Comprehensive Quality Assessment of Brassica napus L. Seeds via HPTLC, LC-QToF, and Anatomical Investigation
Abstract
1. Introduction
2. Results
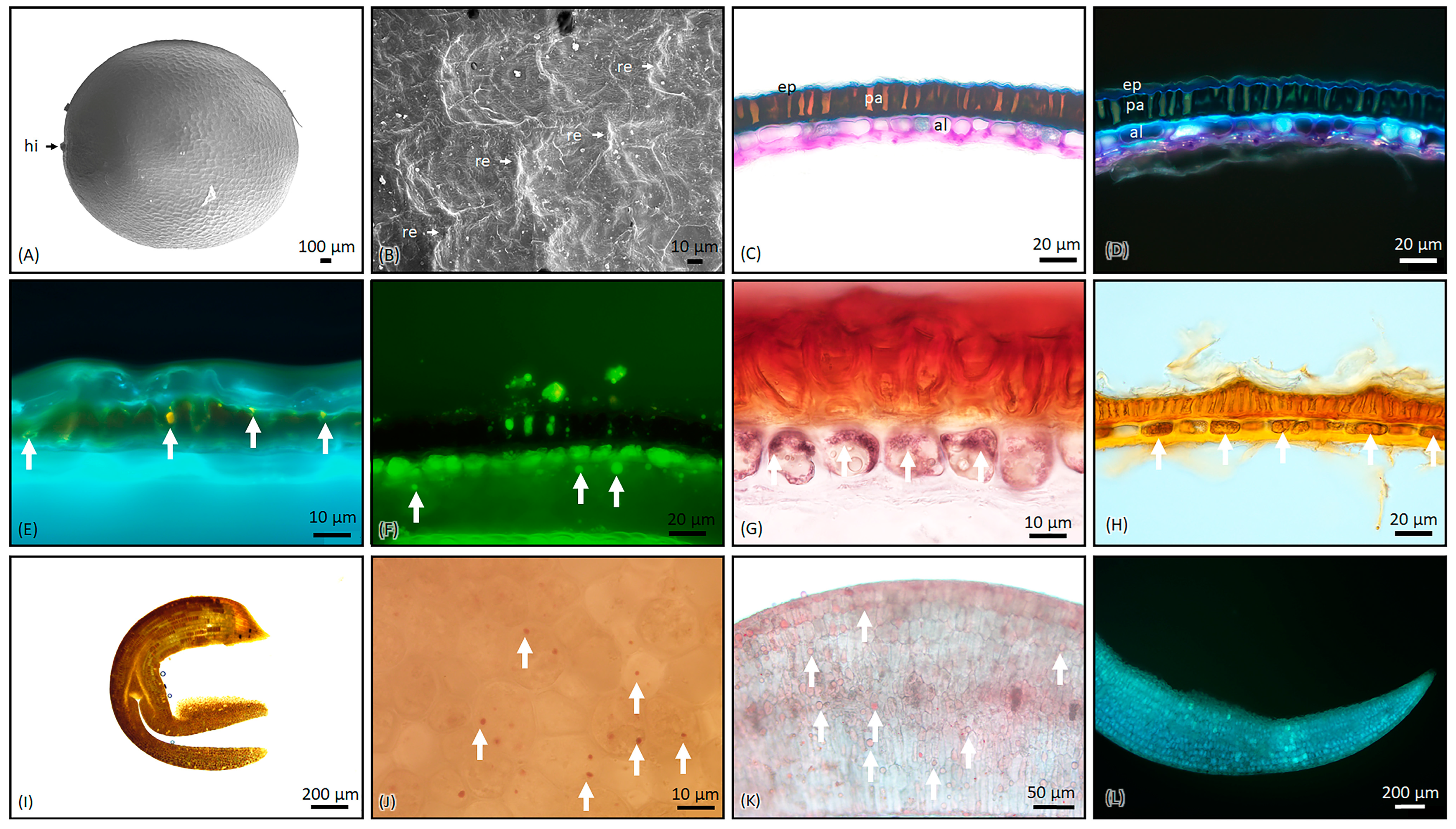
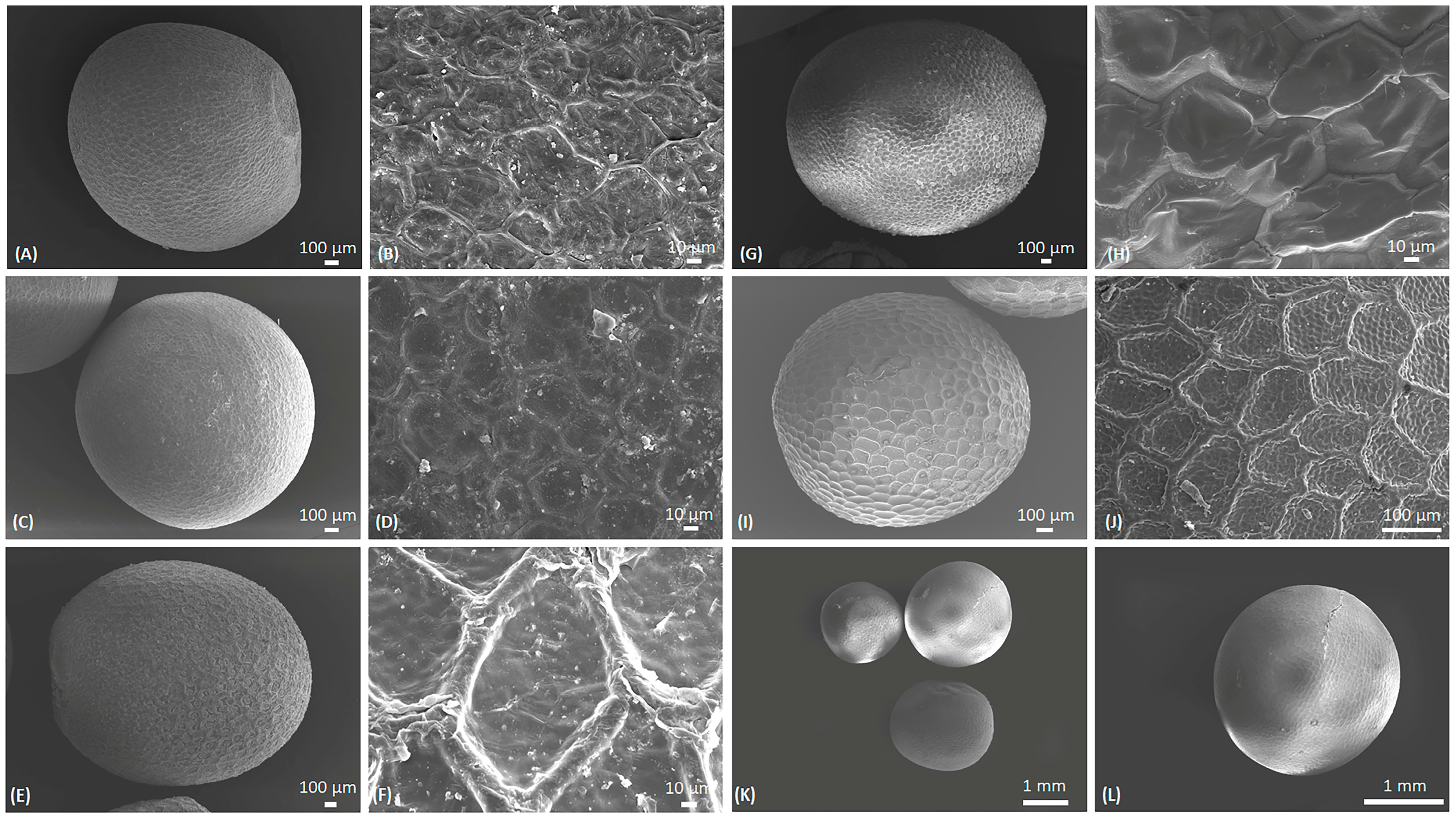
2.1. Microscopic Characterization of B. napus Seeds
2.2. HPTLC Analysis of Brassica Seeds’ Extracts
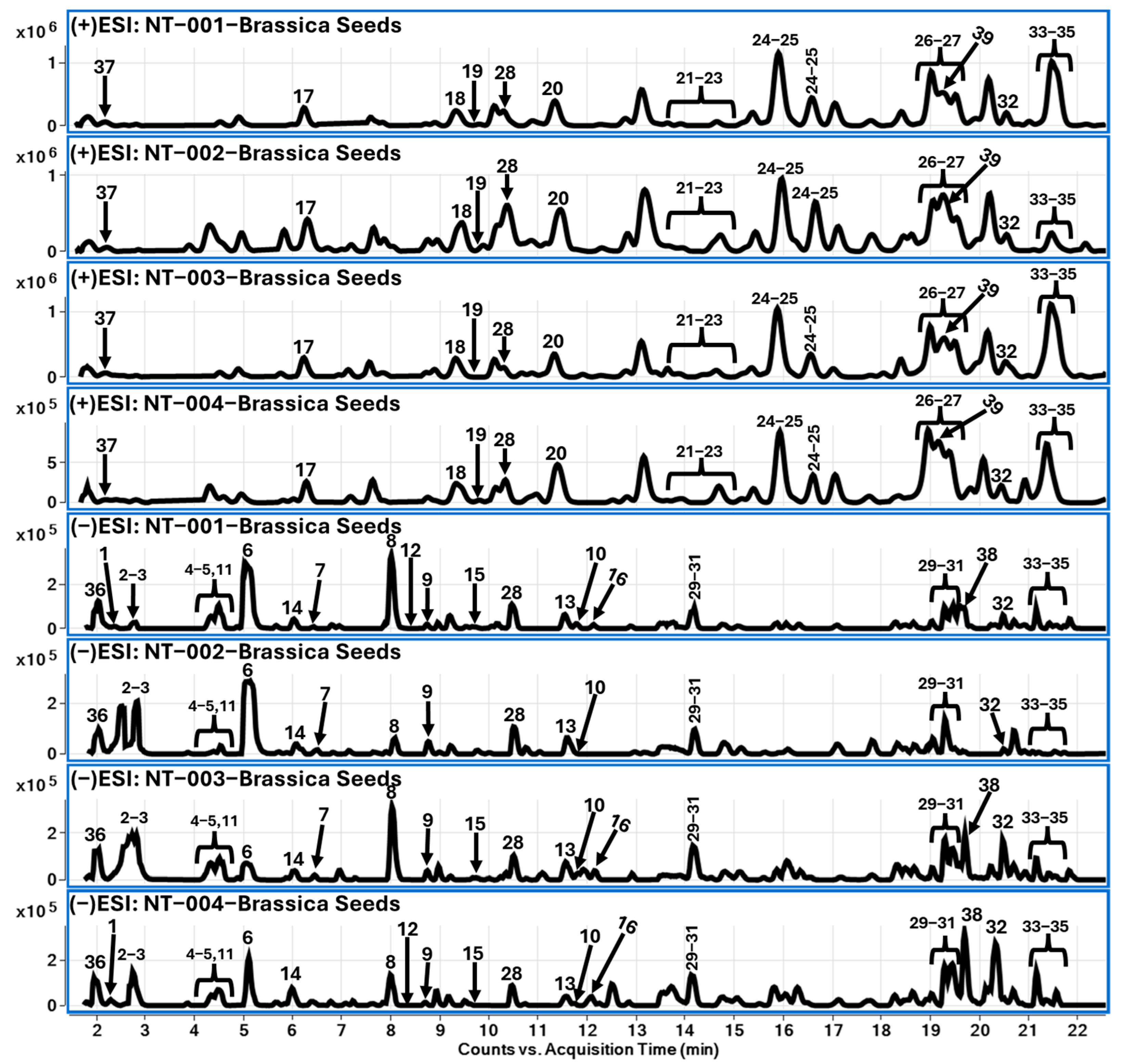
2.3. LC-QToF Analysis of Brassica Seeds
2.3.1. Glucosinolates (1–16)
2.3.2. Choline Derivatives (17–27)
2.3.3. Flavonoids (28–35)
2.3.4. Other Compounds (36–39)
3. Discussion
4. Material and Methods
4.1. Botanical Materials
4.2. Sample Preparation for Microscopy
4.3. Sample Preparation for Scanning Electron Microscopy (SEM)
4.4. Sample Preparation for HPTLC Analysis
4.5. LC-QToF Sample Preparation
Instrumentation and Analytical Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, B.; Quispe, C.; Butnariu, M.; Sarac, I.; Marmouzi, I.; Kamle, M.; Tripathi, V.; Kumar, P.; Bouyahya, A.; Capanoglu, E.; et al. Phytotherapy and Food Applications from Brassica Genus. Phyther. Res. 2021, 35, 3590–3609. [Google Scholar] [CrossRef]
- Paul, S.; Geng, C.; Yang, T.; Yang, Y.; Chen, J. Phytochemical and Health-Beneficial Progress of Turnip (Brassica rapa). J. Food Sci. 2019, 84, 19–30. [Google Scholar] [CrossRef]
