IL-6 Inhibitory Compounds from the Aerial Parts of Piper attenuatum and Their Anticancer Activities on Ovarian Cancer Cell Lines
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
2.2. Screening of Compounds Using Human Embryonic Kidney (HEK)-Blue™ IL-6 Cells
2.3. Anticancer Activity
3. Materials and Methods
3.1. General Experimental Procedures and Chemicals
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data
3.5. Cell Culture
3.6. IL-6 Inhibition Bioassay Using HEK-Blue™ IL-6 Cells
3.7. Cytotoxicity Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menezes, I.C.; Cidade, F.W.; Souza, A.P.; Sampaio, I.C. Isolation and characterization of microsatellite loci in the black pepper, Piper nigrum L. (Piperaceae). Conserv. Genet. Resour. 2009, 1, 209–212. [Google Scholar] [CrossRef]
- Ohlyan, R.; Kandale, A.; Yadav, A. Pharmacognostic evaluation and antibacterial activity of dry fruits of Piper attenuatum Buch-Ham. Int. J. Pharm. Pharm. Sci. 2014, 6, 402–406. [Google Scholar]
- Parvathy, V.A.; Swetha, V.P.; Sheeja, T.E.; Sasikumar, B. A two locus barcode for discriminating Piper nigrum from its related adulterant species. Indian J. Biotechnol. 2018, 17, 346–350. [Google Scholar]
- Pathak, N.; Kumar, R. Piper attenuatum Buch.-Ham. ex Miq.—A review on its macroscopic characters, phytochemistry, medicinal importance, and its comparative study with other Piper species. Curr. Med. Res. Opin. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Reddy, S.D.; Siva, B.; Poornima, B.; Kumar, D.A.; Tiwari, A.K.; Ramesh, U.; Babu, K.S. New free radical scavenging neolignans from fruits of Piper attenuatum. Pharmacogn. Mag. 2015, 11, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper species: A comprehensive review on their phytochemstry, biological activities and applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, G.; Kumar, N.; Sethiya, N.K.; Bisht, D. Diuretic Activity of Ethanol Extract of Piper attenuatum Leaves Might Be Due to the Inhibition of Carbonic Anhydrase Enzyme: An in vivo and in silico Investigation. Clin. Complement. Med. Phamacol. 2024, 4, 100117. [Google Scholar] [CrossRef]
