Targeting NAD Metabolism: Rational Design, Synthesis and In Vitro Evaluation of NAMPT/PARP1 Dual-Target Inhibitors as Anti-Breast Cancer Agents
Abstract
1. Introduction
2. Results and Discussion
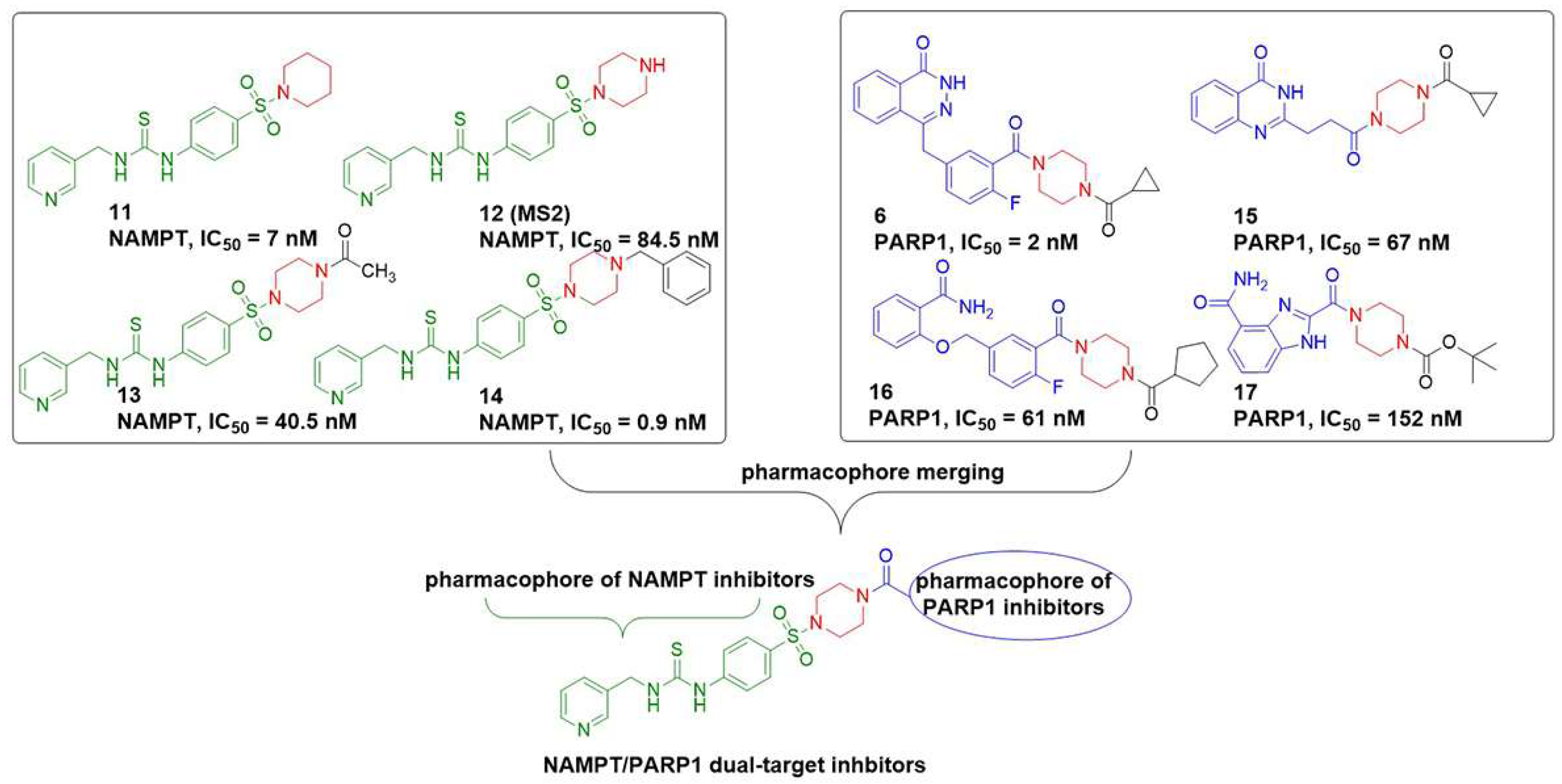
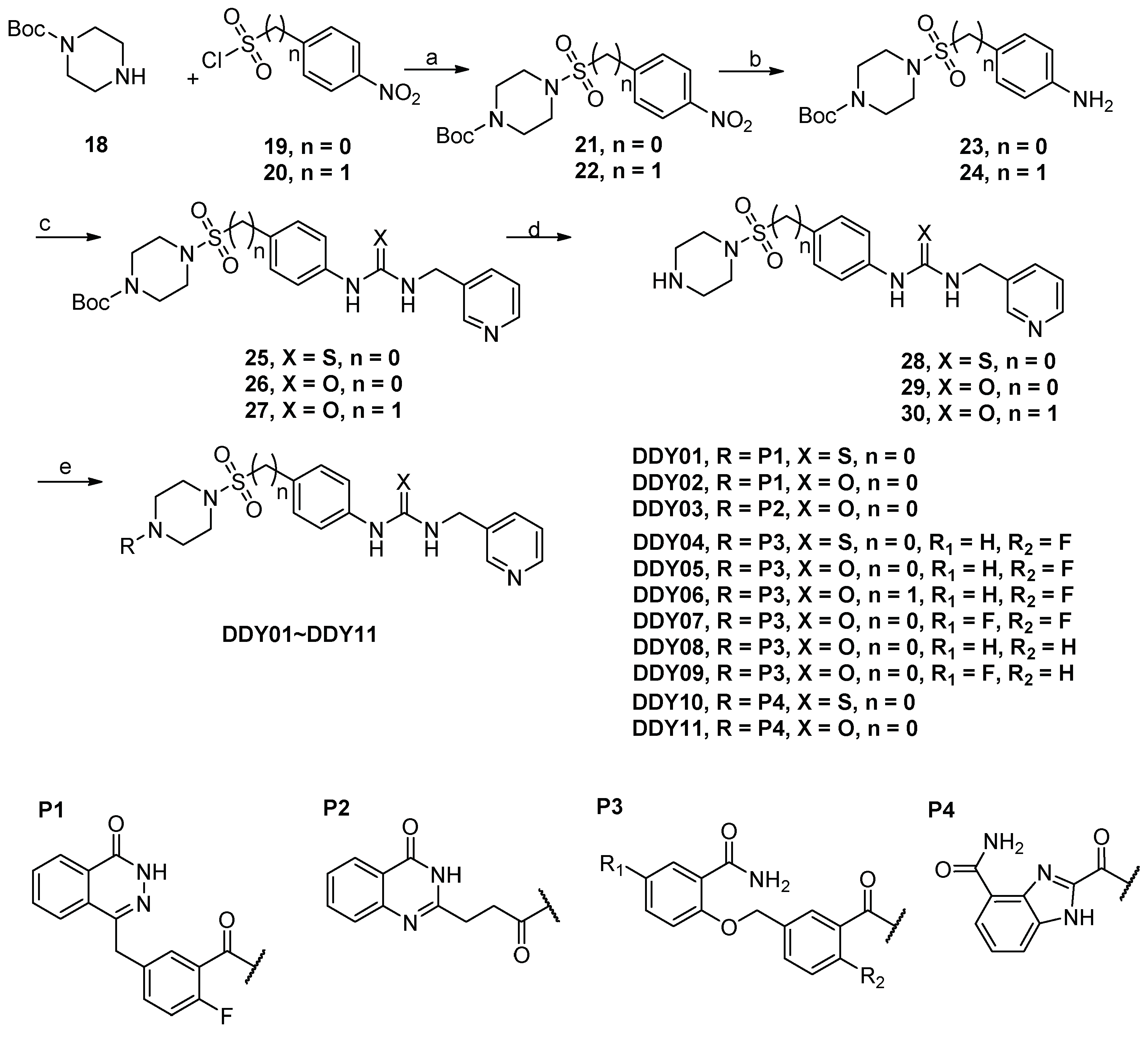
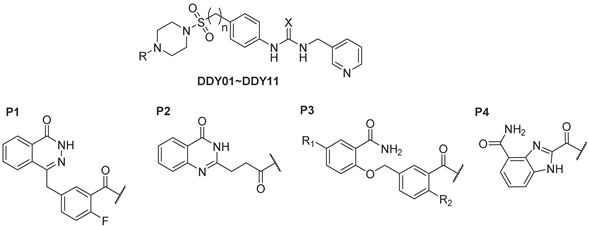
2.1. Design
2.2. Biological Screening
2.2.1. Enzymatic Activities against NAMPT and PARP1
2.2.2. Molecular Docking Study
2.2.3. Cytotoxicity Assay
2.2.4. DDY02 Suppressed Colony Formation
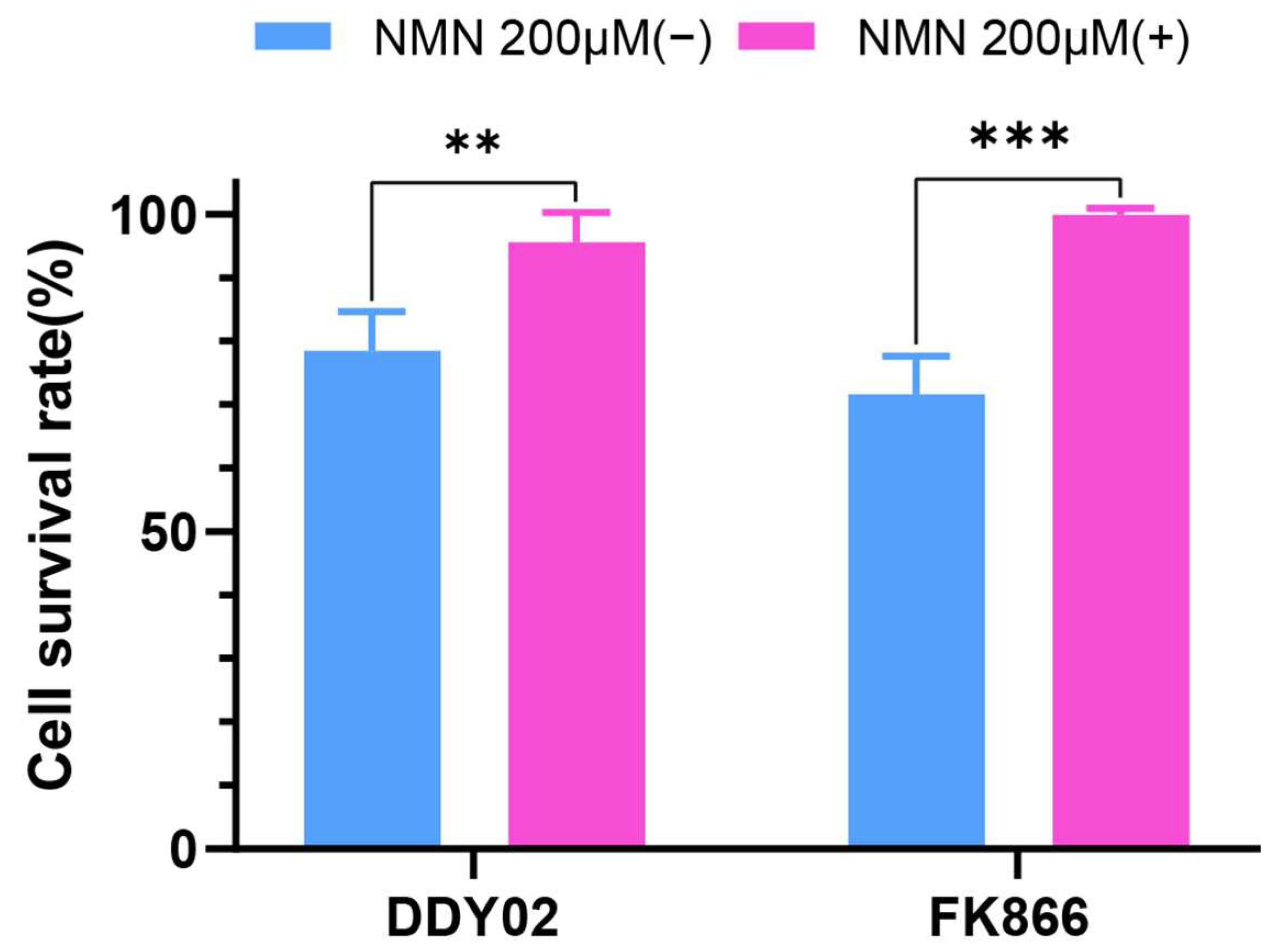
2.2.5. NMN Reversed the Efficacy of Compound DDY02
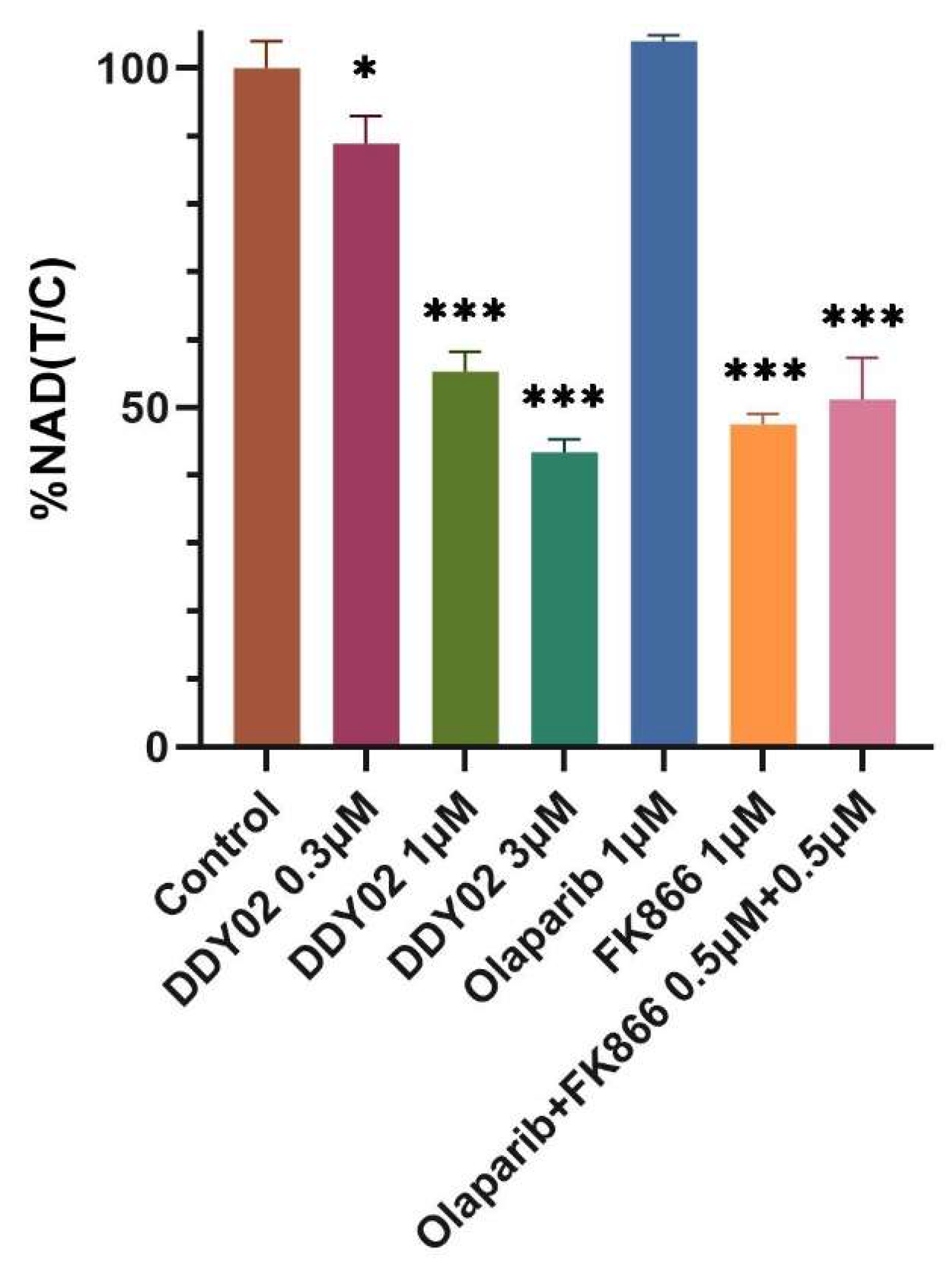
2.2.6. DDY02 Reduced Cellular NAD Levels
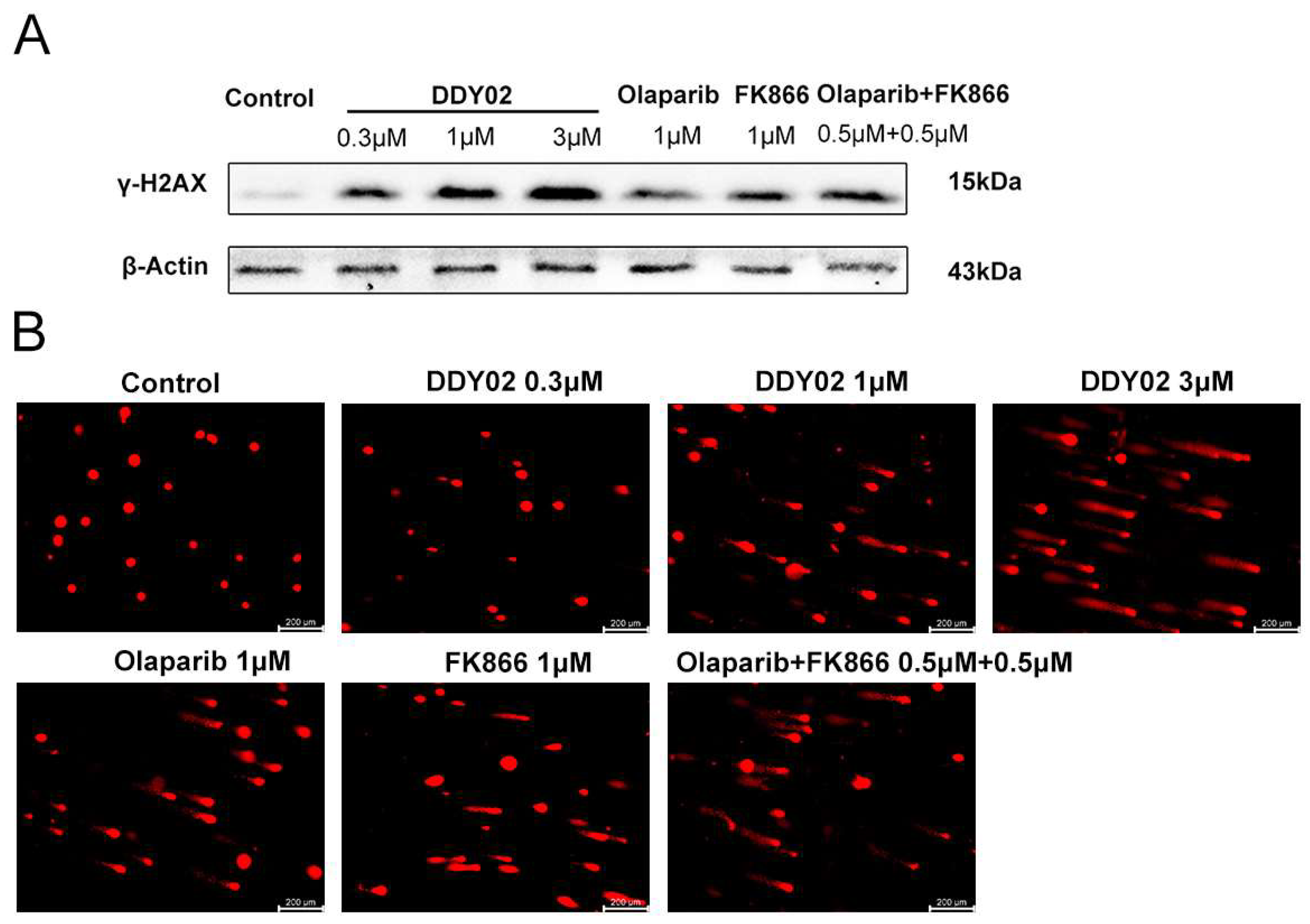
2.2.7. DDY02 Induced DNA Damage
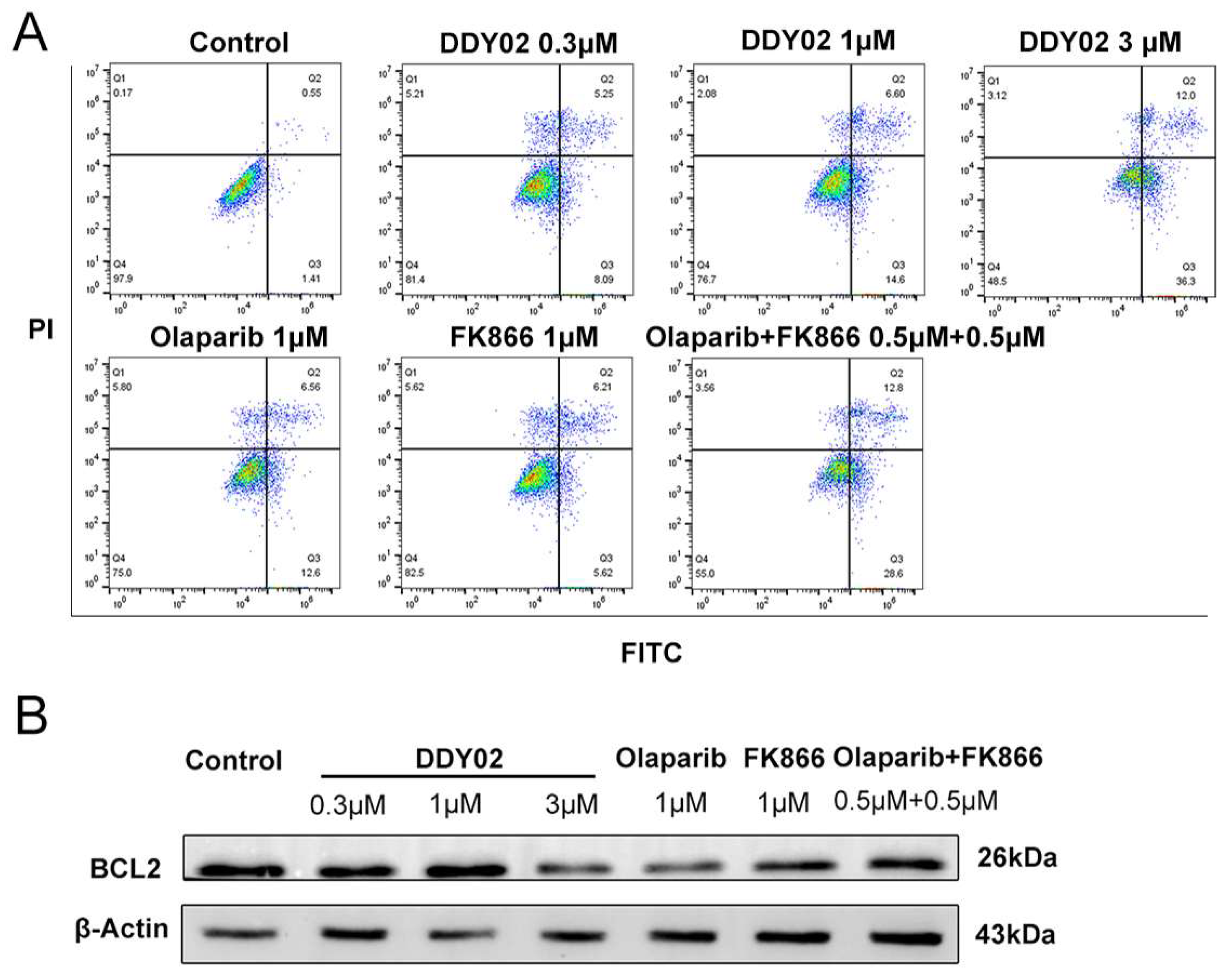
2.2.8. DDY02 Induced Apoptosis
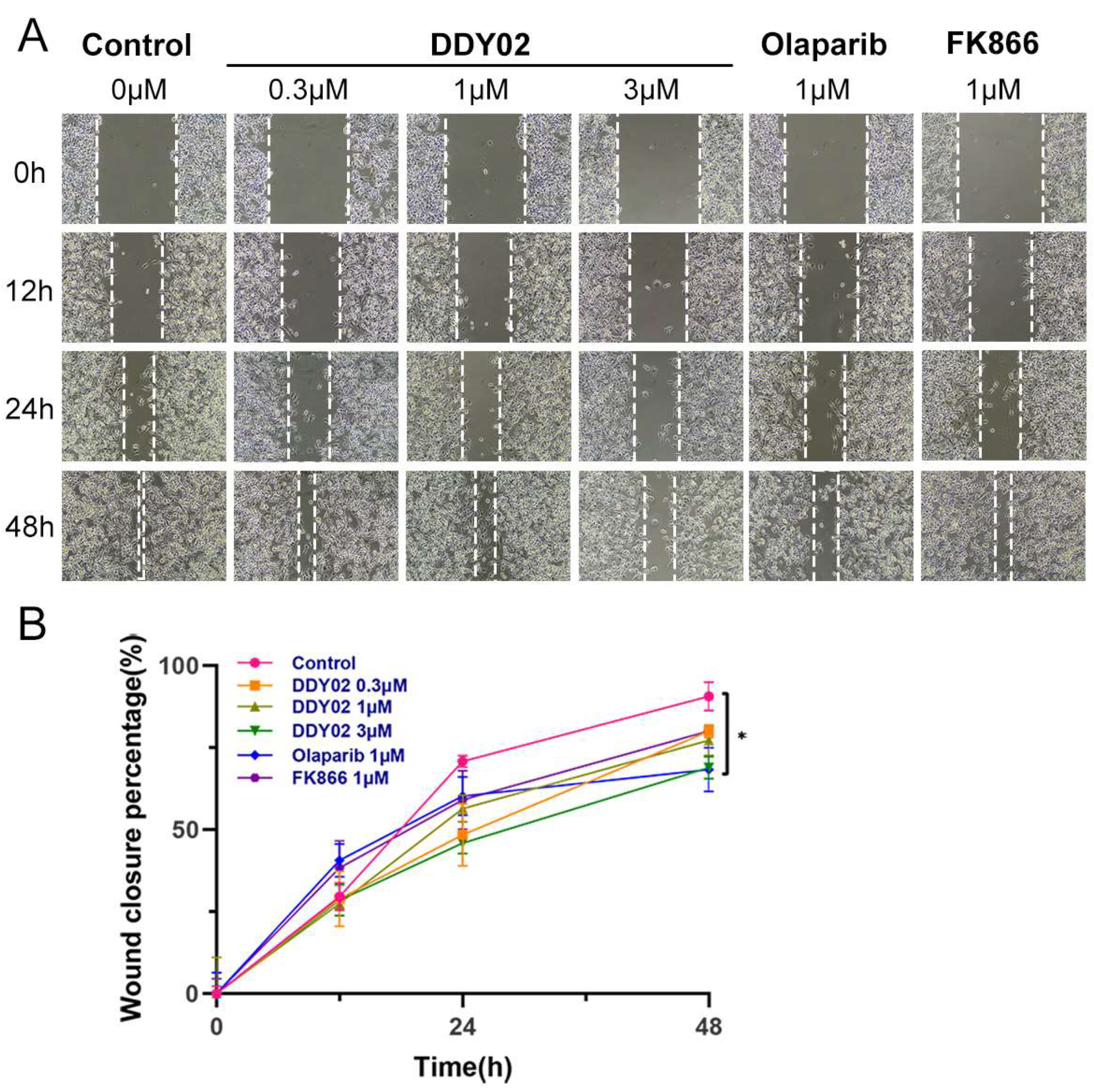
2.2.9. DDY02 Inhibited Cell Migration
3. Materials and Methods
3.1. Chemistry
3.1.1. tert-Butyl 4-((4-Nitrophenyl)sulfonyl)piperazine-1-carboxylate (Compound 21)
3.1.2. tert-Butyl 4-((4-Nitrobenzyl)sulfonyl)piperazine-1-carboxylate (Compound 22)
3.1.3. tert-Butyl 4-((4-Aminophenyl)sulfonyl)piperazine-1-carboxylate (Compound 23)
3.1.4. tert-Butyl 4-((4-Aminobenzyl)sulfonyl)piperazine-1-carboxylate (Compound 24)
3.1.5. tert-Butyl 4-((4-(3-(Pyridin-3-ylmethyl)thioureido)phenyl)sulfonyl)piperazine-1-carboxylate (Compound 25)
3.1.6. tert-Butyl 4-((4-(3-(Pyridin-3-ylmethyl)ureido)phenyl)sulfonyl)piperazine-1-carboxylate (Compound 26)
3.1.7. tert-Butyl 4-((4-(3-(Pyridin-3-ylmethyl)ureido)benzyl)sulfonyl)piperazine-1-carboxylate (Compound 27)
3.1.8. 1-(4-(Piperazin-1-ylsulfonyl)phenyl)-3-(pyridin-3-ylmethyl)thiourea (Compound 28)
3.1.9. 1-(4-(Piperazin-1-ylsulfonyl)phenyl)-3-(pyridin-3-ylmethyl)urea (Compound 29)
3.1.10. 1-(4-((Piperazin-1-ylsulfonyl)methyl)phenyl)-3-(pyridin-3-ylmethyl)urea (Compound 30)
3.1.11. 1-(4-((4-(2-Fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoyl)piperazin-1-yl)sulfonyl)phenyl)-3-(pyridin-3-ylmethyl)thiourea (Compound DDY01)
3.1.12. 1-(4-((4-(2-Fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoyl)piperazin-1-yl)sulfonyl)phenyl)-3-(pyridin-3-ylmethyl)urea (Compound DDY02)
