Enhanced Enzymatic Performance of Immobilized Pseudomonas fluorescens Lipase on ZIF-8@ZIF-67 and Its Application to the Synthesis of Neryl Acetate with Transesterification Reaction
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Preparation Conditions for PFL@ZIF-8@ZIF-67
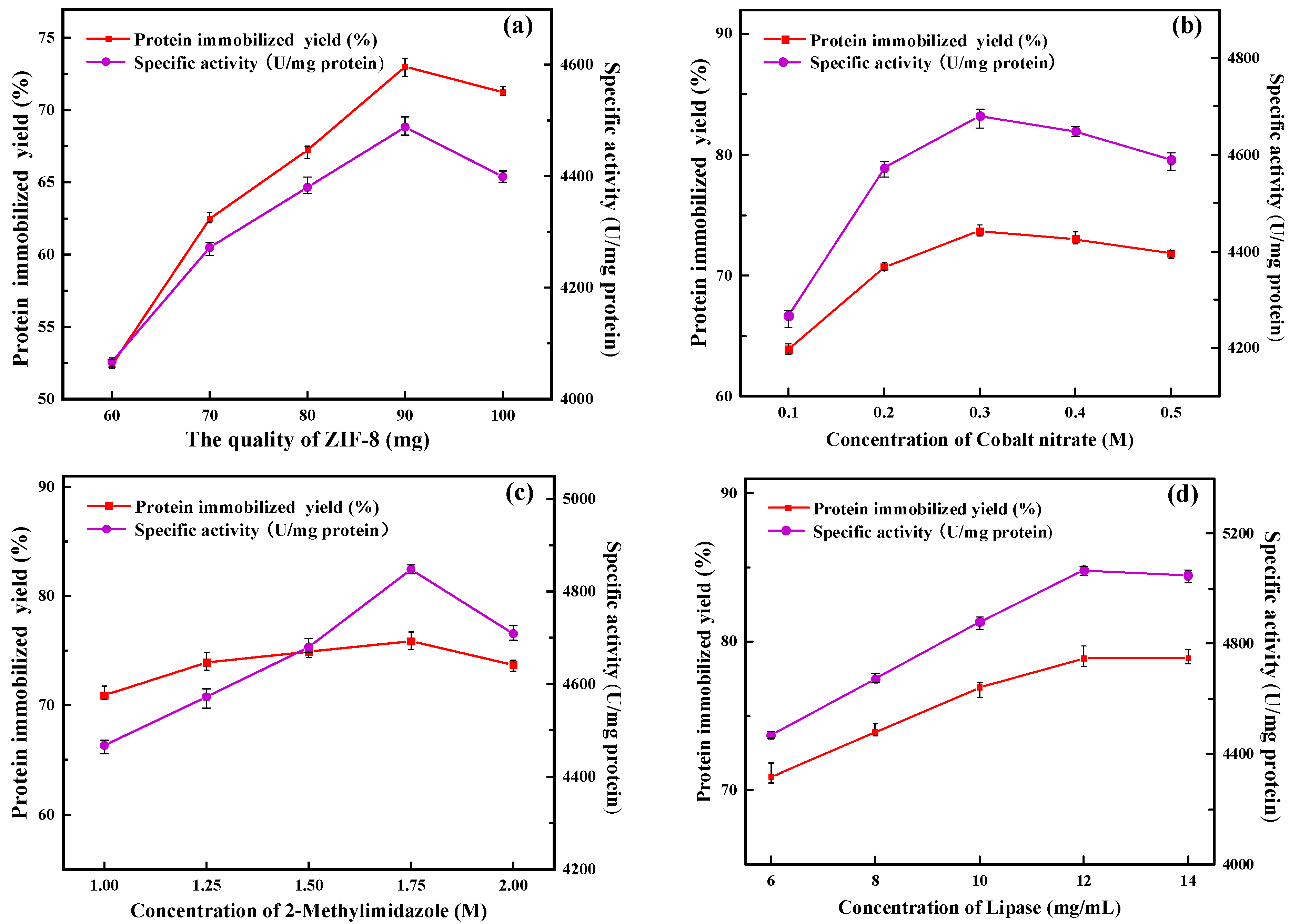
2.1.1. Amount of ZIF-8
2.1.2. Concentration of Cobalt Nitrate
2.1.3. Concentration of 2-Methylimidazole
2.1.4. Concentration of Lipase
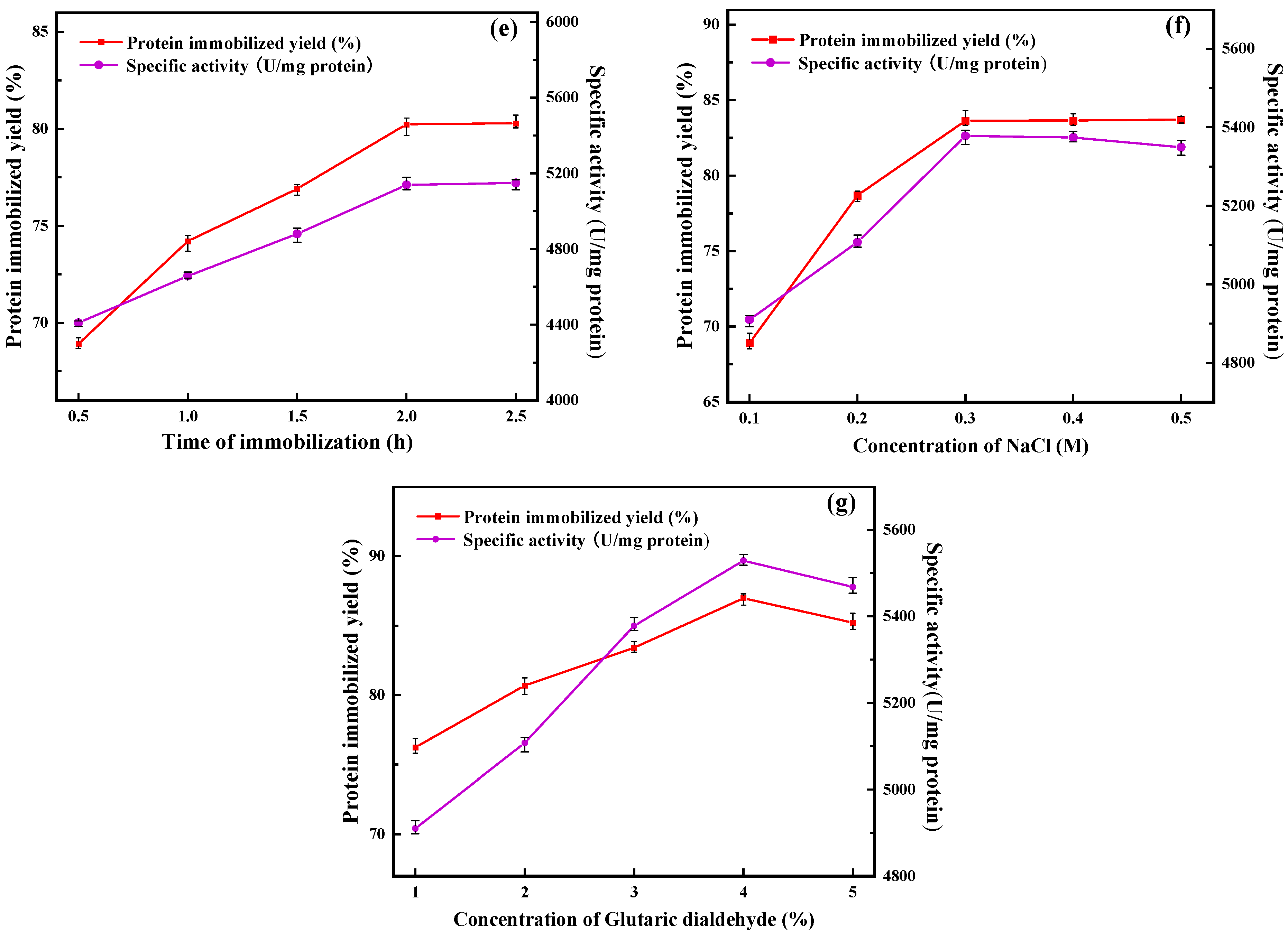
2.1.5. Immobilization Time
2.1.6. Concentration of Sodium Chloride (NaCl)
2.1.7. Concentration of Glutaraldehyde
2.2. Characterization and Kinetics Parameter of PFL@ZIF-8@ZIF-67
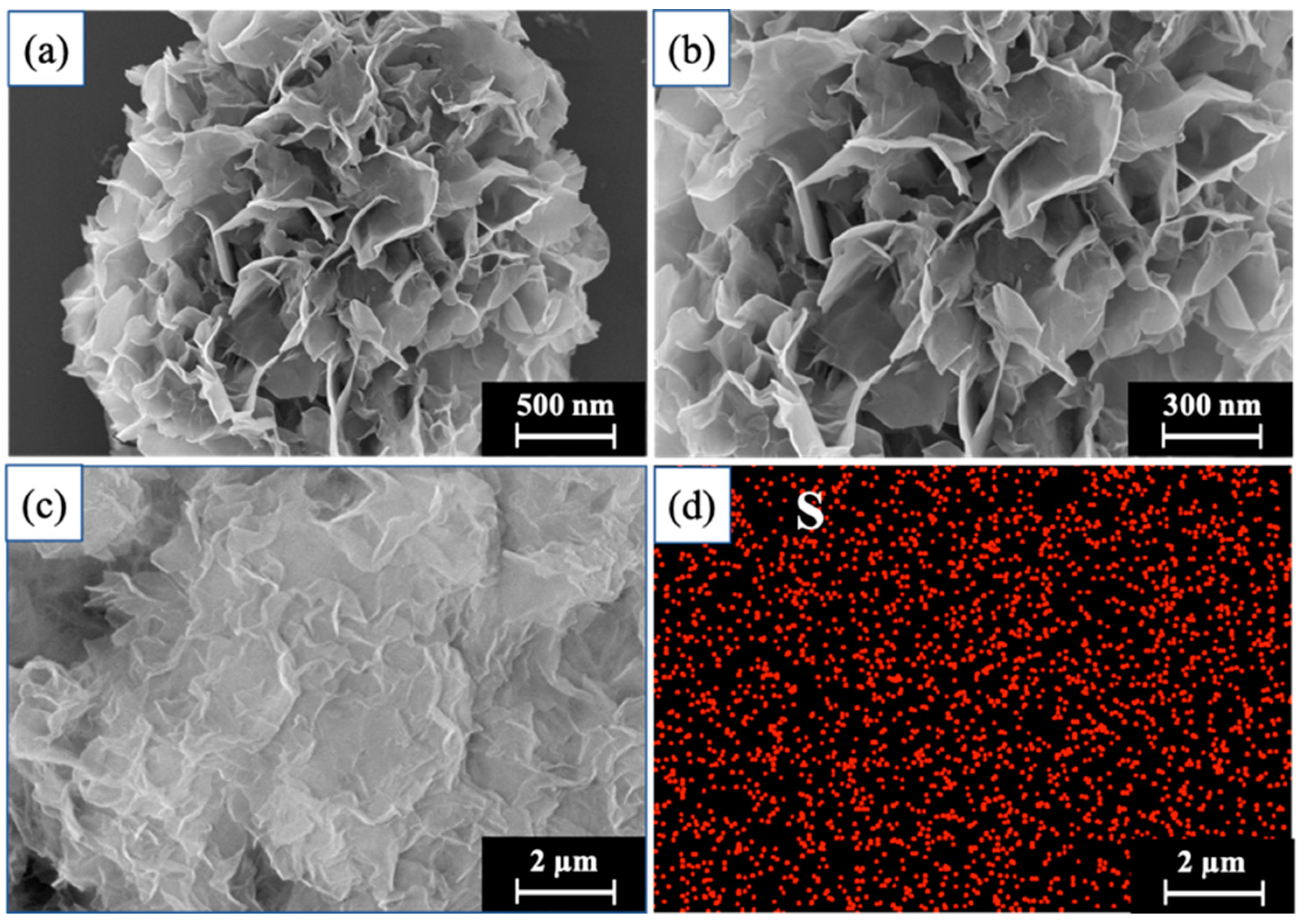
2.2.1. SEM Analysis
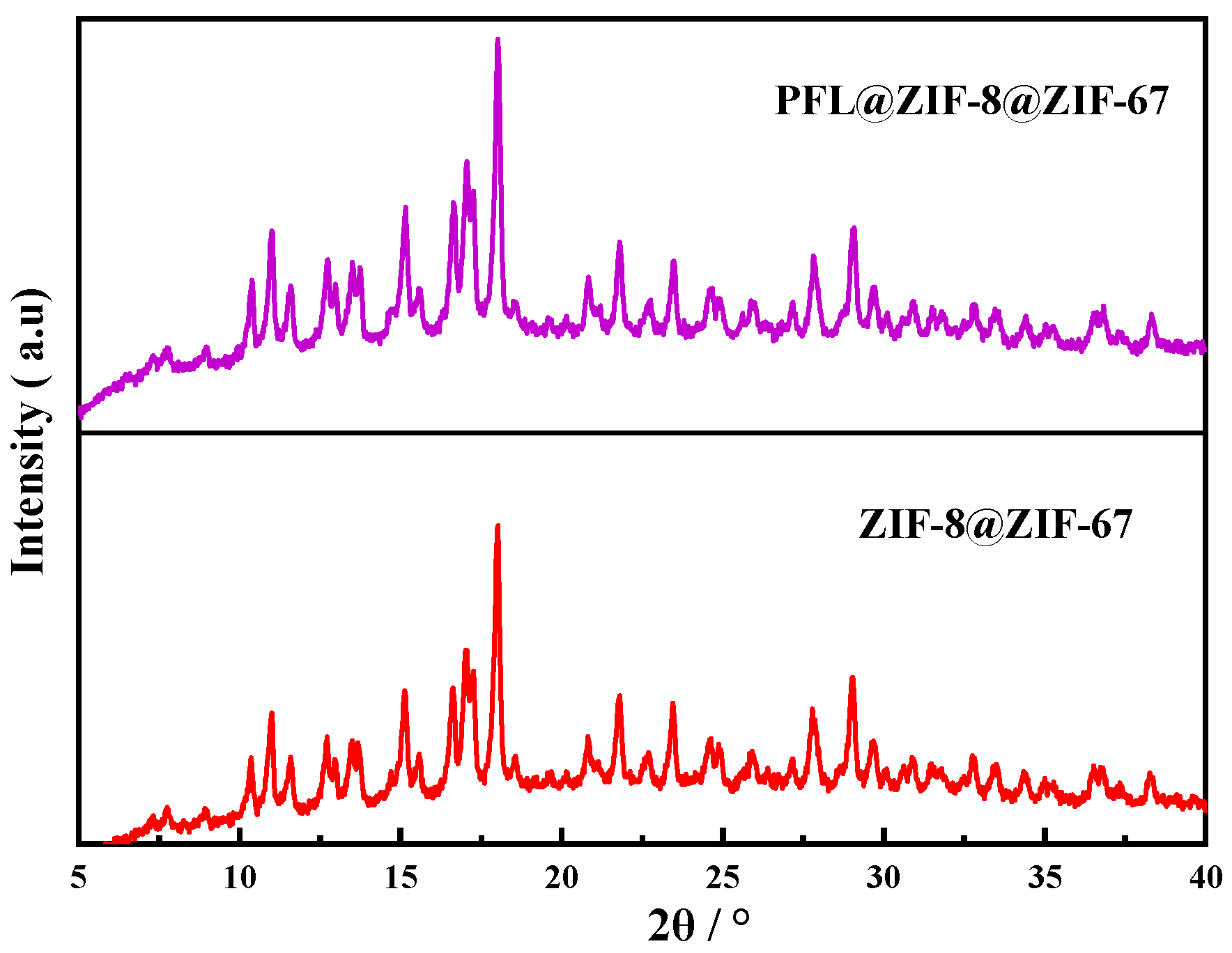
2.2.2. XRD Analysis
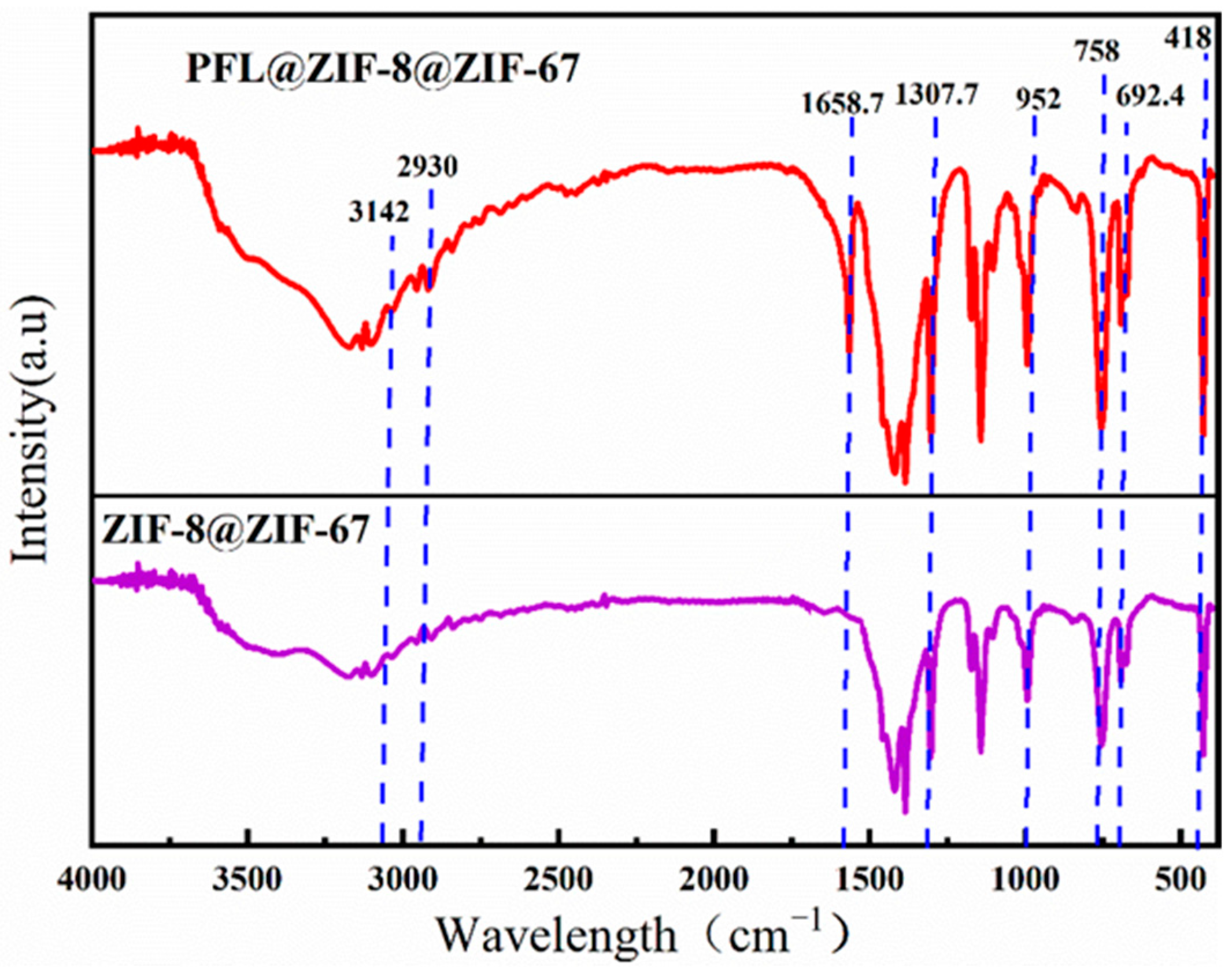
2.2.3. FT-IR Analysis Analysis
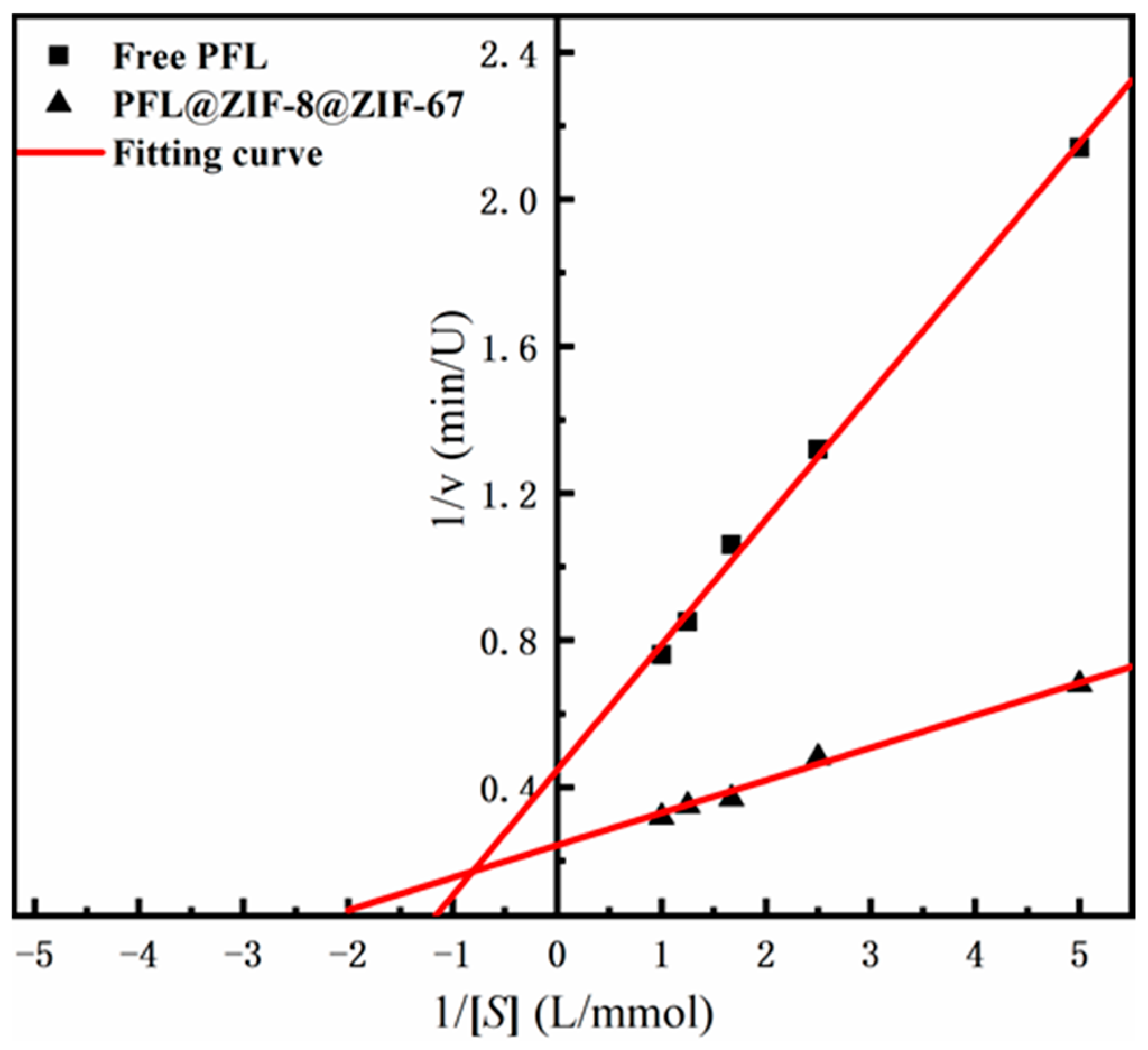
2.2.4. Michaelis–Menten Kinetic Parameters of PFL@ZIF-8@ZIF-67
2.3. Application of PFL@ZIF-8@ZIF-67 in the Synthesis of Neryl Acetate
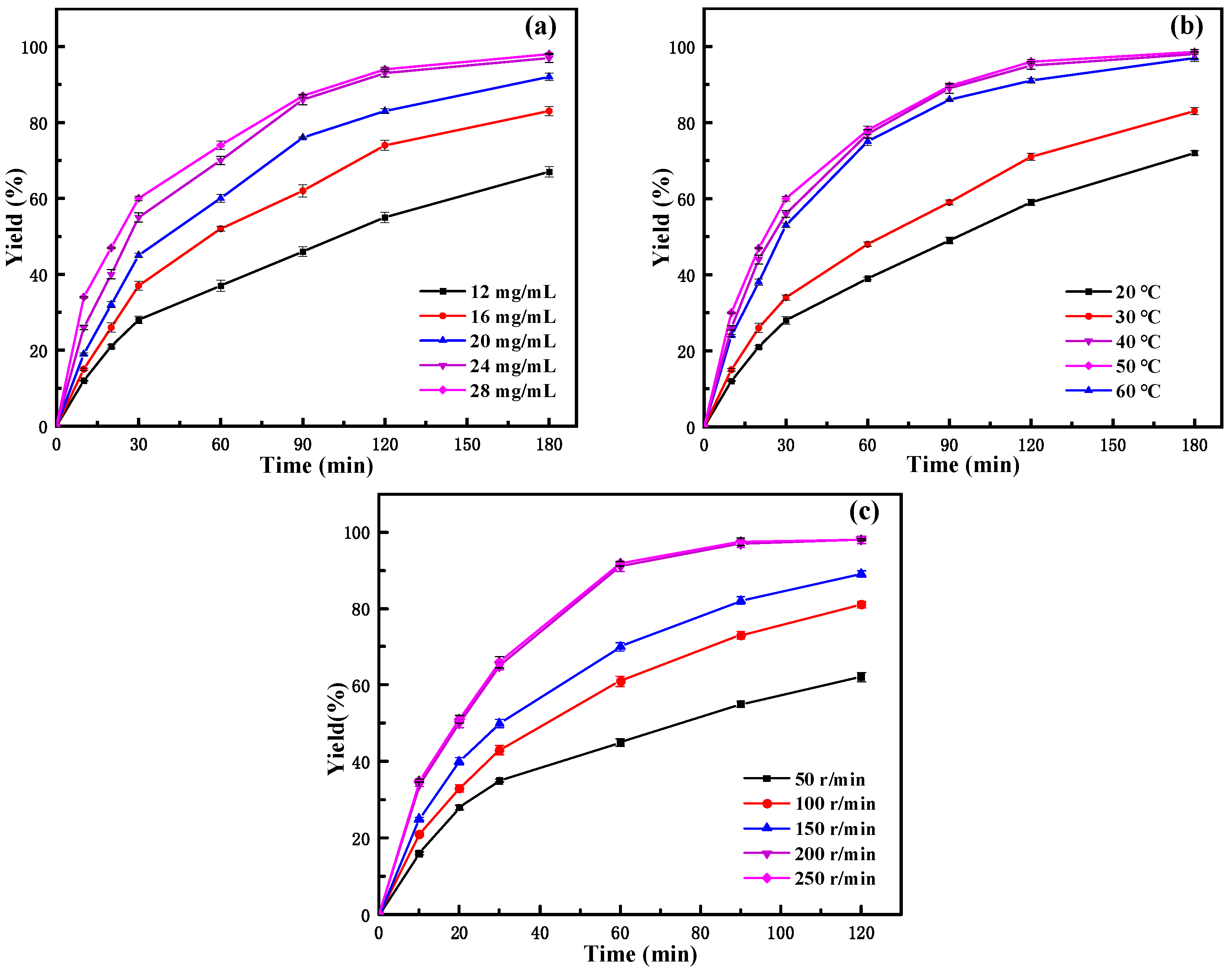
2.3.1. Effect of Immobilized Lipase Amount
2.3.2. Effect of Reaction Temperature
2.3.3. Effect of Rotating Speed
2.4. Stability of PFL@ZIF-8@ZIF-67
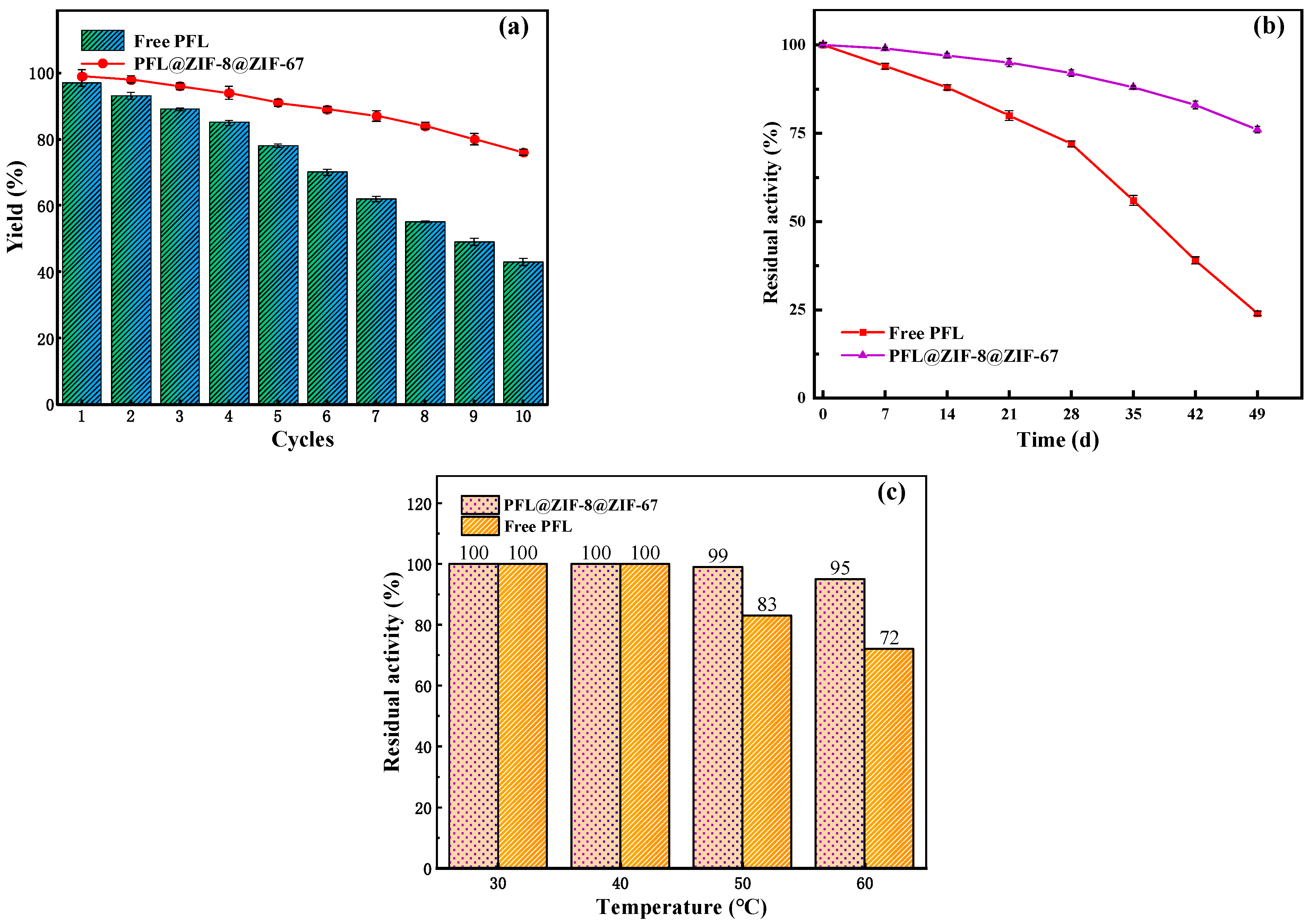
2.4.1. Reusability of PFL@ZIF-8@ZIF-67
2.4.2. Storage Stability of PFL@ZIF-8@ZIF-67
2.4.3. Thermal Stability of PFL@ZIF-8@ZIF-67
3. Materials and Methods
3.1. Experimental Material
3.2. Preparation of PFL@ZIF-8@ZIF-67
3.3. Determination of Protein and Enzyme Activity
3.4. Application of PFL@ZIF-8@ZIF-67 in Synthesis of Neryl Acetate
3.5. Analysis Method of Neryl Acetate
3.6. Characterization
3.7. Kinetics Analysis
3.8. Reusability
3.9. Thermal Stability
