Antimicrobial, Antioxidant, and Antityrosinase Activities of Morina persica L. and Its Isolated Compounds
Abstract
1. Introduction
2. Results
2.1. Isolation Results
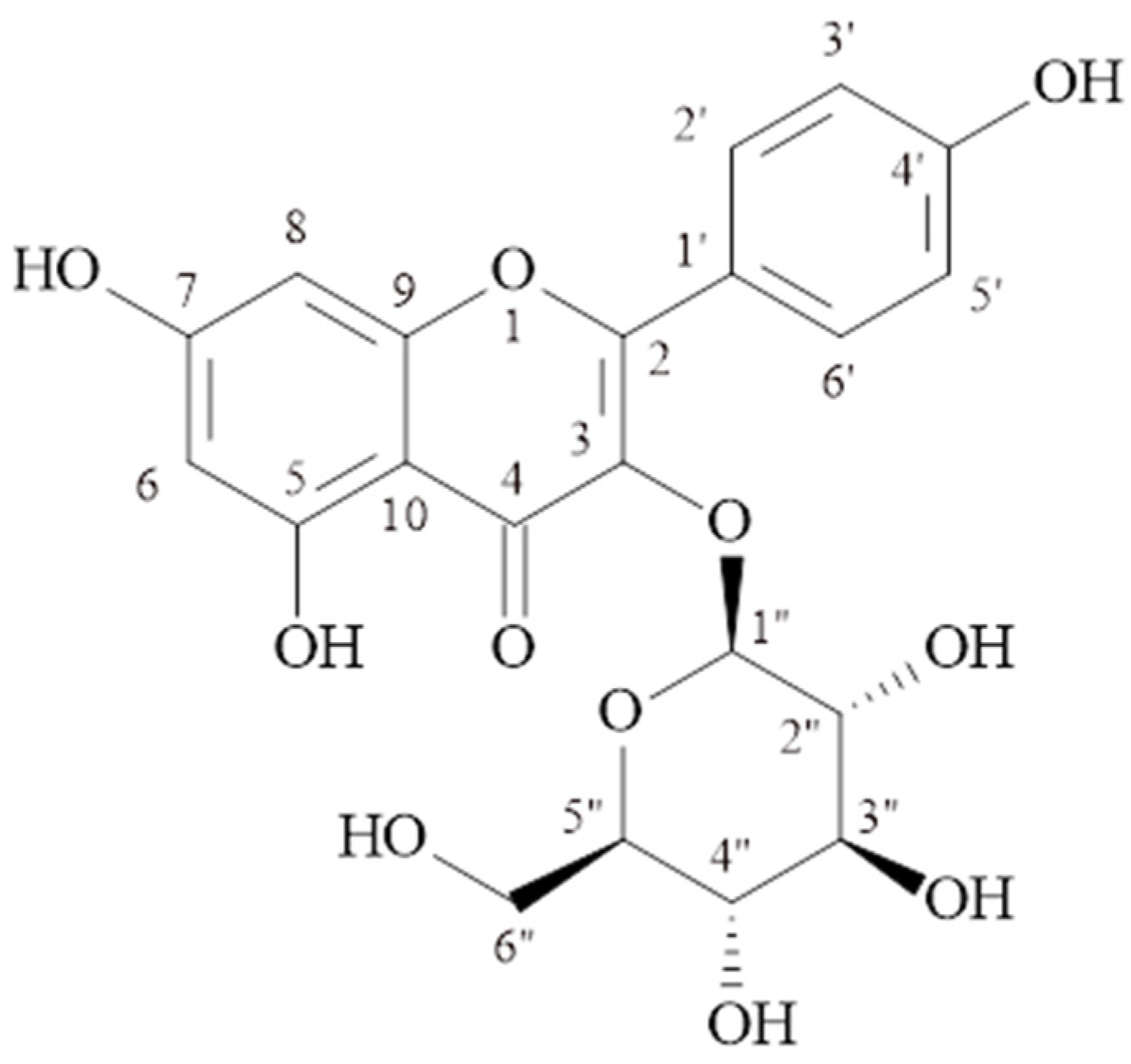
2.1.1. Kaempferol-3-O-β-glucopyranoside (Astragalin) (MP-1)
2.1.2. Quercetin-3-O-rutinoside (Rutin) (MP-2)
2.2. Antimicrobial Activity Results
2.3. Total Phenolic Compound Quantification Results
2.4. DPPH Radical Scavenging Study Results
2.5. ABTS Radical Scavenger Study Results
2.6. Tyrosinase Inhibitor Activity Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction Studies
4.3. Isolation Studies and Structure Determination
4.4. Antimicrobial Studies
4.5. Antioxidant Studies
4.5.1. Determination of Total Phenolic Compound Quantity
4.5.2. DPPH Radical Scavenger Capacity Determination
4.5.3. ABTS+ Radical Scavenger Capacity Determination
4.6. Determination of Tyrosinase Enzyme Inhibition
- A: Phosphate buffer and enzyme
- B: Phosphate buffer
- C: Phosphate buffer, enzyme, and sample
- D: Phosphate buffer and sample
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Varshney, V.K.; Rawat, M.S.M.; Sahrawat, S. Chemical constituents of Morina genus: A comprehensive review. Am. J. Essent. Oil. Nat. Prod. 2013, 1, 1–15. [Google Scholar]
- Yuan, Q.; Zhang, J.; Yao, Z.; Zhou, Q.; Liu, P.; Liu, W.; Liu, H. Prediction of potential distributions of Morina kokonorica and Morina chinensis in China. Ecol. Evol. 2024, 14, e11121. [Google Scholar] [CrossRef] [PubMed]
- Caprifoliaceae Juss., The World Flora Online. Available online: http://www.worldfloraonline.org/taxon/wfo-7000000112 (accessed on 21 May 2024).
- Mocan, A.; Zengin, G.; Uysal, A.; Günes, E.; Mollica, A.; Değirmenci, N.S.; Alpsoy, L.; Aktumsek, A. Biological and chemical insights of Morina persica L.: A source of bioactive compounds with multifunctional properties. J. Funct. Foods 2016, 25, 94–109. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, Z.P.; Xue, C.B.; Wu, W.S. New triterpenoid saponins and neolignans from Morina kokonorica. Helv. Chim. Acta 2009, 92, 536–545. [Google Scholar] [CrossRef]