- Bhandari, S.; Kwak, J.-H. Chemical Composition and Antioxidant Activity in Different Tissues of Brassica Vegetables. Molecules 2015, 20, 1228–1243. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef]
- Ayadi, J.; Debouba, M.; Rahmani, R.; Bouajila, J. Brassica Genus Seeds: A Review on Phytochemical Screening and Pharmacological Properties. Molecules 2022, 27, 6008. [Google Scholar] [CrossRef]
- Beyzi, E.; Gunes, A.; Buyukkilic Beyzi, S.; Konca, Y. Changes in Fatty Acid and Mineral Composition of Rapeseed (Brassica napus Ssp. Oleifera L.) Oil with Seed Sizes. Ind. Crops Prod. 2019, 129, 10–14. [Google Scholar] [CrossRef]
- Tileuberdi, N.; Turgumbayeva, A.; Yeskaliyeva, B.; Sarsenova, L.; Issayeva, R. Extraction, Isolation of Bioactive Compounds and Therapeutic Potential of Rapeseed (Brassica napus L.). Molecules 2022, 27, 8824. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, Y. Polyphenolic Compounds from Rapeseeds (Brassica napus L.): The Major Types, Biofunctional Roles, Bioavailability, and the Influences of Rapeseed Oil Processing Technologies on the Content. Food Res. Int. 2023, 163, 112282. [Google Scholar] [CrossRef]
- Rokosik, E.; Siger, A.; Rudzińska, M.; Dwiecki, K. Antioxidant Activity and Synergism of Canolol and α-Tocopherol in Rapeseed Oil Is Affected by the Presence of Phospholipid Association Colloids. LWT 2020, 133, 110095. [Google Scholar] [CrossRef]
