Wavelength Division Multiplexing-Based High-Sensitivity Surface Plasmon Resonance Imaging Biosensor for High-Throughput Real-Time Molecular Interaction Analysis
Abstract
1. Introduction
2. The Principle of the Wavelength Division Multiplexing (WDM) Algorithm
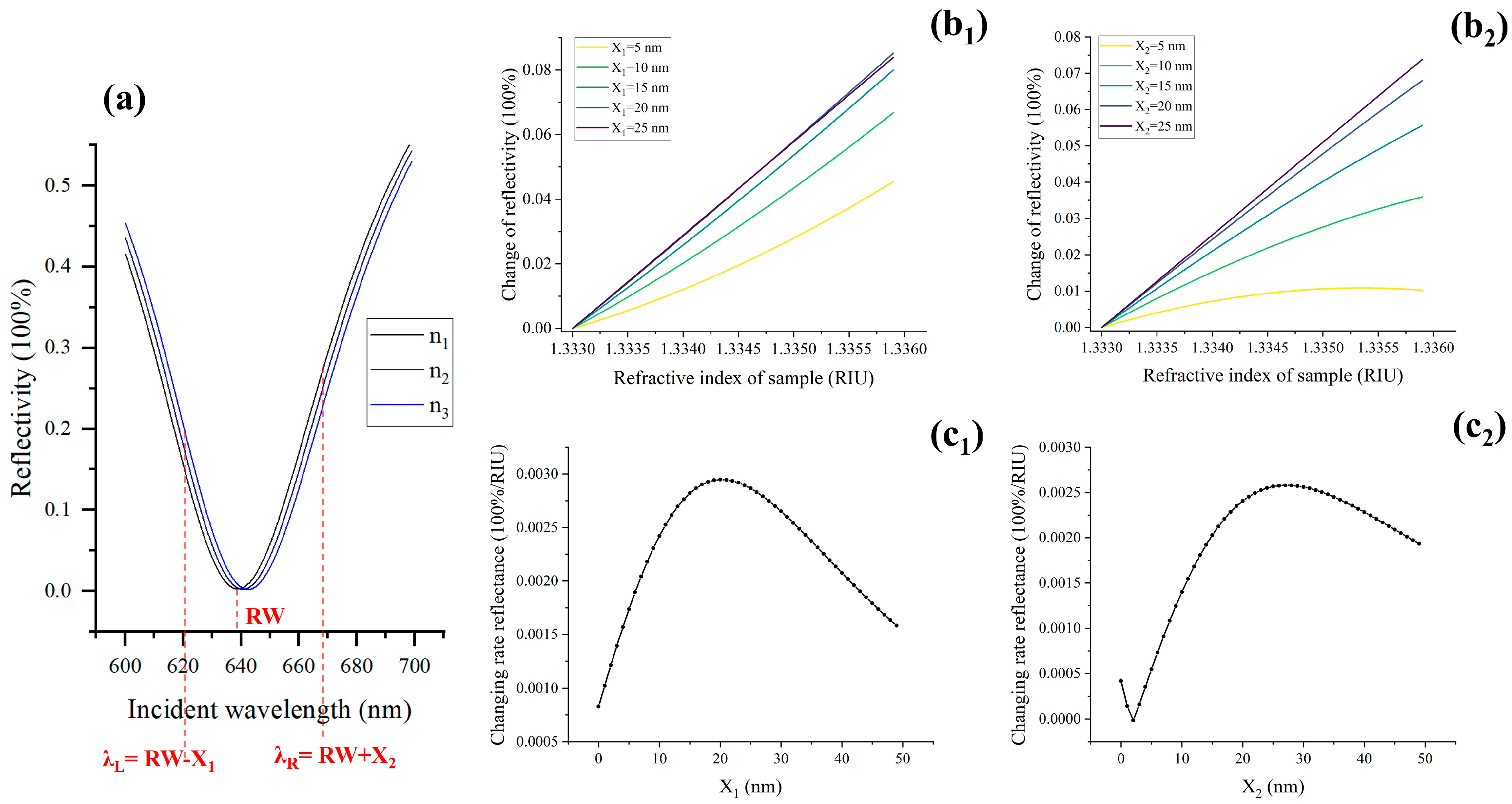
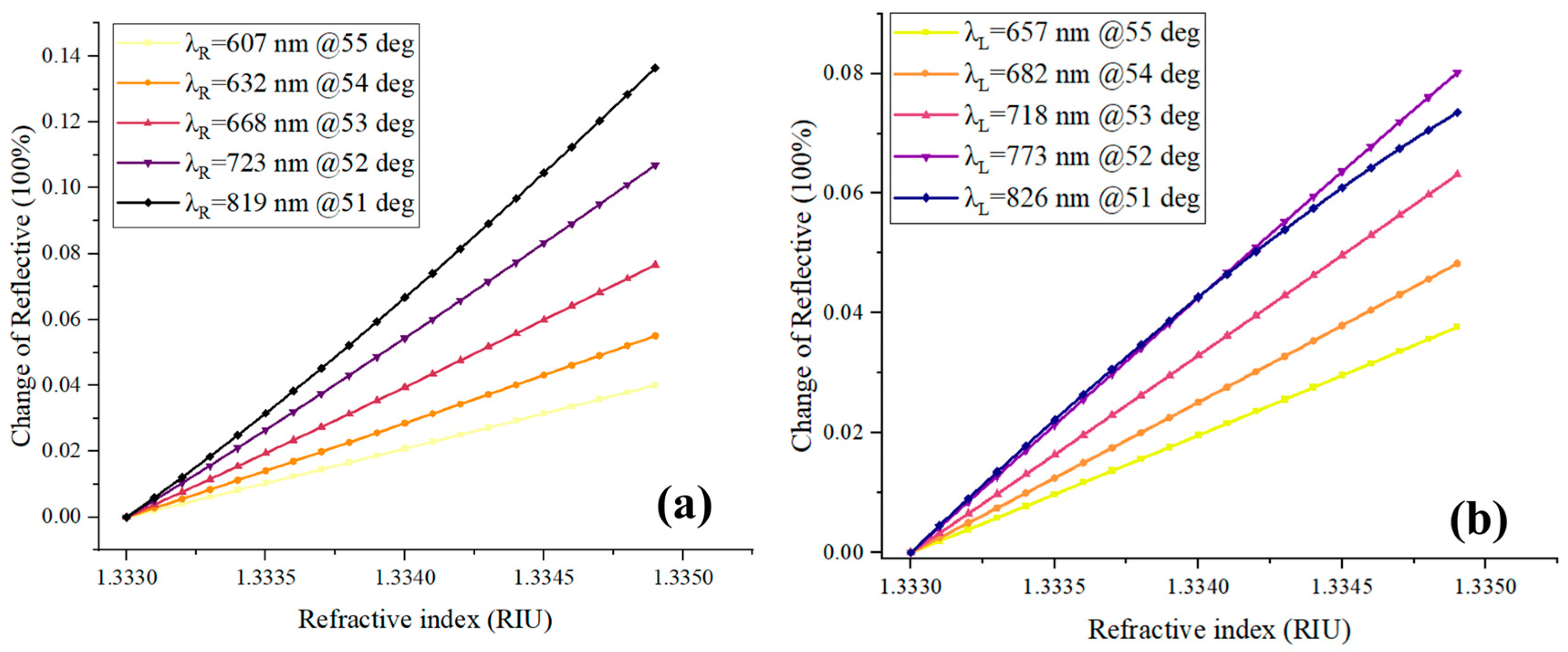
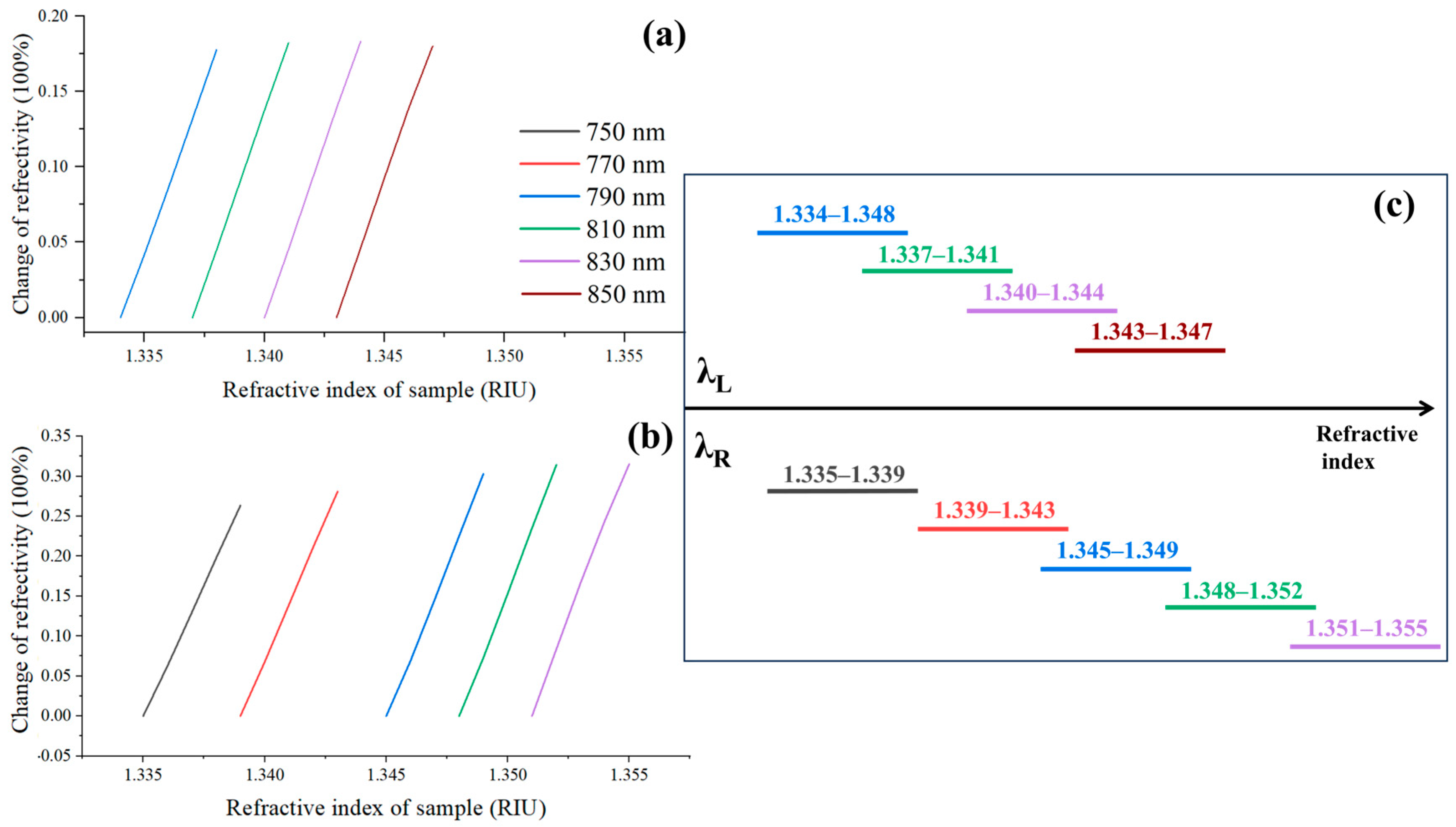
2.1. Selection and Optimization of Excitation Wavelength
2.2. WDM Algorithm
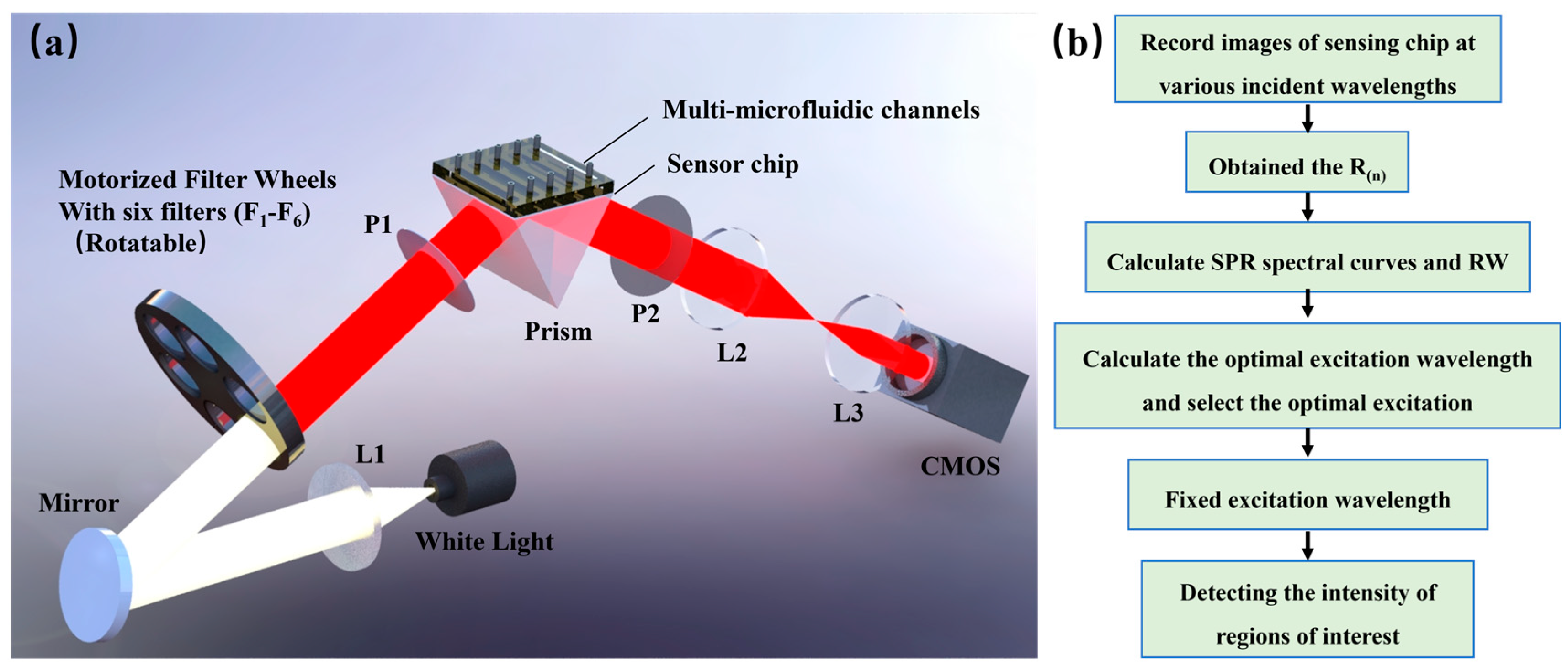
3. System
4. Results and Discussion
4.1. Materials and Chemicals
4.2. System Performance Testing
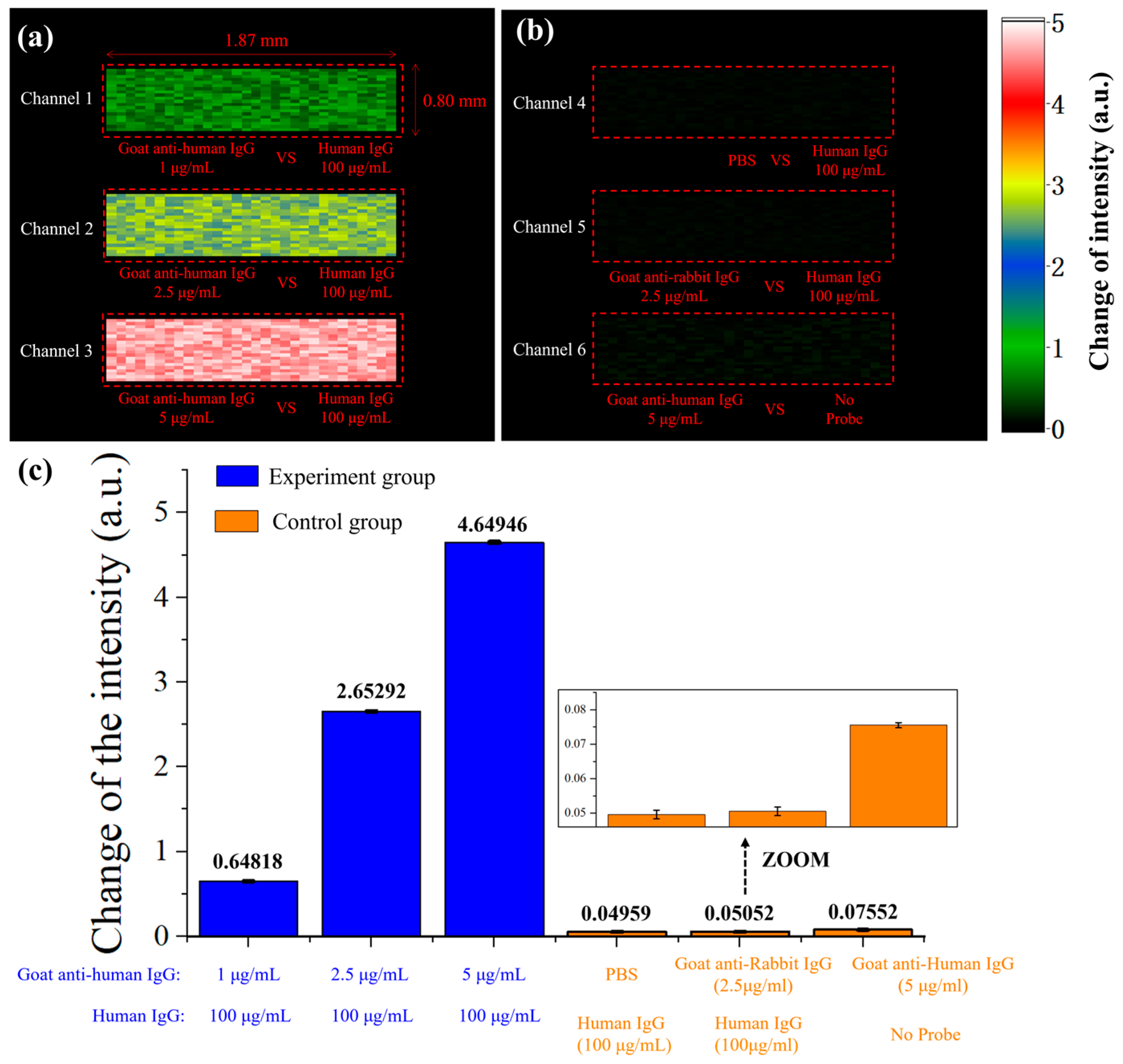
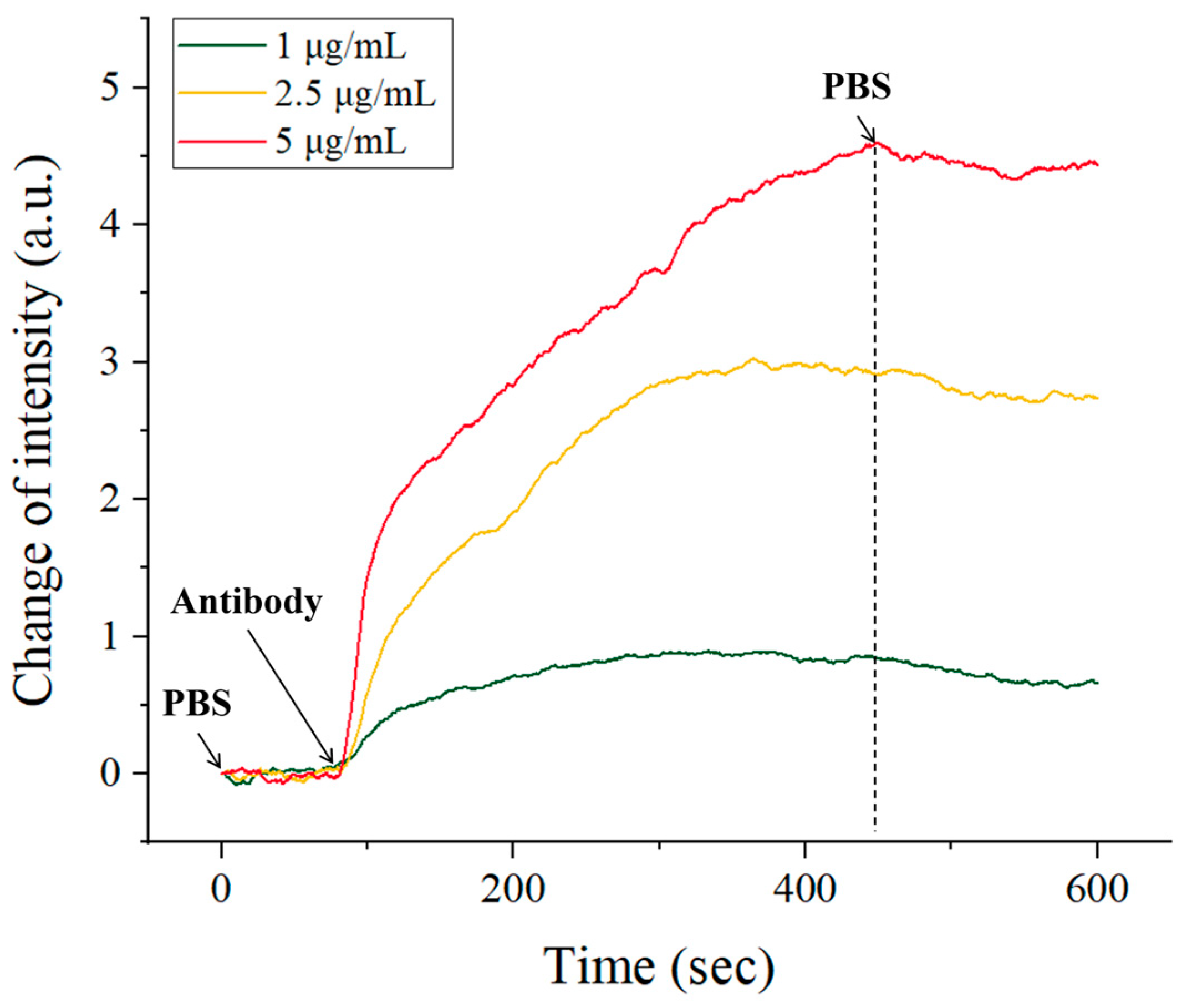
4.3. Detection of Antibody-Antigen Binding
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baiz, C.R.; Blasiak, B.; Bredenbeck, J.; Cho, M.; Choi, J.H.; Corcelli, S.A.; Dijkstra, A.G.; Feng, C.J.; Garrett-Roe, S.; Ge, N.H.; et al. Vibrational Spectroscopic Map, Vibrational Spectroscopy, and Intermolecular Interaction. Chem. Rev. 2020, 120, 7152–7218. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wu, X.; Ding, S.; Lou, X.; Xia, F.; Wang, S.; Hong, Y. Aggregation-Induced Emission Photosensitizers: From Molecular Design to Photodynamic Therapy. J. Med. Chem. 2020, 63, 1996–2012. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, S.A.; Fekete, J.T.; Gyorffy, B. Predictive biomarkers of immunotherapy response with pharmacological applications in solid tumors. Acta Pharmacol. Sin. 2023, 44, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, R.; Bellassai, N.; Jungbluth, V.; Spoto, G. Recent Advances in Antifouling Materials for Surface Plasmon Resonance Biosensing in Clinical Diagnostics and Food Safety. Polymers 2021, 13, 1929. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Liu, Y.; Chang, S.; Chen, H.; Chen, J.H. Surface Plasmonic Sensors: Sensing Mechanism and Recent Applications. Sensors 2021, 21, 5262. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Steed, J.W. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P.; Patil, V.S.; Bhandare, V.V.; Patil, P.P.; Patil, B.M.; Dwivedi, P.S.R.; Bhattacharya, K.; Harish, D.R.; Roy, S. Systems and in vitro pharmacology profiling of diosgenin against breast cancer. Front. Pharmacol. 2022, 13, 1052849. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Mondal, B.; Mukherjee, P.S. Molecular Cavity for Catalysis and Formation of Metal Nanoparticles for Use in Catalysis. Chem. Rev. 2022, 122, 12244–12307. [Google Scholar] [CrossRef]
- Bardelcikova, A.; Soltys, J.; Mojzis, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef]
- Bellissent-Funel, M.C.; Hassanali, A.; Havenith, M.; Henchman, R.; Pohl, P.; Sterpone, F.; van der Spoel, D.; Xu, Y.; Garcia, A.E. Water Determines the Structure and Dynamics of Proteins. Chem. Rev. 2016, 116, 7673–7697. [Google Scholar] [CrossRef]
- Masson, J.F. Surface Plasmon Resonance Clinical Biosensors for Medical Diagnostics. ACS Sens. 2017, 2, 16–30. [Google Scholar] [CrossRef]
- Johnson, S.M.; Javner, C.; Hackel, B.J. Development and Implementation of a Protein–Protein Binding Experiment To Teach Intermolecular Interactions in High School or Undergraduate Classrooms. J. Chem. Educ. 2017, 94, 367–374. [Google Scholar] [CrossRef]
- Tao, J.; Bauer, D.E.; Chiarle, R. Assessing and advancing the safety of CRISPR-Cas tools: From DNA to RNA editing. Nat. Commun. 2023, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Tse Sum Bui, B.; Mier, A.; Haupt, K. Molecularly Imprinted Polymers as Synthetic Antibodies for Protein Recognition: The Next Generation. Small 2023, 19, e2206453. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C. Recent Advancements in Aptamer-Based Surface Plasmon Resonance Biosensing Strategies. Biosensors 2021, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.; Lukaszewski, Z.; Gorodkiewicz, E. Potential of surface plasmon resonance biosensors in cancer detection. J. Pharm. Biomed. Anal. 2021, 194, 113802. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Hejji, L.; Kim, K.H.; Kukkar, D.; Souhail, B.; Bhardwaj, N.; Brown, R.J.C.; Zhang, W. Advances in surface plasmon resonance-based biosensor technologies for cancer biomarker detection. Biosens. Bioelectron. 2022, 197, 113767. [Google Scholar] [CrossRef] [PubMed]
- Puiu, M.; Bala, C. SPR and SPR Imaging: Recent Trends in Developing Nanodevices for Detection and Real-Time Monitoring of Biomolecular Events. Sensors 2016, 16, 870. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.W.; Halter, M.; Tona, A.; Plant, A.L. High resolution surface plasmon resonance imaging for single cells. BMC Cell Biol. 2014, 15, 35. [Google Scholar] [CrossRef]
- Singh, P. SPR Biosensors: Historical Perspectives and Current Challenges. Sens. Actuators B Chem. 2016, 229, 110–130. [Google Scholar] [CrossRef]
- Abid, S.A.; Muneer, A.A.; Al-Kadmy, I.M.S.; Sattar, A.A.; Beshbishy, A.M.; Batiha, G.E.; Hetta, H.F. Biosensors as a future diagnostic approach for COVID-19. Life Sci. 2021, 273, 119117. [Google Scholar] [CrossRef]
