Lavandula angustifolia Essential Oil Inhibits the Ability of Fusobacterium nucleatum to Produce Volatile Sulfide Compounds, a Key Components in Oral Malodor
Abstract
1. Introduction
2. Results
2.1. Essential Oil Extraction and Chemical Analysis
2.2. Minimal Inhibitory Concentration (MIC) Determination
2.3. Volatile Sulfide Compound (VSC) Determination
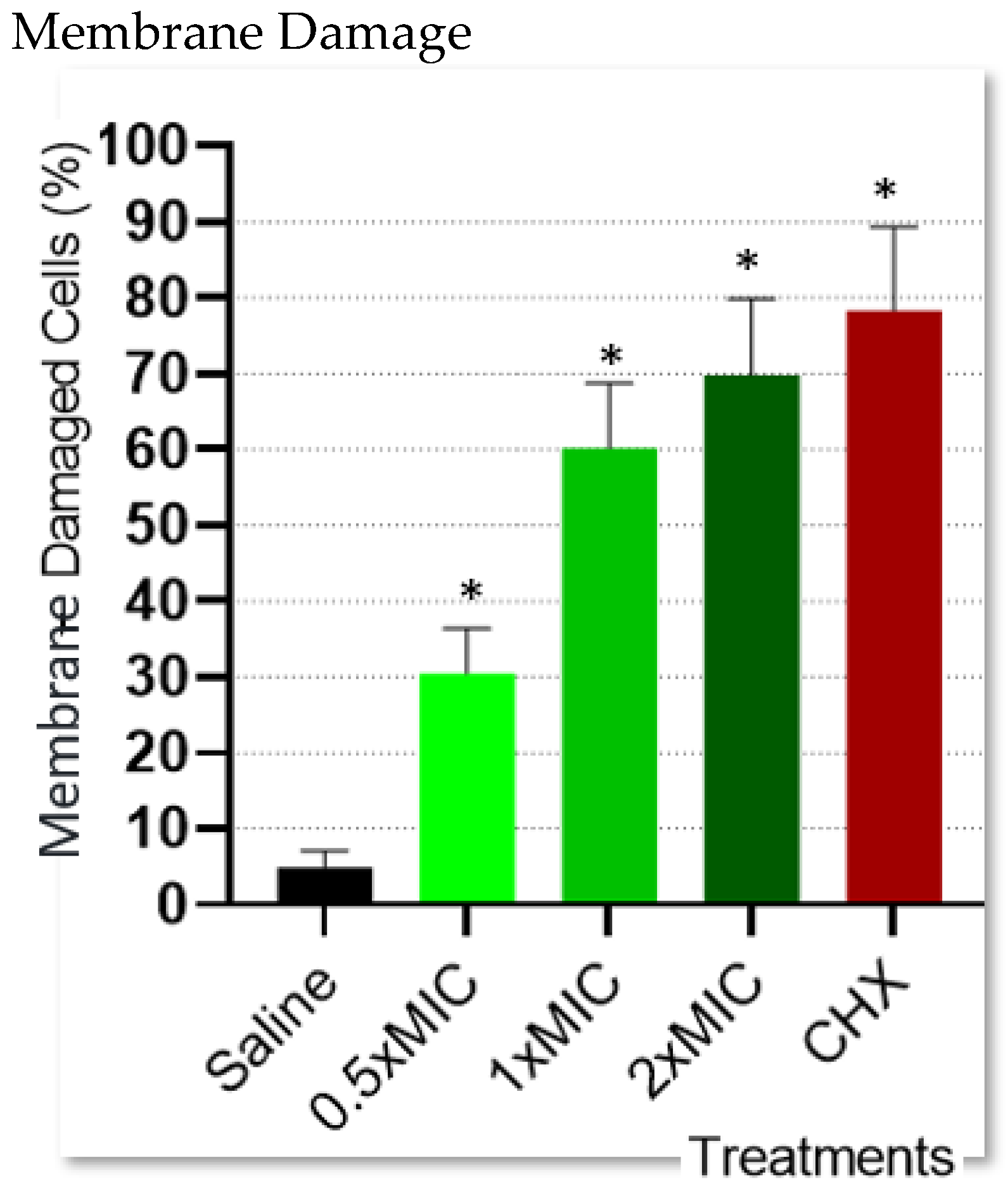
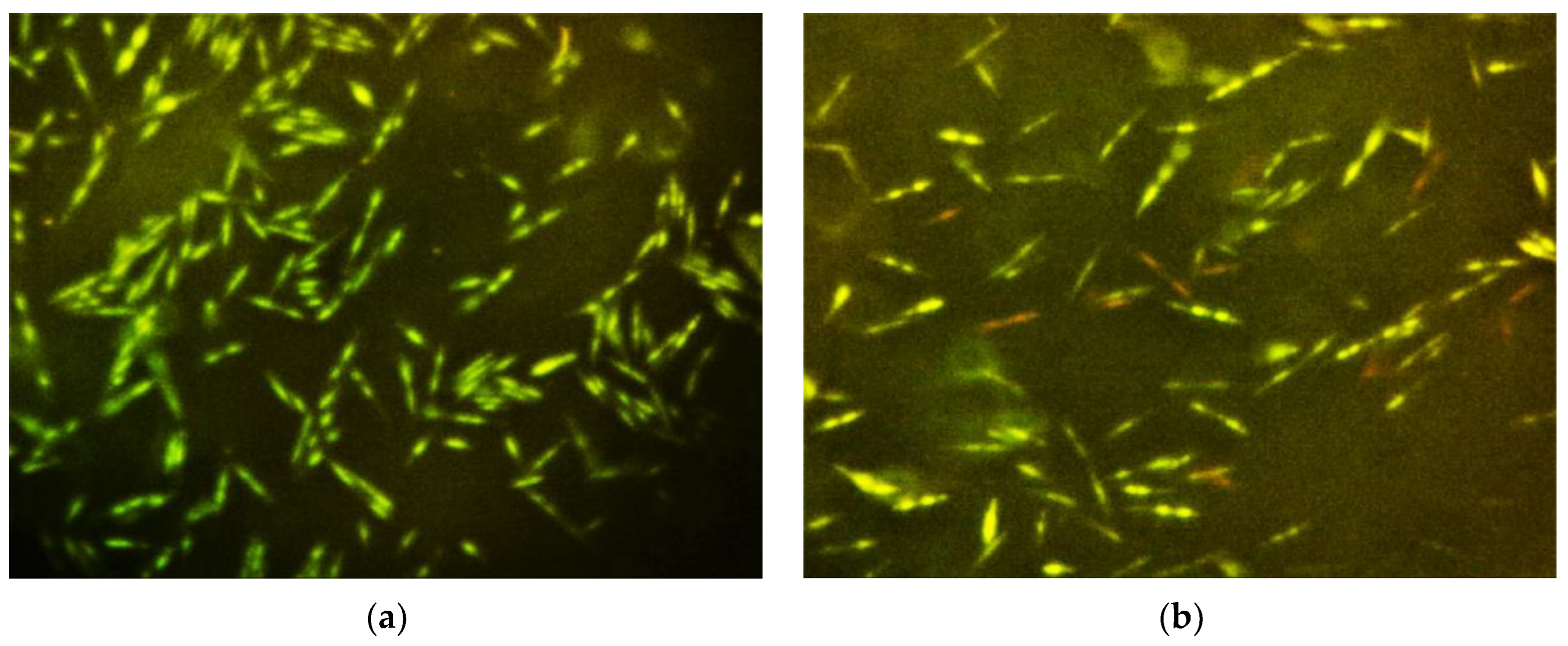
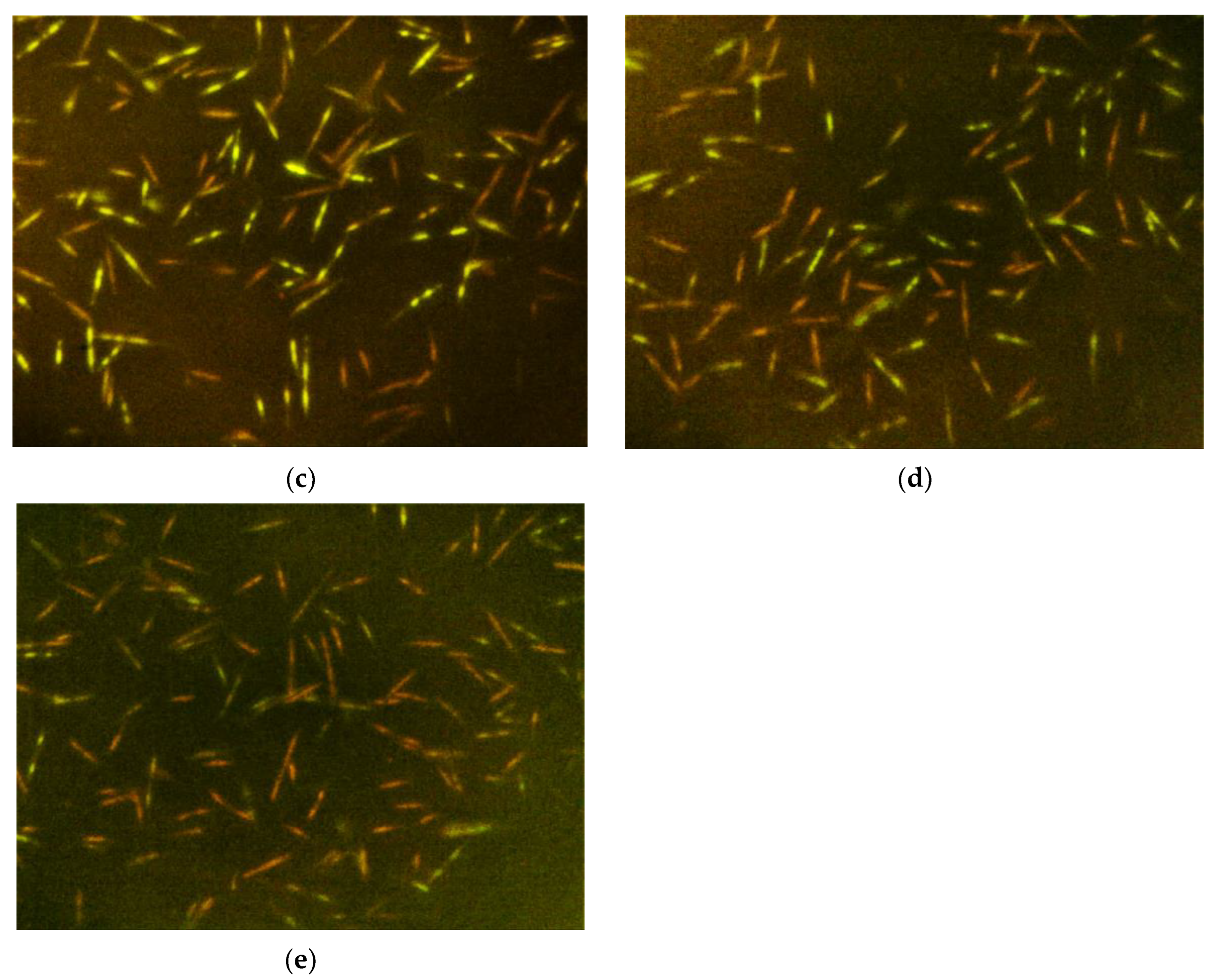
2.4. Bacterial Cell Membrane Integrity Determination
3. Discussion
4. Materials and Methods
4.1. Essential Oil Extraction and Chemical Analysis
4.2. Minimal Inhibitory Concentration (MIC) Determination
4.3. Volatile Sulfide Compound (VSC) Determination
4.4. Bacterial Cell Membrane Integrity Determination
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bornstein, M.M.; Kislig, K.; Hoti, B.B.; Seemann, R.; Lussi, A. Prevalence of halitosis in the population of the city of Bern, Switzerland: A study comparing self-reported and clinical data. Eur. J. Oral Sci. 2009, 117, 261–267. [Google Scholar] [CrossRef]
- Liu, X.N.; Shinada, K.; Chen, X.C.; Zhang, B.X.; Yaegaki, K.; Kawaguchi, Y. Oral malodor-related parameters in the Chinese general population. J. Clin. Periodontol. 2006, 33, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J.; Kazor, C. Microbiology and treatment of halitosis. Periodontology 2000 2002, 28, 256–279. [Google Scholar] [CrossRef]
- Yasukawa, T.; Ohmori, M.; Sato, S. The relationship between physiologic halitosis and periodontopathic bacteria of the tongue and gingival sulcus. Odontology 2010, 98, 44–51. [Google Scholar] [CrossRef]
- Suzuki, N.; Yoneda, M.; Takeshita, T.; Hirofuji, T.; Hanioka, T. Induction and inhibition of oral malodor. Mol. Oral Microbiol. 2019, 34, 85–96. [Google Scholar] [CrossRef]
