Synergistic Theoretical and Experimental Insights into NH4+-Enhanced Vanadium Oxide Cathodes for Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
2. Results and Discussion
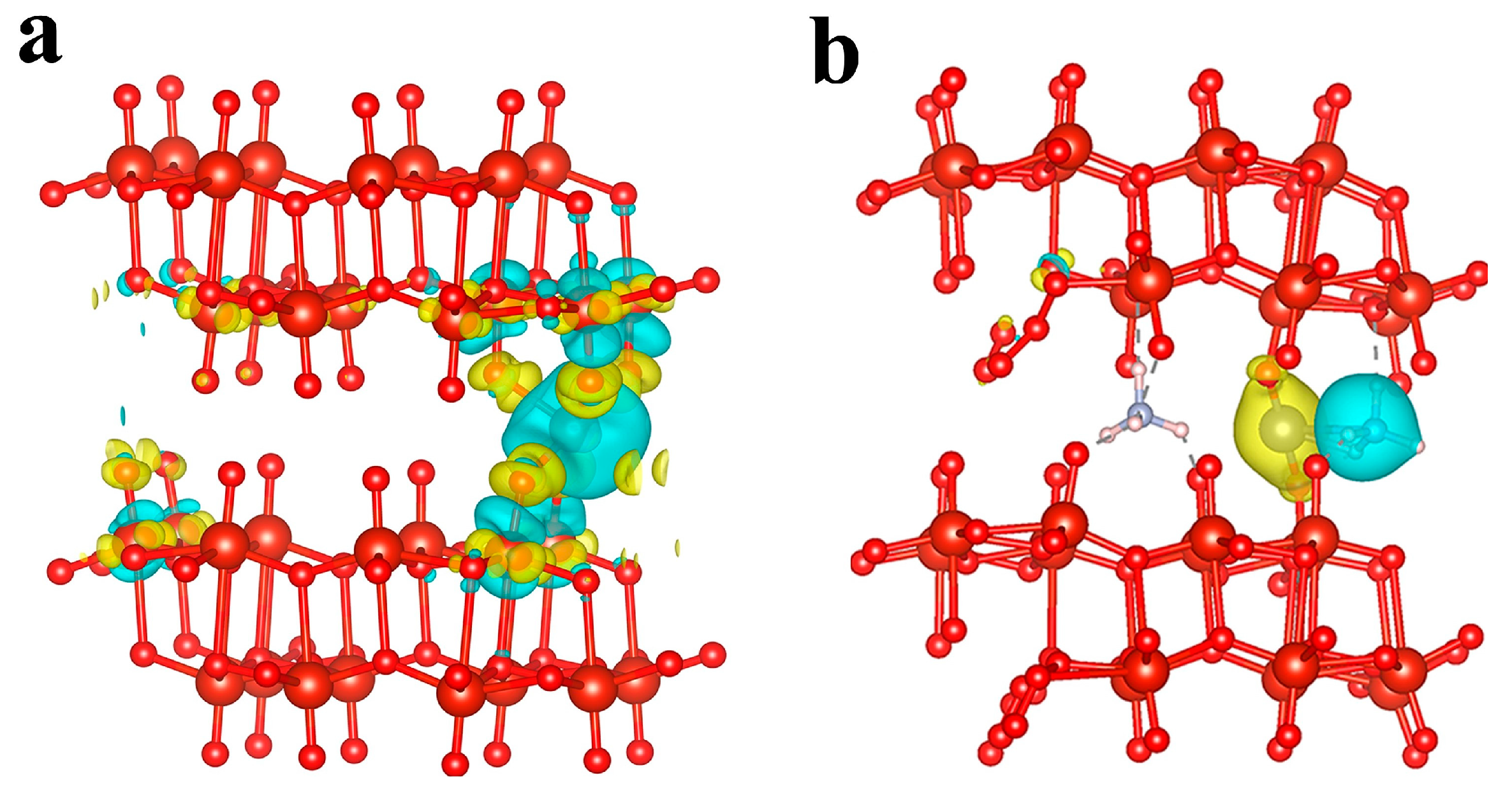
2.1. DFT Calculations
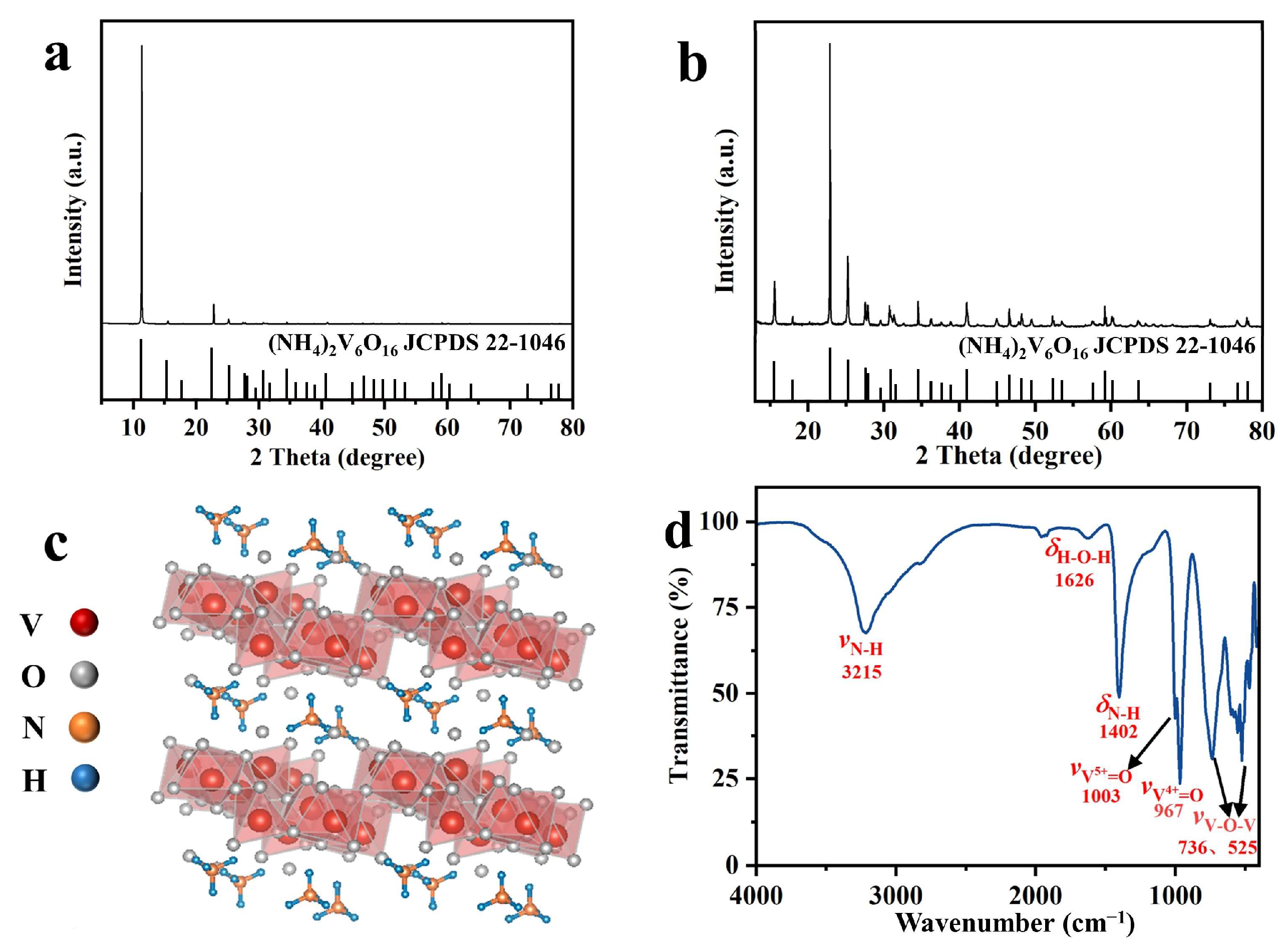
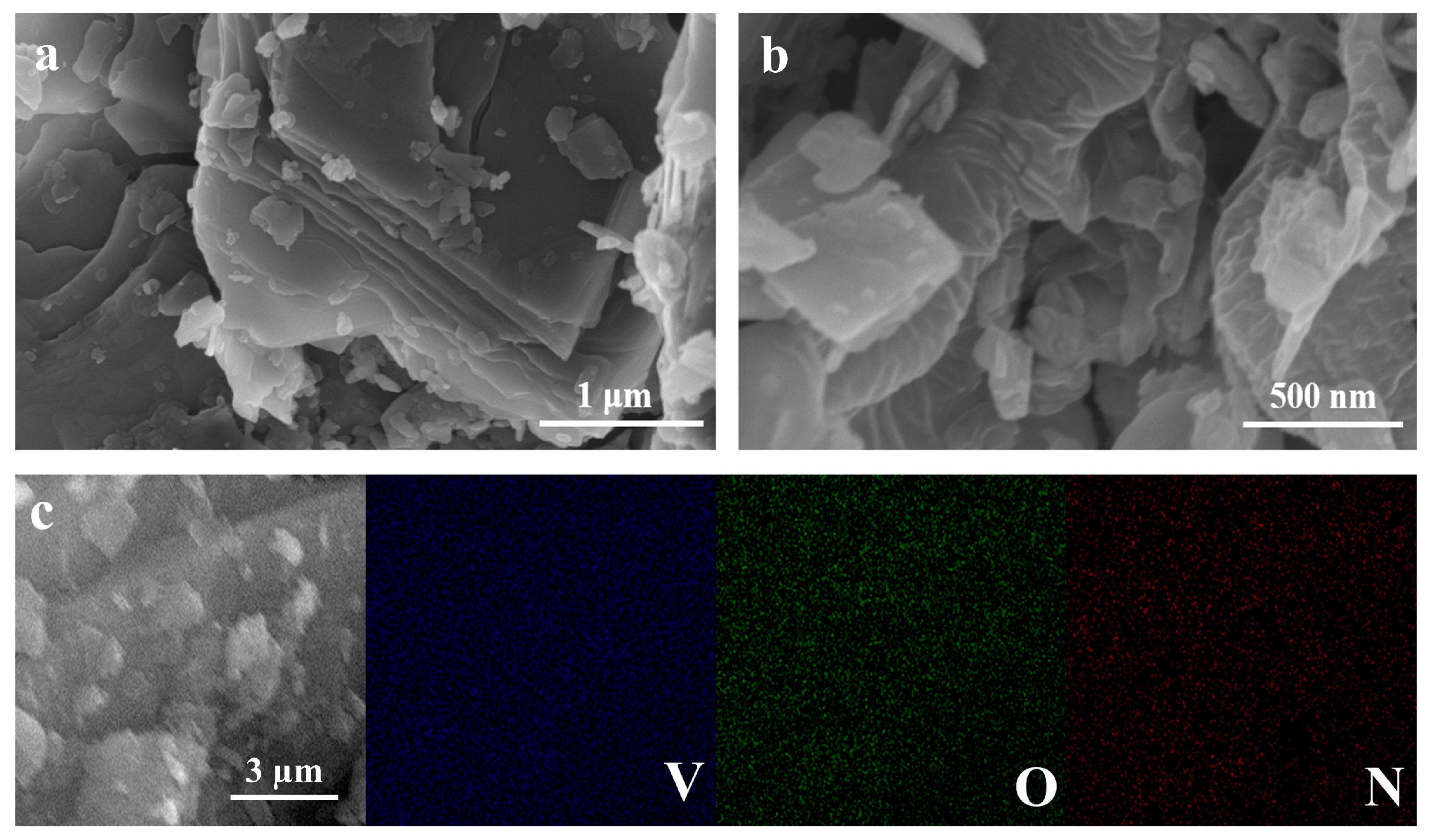
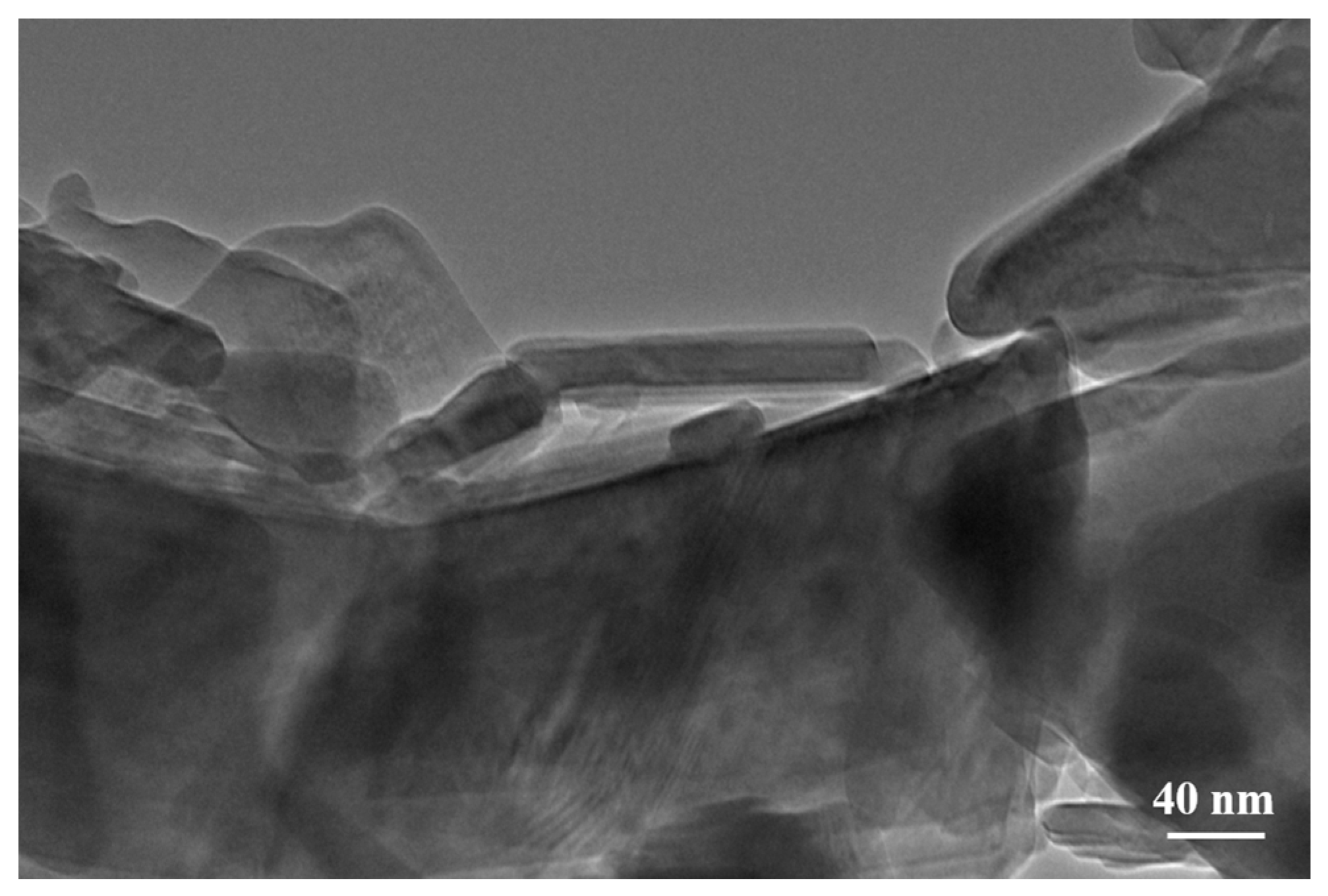
2.2. Morphological Characterization
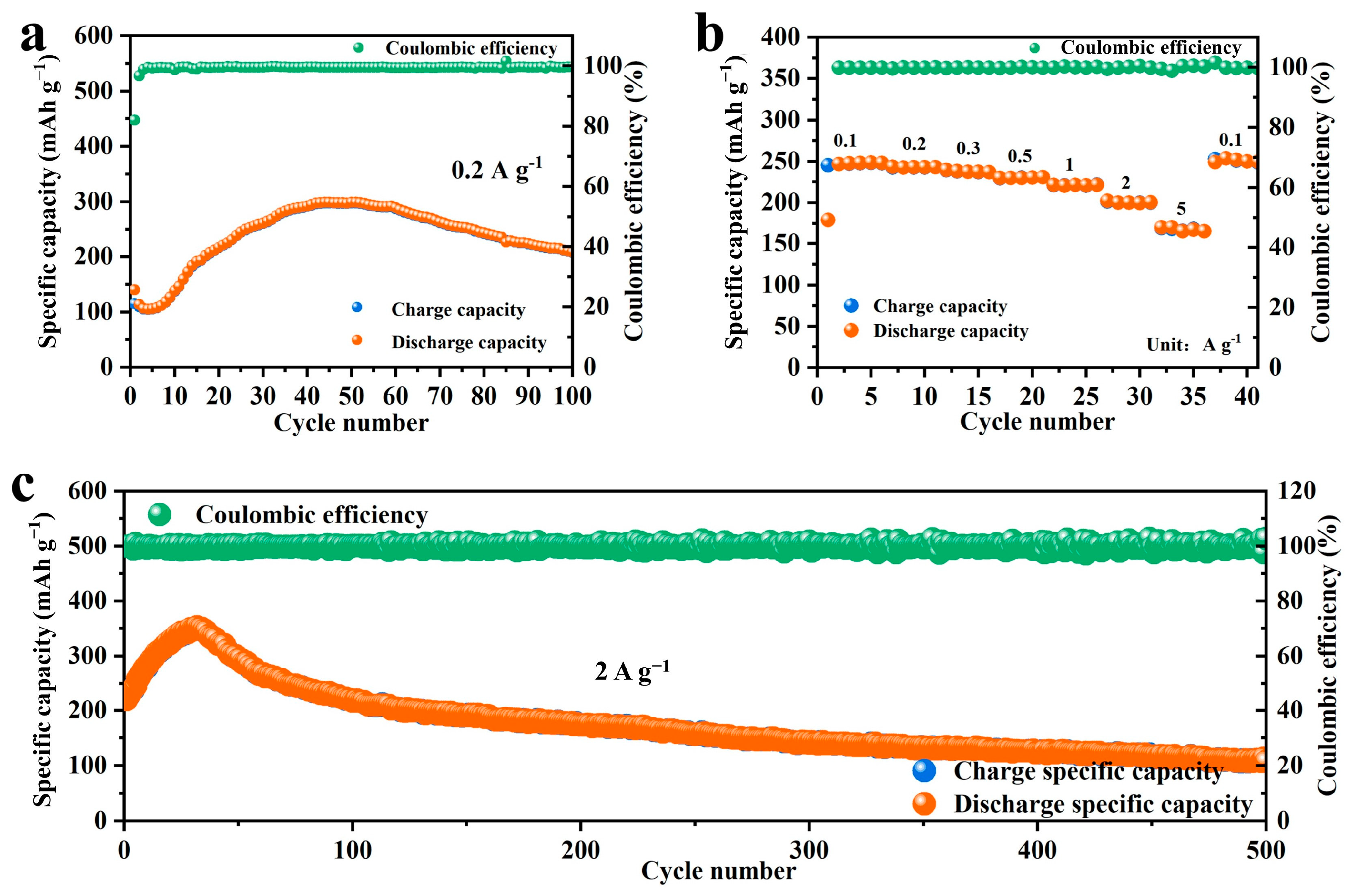
2.3. Electrochemical Properties Characterization
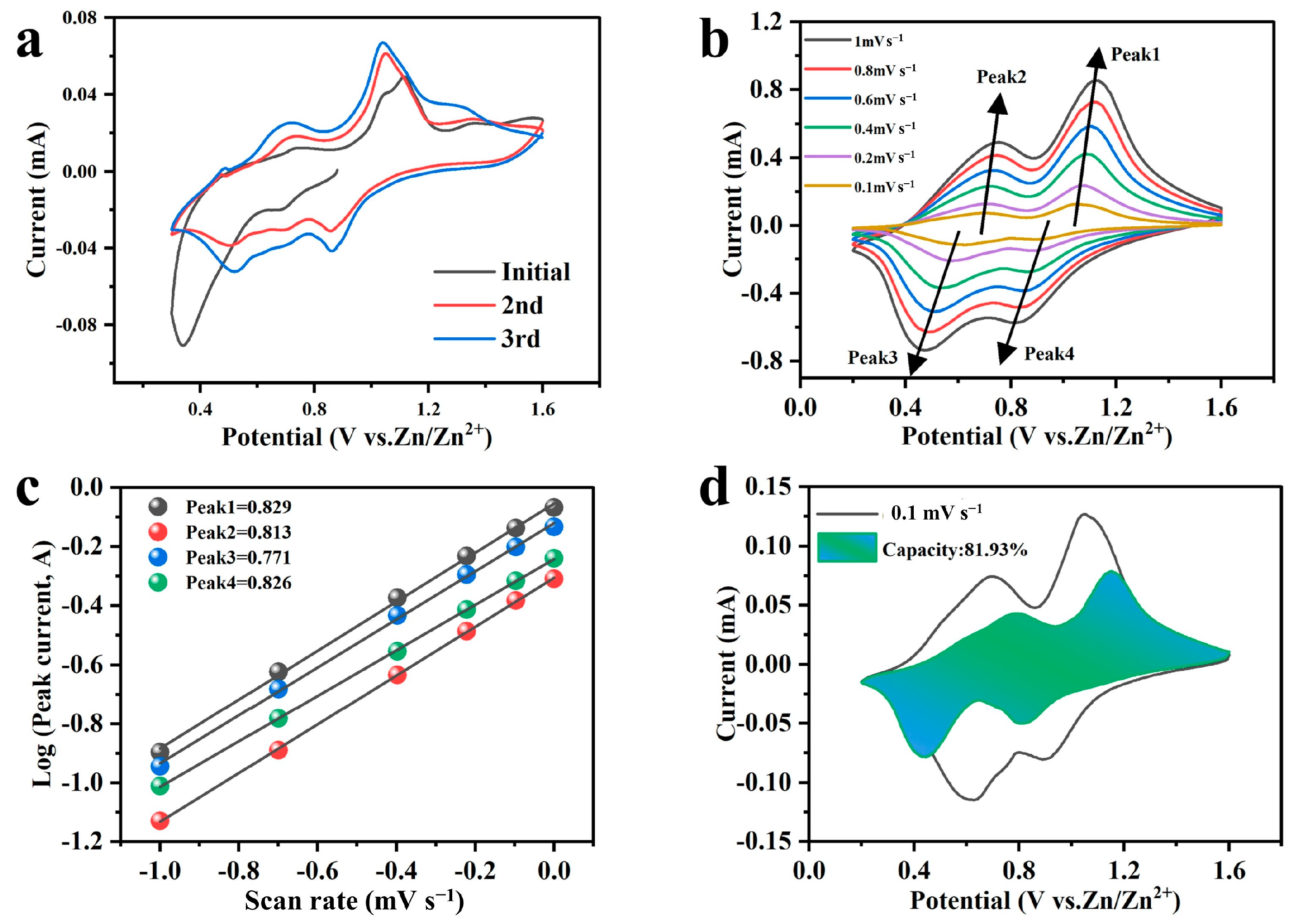
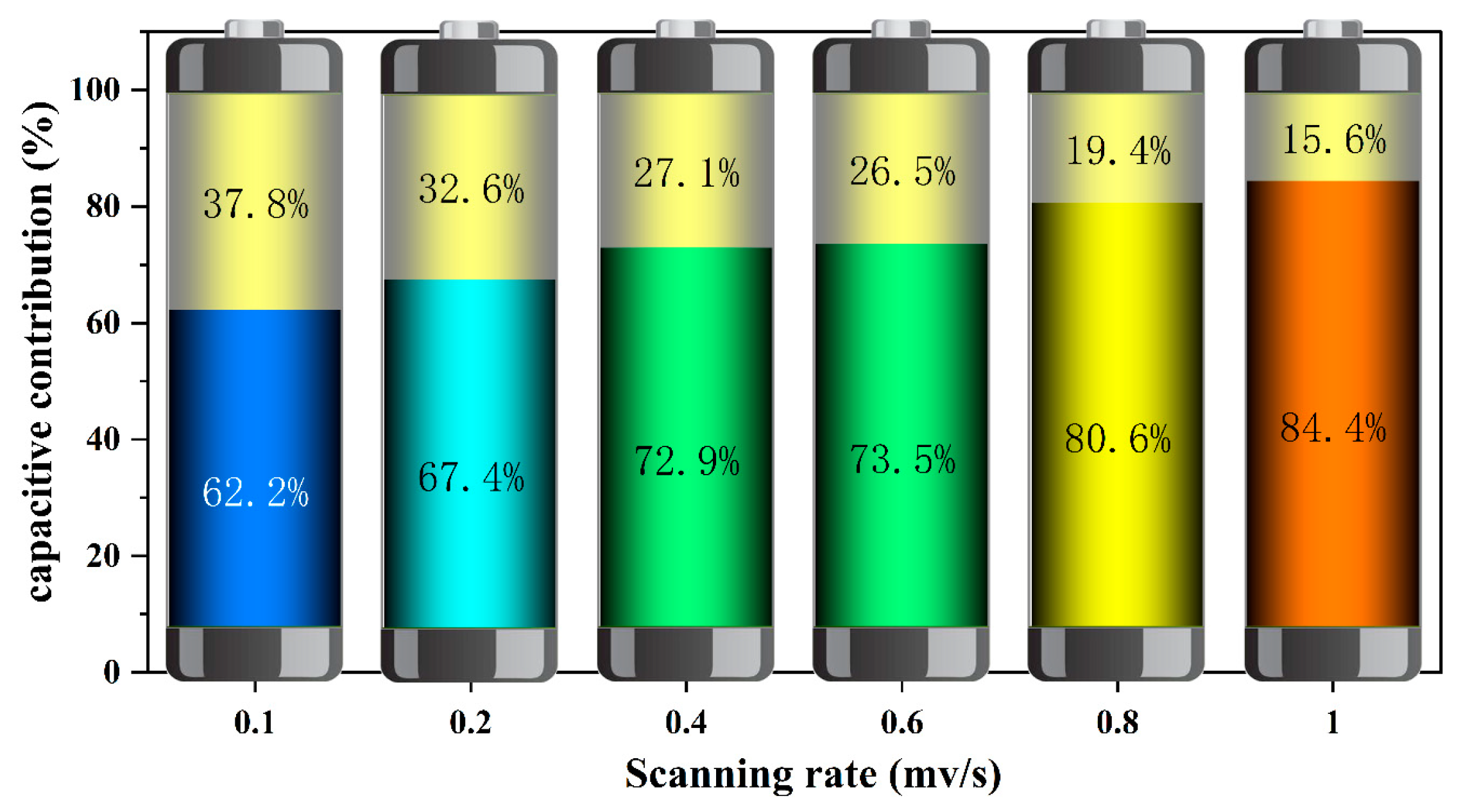
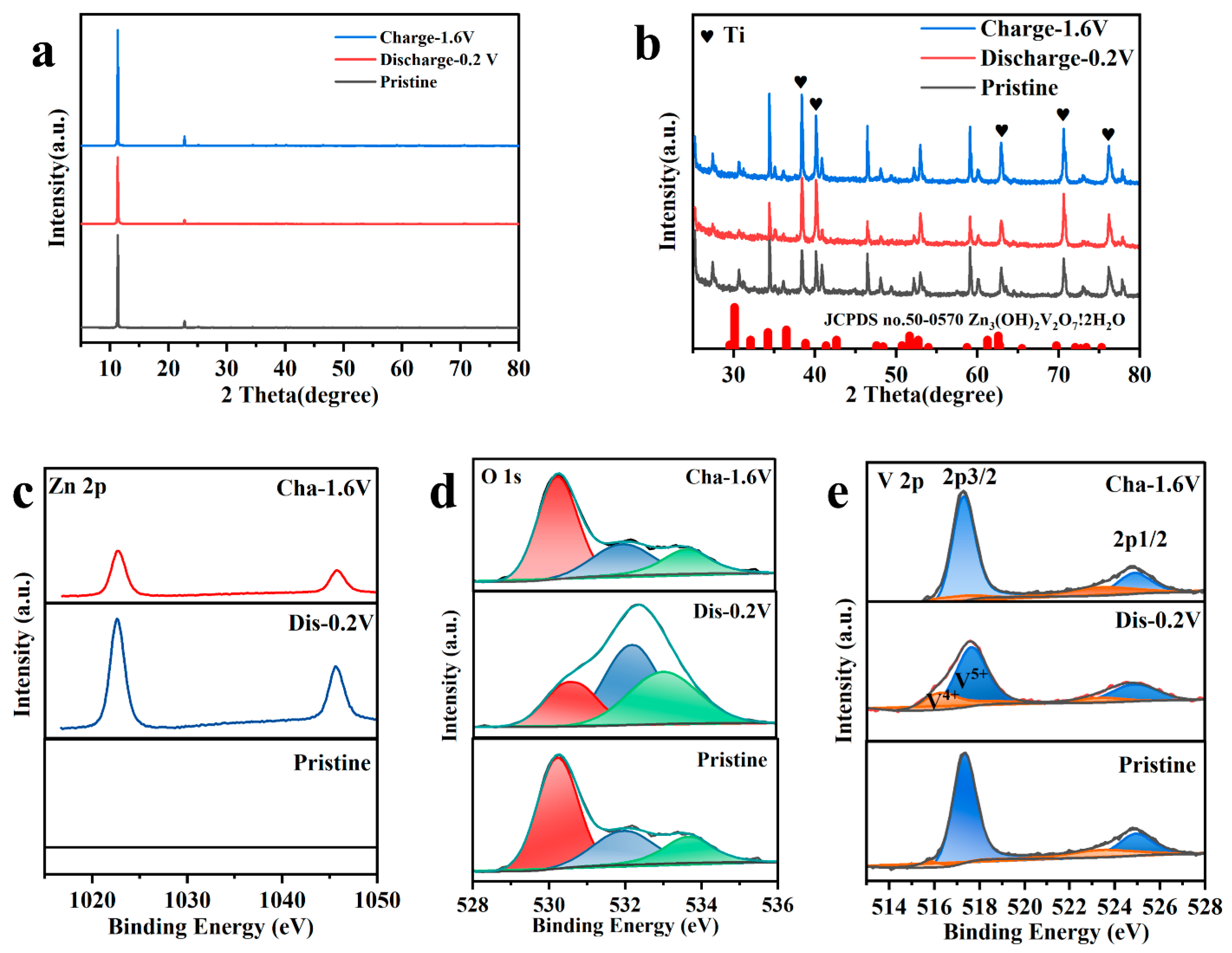
2.4. Storage Mechanism of Zn2+
3. Materials and Methods
3.1. Calculation Method
3.2. Preparation of Material
3.3. Materials Characterization
3.4. Electrode Fabrication
3.5. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chao, D.; Zhou, W.; Xie, F.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S.Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, T.; Zhang, M. Advances in intelligent regeneration of cathode materials for sustainable lithium-ion batteries. Adv. Energy Mater. 2022, 12, 2201526. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Luo, L.; Fan, Y.; Du, Z. A review on thermal management of lithium-ion batteries for electric vehicles. Energy 2022, 238, 121652. [Google Scholar] [CrossRef]