- Ozgen, R. Pharmacognostic Studies on Morina persica L. Master’s Thesis, Ataturk University Institute of Health Sciences, Turkey, 2023. [Google Scholar]
- Dolarslan, M.; Gul, E.; Dinç, B.G. Ethnobotanical, Morphological and Ecological Characteristics of Morina persica L. (Morinaceae). In Science Horizons: Nanotechnology, Plant Diversity, Microbial Insights, and Sustainable Solutions, 1st ed.; Yalçın, D., Ed.; Bidge Publications: Ankara, Turkey, 2023; pp. 105–117. [Google Scholar]
- Shah, A.; Abass, G.; Sharma, M.P. Ethnobotanical study of some medicinal plants from tehsil BudhaL, District Rajouri, (Jammu and Kashmir). Int. Multidiscip. Res. J. 2012, 2, 5–6. [Google Scholar]
- Pettinari, C.; Pettinari, R.; Di Nicola, C.; Tombesi, A.; Scuri, S.; Marchetti, F. Antimicrobial MOFs. Coord. Chem. Rev. 2021, 446, 214121. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Sulaiman, A.M.; Chee, J.Y.; Gazzali, A.M.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutterıdge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015; p. 905. [Google Scholar]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef]
- Tram, N.C.T.; Son, N.T.; Thao, D.T.; Cuong, N.M. Kaempferol and kaempferol glycosides from Phyllanthus acidus leaves. Vietnam J. Chem. 2016, 54, 790–793. [Google Scholar]
- Wei, Y.; Xie, Q.; Fisher, D.; Sutherland, I.A. Separation of patuletin-3-O-glucoside, astragalin, quercetin, kaempferol and isorhamnetin from Flaveria bidentis (L.) Kuntze by elution-pump-out high-performance counter-current chromatography. J. Chromatogr. A 2011, 1218, 6206–6211. [Google Scholar] [CrossRef] [PubMed]
- Al-Majmaie, S.; Nahar, L.; Sharples, G.P.; Wadi, K.; Sarker, S.D. Isolation and antimicrobial activity of rutin and its derivatives from Ruta chalepensis (Rutaceae) growing in Iraq. Rec. Nat. Prod. 2019, 13, 64–70. [Google Scholar] [CrossRef]
- Petrus, J.A.; Hemalatha, S.S.; Suguna, G. Isolation and Characterisation of the Antioxidant Phenolic Metabolites of Boerhaavia erecta L. Leaves. J. Pharm. Sci. Res. 2012, 4, 1856–1861. [Google Scholar]