- Nakano, Y.; Yoshimura, M.; Koga, T. Correlation between oral malodor and periodontal bacteria. Microbes Infect. 2002, 4, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Stokowa-Sołtys, K.; Wojtkowiak, K.; Jagiełło, K. Fusobacterium nucleatum—Friend or foe? J. Inorg. Biochem. 2021, 224, 111586. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Zijnge, V.; van Leeuwen, M.B.M.; Degener, J.E.; Abbas, F.; Thurnheer, T.; Gmür, R.; Harmsen, H.J.M. Oral biofilm architecture on natural teeth. PLoS ONE 2010, 5, e9321. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Tanner, A.; Paster, B.; Lu, S.; Kanasi, E.; Kent, R.; Van Dyke, T.; Sonis, S. Subgingival and tongue microbiota during early periodontitis. J. Dent. Res. 2006, 85, 318–323.74. [Google Scholar] [CrossRef]
- Matsui, M.; Chosa, N.; Shimoyama, Y.; Minami, K.; Kimura, S.; Kishi, M. Effects of tongue cleaning on bacterial flora in tongue coating and dental plaque: A crossover study. BMC Oral Health 2014, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Fedorowicz, Z.; Aljufairi, H.; Nasser, M.; Outhouse, T.L.; Pedrazzi, V. Mouth rinses for the treatment of halitosis. Cochrane Database Syst. Rev. 2008, 4, CD006701. [Google Scholar]
- Sterer, N.; Rubinstein, Y. Effect of various natural medicinals on salivary protein putrefaction and malodor production. Quintessence Int. 2006, 37, 653–658. [Google Scholar]
- Jeffet, U.; Dagan, N.; Sterer, N. Effect of sublethal blue light on herbal extract activity against volatile sulfide compound production by Fusobacterium nucleatum. Photochem. Photobiol. 2021, 97, 443–447. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Abou Baker, D.H.; Amarowicz, R.; Kandeil, A.; Ali, M.A.; Ibrahim, E.A. Antiviral activity of Lavandula angustifolia L. and Salvia officinalis L. essential oils against avian influenza H5N1 virus. J. Agric. Food Res. 2021, 4, 100135. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, S.; Jaime, L.; García-Risco, M.R.; Lopez-Hazas, M.; Reglero, G. Supercritical fluid extraction as an alternative process to obtain antiviral agents from thyme species. Ind. Crop. Prod. 2014, 52, 475–480. [Google Scholar] [CrossRef]
