Characterization and Performance of Peanut Shells in Caffeine and Triclosan Removal in Batch and Fixed-Bed Column Tests
Abstract
1. Introduction
2. Results and Discussion
2.1. Peanut Shell Characterization
2.1.1. Physicochemical Composition
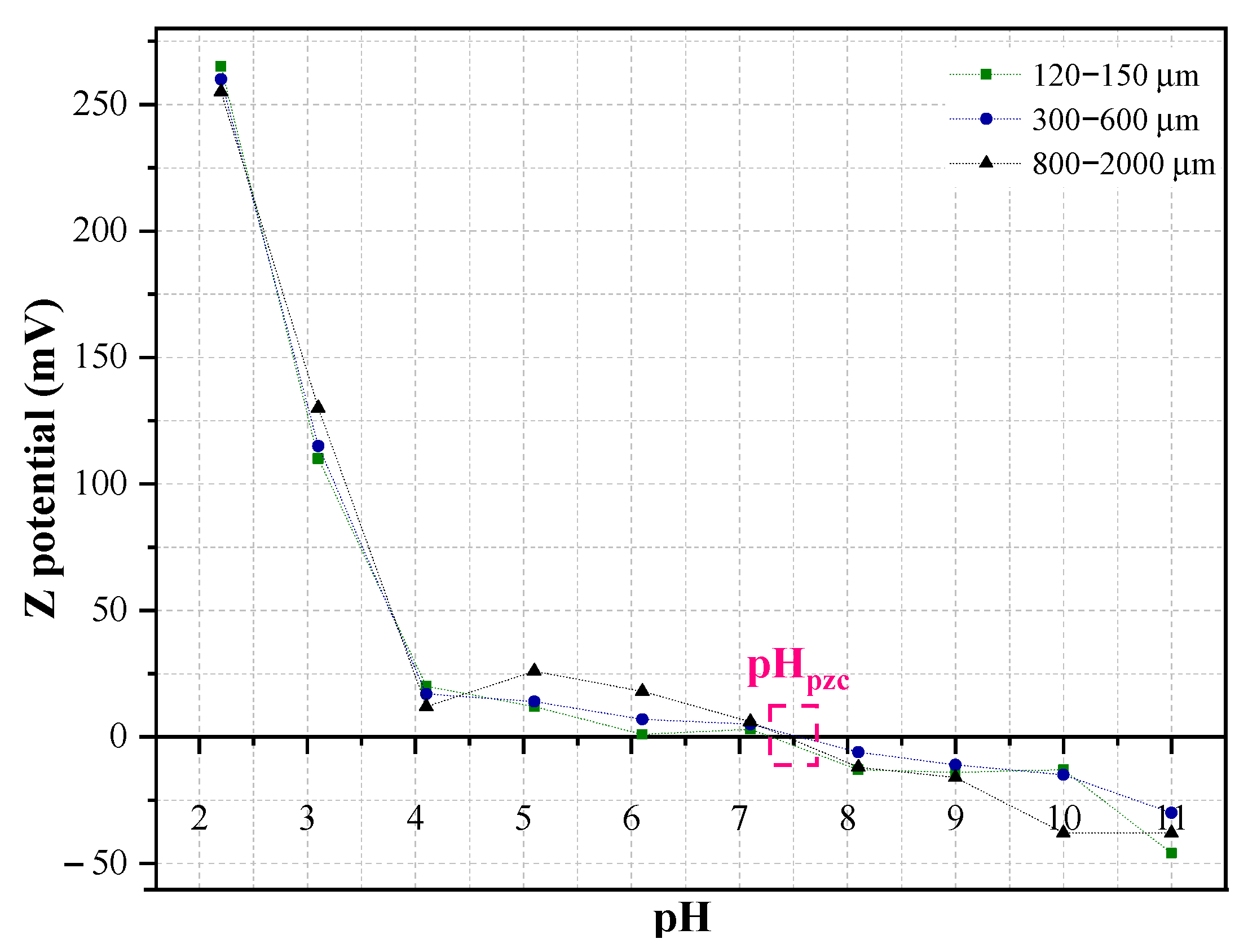
2.1.2. Point of Zero Charge
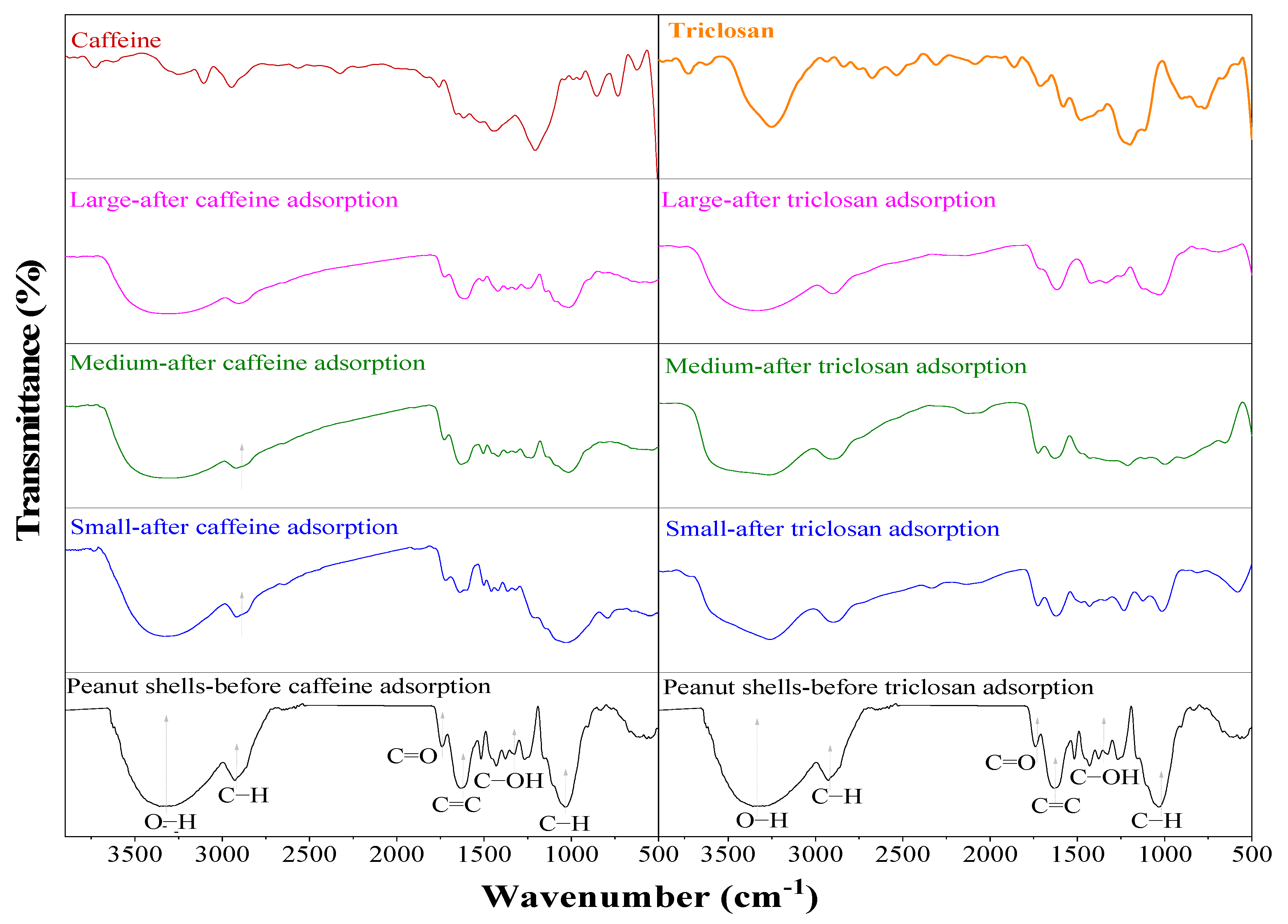
2.1.3. FTIR Analysis
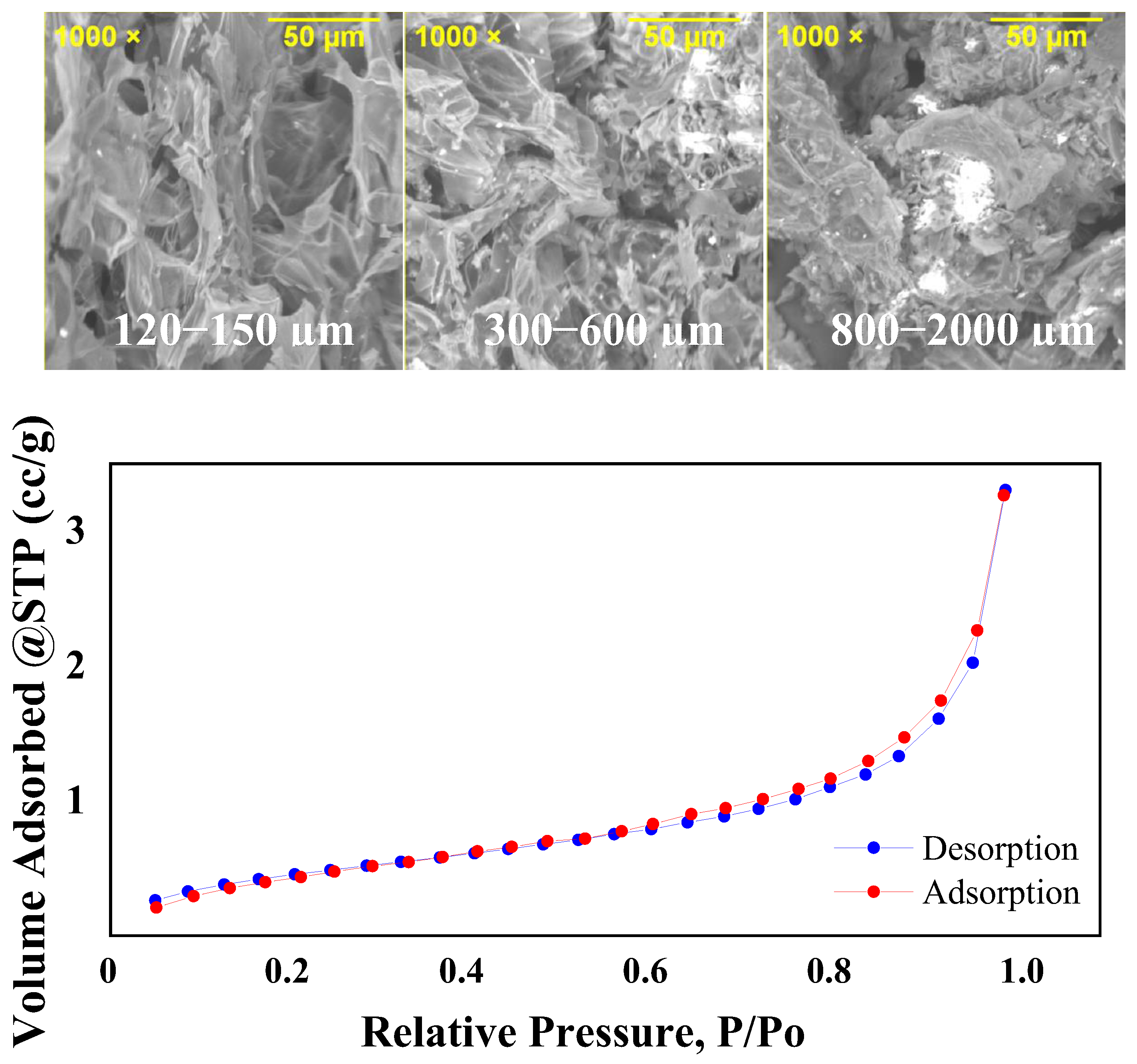
2.1.4. Morphological Characterization
2.2. Adsorption Tests
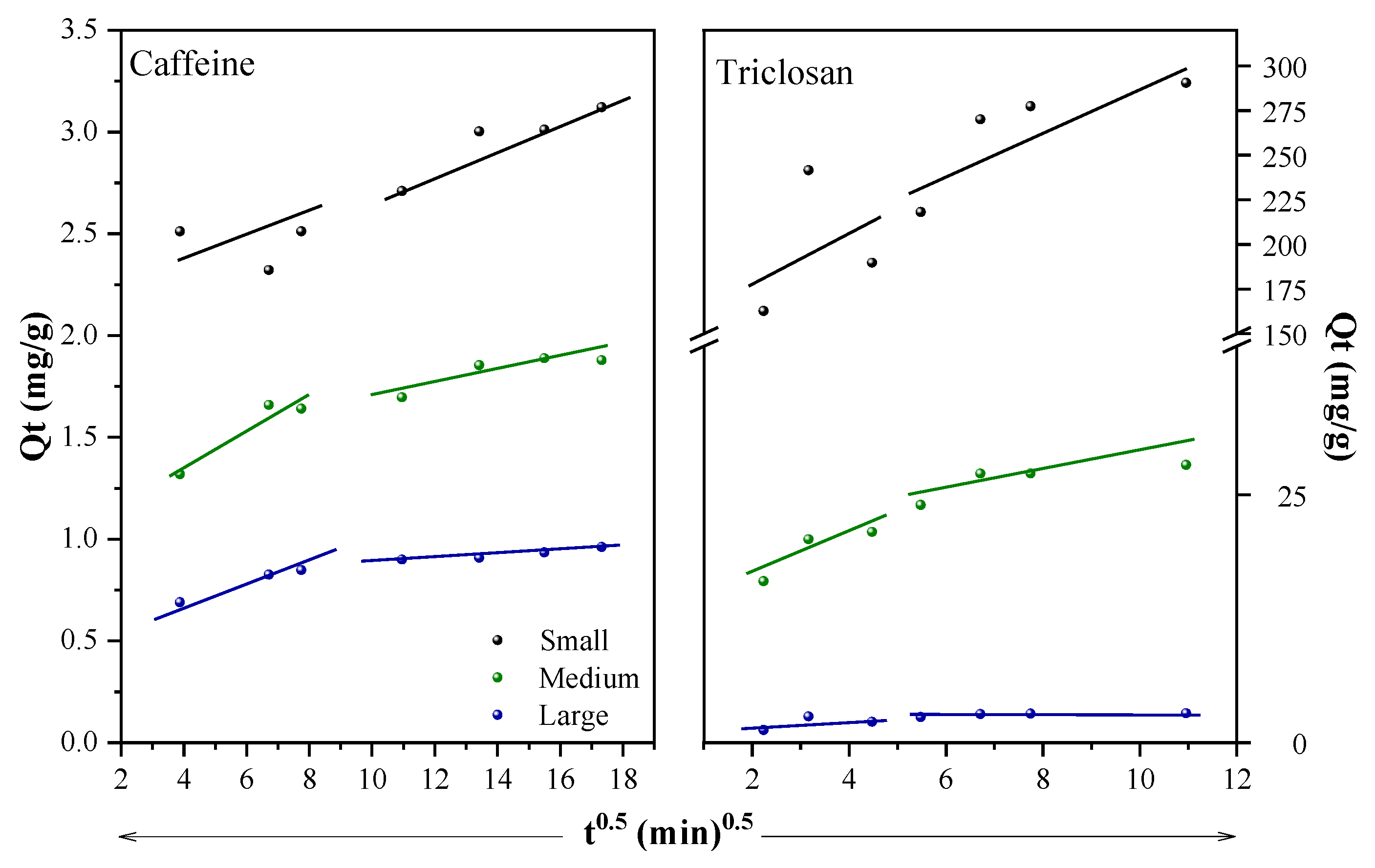
2.3. Adsorption Kinetics and Isotherm Models
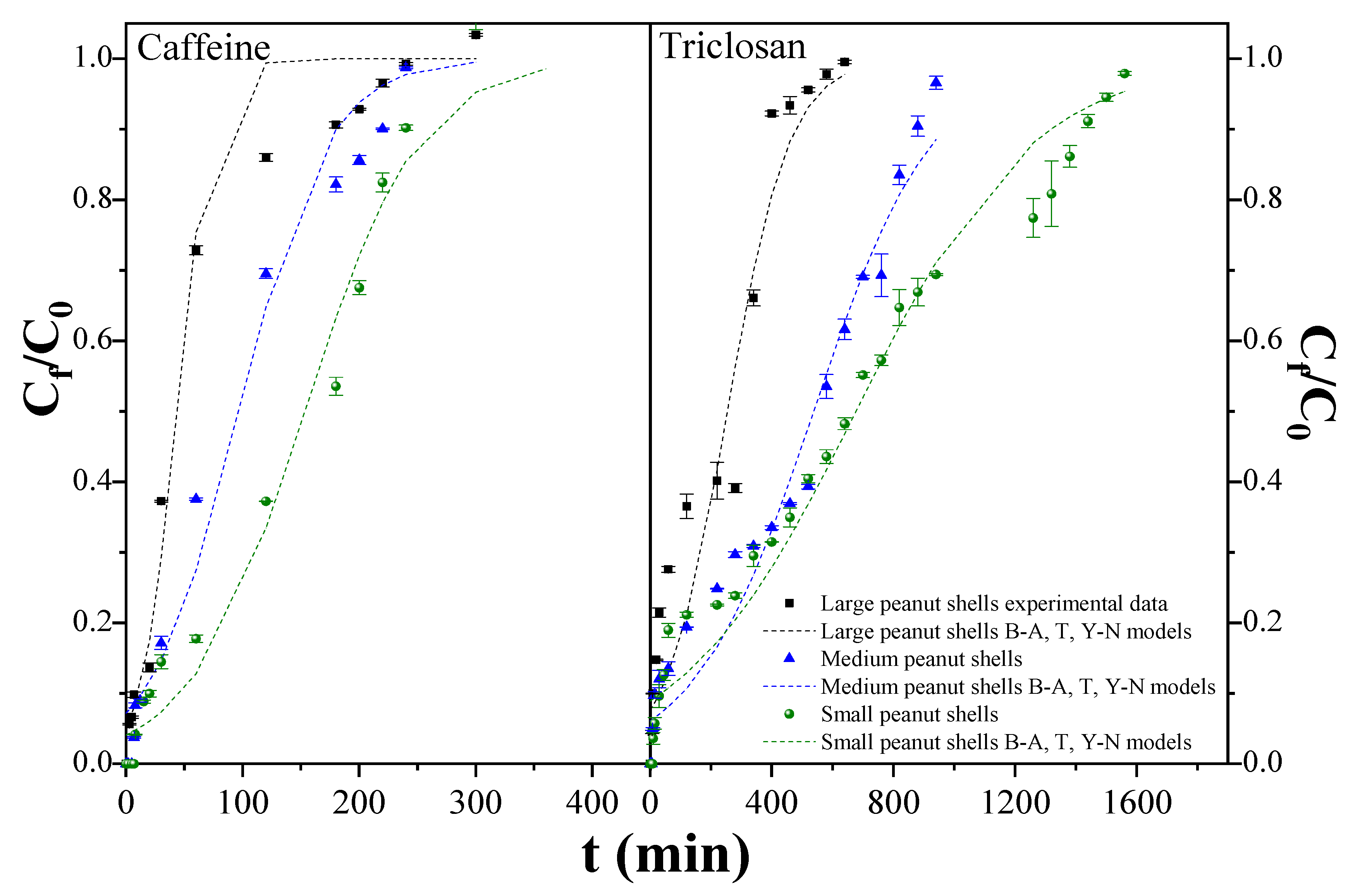
2.4. Fixed-Bed Columns
2.5. Alternatives for Saturated Peanut Shells
3. Materials and Methods
3.1. Materials
Peanut Shell Conditioning
3.2. Experimental Model
3.2.1. Batch Adsorption Tests
3.2.2. Fixed-Bed Column Tests
3.3. Analytical/Instrumental Methods
3.3.1. Peanut Shell Characterization
3.3.2. Caffeine and Triclosan Concentrations before/after Adsorption Tests