3.1.13. 1-(4-((4-(3-(4-Oxo-3,4-dihydroquinazolin-2-yl)propanoyl)piperazin-1-yl)sulfonyl)phenyl)-3-(pyridin-3-ylmethyl)urea (Compound DDY03)
3.1.14. 2-((4-Fluoro-3-(4-((4-(3-(pyridin-3-ylmethyl)thioureido)phenyl)sulfonyl)piperazine-1-carbonyl)benzyl)oxy)benzamide (Compound DDY04)
3.1.15. 2-((4-Fluoro-3-(4-((4-(3-(pyridin-3-ylmethyl)ureido)phenyl)sulfonyl)piperazine-1-carbonyl)benzyl)oxy)benzamide (Compound DDY05)
3.1.16. 2-((4-Fluoro-3-(4-((4-(3-(pyridin-3-ylmethyl)ureido)benzyl)sulfonyl)piperazine-1-carbonyl)benzyl)oxy)benzamide (Compound DDY06)
3.1.17. 5-Fluoro-2-((4-fluoro-3-(4-((4-(3-(pyridin-3-ylmethyl)ureido)phenyl)sulfonyl)piperazine-1-carbonyl)benzyl)oxy)benzamide (Compound DDY07)
3.1.18. 2-((3-(4-((4-(3-(Pyridin-3-ylmethyl)ureido)phenyl)sulfonyl)piperazine-1-carbonyl)benzyl)oxy)benzamide (Compound DDY08)
3.1.19. 5-Fluoro-2-((3-(4-((4-(3-(pyridin-3-ylmethyl)ureido)phenyl)sulfonyl)piperazine-1-carbonyl)benzyl)oxy)benzamide (Compound DDY09)
3.1.20. 2-(4-((4-(3-(Pyridin-3-ylmethyl)thioureido)phenyl)sulfonyl)piperazine-1-carbonyl)-1H-benzo[d]imidazole-4-carboxamide (Compound DDY10)
3.1.21. 2-(4-((4-(3-(Pyridin-3-ylmethyl)ureido)phenyl)sulfonyl)piperazine-1-carbonyl)-1H-benzo[d]imidazole-4-carboxamide (Compound DDY11)
3.2. Molecule Docking
3.3. Determination of Inhibition Rates and IC50 Values against NAMPT and PARP1
3.4. Cell Culture
3.5. Cell Viability Assay
3.6. Western Blot Analysis
3.7. Apoptosis Assays
3.8. Clonogenic Assay
3.9. Cell Migration Assay
3.10. Comet Assay
3.11. NAD Level Determination Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaku, K.; Okabe, K.; Hikosaka, K.; Nakagawa, T. NAD Metabolism in Cancer Therapeutics. Front. Oncol. 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Navas, L.E.; Carnero, A. Nicotinamide Adenine Dinucleotide (NAD) Metabolism as a Relevant Target in Cancer. Cells 2022, 11, 2627. [Google Scholar] [CrossRef] [PubMed]
- Navas, L.E.; Carnero, A. NAD metabolism, stemness, the immune response, and cancer. Signal Transduct. Target. Ther. 2021, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Chiarugi, A.; Dolle, C.; Felici, R.; Ziegler, M. The NAD metabolome—A key determinant of cancer cell biology. Nat. Rev. Cancer 2012, 12, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Heske, C.M. Beyond Energy Metabolism: Exploiting the Additional Roles of NAMPT for Cancer Therapy. Front. Oncol. 