- Zaha, D.C.; Bungau, S.; Aleya, S.; Tit, D.M.; Vesa, C.M.; Popa, A.R.; Pantis, C.; Maghiar, O.A.; Bratu, O.G.; Furau, C.; et al. What antibiotics for what pathogens? The sensitivity spectrum of isolated strains in an intensive care unit. Sci. Total Environ. 2019, 687, 118–127. [Google Scholar] [CrossRef]
- Zaha, D.C.; Bungau, S.; Uivarosan, D.; Tit, D.M.; Maghiar, T.A.; Maghiar, O.; Pantis, C.; Fratila, O.; Rus, M.; Vesa, C.M. Antibiotic Consumption and Microbiological Epidemiology in Surgery Departments: Results from a Single Study Center. Antibiotics 2020, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Karadag, E.; Samancioglu, S.; Ozden, D.; Bakir, E. Effects of aromatherapy on sleep quality and anxiety of patients. Nurs. Crit. Care 2017, 22, 105–112. [Google Scholar] [CrossRef]
- Vakilian, K.; Atarha, M.; Bekhradi, R.; Chaman, R. Healing advantages of lavender essential oil during episiotomy recovery: A clinical trial. Complement. Ther. Clin. Pract. 2011, 17, 50–53. [Google Scholar] [CrossRef]
- Evandri, M.; Battinelli, L.; Daniele, C.; Mastrangelo, S.; Bolle, P.; Mazzanti, G. The antimutagenic activity of Lavandula angustifolia (lavender) essential oil in the bacterial reverse mutation assay. Food Chem. Toxicol. 2005, 43, 1381–1387. [Google Scholar] [CrossRef]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of lavender (Lavandula angustifolia) essential oil on acute inflammatory response. Evid. Based Complement. Altern. Med. 2018, 2018, 1413940. [Google Scholar] [CrossRef]
- Woronuk, G.; Demissie, Z.; Rheaut, M.; Mahmoud, S. Biosynthesis, and therapeutic properties of Lavandula essential oil constituents. Planta Med. 2011, 77, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Dobler, D.; Runkel, F.; Schmidts, T. Effect of essential oils on oral halitosis treatment: A review. Eur. J. Oral Sci. 2020, 128, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Bersan, S.M.F.; Galvão, L.C.C.; Goes, V.F.F.; Sartoratto, A.; Figueira, G.M.; Rehder, V.L.G.; Alencar, S.M.; Duarte, R.M.T.; Rosalen, P.L.; Duarte, M.C.T. Action of essential oils from Brazilian native and exotic medicinal species on oral biofilms. BMC Complement. Altern. Med. 2014, 14, 451. [Google Scholar] [CrossRef]
