From Stem to Spectrum: Phytochemical Characterization of Five Equisetum Species and Evaluation of Their Antioxidant Potential
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Comparison of Five Equisetum Species
2.1.1. Hydroxybenzoic Acids
2.1.2. Hydroxycinnamic Acid Derivatives
2.1.3. Flavonoids
2.1.4. Stilbenoids
2.1.5. Further Minor Compounds
2.1.6. Phytochemical Comparison of the Equisetum Species
| Compound No. | tR [min] | Peak Assignment | Compound Class | λmax [nm] | Mass Spectrometric Data [m/z]/ [M-H]− | Reference | Detection | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS1 (Pseudomolecular Ion/Species) | MS² | MS³ | MS4 | EA | ET | EP | ES | EH | ||||||
| 1 | 2.0 | Sucrose dimer | Others | ND [a] | 683 | 341 | 179, 142, 113 | [27] | × | × | √ | √ | √ | |
| 2 | 13.1 | p-Hydroxybenzoic acid-O-hexoside Dimer | HBA | 206, 360 | 599 | 299 | 137 | [28] | × | √ | × | × | × | |
| 3 | 13.3 | Dihydroxybenzoic acid hexoside isomer | HBA | 206, 362 | 315 | 153 | 109 | [28] | × | × | × | × | √ | |
| 4 | 13.4 | p-Hydroxybenzoic acid O-hexoside | HBA | 252 | 299 | 137 | [28] | × | × | √ | × | × | ||
| 5 | 13.7 | Palustrine [c] | Others | 360, 380 | 310 | 157 | [9] | × | × | √ | × | × | ||
| 6 | 14.0 | Dihydroxybenzoic acid hexoside isomer | HBA | 206, 316, 360 | 315 | 153 | 109 | [28] | × | × | √ | √ | × | |
| 7 | 14.1 | 3′,4′-Dihydroxypropiophenone-3-O-glucoside | Others | 360, 388 | 327 | 165, 137 | t. a. | × | √ | × | × | × | ||
| 8 | 15.5 | Vanillic acid hexose isomer | HBA | 206, 228, 282 | 329 | 167, 151 | [29] | × | × | × | √ | √ | ||
| 9 | 15.8 | Caffeoylputrescine | Others | 238, 318 | 249 | 207, 178, 135 | t. a. | × | √ | × | × | × | ||
| 10 | 16.1 | Acetylspermidine | Others | 390 | 188 | 146 | 118 | t. a. [9] | × | × | √ | × | × | |
| 11 | 16.2 | Hydroxyphenylethyl-coumaroyl-hexoside | HCA | ND [a] | 445 | 137, 179 | t. a. | × | √ | × | × | × | ||
| 12 | 17.6 | Caffeoyl hexose | HCA | 294, 310, 316 | 341 | 179 | 135 | [29] | √ | × | √ | × | √ | |
| 13 | 17.7 | Dihydrochalcone C-hexoside | Others | ND [a] | 329 | 209 | 167, 125, 191 | t. a. | × | √ | × | × | × | |
| 14 | 17.8 | Vanillic acid hexose isomer | HBA | 206, 228, 282 | 329 | 167 | [29] | × | × | × | √ | × | ||
| 15 | 18.1 | Caffeoyl-coumaroyl-hexoside | HCA | 382 | 487 | 341, 179, 135 | 159 | t. a. | × | √ | × | × | × | |
| 16 | 18.8 | Caftaric acid | HCA | 206 | 311 | 149, 179, 135 | [30] [31] | × | × | × | √ | × | ||
| 17 | 19.3 | Hydrocaffeic acid hexoside | HCA | 202, 384 | 343 | 181 | 137 | [29] | × | √ | × | √ | × | |
| 18 | 19.5 | Quercetin-tri-O-hexoside isomer | FA | 274, 326 [f] | 787 | 625 | 300 | 255 | [32] [23] | × | × | √ | × | × |
| 19 | 19.5 | Feruloyl hexose Dimer | HCA | 216, 232, 288, 308 | 711 | 355 | 193 | [29] | √ | × | × | × | √ | |
| 20 | 19.7 | Feruloyl hexose isomer | HCA | 316 | 401 [d] | 355 | 193 | 149 | t. a. | × | √ | × | × | × |
| 21 | 19.8 | Caffeoyl hexose isomer | HCA | 230, 292 | 341 | 179 | 135 | [29] | √ | × | × | × | × | |
| 22 | 20.2 | Caftaric acid dimer | HCA | 220, 328 | 623 | 311 | 179, 149, 135 | [30] [31] | √ | × | × | √ | × | |
| 23 | 20.5 | Hydro-Ferulic acid-hexoside | HCA | 266, 320, 324 | 357 | 195 | 136 | [29] | × | √ | × | × | √ | |
| 24 | 20.9 | Kaempferol 3-diglucoside-7-glucoside | FA | 266, 346 | 771 | 609 | 284, 429 | 255 | [23] | × | × | √ | × | × |
| 25 | 21.7 | 18-Desoxypalustrine [c] | Others | ND [a] | 294 | 157, 251 | t. a. [9] | × | × | √ | × | × | ||
| 26 | 23.0 | Myricetin dihexoside | FA | 360 | 641 | 479 | 317 | 271, 244 | t. a. [33] | × | × | √ | × | × |
| 27 | 23.0 | Feruloyl hexose dimer | HCA | 292 | 711 | 355 | 193, 149 | t. a. [29] | × | × | × | × | √ | |
| 28 | 23.1 | Feruloyl hexose isomer | HCA | 304 | 355 | 193 | 149, 178 | [29] | √ | × | × | × | × | |