- Bockova, M.; Slaby, J.; Springer, T.; Homola, J. Advances in Surface Plasmon Resonance Imaging and Microscopy and Their Biological Applications. Annu. Rev. Anal. Chem. 2019, 12, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Yesudasu, V.; Pradhan, H.S.; Pandya, R.J. Recent progress in surface plasmon resonance based sensors: A comprehensive review. Heliyon 2021, 7, e06321. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Hu, R.; Wang, L.; Gu, D.; He, J.; Wu, S.-Y.; Ho, H.-P.; Li, X.; Qu, J.; Gao, B.Z.; et al. Recent advances in surface plasmon resonance imaging: Detection speed, sensitivity, and portability. Nanophotonics 2017, 6, 1017–1030. [Google Scholar] [CrossRef]
- Deng, S.; Wang, P.; Yu, X. Phase-Sensitive Surface Plasmon Resonance Sensors: Recent Progress and Future Prospects. Sensors 2017, 17, 2819. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.K. A simple and inexpensive surface plasmon resonance setup for phase detection using rotating analyser ellipsometric method. Laser Phys. 2020, 30, 026202. [Google Scholar] [CrossRef]
- Kashif, M.; Mokhtar, M.H.H.; Azeman, N.H.; Hashim, F.H.; Arsad, N.; Abushagur, A.A.G.; Bakar, A.A.A. Phase-interrogated surface plasmon resonance sensor based on laser feedback interferometry. Opt. Lasers Eng. 2021, 141, 106564. [Google Scholar] [CrossRef]
