7, 2.9 Hz, 1H, 5-H), 4.29 (t, J = 5.1 Hz, 1H, OH), 3.85 (hept, J = 6.8 Hz, 1H, 7-H), 3.35 (td, J = 6.5, 5.2 Hz, 2H, 15-H), 2.79 (td, J = 7.0, 5.7 Hz, 2H, 9-H), 1.40–1.30 (m, 4H, 10-H, 14-H), 1.25–1.11 (m, 12H, 8-H, 8′-H, 11-H, 12-H, 13-H) ppm; 13C NMR:δ = 147.7 (C-2), 138.1 (C-1), 132.5 (C-4), 128.0 (C-6), 127.9 (C-4), 125.8 (C-5), 60.7 (C-15), 42.4 (C-9), 32.4 (C-14), 29.2 (C-10), 28.4 (C-12), 28.3 (C-7), 26.0 (C-11), 25.3 (C-13), 23.9 (C-8, C-8′) ppm; MS: m/z = 336.3 (100%, [M + Na]+); anal. calcd. for C16H27NSO3 (313.46): C 61.31, H 8.68, N 4.47; found: C 61.07, H 9.02, N 4.16.
4.2.96. 7-[(2-Isopropylphenyl)sulfonamido]heptyl Sulfamate (47b)
Applying GPB: from 47a (200 mg, 0.64 mmol): 47b (131 mg, 52%); oil; Rf = 0.76 (CHCl3/EtOAc, 2:3); UV–Vis: 224 nm (4.04); IR: ν = 3283m, 2933w, 2862w, 1593vw, 1570w, 1474w, 1444w, 1361s, 1315s, 1202w, 1179s, 1162s, 1148vs, 1115m, 1083m, 1071m, 1056m, 1031w, 924s, 815m, 764s, 724w, 687m, 596s, 563s, 550vs, 444w cm−1; 1H NMR:δ = 7.80–7.75 (m, 1H, 3-H), 7.68 (t, J = 5.7 Hz, 1H, NH), 7.61–7.54 (m, 2H, 4-H, 6-H), 7.37 (s, 2H, NH2), 7.34 (ddd, J = 7.9, 5.9, 2.8 Hz, 1H, 5-H), 3.98 (t, J = 6.5 Hz, 2H, 15-H), 3.84 (hept, J = 6.9 Hz, 1H, 7-H), 2.79 (td, J = 7.0, 5.7 Hz, 2H, 9-H), 1.63–1.52 (m, 2H, 14-H), 1.42–1.31 (m, 2H, 10-H), 1.21 (d, J = 6.8 Hz, 6H, 8-H, 8′-H), 1.31–1.13 (m, 6H, 11-H, 12-H, 13-H) ppm; 13C NMR:δ = 147.7 (C-2), 138.1 (C-1), 132.6 (C-4), 128.0 (C-3), 127.9 (C-6), 125.8 (C-5), 68.9 (C-15), 42.3 (C-9), 29.1 (C-10), 28.3 (C-7), 28.2 (C-14), 28.0 (C-12), 25.8 (C-11), 24.9 (C-13), 23.9 (C-8) ppm; MS: m/z = 415.6 (100%, [M + Na]+); anal. calcd. for C16H28N2S2O5 (392.53): C 48.96, H 7.19, N 7.14; found: C 48.75, H 7.37, N 6.87.
4.2.97. N-(8-Hydroxyoctyl)-2-isopropylbenzene Sulfonamide (48a)
Applying GPA: from 2-isopropylbenzenesulfonyl chloride (500 mg, 2.29 mmol) and 8-amino-octanol (498 mg, 3.43 mmol): 48a (720 mg, 96%); oil; Rf = 0.34 (petrolether/EtOAc, 2:3); UV–Vis: 224 nm (3.90); IR: ν = 3498w, 3294w, 2929m, 2856m, 1474m, 1444m, 1385w, 1363w, 1316s, 1204w, 1148vs, 1115m, 1080m, 1071m, 1056s, 1031m, 890w, 763s, 723w, 686m, 595vs, 565vs, 545s, 450w, 419vw cm−1; 1H NMR:δ = 7.80–7.75 (m, 1H, 3-H), 7.66 (s, 1H, NH), 7.60–7.54 (m, 2H, 4-H, 6-H), 7.33 (ddd, J = 8.0, 5.8, 2.8 Hz, 1H, 5-H), 4.30 (t, J = 5.2 Hz, 1H, OH), 3.84 (hept, J = 6.8 Hz, 1H, 7-H), 3.38–3.33 (m, 2H, 16-H), 2.78 (t, J = 7.1 Hz, 2H, 9-H), 1.41–1.29 (m, 4H, 10-H, 15-H), 1.21 (d, J = 6.8 Hz, 6H, 8-H, 8′-H), 1.18–1.12 (m, 8H, 11-H, 12-H, 13-H, 14-H) ppm; 13C NMR:δ = 147.7 (C-2), 138.1 (C-1), 132.5 (C-4), 128.0 (C-3), 127.9 (C-6), 125.8 (C-5), 60.7 (C-16), 42.4 (C-9), 32.5 (C-15), 29.2 (C-13), 28.8 (C-10), 28.5 (C-12), 28.3 (C-7), 25.9 (C-11), 25.4 (C-14) 23.9 (C-8) ppm; MS: m/z = 350.3 (100%, [M + Na]+); anal. calcd. for C17H29NSO3 (327.48): C 62.35, H 8.93, N 4.28; found: C 62.6, H 9.24, N 3.96.
4.2.98. 8-[(2-Isopropylphenyl)sulfonamido]octyl Sulfamate (48b)
Applying GPB: from 47a (200 mg, 0.61 mmol): 47b (202 mg, 82%); oil; Rf = 0.82 (CHCl3/EtOAc, 2:3); UV–Vis: 224 nm (3.86); IR: ν = 3285w, 2931m, 2858w, 1593vw, 1570w, 1474w, 1444w, 1362s, 1315s, 1202w, 1179s, 1161s, 1149vs, 1115m, 1056m, 1031w, 925s, 819w, 764s, 724w, 687m, 596s, 564s, 550s, 450w, 446w cm−1; 1H NMR:δ = 7.80–7.75 (m, 1H, 3-H), 7.67 (t, J = 5.7 Hz, 1H, NH), 7.60–7.54 (m, 2H, 4-H, 6-H), 7.37 (s, 2H, NH2), 7.34 (ddd, J = 7.9, 5.9, 2.8 Hz, 1H, 5-H), 3.99 (t, J = 6.5 Hz, 2H, 16-H), 3.84 (hept, J = 6.8 Hz, 1H, 7-H), 2.79 (td, J = 7.0, 5.7 Hz, 2H, 9-H), 1.63–1.55 (m, 2H, 15-H), 1.39–1.31 (m, 2H, 10-H), 1.30–1.24 (m, 2H, 14-H), 1.21 (d, J = 6.8 Hz, 6H, 8-H, 8′-H), 1.22–1.14 (m, 6H, 11-H, 12-H, 13-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 147.7 (C-2), 138.1 (C-1), 132.5 (C-4), 128.0 (C-3), 127.9 (C-6), 125.8 (C-5), 69.0 (C-16), 42.3 (C-9), 29.2 (C-15), 28.4 (C-10), 28.3 (C-7, C-13), 28.3 (C-12), 25.9 (C-11), 24.9 (C-14), 23.9 (C-8) ppm; MS: m/z = 429.6 (100%, [M + Na]+); anal. calcd. for C17H30N2S2O5 (406.56): C 50.22, H 7.44, N 6.89; found: C 49.96, H 7.70, N 6.55.
4.2.99. 4-(tert-Butyl)-N-(2-hydroxyethyl)benzene Sulfonamide (49a) [477483-08-2]
Applying GPA: from 4-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 2-amino-ethanol (197 mg, 3.22 mmol): 49a (435 mg, 79%); white solid; Rf = 0.20 (petrolether/EtOAc, 2:3); m.p. = 75–77 °C; UV–Vis: 228 nm (4.10); IR: ν = 3522w, 3162w, 3072vw, 2952w, 2903w, 2891w, 2868w, 1596w, 1463w, 1450w, 1432w, 1400w, 1364w, 1354vw, 1325s, 1308m, 1291w, 1261w, 1207vw, 1198w, 1161vs, 1115m, 1087m, 1071m, 1057s, 1016w, 955m, 905w, 844w, 831vw, 823m, 763s, 726m, 624s, 580vs, 550s, 515vw, 498vw, 473w, 457w, 421w, 411w cm−1; 1H NMR:δ = 7.75–7.70 (m, 2H, 2-H, 2′-H), 7.63–7.58 (m, 2H, 3-H, 3′-H), 7.50 (s, 1H, NH), 4.66 (t, J = 5.5 Hz, 1H, OH), 3.37 (q, J = 6.0 Hz, 2H, 8-H), 2.77 (t, J = 6.4 Hz, 2H, 7-H), 1.30 (s, 9H, 6-H, 6′-H, 6′-H) ppm; 13C NMR:δ = 155.2 (C-4), 137.7 (C-1), 126.4 (C-2), 125.9 (C-3), 59.9 (C-8), 45.1 (C-7), 34.8 (C-5), 30.8 (C-6) ppm; MS: m/z = 280.4 (100%, [M + Na]+); anal. calcd. for C12H19NSO3 (257.35): C 56.01, H 7.44, N 5.44; found: C 55.76, H 7.72, N 5.16.
4.2.100. 2-[(4-(tert-Butyl)phenyl)sulfonamido]ethyl Sulfamate (49b)
Applying GPB: from 49a (200 mg, 0.78 mmol): 49b (228 mg, 87%); white solid; Rf = 0.60 (CHCl3/EtOAc, 2:3); m.p. = 92–93 °C; UV–Vis: 228 nm (4.02); IR: ν = 3392w, 3292m, 3267m, 3075vw, 2951w, 2902w, 2865w, 1596w, 1542w, 1462w, 1445m, 1434w, 1401w, 1375s, 1325s, 1309m, 1292w, 1268w, 1228vw, 1197w, 1181s, 1159s, 1115m, 1088s, 1021m, 954s, 923s, 853s, 841s, 833m, 786m, 773m, 751m, 656m, 621m, 601vs, 578m, 549vs, 487m, 461w, 427w, 413w cm−1; 1H NMR:δ = 7.84 (t, J = 6.0 Hz, 1H, NH), 7.77–7.71 (m, 2H, 2-H, 2′-H), 7.64–7.60 (m, 2H, 3-H, 3′-H), 7.50 (s, 2H, NH2), 4.01 (t, J = 5.7 Hz, 2H, 8-H), 3.03 (q, J = 5.6 Hz, 2H, 7-H), 1.31 (s, 9H, 6-H, 6′-H, 6′-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 155.9 (C-1), 137.8 (C-4), 126.8 (C-2), 126.5 (C-3), 68.0 (C-8), 42.0 (C-7), 35.3 (C-5), 31.3 (C-6) ppm; MS: m/z = 359.4 (100%, [M + Na]+); anal. calcd. for C12H20N2S2O5 (336.42): C 42.84, H 5.99, N 8.33; found: C 42.55, H 6.25, N 8.03.
4.2.101. 4-(tert-Butyl)-N-(3-hydroxypentyl)benzene Sulfonamide (50a) [1017436-75-7]
Applying GPA: from 4-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 3-amino-propanol (242 mg, 3.22 mmol): 50a (530 mg, 91%); white solid; Rf = 0.20 (petrolether/EtOAc, 2:3); m.p. = 63–65 °C; UV–Vis: 228 nm (4.15); IR: ν = 3311m, 3241m, 2963m, 2870w, 1597w, 1472w, 1426m, 1402m, 1363w, 1334m, 1313s, 1292m, 1270w, 1203w, 1160vs, 1112m, 1088m, 1069m, 1027m, 1008m, 960m, 908w, 847w, 834m, 826m, 756s, 704m, 625s, 578vs, 550s, 512m, 487m, 475w, 461w cm−1; 1H NMR:δ = 7.73–7.68 (m, 2H, 2-H, 2′-H), 7.63–7.58 (m, 2H, 3-H, 3′-H), 7.43 (s, 1H, NH), 4.40 (s, 1H, OH), 3.37 (td, J = 6.2, 2.8 Hz, 2H, 9-H), 2.77 (t, J = 7.3 Hz, 2H, 7-H), 1.58–1.49 (m, 2H, 8-H), 1.30 (s, 9H, 6-H, 6′-H, 6′-H) ppm; 13C NMR:δ = 155.2 (C-4), 137.6 (C-1), 126.4 (C-2), 126.0 (C-3), 58.1 (C-9), 40.0 (C-7), 34.8 (C-5), 32.4 (C-8), 30.8 (C-6) ppm; MS: m/z = 294.1 (100%, [M + Na]+); anal. calcd. for C13H21NSO3 (271.38): C 57.54, H 7.80, N 5.16; found: C 57.21, H 8.03, N 5.96.
4.2.102. 3-[(4-(tert-Butyl)phenyl)sulfonamido]propyl Sulfamate (50b)
Applying GPB: from 50a (200 mg, 0.74 mmol): 50b (251 mg, 97%); white solid; Rf = 0.61 (CHCl3/EtOAc, 2:3); m.p. = 85–86 °C; UV–Vis: 228 nm (4.18); IR: ν = 3338w, 3293w, 3252m, 3121vw, 2964w, 2906w, 2870vw, 1597w, 1571w, 1476w, 1463w, 1437w, 1421vw, 1401w, 1373s, 1363s, 1329m, 1318s, 1294m, 1270w, 1242vw, 1169s, 1156vs, 1133w, 1111m, 1085m, 1060w, 1016vw, 982s, 949s, 891m, 877w, 839m, 827s, 757s, 678m, 631m, 595m, 589m, 573s, 564s, 548vs, 501w, 491m, 463w, 413vw cm−1; 1H NMR:δ = 7.74–7.69 (m, 2H, 2-H, 2′-H), 7.64–7.58 (m, 3H, 3-H, 3′-H, NH), 7.41 (s, 2H, NH2), 4.03 (td, J = 6.7, 5.0 Hz, 2H, 9-H), 2.81 (td, J = 7.1, 5.8 Hz, 2H, 7-H), 1.78 (p, J = 6.7 Hz, 2H, 8-H), 1.31 (s, 9H, 6-H, 6′-H, 6′-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 155.4 (C-4), 137.4 (C-1), 126.3 (C-2), 126.1 (C-3), 66.5 (C-9), 39.2 (C-7), 34.8 (C-5), 30.8 (C-6), 28.8 (C-8) ppm; MS: m/z = 373.4 (100%, [M + Na]+); anal. calcd. for C13H22N2S2O5 (350.45): C 44.56, H 6.33, N 7.99; found: C 44.21, H 6.65, N 7.68.
4.2.103. 4-(tert-Butyl)-N-(4-hydroxybutyl)benzene Sulfonamide (51a) [1082935-75-8]
Applying GPA: from 4-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 4-amino-butanol (287 mg, 3.22 mmol):
51a (518 mg, 84%) [
118]; white solid; R
f = 0.20 (petrolether/EtOAc, 2:3); m.p. = 58–60 °C; UV–Vis: 228 nm (4.15); IR: ν = 3452
w, 3248
w, 3115
w, 2962
w, 2944
m, 2867
w, 1594
w, 1471
w, 1463
w, 1435
w, 1400
w, 1364
w, 1316
s, 1291
m, 1267
w, 1197
w, 1154
s, 1111
m, 1084
m, 1065
m, 1038
m, 1018
w, 988
w, 909
w, 839
m, 753
m, 735
w, 685
w, 627
s, 581
vs, 549
s, 514
w, 489
w, 472
w, 464
w cm
−1;
1H NMR:δ = 7.73–7.68 (
m, 2H, 2-H, 2′-H), 7.62–7.58 (
m, 2H, 3-H, 3′-H), 7.47 (
s, 1H, NH), 4.35 (
s, 1H, OH), 3.35–3.29 (
m, 2H, 10-H), 2.75–2.69 (
m, 2H, 7-H), 1.44–1.33 (
m, 4H, 8-H, 9-H), 1.30 (
s, 9H, 6-H, 6′-H, 6′-H) ppm;
13C NMR:δ = 155.1 (C-4), 137.8 (C-1), 126.3 (C-2), 125.9 (C-3), 60.2 (C-10), 42.5 (C-7), 34.8 (C-5), 30.8 (C-6), 29.5 (C-9), 25.8 (C-8) ppm; MS:
m/
z = 308.3 (100%, [M + Na]
+); anal. calcd. for C
14H
23NSO
3 (285.40): C 58.92, H 8.12, N 4.91; found: C 59.76, H 8.32, N 4.67.