| 29 | 23.2 | Methyl-phloretic acid glucoside | HCA | 200, 280 | 343 | 181 | 166 | t. a. | × | × | × | √ | × | |
| 30 | 23.4 | Caffeoyl hexose isomer | HCA | 382 | 341 | 179 | [29] | × | √ | × | × | × | ||
| 31 | 23.5 | Feruloyl hexose isomer | HCA | 204, 310 | 355 | 193 | 178, 134, 149 | [29] | × | × | √ | √ | × | |
| 32 | 23.9 | Quercetin diglucoside isomer | FA | 256, 352 | 625 | 463 | 301 | 271 | [29] | √ | × | × | × | × |
| 33 | 24.1 | Quercetin-tri-O-hexoside isomer | FA | 270, 352 | 787 | 625 | 300 | 255, 271 | [32] | × | × | × | × | √ |
| 34 | 24.6 | Coutaric acid | HCA | 206, 228, 310 | 295 | 149, 163 | 131 | [30] | × | × | √ | √ | × | |
| 35 | 25.3 | Quercetin-di-O-hexoside isomer | FA | 258, 352 | 625 | 463 | 301, 343 | 271, 255 | [34] | × | × | √ | × | × |
| 36 | 26.5 | Quercetin-p-coumaroyl-di-hexoside | FA | 266, 358 | 771 | 609 | 301, 271 | 256, 151 | [b] t. a. | × | × | √ | × | × |
| 37 | 26.8 | p-Coumaroyl-pentose | HCA | 204, 310 | 295 | 163 | 119 | [35] | × | × | √ | √ | × | |
| 38 | 29.0 | 1-O-Sinapoyl-glucoside isomer | HCA | 230, 284, 360, 380 | 433 | 387 | 163 | [30] [36] | × | √ | × | × | × | |
| 39 | 29.2 | Feruloyl-pentose isomer | HCA | 322 | 325 | 193 | 134 | [35] | × | × | × | √ | × | |
| 40 | 29.7 | Caffeoylmalic acid dimer isomere | HCA | 218, 326 | 591 | 295 | 179 | [37] | × | √ | × | × | × | |
| 41 | 29.8 | Caffeoylmalic acid | HCA | 220, 326 | 295 | 179, 133,115 | [30] | √ | × | × | × | × | ||
| 42 | 30.0 | Methyl-kaempferol dihexoside isomer | FA | 230, 274 [f] | 625 | 463 | 300 | 255, 271 | [30] [38] | × | × | √ | × | × |
| 43 | 30.8 | Kaempferol-O-dihexoside isomer | FA | 264, 342 | 609 | 447 | 284 | 255 | [30] | √ | √ | × | × | × |
| 44 | 31.2 | Caffeoylmalic acid dimer isomere | HCA | 242, 328 | 591 | 295 | 179, 133 | [30] | × | × | √ | × | × | |
| 45 | 31.4 | Methyl-kaempferol dihexoside isomer | FA | 280 [f] | 419 | 299 | 255 | [30] [38] | × | √ | × | × | × | |
| 46 | 31.5 | Feruloyl-sulfonyl-malate-hexoside | HCA | 234, 324 | 551 | 389, 193 | 134, 149 | t. a. | × | × | × | × | √ | |
| 47 | 31.7 | Feruloyl-pentose isomer | HCA | 326 | 325 | 193 | 134 | [35] | × | × | × | √ | × | |
| 48 | 32.1 | Kaempferol-O-dihexoside isomer | FA | 264 [f] | 609 | 447 | 284 | 255 | [23] | × | × | √ | × | × |
| 49 | 32.5 | Kaempferol-coumaroyl diglucoside | FA | 266, 346 | 755 | 593 | 285 | 257 | [29] | × | √ | × | × | × |
| 50 | 32.8 | Vicenin 2 | FA | 328 [f] | 593 | 473, 383, 353 | 297, 191 | [24] | √ | × | × | × | × | |
| 51 | 33.4 | Kaempferol-coumaroyl diglucoside isomer | FA | 266, 346 | 755 | 593 | 285 | 257 | [29] | × | × | √ | × | × |
| 52 | 33.8 | Maclurin-O-hexoside isomer | Others | 260, 374 | 423 | 261, 287 | 217 | t. a. | × | × | × | × | √ | |
| 53 | 34.2 | 1-O-Sinapoyl-glucoside isomer | HCA | ND [a] | 431 [d] | 385 | 205, 179 | [30] | × | × | × | × | √ | |
| 54 | 35.0 | Feruloyl-sulfonyl-malate-hexoside isomer | HCA | 310 | 551 | 389 | 193 | t. a. | × | × | × | × | √ | |
| 55 | 35.2 | Maclurin-O-hexoside isomer | Others | 228, 370 | 423 | 261 | 217 | t. a. | × | × | √ | × | × | |
| 56 | 36.9 | Quercetin-acetyl-di-hexoside | FA | 266 [f] | 651 | 489 | 285 | 255 | t. a. | √ | × | × | × | × |
| 57 | 37.5 | Kaempferol-3-O-6″-malonyl-diglucoside | FA | 266, 344 | 695 | 651 | 489 | 284 | [39] | × | × | × | √ | × |
| 58 | 38.1 | Caffeoyl derivative | HCA | 308 | 504 | 179, 342 | 135 | [40] | × | × | × | × | √ | |
| 59 | 38.3 | Hydrocaffeic acid-acetyl-hexoside | HCA | ND [a] | 385 | 325 | 181 | 166 | t. a. | × | × | × | √ | × |
| 60 | 38.9 | Ferulic acid (6-acetyl-hexoside) | HCA | 308 | 397 | 193 | 149, 134 | t. a. [29] | × | × | × | × | √ | |
| 61 | 39.1 | Quercetin-diglucoside isomer | FA | 352 | 625 | 505 | 343, 300 | 271 | [29] | √ | × | × | × | × |
| 62 | 39.2 | Phloridzin | Others | ND | 435 | 273 | 255 | 107, 149 | [41] | × | √ | × | √ | × |