- Soni, G.; Sharma, S.; Dangi, N. In silico molecular docking study and protective effect of Piper attenuatum on aspirin induced gastric ulcer in rats. Curr. Chem. Lett. 2023, 12, 705–720. [Google Scholar] [CrossRef]
- Gaurav, S.; Jeyabalan, G.; Anil, A. Pharmacognostical, Phytochemical and Pharmacological Review of Piper attenuatum (B. HAM) and Caesalpinia crista (LINN). Int. J. Health Biol. Sci. 2021, 3, 7–13. [Google Scholar]
- James, N.E.; Woodman, M.; Ribeiro, J.R. Prognostic immunologic signatures in epithelial ovarian cancer. Oncogene. 2022, 41, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Browning, L.; Patel, M.R.; Horvath, E.B.; Tawara, K.; Jorcyk, C.L. IL-6 and ovarian cancer: Inflammatory cytokines in promotion of metastasis. Cancer Manag. Res. 2018, 10, 6685–6693. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.; Matte, I.; Rancourt, C.; Piché, A. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer 2011, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.; Chen, M.W.; Hsiao, M.; Wang, S.; Chen, C.A.; Hsiao, S.M.; Chang, J.S.; Lai, T.C.; Rose-John, S.; Kuo, M.L.; et al. IL-6 Trans-signaling in formation and progression of malignant ascites in ovarian cancer soluble IL-6Rα in ovarian cancer. Cancer Res. 2011, 71, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Coward, J.; Kulbe, H.; Chakravarty, P.; Leader, D.; Vassileva, V.; Leinster, D.A.; Thompson, R.; Schioppa, T.; Nemeth, J.; Vermeulen, J.; et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin. Cancer Res. 2011, 17, 6083–6096. [Google Scholar] [CrossRef]
- Schilling, W.; Zhang, Y.; Riemer, D.; Das, S. Visible-light-mediated dearomatisation of indoles and pyrroles to pharmaceuticals and pesticides. Chemistry 2020, 26, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Qiu, S.; Chen, H.C.; Zhang, D.; Lu, Y.L.; Chen, X.L. Maleimide structure: A promising scaffold for the development of antimicrobial agents. J. Asian Nat. Prod. Res. 2022, 24, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, B.; Chen, X.; Lin, Z.; Li, G.; Lu, Y.; Huang, H. Design, synthesis and biological evaluation of alkynyl-containing maleimide derivatives for the treatment of drug-resistant tuberculosis. Bioorg. Chem. 2023, 131, 106250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kong, Y.; Shao, X.; Li, Z. Design, synthesis, and insecticidal activities of novel N-pyridylpyrazole amide serivatives containing a maleimide. Chem. Biodivers. 2023, 20, e202300237. [Google Scholar] [CrossRef]