4.2.104. 4-[(4-(tert-Butyl)phenyl)sulfonamido]butyl Sulfamate (51b)
Applying GPB: from 51a (200 mg, 0.7 mmol): 51b (218 mg, 85%); white solid; Rf = 0.63 (CHCl3/EtOAc, 2:3); m.p. = 100–102 °C; UV–Vis: 228 nm (4.12); IR: ν = 3358m, 3288m, 3262w, 2965w, 1597vw, 1558w, 1483vw, 1476w, 1427w, 1399w, 1383w, 1368s, 1338m, 1321s, 1310m, 1292w, 1269w, 1205w, 1176s, 1158vs, 1122w, 1114m, 1108m, 1089m, 1065m, 1044w, 970s, 941w, 919s, 902s, 838m, 826s, 809w, 754m, 750m, 660s, 628s, 580s, 552vs, 533m, 514w, 507m, 413w cm−1; 1H NMR:δ = 7.74–7.68 (m, 2H, 2-H, 2′-H), 7.63–7.58 (m, 2H, 3-H, 3′-H), 7.54 (t, J = 5.9 Hz, 1H, NH), 7.38 (s, 2H, NH2), 3.96 (t, J = 6.3 Hz, 2H, 10-H), 2.79–2.71 (m, 2H, 7-H), 1.66–1.56 (m, 2H, 9-H), 1.50–1.40 (m, 2H, 8-H), 1.30 (s, 9H, 6-H, 6′-H, 6′-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 155.2 (C-4), 137.7 (C-1), 126.3 (C-2), 126.0 (C-3), 68.5 (C-10), 42.0 (C-7), 34.8 (C-5), 30.8 (C-6), 25.6 (C-9), 25.4 (C-8) ppm; MS: m/z = 387.4 (100%, [M + Na]+); anal. calcd. for C14H24N2S2O5 (364.48): C 46.14, H 6.64, N 7.69; found: C 45.86, H 6.98, N 7.42.
4.2.105. 4-(tert-Butyl)-N-(5-hydroxypentyl)benzene Sulfonamide (52a) [1925550-01-1]
Applying GPA: from 4-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 5-amino-pentanol (332 mg, 3.22 mmol): 52a (603 mg, 93%); white solid; Rf = 0.20 (petrolether/EtOAc, 2:3); m.p. = 53–54 °C; UV–Vis: 228 nm (4.02); IR: ν = 3286m, 2933m, 2863w, 1597w, 1473w, 1462w, 1424m, 1399w, 1362w, 1331m, 1318s, 1292m, 1268w, 1199w, 1158s, 1112m, 1088m, 1048s, 1016w, 887m, 843m, 826m, 755m, 735w, 689m, 638m, 630s, 573vs, 552s, 525m, 502w cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 7.72–7.68 (m, 2H, 2-H, 2′-H), 7.62–7.58 (m, 2H, 3-H, 3′-H), 7.46 (t, J = 5.7 Hz, 1H, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.34–3.29 (m, 2H, 11-H), 2.73–2.68 (m, 2H, 7-H), 1.39–1.31 (m, 4H, 8-H, 10-H), 1.30 (s, 9H, 6-H, 6′-H, 6′-H), 1.26–1.15 (m, 2H, 9-H) ppm; 13C NMR:δ = 155.2 (C-4), 137.8 (C-1), 126.3 (C-2), 125.9 (C-3), 60.5 (C-11), 42.6 (C-7), 34.8 (C-5), 32.0 (C-10), 30.8 (C-6), 28.9 (C-8), 22.6 (C-9) ppm; MS: m/z = 322.4 (100%, [M + Na]+); anal. calcd. for C15H25NSO3 (299.43): C 60.17, H 8.42, N 4.68; found: C 59.87, H 8.70, N 4.39.
4.2.106. 5-[(4-(tert-Butyl)phenyl)sulfonamido]pentyl Sulfamate (52b)
Applying GPB: from 52a (500 mg, 2.15 mmol): 52b (196 mg, 78%); white solid; Rf = 0.65 (CHCl3/EtOAc, 2:3); m.p. = 88–89 °C; UV–Vis: 228 nm (4.17); IR: ν = 3356m, 3278m, 3254m, 2968w, 2954w, 2860w, 1597w, 1557w, 1475w, 1463w, 1427w, 1398w, 1363s, 1326s, 1311m, 1292w, 1269w, 1175s, 1156vs, 1124w, 1111m, 1089m, 1068w, 1032m, 1000m, 965s, 918s, 900s, 852w, 840m, 821s, 775m, 754m, 738w, 627s, 579vs, 552vs, 533s, 520m, 501m, 443w, 412w cm−1; 1H NMR:δ = 7.73–7.68 (m, 2H, 2-H, 2′-H), 7.63–7.57 (m, 2H, 3-H, 3′-H), 7.49 (t, J = 5.9 Hz, 1H, NH), 7.37 (s, 2H, NH2), 3.96 (t, J = 6.4 Hz, 2H, 11-H), 2.72 (q, J = 6.6 Hz, 2H, 7-H), 1.55 (p, J = 6.7 Hz, 2H, 10-H), 1.44–1.35 (m, 2H, 8-H), 1.30 (s, 11H, 6-H, 6′-H, 6′-H, 9-H) ppm; 13C NMR:δ = 155.2 (C-4), 137.7 (C-1), 126.3 (C-2), 126.0 (C-3), 68.8 (C-11), 42.3 (C-7), 34.8 (C-5), 30.8 (C-6), 28.5 (C-8), 27.8 (C-10), 22.2 (C-9) ppm; MS: m/z = 401.6 (100%, [M + Na]+); anal. calcd. for C15H26N2S2O5 (378.50): C 47.60, H 6.92, N 7.40; found: C 47.32, H 7.24, N 7.09.
4.2.107. 4-(tert-Butyl)-N-(6-hydroxyhexyl)benzene Sulfonamide (53a) [1787706-38-0]
Applying GPA: from 4-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 6-amino-hexanol (378 mg, 3.22 mmol):
53a (635 mg, 94%) [
119,
120]; white solid; R
f = 0.45 (petrolether/EtOAc 1: 1); m.p. = 53–54 °C; UV–Vis: 229 nm (4.21); IR: ν = 3504
br, 3281
br, 2935
m, 2864
w, 1596
w, 1475
w, 1462
w, 1398
w, 1365
w, 1321
s, 1269
w, 1198
w, 1157
vs, 1112
s, 1087
s, 1056
m, 1026
w, 1014
w, 835
m, 754
m, 726
w, 734
w, 626
s, 581
s, 549
s, 455
w cm
−1;
1H NMR (500 MHz, DMSO-
d6): δ = 7.72–7.68 (
m, 2H, 2-H, 2′-H), 7.62–7.58 (
m, 2H, 3-H, 3′-H), 7.49–7.44 (
m, 1H, NH), 4.29 (
t,
J = 5.1 Hz, 1H, OH), 3.36–3.32 (
m, 2H, 12-H), 2.71 (
td,
J = 7.2, 3.7 Hz, 2H, 7-H), 1.37–1.31 (
m, 4H, 8-H, 11-H), 1.30 (
s, 9H, 6-H, 6′-H, 6′-H), 1.22–1.13 (
m, 4H, 9-H, 10-H) ppm;
13C NMR:δ = 155.2 (C-4), 137.8 (C-1), 126.3 (C-2), 125.9 (C-3), 60.6 (C-12), 42.5 (C-7), 34.8 (C-5), 32.3 (C-11), 30.8 (C-6), 29.0 (C-8), 25.9 (C-10), 25.0 (C-9) ppm; MS:
m/
z = 336.2 (100%, [M + Na]
+); anal. calcd. for C
16H
27NSO
3 (313.46): C 61.31, H 8.68, N 4.47; found: C 59.97, H 8.98, N 4.16.
4.2.108. 6-[(4-(tert-Butyl)phenyl)sulfonamido]hexyl Sulfamate (53b)
Applying GPB: from 53a (196 mg, 0.62 mmol): 53b (152 mg, 62%); white solid; Rf = 0.69 (CHCl3/EtOAc, 2:3); m.p. = 89–90 °C; UV–Vis: 228 nm (4.12); IR: ν = 3364w, 3292m, 3253m, 2964w, 2952w, 2941w, 2863w, 1596w, 1571w, 1481w, 1427w, 1399w, 1364s, 1338m, 1323s, 1308m, 1293w, 1285w, 1270w, 1158vs, 1110m, 1088m, 1062m, 1023m, 1011w, 963m, 942m, 897m, 884m, 826s, 805w, 755m, 733w, 658s, 633m, 577s, 568s, 552m, 536m, 528m, 504w cm−1; 1H NMR:δ = 7.70–7.65 (m, 2H, 2-H, 2′-H), 7.60–7.55 (m, 2H, 3-H, 3′-H), 7.45 (t, J = 5.9 Hz, 1H, NH), 7.34 (s, 2H, NH2), 3.94 (t, J = 6.5 Hz, 2H, 12-H), 2.69 (q, J = 6.7 Hz, 2H, 7-H), 1.57–1.48 (m, 2H, 11-H), 1.37–1.29 (m, 2H, 8-H), 1.28 (s, 9H, 6-H, 6′-H, 6′-H), 1.25–1.17 (m, 4H, 9-H, 10-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 155.2 (C-4), 137.8 (C-1), 126.3 (C-2), 125.9 (C-3), 68.9 (C-12), 42.4 (C-7), 34.8 (C-5), 30.8 (C-6), 28.8 (C-11), 28.1 (C-8), 25.5 (C-10), 24.6 (C-9) ppm; MS: m/z = 415.6 (100%, [M + Na]+); anal. calcd. for C16H28N2S2O5 (392.53): C 48.96, H 7.19, N 7.14; found: C 48.71, H 7.32, N 6.91.
4.2.109. 4-(tert-Butyl)-N-(7-hydroxyheptyl)benzene Sulfonamide (54a)
Applying GPA: from 4-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 7-amino-heptanol (423 mg, 3.22 mmol): 54a (688 mg, 98%); white solid; Rf = 0.27 (petrolether/EtOAc, 2:3); m.p. = 51–52 °C; UV–Vis: 228 nm (4.20); IR: ν = 3296w, 3248m, 2927m, 2856m, 1597w, 1475w, 1464w, 1436w, 1401m, 1364w, 1319s, 1292m, 1269w, 1202w, 1156s, 1113m, 1089m, 1059s, 1030m, 1015w, 1006w, 891w, 843w, 831m, 758m, 695m, 627m, 573vs, 551s, 524w, 509w cm−1; 1H NMR:δ = 7.73–7.68 (m, 2H, 2-H, 2′-H), 7.62–7.57 (m, 2H, 3-H, 3′-H), 7.49–7.43 (m, 1H, NH), 4.29 (t, J = 5.1 Hz, 1H, OH), 3.34 (td, J = 6.8, 5.3 Hz, 2H, 13-H), 2.71 (q, J = 6.4 Hz, 2H, 7-H), 1.40–1.31 (m, 4H, 8-H, 12-H), 1.30 (s, 9H, 6-H, 6′-H, 6′-H), 1.23–1.13 (m, 6H, 9-H, 10-H, 11-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 155.1 (C-4), 137.8 (C-1), 126.3 (C-2), 125.9 (C-3), 60.6 (C-13), 42.5 (C-7), 34.8 (C-5), 32.4 (C-12), 30.8 (C-6), 28.9 (C-8), 28.4 (C-10), 26.0 (C-9), 25.3 (C-11) ppm; MS: m/z = 350.1 (100%, [M + Na]+); anal. calcd. for C17H29NSO3 (327.48): C 62.35, H 8.93, N 4.28; found: C 62.06, H 4.55, N 3.96.
4.2.110. 7-[(4-(tert-Butyl)phenyl)sulfonamido]heptyl Sulfamate (54b)
Applying GPB: from 54a (300 mg, 0.92 mmol): 54b (269 mg, 72%); white solid; Rf = 0.75 (CHCl3/EtOAc, 2:3); m.p. = 95–96 °C; UV–Vis: 228 nm (4.10); IR: ν = 3337w, 3248m, 3125vw, 2953w, 2931m, 2856w, 1596w, 1572w, 1476w, 1463w, 1432w, 1397w, 1373s, 1366s, 1324s, 1315s, 1311s, 1291m, 1269w, 1202vw, 1169s, 1158vs, 1121w, 1111m, 1088m, 1074w, 1057m, 1047w, 1015w, 1000m, 972m, 949s, 895m, 854vw, 833m, 824s, 775w, 760s, 726m, 699s, 626m, 590m, 575vs, 564s, 550s, 535m, 529m, 517m, 502w, 477w, 411vw cm−1; 1H NMR:δ = 7.72–7.68 (m, 2H, 2-H, 2′-H), 7.62–7.58 (m, 2H, 3-H, 3′-H), 7.47 (t, J = 5.8 Hz, 1H, NH), 7.37 (s, 2H, NH2), 3.98 (t, J = 6.5 Hz, 2H, 13-H), 2.71 (td, J = 7.0, 5.9 Hz, 2H, 7-H), 1.63–1.54 (m, 2H, 12-H), 1.30 (s, 9H, 6-H, 6′-H, 6′-H), 1.39–1.16 (m, 8H, 8-H, 9-H, 10-H, 11-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 155.2 (C-4), 137.8 (C-1), 126.3 (C-2), 125.9 (C-3), 68.9 (C-13), 42.5 (C-7), 34.8 (C-5), 30.8 (C-6), 28.9 (C-8), 28.2 (C-12), 28.0 (C-10), 25.8 (C-11), 24.9 (C-9) ppm; MS: m/z = 429.2 (100%, [M + Na]+); anal. calcd. for C17H30N2S2O5 (406.56): C 50.22, H 7.44, N 6.89; found: C 49.96, H 7.77, N 6.53.
4.2.111. 4-(tert-Butyl)-N-(8-hydroxyoctyl)benzene Sulfonamide (55a)
Applying GPA: from 4-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 8-amino-octanol (468 mg, 3.22 mmol): 55a (653 mg, 89%); white solid; Rf = 0.30 (petrolether/EtOAc, 2:3); m.p. = 69–71 °C; UV–Vis: 228 nm (4.13); IR: ν = 3263m, 2927m, 2854m, 1597w, 1479w, 1467w, 1428m, 1401w, 1363w, 1325s, 1292m, 1270w, 1202w, 1156vs, 1122w, 1111m, 1088m, 1057m, 1046m, 1016w, 999w, 950vw, 896w, 881w, 841w, 827m, 757s, 736w, 723w, 675m, 634m, 575vs, 550s, 535w, 525w, 511m cm−1; 1H NMR:δ = 7.72–7.68 (m, 2H, 2-H, 2′-H), 7.62–7.58 (m, 2H, 3-H, 3′-H), 7.48–7.43 (m, 1H), 4.30 (t, J = 5.2 Hz, 1H), 3.35 (td, J = 6.6, 5.1 Hz, 2H, 14-H), 2.71 (q, J = 6.5 Hz, 2H, 7-H), 1.41–1.32 (m, 4H, 8-H, 13-H), 1.30 (s, 9H, 6-H, 6′-H, 6′-H), 1.26–1.12 (m, 8H, 9-H, 10-H, 11-H, 12-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 155.1 (C-4), 137.8 (C-1), 126.3 (C-2), 125.9 (C-3), 60.7 (C-14), 42.5 (C-7), 34.8 (C-5), 32.5 (C-13), 30.8 (C-6), 28.9 (C-8), 28.7 (C-11), 28.6 (C-10), 25.9 (C-9), 25.4 (C-12) ppm; MS: m/z = 364.4 (100%, [M + Na]+); anal. calcd. for C18H31NSO3 (341.51): C 63.31, H 9.15, N 4.10; found: C 63.11, H 4.40, N 3.86.
4.2.112. 8-[(4-(tert-Butyl)phenyl)sulfonamido]octyl Sulfamate (55b)
Applying GPB: from 55a (200 mg, 0.59 mmol): 55b (240 mg, 98%); white solid; Rf = 0.79 (CHCl3/EtOAc, 2:3); m.p. = 79–81 °C; UV–Vis: 228 nm (4.10); IR: ν = 3334w, 3256m, 2950w, 293m, 2856w, 1596w, 1476w, 1463w, 1432w, 1392w, 1370s, 1360s, 1328s, 1314s, 1312s, 1285m, 1285w, 1169s, 1154vs, 1120w, 1110m, 1090m, 1060m, 1041w, 1011w, 1005m, 970m, 949s, 891m, 830m, 825s, 778w, 762s, 732m, 698s, 626m, 591m, 576vs, 562s, 555s, 531m, 530m, 514m cm−1; 1H NMR:δ = 7.72–7.68 (m, 2H, 2-H, 2′-H), 7.62–7.58 (m, 2H, 3-H, 3′-H), 7.46 (t, J = 5.8 Hz, 1H, NH), 7.37 (s, 2H, NH2), 3.99 (t, J = 6.5 Hz, 2H, 14-H), 2.71 (td, J = 7.0, 5.8 Hz, 2H, 7-H), 1.59 (dt, J = 8.2, 6.5 Hz, 2H, 13-H), 1.39–1.24 (m, 4H, 8-H, 12-H), 1.30 (s, 9H, 6-H, 6′-H, 6′-H), 1.26–1.12 (m, 6H, 9-H, 10-H, 11-H) ppm; 13C NMR:δ = 155.2 (C-4), 137.8 (C-1), 126.3 (C-2), 125.9 (C-3), 68.9 (C-14), 42.5 (C-7), 34.8 (C-5), 30.8 (C-6), 28.9 (C-13), 28.4 (C-11), 28.3 (C-8), 28.3 (C-10), 25.9 (C-12), 24.9 (C-9) ppm; MS: m/z = 443.8 (100%, [M + Na]+); anal. calcd. for C18H32N2S2O5 (420.58): C 51.40, H 7.67, N 6.66; found: C 51.17, H 7.90, N 6.38.