- Zhu, G.-L.; Zhao, C.-Z.; Huang, J.-Q.; He, C.; Zhang, J.; Chen, S.; Xu, L.; Yuan, H.; Zhang, Q. Fast charging lithium batteries: Recent progress and future prospects. Small 2019, 15, 1805389. [Google Scholar] [CrossRef] [PubMed]
- Kotal, M.; Jakhar, S.; Roy, S.; Sharma, H.K. Cathode materials for rechargeable lithium batteries: Recent progress and future prospects. J. Energy Storage 2022, 47, 103534. [Google Scholar] [CrossRef]
- Chen, S.P.; Lv, D.; Chen, J.; Zhang, Y.H.; Shi, F.N. Review on defects and modification methods of LiFePO4 cathode material for lithium-ion batteries. Energy Fuels 2022, 36, 1232–1251. [Google Scholar] [CrossRef]
- Aizudin, M.; Fu, W.; Pottammel, R.P.; Dai, Z.; Wang, H.; Rui, X.; Zhu, J.; Li, C.C.; Wu, X.-L.; Ang, E.H. Recent advancements of graphene-based materials for zinc-based batteries: Beyond lithium-ion batteries. Small 2024, 20, 2305217. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, Y.; Cai, X.; Liu, C.; Wang, Z.; Ye, H.; Pan, Y.; Jia, D.; Lin, H. Boosting zinc storage performance of Li3VO4 cathode material for aqueous zinc ion batteries via carbon-incorporation: A study combining theory and experiment. Small 2024, 20, 2305762. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Kubota, K.; Hameed, A.S.; Komaba, S. Research development on K-ion batteries. Chem. Rev. 2020, 120, 6358–6466. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, X.; Luo, X.; Indris, S.; Sarapulova, A.; Bauer, M.; Wang, Z.; Knapp, M.; Ehrenberg, H.; Wei, Y.; et al. High-voltage aqueous Mg-ion batteries enabled by solvation structure reorganization. Adv. Funct. Mater. 2022, 19, 2110674. [Google Scholar] [CrossRef]