| 63 | 39.2 | Malic acid p-coumarate | HCA | 308 | 279 | 163, 133, 119 | [42] | × | × | √ | × | × | ||
| 64 | 39.6 | Kaempferol-acetyl-diglucoside | FA | 268, 346 | 651 | 489, 285 | 255 | [29] | × | √ | × | × | × | |
| 65 | 39.6 | p-Coumaric acid | HCA | 228, 312 | 163 | 119 | [36] | × | × | √ | × | × | ||
| 66 | 40.2 | Quercetin-3-glucoside-7-rhamnoside | FA | 204 [f] | 609 | 447, 301 | 271, 151 | [43] | × | × | × | √ | × | |
| 67 | 40.9 | Maclurin-malonyl-hexoside | Others | 228, 274, 360 | 423, 508 | 287, 261 | 99, 153 | t. a. | × | × | √ | × | × | |
| 68 | 41.4 | Quercetin-(caffeoyl)-glucoside | FA | 230, 370 | 625 | 301 | 151, 178.44 | [32] | × | × | √ | × | × | |
| 69 | 42.3 | Ferulic acid | HCA | 326 | 193 | 134, 178 | [36] | √ | × | × | × | √ | ||
| 70 | 43.0 | Genkwanin-6-C-hexoside | FA | 232, 250, 298 [f] | 509 | 463, 283 | 268 | t. a. | √ | × | × | × | × | |
| 71 | 43.0 | Myricetin-glucoside | FA | 260, 382 | 479 | 317 | 299 | 271(M-H)2- | [33] | × | × | √ | × | × |
| 72 | 43.1 | Caffeic acid/cinnamic acid dimer | HCA | 204, 314 | 455 | 309 | 112, 19 | t. a. | × | × | × | √ | × | |
| 73 | 43.4 | Quercetin 3-O-(4″-O-acetyl) rutinoside | FA | 356 | 695 [e] | 651 | 505, 301 | 271 | [44] | × | × | × | √ | × |
| 74 | 43.6 | 4-Deoxyphloridzin | Others | 204, 268, 346 | 465, 419 | 257 | 239 | 195 | [41] | × | √ | × | × | × |
| 75 | 43.7 | Kaempferol-caffeoyl-hexoside | FA | 266, 346sh | 609 | 285, 429 | 255 | t. a. | √ | × | √ | × | √ | |
| 76 | 44.4 | Kaempferol-3-hexoside-7-rhamnoside | FA | 266, 346 | 593 | 447, 285 | 284 | 255 | [43] | × | × | × | √ | × |
| 77 | 44.7 | unknown | - | 310 | 429 | 215, 149 | - | × | × | × | × | √ | ||
| 78 | 45.2 | Di-caffeoyl-cinnamic acid | HCA | 234, 282sh | 429, 489 | 265, 309 | 147 | t. a. | × | × | × | √ | × | |
| 79 | 45.5 | Caffeoyl hexose isomer | HCA | 310 | 342 | 180, 222,252, 282 | 207, 135 | [29] | √ | × | × | × | × | |
| 80 | 45.5 | Apigenin 6-C-hexoside | FA | ND [a] [f] | 449 | 269 | 207, 251 | t. a. | × | × | × | × | √ | |
| 81 | 45.8 | Quercetin-3-O-rutinoside (rutin) | FA | 258, 356 | 609 | 301 | 271, 178, 255 | [29] [34] | × | × | √ | √ | × | |
| 82 | 46.3 | Quercetin-O-hexoside | FA | 204, 256sh, 352sh | 463 | 301 | 178, 271, 255, 151 | [45] | √ | × | × | × | × | |
| 83 | 46.9 | Hyperoside (Quercetin 3-O-galactoside) | FA | 204, 228, 278sh [f] | 463 | 301 | 271, 151, 178 228 | [30] | × | × | × | √ | × | |
| 84 | 47.0 | 2″-O-Galloylvitexin | FA | 280 [f] | 583, 415 | 313 | 269 | t. a. | × | √ | × | × | × | |
| 85 | 47.3 | Ononin | FA | 322 [f] | 429 | 267 | 223 | 145 | t. a. | √ | × | × | × | × |
| 86 | 47.9 | Kaempferol-3-acetyl-glucoside-7-rhamnoside isomere | FA | 266, 346 | 679 [e] | 635 | 489 | 285 | t. a. [43] | × | × | × | √ | × |
| 87 | 48.1 | Apigenin-7-O-glucoside | FA | 330 [f] | 431 | 269 | 183, 149 | [46] | √ | × | × | × | × | |
| 88 | 48.9 | Dihydro-Pterostilbene-(6-malonyl-hexoside) | Stilbenoid | 280 | 505, 461 | 257 | 239,165,137,93 | t. a. [26] | × | √ | × | × | × | |
| 89 | 48.9 | Kaempferol-3-acetyl-glucoside-7-rhamnoside isomere | FA | 204, 230, 278 [f] | 635 | 489 | 284 | 255 | t. a. [43] | × | × | × | √ | × |
| 90 | 49.5 | Quercetin 3-(6″-acetylglucoside) | FA | 210, 256, 352 | 505 | 301 | 255, 178, 151 | [45] | √ | × | × | × | × | |
| 91 | 49.6 | Alkaloid | - | ND [a] | 473 | 160 | 112 | - | × | × | × | × | √ | |
| 92 | 50.0 | Kaempferol-coumaroyl glucoside | FA | 280 [f] | 593 | 285 | 257 | 226 | [29] | × | √ | × | × | × |
| 93 | 50.0 | Quercetin-O-hexoside isomer | FA | 236, 276sh [f] | 463 | 301 | 255, 271 | [34] | × | × | √ | × | × | |
| 94 | 50.4 | Quercetin-3-acetyl-glucoside isomer | FA | 204, 356 | 505 | 301 | 271, 255 | [45] | × | × | × | √ | × | |
| 95 | 50.7 | Kaempferol-coumaroyl glucoside | FA | 266, 346sh | 593 | 285 | 255, 229, 178 | [29] | × | × | √ | × | × | |