4.2.113. 4-(tert-Butyl)-N-(9-hydroxynonyl)benzene Sulfonamide (56a)
Applying GPA: from 4-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 9-amino-nonanol (513 mg, 3.22 mmol): 56a (636 mg, 83%); white solid; Rf = 0.78 (CHCl3/EtOAc, 2:3); m.p. = 69–70 °C; UV–Vis: 228 nm (4.07); IR: ν = 3459vw, 3285m, 2963w, 2925m, 2856m, 1598w, 1484vw, 1471m, 1438w, 1400w, 1363w, 1323vs, 1294m, 1270w, 1243vw, 1199vw, 1154vs, 1120w, 1110m, 1090s, 1072s, 1045m, 1037m, 1013m, 973w, 902w, 863m, 836m, 820m, 776w, 754m, 723w, 681m, 627m, 574vs, 551s, 534m, 521m, 473vw, 461w, 438w cm−1; 1H NMR:δ = 7.73–7.67 (m, 2H, 2-H, 2′-H), 7.62–7.57 (m, 2H, 3-H, 3′-H), 7.46 (t, J = 5.8 Hz, 1H, NH), 4.29 (td, J = 5.2, 1.0 Hz, 1H, OH), 3.36 (td, J = 6.6, 5.1 Hz, 2H, 15-H), 2.71 (td, J = 7.0, 5.8 Hz, 2H, 7-H), 1.43–1.35 (m, 2H, 14-H), 1.35–1.31 (m, 2H, 8-H), 1.30 (s, 9H, 6-H, 6′-H, 6′-H), 1.27–1.11 (m, 10H, 9-H, 10-H, 11-H, 12-H, 13-H) ppm; 13C NMR:δ = 155.1 (C-4), 137.8 (C-1), 126.3 (C-2), 125.9 (C-3), 60.7 (C-15), 42.5 (C-7), 34.8 (C-5), 32.5 (C-14), 30.8 (C-6), 28.9 (C-8), 28.9 (C-12), 28.8 (C-10), 28.5 (C-11), 26.0 (C-9), 25.4 (C-13) ppm; MS: m/z = 378.1 (100%, [M + Na]+); anal. calcd. for C19H33NSO3 (355.54): C 64.19, H 9.36, N 3.94; found: C 63.76, H 9.51, N 3.68.
4.2.114. 9-[(4-(tert-Butyl)phenyl)sulfonamido]nonyl Sulfamate (56b)
Applying GPB: from 56a (200 mg, 0.56 mmol): 56b (187 mg, 76%); white solid; Rf = 0.88 (CHCl3/EtOAc, 2:3); m.p. = 106–107 °C; UV–Vis: 228 nm (4.06); IR: ν = 3358w, 3270m, 3124vw, 2963w, 2918w, 2853w, 1597w, 1571w, 1475w, 1466w, 1432w, 1397w, 1376s, 1314s, 1295m, 1272w, 1201vw, 1181m, 1157vs, 1111m, 1090m, 1062w, 1033m, 1015vw, 991w, 968m, 933m, 904w, 882w, 837w, 818s, 778vw, 758m, 733vw, 721w, 692m, 628w, 590m, 576s, 566s, 551m, 537m, 529w, 513w, 484m, 456vw, 411vw cm−1; 1H NMR:δ = 7.72–7.68 (m, 2H, 2-H, 2′-H), 7.62–7.58 (m, 2H, 3-H, 3′-H), 7.46 (t, J = 5.8 Hz, 1H, NH), 7.37 (s, 2H, NH2), 3.99 (t, J = 6.5 Hz, 2H, 15-H), 2.71 (td, J = 7.0, 5.8 Hz, 2H, 7-H), 1.65–1.55 (m, 2H, 14-H), 1.39–1.25 (m, 4H, 8-H, 13-H), 1.30 (s, 9H, 6-H, 6′-H, 6′-H), 1.26–1.12 (m, 8H, 9-H, 10-H, 11-H, 12-H) ppm; 13C NMR:δ = 155.16 (C-4), 137.82 (C-1), 126.31 (C-2), 125.92 (C-3), 68.96 (C-15), 42.48 (C-7), 34.78 (C-5), 30.80 (C-6), 28.92 (C-14), 28.71 (C-8), 28.43 (C-10), 28.39 (C-12), 28.29 (C-11), 25.94 (C-9), 25.01 (C-13) ppm; MS: m/z = 457.1 (100%, [M + Na]+); anal. calcd. for C19H34N2S2O5 (434.61): C 52.51, H 7.89, N 6.45; found: C 52.31, H 8.02, N 6.15.
4.2.115. 4-(tert-Butyl)-N-(10-hydroxydecyl)benzene Sulfonamide (57a)
Applying GPA: from 4-(tert-butyl)benzenesulfonyl chloride (300 mg, 1.29 mmol) and 10-amino-decanol (335 mg, 1.93 mmol): 57a (415 mg, 87%); white solid; Rf = 0.77 (CHCl3/EtOAc, 2:3); m.p. = 75–76 °C; UV–Vis: 228 nm (4.13); IR: ν = 3497w, 3131w, 2961w, 2915s, 2876w, 2849m, 1596w, 1476w, 1465m, 1447w, 1441w, 1398m, 1363w, 1317s, 1289m, 1267w, 1198w, 1154vs, 1110s, 1091m, 1074s, 1043w, 1031w, 1020m, 915m, 843m, 826m, 756m, 721w, 635s, 580vs, 554m, 521m, 505w, 468m cm−1; 1H NMR:δ = 7.72–7.68 (m, 2H, 2-H, 2′-H), 7.62–7.57 (m, 2H, 3-H, 3′-H), 7.46 (t, J = 5.7 Hz, 1H, NH), 4.30 (td, J = 5.2, 1.0 Hz, 1H, OH), 3.36 (td, J = 6.5, 5.1 Hz, 2H, 16-H), 2.74–2.67 (m, 2H, 7-H), 1.43–1.35 (m, 2H, 15-H), 1.36–1.26 (m, 2H, 8-H), 1.30 (s, 9H, 6-H, 6′-H, 6′-H), 1.27–1.11 (m, 12H, 9-H, 10-H, 11-H, 12-H, 13-H, 14-H) ppm; 13C NMR:δ = 155.1 (C-4), 137.9 (C-1), 126.3 (C-2), 125.9 (C-3), 60.7 (C-16), 42.5 (C-7), 34.8 (C-5), 32.5 (C-15), 30.8 (C-6), 29.0 (C-8), 28.9 (C-10, C-13), 28.8 (C-11), 28.5 (C-12), 26.0 (C-9), 25.5 (C-14) ppm; MS: m/z = 457.1 (100%, [M + Na]+); anal. calcd. for C20H35NSO3 (369.56): C 65.00, H 9.55, N 3.79; found: C 64.76, H 9.76, N 3.46.
4.2.116. 10-[(4-(tert-Butyl)phenyl)sulfonamido]decyl Sulfamate (57b)
Applying GPB: from 57a (180 mg, 0.48 mmol): 57b (140 mg, 64%); white solid; Rf = 0.87 (CHCl3/EtOAc, 2:3); m.p. = 92–93 °C; UV–Vis: 228 nm (4.11); IR: ν = 3354w, 3294w, 3264m, 2920m, 2852m, 1475w, 1466w, 1433w, 1393w, 1366s, 1319s, 1292w, 1158vs, 1113m, 1089m, 1055m, 1025w, 937m, 895w, 835m, 824m, 756m, 721w, 670m, 628m, 578s, 552s, 532m cm −1; 1H NMR:δ = 7.72–7.67 (m, 2H, 2-H, 2′-H), 7.62–7.57 (m, 2H, 3-H, 3′-H), 7.45 (t, J = 5.8 Hz, 1H, NH), 7.37 (s, 2H, NH2), 4.00 (t, J = 6.5 Hz, 2H, 16-H), 2.74–2.67 (m, 2H, 7-H), 1.61 (p, J = 6.6 Hz, 2H, 15-H), 1.37–1.26 (m, 2H, 8-H), 1.30 (s, 9H, 6-H, 6′-H, 6′-H), 1.27–1.11 (m, 12H, 9-H, 10-H, 11-H, 12-H, 13-H, 14-H) ppm; 13C NMR:δ = 155.2 (C-4), 137.8 (C-1), 126.3 (C-2, 2′), 125.9 (C-3, 3′), 69.0 (C-16), 42.5 (C-7), 34.8 (C-5), 30.8 (C-6, 6′, 6′), 28.9 (C-8), 28.8 (C-11), 28.8 (C-15), 28.5 (C-10), 28.5 (C-13), 28.3 (C-12), 26.0 (C-14), 25.0 (C-9) ppm; MS: m/z = 457.1 (100%, [M + Na]+); anal. calcd. for C20H36N2S2O5 (448.64): C 53.54, H 8.09, N 6.24; found: C 53.34, H 8.37, N 5.99.
4.2.117. 3-(tert-Butyl)-N-(2-hydroxyethyl)benzene Sulfonamide (58a)
Applying GPA: from 3-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 2-amino-ethanol (197 mg, 3.22 mmol): 58a (522 mg, 94%); oil; Rf = 0.20 (petrolether/EtOAc, 2:3); UV–Vis: 224 nm (4.04); IR: ν = 3500w, 3276w, 2962w, 2872w, 1481m, 1460w, 1416m, 1398w, 1366w, 1325m, 1307s, 1267w, 1205vw, 1156vs, 1125m, 1096m, 1057m, 998vw, 949m, 896w, 865w, 796m, 775w, 696s, 678m, 625m, 586vs, 545m, 534m, 524m, 481w, 456w cm−1; 1H NMR:δ = 7.82–7.80 (m, 1H, 2-H), 7.67 (ddd, J = 7.8, 2.0, 1.1 Hz, 1H, 6-H), 7.62 (ddd, J = 7.8, 1.8, 1.1 Hz, 1H, 4-H), 7.58 (s, 1, NH), 7.51 (td, J = 7.8, 0.5 Hz, 1H, 5-H), 4.67 (t, J = 5.5 Hz, 1H, OH), 3.37 (q, J = 6.1 Hz, 2H, 10-H), 2.79 (t, J = 6.4 Hz, 2H, 9-H), 1.31 (s, 9H, 8-H, 8′-H, 8′-H) ppm; 13C NMR:δ = 151.9 (C-3), 140.4 (C-1), 129.3 (C-4), 128.9 (C-5), 123.7 (C-2), 122.9 (C-6), 59.9 (C-10), 45.1 (C-9), 34.7 (C-7), 30.9 (C-8) ppm; MS: m/z = 280.3 (100%, [M + Na]+); anal. calcd. for C12H19NSO3 (257.35): C 56.01, H 7.44, N 5.44; found: C 55.76, H 7.69, N 55.16.
4.2.118. 2-[(3-(tert-Butyl)phenyl)sulfonamido]ethyl Sulfamate (58b)
Applying GPB: from 58a (200 mg, 0.78 mmol): 58b (194 mg, 74%); oil; Rf = 0.68 (CHCl3/EtOAc, 2:3); UV–Vis: 224 nm (4.02); IR: ν = 3276w, 2963w, 2871w, 1560w, 1482w, 1460w, 1417w, 1365s, 1326m, 1309m, 1288w, 1267w, 1179s, 1156vs, 1125m, 1097m, 1021m, 998w, 925s, 867w, 797w, 775m, 753m, 695m, 678m, 626m, 585s, 549s, 493w, 434w cm−1; 1H NMR:δ = 7.94–7.87 (m, 1H, NH), 7.81–7.78 (m, 1H, 2-H), 7.73–7.67 (m, 1H, 6-H), 7.65–7.61 (m, 1H, 4-H), 7.56–7.47 (m, 3H, 5-H, NH2), 4.00 (t, J = 5.7 Hz, 2H, 10-H), 3.08–2.99 (m, 2H, 9-H), 1.32 (s, 9H, 8-H, 8′-H, 8′-H) ppm; 13C NMR:δ = 152.1 (3), 140.0 (1), 129.6 (4), 129.0 (5), 123.7 (2), 122.8 (6), 67.5 (10), 41.5 (9), 34.7 (7), 30.9 (8, 8′, 8′) ppm; MS: m/z = 359.6 (100%, [M + Na]+); anal. calcd. for C12H20N2S2O5 (336.42): C 42.84, H 5.99, N 8.33; found: C 42.63, H 6.24, N 8.01.
4.2.119. 3-(tert-Butyl)-N-(3-hydroxypropyl)benzene Sulfonamide (59a)
Applying GPA: from 3-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 3-amino-propanol (242 mg, 3.22 mmol): 59a (572 mg, 98%); oil; Rf = 0.23 (petrolether/EtOAc, 2:3); UV–Vis: 224 nm (4.15); IR: ν = 3501w, 3276w, 2961m, 2873w, 1481m, 1460w, 1416w, 1398w, 1366w, 1324m, 1307s, 1267w, 1178w, 1155vs, 1125m, 1084m, 1069m, 1008w, 998w, 960w, 876w, 798m, 774m, 696s, 678m, 625m, 586vs, 534m, 491m, 460w cm−1; 1H NMR:δ = 7.79 (td, J = 1.9, 0.5 Hz, 1H, 2-H), 7.67 (ddd, J = 7.8, 2.0, 1.1 Hz, 1H, 6-H), 7.61 (ddd, J = 7.7, 1.8, 1.2 Hz, 1H, 4-H), 7.52 (td, J = 7.5, 1.8 Hz, 2H, 5-H, NH), 4.39 (t, J = 5.1 Hz, 1H, OH), 3.36 (td, J = 6.2, 4.5 Hz, 2H, 11-H), 2.79 (td, J = 7.5, 3.6 Hz, 2H, 9-H), 1.55–1.46 (m, 2H, 10-H), 1.31 (s, 9H, 8-H, 8′-H, 8′-H) ppm; 13C NMR:δ = 151.9 (C-3), 140.3 (C-1), 129.3 (C-4), 128.9 (C-5), 123.7 (C-2), 122.9 (C-6), 58.0 (C-11), 40.0 (C-9), 34.7 (C-7), 32.3 (C-10), 30.9 (C-8) ppm; MS: m/z = 294.1 (100%, [M + Na]+); anal. calcd. for C13H21NSO3 (271.38): C 57.54, H 7.80, N 5.16; found: C 57.16, H 8.03, N 4.91.
4.2.120. 3-[(3-(tert-Butyl)phenyl)sulfonamido]propyl Sulfamate (59b)
Applying GPB: from 59a (500 mg, 2.15 mmol): 59b (212 mg, 82%); oil; Rf = 0.68 (CHCl3/EtOAc, 2:3); UV–Vis: 224 nm (3.85); IR: ν = 3274w, 2964w, 1482w, 1417w, 1364s, 1325m, 1308m, 1177s, 1156vs, 1125m, 1096m, 939m, 798m, 772m, 696m, 678m, 625m, 585s, 550s, 536m, 496m cm−1; 1H NMR:δ = 7.79 (t, J = 1.9 Hz, 1H, 2-H), 7.72–7.59 (m, 3H, 4-H, 6-H, NH), 7.53 (t, J = 7.8 Hz, 1H, 5-H), 7.41 (s, 2H, NH2), 4.02 (t, J = 6.3 Hz, 2H, 11-H), 2.81 (t, J = 7.2 Hz, 2H, 9-H), 1.75 (p, J = 6.7 Hz, 2H, 10-H), 1.31 (s, 9H, 8-H, 8′-H, 8′-H) ppm; 13C NMR:δ = 152.1 (C-3), 140.1 (C-1), 129.5 (C-4), 129.0 (C-5), 123.7 (C-2), 122.8 (C-6), 66.5 (C-11), 39.2 (C-9), 34.7 (C-7), 30.9 (C-8), 28.7 (C-10) ppm; MS: m/z = 373.5 (100%, [M + Na]+); anal. calcd. for C13H22N2S2O5 (350.45): C 44.56, H 6.33, N 7.99; found: C 44.16, H 6.68, N 7.51.