3.10. Storage Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Pan, Q.S.; Qian, X.K.; Zhou, X.L.; Wang, Y.J.; He, R.J.; Wang, L.T.; Li, Y.R.; Huo, H.; Sun, C.G.; et al. Discovery of triterpenoids as potent dual inhibitors of pancreatic lipase and human carboxylesterase 1. J. Enzym. Inhib. Med. Chem. 2022, 37, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, S.A.; Hamayun, M.; Lee, I.-J. The recent advances in the utility of microbial lipases: A review. Microorganisms 2023, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Raffee, F.; Rezaee, M. Different strategies for the lipase immobilization on the chitosan based supports and their applications. Int. J. Biol. Macromol. 2021, 179, 170–195. [Google Scholar] [CrossRef] [PubMed]
- Facin, B.R.; Melchiors, M.S.; Valerio, A.; Oliveira, J.V.; de Oliveira, D. Driving immobilized lipases as biocatalysts: 10 years state of the art and future prospects. Ind. Eng. Chem. Res. 2019, 58, 5358–5378. [Google Scholar] [CrossRef]
- Salgado, C.A.; dos Santos, C.I.A.; Vanetti, M.C.D. Microbial lipases: Propitious biocatalysts for the food industry. Food Biosci. 2022, 45, 101509. [Google Scholar] [CrossRef]
- Verger, R. ‘Interfacial activation’ of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Schmid, R.R.; Verger, D. Lipases: Interfacial enzymes with attractive applications. Angew. Chem. Int. Ed. 1998, 37, 1608–1633. [Google Scholar] [CrossRef]
- Kim, K.K.; Song, H.K.; Shin, D.H.; Hwang, K.Y.; Suh, S.W. The crystal structure of a triacylglycerol lipase from Pseudomonas cepacia reveals a highly open conformation in the absence of a bound inhibitor. Structure 1997, 5, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.P.; Zhi, G.Y.; Han, L.; Chen, Q.; Zhang, D.H. Biosynthesis of benzyl cinnamate using an efficient immobilized lipase entrapped in nano-molecular cages. Food Chem. 2021, 364, 130428. [Google Scholar] [CrossRef]
- Remonatto, D.; Miotti, R.H.; Monti, R.; Bassan, J.C.; de Paula, A.V. Applications of immobilized lipases in enzymatic reactors: A review. Process Biochem. 2022, 114, 1–20. [Google Scholar] [CrossRef]