- Güneş, F. Seed Morphology and Their Systematic Importance of Lathyrus Taxa Belonging to Platystylis (=Lathyrostylis) Section (Fabaceae) from Turkey. Afr. J. Agric. Res. 2012, 7, 265–277. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Rao, A.S. Estimation of Rutin in Ethanolic Extract of Brassica oleracea L. var Capitata. Leaves by HPTLC Method. Int. J. Sci. Res. 2012, 2, 421–422. [Google Scholar] [CrossRef]
- Shafaei, A.; Hill, C.R.; Hodgson, J.M.; Blekkenhorst, L.C.; Boyce, M.C. Simultaneous Extraction and Quantitative Analysis of S-Methyl-l-Cysteine Sulfoxide, Sulforaphane and Glucosinolates in Cruciferous Vegetables by Liquid Chromatography Mass Spectrometry. Food Chem. X 2024, 21, 101065. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Sharaf Eldin, M.G.; Kassem, H.; Abou el Fetouh, M. Metabolome Classification of Brassica napus L. Organs via UPLC–QTOF–PDA–MS and Their Anti-oxidant Potential. Phytochem. Anal. 2013, 24, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Lin, Q.; Ma, C.; Ju, Z.; Wang, C. Metabolic Profiling and Pharmacokinetic Studies of Sinapine Thiocyanate by UHPLC-Q/TOF-MS and UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2022, 207, 114431. [Google Scholar] [CrossRef]