3.4. Data Analysis
3.4.1. Adsorption Kinetics and Isotherm Study
3.4.2. Breakthrough Curve Models
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almeida-Naranjo, C.E.; Guerrero, V.H.; Villamar-Ayala, C.A. Emerging Contaminants and Their Removal from Aqueous Media Using Conventional/Non-Conventional Adsorbents: A Glance at the Relationship between Materials, Processes and Technologies. Water 2023, 15, 1626. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Aldás, M.B.; Cabrera, G.; Guerrero, V.H. Caffeine Removal from Synthetic Wastewater Using Magnetic Fruit Peel Composites: Material Characterization, Isotherm and Kinetic Studies. Environ. Chall. 2021, 5, 100343. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment Technologies for Emerging Contaminants in Water: A Review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, J.; Jiang, Y.; Shao, Y. High Concentration and High Dose of Disinfectants and Antibiotics Used during the COVID-19 Pandemic Threaten Human Health. Environ. Sci. Eur. 2021, 33, 11. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Katsouromalli, A.; Pashalidis, I. Oxidized Biochar Obtained from Pine Needles as a Novel Adsorbent to Remove Caffeine from Aqueous Solutions. J. Mol. Liq. 2020, 304, 112661. [Google Scholar] [CrossRef]
- Triwiswara, M.; Lee, C.G.; Moon, J.K.; Park, S.J. Adsorption of Triclosan from Aqueous Solution onto Char Derived from Palm Kernel Shell. Desalination Water Treat. 2020, 177, 71–79. [Google Scholar] [CrossRef]
- Toniciolli Rigueto, V.C.; Torres, M.; Favretto, C.; Stefanello, J.; Barbosa, V.; Steffanelo, J. Alternative Techniques for Caffeine Removal from Wastewater: An Overview of Opportunities and Challenges. J. Water Process Eng. 2020, 35, 101231. [Google Scholar] [CrossRef]
- Cho, E.J.; Kang, J.K.; Moon, J.K.; Um, B.H.; Lee, C.G.; Jeong, S.; Park, S.J. Removal of Triclosan from Aqueous Solution via Adsorption by Kenaf-derived Biochar: Its Adsorption Mechanism Study via Spectroscopic and Experimental Approaches. J. Environ. Chem. Eng. 2021, 9, 106343. [Google Scholar] [CrossRef]
- Couto, O.M.; Matos, I.; da Fonseca, I.M.; Arroyo, P.A.; da Silva, E.A.; de Barros, M.A.S.D. Effect of Solution PH and Influence of Water Hardness on Caffeine Adsorption onto Activated Carbons. Can. J. Chem. Eng. 2015, 93, 68–77. [Google Scholar] [CrossRef]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A Review on Agro-Industrial Waste (AIW) Derived Adsorbents for Water and Wastewater Treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Frutos, M.; Tejedor, J.; Cuestas, J.; Valenzuela, F.; Rivadeneira, M.I.; Villamar, C.A.; Guerrero, V.H. Caffeine Adsorptive Performance and Compatibility Characteristics (Eisenia Foetida Savigny) of Agro-Industrial Residues Potentially Suitable for Vermifilter Beds. Sci. Total Environ. 2021, 801, 149666. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 12 April 2024).