| 96 | 51.2 | Kaempferol glucoside | FA | 280 [f] | 447 | 285 | 255 | [29] | × | √ | × | × | × | |
| 97 | 51.6 | Quercetin 3-(6″-acetylglucoside) isomer | FA | 204, 256, 352 | 505 | 445, 301 | 271, 255, 151 | [45] | √ | × | × | × | × | |
| 98 | 52.1 | Quercetagetin-malonyl-hexoside | FA | 260, 384 | 565 | 521 | 317 | 299, 271, 255 | t. a. | × | × | √ | × | × |
| 99 | 52.1 | Quercetin-3-acetyl-glucoside isomer | FA | 232, 274sh, 360 | 505 | 301 | 271 | [45] | × | × | × | √ | × | |
| 100 | 52.2 | Methyl-Kaempferol-acetyl-glucoside | FA | 342 | 505 | 300 | 271, 255, 151 | [30] [38] | √ | × | × | × | × | |
| 101 | 52.7 | Schaftoside/ Isoschaftoside isomer (Apigenin-glucoside-arabinoside) | FA | 280 [f] | 563 | 503 | 341 | 311 | [24] | × | √ | × | × | × |
| 102 | 53.4 | Schaftoside/Isoschaftoside isomer (Apigenin-glucoside-arabinoside) | FA | 280 [f] | 563 | 503 | 341 | 311 | [24] | × | √ | × | × | × |
| 103 | 53.7 | Quercetin-malonyl-hexoside | FA | 256, 370 | 505, 549 | 301 | 151, 178, 205, 255 | (b) [b] t. a. | × | × | √ | × | × | |
| 104 | 54.3 | Dicaffeoyltartaric acid isomer | HCA | 244, 220, 328 | 473 | 311 | 179, 149, 135 | 87 | [47] [30] | √ | × | × | × | × |
| 105 | 55.1 | Flavonol C-hexoside isomer | FA | ND [a] [f] | 431 | 251 | 207, 163 | - | × | × | × | × | √ | |
| 106 | 56.2 | Dicaffeoyltartaric acid isomer | HCA | 238, 324 | 473 | 311 | 179, 149, 135 | [47] [30] | √ | × | × | × | × | |
| 107 | 56.5 | N-Formylpalustrine | Others | 336 | 635 | 468 | 244, 338 | 161, 201 | [9] | × | × | √ | × | × |
| 108 | 57.0 | Flavonol C-hexoside isomer | FA | ND [a] [f] | 431 | 251 | 207, 163 | - | × | × | × | × | √ | |
| 109 | 57.9 | N-Formylpalustrine isomer | Others | 336 | 635 | 468 | 244, 338 | 227, 202 | [9] | × | × | √ | × | × |
| 110 | 58.5 | Formononetin-acetyl-hexoside | FA | 312 [f] | 471, 413 | 267 | 223 | t. a. | √ | × | × | × | × | |
| 111 | 59.2 | Lunularic acid-hexoside | Stilbenoid | 236 | 419 | 257 | 213 | t. a. | × | × | × | √ | × | |
| 112 | 61.1 | Formononetin-malonyl-hexoside | FA | 322 [f] | 515 | 267 | 161 | t. a. | √ | × | × | × | × | |
| 113 | 63.6 | Lunularic acid- malonyl-hexoside | Stilbenoid | 238 | 505 | 461 | 213 | t. a. | × | × | × | √ | × | |
| 114 | 64.8 | Caffeic acid derivative | HCA | ND [a] | 457 | 179 | 119 | t. a. | × | × | × | × | √ | |
| 115 | 66.0 | 1,3-Dihydroxyanthraquinones acetyl-hexoside | Others | 224 | 443 | 401 | 239 | t. a. [48] | × | √ | × | √ | × | |
| 116 | 66.8 | Sinapoyl malate-hexosyl-pentoside | HCA | 230, 280sh | 635 | 501, 339 | 324 | 309 | t. a. | × | √ | × | × | × |
| 117 | 68.1 | Dicaffeoyl-quinic acid (Cynarin) | HCA | 234 | 515 | 335 | 291, 179 | t. a. | × | √ | × | × | × | |
| E. arvense | E. hyemale | E. palustre | E. sylvaticum | E. telmateia |
|---|---|---|---|---|
| Flavonoid acetyl-glycosides (quercetin derivative | Flavonoid tri-glycosides (e.g., quercetin tri-O-hexoside *) | Flavonoid tri-glycosides (e.g., quercetin tri-O-hexoside *) | Flavonoid acetyl-glycosides (kaempferol derivative) | Flavonoid acetyl-glycosides (kaempferol derivative) |
| Dicaffeoyltartaric acid | Ferulic acid derivatives | Myricetin di-hexoside * | Stilbenoid * (e.g., lunularic acid derivatives) | Stilbenoid * (e.g., pterostilbene derivative) |
| Vicenin 2 * |
2.2. Total Phenolic Content and Antioxidant Potential of Equisetum Extracts
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and Extraction
3.3. RP-HPLC-DAD-ESI-MSn Analysis
3.4. Folin–Ciocalteu Assay for the Determination of Total Phenolic Contents
- (a)
- Preparation of calibration standard solutions.
- (b)
- Preparation of sample test solutions.
- (c)
- Measurement.
3.5. 2.2-Diphenyl-1-picrylhydrazyl (DPPH) Assay for the Determination of Antioxidant Activity
- (a)
- Preparation of the DPPH solution
- (b)
- Preparation of calibration standard solutions.
- (c)
- Preparation of sample solutions.