- Price, K.R.; Casuscelli, F.; Colquhoun, I.J.; Rhodes, M.J.C. Hydroxycinnamic Acid Esters from Broccoli Florets. Phytochemistry 1997, 45, 1683–1687. [Google Scholar] [CrossRef]
- Baumert, A.; Milkowski, C.; Schmidt, J.; Nimtz, M.; Wray, V.; Strack, D. Formation of a Complex Pattern of Sinapate Esters in Brassica Napus Seeds, Catalyzed by Enzymes of a Serine Carboxypeptidase-like Acyltransferase Family? Phytochemistry 2005, 66, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Sun, N.; Huang, H.; Xie, J.; Chen, Y.; Hu, X.; Hu, X.; Dong, R.; Yu, Q. Catabolism of Polyphenols Released from Mung Bean Coat and Its Effects on Gut Microbiota during in vitro Simulated Digestion and Colonic Fermentation. Food Chem. 2022, 396, 133719. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Sousa, C.; Pereira, D.; Valentao, P.; Taveira, M.; Martins, A.; Pereira, J.; Seabra, R.; Andrade, P. Screening of Antioxidant Phenolic Compounds Produced by In Vitro Shoots of Brassica Oleracea L. Var. Costata DC. Comb. Chem. High Throughput Screen. 2009, 12, 230–240. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Consensus Document on the Biology of the Brassica Crops (Brassica Spp.). Series on Harmonisation of Regulatory Oversight of Biotechnology, No 54. 2012, 142. Available online: https://www.oecd.org/science/biotrack/27531440.pdf (accessed on 19 June 2024).