2020, 9, 1514. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wang, L.; Wang, T.; Yang, J.; Zheng, S.; Tong, J.; Jiang, S.; Zhang, X.; Zhang, K. Recent advances of targeting nicotinamide phosphoribosyltransferase (NAMPT) for cancer drug discovery. Eur. J. Med. Chem. 2023, 258, 115607. [Google Scholar] [CrossRef]
- Wei, Y.; Xiang, H.; Zhang, W. Review of various NAMPT inhibitors for the treatment of cancer. Front. Pharmacol. 2022, 13, 970553. [Google Scholar] [CrossRef]
- Galli, U.; Travelli, C.; Massarotti, A.; Fakhfouri, G.; Rahimian, R.; Tron, G.C.; Genazzani, A.A. Medicinal chemistry of nicotinamide phosphoribosyltransferase (NAMPT) inhibitors. J. Med. Chem. 2013, 56, 6279–6296. [Google Scholar] [CrossRef]
- Korotchkina, L.; Kazyulkin, D.; Komarov, P.G.; Polinsky, A.; Andrianova, E.L.; Joshi, S.; Gupta, M.; Vujcic, S.; Kononov, E.; Toshkov, I.; et al. OT-82, a novel anticancer drug candidate that targets the strong dependence of hematological malignancies on NAD biosynthesis. Leukemia 2020, 34, 1828–1839. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Liu, F.; Li, S.; Shi, D. Rational Multitargeted Drug Design Strategy from the Perspective of a Medicinal Chemist. J. Med. Chem. 2021, 64, 10581–10605. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Huang, Y.H.; Chen, S.Q.; Wu, S.C.; Dong, G.Q.; Sheng, C.Q. Nicotinamide Phosphoribosyltransferase (NAMPT) Is a New Target of Antitumor Agent Chidamide. ACS Med. Chem. Lett. 2020, 11, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.J.; Szabo, C. Poly(ADP-ribose) polymerase inhibition: Past, present and future. Nat. Rev. Drug Discov. 2020, 19, 711–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Wang, P.Y.; Wang, Y.T.; Yang, G.F.; Zhang, A.; Miao, Z.H. An Update on Poly(ADP-ribose)polymerase-1 (PARP-1) Inhibitors: Opportunities and Challenges in Cancer Therapy. J. Med. Chem. 2016, 59, 9575–9598. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.X.; Jiang, T.; Long, J.; Ma, Z.Y.; Lu, A.P.; Cheng, Y.; Cao, D.S. The ups and downs of Poly(ADP-ribose) Polymerase-1 inhibitors in cancer therapy-Current progress and future direction. Eur. J. Med. Chem. 2020, 203, 112570. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Li, H.; Chen, L.; Yao, D. Dual-target inhibitors of PARP1 in cancer therapy: A drug discovery perspective. Drug Discov. Today 2023, 28, 103607. [Google Scholar] [CrossRef]