- Ng, S.P.; Wu, C.M.; Wu, S.Y.; Ho, H.P. White-light spectral interferometry for surface plasmon resonance sensing applications. Opt. Express 2011, 19, 4521–4527. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, Y.; Gu, D.; Zhang, K.; Qu, J.; He, J.; Li, X.; Wu, S.Y.; Ho, H.P.; Somekh, M.G.; et al. Wavelength-multiplexing phase-sensitive surface plasmon imaging sensor. Opt. Lett. 2013, 38, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhou, J.; Xiao, X.; Wang, L.; Qu, J.; Li, X.; Gao, B.Z.; Shao, Y. A Speckle-Free Angular Interrogation SPR Imaging Sensor Based on Galvanometer Scan and Laser Excitation. Plasmonics 2019, 14, 1497–1504. [Google Scholar] [CrossRef]
- Wang, D.; Chuen Loo, J.F.; Lin, W.; Geng, Q.; Shan Ngan, E.K.; Kong, S.K.; Yam, Y.; Chen, S.-C.; Ho, H.P. Development of a sensitive DMD-based 2D SPR sensor array using single-point detection strategy for multiple aptamer screening. Sens. Actuators B Chem. 2020, 305, 127240. [Google Scholar] [CrossRef]
- Thadson, K.; Sasivimolkul, S.; Suvarnaphaet, P.; Visitsattapongse, S.; Pechprasarn, S. Measurement precision enhancement of surface plasmon resonance based angular scanning detection using deep learning. Sci. Rep. 2022, 12, 2052. [Google Scholar] [CrossRef]