- Warwick, S.I.; Francis, A.; Mulligan, G.A.; Canadian Biodiversity Information Facility. Species Bank. Brassicaceae of Canada. 2014. Available online: http://www.cbif.gc.ca/eng/species-bank/brassicaceae-of canada/?id=1370403267260 (accessed on 20 April 2023).
- Caeseele, L.V.; Mills, J.T.; Sumner, M.; Gillespie, R. Cytological Study of Palisade Development in the Seed Coat of Candle Canola. Can. J. Bot. 1982, 60, 2469–2475. [Google Scholar] [CrossRef]
- Vaughan, J.G. The Structure and Utilization of Oil Seeds; Chapman & Hall: London, UK, 1970; ISBN 9780412097904. [Google Scholar]
- Rahman, M.; McVetty, P. A Review of Brassica Seed Color. Can. J. Plant Sci. 2011, 91, 437–446. [Google Scholar] [CrossRef]
- Shirley, B.W. Flavonoids in Seeds and Grains: Physiological Function, Agronomic Importance and the Genetics of Biosynthesis. Seed Sci. Res. 1998, 8, 415–422. [Google Scholar] [CrossRef]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.-M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and Biochemistry of Seed Flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef]
- Leung, J.; Fenton, T.W.; Mueller, M.M.; Clandinin, O.R. Condensed Tannins of Rapeseed Meal. J. Food Sci. 1979, 44, 1313–1317. [Google Scholar] [CrossRef]
- Bouchereau, A.; Hamelin, J.; Lamour, I.; Renard, M.; Larher, F. Distribution of Sinapine and Related Compounds in Seeds of Brassica and Allied Genera. Phytochemistry 1991, 30, 1873–1881. [Google Scholar] [CrossRef]
- Yang, S.-C.; Arasu, M.V.; Chun, J.-H.; Jang, Y.-S.; Lee, Y.-H.; Kim, I.H.; Lee, K.-T.; Hong, S.-T.; Kim, S.-J. Identification and Determination of Phenolic Compounds in Rapeseed Meals (Brassica napus L.). J. Agric. Chem. Environ. 2015, 04, 14–23. [Google Scholar] [CrossRef]
- Ishida, M.; Chiba, I.; Okiyama, Y.; Takahata, Y.; Kaizuma, N. Separation and Identification of Desulfoglucosinolates in Japanese Rapessed by LC/APCI-MS. Japan Agric. Res. Q. 1997, 31, 73–80. [Google Scholar]
- Avato, P.; Argentieri, M.P. Brassicaceae: A Rich Source of Health Improving Phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Parikh, H.; Pandita, N.; Khanna, A. Phytoextract of Indian Mustard Seeds Acts by Suppressing the Generation of ROS against Acetaminophen-Induced Hepatotoxicity in HepG2 Cells. Pharm. Biol. 2015, 53, 975–984. [Google Scholar] [CrossRef]
- Danlami, U. Phytochemical, Nutritional and Antimicrobial Evaluations of the Aqueous Extract of Brassica nigra (Brassicaceae) Seeds. Am. J. Appl. Chem. 2016, 4, 161. [Google Scholar] [CrossRef]
- Ogidi, O.I.; Omu, O.; Ezeagba, P.A. Ethno Pharmacologically Active Components of Brassica juncea (Brown Mustard) Seeds. Int. J. Pharm. Res. Dev. 2019, 1, 09–13. [Google Scholar] [CrossRef]
- Sontakke, K.S.; Shinde, S.L. Evaluation of the Phytochemical Potential of Brassica juncea L. Seeds. Vidyabharati Int. Interdiscip. Res. J. 2020, 10, 25–29. [Google Scholar]
- Johansen, D.A. Plant Microtechnique, 1st ed.; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1940; 523p. [Google Scholar]