- Martínez-Morcillo, F.J.; Cantón-Sandoval, J.; Martínez-Navarro, F.J.; Cabas, I.; Martínez-Vicente, I.; Armistead, J.; Hatzold, J.; López-Muñoz, A.; Martínez-Menchón, T.; Corbalán-Vélez, R.; et al. NAMPT-derived NAD+ fuels PARP1 to promote skin inflammation through parthanatos cell death. PLoS Biol. 2021, 19, e3001455. [Google Scholar] [CrossRef]
- Bajrami, I.; Kigozi, A.; Van, W.A.; Brough, R.; Frankum, J.; Lord, C.J.; Ashworth, A. Synthetic lethality of PARP and NAMPT inhibition in triple-negative breast cancer cells. EMBO Mol. Med. 2012, 4, 1087–1096. [Google Scholar] [CrossRef]
- Heske, C.M.; Davis, M.I.; Baumgart, J.T.; Wilson, K.; Gormally, M.V.; Chen, L.; Zhang, X.; Ceribelli, M.; Duveau, D.Y.; Guha, R.; et al. Matrix Screen Identifies Synergistic Combination of PARP Inhibitors and Nicotinamide Phosphoribosyltransferase (NAMPT) Inhibitors in Ewing Sarcoma. Clin. Cancer Res. 2017, 23, 7301–7311. [Google Scholar] [CrossRef]
- Sauriol, A.; Carmona, E.; Udaskin, M.L.; Radulovich, N.; Leclerc-Desaulniers, K.; Rottapel, R.; Oza, A.M.; Lheureux, S.; Provencher, D.M.; Mes-Masson, A.-M. Inhibition of nicotinamide dinucleotide salvage pathway counters acquired and intrinsic poly(ADP-ribose) polymerase inhibitor resistance in high-grade serous ovarian cancer. Sci. Rep. 2023, 13, 3334. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, X.; Yang, C.; Miao, Z.; Song, S.; Huan, X.; Chen, Y.; Ding, J. Phthalazone Derivative and Preparation Method and Application Thereof. CN110194762. 10 September 2021. [Google Scholar]
- Jiang, S.; Zhang, K.; Liao, J.; Wang, D.; Ni, Y.; Wang, T.; Zhang, X. Preparation of Phthalozinone Compounds and Its Medical Application. CN113788827. 25 April 2023. [Google Scholar]
- Zheng, X.; Bauer, P.; Baumeister, T.; Buckmelter, A.J.; Caligiuri, M.; Clodfelter, K.H.; Han, B.; Ho, Y.-C.; Kley, N.; Lin, J.; et al. Structure-Based Identification of Ureas as Novel Nicotinamide Phosphoribosyltransferase (Nampt) Inhibitors. J. Med. Chem. 2013, 56, 4921–4937. [Google Scholar] [CrossRef]