- Arana-Pena, S.; Carballares, D.; Morellon-Sterling, R.; Rocha-Martin, J.; Fernandez-Lafuente, R. The combination of covalent and ionic exchange immobilizations enables the coimmobilization on vinyl sulfone activated supports and the reuse of the most stable immobilized enzyme. Int. J. Biol. Macromol. 2022, 199, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Holla, V.; Karkeszova, K.; Antosova, M.; Polakovic, M. Transglycosylation properties of a Kluyveromyces lactis enzyme preparation: Production of tyrosol β-fructoside using free and immobilized enzyme. Process Biochem. 2021, 110, 168–175. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.R.; Zhang, X.Y.; Gu, Z.; Wu, G.L. MOF-derived Ni-Co bimetal/porous carbon composites as electromagnetic wave absorber. Adv. Compos. Hybrid. Mater. 2022, 6, 28. [Google Scholar] [CrossRef]
- Ren, M.Z.; Zhang, Y.J.; Yu, L.L.; Qu, L.B.; Li, Z.H.; Zhang, L. A Co-based MOF as nanozyme with enhanced oxidase-like activity for highly sensitive and selective colorimetric differentiation of aminophenol isomers. Talanta 2022, 255, 124291. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.Z.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.L.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.C. Enzyme-MOF (metal-organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.H.; Shim, H.S.; Ha, J.; Moon, H.R. MOF-on-MOF Architectures: Applications in Separation, Catalysis, and Sensing. Bull. Korean Chem. Soc. 2021, 42, 956–969. [Google Scholar] [CrossRef]
- Mao, J.W.; Meng, Q.; Xu, Z.H.; Xu, L.S.; Fan, Z.; Zhang, G.L. MOF-on-MOF heterojunction-derived Co3O4-CuCo2O4 microflowers for low-temperature catalytic oxidation. Chem. Commun. 2022, 58, 13600–13603. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, X.M.; Xue, W.J.; Nie, L.; Huang, H.L.; Zhong, C.L. Engineering the direct Z-scheme systems over lattice intergrown of MOF-on-MOF for selective CO2 photoreduction to CO. AIChE J. 2022, 69, e17906. [Google Scholar] [CrossRef]