- (d)
- Measurement.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lubienski, M. Die Schachtelhalme (Equisetaceae, Pteridophyta) der Flora Deutschlands—Ein aktualisierter Bestimmungsschlüssel. Veröff. Boch. Bot. Ver. 2010, 2, 82–100. [Google Scholar]
- Christenhusz, M.J.M.; Bangiolo, L.; Chase, M.W.; Fay, M.F.; Husby, C.; Witkus, M.; Viruel, J. Phylogenetics, classification and typification of extant horsetails (Equisetum, Equisetaceae). Bot. J. Linn. Soc. 2019, 189, 311–352. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Chase, M.W.; Fay, M.F.; Hidalgo, O.; Leitch, I.J.; Pellicer, J.; Viruel, J. Biogeography and genome size evolution of the oldest extant vascular plant genus, Equisetum (Equisetaceae). Ann. Bot. 2021, 127, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Elgorriaga, A.E.A. Origin of Equisetum: Evolution of horsetails (Equisetales) within the major euphyllophyte clade Sphenopsida. Am. J. Bot. 2018, 105, 1286–1303. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.W.; Puttick, M.N.; Donoghue, P.C.J. Origin of horsetails and the role of whole-genome duplication in plant macroevolution. Proc. Biol. Sci. 2019, 286, 20191662. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, J.; Jenipher, C.; Sriramavaratharajan, V.; Gurav, S.S.; Gandhi, G.R.; Ravichandran, K.; Ayyanar, M. Genus Equisetum L: Taxonomy, toxicology, phytochemistry and pharmacology. J. Ethnopharmacol. 2023, 314, 116630. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Flores, J.A.; Stubbs, T.L.; Benton, M.J. Macroevolutionary patterns in Rhynchocephalia: Is the tuatara (Sphenodon punctatus) a living fossil? Palaeontology 2017, 60, 319–328. [Google Scholar] [CrossRef]
- Eghlima, G.; Esmaeili, H.; Frzaneh, M.; Mirjalili, M.H. Multivariate analysis of Equisetum arvense L. ecotypes based on silicon content, phytochemical and morphological characterization. Silicon 2023, 16, 115–122. [Google Scholar] [CrossRef]
- Cramer, L.; Ernst, L.; Lubienski, M.; Papke, U.; Schiebel, H.-M.; Jerz, G.; Beuerle, T. Structural and quantitative analysis of Equisetum alkaloids. Phytochemistry 2015, 116, 269–282. [Google Scholar] [CrossRef]
- The European Pharmacopoeia Commission. Pharmacopoea Europaea, G.E. Schachtelhaltmkraut; Deutscher Apotheker Verlag: Eschborn, Germany, 2020; pp. 2401–2403. [Google Scholar]
- Gründemann, C.; Lengen, K.; Sauer, B.; Garcia-Käufer, M.; Zehl, M.; Huber, R. Equisetum arvense (common horsetail) modulates the function of inflammatory immunocompetent cells. BMC Complement. Altern. Med. 2014, 14, 283. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products. European Union Herbal Monograph on Equisetum arvense L.; European Medicines Agency: Amsterdam, The Netherlands, 2016.
- Yeganegi, M.; Tabatabaei Yazdi, F.; Mortazavi, S.A.; Asili, J.; Alizadeh Behbahani, B.; Beigbabaei, A. Equisetum telmateia extracts: Chemical compositions, antioxidant activity and antimicrobial effect on the growth of some pathogenic strain causing poisoning and infection. Microb. Pathog. 2018, 116, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Alves, C.F.; Bonez, P.C.; de Souza, M.E.; Da Cruz, R.C.; Boligon, A.A.; Piana, M.; Brum, T.F.; Rossi, G.G.; Da Jesus, R.S.; Grando, T.H.; et al. Antimicrobial, antitrypanosomal and antibiofilm activity of Equisetum hyemale. Microb. Pathog. 2016, 101, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, P.; Liu, Q.; Cheng, X.; Zhou, Y.; Xiao, Y. Cell cycle arrest and cell apoptosis induced by Equisetum hyemale extract in murine leukemia L1210 cells. J. Ethnopharmacol. 2012, 144, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, I.; Yesilada, E.; Ito, S. An anti-ulcerogenic flavonol diglucoside from Equisetum palustre L. J. Ethnopharmacol. 