- Li, R.; Zhang, Y.; Chu, W.; Chen, Z.; Wang, J. Adsorptive Removal of Antibiotics from Water Using Peanut Shells from Agricultural Waste. RSC Adv. 2018, 8, 13546–13555. [Google Scholar] [CrossRef]
- Taşar, Ş.; Kaya, F.; Özer, A. Biosorption of Lead(II) Ions from Aqueous Solution by Peanut Shells: Equilibrium, Thermodynamic and Kinetic Studies. J. Enviromental Chem. Eng. 2014, 2, 1018–1026. [Google Scholar] [CrossRef]
- Tanyildizi, M.S. Modeling of Adsorption Isotherms and Kinetics of Reactive Dye from Aqueous Solution by Peanut Hull. Chem. Eng. J. 2011, 168, 1234–1240. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Li, B. Adsorption of Hg2+ and Cd2+ by Ethylenediamine Modified Peanut Shells. Carbohydr. Polym. 2010, 81, 335–339. [Google Scholar] [CrossRef]
- Quesada, H.B.; de Araújo, T.P.; Cusioli, L.F.; de Barros, M.A.S.D.; Gomes, R.G.; Bergamasco, R. Caffeine Removal by Chitosan/Activated Carbon Composite Beads: Adsorption in Tap Water and Synthetic Hospital Wastewater. Chem. Eng. Res. Des. 2022, 184, 1–12. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortíz, A.; Ortega-Toro, R. Batch and Packed Bed Column Study for the Removal of Cr (Vi) and Ni (Ii) Using Agro-Industrial Wastes. Appl. Sci. 2021, 11, 9355. [Google Scholar] [CrossRef]
- Diniz, V.; Rath, S. Adsorption of Aqueous Phase Contaminants of Emerging Concern by Activated Carbon: Comparative Fixed-Bed Column Study and in Situ Regeneration Methods. J. Hazard. Mater. 2023, 459, 132197. [Google Scholar] [CrossRef]
- Campos, N.F.; Sales, D.C.S.; Rodríguez-Díaz, J.M.; Barbosa, C.M.B.M.; Duarte, M.M.M.B. Adsorption of Naphthenic Acids on Peanut Shell Activated Carbon: Batch and Fixed-Bed Column Study of the Process. Chem. Eng. Res. Des. 2022, 188, 633–644. [Google Scholar] [CrossRef]
- Messina, L.I.G.; Bonelli, P.R.; Cukierman, A.L. Copyrolysis of Peanut Shells and Cassava Starch Mixtures: Effect of the Components Proportion. J. Anal. Appl. Pyrolysis 2015, 113, 508–517. [Google Scholar] [CrossRef]
- Lochananon, W.; Chatsiriwech, D. Effect of Phosphoric Acid Concentration on Properties of Peanut Shell Adsorbents. J. Ind. Eng. Chem. 2008, 14, 84–88. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current Status, Occurrence, Environmental Risks and Bioaccumulation Potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684. [Google Scholar] [CrossRef]
- Ramírez Franco, J.H.; Enríquez Enríquez, M.K. Remoción de Plomo (II) Usando Lignina Obtenida a Partir Del Procesamiento Del Seudotallo de Plátano. Acta Agron. 2015, 64, 209–213. [Google Scholar] [CrossRef]
- .Al-Qodah, Z.; Yahya, M.A.; Al-Shannag, M. On the Performance of Bioadsorption Processes for Heavy Metal Ions Removal by Low-Cost Agricultural and Natural by-Products Bioadsorbent: A Review. Desalination Water Treat. 2017, 85, 339–357. [Google Scholar] [CrossRef]
- Pholosi, A.; Ofomaja, A.E.; Naidoo, E.B. Effect of Chemical Extractants on the Biosorptive Properties of Pine Cone Powder: Influence on Lead(II) Removal Mechanism. J. Saudi Chem. Soc. 2013, 17, 77–86. [Google Scholar] [CrossRef]
- Wang, S.; Nam, H.; Nam, H. Preparation of Activated Carbon from Peanut Shell with KOH Activation and Its Application for H2S Adsorption in Confined Space. J. Environ. Chem. Eng. 2020, 8, 103683. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of Sorbent Point Zero Charge: Usefulness in Sorption Studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Ali, I.; Imanova, G.T.; Albishri, H.M.; Alshitari, W.H.; Locatelli, M.; Siddiqui, M.N.; Hameed, A.M. An Ionic-Liquid-Imprinted Nanocomposite Adsorbent: Simulation, Kinetics and Thermodynamic Studies of Triclosan Endocrine Disturbing Water Contaminant Removal. Molecules 2022, 27, 5358. [Google Scholar] [CrossRef]
- Zhu, C.S.; Wang, L.P.; Chen, W. bin Removal of Cu(II) from Aqueous Solution by Agricultural by-Product: Peanut Hull. J. Hazard. Mater. 2009, 168, 739–746. [Google Scholar] [CrossRef]
- Wei, Q.; Li, X.; Zhang, J.; Hu, B.; Zhu, W.; Liang, W.; Sun, K. Full-Size Pore Structure Characterization of Deep-Buried Coals and Its Impact on Methane Adsorption Capacity: A Case Study of the Shihezi Formation Coals from the Panji Deep Area in Huainan Coalfield, Southern North China. J. Pet. Sci. Eng. 2019, 173, 975–989. [Google Scholar] [CrossRef]
- Sultan, M.; Miyazaki, T.; Koyama, S. Optimization of Adsorption Isotherm Types for Desiccant Air-Conditioning Applications. Renew. Energy 2018, 121, 441–450. [Google Scholar] [CrossRef]
- Santaeufemia, S.; Abalde, J.; Torres, E. Eco-Friendly Rapid Removal of Triclosan from Seawater Using Biomass of a Microalgal Species: Kinetic and Equilibrium Studies. J. Hazard. Mater. 2019, 369, 674–683. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Silva, M.G.C.; Vieira, M.G.A. Equilibrium and Kinetic Studies of Caffeine Adsorption from Aqueous Solutions on Thermally Modified Verde-Lodo Bentonite. Appl. Clay Sci. 2019, 168, 366–373. [Google Scholar] [CrossRef]
- Khoriha, N.; Mohd, E.; Hadibarata, T. Triclosan Removal by Adsorption Using Activated Carbon Derived from Waste Biomass: Isotherms and Kinetic Studies. J. Chem. Soc. Dalton Trans. 2018, 65, 951–959. [Google Scholar]
- Fakioğlu, M.; Kalpaklı, Y. Mechanism and Behavior of Caffeine Sorption: Affecting Factors. RSC Adv. 2022, 12, 26504–26513. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.; Hosseini-bandegharaei, A.; Chao, H. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J.C. Thermodynamic Study of Triclosan Adsorption from Aqueous Solutions on Activated Carbon: Modelling of Experimental Adsorption Isotherm and Calorimetry Data. J. Therm. Anal. Calorim. 2020, 139, 913–921. [Google Scholar] [CrossRef]