- Sereda, A.; Moreau, J.; Canva, M.; Maillart, E. High performance multi-spectral interrogation for surface plasmon resonance imaging sensors. Biosens. Bioelectron. 2014, 54, 175–180. [Google Scholar] [CrossRef]
- Li, J.; Han, D.; Zeng, J.; Deng, J.; Hu, N.; Yang, J. Multi-channel surface plasmon resonance biosensor using prism-based wavelength interrogation. Opt. Express 2020, 28, 14007–14017. [Google Scholar] [CrossRef]
- Zeng, Y.; Nie, Z.; Kai, D.; Chen, J.; Shao, Y.; Kong, W.; Yuan, Z.; Ho, H.P.; Zhang, F. Quasi-phase extraction-based surface plasmon resonance imaging method for coffee ring effect monitoring and biosensing. Anal. Bioanal. Chem. 2023, 415, 5735–5743. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, J.; Wang, X.; Cai, Z.; Shao, Y. Wavelength-scanning surface plasmon resonance microscopy: A novel tool for real time sensing of cell-substrate interactions. Biosens. Bioelectron. 2019, 145, 111717. [Google Scholar] [CrossRef]
- Piliarik, M.; Homola, J. Surface plasmon resonance (SPR) sensors: Approaching their limits? Opt. Express 2009, 17, 16505–16517. [Google Scholar] [CrossRef]