- Xie, Z.; Liang, Z.; Xie, C.; Zhao, M.; Yu, X.; Yang, M.; Huang, J.; Xu, X. Separation and Purification of Rosmarinic Acid and Rutin from Glechoma hederacea L. using High-Speed Counter-Current Chromatography. Sep. Sci. Technol. 2014, 49, 588–593. [Google Scholar] [CrossRef]
- Tasdemir, D.; Brun, R.; Perozzo, R.; Dönmez, A.A. Evaluation of Antiprotozoal and Plasmodial Enoyl-ACP Reductase Inhibition Potential of Turkish Medicinal Plants. Phytother. Res. 2005, 19, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, F.O.; Oyedeji, O.; Osundahunsi, M.T. Antimicrobial and Antioxidant Properties of Kaempferol-3-O-glucoside and 1-(4-Hydroxyphenyl)-3-phenylpropan-1-one Isolated from the Leaves of Annona muricata (Linn.). J. Pharm. Res. Int. 2019, 26, 1–13. [Google Scholar] [CrossRef]
- Al-Shabibi, M.H.S.; Al-Touby, S.S.J.; Hossain, M.A. Isolation, characterization and prediction of biologically active glycoside compounds quercetin-3-rutinoside from the fruits of Ficus sycomorus. Carbohydr. Res. 2022, 511, 108483. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; El-Din, M.I.G.; Hritcu, L.; Eldahshan, O.A. Insights on the Inhibitory Power of Flavonoids on Tyrosinase Activity: A Survey from 2016 to 2021. Molecules 2021, 26, 7546. [Google Scholar] [CrossRef]
- Elgamal, A.M.; El Raey, M.A.; Gaara, A.; Abdelfattah, M.A.O.; Sobeh, M. Phytochemical profiling and anti-aging activities of Euphorbia retusa extract: In silico and in vitro studies. Arab. J. Chem. 2021, 14, 103159. [Google Scholar] [CrossRef]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Sholihah, I.A.; Raif, M.A.; Kunardi, S.; Million, H.; Widowati, W. Antioxidant and antiaging activity of rutin and caffeic acid. Pharmaciana 2020, 10, 147. [Google Scholar] [CrossRef]
- Gözcü, S.; Uğan, R.A.; Özbek, H.; Gündoğdu, B.; Güvenalp, Z. Evaluation of antidiabetic and antioxidant effects of Polygonum cognatum Meisn. and phytochemical analysis of effective extracts. BMC 2024, 38, e5809. [Google Scholar] [CrossRef] [PubMed]
- M100-S27; Performance Standards for Antimicrobial Susceptibility Testing, Twenty-Seventh Informational Supplement. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2017.