- Sharipova, A.A.; Aidarova, S.B.; Bekturganova, N.E.; Tleuova, A.; Schenderlein, M.; Lygina, O.; Lyubchik, S.; Miller, R. Triclosan as Model System for the Adsorption on Recycled Adsorbent Materials. Colloids Surf. A Physicochem. Eng. Asp. 2016, 505, 193–196. [Google Scholar] [CrossRef]
- Choudhary, V.; Philip, L. Sustainability Assessment of Acid-Modified Biochar as Adsorbent for the Removal of Pharmaceuticals and Personal Care Products from Secondary Treated Wastewater. J. Environ. Chem. Eng. 2022, 10, 107592. [Google Scholar] [CrossRef]
- Khezami, L.; Chetouani, A.; Taouk, B.; Capart, R. Production and Characterisation of Activated Carbon from Wood Components in Powder: Cellulose, Lignin, Xylan. Powder Technol. 2005, 157, 48–56. [Google Scholar] [CrossRef]
- Portinho, R.; Zanella, O.; Féris, L.A. Grape Stalk Application for Caffeine Removal through Adsorption. J. Environ. Manag. 2017, 202, 178–187. [Google Scholar] [CrossRef]
- Tomul, F.; Arslan, Y.; Kabak, B.; Tran, H.N.; Chi, H. Adsorption Process of Naproxen onto Peanut Shells-Derived Biosorbent: Important Role of n-π Interaction and van Der Waals Force. J. Chem. Technol. Biotechnol. 2020, 96, 869–880. [Google Scholar] [CrossRef]
- Mohd Khori, N.K.E.; Hadibarata, T.; Elshikh, M.S.; Al-Ghamdi, A.A.; Salmiati; Yusop, Z. Triclosan Removal by Adsorption Using Activated Carbon Derived from Waste Biomass: Isotherms and Kinetic Studies. J. Chin. Chem. Soc. 2018, 65, 951–959. [Google Scholar] [CrossRef]
- Triwiswara, M.; Kang, J.K.; Moon, J.K.; Lee, C.G.; Park, S.J. Removal of Triclosan from Aqueous Solution Using Thermally Treated Rice Husks. Desalination Water Treat. 2020, 202, 317–326. [Google Scholar] [CrossRef]
- Román, S.; Ledesma, B.; Álvarez, A.; Herdes, C. Towards Sustainable Micro-Pollutants’ Removal from Wastewaters: Caffeine Solubility, Self-Diffusion and Adsorption Studies from Aqueous Solutions into Hydrochars. Mol. Phys. 2018, 116, 2129–2141. [Google Scholar] [CrossRef]
- Al-shawabkeh, A.F.; Omar, W.; Hasseine, A.; Al-amayreh, M. Experimental study of the application of date palm trunk fiber as biosorbent for removal cadmium using a fixed bed column: Investigation of the influence of particle size. Desalination Water Treat. 2021, 223, 328–334. [Google Scholar] [CrossRef]
- Sivarajasekar, N.; Mohanraj, N.; Baskar, R.; Sivamani, S. Fixed-Bed Adsorption of Ranitidine Hydrochloride Onto Microwave Assisted—Activated Aegle Marmelos Correa Fruit Shell: Statistical Optimization and Breakthrough Modelling. Arab. J. Sci. Eng. 2018, 43, 2205–2215. [Google Scholar] [CrossRef]
- Iheanacho, O.C.; Nwabanne, J.T.; Obi, C.C.; Onu, C.E. Packed Bed Column Adsorption of Phenol onto Corn Cob Activated Carbon: Linear and Nonlinear Kinetics Modeling. S. Afr. J. Chem. Eng. 2021, 36, 80–93. [Google Scholar] [CrossRef]
- Nwabanne, J.T.; Iheanacho, O.C.; Obi, C.C.; Onu, C.E. Linear and Nonlinear Kinetics Analysis and Adsorption Characteristics of Packed Bed Column for Phenol Removal Using Rice Husk-Activated Carbon. Appl. Water Sci. 2022, 12, 91. [Google Scholar] [CrossRef]
- Hu, Q.; Xie, Y.; Zhang, Z. Modification of Breakthrough Models in a Continuous-Flow Fixed-Bed Column: Mathematical Characteristics of Breakthrough Curves and Rate Profiles. Sep. Purif. Technol. 2020, 238, 116399. [Google Scholar] [CrossRef]
- Omitola, O.B.; Abonyi, M.N.; Akpomie, K.G.; Dawodu, F.A. Adams-Bohart, Yoon-Nelson, and Thomas Modeling of the Fix-Bed Continuous Column Adsorption of Amoxicillin onto Silver Nanoparticle-Maize Leaf Composite. Appl. Water Sci. 2022, 12, 94. [Google Scholar] [CrossRef]
- Yanala, S.R.; Pagilla, K.R. Use of Biochar to Produce Reclaimed Water for Irrigation Use. Chemosphere 2020, 251, 126403. [Google Scholar] [CrossRef]
- Omorogie, M.O.; Babalola, J.O.; Unuabonah, E.I. Regeneration Strategies for Spent Solid Matrices Used in Adsorption of Organic Pollutants from Surface Water: A Critical Review. Desalination Water Treat. 2014, 57, 518–544. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, Regeneration and Sustainable Management of Spent Adsorbents from Wastewater Treatment Streams: A Review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A Comprehensive Review on the Chemical Regeneration of Biochar Adsorbent for Sustainable Wastewater Treatment. npj Clean. Water 2022, 5, 29. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Gallegos, E.; Domínguez, E.; Gutiérrez, P.; Valle, V.; Aguilar, A.D.; Debut, A.; Vasco, C. From Renewable Biomass to Water Purification Systems: Oil Palm Empty Fruit Bunch as Bio-Adsorbent for Domestic Wastewater Remediation and Methylene Blue Removal. Water 2023, 15, 4116. [Google Scholar] [CrossRef]
- Rial, J.B.; Ferreira, M.L. Potential Applications of Spent Adsorbents and Catalysts: Re-Valorization of Waste. Sci. Total Environ. 2022, 823, 153370. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Darío Aguilar, A.; Valle, V.; Report, S. A Circular Bioeconomy Approach for Post-Bioadsorbent Materials from the Removal of Domestic Wastewater Contaminants as Potential. Reinforcements 2024. [Google Scholar] [CrossRef]
- Sbardella, L.; Comas, J.; Fenu, A.; Rodriguez-Roda, I.; Weemaes, M. Advanced Biological Activated Carbon Filter for Removing Pharmaceutically Active Compounds from Treated Wastewater. Sci. Total Environ. 2018, 636, 519–529. [Google Scholar] [CrossRef]
- ASTM D4442-2020; Standard Test Methods for Direct Moisture Content Measurement of Wood and Wood-Based Materials. American Society for Testing Materials: West Conshohocken, PA, USA, 2020.