- Yao, M.S.; Xiu, J.W.; Huang, Q.Q.; Li, W.H.; Wu, W.W.; Wu, A.Q.; Cao, L.A.; Deng, W.H.; Wang, G.E.; Xu, G. Van der waals heterostructured MOF-on-MOF thin films: Cascading functionality to realize advanced chemiresistive sensing. Angew. Chem. Int. Ed. 2019, 58, 4915–14919. [Google Scholar]
- Xiao, L.L.; Zheng, S.Y.; Yang, K.X.; Duan, J.X.; Jiang, J. The construction of CoP nanoparticles coated with carbon layers derived from core-shell bimetallic MOF for electrochemical detection of dopamine. Microchem. J. 2021, 168, 106432. [Google Scholar] [CrossRef]
- Cao, Q.; Xiao, Y.S.; Liu, N.; Huang, R.; Ye, C.; Huang, C.H.; Liu, H.; Han, G.; Wu, L.D. Synthesis of Yolk/Shell Heterostructures MOF@MOF as Biomimetic Sensing Platform for Catechol Detection. Sens. Actuators B 2020, 370, 132503. [Google Scholar] [CrossRef]
- Zeferino, R.C.F.; Piaia, V.A.A.; Orso, V.T.; Pinheiro, V.M.; Zanetti, M.; Colpani, G.L.; Padoin, N.; Soares, C.; Fiori, M.A.; Riella, H.G. Neryl acetate synthesis from nerol esterification with acetic anhydride by heterogeneous catalysis using ion exchange resin. J. Ind. Eng. Chem. 2022, 105, 121–131. [Google Scholar] [CrossRef]
- Singh, A.P.; Arya, H.; Singh, V.; Kumar, P.; Gautam, H.K. Identification of natural inhibitors to inhibit C. acnes lipase through docking and simulation studies. J. Mol. Model. 2022, 28, 281. [Google Scholar] [CrossRef] [PubMed]
- Parreira, L.A.; Azevedo, A.F.; Menini, L.; Gusevskaya, E.V. Functionalization of the naturally occurring linalool and nerol by the palladium catalyzed oxidation of their trisubstituted olefinic bonds. J. Mol. Catal. A Chem. 2017, 426, 429–434. [Google Scholar] [CrossRef]
- Lozano, P.; Bernal, J.M.; Navarro, A. A clean enzymatic process for producing flavour esters by direct esterification in switchable ionic liquid/solid phases. Green Chem. 2012, 14, 3026–3033. [Google Scholar] [CrossRef]
- Sun, W.Y.; Xiong, J.; Xu, H.H.; Ma, M.Y.; Hu, Y.Y. Synthesis of neryl acetate by free lipase-catalyzed transesterification in organic solvents and its kinetics. Food Sci. Technol. 2022, 42, e26522. [Google Scholar] [CrossRef]