- Zhou, T.; Han, Q.; Xie, L.; Yang, X.; Zhu, L.; Cao, X. Recent developments and challenges of vanadium oxides (VxOy) cathodes for aqueous zinc-ion batteries. Chem. Rec. 2022, 22, e202100275. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Deng, R.; Wang, Z.; Wang, Y.; Huang, G.; Wang, J.; Pan, F. The challenges and perspectives of developing solid-state electrolytes for rechargeable multivalent battery. J. Solid State Electrochem. 2023, 27, 1291–1327. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Wang, H.; Cui, F.; Zhu, G. Integrated pyrazine-based porous aromatic frameworks/carbon nanotube composite as cathode materials for aqueous zinc ion batteries. Chem. Eng. J. 2022, 450, 138051. [Google Scholar] [CrossRef]
- Zeng, Y.; Luan, D.; Lou, X.W. Recent advances in electrode engineering strategies for aqueous Zn-based batteries. Chem 2023, 9, 1118–1146. [Google Scholar] [CrossRef]
- Li, Z.; Tan, J.; Wang, Y.; Gao, C.; Wang, Y.; Ye, M.; Shen, J. Building better aqueous Zn-organic batteries. Energy Environ. Sci. 2023, 16, 2398–2431. [Google Scholar] [CrossRef]
- Li, G.; Sun, L.; Zhang, S.; Zhang, C.; Jin, H.; Davey, K.; Liang, G.; Liu, S.; Mao, J.; Guo, Z. Developing cathode materials for aqueous zinc ion batteries: Challenges and practical prospects. Adv. Funct. Mater. 2024, 29, 2301291. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Wei, C.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Recent advances and perspectives of Zn-metal free “rocking-chair”-type Zn-ion batteries. Adv. Energy Mater. 2021, 11, 2002529. [Google Scholar] [CrossRef]
- Selvakumaran, D.; Pan, A.; Liang, S.; Cao, G. A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries. J. Mater. Chem. A 2019, 7, 18209–18236. [Google Scholar] [CrossRef]
- Tan, Y.; An, F.; Liu, Y.; Li, S.; He, P.; Zhang, N.; Li, P.; Qu, X. Reaction kinetics in rechargeable zinc-ion batteries. J. Power Sources 2021, 492, 229655. [Google Scholar] [CrossRef]
- Shang, W.; Yu, W.; Liu, Y.; Li, R.; Dai, Y.; Cheng, C.; Tan, P.; Ni, M. Rechargeable alkaline zinc batteries: Progress and challenges. Energy Storage Mater. 2020, 31, 44–57. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.-C.; Guo, Y.-F.; Wang, P.-F.; Zhu, Y.-R.; Yi, T.-F. Insights on rational design and energy storage mechanism of Mn-based cathode materials towards high performance aqueous zinc-ion batteries. Coord. Chem. Rev. 2023, 479, 215009. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Xi, B.; Chen, W.; Jia, Y.; Feng, J.; Xiong, S. Advances and perspectives of cathode storage chemistry in aqueous zinc-ion batteries. ACS Nano 2021, 15, 9244–9272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, P.; Liang, J.; Xia, X.; Ren, L.; Song, L.; Liu, W.; Sun, X. Uncovering sulfur doping effect in MnO2 nanosheets as an efficient cathode for aqueous zinc ion battery. Energy Storage Mater. 2022, 47, 424–433. [Google Scholar] [CrossRef]