4.2.121. 3-(tert-Butyl)-N-(4-hydroxybutyl)benzene Sulfonamide (60a)
Applying GPA: from 3-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 4-amino-butanol (287 mg, 3.22 mmol): 60a (589 mg, 96%); oil; Rf = 0.20 (petrolether/EtOAc, 2:3); UV–Vis: 224 nm (3.90); IR: ν = 3499w, 3280w, 2959m, 2870w, 1481m, 1460w, 1416w, 1398w, 1366w, 1324m, 1307m, 1267w, 1156vs, 1125m, 1088m, 1056m, 1034w, 998w, 872w, 797m, 773w, 696s, 678m, 626m, 586vs, 535m, 520m, 496w, 464w cm−1; 1H NMR:δ = 7.81–7.78 (m, 1H, 2-H), 7.66 (ddd, J = 7.8, 2.0, 1.1 Hz, 1H, 6-H), 7.61 (ddd, J = 7.8, 1.8, 1.1 Hz, 1H, 4-H), 7.56–7.48 (m, 2H, 5-H, NH), 4.35 (s, 1H, OH), 3.37–3.27 (m, 2H, 12-H), 2.77–2.70 (m, 2H, 9-H), 1.43–1.32 (m, 4H, 10-H, 11-H), 1.31 (s, 9H, 8-H, 8′-H, 8′-H) ppm; 13C NMR:δ = 151.9 (C-3), 140.5 (C-1), 129.2 (C-4), 128.9 (C-5), 123.7 (C-2), 122.9 (C-6), 60.2 (C-12), 42.5 (C-9), 34.7 (C-7), 30.9 (C-8), 29.5 (C-11), 25.7 (C-10) ppm; MS: m/z = 308.4 (100%, [M + Na]+); anal. calcd. for C14H23NSO3 (285.40): C 58.92, H 8.12, N 4.91; found: C 58.68, H 8.33, N 4.78.
4.2.122. 4-[(3-(tert-Butyl)phenyl)sulfonamido]butyl Sulfamate (60b)
Applying GPB: from 60a (183 mg, 0.64 mmol): 60b (206 mg, 88%); oil; Rf = 0.69 (CHCl3/EtOAc, 2:3); UV–Vis: 225 nm (3.85); IR: ν = 3276w, 2962w, 2872w, 1481w, 1460w, 1438w, 1415w, 1366m, 1329m, 1308m, 1287w, 1266w, 1180m, 1157vs, 1125m, 1098m, 1089m, 1068m, 1010w, 996w, 923m, 865w, 799m, 778m, 741w, 698s, 677s, 631m, 588vs, 552s, 535m, 458vw cm−1; 1H NMR:δ = 7.78 (t, J = 1.9 Hz, 1H, 2-H), 7.74–7.58 (m, 3H, 4-H, 6-H, NH), 7.52 (t, J = 7.7 Hz, 1H, 5-H), 7.38 (s, 2H, NH2), 3.95 (t, J = 6.3 Hz, 2H, 12-H), 2.76 (q, J = 6.6 Hz, 2H, 9-H), 1.66–1.56 (m, 2H, 11-H), 1.48–1.39 (m, 2H, 10-H), 1.32 (s, 9H, 8-H, 8′-H, 8′-H) ppm; 13C NMR:δ = 152.0 (C-3), 140.4 (C-1), 129.3 (C-4), 129.0 (C-5), 123.7 (C-6), 122.8 (C-2), 34.7 (C-7), 30.9 (C-8), 25.6 (C-11), 25.4 (C-10) ppm; MS: m/z = 387.1 (100%, [M + Na]+); anal. calcd. for C14H24N2S2O5 (364.48): C 46.14, H 6.64, N 7.69; found: C 45.85, H 6.99, N 7.41.
4.2.123. 3-(tert-Butyl)-N-(5-hydroxypentyl)benzene Sulfonamide (61a)
Applying GPA: from 3-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 5-amino-pentanol (332 mg, 3.22 mmol): 61a (610 mg, 95%); oil; Rf = 0.23 (petrolether/EtOAc, 2:3); UV–Vis: 224 nm (3.90); IR: ν = 3503w, 3278w, 2939m, 2868w, 1481m, 1459w, 1416w, 1398w, 1366w, 1325m, 1307m, 1267w, 1156vs, 1125m, 1087m, 1040m, 998w, 898w, 797w, 774w, 697s, 678m, 626m, 586vs, 534m, 501w, 478w, 461w cm−1; 1H NMR:δ = 7.79 (td, J = 1.9, 0.5 Hz, 1H, 2-H), 7.66 (ddd, J = 7.8, 2.0, 1.1 Hz, 1H, 6-H), 7.61 (ddd, J = 7.7, 1.8, 1.2 Hz, 1H, 4-H), 7.55–7.48 (m, 2H, 5-H, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.35–3.27 (m, 2H, 13-H), 2.72 (q, J = 6.2 Hz, 2H, 9-H), 1.38–1.31 (m, 4H, 10-H, 12-H), 1.31 (s, 9H, 8-H, 8′-H, 8′-H), 1.26–1.17 (m, 2H, 11-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 151.9 (C-3), 140.5 (C-1), 129.2 (C-4), 128.9 (C-5), 123.7 (C-2), 122.8 (C-6), 60.5 (C-13), 42.6 (C-9), 34.7 (C-7), 32.0 (C-12), 30.9 (C-8), 28.8 (C-10), 22.6 (C-11) ppm; MS: m/z = 322.3 (100%, [M + Na]+); anal. calcd. for C15H25NSO3 (299.43): C 60.17, H 8.42, N 4.68; found: C 59.86, H 8.85, N 4.32.
4.2.124. 5-[(3-(tert-Butyl)phenyl)sulfonamido]pentyl Sulfamate (61b)
Applying GPB: from 61a (200 mg, 0.67 mmol): 61b (150 mg, 59%); oil; Rf = 0.69 (CHCl3/EtOAc, 2:3); UV–Vis: 224 nm (4.15); IR: ν = 3420w, 3368w, 3287m, 2920m, 2859w, 2869w, 1478w, 1410m, 1388w, 1332m, 1315w, 1299w, 1152vs, 1078m, 1062m, 1013m, 963w, 900m, 817s, 735w, 701w, 668s, 570s, 552s, 516m, 464w, 415w cm−1; 1H NMR:δ = 7.91–7.83 (m, 2H, 2-H, NH), 7.66 (dd, J = 8.1, 1.4 Hz, 1H, 6-H), 7.52 (ddd, J = 8.1, 7.2, 1.5 Hz, 1H, 4-H), 7.43–7.40 (m, 1H, 5-H), 7.39–7.37 (m, 2H, NH2), 3.99 (t, J = 6.5 Hz, 2H, 13-H), 2.86–2.78 (m, 2H, 9-H), 1.65–1.55 (m, 2H, 12-H), 1.51 (s, 9H, 8-H, 8′-H, 8′-H), 1.49–1.45 (m, 2H, 10-H), 1.41–1.30 (m, 2H, 11-H) ppm; 13C NMR:δ = 148.9 (C-3), 140.6 (C-1), 131.8 (C-4), 129.4 (C-5), 129.0 (C-2), 126.4 (C-6), 68.9 (C-13), 42.6 (C-9), 36.7 (C-7), 31.8 (C-8), 28.7 (C-12), 27.9 (C-10), 22.3 (C-11) ppm; MS: m/z = 401.6 (100%, [M + Na]+); anal. calcd. for C15H26N2S2O5 (378.50): C 47.60, H 6.92, N 7.40; found: C 47.38, H 7.17, N 7.36.
4.2.125. 3-(tert-Butyl)-N-(6-hydroxyhexyl)benzene Sulfonamide (62a)
Applying GPA: from 3-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 6-amino-hexanol (378 mg, 3.22 mmol): 62a (621 mg, 92%); oil; Rf = 0.25 (petrolether/EtOAc, 2:3); UV–Vis: 225 nm (3.94); IR: ν = 3505vw, 3278w, 2935m, 2864w, 1597vw, 1481w, 1460w, 1416w, 1397w, 1366w, 1325m, 1307m, 1287w, 1267w, 1156vs, 1125m, 1097m, 1086m, 1073m, 1055m, 899w, 869w, 797m, 773w, 697s, 678m, 626m, 587vs, 534m, 500w, 469w, 457w, 445w cm−1; 1H NMR:δ = 7.80–7.78 (m, 1H, 2-H), 7.66 (ddd, J = 7.8, 2.0, 1.1 Hz, 1H, 6-H), 7.60 (ddd, J = 7.7, 1.8, 1.2 Hz, 1H, 4-H), 7.55–7.48 (m, 2H, 5-H, NH), 4.29 (t, J = 5.1 Hz, 1H, OH), 3.36–3.30 (m, 2H, 14-H), 2.72 (td, J = 7.0, 5.7 Hz, 2H, 9-H), 1.37–1.32 (m, 4H, 10-H, 13-H), 1.31 (s, 9H, 8-H, 8′-H, 8′-H), 1.22–1.12 (m, 4H, 11-H, 12-H) ppm; 13C NMR:δ = 151.9 (C-3), 140.5 (C-1), 129.2 (C-4), 128.9 (C-5), 123.7 (C-2), 122.8 (C-6), 60.6 (C-14), 42.5 (C-9), 34.7 (C-7), 32.3 (C-13), 30.9 (C-8), 28.9 (C-10), 25.9 (C-11), 25.0 (C-12) ppm; MS: m/z = 336.2 (100%, [M + Na]+); anal. calcd. for C16H27NSO3 (313.46): C 61.31, H 8.68, N 4.47; found: C 61.07, H 9.01, N 4.15.
4.2.126. 6-[(3-(tert-Butyl)phenyl)sulfonamido]hexyl Sulfamate (62b)
Applying GPB: from 62a (200 mg, 0.64 mmol): 62b (160 mg, 64%); oil; Rf = 0.73 (CHCl3/EtOAc, 2:3); UV–Vis: 225 nm (3.84); IR: ν = 3276w, 2960w, 2867w, 1561vw, 1481w, 1462w, 1417w, 1364s, 1325m, 1307m, 1267w, 1177s, 1156vs, 1125m, 1098m, 1088m, 923s, 798m, 773w, 725w, 697m, 678m, 626m, 587vs, 551s, 535m, 465w cm−1; 1H NMR:δ = 7.80–7.78 (m, 1H, 2-H), 7.67 (ddd, J = 7.8, 2.1, 1.2 Hz, 1H, 6-H), 7.62–7.58 (m, 1H, 4-H), 7.57–7.48 (m, 2H, 5-H, NH), 7.36 (s, 2H, NH2), 3.96 (t, J = 6.5 Hz, 2H, 14-H), 2.73 (q, J = 6.6 Hz, 2H, 9-H), 1.60–1.50 (m, 2H, 13-H), 1.39–1.28 (m, 2H, 10-H), 1.31 (s, 9H, 8-H, 8′-H, 8′-H), 1.27–1.17 (m, 4H, 11-H, 12-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 151.9 (C-3), 140.5 (C-1), 129.2 (C-4), 128.9 (C-5), 123.7 (C-2), 122.8 (C-6), 68.9 (C-14), 42.4 (C-9), 34.7 (C-7), 30.9 (C-8), 28.8 (C-13), 28.2 (C-10), 25.5 (C-12), 24.6 (C-11) ppm; MS: m/z = 415.6 (100%, [M + Na]+); anal. calcd. for C16H28N2S2O5 (392.53): C 48.96, H 7.19, N 7.14; found: C 48.71, H 7.32, N 6.84.
4.2.127. 3-(tert-Butyl)-N-(7-hydroxyheptyl)benzene Sulfonamide (63a)
Applying GPA: from 3-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 7-amino-heptanol (422 mg, 3.22 mmol): 63a (635 mg, 90%); oil; Rf = 0.25 (petrolether/EtOAc, 2:3); UV–Vis: 225 nm (4.00); IR: ν = 3503vw, 3279w, 2932m, 2860w, 1481w, 1461w, 1416w, 1397w, 1366w, 1325m, 1308m, 1267w, 1156vs, 1125m, 1098m, 1087m, 1056m, 999w, 897w, 797w, 773w, 724w, 697s, 678m, 626m, 587vs, 534m, 500w, 461w, 445w cm−1; 1H NMR:δ = 7.80–7.77 (m, 1H, 2-H), 7.66 (ddd, J = 7.8, 2.0, 1.1 Hz, 1H, 6-H), 7.60 (ddd, J = 7.7, 1.8, 1.1 Hz, 1H, 4-H), 7.55–7.47 (m, 2H, 5-H, NH), 4.29 (t, J = 5.1 Hz, 1H, OH), 3.40–3.31 (m, 2H, 15-H), 2.76–2.68 (m, 2H, 9-H), 1.40–1.32 (m, 4H, 10-H, 14-H), 1.31 (s, 9H, 8-H, 8′-H, 8′-H), 1.24–1.09 (m, 6H, 11-H, 12-H, 13-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 151.8 (C-3), 140.5 (C-1), 129.2 (C-4), 128.9 (C-5), 123.7 (C-2), 122.8 (C-6), 60.7 (C-15), 42.5 (C-9), 34.7 (C-7), 32.4 (C-14), 30.9 (C-8), 28.8 (C-10), 28.4 (C-12), 26.0 (C-11), 25.3 (C-13) ppm; MS: m/z = 350.3 (100%, [M + Na]+); anal. calcd. for C17H29NSO3 (327.48): C 62.35, H 8.93, N 4.28; found: C 62.03, H 9.21, N 3.96.
4.2.128. 7-[(3-(tert-Butyl)phenyl)sulfonamido]heptyl Sulfamate (63b)
Applying GPB: from 63a (200 mg, 0.61 mmol): 63b (134 mg, 54%); oil; Rf = 0.78 (CHCl3/EtOAc, 2:3); UV–Vis: 225 nm (4.10); IR: ν = 3277w, 2937w, 2862w, 1562w, 1481w, 1417w, 1364s, 1325m, 1307m, 1267w, 1177s, 1156vs, 1125m, 1098m, 1088m, 923s, 799m, 773m, 724w, 697m, 678m, 626m, 587vs, 551s, 495w, 460w, 443vw cm−1; 1H NMR:δ = 7.79 (td, J = 1.9, 0.5 Hz, 1H, 2-H), 7.67 (ddd, J = 7.8, 2.0, 1.1 Hz, 1H, 6-H), 7.60 (ddd, J = 7.7, 1.8, 1.2 Hz, 1H, 4-H), 7.56–7.49 (m, 2H, 5-H, NH), 7.37 (s, 2H, NH2), 3.98 (t, J = 6.5 Hz, 2H, 15-H), 2.73 (td, J = 6.9, 5.8 Hz, 2H, 9-H), 1.61–1.53 (m, 2H, 14-H), 1.31 (s, 9H, 8-H, 8′-H, 8′-H), 1.35–1.28 (m, 2H, 10-H), 1.27–1.14 (m, 6H, 11-H, 12-H, 13-H) ppm; 13C NMR:δ = 151.9 (C-3), 140.5 (C-1), 129.2 (C-4), 128.9 (C-5), 123.7 (C-2), 122.8 (C-6), 68.9 (C-15), 42.5 (C-9), 34.7 (C-7), 30.9 (C-8), 28.8 (C-14), 28.2 (C-11), 28.0 (C-10), 25.8 (C-13), 24.9 (C-12) ppm; MS: m/z = 429.7 (100%, [M + Na]+); anal. calcd. for C17H30N2S2O5 (406.56): C 50.22, H 7.44, N 6.89; found: C 49.96, H 7.79, N 6.65.
4.2.129. 3-(tert-Butyl)-N-(8-hydroxyoctyl)benzene Sulfonamide (64a)
Applying GPA: from 3-(tert-butyl)benzenesulfonyl chloride (500 mg, 2.15 mmol) and 8-amino-octanol (468 mg, 3.22 mmol): 64a (703 mg, 96%); oil; Rf = 0.30 (petrolether/EtOAc, 2:3); UV–Vis: 225 nm (3.92); IR: ν = 3503vw, 3279w, 2930m, 2857m, 1481w, 1461w, 1416w, 1397w, 1366w, 1325m, 1308m, 1267w, 1157vs, 1125m, 1098m, 1086m, 998vw, 898w, 797m, 774w, 722w, 697s, 678m, 626m, 587vs, 534m, 515m cm−1; 1H NMR:δ = 7.79 (td, J = 1.9, 0.5 Hz, 1H, 2-H), 7.66 (ddd, J = 7.8, 2.0, 1.1 Hz, 1H, 6-H), 7.60 (ddd, J = 7.7, 1.8, 1.1 Hz, 1H, 4-H), 7.54–7.48 (m, 2H, 5-H, NH), 4.29 (t, J = 5.1 Hz, 1H, OH), 3.38–3.33 (m, 2H, 16-H), 2.72 (td, J = 6.9, 2.7 Hz, 2H, 9-H), 1.41–1.32 (m, 4H, 10-H, 15-H), 1.31 (s, 9H, 8-H, 8′-H, 8′-H), 1.25–1.09 (m, 8H, 11-H, 12-H, 13-H, 14-H) ppm; 13C NMR:δ = 151.9 (C-3), 140.6 (C-1), 129.2 (C-4), 128.9 (C-5), 123.7 (C-2), 122.9 (C-6), 60.7 (C-16), 42.5 (C-9), 34.7 (C-7), 32.5 (C-15), 30.9 (C-8), 28.8 (C-10), 28.8 (C-12), 28.6 (C-13), 25.9 (C-11), 25.4 (C-14) ppm; MS: m/z = 364.4 (100%, [M + Na]+); anal. calcd. for C18H31NSO3 (341.51): C 63.31, H 9.15, N 4.10; found: C 63.07, H 9.42, N 3.91.