- Xu, T.-Y.; Zhang, S.-L.; Dong, G.-Q.; Liu, X.-Z.; Wang, X.; Lv, X.-Q.; Qian, Q.-J.; Zhang, R.-Y.; Sheng, C.-Q.; Miao, C.-Y. Discovery and characterization of novel small-molecule inhibitors targeting nicotinamide phosphoribosyltransferase. Sci. Rep. 2015, 5, 10043. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Chen, W.; Wang, X.; Yang, X.; Xu, T.; Wang, P.; Zhang, W.; Rao, Y.; Miao, C.; Sheng, C. Small Molecule Inhibitors Simultaneously Targeting Cancer Metabolism and Epigenetics: Discovery of Novel Nicotinamide Phosphoribosyltransferase (NAMPT) and Histone Deacetylase (HDAC) Dual Inhibitors. J. Med. Chem. 2017, 60, 7965–7983. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dong, G.; Wu, Y.; Zhang, W.; Miao, C.; Sheng, C. Dual NAMPT/HDAC Inhibitors as a New Strategy for Multitargeting Antitumor Drug Discovery. ACS Med. Chem. Lett. 2018, 9, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Wu, Y.; Cheng, J.; Chen, L.; Liu, R.; Ding, Y.; Wu, S.; Ma, J.; Sheng, C. Ispinesib as an Effective Warhead for the Design of Autophagosome-Tethering Chimeras: Discovery of Potent Degraders of Nicotinamide Phosphoribosyltransferase (NAMPT). J. Med. Chem. 2022, 65, 7619–7628. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pu, C.; Fu, Y.; Dong, G.; Huang, M.; Sheng, C. NAMPT-targeting PROTAC promotes antitumor immunity via suppressing myeloid-derived suppressor cell expansion. Acta Pharm. Sin. B 2022, 12, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Cheng, J.; He, S.; Fang, Y.; Huang, M.; Sheng, C.; Dong, G. Discovery of Highly Potent Nicotinamide Phosphoribosyltransferase Degraders for Efficient Treatment of Ovarian Cancer. J. Med. Chem. 2023, 66, 1048–1062. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Dong, G.; Wu, Y.; Dong, G.; Du, L.; Li, M.; Sheng, C. Fluorescent and theranostic probes for imaging nicotinamide phosphoribosyl transferase (NAMPT). Eur. J. Med. Chem. 2023, 248, 115080. [Google Scholar] [CrossRef] [PubMed]
- Menear, K.A.; Adcock, C.; Alonso, F.C.; Blackburn, K.; Copsey, L.; Drzewiecki, J.; Fundo, A.; Le Gall, A.; Gomez, S.; Javaid, H.; et al. Novel alkoxybenzamide inhibitors of poly(ADP-ribose) polymerase. Bioorg. Med. Chem. Lett. 2008, 18, 3942–3945. [Google Scholar] [CrossRef]