- ASTM D1110-21; Standard Test Methods for Water Solubility of Wood. American Society for Testing Materials: West Conshohocken, PA, USA, 2021.
- ASTM D1107-96-2021; Standard Test Methods for Ethanol-Toluene Solubility of Wood. American Society for Testing Materials: West Conshohocken, PA, USA, 2021.
- ASTM D1109-2022; Standard Test Method for 1 % Sodium Hydroxide Solubility of Wood. American Society for Testing Materials: West Conshohocken, PA, USA, 2022.
- ASTM D1106-2021; Standard Test Method for Acid-Insoluble Lignin in Wood. American Society for Testing Materials: West Conshohocken, PA, USA, 2021.
- ASTM D1102-84-2021; Standard Test Method for Ash in Wood. American Society for Testing Materials: West Conshohocken, PA, USA, 2021.
- ASTM E872-19; Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. American Society for Testing Materials: West Conshohocken, PA, USA, 2019.


| Parameter | Peanut Shells Composition (%) | TGA Analysis | ||
|---|---|---|---|---|
| A [22] | B [23] | Current Study | ||
| Humidity | 6.5 | - | 8.9 ± 0.1 | 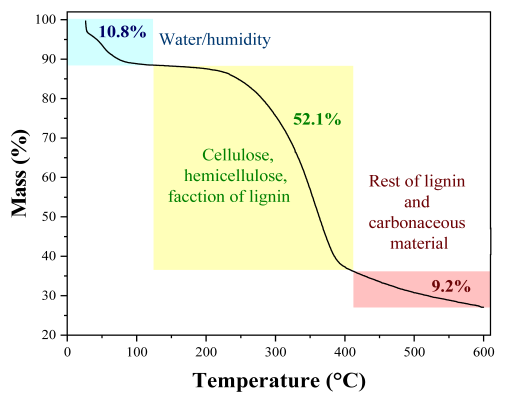 |
| Lignin | 30.9 | 31.6 | 36.2 ± 4.4 | |
| Cellulose | 54.6 | 34.7 | 19.4 ± 5.9 | |
| Hemicellulose | 14.5 | 9.0 | 26.1 ± 1.7 | |
| Extractives | - | 13.8 | 15.2 ± 0.4 | |
| Volatile material | 68.8 | 76.0 | 74.1 ± 0.4 | |
| Ash | 5.5 | 5.5 | 3.1 ± 0.1 | |
| Fixed carbon | 19.20 | - | 13.8 ± 0.4 | |
| Model | Caffeine | Triclosan | |||||
|---|---|---|---|---|---|---|---|
| Particle Size (µm) | |||||||
| 120–150 | 300–600 | 800–2000 | 120–150 | 300–600 | 800–2000 | ||
| qexp (mg/g) | 3.045 | 1.877 | 0.938 | 277.969 | 27.432 | 2.914 | |
| Pseudo-first-order | Qe (mg/g) | 2.993 | 1.782 | 0.901 | 255.350 | 25.863 | 2.907 |
| K1 (1/min) | 0.031 | 0.086 | 0.092 | 0.154 | 0.174 | 0.081 | |
| R2 | 0.868 | 0.757 | 0.772 | 0.509 | 0.737 | 0.927 | |
| SSE | 0.067 | 0.059 | 0.011 | 67.245 | 27.087 | 0.159 | |
| χ2 | 0.004 | 0.005 | 0.002 | 4.805 | 0.189 | 0.011 | |
| Pseudo-second order | Qe (mg/g) | 3.267 | 1.892 | 0.951 | 289.324 | 28.573 | 3.270 |
| K2 (g/(g min)) | 0.016 | 0.077 | 1.134 | 0.001 | 0.008 | 0.036 | |
| R2 | 0.964 | 0.929 | 0.961 | 0.766 | 0.918 | 0.969 | |
| SSE | 0.018 | 0.017 | 0.011 | 31.991 | 8.455 | 0.067 | |
| χ2 | 0.001 | 0.001 | 0.002 | 2.280 | 0.059 | 0.005 | |
| Elovich | α (g/(g min)) | 2.721 | 23.043 | 27.544 | 235.956 | 46.928 | 1.155 |
| Β mg/g | 2.421 | 5.501 | 11.924 | 0.022 | 0.246 | 1.685 | |
| R2 | 0.976 | 0.933 | 0.966 | 0.891 | 0.941 | 0.919 | |
| SSE | 0.008 | 0.069 | 9.420 × 10−4 | 87.988 | 7.110 | 0.051 | |
| χ2 | 0.003 | 0.003 | 0.342 × 10−4 | 3.729 | 1.515 | 0.044 | |
| Diffusion | Kp1 (mg/(g min1/2)) | −0.014 | 0.091 | 0.042 | 8.634 | 2.116 | 0.321 |
| C1 (mg/g) | 2.534 | 0.984 | 0.530 | 169.595 | 12.385 | 0.953 | |
| R2 | 0.866 | 0.907 | 0.984 | 0.583 | 0.783 | 0.449 | |
| SSE | 0.022 | 0.007 | 2.376 × 10−4 | 3.029 | 3.129 | 0.683 | |
| Kp2 (mg/(g min1/2)) | 0.060 | 0.029 | 0.010 | 11.083 | 0.603 | 0.059 | |
| C2 (mg/g) | 2.106 | 1.420 | 0.786 | 178.469 | 21.913 | 2.384 | |
| R2 | 0.867 | 0.7557 | 0.931 | 0.669 | 0.631 | 0.594 | |
| SSE | 0.012 | 0.006 | 1.605 × 10−4 | 1.004 | 3.510 | 0.039 | |
| Model | Caffeine | Triclosan | |||||
|---|---|---|---|---|---|---|---|
| Particle Size (µm) | |||||||
| 120–150 | 300–600 | 800–2000 | 120–150 | 300–600 | 800–2000 | ||
| qexp (mg/g) | 3.649 | 2.293 | 0.993 | 238.906 | 31.849 | 3.882 | |
| Langmuir | qmax (mg/g) | 3.522 | 3.147 | 1.490 | 253.559 | 32.049 | 4.041 |
| KL (L/mg) | 2.619 | 0.289 | 0.139 | 1.317 | 1.396 | 0.707 | |