2009, 121, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tian, Y.; Sugimoto, S.; Yamano, Y.; Kawakami, S.; Otsuka, H.; Matsunami, K. Four new glucosides from the aerial parts of Equisetum sylvaticum. J. Nat. Med. 2022, 76, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Juurlink, B.H.J.; Azouz, H.J.; Aldalati, A.M.Z.; AlTinawi, B.M.H.; Ganguly, P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr. J. 2014, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Taher, E.A.; Sheikh, B.Y.; Anjum, S.; Saeed, A.; AlAjmi, M.F.; Moustafa, M.S.; Al-Mousawi, S.M.; Farag, M.A.; Hegazy, M.E.F.; et al. Hydroxycinnamic acids: Natural sources, biosynthesis, possible biological activities, and roles in islamic medicine. Stud. Nat. Prod. Chem. 2018, 55, 269–292. [Google Scholar] [CrossRef]
- Bengoechea, L.; Hernández, T.; Quesada, C.; Bartolomé, B.; Estrella, I.; Gómez-Cordovés, C. Structure of hydroxycinnamic acid derivatives established by high-performance liquid chromatography with photodiode-array detection. Chromatographia 1995, 41, 94–98. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Taniguchi, M.; LaRocca, C.A.; Bernat, J.D.; Lindsey, J.S. Digital database of absorption spectra of diverse flavonoids enables structural comparisons and quantitative evaluations. J. Nat. Prod. 2023, 86, 1087–1119. [Google Scholar] [CrossRef]
- Llorach, R.; Gil-Izquierdo, A.; Ferreres, F.; Tomás-Barberán, F.A. HPLC-DAD-MS/MS ESI characterization of unusual highly glycosylated acylated flavonoids from cauliflower (Brassica oleracea L. var. botrytis) agroindustrial byproducts. J. Agric. Food Chem. 2003, 51, 3895–3899. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Z.; Liao, X.; Zhou, C.; Xie, Z.; Zhu, S.; Wei, G.; Huang, Y. Identification of C-glycosyl flavones by high performance liquid chromatography electrospray ionization mass spectrometry and quantification of five main C-glycosyl flavones in Flickingeria fimbriata. BMC Chem. 2019, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Akinwumi, B.C.; Bordun, K.-A.M.; Anderson, H.D. Biological activities of stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, J.; Dong, P.; Li, Y.; Cui, Y.; Li, H.; Li, H.; Zhang, J.; Wang, S.; Dai, L. Comprehensive analysis of pterostilbene metabolites in vivo and in vitro using a UHPLC-Q-Exactive plus mass spectrometer with multiple data-mining methods. ACS Omega 2022, 7, 38561–38575. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lao, J.; Zhou, R.; He, W.; Qin, Y.; Zhong, C.; Xie, J.; Liu, H.; Wan, D.; Zhang, S.; et al. Simultaneous identification and dynamic analysis of saccharides during steam processing of rhizomes of Polygonatum cyrtonema by HPLC-QTOF-MS/MS. Molecules 2018, 23, 2855. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. High-throughput screening and characterization of phenolic compounds in stone fruits waste by LC-ESI-QTOF-MS/MS and their potential antioxidant activities. Antioxidants 2021, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Rodríguez-Pérez, C.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comprehensive, untargeted, and qualitative RP-HPLC-ESI-QTOF/MS2 metabolite profiling of green asparagus (Asparagus officinalis). J. Food Compos. Anal. 2016, 46, 78–87. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Pikula, K.; Manakov, Y.; Ercisli, S.; Derbush, I.; Kislin, E.; Seryodkin, I.; Sabitov, A.; Kalenik, T.; et al. LC-MS/MS screening of phenolic compounds in wild and cultivated grapes Vitis amurensis Rupr. Molecules 2021, 26, 3650. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Lima, N.; Vallverdú-Queralt, A.; Meudec, E.; Pinasseau, L.; Verbaere, A.; Bordignon-Luiz, M.T.; Le Guernevé, C.; Cheynier, V.; Sommerer, N. Quantification of hydroxycinnamic derivatives in wines by UHPLC-MRM-MS. Anal. Bioanal. Chem. 2018, 410, 3483–3490. [Google Scholar] [CrossRef]