- Ait Said, L. Chemical composition and antibacterial activity of Lavandula coronopifolia essential oil against antibiotic-resistant bacteria. Nat. Prod. Res. 2015, 29, 582–585. [Google Scholar] [CrossRef]
- Gursoy, U.K.; Gursoy, M.; Gursoy, O.V.; Cakmakci, L.; Könönen, E.; Uitto, V.-J. Anti-biofilm properties of Satureja hortensis L. essential oil against periodontal pathogens. Anaerobe 2009, 15, 164–167. [Google Scholar] [CrossRef]
- Takarada, K.; Kimizura, R.; Takahashi, N.; Honma, K.; Okuda, K.; Kato, T. A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol. Immunol. 2004, 19, 61–64. [Google Scholar] [CrossRef]
- Leong, W.H.; Lai, K.S.; Lim, S.E. Combination Therapy Involving Lavandula angustifolia and Its Derivatives in Exhibiting Antimicrobial Properties and Combatting Antimicrobial Resistance: Current Challenges and Future Prospects. Processes 2021, 9, 609. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Haas, B.; Grenier, D. Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum. Sci. Rep. 2017, 7, 44815. [Google Scholar] [CrossRef] [PubMed]
- Sterer, N.; Nuas, S.; Mizrahi, B.; Goldenberg, C.; Weiss, E.I.; Domb, A.; Davidi, M.P. Oral malodor reduction by a palatal mucoadhesive tablet containing herbal formulation. J. Dent. 2008, 36, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Lagha, A.B.; Vaillancourt, K.; Huacho, P.M.; Grenier, D. Effects of Labrador Tea, Peppermint, and Winter Savory Essential Oils on Fusobacterium nucleatum. Antibiotics 2020, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Białoń, M.; Krzyśko-Łupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P.P. Chemical Composition of Two Different Lavender Essential Oils and Their Effect on Facial Skin Microbiota. Molecules 2019, 24, 3270. [Google Scholar] [CrossRef] [PubMed]
- Garzoli, S.; Turchetti, G.; Giacomello, P.; Tiezzi, A.; Masci, V.L.; Ovidi, E. Liquid and Vapour Phase of Lavandin (Lavandula intermedia) Essential Oil: Chemical Composition and Antimicrobial Activity. Molecules 2019, 24, 2701. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2020. [Google Scholar]