- Menear, K.A.; Adcock, C.; Boulter, R.; Cockcroft, X.L.; Copsey, L.; Cranston, A.; Dillon, K.J.; Drzewiecki, J.; Garman, S.; Gomez, S.; et al. 4-[3-(4-cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin- 1-one: A novel bioavailable inhibitor of poly(ADP-ribose) polymerase-1. J. Med. Chem. 2008, 51, 6581–6591. [Google Scholar] [CrossRef]
- Giannini, G.; Battistuzzi, G.; Vesci, L.; Milazzo, F.M.; De Paolis, F.; Barbarino, M.; Guglielmi, M.B.; Carollo, V.; Gallo, G.; Artali, R.; et al. Novel PARP-1 inhibitors based on a 2-propanoyl-3H-quinazolin-4-one scaffold. Bioorg. Med. Chem. Lett. 2014, 24, 462–466. [Google Scholar] [CrossRef]
- Zhou, J.; Ji, M.; Zhu, Z.; Cao, R.; Chen, X.; Xu, B. Discovery of 2-substituted 1H-benzo d immidazole-4-carboxamide derivatives as novel poly(ADP-ribose)polymerase-1 inhibitors with in vivo anti-tumor activity. Eur. J. Med. Chem. 2017, 132, 26–41. [Google Scholar] [CrossRef]
- Lu, G.; Nie, W.; Xin, M.; Meng, Y.; Gu, J.; Miao, H.; Cheng, X.; Chan, A.S.C.; Zou, Y. Design, synthesis, biological evaluation and molecular docking study of novel urea-based benzamide derivatives as potent poly(ADP-ribose) polymerase-1 (PARP-1) inhibitors. Eur. J. Med. Chem. 2022, 243, 114790. [Google Scholar] [CrossRef]
- Bouchalova, K.; Svoboda, M.; Kharaishvili, G.; Vrbkova, J.; Bouchal, J.; Trojanec, R.; Koudelakova, V.; Radova, L.; Cwiertka, K.; Hajduch, M.; et al. BCL2 is an independent predictor of outcome in basal-like triple-negative breast cancers treated with adjuvant anthracycline-based chemotherapy. Tumor Biol. 2015, 36, 4243–4252. [Google Scholar] [CrossRef]
- Lindner, A.U.; Lucantoni, F.; Varešlija, D.; Resler, A.; Murphy, B.M.; Gallagher, W.M.; Hill, A.D.K.; Young, L.S.; Prehn, J.H.M. Low cleaved caspase-7 levels indicate unfavourable outcome across all breast cancers. J. Mol. Med. 2018, 96, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, J.; Hoffarth, S.; Huber, C.; Schuler, M. The DNA damage-induced decrease of Bcl-2 is secondary to the activation of apoptotic effector caspases. Oncogene 2003, 22, 6852–6856. [Google Scholar] [CrossRef] [PubMed]
- Daniels, V.W.; Zoeller, J.J.; van Gastel, N.; McQueeney, K.E.; Parvin, S.; Potter, D.S.; Fell, G.G.; Ferreira, V.G.; Yilma, B.; Gupta, R.; et al. Metabolic perturbations sensitize triple-negative breast cancers to apoptosis induced by BH3 mimetics. Sci. Signal 2021, 14, eabc7405. [Google Scholar] [CrossRef]