| R2 | 0.838 | 0.993 | 0.977 | 0.947 | 0.838 | 0.959 | |
| SSE | 0.186 | 0.012 | 0.014 | 9.627 | 6.338 | 0.100 | |
| χ2 | 0.016 | 0.001 | 0.005 | 0.995 | 0.548 | 0.008 | |
| Freundlich | KF ((mg/g)/ (mg/L))n | 2.370 | 0.804 | 0.225 | 145.638 | 18.328 | 1.846 |
| 1/n | 0.196 | 0.492 | 0.582 | 0.176 | 0.168 | 0.280 | |
| R2 | 0.976 | 0.980 | 0.995 | 0.719 | 0.561 | 0.985 | |
| SSE | 0.028 | 0.033 | 0.003 | 510.919 | 17.161 | 0.036 | |
| χ2 | 0.002 | 0.004 | 0.001 | 5.288 | 1.490 | 0.003 | |
| Sips | qmax (mg/g) | 6.899 | 3.273 | 1.978 | 238.975 | 29.647 | 7.376 |
| KS (L/mg) | 0.141 | 0.264 | 0.072 | 1.359 | 1.585 | 0.083 | |
| 1/n | 0.325 | 0.959 | 0.802 | 1.737 | 3.645 | 0.456 | |
| R2 | 0.958 | 0.993 | 0.987 | 0.997 | 0.973 | 0.989 | |
| SSE | 0.049 | 0.012 | 0.008 | 5.335 | 1.072 | 0.028 | |
| χ2 | 0.004 | 0.001 | 0.003 | 0.055 | 0.094 | 0.002 | |
| Parameter | Contaminants | ||||||
|---|---|---|---|---|---|---|---|
| Particle Size (µm) | |||||||
| Caffeine | Triclosan | ||||||
| 120–150 | 300–600 | 800–2000 | 120–150 | 300–600 | 800–2000 | ||
| Mass (g) | 0.80 | 1.40 | 1.43 | 0.80 | 1.40 | 1.43 | |
| Vc (L) | 3.14 × 10−3 | ||||||
| EBTC (day) | 0.44 | ||||||
| FBU (%) | 77.41 | 50.81 | 37.42 | 47.86 | 36.52 | 23.76 | |
| hMTZ (cm) | 0.90 | 1.97 | 2.50 | 1.14 | 2.54 | 3.05 | |
| C/C0 = 0.1 | tb (min) | 20.20 | 12.20 | 7.00 | 30.20 | 15.20 | 8.20 |
| Vb (mL) | 10.10 | 6.10 | 3.50 | 15.10 | 7.60 | 4.10 | |
| qb (mg/g) | 0.26 | 0.16 | 0.07 | 0.80 | 0.27 | 0.12 | |
| C/C0 = 0.9 | ts (min) | 240.20 | 220.20 | 180.2 | 1440.20 | 880.20 | 400.20 |
| qs (mg/g) | 0.33 | 0.31 | 0.19 | 1.12 | 0.74 | 0.49 | |
| Model | Contaminants | ||||||
|---|---|---|---|---|---|---|---|
| Caffeine | Triclosan | ||||||
| Particle Size (µm) | |||||||
| 120–150 | 300–600 | 800–2000 | 120–150 | 300–600 | 800–2000 | ||
| Bohart–Adams | KAB (L/(min-mg)) | 6.361 × 10−4 | 8.184 × 10−4 | 2.072 × 10−3 | 7.655 × 10−5 | 1.134 × 10−4 | 2.193 × 10−4 |
| N0 (mg/L) | 700.941 | 442.586 | 197.856 | 4293.136 | 3411.516 | 1612.556 | |
| Thomas | q0 (mg/g) | 0.716 | 0.453 | 0.203 | 143.438 | 2.511 | 1.187 |
| kTH (mg/min) | 1.919 | 2.475 | 6.335 | 0.006 | 0.475 | 0.920 | |
| Yoon–Nelson | kYN (min−1) | 0.020 | 0.026 | 0.068 | 0.003 | 0.005 | 0.010 |
| Τ (min) | 153.751 | 97.152 | 43.427 | 677.226 | 538.153 | 254.545 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida-Naranjo, C.E.; Frutos, M.; Guerrero, V.H.; Villamar-Ayala, C. Characterization and Performance of Peanut Shells in Caffeine and Triclosan Removal in Batch and Fixed-Bed Column Tests. Molecules 2024, 29, 2923. https://doi.org/10.3390/molecules29122923
Almeida-Naranjo CE, Frutos M, Guerrero VH, Villamar-Ayala C. Characterization and Performance of Peanut Shells in Caffeine and Triclosan Removal in Batch and Fixed-Bed Column Tests. Molecules. 2024; 29(12):2923. https://doi.org/10.3390/molecules29122923
Chicago/Turabian StyleAlmeida-Naranjo, Cristina E., Mayra Frutos, Victor H. Guerrero, and Cristina Villamar-Ayala. 2024. "Characterization and Performance of Peanut Shells in Caffeine and Triclosan Removal in Batch and Fixed-Bed Column Tests" Molecules 29, no. 12: 2923. https://doi.org/10.3390/molecules29122923
APA StyleAlmeida-Naranjo, C. E., Frutos, M., Guerrero, V. H., & Villamar-Ayala, C. (2024). Characterization and Performance of Peanut Shells in Caffeine and Triclosan Removal in Batch and Fixed-Bed Column Tests. Molecules, 29(12), 2923. https://doi.org/10.3390/molecules29122923