- Fan, X.; Xiang, K.; Zhou, W.; Deng, W.; Zhu, H.; Chen, L.; Chen, H. A novel improvement strategy and a comprehensive mechanism insight for α-MnO2 energy storage in rechargeable aqueous zinc-ion batteries. Carbon Energy 2024, e536. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, K.; Zhou, W.; Deng, W.; Zhu, H.; Chen, H. Investigations on tunnel-structure MnO2 for utilization as a high-voltage and long-life cathode material in aqueous ammonium-ion and hybrid-ion batteries. Small 2024, 20, 2308741. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miao, R.; Yang, Y.; Han, L.; Han, Q. A zinc-ion battery-type self-powered strain sensing system by using a high-performance ionic hydrogel. Soft Matter 2023, 19, 8022–8032. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lu, M.; Wang, B.; Cheng, H.; Yang, H.; Cai, D.; Han, W.; Fan, H.J. High-mass loading V3O7·H2O nanoarray for Zn-ion battery: New synthesis and two-stage ion intercalation chemistry. Nano Energy 2021, 83, 105835. [Google Scholar] [CrossRef]
- Lv, T.; Peng, Y.; Zhang, G.; Liang, S.; Yang, Z.; Yang, S.; Pang, H. How about vanadium-based compounds as cathode materials for aqueous zinc ion batteries? Adv. Sci. 2023, 10, 2206907. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Liu, J.