- Xue, W.; Yang, G.; Bi, S.; Zhang, J.Y.; Hou, Z.L. Construction of caterpillar-like hierarchically structured Co/MnO/CNTs derived from MnO2/ZIF-8@ZIF-67 for electromagnetic wave absorption. Carbon 2021, 173, 521–527. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, K.; Liu, S.J.; Cao, X.; Wu, K.L.; Cheong, W.C.; Chen, Z.; Wang, Y.; Li, Y.; Liu, Y.Q.; et al. Core-shell ZIF-8@ZIF-67 derived CoP nanoparticles-embedded N-doped carbon nanotube hollow polyhedron for efficient over-all water splitting. J. Am. Chem. Soc. 2018, 140, 2610–2618. [Google Scholar] [CrossRef]
- Lu, L.D.; Zhu, L.Q.; Zhu, G.X.; Dong, M.L.; Zeng, Z.M. ZIF-8/lipase coated tapered optical fiber biosensor for the detection of triacylglycerides. IEEE Sens. J. 2020, 20, 14173–14180. [Google Scholar] [CrossRef]
- Vaidya, L.B.; Nadar, S.S.; Rathod, V.K. Entrapment of surfactant modified lipase within zeolitic imidazolate framework (ZIF)-8. Int. J. Biol. Macromol. 2020, 146, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Li, K.; Wang, J.H.; Xu, L.; Yan, Y.J. Hierarchical ZIF-8 toward immobilizing Burkholderia cepacia lipase for application in biodiesel preparation. Int. J. Mol. Sci. 2018, 19, 1424. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.J.; Zhang, X.; Yang, C.L.; Naseer, S.; Zhang, X.T.; Ouyang, J.; Li, D.L.; Yang, J.F. The effects of NaCl on enzyme encapsulation by zeolitic imidazolate frameworks-8. Enzym. Microb. Technol. 2019, 122, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2013, 4, 1583–1600. [Google Scholar] [CrossRef]
- Kohsari, I.; Shariatinia, Z.; Pourmortazavi, S.M. Antibacterial electrospun chitosan-polyethylene oxide nanocomposite mats containing ZIF-8 nanoparticles. Int. J. Biol. Macromol. 2016, 91, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Tasarin, S.; Panawong, C.; Sumranjit, J.; Budsombat, S. Enhancement of proton conductivity of crosslinked poly(vinyl alcohol) through introduction of zeolitic imidazolate framework-8 and imidazole. Int. J. Hydrog. Energy 2021, 46, 36969–36981. [Google Scholar] [CrossRef]