- Karahisar, E.; Tugay, O.; Orhan, I.E.; Sezer Senol Deniz, F.; Vlad Luca, S.; Skalicka-Wozniak, K.; Sahin, M. Metabolite profiling by hyphenated liquid chromatographic mass spectrometric technique (HPLC-DAD-ESI-Q-TOF-MS/MS) and neurobiological potential of Haplophyllum sahinii and H. vulcanicum extracts. Chem. Biodivers. 2019, 16, e1900333. [Google Scholar] [CrossRef]
- Tedasen, A.; Dokduang, S.; Sukpondma, Y.; Lailerd, N.; Madla, S.; Sriwiriyajan, S.; Rattanaburee, T.; Tipmanee, V.; Graidist, P. (-)-Kusunokinin inhibits breast cancer in N-nitrosomethylurea-induced mammary tumor rats. Eur. J. Pharmacol. 2020, 882, 173311. [Google Scholar] [CrossRef]
- Tanoguchi, M.; Hosono, E.; Kitaoka, M.; Arimoto, M.; Yamaguchi, H. Studies on the constituents of the seeds of Hernandia ovigera L. IX. Identification of two dibenzylbutyrolactone-type lignans and an attempt of conversion into phenyltetralin-type lignan. Chem. Pharm. Bull. 1991, 39, 1873–1876. [Google Scholar] [CrossRef][Green Version]
- Sriwiriyajan, S.; Sukpondma, Y.; Srisawat, T.; Madla, S.; Graidist, P. (-)-Kusunokinin and piperloguminine from Piper nigrum: An alternative option to treat breast cancer. Biomed. Pharmacother. 2017, 92, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Ahn, E.M.; Akao, T.; Abdel-Hafez, A.A.; Nakamura, N.; Hattori, M. Transformation of arctiin to estrogenic and antiestrogenic substances by human intestinal bacteria. Chem. Pharm. Bull. 2003, 51, 378–384. [Google Scholar] [CrossRef] [PubMed]
- De Araujo-Junior, J.X.; Da-Cunha, E.V.; Chaves, M.C.D.O.; Gray, A.I. Piperdardine, a piperidine alkaloid from Piper tuberculatum. Phytochemistry 1997, 44, 559–561. [Google Scholar] [CrossRef]
- Abdubakiev, S.; Li, H.; Lu, X.; Li, J.; Aisa, H.A. N-Alkylamides from Piper longum L. and their stimulative effects on the melanin content and tyrosinase activity in B16 melanoma cells. Nat. Prod. Res. 2020, 34, 2510–2513. [Google Scholar] [CrossRef] [PubMed]