- Folin, O.; Denis, W. On phosphotungstic-phosphomolybdic compounds as colour reagents. J. Biol. Chem. 1912, 12, 239–243. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Gulcin, I.; Tel, A.Z.; Kirecci, E. Antioxidant, Antimicrobial, Antifungal, and Antiradical Activities of Cyclotrichium niveum (BOISS.) Manden and Schen. Int. J. Food Prop. 2008, 11, 450–471. [Google Scholar] [CrossRef]
- Blois, M. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]


| Escherichia coli (ATCC 25922) | Klebsiella pneumoniae (ATCC 700603) | Pseudomonas aeruginosa (ATCC 9027) | Staphylococcus aureus (ATCC 29213) | Enterococcus faecalis (ATCC 29212) | |
|---|---|---|---|---|---|
| Dichloromethane | 64 | 16 | 512 | 1 | 2 |
| n-Hexane | 32 | 64 | 1024 | 1 | 4 |
| Ethyl acetate | 32 | 64 | 1024 | 8 | 64 |
| Aqueous | 128 | 256 | 1024 | 4 | 32 |
| Methanol | 4 | 256 | 1024 | 4 | 8 |
| MP-1 | 32 | 1024 | 1024 | 1 | 4 |
| MP-2 | 128 | 1024 | 1024 | 1 | 1 |
| Sulfamerazine | 1600 | 1600 | 1600 | 6.25 | 3.12 |
| Extract | Total Phenolic Compound (μg GAE/mg Extract) |
|---|---|
| Methanol | 49.29 ± 0.105 |
| n-Hexane | 22.24 ± 0.080 |
| Dichloromethane | 53.23 ± 0.152 |
| Ethyl acetate | 169.24 ± 0.229 |
| Aqueous | 46.71 ± 0.059 |
| % Inhibition (50 μg/mL) | |
|---|---|
| Methanol | 11.80 ± 0.021 |
| n-Hexane | 5.50 ± 0.003 |
| Dichloromethane | 10.65 ± 0.041 |
| Ethyl acetate | 38.80 ± 0.136 |
| Aqueous | 10.25 ± 0.066 |
| MP-1 | 43.88 ± 0.106 |
| MP-2 | 19.00 ± 0.023 |
| Trolox | 93.38 ± 0.209 |
| % Inhibition (50 μg/mL) | |
|---|---|
| Methanol | 40.92 ± 0.198 |
| n-Hexane | 15.99 ± 0.034 |
| Dichloromethane | 28.70 ± 0.148 |
| Ethyl acetate | 98.92 ± 0.341 |
| Aqueous | 42.88 ± 0.127 |
| MP-1 | 93.10 ± 0.152 |
| MP-2 | 68.59 ± 0.211 |
| Trolox | 98.71 ± 0.301 |
| % Inhibition (200 μg/mL) | |
|---|---|
| Methanol | 57.14 ± 0.184 |
| n-Hexane | 31.03 ± 0.197 |
| Dichloromethane | 51.72 ± 0.203 |
| Ethyl acetate | 20.35 ± 0.136 |
| Aqueous | 17.24 ± 0.105 |
| Kojic acid | 90.48 ± 0.312 |
| IC50 (μg/mL) | |
|---|---|
| MP-1 | 297.9 ± 1.2 |
| MP-2 | 115.2 ± 0.8 |
| Kojic acid | 14.6 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özgen, R.; Sezen Karaoğlan, E.; Sevindik, H.G.; Hancı, H.; Kazaz, C. Antimicrobial, Antioxidant, and Antityrosinase Activities of Morina persica L. and Its Isolated Compounds. Molecules 2024, 29, 3017. https://doi.org/10.3390/molecules29133017
Özgen R, Sezen Karaoğlan E, Sevindik HG, Hancı H, Kazaz C. Antimicrobial, Antioxidant, and Antityrosinase Activities of Morina persica L. and Its Isolated Compounds. Molecules. 2024; 29(13):3017. https://doi.org/10.3390/molecules29133017
Chicago/Turabian StyleÖzgen, Rıdvan, Esen Sezen Karaoğlan, Handan Gökben Sevindik, Hayrunisa Hancı, and Cavit Kazaz. 2024. "Antimicrobial, Antioxidant, and Antityrosinase Activities of Morina persica L. and Its Isolated Compounds" Molecules 29, no. 13: 3017. https://doi.org/10.3390/molecules29133017
APA StyleÖzgen, R., Sezen Karaoğlan, E., Sevindik, H. G., Hancı, H., & Kazaz, C. (2024). Antimicrobial, Antioxidant, and Antityrosinase Activities of Morina persica L. and Its Isolated Compounds. Molecules, 29(13), 3017. https://doi.org/10.3390/molecules29133017