- Lv, B.Z.; Qi, W.J.; Luo, M.S.; Liu, Q.L.; Guo, L. Fischer-tropsch synthesis: ZIF-8@ZIF-67-derived cobalt nanoparticle-embedded nanocage catalysts. Ind. Eng. Chem. Res. 2020, 59, 12352–12359. [Google Scholar]
- Li, Y.; Zhou, K.; He, M.; Yao, J.F. Synthesis of ZIF-8 and ZIF-67 using mixed-base and their dye adsorption. Microporous Mesoporous Mater. 2016, 234, 287–292. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Ding, L.; Wang, X.M.; Yang, X.M.; He, J.B.; Yang, B.J.; Wang, B.N.; Zhang, D.W.; Li, Z.W. Yolk-shell ZIF-8@ZIF-67 derived Co3O4@NiCo2O4 catalysts with effective electrochemical properties for Li-O2 batteries. J. Alloys Compd. 2021, 861, 157945. [Google Scholar] [CrossRef]
- Guo, Z.; Bai, S.; Sun, Y. Preparation and characterization of immobilized lipase on magnetic hydrophobic microspheres. Enzym. Microb. Technol. 2003, 32, 776–782. [Google Scholar] [CrossRef]
- Suo, H.B.; Xu, L.L.; Xu, C.; Qiu, X.; Huang, H.; Hu, Y. Enhanced catalytic performance of lipase covalently bonded on ionic liquids modified magnetic alginate composites. J. Colloid. Interface Sci. 2019, 553, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Dong, X.Y.; Guo, Z.; Sun, Y. Remarkably enhanced activity and substrate affinity of lipase covalently bonded on zwitterionic polymer-grafted silicananoparticles. J. Colloid. Interface Sci. 2018, 519, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Cong, F.; Xu, Y.; Kang, J.; Zhang, Y.; Zhou, M.; Xing, K.; Zhang, G.; Pan, H. Synthesis of cinnamyl acetate catalysed by highly reusable cotton-immobilized Pseudomonas fuorescens lipase. Biocatal. Biotransfor. 2018, 36, 332–339. [Google Scholar] [CrossRef]
- De Castro, H.F.; De Oliveira, P.C.; Pereira, E.B. Evaluation of different approaches for lipase catalysed synthesis of citronellyl acetate. Biotech. Lett. 1997, 19, 229–232. [Google Scholar] [CrossRef]