- Knapp, H.; Weigand, C.; Gloser, J.; Winterhalter, P. 2-Hydroxy-2,6,10,10-tetramethyl-1-oxaspiro [4.5]dec-6-en-8-one: Precursor of 8,9-dehydrotheaspirone in white-fleshed nectarines. J. Agric. Food Chem. 1997, 45, 1309–1313. [Google Scholar] [CrossRef]
- Mori, K.; Khlebnikov, V. Carotenoids and degraded carotenoids, VIII-Synthesis of (+)-dihydroactinidiolide, (+)- and (−)-actinidiolide, (+)- and (−)-loliolide as well as (+)- and (−)-epiloliolide. Liebigs Ann. Chem. 1993, 1993, 77–82. [Google Scholar] [CrossRef]
- Lin, C.F.; Hwang, T.L.; Chien, C.C.; Tu, H.Y.; Lay, H.L. A new hydroxychavicol dimer from the roots of Piper betle. Molecules 2013, 18, 2563–2570. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, C.J.; Deng, X.H.; Zhu, J.Y.; Qin, L.P. Alkaloids from the aerial part of Piper flaviflorum. Chem. Nat. Compd. 2014, 50, 394–396. [Google Scholar] [CrossRef]
- Elbermawi, A.; Halim, A.F.; Mansour, E.S.; Ahmad, K.F.; Elsbaey, M.; Ashour, A.; Amen, Y.; El-Gamil, M.M.; Tomofumi, M.; Shimizu, K. Lycium schweinfurthii: New secondary metabolites and their cytotoxic activities. Nat. Prod. Res. 2022, 36, 5134–5141. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.D.A.O.; Oliveira, M.M.; Faleiro, F.L.; Scariot, D.B.; Boeing, J.S.; Visentainer, J.V.; Romagnolo, M.B.; Nakamura, C.V.; Truiti, M.D.C.T. Antileishmanial and antioxidant potential of fractions and isolated compounds from Nectandra cuspidata. Nat. Prod. Res. 2018, 32, 2825–2828. [Google Scholar] [CrossRef]
- Phan, V.K.; Nguyen, X.C.; Nguyen, X.N.; Vu, K.T.; Ninh, K.B.; Chau, V.M.; Bui, H.T.; Truong, N.H.; Lee, S.H.; Jang, H.D.; et al. Antioxidant activity of a new C-glycosylflavone from the leaves of Ficus microcarpa. Bioorg. Med. Chem. Lett. 2011, 21, 633–637. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Bao, Y.; Chen, K.; Xu, L.; Su, H.; Kuang, Y.; Wang, Z.; Qiao, X.; Ye, M. Characterization of a highly selective 2″-O-galactosyltransferase from Trollius chinensis and structure-guided engineering for improving UDP-glucose selectivity. Org. Lett. 2021, 23, 9020–9024. [Google Scholar] [CrossRef]
- Hsu, E.J.; Cao, X.; Moon, B.; Bae, J.; Sun, Z.; Liu, Z.; Fu, Y.X. A cytokine receptor-masked IL2 prodrug selectively activates tumor-infiltrating lymphocytes for potent antitumor therapy. Nat. Commun. 2021, 12, 2768. [Google Scholar] [CrossRef]