-H.; Gao, Y.; Cao, X.; Zhan, C.; Wang, Y.; Wang, S.; Chou, S.-L.; Dou, S.-X.; et al. Vanadium-based cathodes for aqueous zinc-ion batteries: Mechanism, design strategies and challenges. Energy Storage Mater. 2022, 50, 21–46. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, P.; Li, X.; Liu, J.; Liu, H.; Sun, B.; Pan, K.; Song, K.; Cheng, H. Heterojunction tunnelled vanadium-based cathode materials for high-performance aqueous zinc ion batteries. J. Colloid Interface Sci. 2024, 665, 564–572. [Google Scholar] [CrossRef]
- Liu, N.; Li, B.; He, Z.; Dai, L.; Wang, H.; Wang, L. Recent advances and perspectives on vanadium- and manganese-based cathode materials for aqueous zinc ion batteries. J. Energy Chem. 2021, 59, 134–159. [Google Scholar] [CrossRef]
- Zampardi, G.; Mantia, F.L. Prussian blue analogues as aqueous Zn-ion batteries electrodes: Current challenges and future perspectives. Curr. Opin. Electrochem. 2020, 21, 84–92. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, L.; Xie, L.; Han, Q.; Yang, X.; Chen, L.; Wang, G.; Cao, X. Cathode materials for aqueous zinc-ion batteries: A mini review. J. Colloid Interface Sci. 2022, 605, 828–850. [Google Scholar] [CrossRef]
- Sun, T.; Fan, H.J. Understanding cathode materials in aqueous zinc–organic batteries. Curr. Opin. Electrochem. 2021, 30, 100799. [Google Scholar] [CrossRef]