- Détár, E.; Németh, É.Z.; Gosztola, B.; Demján, I.; Pluhár, Z. Effects of variety and growth year on the essential oil properties of lavender (Lavandula angustifolia Mill.) and lavandin (Lavandula x intermedia Emeric ex Loisel.). Biochem. Syst. Ecol. 2020, 90, 104020. [Google Scholar] [CrossRef]
- Bejar, E. Adulteration of english lavender (Lavandula angustifolia) essential oil. J. Pharm. Biomed. Anal. 2020, 199, 114050. [Google Scholar]
- Lane, W.A.; Mahmoud, S.S. Composition of essential oil from Lavandula angustifolia and L. intermedia varieties grown in British Columbia, Canada. Nat. Prod. Commun. 2008, 3, 1361–1366. [Google Scholar] [CrossRef]
- Karaca, N.; Şener, G.; Demirci, B.; Demirci, F. Synergistic antibacterial combination of Lavandula latifolia Medik. essential oil with camphor. Z. Naturforsch. C. J. Biosci. 2020, 76, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Benabdelkader, T.; Zitouni, A.; Guitton, Y.; Jullien, F.; Maitre, D.; Casabianca, H.; Legendre, L.; Kameli, A. Essential oils from wild populations of Algerian Lavandula stoechas L.: Composition, chemical variability, and in vitro biological properties. Chem. Biodivers. 2011, 8, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Dagli, N.; Dagli, R.; Mahmoud, R.S.; Baroudi, K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int. Soc. Prevent. Community Dent. 2015, 5, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Waczka, M.; Duda-Madej, A.; Grabarczyk, M.; Winska, K. Natural Compounds in the Battle against Microorganisms—Linalool. Molecules 2022, 27, 6928. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Lim, Y.K.; Freire, M.O.; Cho, E.; Jin, D.; Kook, J.K. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe 2012, 18, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food 2013, 12, 40–53. [Google Scholar] [CrossRef]

| Compound | Retention Time (min) | Quantification 1 |
|---|---|---|
| Camphene | 10.911 | 0.72 |
| p-Cymene | 14.57 | 0.22 |