- Park, S.A.; Seo, Y.J.; Kim, L.K.; Kim, H.J.; Yoon, K.D.; Hoe, T.H. Butein inhibits cell growth by blocking the IL-6/IL-6Rα interaction in human ovarian cancer and by regulation of the IL-6/STAT3/FoxO3a pathway. Int. J. Mol. Sci. 2023, 24, 6038. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Kim, L.K.; Park, H.M.; Kim, H.J.; Heo, T.H. Inhibition of GP130/STAT3 and EMT by combined bazedoxifene and paclitaxel treatment in ovarian cancer. Oncol. Rep. 2022, 47, 52. [Google Scholar] [CrossRef]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy resistance in advanced ovarian cancer patients. Biomark. Cancer 2019, 11, 1179299X19860815. [Google Scholar] [CrossRef]
- Murata, M.; Nakai, Y.; Kawazu, K.; Ishizaka, M.; Kajiwara, H.; Abe, H.; Takeuchi, K.; Ichinose, Y.; Mitsuhara, I.; Mochizuki, A.; et al. Loliolide, a carotenoid metabolite, is a potential endogenous inducer of herbivore resistance. Plant Physiol. 2019, 179, 1822–1833. [Google Scholar] [CrossRef]
- Li, L.L.; Li, Z.; Lou, Y.; Meiners, S.J.; Kong, C.H. (-)-Loliolide is a general signal of plant stress that activates jasmonate-related responses. New Phytol. 2023, 238, 2099–2112. [Google Scholar] [CrossRef]
- Cho, D.H.; Yun, J.H.; Heo, J.; Lee, I.K.; Lee, Y.J.; Bae, S.; Yun, B.S.; Kim, H.S. Identification of loliolide with anti-aging properties from Scenedesmus deserticola JD052. J. Microbiol. Biotechnol. 2023, 33, 1250–1256. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, D.S.; Kim, S.; Lorz, L.R.; Choi, E.; Lim, H.Y.; Hossain, M.A.; Jang, S.; Choi, Y.I.; Park, K.J.; et al. Loliolide presents antiapoptosis and antiscratching effects in human keratinocytes. Int. J. Mol. Sci. 2019, 20, 651. [Google Scholar] [CrossRef] [PubMed]
- Han, E.J.; Fernando, I.P.S.; Kim, H.S.; Lee, D.S.; Kim, A.; Je, J.G.; Seo, M.J.; Jee, Y.H.; Jeon, Y.J.; Kim, S.Y.; et al. (-)-Loliolide isolated from Sargassum horneri suppressed oxidative stress and inflammation by activating Nrf2/HO-1 signaling in IFN-γ/TNF-α-stimulated HaCaT keratinocytes. Antioxidants 2021, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Alves, C.; Martins, A.; Susano, P.; Simões, M.; Guedes, M.; Rehfeldt, S.; Pinteus, S.; Gaspar, H.; Rodrigues, A.; et al. Loliolide, a new therapeutic option for neurological diseases? In vitro neuroprotective and anti-inflammatory activities of a monoterpenoid lactone isolated from Codium tomentosum. Int. J. Mol. Sci. 2021, 22, 1888. [Google Scholar] [CrossRef]