- Rifaï, K.; Idrissou, M.; Penault-Llorca, F.; Bignon, Y.-J.; Bernard-Gallon, D. Breaking down the Contradictory Roles of Histone Deacetylase SIRT1 in Human Breast Cancer. Cancers 2018, 10, 409. [Google Scholar] [CrossRef]
- Schacke, M.; Kumar, J.; Colwell, N.; Hermanson, K.; Folle, G.A.; Nechaev, S.; Dhasarathy, A.; Lafon-Hughes, L. PARP-1/2 Inhibitor Olaparib Prevents or Partially Reverts EMT Induced by TGF-β in NMuMG Cells. Int. J. Mol. Sci. 2019, 20, 518. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef]


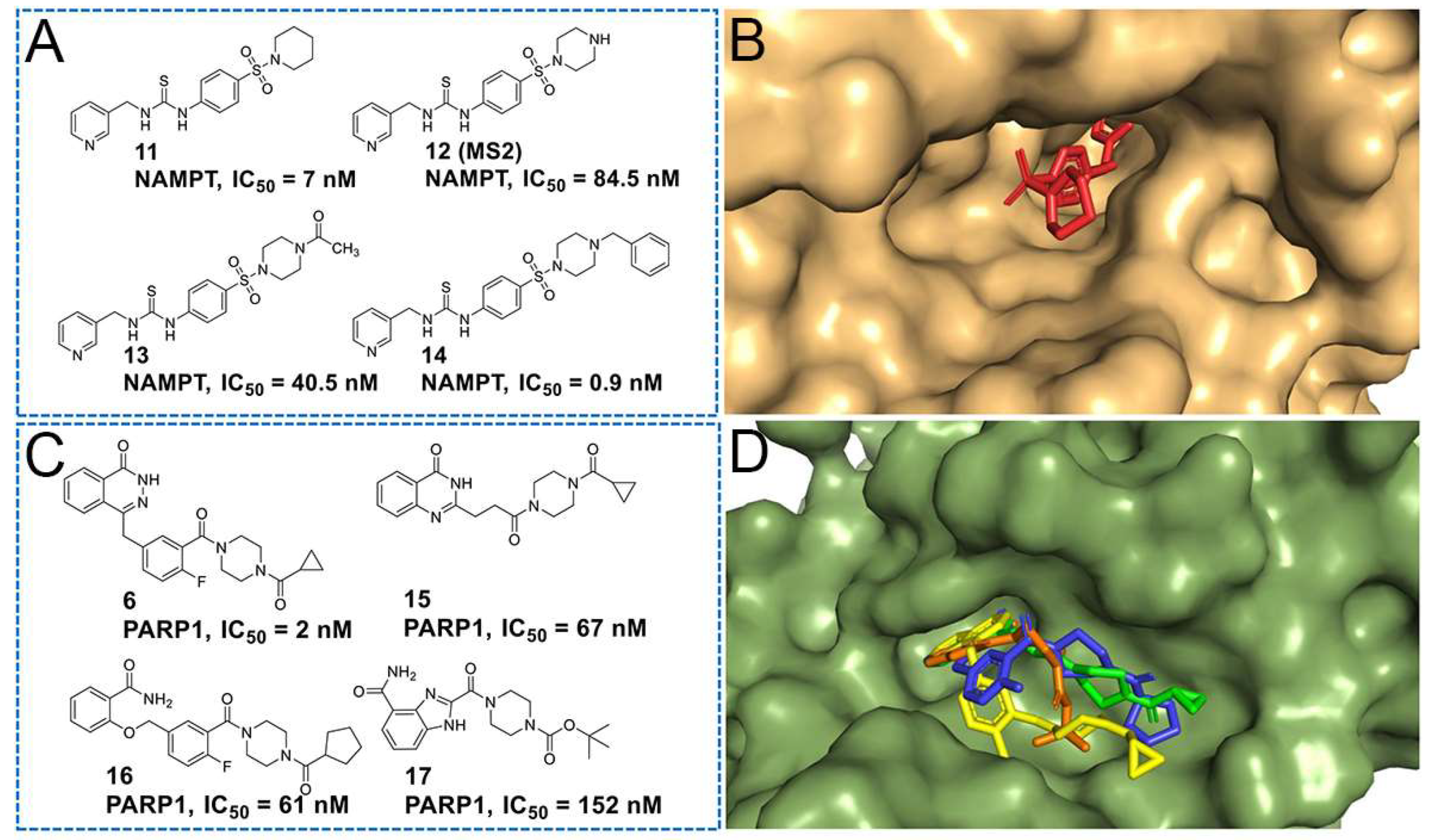



| Compound | R | X | n | R1 | R2 | NAMPT IC50 (μM) a or Inhibition Rate (0.1 μM, %) b | PARP1 IC50 (μM) a or Inhibition Rate (0.5 μM, %) b |
|---|---|---|---|---|---|---|---|
| FK866 | — | — | — | — | — | 0.012 ± 0.004 | — |
| Olaparib | — | — | — | — | — | — | 0.002 ± 0.001 |
| DDY01 | P1 | S | 0 | — | — | 83% | 0.009 ± 0.002 |
| DDY02 | P1 | O | 0 | — | — | 0.010 ± 0.003 | 0.050 ± 0.010 |
| DDY03 | P2 | O | 0 | — | — | 0.055 ± 0.019 | 0.025 ± 0.007 |
| DDY04 | P3 | S | 0 | H | F | 84% | 70% |
| DDY05 | P3 | O | 0 | H | F | 86% | 1.879 ± 0.600 |
| DDY06 | P3 | O | 1 | H | F | 48% | 89% |
| DDY07 | P3 | O | 0 | F | F | 84% | 92% |
| DDY08 | P3 | O | 0 | H | H | 80% | 69% |
| DDY09 | P3 | O | 0 | F | H | 86% | 78% |
| DDY10 | P4 | S | 0 | — | — | 7% | 55% |
| DDY11 | P4 | O | 0 | — | — | 0.034 ± 0.009 | 0.014 ± 0.003 |
| Compound | R | X | n | R1 | R2 | Cytotoxicity IC50 (μM) * | ||
|---|---|---|---|---|---|---|---|---|
| MDA-MB-231 | MDA-MB-468 | MCF-7 | ||||||
| FK866 | — | — | — | — | — | 3.83 ± 0.12 | 4.01 ± 0.21 | 5.8 ± 0.86 |
| Olaparib | — | — | — | — | — | 6.74 ± 0.08 | 6.19 ± 0.09 | 8.68 ± 0.81 |
| FK866 + Olaparib (1:1) | — | — | — | — | — | 3.14 ± 0.09 | 3.59 ± 0.10 | 5.31 ± 0.03 |
| DDY01 | P1 | S | 0 | — | — | 6.17 ± 0.27 | 5.09 ± 0.09 | 8.48 ± 0.18 |
| DDY02 | P1 | O | 0 | — | — | 10.43 ± 0.16 | 4.43 ± 0.04 | 7.61 ± 0.14 |
| DDY03 | P2 | O | 0 | — | — | 10.88 ± 0.68 | 8.17 ± 0.31 | 11.23 ± 0.79 |
| DDY04 | P3 | S | 0 | H | F | 13.48 ± 0.66 | 14.06 ± 0.3 | 13.37 ± 2.41 |
| DDY05 | P3 | O | 0 | H | F | 10.22 ± 0.29 | 8.02 ± 0.74 | >20 |