- Francescato, L.N.; Debenedetti, S.L.; Schwanz, T.G.; Bassani, V.L.; Henriques, A.T. Identification of phenolic compounds in Equisetum giganteum by LC-ESI-MS/MS and a new approach to total flavonoid quantification. Talanta 2013, 105, 192–203. [Google Scholar] [CrossRef]
- Chang, Q.; Wong, Y.-S. Identification of flavonoids in Hakmeitau beans (Vigna sinensis) by high-performance liquid chromatography-electrospray mass spectrometry (LC-ESI/MS). J. Agric. Food Chem. 2004, 52, 6694–6699. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef]
- Verardo, V.; Bonoli, M.; Marconi, E.; Caboni, M.F. Distribution of bound hydroxycinnamic acids and their glycosyl esters in barley (Hordeum vulgare L.) air-classified flour: Comparative study between reversed phase-high performance chromatography-mass spectrometry (RP-HPLC/MS) and spectrophotometric analysis. J. Agric. Food Chem. 2008, 56, 11900–11905. [Google Scholar] [CrossRef] [PubMed]
- Sinosaki, N.; Tonin, A.; Ribeiro, M.; Poliseli, C.; Roberto, S.; Da Silveira, R.; Visentainer, J.; Santos, O.; Meurer, E. Structural study of phenolic acids by triple quadrupole mass spectrometry with electrospray ionization in negative mode and H/D isotopic exchange. J. Braz. Chem. Soc. 2020, 31, 402–408. [Google Scholar] [CrossRef]
- Grevsen, K.; Fretté, X.; Christensen, L.P. Concentration and composition of flavonol glycosides and phenolic acids in aerial parts of stinging nettle (Urtica dioica L.) are affected by high nitrogen fertilization and by harvest time. Eur. J. Hortic. Sci. 2008, 73, 20–27. [Google Scholar]
- Lorenz, P.; Bunse, M.; Klaiber, I.; Conrad, J.; Laumann-Lipp, T.; Stintzing, F.C.; Kammerer, D.R. Comprehensive phytochemical characterization of herbal parts from kidney vetch (Anthyllis vulneraria L.) by LC/MSn and GC/MS. Chem. Biodivers. 2020, 17, e2000485. [Google Scholar] [CrossRef]
- Vieira, M.N.; Winterhalter, P.; Jerz, G. Flavonoids from the flowers of Impatiens glandulifera Royle isolated by high performance countercurrent chromatography. Phytochem. Anal. 2016, 27, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiong, H.; Xu, X.; Xue, X.; Liu, M.; Xu, S.; Liu, H.; Gao, Y.; Zhang, H.; Li, X. Compounds identification in semen Cuscutae by ultra-high-performance liquid chromatography (UPLCs) coupled to electrospray ionization mass spectrometry. Molecules 2018, 23, 1199. [Google Scholar] [CrossRef] [PubMed]
- Hilt, P.; Schieber, A.; Yildirim, C.; Arnold, G.; Klaiber, I.; Conrad, J.; Beifuss, U.; Carle, R. Detection of phloridzin in strawberries (Fragaria x ananassa Duch.) by HPLC-PDA-MS/MS and NMR spectroscopy. J. Agric. Food Chem. 2003, 51, 2896–2899. [Google Scholar] [CrossRef]
- Maciejewska-Turska, M.; Zgórka, G. In-depth phytochemical and biological studies on potential AChE inhibitors in red and zigzag clover dry extracts using reversed-phase liquid chromatography (RP-LC) coupled with photodiode array (PDA) and electron spray ionization-quadrupole/time of flight-mass spectrometric (ESI-QToF/MS-MS) detection and thin-layer chromatography-bioautography. Food Chem. 2022, 375, 131846. [Google Scholar] [CrossRef]
- Rösch, D.; Krumbein, A.; Mügge, C.; Kroh, L.W. Structural investigations of flavonol glycosides from sea buckthorn (Hippophaë rhamnoides) pomace by NMR spectroscopy and HPLC-ESI-MS(n). J. Agric. Food Chem. 2004, 52, 4039–4046. [Google Scholar] [CrossRef]
- Jang, G.H.; Kim, H.W.; Lee, M.K.; Jeong, S.Y.; Bak, A.R.; Lee, D.J.; Kim, J.B. Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC-DAD-QTOF/MS. Saudi J. Biol. Sci. 2018, 25, 1622–1631. [Google Scholar] [CrossRef]
- Bunse, M.; Lorenz, P.; Stintzing, F.C.; Kammerer, D.R. Characterization of secondary metabolites in flowers of Sanguisorba officinalis L. by HPLC-DAD-MSn and GC/MS. Chem. Biodivers. 2020, 17, e1900724. [Google Scholar] [CrossRef]