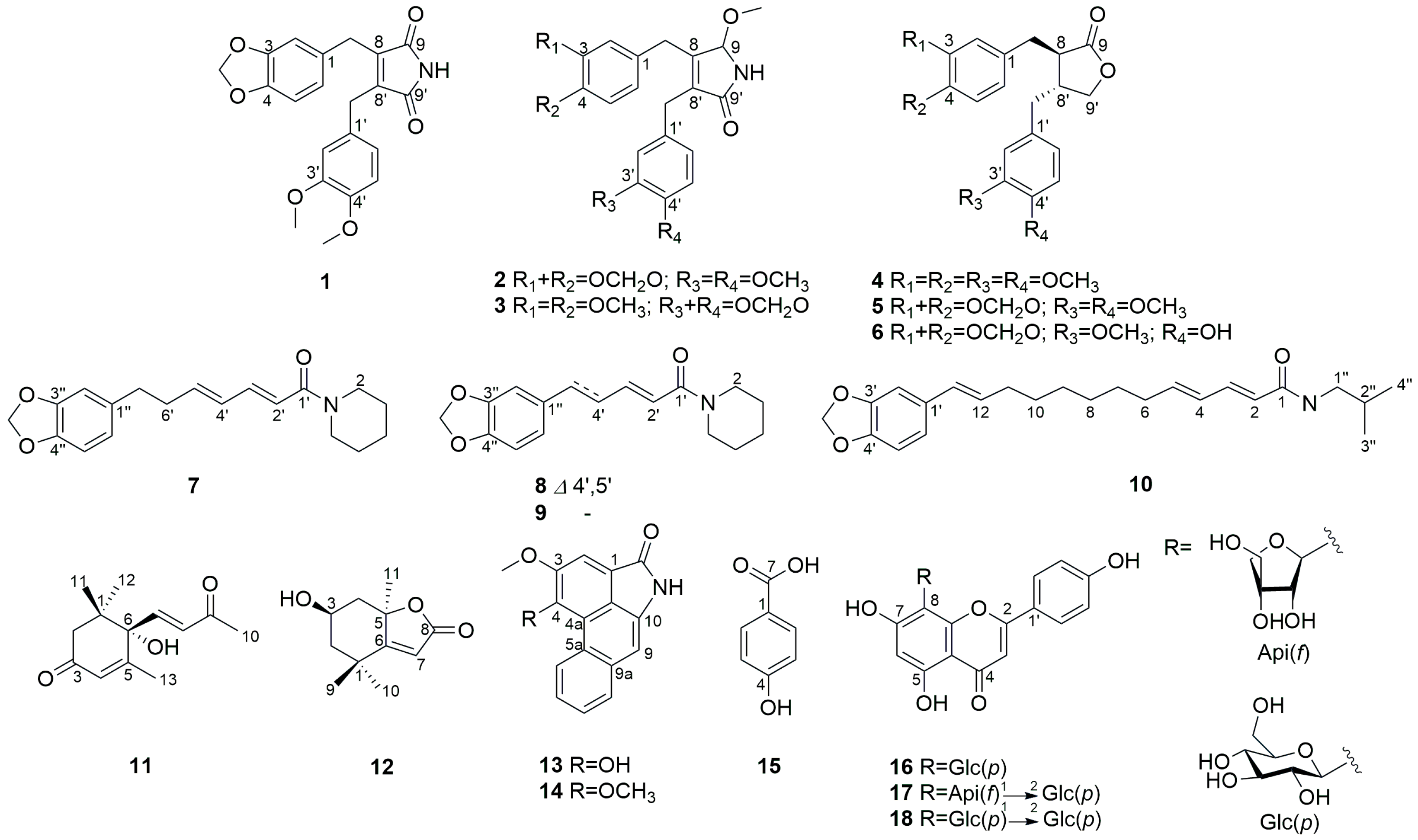
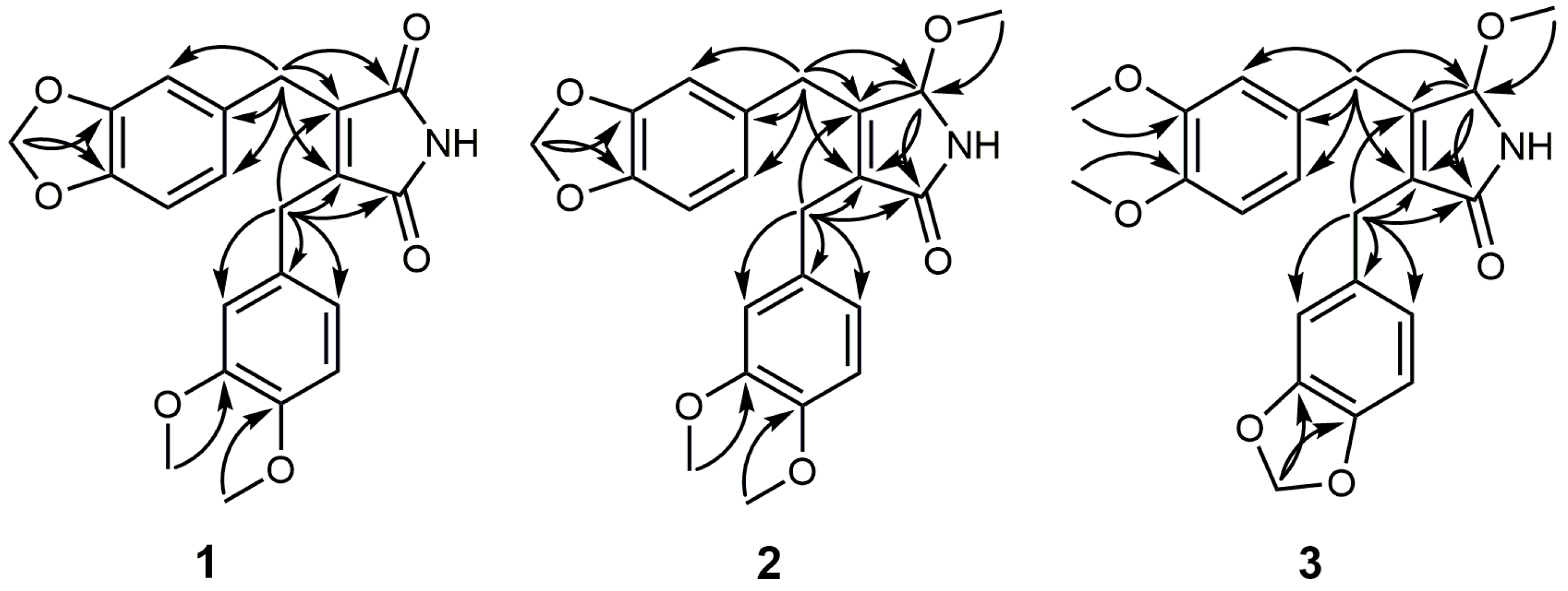
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δc | δH (Mult, J in Hz) | δc | δH (Mult, J in Hz) | δc | δH (Mult, J in Hz) | |
| 1 | 130.3 | 130.9 | 129.5 | |||
| 2 | 109.5 | 6.61 (1H, d, 1.8) | 109.5 | 6.50 (1H, d, 1.8) | 112.0 | 6.49 (1H, d, 1.9) |
| 3 | 148.2 | 148.1 | 149.1 | |||
| 4 | 146.8 | 146.7 | 147.9 | |||
| 5 | 108.7 | 6.69 (1H, d, 8.0) | 108.6 | 6.67 (1H, d, 8.0) | 111.4 | 6.75 (1H, overlap) |
| 6 | 122.0 | 6.59 (1H, dd, 8.0, 1.8) | 122.1 | 6.52 (1H, m) | 121.0 | 6.64 (1H, dd, 8.1, 1.9) |
| 7 | 29.3 | 3.63 (2H, s) | 32.2 | 3.73 (1H, d, 13.6) | 32.1 | 3.79 (1H, d, 14.9) |
| 3.33 (1H, d, 14.8) | 3.38 (1H, d, 14.9) | |||||
| 8 | 141.1 | 152.6 | 152.8 | |||
| 9 | 171.3 | 84.5 | 5.11 (1H, s) | 84.3 | 5.09 (1H, s) | |
| 1′ | 129.0 | 131.4 | 132.5 | |||
| 2′ | 112.3 | 6.64 (1H, d, 2.0) | 111.6 | 6.75 (1H, overlap) | 109.1 | 6.75 (1H, overlap) |
| 3′ | 149.4 | 147.9 | 146.1 | |||
| 4′ | 148.3 | 149.3 | 147.8 | |||
| 5′ | 111.6 | 6.75 (1H, d, 8.0) | 112.2 | 6.79 (1H, brs) | 108.3 | 6.71 (1H, overlap) |
| 6′ | 121.1 | 6.67 (1H, dd, 8.0, 2.0) | 120.7 | 6.75 (1H, overlap) | 121.4 (overlap) | 6.71 (1H, overlap) |
| 7′ | 29.4 | 3.66 (2H, s) | 29.0 | 3.62 (2H, d, 6.5) | 28.8 | 3.66 (1H, d, 14.8) |
| 3.58 (1H, d, 14.8) | ||||||
| 8′ | 140.7 | 134.6 | 133.9 | |||
| 9′ | 171.4 | 173.2 | 173.0 | |||
| 3-OCH3 | 55.8 | 3.74 (3H, s) | ||||
| 4-OCH3 | 55.9 | 3.84 (3H, s) | ||||
| 3′-OCH3 | 56.1 | 3.77 (3H, s) | 56.2 | 3.83 (3H, s) | ||
| 4′-OCH3 | 56.2 | 3.83 (3H, s) | 56.1 | 3.80 (3H, s) | ||
| 9-OCH3 | 51.9 | 3.16 (3H, s) | 51.9 | 3.18 (3H, s) | ||
| -OCH2O | 101.3 | 5.90 (2H, s) | 101.3 | 5.90 (2H, s) | 100.9 | 5.89 (2H, s) |
| Compound | EC50 (µM) a | CC50 (µM) b | TI c |
|---|---|---|---|
| piperamide III (3) | 3.52 ± 0.14 | 28.70 ± 0.19 | 8.16 ± 0.33 |
| (-)-dimethylmatairesinol (4) | 4.06 ± 0.07 | 22.64 ± 0.25 | 5.57 ± 0.32 |
| (6S)-dehydrovomifoliol (11) | 2.12 ± 0.04 | 13.72 ± 0.11 | 6.46 ± 0.15 |