- Dossat, V.; Combes, D.; Marty, A. Efficient lipase catalysed production of a lubricant and surfactant formulation using a continuous solvent-free process. J. Biontechnol. 2002, 97, 117–124. [Google Scholar] [CrossRef]
- Kumari, A.; Mahapatra, P.; Garlapati, V.; Banerjee, R. Enzymatic transesterification of Jatropha oil. Biotechnol. Biofuels 2009, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Zong, Z.; Zhang, S.P.; Zhen, M.L.; Xu, N. Metabolic engineering of Escherichia coli for the production of neryl acetate. Biochem. Eng. J. 2020, 161, 107704. [Google Scholar] [CrossRef]
- He, H.M.; Han, H.B.; Shi, H.; Tian, Y.Y.; Sun, F.X.; Song, Y.; Li, Q.S.; Zhu, G.S. Construction of thermophilic lipase-embedded metal organic frameworks via biomimetic mineralization: A biocatalyst for ester hydrolysis and kinetic resolution. ACS Appl. Mater. Interfaces 2016, 8, 24517–24524. [Google Scholar] [CrossRef]
- Martins, A.B.; Schein, M.F.; Friedrich, J.L.R.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Ultrasound-assisted butyl acetate synthesis catalyzed by Novozym 435: Enhanced activity and operational stability. Ultrason. Sonochem. 2013, 20, 1155–1160. [Google Scholar] [CrossRef]
- Graebin, N.G.; Martins, A.B.; Lorenzoni, A.S.G.; Garcia-Galan, C.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Immobilization of lipase B from Candida antarctica on porous styrene–divinylbenzene beads improves butyl acetate synthesis. Biotechnol. Prog. 2012, 28, 406–412. [Google Scholar] [CrossRef]
- Chiaradia, V.; Valerio, A.; De Oliveira, D.; Araújo, P.H.H.; Sayer, C. Simultaneous single-step immobilization of Candida antarctica lipase B and incorporation of magnetic nanoparticles on poly(urea-urethane) nanoparticles by interfacial miniemulsion polymerization. J. Mol. Catal. B Enzym. 2016, 131, 31–35. [Google Scholar] [CrossRef]