4.2.130. 8-[(3-(tert-Butyl)phenyl)sulfonamido]octyl Sulfamate (64b)
Applying GPB: from 64a (200 mg, 0.59 mmol): 64b (221 mg, 90%); white solid; Rf = 0.83 (CHCl3/EtOAc, 2:3); m.p. = 57–59 °C; UV–Vis: 225 nm (3.99); IR: ν = 3364w, 3279m, 2967w, 2928m, 2858w, 1558w, 1481w, 1475w, 1466w, 1428w, 1399w, 1378s, 1322m, 1310s, 1289m, 1270w, 1178s, 1170s, 1158vs, 1127m, 1099w, 1070w, 1057m, 1037m, 999w, 959vs, 909s, 896s, 820s, 793m, 775m, 761w, 725m, 706m, 692vs, 667m, 628m, 621m, 588vs, 554s, 535s, 510s, 485m cm−1; 1H NMR:δ = 7.79 (td, J = 1.9, 0.5 Hz, 1H, 2-H), 7.67 (ddd, J = 7.8, 2.0, 1.1 Hz, 1H, 6-H), 7.60 (ddd, J = 7.7, 1.8, 1.2 Hz, 1H, 4-H), 7.56–7.48 (m, 2H, 5-H, NH), 7.37 (s, 2H, NH2), 3.99 (t, J = 6.5 Hz, 2H, 16-H), 2.72 (td, J = 7.0, 5.8 Hz, 2H, 9-H), 1.63–1.54 (m, 2H, 15-H), 1.36–1.21 (m, 4H, 10-H, 14-H), 1.31 (s, 9H, 8-H, 8′-H, 8′-H), 1.22–1.11 (m, 6H, 11-H, 12-H, 13-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 151.9 (C-3), 140.5 (C-1), 129.2 (C-4), 128.9 (C-5), 123.7 (C-2), 122.8 (C-6), 68.9 (C-16), 42.5 (C-9), 34.7 (C-7), 30.9 (C-8), 28.8 (C-15), 28.4 (C-13), 28.3 (C-10), 28.3 (C-12), 25.9 (C-11), 24.9 (C-14) ppm; MS: m/z = 443.8 (100%, [M + Na]+); anal. calcd. for C18H32N2S2O5 (420.58): C 51.40, H 7.67, N 6.66; found: C 51.17, H 7.96, N 6.45.
4.2.131. 2-(tert-Butyl)-N-(5-hydroxypentyl)benzene Sulfonamide (65a)
Applying GPA: from 2-(tert-butyl)benzenesulfonyl chloride (330 mg, 1.42 mmol) and 5-amino-pentanol (219 mg, 2.13 mmol): 65a (352 mg, 83%); oil; Rf = 0.23 (petrolether/EtOAc, 2:3); UV–Vis: 221 nm (3.83); IR: ν = 3493vw, 3294w, 3060vw, 2938m, 2867w, 1463w, 1432w, 1400w, 1366w, 1314s, 1260w, 1243w, 1199vw, 1175w, 1152vs, 1128m, 1111m, 1082m, 1057m, 1044m, 934vw, 874vw, 841vw, 760m, 734m, 662m, 592vs, 546s, 475w, 434vw cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 7.89 (dd, J = 8.0, 1.5 Hz, 1H, 3-H), 7.83 (t, J = 5.6 Hz, 1H, NH), 7.66 (dd, J = 8.2, 1.4 Hz, 1H, 6-H), 7.51 (td, J = 7.6, 1.5 Hz, 1H, 4-H), 7.40 (td, J = 7.6, 1.4 Hz, 1H, 5-H), 4.32 (t, J = 5.1 Hz, 1H, OH), 3.36 (td, J = 6.4, 5.0 Hz, 2H, 13-H), 2.81 (q, J = 6.6 Hz, 2H, 9-H), 1.53–1.50 (m, 9H, 8-H, 8′-H, 8′-H), 1.48–1.25 (m, 6H, 10-H, 11-H, 12-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 148.9 (C-2), 140.7 (C-1), 131.7 (C-4), 129.4 (C-3), 128.9 (C-6), 126.4 (C-5), 60.5 (C-13), 42.8 (C-9), 36.7 (C-7), 32.0 (C-12), 31.8 (C-8), 29.1 (C-10), 22.6 (C-11) ppm; MS: m/z = 322.3 (100%, [M + Na]+); anal. calcd. for C15H25NSO3 (299.43): C 60.17, H 8.42, N 4.68; found: C 59.82, H 8.69, N 4.32.
4.2.132. 5-[(2-(tert-Butyl)phenyl)sulfonamido]pentyl Sulfamate (65b)
Applying GPB: from 65a (120 mg, 0.4 mmol): 65b (88 mg, 58%); oil; Rf = 0.66 (CHCl3/EtOAc, 2:3); UV–Vis: 221 nm (3.98); IR: ν = 3283w, 2941w, 2867w, 1566w, 1464w, 1432w, 1362s, 1313s, 1260w, 1243w, 1176s, 1152vs, 1128m, 1111m, 1083w, 1059w, 923s, 839w, 822w, 761s, 733m, 662m, 593vs, 550vs cm−1; 1H NMR:δ = 7.80–7.77 (m, 1H, 6-H), 7.67 (ddd, J = 7.8, 2.0, 1.1 Hz, 1H, 3-H), 7.60 (ddd, J = 7.7, 1.8, 1.2 Hz, 1H, 5-H), 7.56 (t, J = 5.9 Hz, 1H, NH), 7.52 (t, J = 7.8 Hz, 1H, 4-H), 7.37 (s, 2H, NH2), 3.95 (t, J = 6.5 Hz, 2H, 13-H), 2.73 (q, J = 6.6 Hz, 2H, 9-H), 1.58–1.50 (m, 2H, 12-H), 1.42–1.33 (m, 2H, 10-H), 1.31 (s, 9H, 8-H, 8′-H, 8′-H), 1.34–1.22 (m, 2H, 11-H) ppm; 13C NMR:δ = 151.9 (C-2), 140.4 (C-1), 129.3 (C-4), 128.9 (C-3), 123.7 (C-6), 122.8 (C-5), 68.8 (C-13), 42.3 (C-9), 34.7 (C-7), 30.9 (C-8), 28.4 (C-12), 27.8 (C-10), 22.2 (C-11) ppm; MS: m/z = 401.6 (100%, [M + Na]+); anal. calcd. for C15H26N2S2O5 (378.50): C 47.60, H 6.92, N 7.40; found: C 47.26, H 7.17, N 7.13.
4.2.133. 2-(tert-Butyl)-N-(6-hydroxyhexyl)benzene Sulfonamide (66a)
Applying GPA: from 2-(tert-butyl)benzenesulfonyl chloride (330 mg, 1.42 mmol) and 6-amino-hexanol (249 mg, 2.13 mmol): 66a (419 mg, 94%); oil; Rf = 0.30 (petrolether/EtOAc, 2:3); UV–Vis: 221 nm (3.78); IR: ν = 3497vw, 3295w, 2934m, 2863w, 1463w, 1432w, 1400w, 1366w, 1314s, 1260w, 1242w, 1199vw, 1175w, 1152vs, 1128m, 1111m, 1056m, 842vw, 760m, 734m, 662m, 592vs, 546m, 476w cm −1; 1H NMR:δ = 7.89 (dd, J = 8.0, 1.5 Hz, 1H, 3-H), 7.82 (t, J = 5.8 Hz, 1H, NH), 7.66 (dd, J = 8.1, 1.3 Hz, 1H, 6-H), 7.51 (ddd, J = 8.0, 7.2, 1.5 Hz, 1H, 4-H), 7.43–7.37 (m, 1H, 5-H), 4.31 (t, J = 5.2 Hz, 1H, OH), 3.36 (td, J = 6.5, 5.2 Hz, 2H, 14-H), 2.81 (td, J = 7.1, 5.8 Hz, 2H, 9-H), 1.51 (s, 9H, 8-H, 8′-H, 8′-H), 1.48–1.33 (m, 4H, 10-H, 13-H), 1.31–1.19 (m, 4H, 11-H, 12-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 148.8 (C-2), 140.7 (C-1), 131.7 (C-4), 129.4 (C-3), 128.9 (C-6), 126.4 (C-5), 60.6 (C-14), 42.8 (C-9), 36.7 (C-7), 32.4 (C-13), 31.8 (C-8), 29.3 (C-10), 26.0 (C-12), 25.1 (C-11) ppm; MS: m/z = 336.3 (100%, [M + Na]+); anal. calcd. for C16H27NSO3 (313.46): C 61.31, H 8.68, N 4.47; found: C 61.04, H 8.94, N 4.11.
4.2.134. 6-[(2-(tert-Butyl)phenyl)sulfonamido]hexyl Sulfamate (66b)
Applying GPB: from 66a (120 mg, 0.38 mmol): 66b (141 mg, 94%); oil; Rf = 0.70 (CHCl3/EtOAc, 2:3); UV–Vis: 221 nm (4.00); IR: ν = 3283w, 2941w, 2867w, 1566w, 1464w, 1432w, 1362s, 1313s, 1260w, 1243w, 1176s, 1152vs, 1128m, 1111m, 1083w, 1059w, 923s, 839w, 822w, 761s, 733m, 662m, 593vs, 550vs cm −1; 1H NMR:δ = 7.89 (dd, J = 8.0, 1.5 Hz, 1H, 3-H), 7.84 (t, J = 5.8 Hz, 1H, NH), 7.66 (dd, J = 8.1, 1.4 Hz, 1H, 6-H), 7.51 (ddd, J = 7.9, 7.2, 1.5 Hz, 1H, 4-H), 7.44–7.39 (m, 1H, 5-H), 7.38 (s, 2H, NH2), 3.99 (t, J = 6.5 Hz, 2H, 14-H), 2.81 (td, J = 7.0, 5.8 Hz, 2H, 9-H), 1.64–1.54 (m, 2H, 13-H), 1.51 (s, 9H, 8-H, 8′-H, 8′-H), 1.49–1.41 (m, 2H, 10-H), 1.33–1.25 (m, 4H, 11-H, 12-H) ppm; 13C NMR:δ = 148.9 (C-2), 140.7 (C-1), 131.8 (C-4), 129.4 (C-3), 129.0 (C-6), 126.4 (C-5), 68.9 (C-14), 42.7 (C-9), 36.7 (C-7), 31.8 (C-8), 29.1 (C-13), 28.2 (C-10), 25.6 (C-12), 24.6 (C-11) ppm; MS: m/z = 415.6 (100%, [M + Na]+); anal. calcd. for C16H28N2S2O5 (392.53): C 48.96, H 7.19, N 7.14; found: C 48.75, H 7.43, N 6.87.
4.2.135. 2-(tert-Butyl)-N-(7-hydroxyheptyl)benzene Sulfonamide (67a)
Applying GPA: from 2-(tert-butyl)benzenesulfonyl chloride (330 mg, 1.42 mmol) and 7-amino-heptanol (279 mg, 2.13 mmol): 67a (411 mg, 89%); oil; Rf = 0.34 (petrolether/EtOAc, 2:3); UV–Vis: 221 nm (4.03); IR: ν = 3496vw, 3295w, 2931m, 2859w, 1463w, 1432w, 1400w, 1366w, 1315s, 1260w, 1242w, 1199vw, 1175vw, 1152s, 1128m, 1111m, 1057m, 866vw, 840vw, 760m, 734m, 662m, 593vs, 547m, 477w, 428vw cm−1; 1H NMR:δ = 7.90 (dt, J = 8.0, 1.3 Hz, 1H, 3-H), 7.82 (s, 1H, NH), 7.66 (dt, J = 8.1, 1.3 Hz, 1H, 6-H), 7.54–7.47 (m, 1H, 4-H), 7.44–7.37 (m, 1H, 5-H), 4.23 (s, 1H, OH), 3.40–3.33 (m, 2H, 15-H), 2.85–2.76 (m, 2H, 9-H), 1.56–1.48 (m, 9H, 8-H, 8′-H, 8′-H), 1.47–1.33 (m, 4H, 10-H, 14-H), 1.30–1.16 (m, 6H, 11-H, 12-H, 13-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 148.8 (C-2), 140.7 (C-1), 131.7 (C-4), 129.5 (C-3), 128.9 (C-6), 126.4 (C-5), 60.7 (C-15), 42.7 (C-9), 36.7 (C-7), 32.4 (C-14), 31.8 (C-8), 29.2 (C-10), 28.5 (C-12), 26.1 (C-11), 25.4 (C-13) ppm; MS: m/z = 350.3 (100%, [M + Na]+); anal. calcd. for C17H29NSO3 (327.48): C 62.35, H 8.93, N 4.28; found: C 62.02, H 9.25, N 3.96.
4.2.136. 7-[(2-(tert-Butyl)phenyl)sulfonamido]heptyl Sulfamate (67b)
Applying GPB: from 67a (150 mg, 0.46 mmol): 67b (165 mg, 89%); oil; Rf = 0.76 (CHCl3/EtOAc, 2:3); UV–Vis: 221 nm (3.87); IR: ν = 3283w, 2936w, 2862w, 1566w, 1464w, 1432w, 1362s, 1314s, 1177s, 1152vs, 1128m, 1111m, 1083w, 1058w, 1000w, 924s, 838w, 814w, 762s, 734m, 662m, 593vs, 550vs, 500m cm−1; 1H NMR:δ = 7.89 (dd, J = 8.0, 1.5 Hz, 1H, 3-H), 7.83 (t, J = 5.8 Hz, 1H, NH), 7.66 (dd, J = 8.1, 1.3 Hz, 1H, 6-H), 7.51 (td, J = 8.1, 7.7, 1.5 Hz, 1H, 4-H), 7.44–7.38 (m, 1H, 5-H), 7.37 (s, 2H, NH2), 3.99 (t, J = 6.5 Hz, 2H, 15-H), 2.81 (td, J = 7.0, 5.8 Hz, 2H, 9-H), 1.64–1.55 (m, 2H, 14-H), 1.51 (s, 9H, 8-H, 8′-H, 8′-H), 1.48–1.39 (m, 2H, 10-H), 1.35–1.21 (m, 6H, 11-H, 12-H, 13-H) ppm; 13C NMR:δ = 148.8 (C-2), 140.7 (C-1), 131.8 (C-4), 129.5 (C-3), 129.0 (C-6), 126.4 (C-5), 69.0 (C-15), 42.7 (C-9), 36.7 (C-7), 31.8 (C-8), 29.1 (C-14), 28.2 (C-11), 28.0 (C-10), 25.9 (C-13), 25.0 (C-12) ppm; MS: m/z = 429.6 (100%, [M + Na]+); anal. calcd. for C17H30N2S2O5 (406.56): C 50.22, H 7.44, N 6.89; found: C 49.94, H 7.76, N 6.53.
4.2.137. 4-Cyclohexyl-N-(2-hydroxyethyl)benzene Sulfonamide (68a) [919974-61-1]
Applying GPA: from 4-cyclohexylbenzenesulfonyl chloride (500 mg, 1.93 mmol) and 2-amino-ethanol (177 mg, 2.90 mmol): 68a (453 mg, 83%); oil; Rf = 0.56 (CHCl3/EtOAc, 2:3); UV–Vis: 229 nm (4.19); IR: ν = 3514vw, 3264w, 3146w, 2920m, 2848m, 1597w, 1496vw, 1483vw, 1461w, 1451m, 1427w, 1409w, 1348w, 1319s, 1278w, 1260w, 1216vw, 1188w, 1156s, 1134w, 1093s, 1059m, 1036m, 1018w, 996w, 953m, 890w, 864vw, 842w, 826s, 802w, 782vw, 731w, 701s, 631w, 597s, 574vs, 527m, 504w, 491w, 479w, 458m cm−1; 1H NMR:δ = 7.72–7.68 (m, 2H, 2-H, 2′-H), 7.51–7.46 (m, 1H, NH), 7.45–7.40 (m, 2H, 3-H, 3′-H), 4.66 (t, J = 5.6 Hz, 1H, OH), 3.37 (q, J = 6.3 Hz, 2H, 10-H), 2.77 (q, J = 5.9 Hz, 2H, 9-H), 2.64–2.55 (m, 1H, 5-H), 1.84–1.74 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.74–1.66 (m, 1H, 8-Ha), 1.50–1.30 (m, 4H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb), 1.30–1.17 (m, 1H, 8-Hb) ppm; 13C NMR:δ = 152.1 (C-4), 138.0 (C-1), 127.4 (C-2), 126.6 (C-3), 59.9 (C-10), 45.1 (C-9), 43.6 (C-5), 33.5 (C-6), 26.2 (C-7), 25.4 (C-8) ppm; MS: m/z = 305.9 (90%, [M + Na]+); anal. calcd. for C14H21NSO3 (283.39): C 59.34, H 7.47, N 4.94; found: C 59.11, H 7.85, N 4.65.