| (-)-loliolide (12) | 0.91 ± 0.04 | 10.60 ± 0.10 | 11.58 ± 0.14 |
| ficuflavoside (17) | 1.31 ± 0.05 | 32.46 ± 0.45 | 24.77 ± 0.50 |
| vitexin 2″-O-Glc (18) | 0.58 ± 0.07 | 33.80 ± 0.45 | 58.35 ± 0.52 |
| Bazedoxifene d | 1.91 ± 0.43 | 4.07 ± 0.35 | 2.14 ± 0.78 |
| Compound | IC50 (µM) a | |||
|---|---|---|---|---|
| A2780 | A2780-Cis | SKOV3 | SKOV3-TR | |
| piperamide III (3) | 17.54 ± 0.19 | 40.51 ± 1.11 | 15.76 ± 0.15 | 40.98 ± 1.18 |
| (-)-dimethylmatairesinol (4) | 20.56 ± 0.25 | 16.95 ± 0.19 | 20.04 ± 0.18 | 33.01 ± 0.50 |
| (6S)-dehydrovomifoliol (11) | 9.80 ± 0.11 | 14.44 ± 0.21 | 10.69 ± 0.14 | 19.94 ± 0.22 |
| (-)-loliolide (12) | 8.62 ± 0.10 | 16.60 ± 0.18 | 10.71 ± 0.31 | 6.44 ± 0.10 |
| ficuflavoside (17) | 21.80 ± 0.45 | 41.04 ± 0.63 | 26.64 ± 0.30 | 40.34 ± 0.85 |
| vitexin 2″-O-Glc (18) | 40.97 ± 2.34 | 40.90 ± 3.07 | 23.07 ± 0.25 | 30.23 ± 0.48 |
| Bazedoxifene b | 8.20 ± 1.77 | 33.80 ± 0.15 | 7.25 ± 0.62 | 20.32 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Kim, L.K.; Kim, A.; Htwe, K.M.; Heo, T.-H.; Shin, K.J.; Kim, H.J.; Yoon, K.D. IL-6 Inhibitory Compounds from the Aerial Parts of Piper attenuatum and Their Anticancer Activities on Ovarian Cancer Cell Lines. Molecules 2024, 29, 2981. https://doi.org/10.3390/molecules29132981
Kim HJ, Kim LK, Kim A, Htwe KM, Heo T-H, Shin KJ, Kim HJ, Yoon KD. IL-6 Inhibitory Compounds from the Aerial Parts of Piper attenuatum and Their Anticancer Activities on Ovarian Cancer Cell Lines. Molecules. 2024; 29(13):2981. https://doi.org/10.3390/molecules29132981
Chicago/Turabian StyleKim, Hye Jin, Lee Kyung Kim, Anna Kim, Khin Myo Htwe, Tae-Hwe Heo, Kye Jung Shin, Hee Jung Kim, and Kee Dong Yoon. 2024. "IL-6 Inhibitory Compounds from the Aerial Parts of Piper attenuatum and Their Anticancer Activities on Ovarian Cancer Cell Lines" Molecules 29, no. 13: 2981. https://doi.org/10.3390/molecules29132981
APA StyleKim, H. J., Kim, L. K., Kim, A., Htwe, K. M., Heo, T.-H., Shin, K. J., Kim, H. J., & Yoon, K. D. (2024). IL-6 Inhibitory Compounds from the Aerial Parts of Piper attenuatum and Their Anticancer Activities on Ovarian Cancer Cell Lines. Molecules, 29(13), 2981. https://doi.org/10.3390/molecules29132981