| DDY06 | P3 | O | 1 | H | F | 15.36 ± 2.15 | 17.16 ± 1.57 | 7.95 ± 0.34 |
| DDY07 | P3 | O | 0 | F | F | 8.1 ± 0.71 | 7.43 ± 0.23 | 13.47 ± 0.16 |
| DDY08 | P3 | O | 0 | H | H | 11.09 ± 0.19 | 12.89 ± 1.69 | 9.18 ± 0.44 |
| DDY09 | P3 | O | 0 | F | H | 8.7 ± 0.24 | 6.98 ± 0.57 | 11.01 ± 0.82 |
| DDY10 | P4 | S | 0 | — | — | 4.45 ± 0.29 | 6.74 ± 0.16 | 8.83 ± 0.31 |
| DDY11 | P4 | O | 0 | — | — | 13.83 ± 0.33 | 12.22 ± 1.39 | 7.39 ± 0.34 |
| Compound | Cell Survival Rate (3.0 μM, %) * | Cytotoxicity IC50 * |
|---|---|---|
| FK866 | 65.49 ± 0.41 | 4.41 ± 0.22 |
| Olaparib | 91.76 ± 1.78 | 9.61 ± 0.77 |
| Olaparib + FK866 | 65.31 ± 0.03 | — |
| DDY01 | 67.1 ± 1.73 | — |
| DDY02 | 70.28 ± 1.06 | 5.31 ± 0.12 |
| DDY03 | 80.71 ± 1.5 | — |
| DDY04 | 98.38 ± 1.74 | — |
| DDY05 | 88.65 ± 2.41 | — |
| DDY06 | 69.46 ± 2.56 | — |
| DDY07 | 70.73 ± 0.07 | — |
| DDY08 | 72.63 ± 3.46 | — |
| DDY09 | 66.93 ± 2.45 | — |
| DDY10 | 96.87 ± 1.71 | — |
| DDY11 | 82.15 ± 0.21 | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Kong, X.; Chu, X.; Fu, H.; Feng, X.; Zhao, C.; Deng, Y.; Ge, J. Targeting NAD Metabolism: Rational Design, Synthesis and In Vitro Evaluation of NAMPT/PARP1 Dual-Target Inhibitors as Anti-Breast Cancer Agents. Molecules 2024, 29, 2836. https://doi.org/10.3390/molecules29122836
Li Y, Kong X, Chu X, Fu H, Feng X, Zhao C, Deng Y, Ge J. Targeting NAD Metabolism: Rational Design, Synthesis and In Vitro Evaluation of NAMPT/PARP1 Dual-Target Inhibitors as Anti-Breast Cancer Agents. Molecules. 2024; 29(12):2836. https://doi.org/10.3390/molecules29122836
Chicago/Turabian StyleLi, Yingpeng, Xianxiu Kong, Xinhong Chu, Hui Fu, Xinchi Feng, Chengcheng Zhao, Yanru Deng, and Jun Ge. 2024. "Targeting NAD Metabolism: Rational Design, Synthesis and In Vitro Evaluation of NAMPT/PARP1 Dual-Target Inhibitors as Anti-Breast Cancer Agents" Molecules 29, no. 12: 2836. https://doi.org/10.3390/molecules29122836
APA StyleLi, Y., Kong, X., Chu, X., Fu, H., Feng, X., Zhao, C., Deng, Y., & Ge, J. (2024). Targeting NAD Metabolism: Rational Design, Synthesis and In Vitro Evaluation of NAMPT/PARP1 Dual-Target Inhibitors as Anti-Breast Cancer Agents. Molecules, 29(12), 2836. https://doi.org/10.3390/molecules29122836