- Tie, Z.; Niu, Z. Design strategies for high-performance aqueous Zn/organic batteries. Angew. Chem. Int. Ed. 2020, 59, 21293–21303. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Guo, T.; Xiang, K.; Wen, X.; Zhou, W.; Chen, H. Facile construction on flower-like CuS microspheres and their applications for the high-performance aqueous ammonium-ion batteries. Mater. Res. Bull. 2024, 170, 112595. [Google Scholar] [CrossRef]
- Huang, Y.; Xing, L.; Pei, S.; Zhou, W.; Hu, Y.; Deng, W.; Chen, L.; Zhu, H.; Chen, H. Co9S8/CNTs microspheres as superior-performance cathodes in aqueous ammonium-ion batteries. Trans. Nonferrous Met. Soc. China 2023, 33, 3452–3464. [Google Scholar] [CrossRef]
- He, D.; Sun, T.; Wang, Q.; Ma, T.; Zheng, S.; Tao, Z.; Liang, J. Multi-functional potassium ion assists ammonium vanadium oxide cathode for high-performance aqueous zinc-ion batteries. Batteries 2022, 8, 84. [Google Scholar] [CrossRef]
- Tang, B.; Zhou, J.; Fang, G.; Liu, F.; Zhu, C.; Wang, C.; Pan, A.; Liang, S. Engineering the interplanar spacing of ammonium vanadates as a high-performance aqueous zinc-ion battery cathode. J. Mater. Chem. A 2019, 7, 940–945. [Google Scholar] [CrossRef]
- Cui, F.; Hu, F.; Yu, X.; Guan, C.; Song, G.; Zhu, K. In-situ tuning the NH4+ extraction in (NH4)2V4O9 nanosheets towards high performance aqueous zinc ion batteries. J. Power Sources 2021, 492, 229629. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Xu, L.; Gao, Z.; Zheng, J.; Wang, Q.; Meng, C.; Wang, J. Fabrication of (NH4)2V3O8 nanoparticles encapsulated in amorphous carbon for high capacity electrodes in aqueous zinc ion batteries. Chem. Eng. J. 2020, 382, 122844. [Google Scholar] [CrossRef]