4.2.138. 2-[(4-Cyclohexylphenyl)sulfonamido]ethyl Sulfamate (68b)
Applying GPB: from 68a (200 mg, 0.71 mmol): 68b (232 mg, 91%); white solid; Rf = 0.77 (CHCl3/EtOAc, 2:3); m.p. = 109–110 °C; UV–Vis: 229 nm (4.28); IR: ν = 3358m, 3256w, 2922m, 2849w, 1599w, 1549w, 1462w, 1452w, 1412m, 1363s, 1314s, 1279w, 1252vw, 1181s, 1154vs, 1095m, 1078w, 1010m, 998m, 957s, 903m, 828m, 781m, 748s, 723m, 702s, 634w, 610s, 563s, 549vs, 525m, 501m, 465m, 449m cm−1; 1H NMR:δ = 7.82 (t, J = 6.0 Hz, 1H, NH), 7.73–7.69 (m, 2H, 2-H, 2′-H), 7.50 (s, 2H, NH2), 7.47–7.42 (m, 2H, 3-H, 3′-H), 4.00 (t, J = 5.7 Hz, 2H, 10-H), 3.03 (q, J = 5.8 Hz, 2H, 9-H), 2.64–2.55 (m, 1H, 5-H), 1.84–1.76 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.75–1.67 (m, 1H, 8-Ha), 1.49–1.30 (m, 5H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb), 1.30–1.18 (m, 1H, 8-Hb) ppm; 13C NMR:δ = 152.4 (C-1), 137.7 (C-4), 127.5 (C-2), 126.6 (C-3), 67.6 (C-10), 43.6 (C-5), 41.5 (C-9), 33.5 (C-6), 26.2 (C-7), 25.4 (C-8) ppm; MS: m/z = 385.5 (100%, [M + Na]+); anal. calcd. for C14H22N2S2O5 (362.46): C 46.39, H 6.12, N 7.73; found: C 46.16, H 6.38, N 7.41.
4.2.139. 4-Cyclohexyl-N-(3-hydroxypropyl)benzene Sulfonamide (69a) [919974-63-3]
Applying GPA: from 4-cyclohexylbenzenesulfonyl chloride (500 mg, 1.93 mmol) and 3-amino-propanol (218 mg, 2.90 mmol): 69a (470 mg, 82%); white solid; Rf = 0.53 (CHCl3/EtOAc, 2:3); m.p. = 78–79 °C; UV–Vis: 229 nm (4.13); IR: ν = 3471w, 3167w, 2929m, 2851m, 1598w, 1494vw, 1472w, 1463w, 1448w, 1424w, 1407w, 1375vw, 1351vw, 1310s, 1283w, 1230vw, 1185w, 1157vs, 1096m, 1075s, 1005m, 964m, 943w, 876m, 853w, 838w, 827m, 803w, 781w, 740m, 698m, 595vs, 574s, 507m, 481w, 455w cm−1; 1H NMR:δ = 7.72–7.66 (m, 2H, 2-H, 2′-H), 7.47–7.37 (m, 3H, NH, 3-H, 3′-H), 4.39 (t, J = 5.1 Hz, 1H, OH), 3.39–3.33 (m, 2H, 11-H), 2.80–2.72 (m, 2H, 9-H), 2.59 (td, J = 9.4, 4.7 Hz, 1H, 5-H), 1.83–1.75 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.74–1.67 (m, 1H, 8-Ha), 1.52 (dt, J = 13.3, 6.3 Hz, 2H, 10-H), 1.48–1.31 (m, 4H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb), 1.29–1.18 (m, 1H, 8-Hb) ppm; 13C NMR:δ = 152.1 (C-4), 137.9 (C-1), 127.4 (C-2), 126.6 (C-3), 58.1 (C-11), 43.6 (C-5), 40.0 (C-9), 33.5 (C-6), 32.4 (C-10), 26.2 (C-7), 25.4 (C-8) ppm; MS: m/z = 319.9 (90%, [M + Na]+); anal. calcd. for C15H23NSO3 (297.41): C 60.58, H 7.80, N 4.71; found: C 60.44, H 8.02, N 4.54.
4.2.140. 3-[(4-Cyclohexylphenyl)sulfonamido]propyl Sulfamate (69b)
Applying GPB: from 69a (200 mg, 0.67 mmol): 69b (210 mg, 83%); white solid; Rf = 0.74 (CHCl3/EtOAc, 2:3); m.p. = 113–114 °C; UV–Vis: 229 nm (4.27); IR: ν = 3351w, 3283m, 2929m, 2851w, 1598w, 1567w, 1474w, 1438w, 1403w, 1371vs, 1323m, 1307s, 1281w, 1256w, 1177vs, 1152vs, 1093m, 1070m, 1039m, 999w, 944s, 921m, 888m, 836s, 824s, 778w, 756m, 729m, 706s, 654m, 630m, 599s, 593s, 568vs, 548s, 527m, 515m, 506m, 486w, 428w cm−1; 1H NMR:δ = 7.74–7.67 (m, 2H, 2-H, 2′-H), 7.57–7.36 (m, 5H, 3-H, 3′-H, NH2, NH), 4.02 (t, J = 6.3 Hz, 2H, 11-H), 2.80 (t, J = 7.1 Hz, 2H, 9-H), 2.64–2.55 (m, 1H, 5-H), 1.84–1.67 (m, 7H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha, 8-Ha, 10-H), 1.49–1.31 (m, 4H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb), 1.29–1.18 (m, 1H, 8-Hb) ppm; 13C NMR:δ = 152.2 (C-1), 137.7 (C-4), 127.5 (C-2), 126.6 (C-3), 66.5 (C-11), 43.5 (C-5), 39.2 (C-9), 33.5 (C-6), 28.8 (C-10), 26.2 (C-7), 25.4 (C-8) ppm; MS: m/z = 399.5 (100%, [M + Na]+); anal. calcd. for C15H24N2S2O5 (376.49): C 47.85, H 6.43, N 7.44; found: C 47.57, H 6.61, N 7.21.
4.2.141. 4-Cyclohexyl-N-(4-hydroxybutyl)benzene Sulfonamide (70a) [1976415-75-4]
Applying GPA: from 4-cyclohexylbenzenesulfonyl chloride (500 mg, 1.93 mmol) and 4-amino-butanol (258 mg, 2.90 mmol): 70a (487 mg, 81%); white solid; Rf = 0.50 (CHCl3/EtOAc, 2:3); m.p. = 58–60 °C; UV–Vis: 229 nm (4.17); IR: ν = 3477m, 3101w, 2950w, 2922m, 2849m, 1446m, 1429w, 1396w, 1312s, 1257w, 1150vs, 1097m, 1081m, 1030s, 994m, 941m, 916w, 821m, 812w, 786s, 771m, 735m, 694w, 601s, 570vs, 537m, 508w, 485w, 438w, 490m, 476w, 441w cm−1; 1H NMR:δ = 7.71–7.66 (m, 2H, 2-H, 2′-H), 7.48–7.40 (m, 3H, NH, 3-H, 3′-H), 4.34 (s, 1H, OH), 3.33–3.28 (m, 2H, 12-H), 2.71 (t, J = 6.5 Hz, 2H, 9-H), 2.63–2.54 (m, 1H, 5-H), 1.83–1.75 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.75–1.66 (m, 1H, 8-Ha), 1.47–1.30 (m, 8H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb, 10-H, 11-H), 1.29–1.20 (m, 1H, 8-Hb) ppm; 13C NMR:δ = 152.0 (C-1), 138.1 (C-4), 127.4 (C-2), 126.5 (C-3), 60.2 (C-12), 43.6 (C-5), 42.5 (C-9), 33.5 (C-6), 29.5 (C-11), 26.2 (C-7), 25.8 (C-10), 25.4 (C-8) ppm; MS: m/z = 334.3 (100%, [M + Na]+); anal. calcd. for C16H25NSO3 (311.44): C 61.71, H 8.09, N 4.50; found: C 61.53, H 8.24, N 4.16.
4.2.142. 4-[(4-Cyclohexylphenyl)sulfonamido]butyl Sulfamate (70b)
Applying GPB: from 70a (200 mg, 0.64 mmol): 70b (224 mg, 89%); white solid; Rf = 0.69 (CHCl3/EtOAc, 2:3); m.p. = 103–105 °C; UV–Vis: 229 nm (4.25); IR: ν = 3365w, 3308w, 3266w, 2923m, 2850w, 1598w, 1543w, 1466w, 1453w, 1411m, 1388w, 1366s, 1315s, 1279w, 1272w, 1180m, 1157s, 1132m, 1095m, 1065w, 1051m, 1019m, 997w, 958m, 921m, 872w, 827m, 790s, 737m, 710s, 656m, 631m, 597m, 588m, 571s, 550vs, 532m, 508w, 503w, 489w, 478w, 453wa cm−1; 1H NMR:δ = 7.73–7.67 (m, 2H, 2-H, 2′-H), 7.53 (t, J = 5.9 Hz, 1H, NH), 7.46–7.41 (m, 2H, 3-H, 3′-H), 7.38 (s, 2H, NH2), 3.96 (t, J = 6.3 Hz, 2H, 12-H), 2.75 (q, J = 6.7 Hz, 2H, 9-H), 2.64–2.55 (m, 1H, 5-H), 1.84–1.76 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.75–1.67 (m, 1H, 8-Ha), 1.66–1.56 (m, 2H, 11-H), 1.51–1.31 (m, 6H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb, 10-H), 1.30–1.17 (m, 1H, 8-Hb) ppm; 13C NMR:δ = 152.1 (C-1), 138.0 (C-4), 127.4 (C-2), 126.5 (C-3), 68.5 (C-12), 43.6 (C-5), 42.0 (C-9), 33.5 (C-6), 26.2 (C-7), 25.6 (C-10), 25.4 (C-8, C-11) ppm; MS: m/z = 413.7 (100%, [M + Na]+); anal. calcd. for C16H26N2S2O5 (390.51): C 49.21, H 6.71, N 7.17; found: C 48,97, H 6.97, N 6.86.
4.2.143. 4-Cyclohexyl-N-(5-hydroxypentyl)benzene Sulfonamide (71a)
Applying GPA: from 4-cyclohexylbenzenesulfonyl chloride (500 mg, 1.93 mmol) and 5-amino-pentanol (299 mg, 2.90 mmol): 71a (520 mg, 83%); oil; Rf = 0.49 (CHCl3/EtOAc, 2:3); UV–Vis: 229 nm (4.20); IR: ν = 3498vw, 3280w, 2924m, 2852m, 1598w, 1495vw, 1448m, 1410w, 1314s, 1283w, 1188vw, 1153vs, 1093s, 1040m, 999w, 920vw, 894w, 868w, 826m, 781w, 732w, 699s, 594s, 574s, 506m, 443vw, 508w, 485w, 438w, 490m, 476w, 441w cm−1; 1H NMR:δ = 7.71–7.66 (m, 2H, 2-H, 2′-H), 7.46–7.40 (m, 3H, 3-H, 3′-H, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.34–3.28 (m, 2H, 13-H), 2.70 (td, J = 7.0, 3.2 Hz, 2H, 9-H), 2.63–2.54 (m, 1H, 5-H), 1.83–1.76 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.74–1.66 (m, 1H, 8-Ha), 1.48–1.15 (m, 11H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb, 8-Hb, 10-H, 11-H, 12-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 152.0 (C-4), 138.1 (C-1), 127.4 (C-3), 126.5 (C-2), 60.5 (C-13), 43.6 (C-5), 42.6 (C-9), 33.5 (C-6), 32.0 (C-12), 28.9 (C-10), 26.2 (C-7), 25.4 (C-8), 22.6 (C-11) ppm; MS: m/z = 348.4 (100%, [M + Na]+); anal. calcd. for C17H27NSO3 (325.47): C 62.74, H 8.36, N 4.30; found: C 62.51, H 8.63, N 4.17.
4.2.144. 5-[(4-Cyclohexylphenyl)sulfonamido]pentyl Sulfamate (71b)
Applying GPB: from 71a (200 mg, 0.61 mmol): 71b (240 mg, 96%); white solid; Rf = 0.62 (CHCl3/EtOAc, 2:3); m.p. = 112–114 °C; UV–Vis: 229 nm (3.95); IR: ν = 3343w, 3277m, 3240w, 2934m, 2867w, 2848w, 1599w, 1471w, 1449w, 1444w, 1433w, 1404w, 1371s, 1356m, 1309s, 1278w, 1179s, 1154vs, 1093m, 1077w, 1052m, 968s, 920s, 886m, 866w, 821vs, 782w, 777w, 731w, 703s, 661m, 631m, 599s, 584m, 571vs, 558s, 537s, 521m, 504m, 478w cm−1; 1H NMR:δ = 7.71–7.67 (m, 2H, 2-H, 2′-H), 7.48 (t, J = 5.9 Hz, 1H, NH), 7.46–7.41 (m, 2H, 3-H, 3′-H), 7.37 (s, 2H, NH2), 3.96 (t, J = 6.5 Hz, 2H, 13-H), 2.72 (q, J = 6.6 Hz, 2H, 9-H), 2.64–2.55 (m, 1H, 5-H), 1.83–1.76 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.75–1.67 (m, 1H, 8-Ha), 1.60–1.51 (m, 2H, 12-H), 1.49–1.34 (m, 6H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb, 10-H), 1.34–1.21 (m, 3H, 8-Hb, 11-H) ppm; 13C NMR:δ = 152.1 (C-1), 138.0 (C-4), 127.4 (C-2), 126.5 (C-3), 68.8 (C-13), 43.5 (C-5), 42.3 (C-9), 33.5 (C-6), 28.5 (C-12), 27.8 (C-10), 26.2 (C-7), 25.4 (C-8), 22.2 (C-11) ppm; MS: m/z = 427.8 (100%, [M + Na]+); anal. calcd. for C17H28N2S2O5 (404.54): C 50.47, H 6.98, N 6.92; found: C 50.10, H 7.13, N 6.76.
4.2.145. 4-Cyclohexyl-N-(6-hydroxyhexyl)benzene Sulfonamide (72a)
Applying GPA: from 4-cyclohexylbenzenesulfonyl chloride (500 mg, 1.93 mmol) and 6-amino-hexanol (340 mg, 2.90 mmol): 72a (589 mg, 90%); white solid; Rf = 0.48 (CHCl3/EtOAc, 2:3); m.p. = 87–88 °C; UV–Vis: 229 nm (4.14); IR: ν = 3424w, 3365vw, 3261m, 2963vw, 2926m, 2855m, 1599w, 1479w, 1475w, 1462vw, 1451w, 1429m, 1411w, 1358w, 1316s, 1279w, 1187w, 1157s, 1103w, 1092m, 1063m, 1035m, 1016w, 996w, 984vw, 904w, 878w, 865vw, 844w, 827m, 800w, 780w, 735m, 709s, 679m, 598s, 571vs, 534m, 513w, 505w, 497m cm−1; 1H NMR:δ = 7.71–7.66 (m, 2H, 2-H, 2′-H), 7.47–7.39 (m, 3H, NH, 3-H, 3′-H), 4.29 (s, 1H, OH), 3.36–3.29 (m, 2H, 14-H), 2.75–2.66 (m, 2H, 9-H), 2.63–2.55 (m, 1H, 5-H), 1.82–1.75 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.74–1.67 (m, 1H, 8-Ha), 1.48–1.29 (m, 8H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb, 10-H, 13-H), 1.28–1.21 (m, 1H, 8-Hb), 1.21–1.14 (m, 4H, 11-H, 12-H) ppm; 13C NMR:δ = 152.0 (C-4), 138.1 (C-1), 127.4 (C-3), 126.5 (C-2), 60.6 (C-14), 43.6 (C-5), 42.5 (C-9), 33.5 (C-6), 32.3 (C-13), 29.0 (C-10), 26.2 (C-7), 25.9 (C-12), 25.4 (C-8), 25.0 (C-11) ppm; MS: m/z = 361.9 (100%, [M + Na]+); anal. calcd. for C18H29NSO3 (339.49): C 63.68, H 8.61, N 4.13; found: C 63.58, H 8.83, N 3.97.