| Eucalyptol | 14.955 | 17.06 |
| Lavender lactone | 15.094 | 0.41 |
| Cis-Linalool oxide (furanoid) | 16.898 | 6.5 |
| trans-Linalool oxide (furanoid) | 17.68 | 4.5 |
| Linalool | 18.444 | 7.72 |
| Octen-3-yl-acetate | 18.841 | 0.35 |
| Camphor | 20.601 | 33.26 |
| Borneol | 21.756 | 7.21 |
| Trimethyl-6-vinyltetrahydro-2H-pyran-3-ol | 21.945 | 0.42 |
| Terpinen-4-ol | 22.147 | 0.19 |
| Para-cymen-8-ol | 22.481 | 0.11 |
| α-Terpineol | 22.866 | 0.57 |
| Linalyl acetate | 25.592 | 12.18 |
| Bornyl acetate | 26.998 | 0.3 |
| Lavandulyl acetate | 27.106 | 1.43 |
| Neryl acetate | 30.399 | 0.1 |
| Geranyl acetate | 31.257 | 0.24 |
| α-santalene | 32.853 | 0.55 |
| Caryophyllene oxide | 39.288 | 1.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosner, O.; Livne, S.; Bsharat, M.; Dviker, S.; Jeffet, U.; Matalon, S.; Sterer, N. Lavandula angustifolia Essential Oil Inhibits the Ability of Fusobacterium nucleatum to Produce Volatile Sulfide Compounds, a Key Components in Oral Malodor. Molecules 2024, 29, 2982. https://doi.org/10.3390/molecules29132982
Rosner O, Livne S, Bsharat M, Dviker S, Jeffet U, Matalon S, Sterer N. Lavandula angustifolia Essential Oil Inhibits the Ability of Fusobacterium nucleatum to Produce Volatile Sulfide Compounds, a Key Components in Oral Malodor. Molecules. 2024; 29(13):2982. https://doi.org/10.3390/molecules29132982
Chicago/Turabian StyleRosner, Ofir, Shiri Livne, Maria Bsharat, Shir Dviker, Uziel Jeffet, Shlomo Matalon, and Nir Sterer. 2024. "Lavandula angustifolia Essential Oil Inhibits the Ability of Fusobacterium nucleatum to Produce Volatile Sulfide Compounds, a Key Components in Oral Malodor" Molecules 29, no. 13: 2982. https://doi.org/10.3390/molecules29132982
APA StyleRosner, O., Livne, S., Bsharat, M., Dviker, S., Jeffet, U., Matalon, S., & Sterer, N. (2024). Lavandula angustifolia Essential Oil Inhibits the Ability of Fusobacterium nucleatum to Produce Volatile Sulfide Compounds, a Key Components in Oral Malodor. Molecules, 29(13), 2982. https://doi.org/10.3390/molecules29132982