- Lu, J.; Lin, X.; Wang, S.; Xu, X.; Zhou, Y.; Zhang, Y.; Li, Q.; Liu, H. High ionic conductivity and toughness hydrogel electrolyte for high-performance flexible solid-state zinc-ion hybrid supercapacitors enabled by cellulose-bentonite coordination interactions. Green Chem. 2023, 25, 1635–1646. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Xu, J.; Zhang, Y. Synergistic Theoretical and Experimental Insights into NH4+-Enhanced Vanadium Oxide Cathodes for Aqueous Zinc-Ion Batteries. Molecules 2024, 29, 2834. https://doi.org/10.3390/molecules29122834
Lin H, Xu J, Zhang Y. Synergistic Theoretical and Experimental Insights into NH4+-Enhanced Vanadium Oxide Cathodes for Aqueous Zinc-Ion Batteries. Molecules. 2024; 29(12):2834. https://doi.org/10.3390/molecules29122834
Chicago/Turabian StyleLin, He, Jing Xu, and Yu Zhang. 2024. "Synergistic Theoretical and Experimental Insights into NH4+-Enhanced Vanadium Oxide Cathodes for Aqueous Zinc-Ion Batteries" Molecules 29, no. 12: 2834. https://doi.org/10.3390/molecules29122834
APA StyleLin, H., Xu, J., & Zhang, Y. (2024). Synergistic Theoretical and Experimental Insights into NH4+-Enhanced Vanadium Oxide Cathodes for Aqueous Zinc-Ion Batteries. Molecules, 29(12), 2834. https://doi.org/10.3390/molecules29122834