4.2.146. 6-[(4-Cyclohexylphenyl)sulfonamido]hexyl Sulfamate (72b)
Applying GPB: from 72a (200 mg, 0.6 mmol): 72b (186 mg, 75%); white solid; Rf = 0.63 (CHCl3/EtOAc, 2:3); m.p. = 86–88 °C; UV–Vis: 229 nm (3.81); IR: ν = 3370w, 3280m, 2929w, 2854w, 1558w, 1456w, 1356s, 1173vs, 1156s, 1094w, 995s, 922s, 828w, 770vs, 700w, 595m, 571m, 550vs, 493m cm−1; 1H NMR:δ = 7.71–7.66 (m, 2H, 2-H, 2′-H), 7.48–7.41 (m, 3H, NH, 3-H, 3′-H), 7.37 (s, 2H, NH2), 3.97 (t, J = 6.5 Hz, 2H, 14-H), 2.71 (q, J = 6.6 Hz, 2H, 9-H), 2.59 (ddd, J = 11.5, 8.4, 2.8 Hz, 1H, 5-H), 1.84–1.75 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.75–1.66 (m, 1H, 8-Ha), 1.55 (p, J = 6.7 Hz, 2H, 13-H), 1.48–1.30 (m, 6H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb, 10-H), 1.29–1.17 (m, 5H, 8-Hb, 11-H, 12-H); 13C NMR:δ = 152.3 (C-1), 138.2 (C-4), 127.5 (C-2), 126.7 (C-3), 69.1 (C-14), 43.7 (C-9), 42.5 (C-5), 33.7 (C-6), 29.0 (C-13), 28.3 (C-10), 26.3 (C-7), 25.6 (C-8), 24.7 (C-11) ppm; MS: m/z = 441.1 (100%, [M + Na]+), 419.1 (95%, [M + H]+); anal. calcd. for C18H30N2S2O5 (418.57): C 51.65, H 7.22, N 6.69; found: C 51.24, H 7.60, N 6.33.
4.2.147. 4-Cyclohexyl-N-(7-hydroxyheptyl)benzene Sulfonamide (73a)
Applying GPA: from 4-cyclohexylbenzenesulfonyl chloride (500 mg, 1.93 mmol) and 7-amino-heptanol (380 mg, 2.90 mmol): 73a (592 mg, 87%); white solid; Rf = 0.47 (CHCl3/EtOAc, 2:3); m.p. = 67–68 °C; UV–Vis: 229 nm (4.15); IR: ν = 3272m, 2923m, 2852m, 1599w, 1474w, 1463w, 1449w, 1429m, 1409w, 1318s, 1281w, 1186w, 1156vs, 1093m, 1058m, 1034m, 999w, 894w, 867w, 847w, 823m, 780w, 707s, 665m, 632m, 598s, 570vs, 543w, 529m, 506w cm−1; 1H NMR:δ = 7.71–7.66 (m, 2H, 2-H, 2′-H), 7.46–7.40 (m, 3H, NH, 3H-, 3′-H), 4.29 (t, J = 5.1 Hz, 1H, OH), 3.35 (td, J = 6.7, 5.1 Hz, 2H, 15-H), 2.70 (t, J = 7.1 Hz, 2H, 9-H), 2.63–2.54 (m, 1H, 5-H), 1.84–1.75 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.74–1.66 (m, 1H, 8-Ha), 1.48–1.26 (m, 8H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb, 10-H, 14-H), 1.26–1.11 (m, 7H, 8-Hb, 11-H, 12-H, 13-H) ppm; 13C NMR:δ = 152.0 (C-1), 138.2 (C-4), 127.3 (C-2), 126.5 (C-3), 60.7 (C-15), 43.5 (C-5), 42.5 (C-9), 33.5 (C-6), 32.4 (C-14), 28.9 (C-10), 28.4 (C-11), 26.2 (C-7), 26.0 (C-13), 25.4 (C-8), 25.3 (C-12) ppm; MS: m/z = 375.8 (100%, [M + Na]+); anal. calcd. for C19H31NSO3 (353.52): C 64.55, H 8.84, N 3.96; found: C 64.12, H 9.12, N 3.64.
4.2.148. 7-[(4-Cyclohexylphenyl)sulfonamido]heptyl Sulfamate (73b)
Applying GPB: from 73a (200 mg, 0.57 mmol): 73b (201 mg, 82%); white solid; Rf = 0.61 (CHCl3/EtOAc, 2:3); m.p. = 102–103 °C; UV–Vis: 229 nm (4.18); IR: ν = 3345m, 3273m, 3244w, 2928m, 2857w, 1598w, 1562vw, 1474w, 1452w, 1430w, 1411vw, 1394w, 1375s, 1309s, 1281w, 1177s, 1153vs, 1107w, 1093m, 1077vw, 1062m, 1049w, 1006w, 997m, 972s, 923m, 899m, 867vw, 835w, 813s, 781w, 731m, 708s, 668m, 598m, 590m, 568s, 550s, 532m, 515m, 507w cm−1; 1H NMR:δ = 7.71–7.66 (m, 2H, 2-H, 2′-H), 7.47–7.40 (m, 3H, 3-H, 3′-H, NH), 7.37 (s, 2H, NH2), 3.98 (t, J = 6.5 Hz, 2H, 15-H), 2.71 (q, J = 6.6 Hz, 2H, 9-H), 2.63–2.54 (m, 1H, 5-H), 1.84–1.75 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.74–1.67 (m, 1H, 8-Ha), 1.63–1.53 (m, 2H, 14-H), 1.48–1.30 (m, 6H, 6-Hb, 6′-Hb, 7-Hb, 7-Hb, 10-H), 1.29–1.15 (m, 7H, 8-Hb, 11-H, 12-H, 13-H) ppm; 13C NMR:δ = 152.1 (C-1), 138.1 (C-4), 127.4 (C-2), 126.6 (C-3), 69.0 (C-15), 43.6 (C-9), 42.5 (C-5), 33.6 (C-6), 28.9 (C-14), 28.2 (C-11), 28.0 (C-10), 26.2 (C-7), 25.9 (C-13), 25.5 (C-8), 24.9 (C-12) ppm; MS: m/z = 455.8 (100%, [M + Na]+); anal. calcd. for C19H32N2S2O5 (432.59): C 52.75, H 7.46, N 6.48; found: C 52.45, H 7.88, N 6.13.
4.2.149. 4-Cyclohexyl-N-(8-hydroxyoctyl)benzene Sulfonamide (74a)
Applying GPA: from 4-cyclohexylbenzenesulfonyl chloride (500 mg, 1.93 mmol) and 8-amino-octanol (421 mg, 2.90 mmol): 74a (618 mg, 87%); white solid; Rf = 0.45 (CHCl3/EtOAc, 2:3); UV–Vis: 229 nm (4.16); IR: ν = 3277m, 2924s, 2851m, 1599w, 1495vw, 1478w, 1466w, 1449w, 1427m, 1409w, 1318s, 1280w, 1185w, 1157vs, 1133w, 1094m, 1052s, 1016w, 997w, 982w, 901m, 883w, 866w, 849w, 823m, 780w, 755m, 733m, 706s, 651m, 632m, 598s, 570vs, 531m, 507m, 470w cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 7.71–7.67 (m, 2H, 2-H, 2′-H), 7.46–7.42 (m, 2H, 3-H, 3′-H), 7.42–7.41 (m, 1H, NH), 4.29 (t, J = 5.2 Hz, 1H, OH), 3.36 (td, J = 6.6, 5.1 Hz, 2H, 16-H), 2.70 (q, J = 6.6 Hz, 2H, 9-H), 4.31–4.28 (m, 1H, 5-H), 1.83–1.75 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.74–1.67 (m, 1H, 8-Ha), 1.47–1.28 (m, 8H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb, 10-H, 15-H), 1.27–1.10 (m, 9H, 8-Hb, 11-H, 12-H, 13-H, 14-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 152.0 (C-4), 138.2 (C-1), 127.3 (C-2), 126.5 (C-2), 60.7 (C-16), 43.5 (C-5), 42.5 (C-9), 33.5 (C-6), 32.5 (C-15), 28.9 (C-10), 28.8 (C-14), 28.6 (C-11), 26.2 (C-7), 25.9 (C-13), 25.4 (C-8), 25.4 (C-12) ppm; MS: m/z = 390.3 (100%, [M + Na]+); anal. calcd. for C20H33NSO3 (367.55): C 65.36, H 9.05, N 3.81; found: C 65.06, H 9.37, N 3.56.
4.2.150. 8-[(4-Cyclohexylphenyl)sulfonamido]octyl Sulfamate (74b)
Applying GPB: from 74a (200 mg, 0.54 mmol): 74b (226 mg, 93%); white solid; Rf = 0.68 (CHCl3/EtOAc, 2:3); m.p. = 91–93 °C; UV–Vis: 229 nm (4.11); IR: ν = 3367w, 3299w, 3274w, 2953w, 2922m, 2851m, 1598w, 1544w, 1475w, 1463w, 1453w, 1409m, 1396w, 1372s, 1316s, 1183m, 1157s, 1149s, 1106w, 1093m, 1069w, 1057m, 1039m, 1016w, 1004m, 996m, 981s, 944w, 935m, 887m, 857w, 827m, 813s, 782w, 763w, 730m, 712s, 659m, 632w, 589m, 575s, 555vs, 528s, 507m, 481w cm−1; 1H NMR:δ = 7.71–7.66 (m, 2H, 2-H, 2′-H), 7.47–7.40 (m, 3H, 3-H, 3′-H, NH), 7.37 (s, 2H, NH2), 3.99 (t, J = 6.5 Hz, 2H, 16-H), 2.74–2.67 (m, 2H, 9-H), 2.64–2.55 (m, 1H, 5-H), 1.83–1.74 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.74–1.67 (m, 1H, 8-Ha), 1.64–1.55 (m, 2H, 15-H), 1.49–1.12 (m, 15H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb, 8-Hb, 10-H, 11-H, 12-H, 13-H, 14-H) ppm; 13C NMR:δ = 152.0 (C-1), 138.2 (C-4), 127.4 (C-2), 126.5 (C-3), 69.0 (C-16), 43.6 (C-5), 42.5 (C-9), 33.6 (C-6), 28.9 (C-15), 28.4 (C-10), 28.3 (C-13), 28.3 (C-12), 26.2 (C-7), 25.9 (C-11), 25.4 (C-8), 25.0 (C-14) ppm; MS: m/z = 469.9 (100%, [M + Na]+); anal. calcd. for C20H34N2S2O5 (446.62): C 53.79, H 7.67, N 6.27; found: C 53.35, H 7.96, N 5.98.
4.2.151. 4-Cyclohexyl-N-(9-hydroxynonyl)benzene Sulfonamide (75a)
Applying GPA: from 4-cyclohexylbenzenesulfonyl chloride (500 mg, 1.93 mmol) and 9-amino-nonanol (462 mg, 2.90 mmol): 75a (723 mg, 98%); white solid; Rf = 0.84 (CHCl3/EtOAc, 2:3); m.p. = 55–57 °C; UV–Vis: 229 nm (4.11); IR: ν = 3279m, 2922s, 2851m, 1599w, 1475w, 1467w, 1448w, 1426m, 1409w, 1318s, 1281w, 1269w, 1186w, 1157vs, 1095m, 1054m, 1036m, 1016w, 998w, 974w, 902w, 883w, 851vw, 822m, 780w, 733m, 706s, 654m, 632m, 599s, 569vs, 529m, 508w, 472vw, 460vw cm−1; 1H NMR:δ = 7.70–7.66 (m, 2H, 2-H, 2′-H), 7.46–7.40 (m, 3H, NH, 3-H, 3′-H), 4.30 (td, J = 5.1, 1.1 Hz, 1H, OH), 3.36 (td, J = 6.5, 5.1 Hz, 2H, 17-H), 2.71 (q, J = 6.7 Hz, 2H, 9-H), 2.63–2.55 (m, 1H, 5-H), 1.83–1.75 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.74–1.67 (m, 1H, 8-Ha), 1.48–1.27 (m, 6H, 7-Hb, 7′-Hb, 8-Hb, 10-H, 16-H), 1.26–1.11 (m, 11H, 6-Hb, 6′-Hb, 11-H, 12-H, 13-H, 14-H, 15-H) ppm; 13C NMR:δ = 152.0 (C-4), 138.2 (C-1), 127.3 (C-2), 126.5 (C-3), 60.7 (C-17), 43.6 (C-5), 42.5 (C-9), 33.6 (C-6), 32.5 (C-16), 28.9 (C-10, C-14), 28.8 (C-12), 28.5 (C-13), 26.2 (C-7), 26.0 (C-11), 25.5 (C-8), 25.4 (C-15) ppm; MS: m/z = 457.1 (100%, [M + Na]+); anal. calcd. for C21H35NSO3 (381.58): C 54.76, H 7.88, N 6.08; found: C 54.31, H 8.06, N 5.76.
4.2.152. 9-[(4-Cyclohexylphenyl)sulfonamido]nonyl Sulfamate (75b)
Applying GPB: from 75a (200 mg, 0.52 mmol): 75b (190 mg, 79%); white solid; Rf = 0.87 (CHCl3/EtOAc, 2:3); m.p. = 88–89 °C; UV–Vis: 230 nm (4.04); IR: ν = 3379w, 3278m, 2962w, 2924m, 2855m, 1600w, 1542w, 1477w, 1461vw, 1451w, 1424m, 1399w, 1376s, 1326w, 1310s, 1281w, 1214vw, 1186s, 1154vs, 1118w, 1094m, 1078vw, 1060m, 1034m, 993w, 967s, 907s, 885m, 866w, 819vs, 781w, 734w, 704s, 652m, 631m, 598m, 587m, 573s, 555vs, 534m, 524m, 505m, 483m, 454vw cm−1; 1H NMR:δ = 7.68–7.63 (m, 2H, 2-H, 2′-H), 7.44–7.38 (m, 3H, NH, 3-H, 3′-H), 7.34 (s, 2H, NH2), 3.97 (t, J = 6.5 Hz, 2H, 17-H), 2.68 (q, J = 6.7 Hz, 2H, 9-H), 2.61–2.52 (m, 1H, 5-H), 1.82–1.72 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.72–1.63 (m, 1H, 8-Ha), 1.62–1.54 (m, 2H, 16-H), 1.46–1.08 (m, 17H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb, 8-Hb, 10-H, 11-H, 12-H, 13-H, 14-H, 15-H) ppm; 13C NMR:δ = 152.0 (C-4), 138.2 (C-1), 127.4 (C-2), 126.5 (C-3), 69.0 (C-17), 43.6 (C-5), 42.5 (C-9), 33.6 (C-6), 28.9 (C-16), 28.7 (C-10), 28.4 (C-12), 28.4 (C-14), 28.3 (C-13), 26.2 (C-7), 25.9 (C-11), 25.4 (C-8), 25.0 (C-15) ppm; MS: m/z = 457.1 (100%, [M + Na]+); anal. calcd. for C21H36N2S2O5 (460.65): C 54.76, H 7.88, N 6.08; found: C 54.35, H 8.01, N 5.74.
4.2.153. 4-Cyclohexyl-N-(10-hydroxydecyl)benzene Sulfonamide (76a)
Applying GPA: from 4-cyclohexylbenzenesulfonyl chloride (450 mg, 1.74 mmol) and 10-amino-decanol (452 mg, 2.61 mmol): 76a (625 mg, 91%); white solid; Rf = 0.82 (CHCl3/EtOAc, 2:3); m.p. = 71–72 °C; UV–Vis: 229 nm (4.17); IR: ν = 3494w, 3130w, 2915s, 2877w, 2849s, 1598w, 1476w, 1466w, 1448m, 1440m, 1408w, 1397w, 1346w, 1310s, 1288w, 1267w, 1188w, 1150vs, 1114m, 1095m, 1074s, 1043w, 1031w, 1020m, 999w, 973w, 914m, 876w, 865vw, 840w, 825m, 781w, 734w, 720m, 701s, 606vs, 575s, 523m, 505w, 468m cm−1; 1H NMR:δ = 7.70–7.66 (m, 2H, 2-H, 2′-H), 7.46–7.39 (m, 3H, NH, 3-H, 3′-H), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.36 (td, J = 6.6, 5.2 Hz, 2H, 18-H), 2.74–2.67 (m, 2H, 9-H), 2.64–2.54 (m, 1H, 5-H), 1.84–1.75 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.74–1.67 (m, 1H, 8-Ha), 1.47–1.27 (m, 8H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb, 10-H, 17-H), 1.27–1.09 (m, 13H, 8-Hb, 11-H, 12-H, 13-H, 14-H, 15-H, 16-H) ppm;´13C NMR:δ = 152.0 (C-4), 138.2 (C-1), 127.3 (C-2), 126.6 (C-3), 60.7 (C-18), 43.6 (C-5), 42.5 (C-9), 33.6 (C-6), 32.5 (C-17), 29.0 (C-10), 28.9 (C-12), 28.9 (C-15), 28.8 (C-13), 28.5 (C-14), 26.2 (C-7), 26.0 (C-11), 25.5 (C-16), 25.4 (C-8) ppm; MS: m/z = 457.1 (100%, [M + Na]+); anal. calcd. for C22H37NSO3 (395.60): C 66.79, H 9.43, N 3.54; found: C 66.25, H 9.81, N 3.16.