- Severac, E.; Galy, O.; Turon, F.; Pantel, C.A.; Condoret, J.S.; Monsan, P.; Marty, A. Selection of CalB immobilization method to be used in continuous oil transesterification: Analysis of the economical impact. Enzym. Microb. Technol. 2011, 48, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kumari, G.; Jayaramulu, K.; Maji, T.K.; Narayana, C. Temperature induced structural transformations and gas adsorption in the zeolitic imidazolate framework ZIF-8: A Raman study. J. Phys. Chem. A 2013, 117, 11006–11012. [Google Scholar] [CrossRef] [PubMed]
- Ozyilmaz, E.; Ascioglu, S.; Yilmaz, M. Calix[4]arene tetracarboxylic acid-treated lipase immobilized onto metal-organic framework: Biocatalyst for ester hydrolysis and kinetic resolution. Int. J. Biol. Macromol. 2021, 175, 79–86. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Xiong, J.; Xu, H.; Sun, W.; Pan, X.; Cui, S.; Lv, S.; Zhang, Y. Enhanced Enzymatic Performance of Immobilized Pseudomonas fluorescens Lipase on ZIF-8@ZIF-67 and Its Application to the Synthesis of Neryl Acetate with Transesterification Reaction. Molecules 2024, 29, 2922. https://doi.org/10.3390/molecules29122922
Wang Q, Xiong J, Xu H, Sun W, Pan X, Cui S, Lv S, Zhang Y. Enhanced Enzymatic Performance of Immobilized Pseudomonas fluorescens Lipase on ZIF-8@ZIF-67 and Its Application to the Synthesis of Neryl Acetate with Transesterification Reaction. Molecules. 2024; 29(12):2922. https://doi.org/10.3390/molecules29122922
Chicago/Turabian StyleWang, Qi, Jian Xiong, Hanghang Xu, Wenyuan Sun, Xiaoxu Pan, Shixin Cui, Siting Lv, and Yinling Zhang. 2024. "Enhanced Enzymatic Performance of Immobilized Pseudomonas fluorescens Lipase on ZIF-8@ZIF-67 and Its Application to the Synthesis of Neryl Acetate with Transesterification Reaction" Molecules 29, no. 12: 2922. https://doi.org/10.3390/molecules29122922
APA StyleWang, Q., Xiong, J., Xu, H., Sun, W., Pan, X., Cui, S., Lv, S., & Zhang, Y. (2024). Enhanced Enzymatic Performance of Immobilized Pseudomonas fluorescens Lipase on ZIF-8@ZIF-67 and Its Application to the Synthesis of Neryl Acetate with Transesterification Reaction. Molecules, 29(12), 2922. https://doi.org/10.3390/molecules29122922