- Brundrett, M.C.; Kendrick, B.; Peterson, C.A. Efficient Lipid Staining in Plant Material with Sudan Red 7B or Fluoral Yellow 088 in Polyethylene Glycol-Glycerol. Biotech. Histochem. 1991, 66, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Yoder, L.R.; Mahlberg, P.G. Reactions of Alkaloid and Histochemical Indicators in Laticifers and Specialized Parenchyma Cells of Catharanthus roseus (Apocynaceae). Am. J. Bot. 1976, 63, 1167. [Google Scholar] [CrossRef]
- John Adams, S.; Kuruvilla, G.R.; Krishnamurthy, K.V.; Nagarajan, M.; Venkatasubramanian, P. Pharmacognostic and Phytochemical Studies on Ayurvedic Drugs Ativisha and Musta. Rev. Bras. Farmacogn. 2013, 23, 398–409. [Google Scholar] [CrossRef]
- Heslop-Harrison, Y.; Heslop-Harrison, J. The Digestive Glands of Pinguicula: Structure and Cytochemistry. Ann. Bot. 1981, 47, 293–319. [Google Scholar] [CrossRef]
- Ursache, R.; Andersen, T.G.; Marhavý, P.; Geldner, N. A Protocol for Combining Fluorescent Proteins with Histological Stains for Diverse Cell Wall Components. Plant J. 2018, 93, 399–412. [Google Scholar] [CrossRef]
- Adams, S.J.; Senthil Kumar, T.; Muthuraman, G.; Majeed, A. Distribution, Morphology, Anatomy and Histochemistry of Crepidium Acuminatum. Mod. Phytomorphol. 2018, 12, 15–32. [Google Scholar] [CrossRef]
- Matteini, P.; Agati, G.; Pinelli, P.; Goti, A. Modes of Complexation of Rutin with the Flavonoid Reagent Diphenylborinic Acid 2-Aminoethyl Ester. Monatshefte Chem. Chem. Mon. 2011, 142, 885–893. [Google Scholar] [CrossRef]
- Ferrara, B.T.; Thompson, E.P. A Method for Visualizing Fluorescence of Flavonoid Therapeutics In Vivo in the Model Eukaryote Dictyostelium Discoideum. Biotechniques 2019, 66, 65–71. [Google Scholar] [CrossRef] [PubMed]


| # | RT (min) | Compound Name [Ref] | Mol. Formula | Exact Mass [M] | [M+H]+ | Fragment Ions (Positive Mode) | [M-H]− | Fragment Ions (Negative Mode) | Samples | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NT-001 | NT-002 | NT-003 | NT-004 | |||||||||
| Aliphatic glucosinolates [12] | ||||||||||||
| 1 | 2.40 | Glucoraphanin | C12H23NO10S3 | 437.0484 | - | - | 436.0421 (436.0411) * | 275.0298 [M-H-Glc]−, 96.9590 [HSO4]−, 74.9908 [OH-N=C=S]− | + | + | + | + |
| 2 | 2.80 | Progoitrin | C11H19NO10S2 | 389.0450 | - | - | 388.0383 (388.0378) | 259.0155, 241.0024, 135.9690, 96.9600 [HSO4]−, 74.9910 [OH-N=C=S]− | + | + | + | + |
| 3 | 2.89 | Sinigrin | C10H17NO9S2 | 359.0345 | - | - | 358.0277 (358.0272) | 259.0125, 241.0029, 96.9599 [HSO4]−, 74.9910 [OH-N=C=S]− | + | + | + | + |
| 4 | 4.36 | Glucoallysin | C13H25NO10S3 | 451.0641 | - | - | 450.0572 (450.0568) | 259.0132, 112.9854, 96.9600 [HSO4]− | + | + | + | + |
| 5 | 4.52 | Gluconapoleiferin | C12H21NO10S2 | 403.0607 | - | - | 402.0540 (402.0534) | 259.0116, 96.9601 [HSO4]−, 74.9909 [OH-N=C=S]− | + | + | + | + |
| 6 | 5.23 | Gluconapin | C11H19NO9S2 | 373.0501 | - | - | 372.0428 (372.0425) | 274.9895, 259.0135, 241.0033, 130.0329, 96.9600 [HSO4]−, 74.9912 [OH-N=C=S]− | + | + | + | + |
| 7 | 6.50 | Glucoiberverin | C11H20NO9S3− | 406.0300 [M]− | - | - | 406.0309 (406.0300) | 96.9608 [HSO4]−, 74.9918 [OH-N=C=S]− | + | + | + | ND |