- Plazonić, A.; Bucar, F.; Males, Z.; Mornar, A.; Nigović, B.; Kujundzić, N. Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules 2009, 14, 2466–2490. [Google Scholar] [CrossRef]
- Boeing, T.; Moreno, K.G.T.; Gasparotto Junior, A.; Da Mota Silva, L.; de Souza, P. Phytochemistry and pharmacology of the genus Equisetum (Equisetaceae): A narrative review of the species with therapeutic potential for kidney diseases. Hindawi 2021. [Google Scholar] [CrossRef]
- Dou, Z.; Dai, Y.; Zhou, Y.; Wang, S. Quality evaluation of rhubarb based on qualitative analysis of the HPLC fingerprint and UFLC-Q-TOF-MS/MS combined with quantitative analysis of eight anthraquinone glycosides by QAMS. Biomed. Chromatogr. 2021, 35, e5074. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Apak, R. Antioxidant activity/capacity measurement. 1. classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The chemistry behind the Folin-Ciocalteu method for the estimation of (poly)phenol content in food: Total phenolic intake in a Mediterranean dietary pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 2. hydrogen atom transfer (HAT)-based, mixed-mode (electron transfer (ET)/HAT), and lipid peroxidation assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef]
- Meščić Macan, A.; Gazivoda Kraljević, T.; Raić-Malić, S. Therapeutic perspective of vitamin C and its derivatives. Antioxidants 2019, 8, 247. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, C.Y. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit. Rev. Food Sci. Nutr. 2004, 44, 253–273. [Google Scholar] [CrossRef]


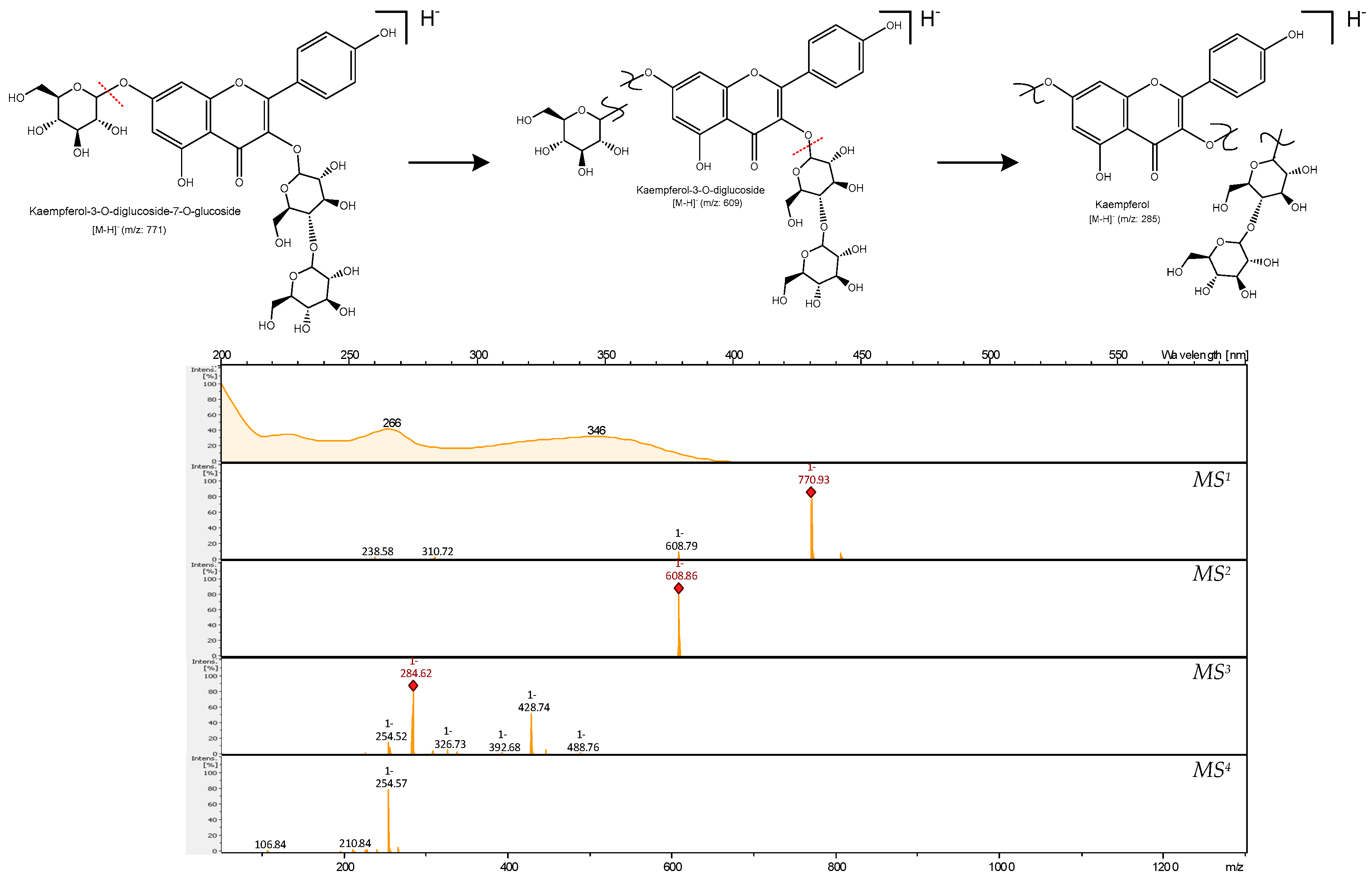

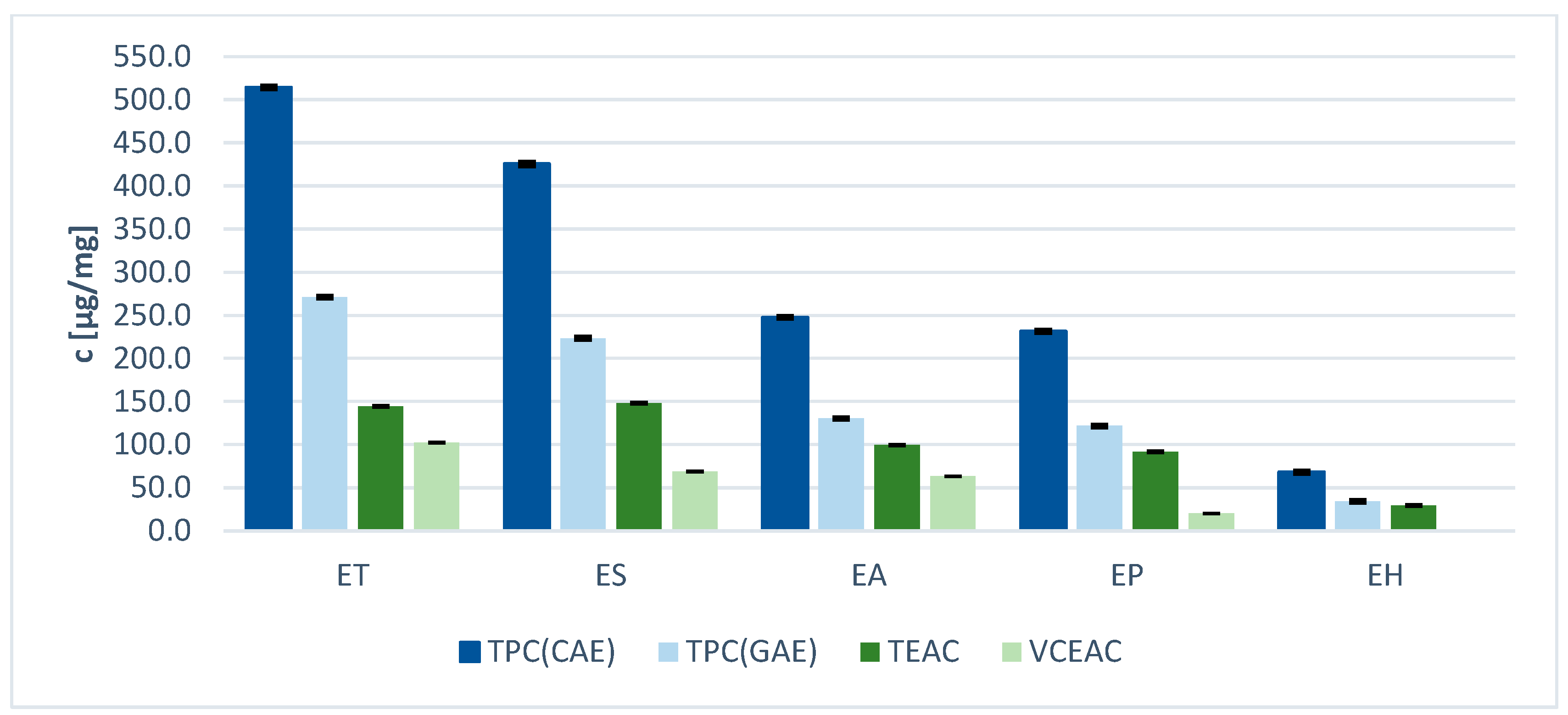
| Sample | TPC [µg GAE/mg Ex] | TPC µg [CAE/mg Ex] |
|---|---|---|
| Equisetum telmateia | 270.9 ± 0.50 | 514.1 ± 0.94 |
| Equisetum sylvaticum | 223.4 ± 0.69 | 425.5 ± 1.28 |
| Equisetum arvense | 130.4 ± 0.19 | 247.6 ± 0.36 |
| Equisetum palustre | 121.8 ± 0.34 | 231.5 ± 0.64 |
| Equisetum hyemale | 34.2 ± 0.44 | 68.3 ± 0.81 |
| Sample | VCEAC [µg Vit C/mg Ex] | TEAC [µg Trolox/mg Ex] |
|---|---|---|
| Equisetum sylvaticum | 102.5 ± 0.42 | 148.3 ± 0.61 |
| Equisetum telmateia | 99.8 ± 0.35 | 144.4 ± 0.51 |
| Equisetum arvense | 68.9 ± 0.24 | 99.6 ± 0.35 |
| Equisetum palustre | 63.5 ± 0.18 | 91.8 ± 0.27 |
| Equisetum hyemale | 20.5 ± 0.32 | 29.5 ± 0.47 |
| Sample | [%] | [%] | [%] | [%] |
|---|---|---|---|---|
| Equisetum telmateia | 38.6 | 21.9 | 0.1 | 60.6 |
| Equisetum sylvaticum | 29.9 | 38.7 | 1.1 | 69.7 |
| Equisetum arvense | 46.2 | 42.6 | 0.0 | 88.8 |
| Equisetum palustre | 64.3 | 20.4 | 0.1 | 84.8 |
| Equisetum hyemale | 2.3 | 88.9 | 0.3 | 91.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosrati Gazafroudi, K.; Mailänder, L.K.; Daniels, R.; Kammerer, D.R.; Stintzing, F.C. From Stem to Spectrum: Phytochemical Characterization of Five Equisetum Species and Evaluation of Their Antioxidant Potential. Molecules 2024, 29, 2821. https://doi.org/10.3390/molecules29122821
Nosrati Gazafroudi K, Mailänder LK, Daniels R, Kammerer DR, Stintzing FC. From Stem to Spectrum: Phytochemical Characterization of Five Equisetum Species and Evaluation of Their Antioxidant Potential. Molecules. 2024; 29(12):2821. https://doi.org/10.3390/molecules29122821
Chicago/Turabian StyleNosrati Gazafroudi, Khadijeh, Lilo K. Mailänder, Rolf Daniels, Dietmar R. Kammerer, and Florian C. Stintzing. 2024. "From Stem to Spectrum: Phytochemical Characterization of Five Equisetum Species and Evaluation of Their Antioxidant Potential" Molecules 29, no. 12: 2821. https://doi.org/10.3390/molecules29122821
APA StyleNosrati Gazafroudi, K., Mailänder, L. K., Daniels, R., Kammerer, D. R., & Stintzing, F. C. (2024). From Stem to Spectrum: Phytochemical Characterization of Five Equisetum Species and Evaluation of Their Antioxidant Potential. Molecules, 29(12), 2821. https://doi.org/10.3390/molecules29122821