4.2.154. 10-[(4-Cyclohexylphenyl)sulfonamido]decyl Sulfamate (76b)
Applying GPB: from 76a (180 mg, 0.46 mmol): 76b (161 mg, 75%); white solid; Rf = 0.9 (CHCl3/EtOAc, 2:3); m.p. = 100–101 °C; UV–Vis: 229 nm (4.33); IR: ν = 3369w, 3298w, 3275w, 2953w, 2919m, 2849m, 1598w, 1543w, 1475w, 1464w, 1454w, 1409w, 1396w, 1373s, 1317s, 1183m, 1157s, 1150s, 1093m, 1060w, 1052m, 1037w, 1021m, 1001m, 978m, 937m, 915w, 882m, 845w, 827m, 815s, 798m, 782w, 763w, 731m, 713s, 661m, 632w, 591m, 575s, 555vs, 530m, 504w, 476w, 471w, 417w cm−1; 1H NMR:δ = 7.71–7.66 (m, 2H, 2-H, 2′-H), 7.46–7.40 (m, 3H, NH, 3-H, 3′-H), 7.37 (s, 2H, NH2), 4.00 (t, J = 6.5 Hz, 2H, 18-H), 2.71 (q, J = 6.7 Hz, 2H, 9-H), 2.63–2.54 (m, 1H, 5-H), 1.83–1.75 (m, 4H, 6-Ha, 6′-Ha, 7-Ha, 7′-Ha), 1.74–1.67 (m, 1H, 8-Ha), 1.61 (p, J = 6.5 Hz, 2H, 17-H), 1.48–1.37 (m, 3H, 8-Hb, 10-H), 1.36–1.12 (m, 16H, 6-Hb, 6′-Hb, 7-Hb, 7′-Hb, 11-H, 12-H, 13-H, 14-H, 15-H, 16-H) ppm; 13C NMR:δ = 152.0 (C-4), 138.2 (C-1), 127.4 (C-2), 126.5 (C-3), 69.0 (C-18), 43.6 (C-5), 42.5 (C-9), 33.6 (C-6), 28.9 (C-10), 28.8 (C-13), 28.8 (C-17), 28.5 (C-12), 28.5 (C-15), 28.3 (C-14), 26.2 (C-7), 26.0 (C-11), 25.4 (C-8), 25.1 (C-16) ppm; MS: m/z = 457.1 (100%, [M + Na]+); anal. calcd. for C22H38N2S2O5 (474.68): C 55.67, H 8.07, N 5.90; found: C 55.25, H 8.41, N 5.65.
4.2.155. 4-(Adamantan-1-yl)-N-(8-hydroxyoctyl)benzene Sulfonamide (77a)
Applying GPA: from 4-(adamantan-1-yl)benzenesulfonyl chloride (300 mg, 0.96 mmol) and 8-amino-octanol (212 mg, 1.45 mmol): 77a (363 mg, 90%); white solid; Rf = 0.70 (CHCl3/EtOAc, 2:3); m.p. = 101–102 °C; UV–Vis: 231 nm (4.15); IR: ν = 3463w, 3147w, 2917s, 2903m, 2849m, 1477w, 1465w, 1447w, 1399w, 1381w, 1367w, 1360w, 1345w, 1316s, 1304m, 1291m, 1158s, 1095m, 1085m, 1058m, 1029m, 1014w, 975w, 957w, 951w, 939m, 846w, 832w, 806m, 774w, 742m, 721w, 683m, 674m, 599s, 579vs, 534m, 483m cm−1; 1H NMR:δ = 7.73–7.69 (m, 2H, 2-H, 2′-H), 7.58–7.54 (m, 2H, 3-H, 3′-H), 7.44 (t, J = 5.8 Hz, 1H, NH), 4.29 (td, J = 5.1, 1.0 Hz, 1H, OH), 3.35 (td, J = 6.6, 5.2 Hz, 2H, 22-H), 2.74–2.66 (m, 2H, 15-H), 2.10–2.03 (m, 3H, 7-H, 9-H, 11-H), 1.88 (d, J = 3.0 Hz, 6H, 6-H, 12-H, 13-H), 1.79–1.69 (m, 6H, 8-H, 10-H, 14-H), 1.42–1.27 (m, 4H, 16-H, 21-H), 1.26–1.10 (m, 8H, 17-H, 18-H, 19-H, 20-H) ppm; 13C NMR:δ = 155.2 (C-4), 137.8 (C-1), 126.4 (C-2), 125.5 (C-3), 60.7 (C-22), 42.5 (C-15), 42.2 (C-12, C-6, C-13), 36.2 (C-5), 36.0 (C-10, C-8, C-14), 32.5 (C-21), 28.9 (C-16), 28.8 (C-18), 28.6 (C-19), 28.2 (C-7, C-9, C-11), 25.9 (C-17), 25.4 (C-20) ppm; MS: m/z = 457.1 (100%, [M + Na]+); anal. calcd. for C24H37NSO3 (419.62): C 68.70, H 8.89, N 3.34; found: C 68.47, H 9.19, N 2.96.
4.2.156. 8-[(4-(Adamantan-1-yl)phenyl)sulfonamido]octyl Sulfamate (77b)
Applying GPB: from 77a (190 mg, 0.42 mmol): 77b (147 mg, 66%); white solid; Rf = 0.87 (CHCl3/EtOAc, 2:3); m.p. = 85–86 °C; UV–Vis: 231 nm (4.20); IR: ν = 3360w, 3254m, 2929m, 2899s, 2847m, 1568w, 1448m, 1398w, 1361m, 1344w, 1316vs, 1293m, 1183m, 1145vs, 1111w, 1092s, 1076m, 1065m, 1044w, 1031w, 1013m, 999w, 982w, 976w, 964m, 953m, 933s, 906m, 898m, 877m, 861w, 837w, 795s, 773w, 756m, 744s, 730w, 721m, 694s, 665w, 633w, 595s, 575vs, 551vs, 537m, 526m, 505w, 480m, 473m cm−1; 1H NMR:δ = 7.73–7.68 (m, 2H, 2-H, 2′-H), 7.59–7.54 (m, 2H, 3-H, 3′-H), 7.45 (t, J = 5.9 Hz, 1H, NH), 7.37 (s, 2H, NH2), 3.99 (t, J = 6.5 Hz, 2H, 22-H), 2.71 (q, J = 6.7 Hz, 2H, 15-H), 2.07 (p, J = 3.1 Hz, 3H, 7-H, 9-H, 11-H), 1.88 (d, J = 2.9 Hz, 6H, 6-H, 12-H, 13-H), 1.79–1.69 (m, 6H, 8-H, 10-H, 14-H), 1.64–1.55 (m, 2H, 21-H), 1.37–1.25 (m, 2H, 16-H), 1.23–1.13 (m, 8H, 17-H, 18-H, 19-H, 20-H) ppm; 13C NMR:δ = 155.2 (C-4), 137.8 (C-1), 126.4 (C-2), 125.5 (C-3), 69.0 (C-22), 42.5 (C-15), 42.2 (C-6, C-12, C-13), 36.2 (C-5), 36.0 (C-8, C-10, C-14), 28.9 (C-21), 28.4 (C-19), 28.3 (C-16), 28.3 (C-18), 28.2 (C-7, C-9, C-11), 25.9 (C-17), 25.0 (C-20) ppm; MS: m/z = 457.1 (100%, [M + Na]+); anal. calcd. for C24H38N2S2O5 (498.70): C 57.80, H 7.68, N 5.62; found: C 57.64, H 7.43, N 5.45.
4.2.157. 4-(Adamantan-1-yl)-N-(9-hydroxynonyl)benzene Sulfonamide (78a)
Applying GPA: from 4-(adamantan-1-yl)benzenesulfonyl chloride (150 mg, 0.48 mmol) and 9-amino-nonanol (115 mg, 0.72 mmol): 78a (201 mg, 96%); white solid; Rf = 0.72 (CHCl3/EtOAc, 2:3); m.p. = 84–85 °C; UV–Vis: 231 nm (4.28); IR: ν = 3503vw, 3281w, 2903s, 2849m, 1597w, 1496vw, 1449m, 1400w, 1319s, 1293m, 1157vs, 1094m, 1031m, 1014w, 976w, 839w, 806m, 752m, 741m, 674s, 596vs, 576s, 475w cm−1; 1H NMR:δ = 7.71 (d, J = 8.6 Hz, 2H, 2-H, 2′-H), 7.56 (d, J = 8.6 Hz, 2H, 3-H, 3′-H), 7.44 (t, J = 5.8 Hz, 1H, NH), 4.29 (t, J = 5.1 Hz, 1H, OH), 3.40–3.33 (m, 2H, 23-H), 2.71 (q, J = 6.8 Hz, 2H, 15-H), 2.07 (s, 3H, 7-H, 9-H, 11-H), 1.88 (d, J = 2.7 Hz, 6H, 6-H, 12-H, 13-H), 1.79–1.69 (m, 6H, 8-H, 10-H, 14-H), 1.43–1.35 (m, 2H, 22-H), 1.34–1.27 (m, 2H, 16-H), 1.27–1.10 (m, 10H, 17-H, 18-H, 19-H, 20-H, 21-H) ppm; 13C NMR:δ = 155.2 (C-4), 137.9 (C-1), 126.4 (C-2), 125.5 (C-3), 60.7 (C-23), 42.5 (C-15), 42.2 (C-6, C-12, C-13), 36.2 (C-5), 36.0 (C-8, C-10, C-14), 32.5 (C-22), 28.9 (C-16, C-18), 28.9 (C-20), 28.5 (C-19), 28.2 (C-7, C-9, C-11), 26.0 (C-17), 25.5 (C-21) ppm; MS: m/z = 457.1 (100%, [M + Na]+); anal. calcd. for C25H39NSO3 (433.65): C 69.24, H 9.07, N 3.23; found: C 68.97, H 9.33, N 2.95.
4.2.158. 9-[(4-(Adamantan-1-yl)phenyl)sulfonamido]nonyl Sulfamate (78b)
Applying GPB: from 78a (110 mg, 0.25 mmol): 78b (110 mg, 84%); white solid; Rf = 0.92 (CHCl3/EtOAc, 2:3); m.p. = 94–96 °C; UV–Vis: 231 nm (4.19); IR: ν = 3347w, 3269w, 2922m, 2903m, 2850m, 1429w, 1401w, 1370m, 1317s, 1297m, 1176m, 1156vs, 1117w, 1103w, 1093w, 1076w, 1059w, 1033w, 1013w, 963m, 926m, 879w, 848w, 839w, 823m, 805m, 775w, 747m, 729w, 697m, 648w, 598s, 572s, 564s, 534w, 522w, 513w, 484w cm−1; 1H NMR:δ = 7.71 (d, J = 8.2 Hz, 2H, 2-H, 2′-H), 7.57 (d, J = 8.2 Hz, 2H, 3-H, 3′-H), 7.44 (t, J = 5.9 Hz, 1H, NH), 7.37 (s, 2H, NH2), 3.99 (t, J = 6.5 Hz, 2H, 23-H), 2.71 (q, J = 6.7 Hz, 2H, 15-H), 2.07 (s, 3H, 7-H, 9-H, 11-H), 1.88 (d, J = 2.9 Hz, 6H, 6-H, 12-H, 13-H), 1.74 (s, 6H, 8-H, 10-H, 14-H), 1.60 (p, J = 6.7 Hz, 2H, 22-H), 1.37–1.27 (m, 4H, 16-H, 21-H), 1.27–1.11 (m, 8H, 17-H, 18-H, 19-H, 20-H) ppm; 13C NMR:δ = 155.7 (C-4), 138.3 (C-1), 126.8 (C-2), 126.0 (C-3), 69.4 (C-23), 42.9 (C-15), 42.7 (C-6, C-12, C-13), 36.6 (C-5), 36.5 (C-8, C-10, C-14), 29.4 (C-22), 29.2 (C-16), 28.9 (C-18), 28.9 (C-20), 28.8 (C-19), 26.4 (C-17), 25.5 (C-21) ppm; MS: m/z = 457.1 (100%, [M + Na]+); anal. calcd. for C25H40N2S2O5 (512.72): C 58.56, H 7.86, N 5.46; found: C 58.27, H 8.05, N 5.16.
4.2.159. 4-(Adamantan-1-yl)-N-(10-hydroxydecyl)benzene Sulfonamide (79a)
Applying GPA: from 4-(adamantan-1-yl)benzenesulfonyl chloride (300 mg, 0.97 mmol) and 10-amino-decanol (251 mg, 1.45 mmol): 79a (377 mg, 87%); white solid; Rf = 0.74 (CHCl3/EtOAc, 2:3); m.p. = 76–79 °C; UV–Vis: 231 nm (4.20); IR: ν = 3496vw, 3282w, 2903s, 2849s, 1597w, 1449m, 1400w, 1369w, 1320s, 1293m, 1157vs, 1094m, 1056m, 1031m, 1014w, 976w, 881vw, 839w, 806m, 740m, 721w, 674s, 596vs, 577s, 495w, 487w, 476w, 451vw cm−1; 1H NMR:δ = 7.73–7.68 (m, 2H, 2-H, 2′-H), 7.59–7.53 (m, 2H, 3-H, 3′-H), 7.44 (t, J = 5.8 Hz, 1H, NH), 4.30 (t, J = 5.1 Hz, 1H, OH), 3.36 (td, J = 6.5, 5.2 Hz, 2H, 24-H), 2.71 (q, J = 6.6 Hz, 2H, 15-H), 2.09–2.03 (m, 3H, 7-H, 9-H, 11-H), 1.88 (d, J = 3.0 Hz, 6H, 6-H, 12-H, 13-H), 1.73 (d, J = 4.4 Hz, 6H, 8-H, 10-H, 14-H), 1.43–1.34 (m, 2H, 23-H), 1.34–1.26 (m, 2H, 16-H), 1.26–1.09 (m, 12H, 17-H, 18-H, 19-H, 20-H, 21-H, 22-H) ppm; 13C NMR:δ = 155.2 (C-4), 137.9 (C-1), 126.4 (C-2), 125.5 (C-3), 60.7 (C-24), 42.5 (C-15), 42.2 (C-6, C-12, C-13), 36.2 (C-5), 36.0 (C-8, C-10, C-14), 32.5 (C-23), 29.0 (C-16), 28.9 (C-19), 28.9 (C-21), 28.8 (C-20), 28.5 (C-18), 28.2 (C-7, C-9, C-11), 26.0 (C-17), 25.5 (C-22) ppm; MS: m/z = 457.1 (100%, [M + Na]+); anal. calcd. for C26H41NSO3 (447.68): C 69.76, H 9.23, N 3.13; found: C 69.41, H 9.55, N 2.94.
4.2.160. 10-[(4-(Adamantan-1-yl)phenyl)sulfonamido]decyl Sulfamate (79b)
Applying GPB: from 79a (170 mg, 0.38 mmol): 79b (92 mg, 46%); white solid; Rf = 0.85 (CHCl3/EtOAc, 2:3); m.p. = 87–89 °C; UV–Vis: 231 nm (4.18); IR: ν = 3291w, 2919m, 2903m, 2850m, 1597vw, 1567vw, 1563vw, 1471w, 1450w, 1426w, 1400w, 1366m, 1322s, 1294m, 1179m, 1158vs, 1119w, 1103w, 1093m, 1064w, 1051w, 1031w, 1014w, 954m, 936m, 880w, 831m, 805m, 775w, 762w, 747m, 731w, 722w, 682m, 628w, 597s, 572s, 561s, 523w, 474w cm−1; 1H NMR:δ = 7.74–7.69 (m, 2H, 2-H, 2′-H), 7.59–7.54 (m, 2H, 3-H, 3′-H), 7.44 (t, J = 5.8 Hz, 1H, NH), 7.37 (s, 2H, NH2), 4.00 (t, J = 6.5 Hz, 2H, 24-H), 2.71 (q, J = 6.6 Hz, 2H, 15-H), 2.07 (p, J = 3.1 Hz, 3H, 7-H, 9-H, 11-H), 1.88 (d, J = 2.9 Hz, 6H, 6-H, 12-H, 13-H), 1.80–1.68 (m, 6H, 8-H, 10-H, 14-H), 1.61 (p, J = 6.6 Hz, 2H, 23-H), 1.36–1.27 (m, 4H, 16-H, 22-H), 1.27–1.11 (m,10H, 17-H, 18-H, 19-H, 20-H, 21-H) ppm; 13C NMR:δ = 155.6 (C-4), 138.3 (C-1), 126.8 (C-2), 126.0 (C-3), 69.4 (C-24), 42.9 (C-15), 42.7 (C-6, C-12, C-13), 36.6 (C-5), 36.5 (C-8, C-10, C-14), 29.4 (C-16), 29.3 (C-19), 29.3 (C-23), 29.0 (C-18), 28.9 (C-21), 28.8 (C-20), 28.6 (C-7, C-9, C-11), 26.4 (C-17), 25.5 (C-22) ppm; MS: m/z = 457.1 (100%, [M + Na]+); anal. calcd. for C26H42N2S2O5 (526.75): C 59.29, H 8.04, N 5.32; found: C 58.97, H 8.31, N 5.03.