| 8 | 8.06 | Glucobrassicanapin | C12H21NO9S2 | 387.0658 | - | - | 386.0594 (386.0585) | 274.9950, 259.0120, 144.0484, 96.9604 [HSO4]−, 74.9912 [OH-N=C=S]− | + | + | + | + |
| 9 | 8.78 | Glucoerucin | C12H23NO9S3 | 421.0535 | - | - | 420.0459 (420.0462) | 96.9597 [HSO4]−, 74.9916 [OH-N=C=S]− | + | + | + | + |
| 10 | 11.80 | Glucoberteroin | C13H24NO9S3− | 434.0613 [M]− | - | - | 434.0609 (434.0613) | 96.9598 [HSO4]−, 74.9911 [OH-N=C=S]− | + | + | + | + |
| Aromatic glucosinolates [12] | ||||||||||||
| 11 | 4.38 | Sinalbin | C14H19NO10S2 | 425.0450 | - | - | 424.0382 (424.0378) | 241.0014, 96.9599 [HSO4]−, 74.9904 [OH-N=C=S]− | + | + | + | tr |
| 12 | 8.40 | Glucotropaeolin | C14H19NO9S2 | 409.0501 | - | - | 408.0430 (408.0428) | 96.9593 [HSO4]−, 74.9901 [OH-N=C=S]− | + | ND | ND | + |
| 13 | 11.54 | Gluconasturtiin | C15H21NO9S2 | 423.0658 | - | - | 422.0589 (422.0585) | 259.0113, 96.9599 [HSO4]−, 74.9913 [OH-N=C=S]− | + | + | + | + |
| Indole glucosinolates [12] | ||||||||||||
| 14 | 6.05 | 4-Hydroxy-glucobrassicin | C16H20N2O10S2 | 464.0559 | - | - | 463.0496 (463.0487) | 285.0190, 259.0133, 221.0360, 96.9601 [HSO4]−, 74.9908 [OH-N=C=S]− | + | + | + | + |
| 15 | 9.65 | Glucobrassicin | C16H20N2O9S2 | 448.0610 | - | - | 447.0546 (447.0537) | 96.9606 [HSO4]−, 74.9910 [OH-N=C=S]− | + | tr | + | + |
| 16 | 12.10 | 4-Methoxy-glucobrassicin/Neoglucobrassicin | C17H22N2O10S2 | 478.0716 | - | - | 477.0657 (477.0643) | 96.9607 [HSO4]−, 74.9919 [OH-N=C=S]− | + | tr | + | + |
| Choline derivatives [13] | ||||||||||||
| 17 | 6.28 | Vanilloyl-choline-hexoside | C19H29NO9 | 415.1842 | 416.1914 (416.1915) * [M]+ | 223.1436, 194.1166, 138.0908, 118.0860 | - | - | + | + | + | + |
| 18 | 9.34 | Sinapoylcholine-hexoside | C22H34NO10+ | 472.2177 | 472.2179 (472.2177) [M]+ | 261.1310, 251.0914, 239.1489, 207.0651, 175.0387 | - | - | + | + | + | + |
| 19 | 9.59 | tr | tr | tr | tr | |||||||
| 20 | 11.30 | Benzoylcholine | C12H18NO2+ | 208.1338 | 208.1335 [M]+ | 149.0590 [M-NH(CH3)]+, 105.0330 [M-NH(CH3)-C2H4O]+, 77.00385 [M-NH(CH3)-C2H4O-CO]+ | - | - | + | + | + | + |
| 21 | 13.76 | Sinapine/sinapine isomers [14] | C16H24NO5+ | 310.1654 | 310.1654 [M]+ | 251.0906 [M-NH(CH3)]+, 207.0637 [M-NH(CH3)-C2H4O]+, 175.0382 [M-NH(CH3)-C2H4O-CH3OH]+, 147.0433 [M-NH(CH3)-C2H4O-CH3OH-CO]+, 119.0484 [M-NH(CH3)-C2H4O-CH3OH-2CO]+, 91.0536 [M-NH(CH3)-C2H4O-CH3OH-3CO]+ | - | - | ++ | ++ | ++ | ++ |
| 22 | 14.00 | + | + | + | + | |||||||
| 23 | 14.80 | + | + | + | + | |||||||
| 24 | 15.95 | Feruloyl choline furulyl ester | C25H34NO8+ | 476.2279 | 476.2276 (476.2279) [M]+ | 310.1641, 221.0803, 177.0541, 145.0279 | - | - | + | + | + | + |
| 25 | 16.62 | + | + | + | + | |||||||
| 26 | 18.94 | Sinapoyl choline feruloyl ester | C26H36NO9+ | 506.2390 | 506.2379 (506.2390) [M]+ | 251.0926, 207.0657, 175.0396, 147.0447, 104.1069 | - | - | + | + | + | + |
| 27 | 19.48 | + | + | + | + | |||||||
| Flavonoids [15,16] | ||||||||||||
| 28 | 10.40 | Kaempferol-sophoroside-glucoside | C33H40O21 | 772.2062 | 795.1941 (795.1954) [M+Na]+ | 611.1568 [M+H+Glc]+, 449.1059 [M+H+2Glc]+, 287.0530 [M+H+3Glc]+ | 771.1988 (771.1989) | 609.1471 [M-H-Glc]−, 447.0901 [M-H-2Glc]−, 285.0389 [M-H-3Glc]− | + | + | + | + |
| 29 | 14.14 | Kaempferol-(sinapoylglucoside)-sophoroside | C44H50O25 | 978.2641 | 1001.2539 (1001.2533) [M+Na]+ | 611.1604, 287.0558 | 977.2560 (977.2568) | 815.2058, 609.1506, 465.0731, 307.0863 | + | + | + | + |
| 30 | 19.03 | C44H50O25 | 978.2641 | 815.2058, 653.1535, 447.0937, 285.0382 | + | + | + | + | ||||
| 31 | 19.57 | C44H50O25 | 978.2641 | 815.2087, 609.1491, 545.0942, 285.0412 | + | tr | + | + | ||||
| 32 | 20.34 | Disinapoyl-gentiobiose | C34H42O19 | 754.2320 | 777.2216 (777.2213) [M+Na]+ | 553.1532, 411.1420, 207.0648 | 753.2243 (753.2248) | 547.1661, 205.0504 | + | + | + | + |
| 33 | 21.20 | Trisinapoyl-gentiobiose | C45H52O23 | 960.2899 | 983.2781 (983.2792) [M+Na]+ | 759.2098 [M+Na-Sinapic acid]+, 631.1443, 615.1676, 369.1187, 207.0654, 175.0392 | 959.2819 (959.2827) | 591.1734, 427.1225, 247.0612, 223.0620, 205.0509 | + | + | + | + |
| 34 | 21.60 | ND | ND | tr | + | |||||||
| 35 | 21.85 | + | + | tr | ||||||||
| Others | ||||||||||||
| 36 | 2.00 | Sucrose (Carbohydrate) | C12H22O11 | 342.1162 | - | - | 341.1089 (341.1095) | 179.0557, 161.0457, 119.0345, 89.0242, 59.0139 | + | + | + | + |
| 37 | 2.10 | Glutamyl-methionine sulfoxide (Dipeptide) | C10H18N2O6S | 294.0886 | 295.0957 (295.0958) | 133.0427, 104.1068 | - | - | + | + | + | + |
| 38 | 19.68 | Sinapoyl malic acid (HCA) [17] | C15H16O9 | 340.0794 | - | - | 339.0726 (339.0722) | 223.0610, 149.0239 | + | + | + | + |
| 39 | 19.32 | Cyclic (diferulic acid/spermidine) conjugate (Spermidine amide) [16] | C27H33N3O6 | 495.2369 | 496.2437 (496.2442) | 328.2470, 175.0382 | 494.2303 (494.2297) 530.2081 (530.2063) [M+Cl]- | - | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tileuberdi, N.; Katragunta, K.; Adams, S.J.; Aldana-Mejía, J.A.; Omarbekova, A.; Avula, B.; Khan, I.A.; Turgumbayeva, A.; Ross, S.A. Comprehensive Quality Assessment of Brassica napus L. Seeds via HPTLC, LC-QToF, and Anatomical Investigation. Molecules 2024, 29, 2965. https://doi.org/10.3390/molecules29132965
Tileuberdi N, Katragunta K, Adams SJ, Aldana-Mejía JA, Omarbekova A, Avula B, Khan IA, Turgumbayeva A, Ross SA. Comprehensive Quality Assessment of Brassica napus L. Seeds via HPTLC, LC-QToF, and Anatomical Investigation. Molecules. 2024; 29(13):2965. https://doi.org/10.3390/molecules29132965
Chicago/Turabian StyleTileuberdi, Nazym, Kumar Katragunta, Sebastian John Adams, Jennyfer A. Aldana-Mejía, Ardak Omarbekova, Bharathi Avula, Ikhlas A. Khan, Aknur Turgumbayeva, and Samir A. Ross. 2024. "Comprehensive Quality Assessment of Brassica napus L. Seeds via HPTLC, LC-QToF, and Anatomical Investigation" Molecules 29, no. 13: 2965. https://doi.org/10.3390/molecules29132965
APA StyleTileuberdi, N., Katragunta, K., Adams, S. J., Aldana-Mejía, J. A., Omarbekova, A., Avula, B., Khan, I. A., Turgumbayeva, A., & Ross, S. A. (2024). Comprehensive Quality Assessment of Brassica napus L. Seeds via HPTLC, LC-QToF, and Anatomical Investigation. Molecules, 29(13), 2965. https://doi.org/10.3390/molecules29132965