- Zhou, X.L.; Yang, Y.Z.; Wang, S.P.; Liu, X.W. Surface Plasmon Resonance Microscopy: From Single-Molecule Sensing to Single-Cell Imaging. Angew. Chem. Int. Ed. 2020, 59, 1776–1785. [Google Scholar] [CrossRef]
- Zybin, A.; Grunwald, C.; Mirsky, V.M.; Kuhlmann, J.; Wolfbeis, O.S.; Niemax, K. Double-wavelength technique for surface plasmon resonance measurements: Basic concept and applications for single sensors and two-dimensional sensor arrays. Anal. Chem. 2005, 77, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhou, J.; Sang, W.; Kong, W.; Qu, J.; Ho, H.P.; Zhou, K.; Gao, B.Z.; Chen, J.; Shao, Y. High-Sensitive Surface Plasmon Resonance Imaging Biosensor Based on Dual-Wavelength Differential Method. Front. Chem. 2021, 9, 801355. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Niu, Z.; Liu, L.; Zeng, Y.; Ma, L.; Nie, Z.; Tian, Z.; Kai, D.; Zhang, F.; Liu, G.; et al. Intensity Interrogation-Based High-Sensitivity Surface Plasmon Resonance Imaging Biosensor for Apoptosis Detection in Cancer. Biosensors 2023, 13, 946. [Google Scholar] [CrossRef]
- Saha, F.B.K.; Gizeli, E. Comparative Study of IgG Binding to Proteins G and A: Nonequilibrium Kinetic and Binding Constant Determination with the Acoustic Waveguide Device. Anal. Chem. 2003, 15, 835–842. [Google Scholar] [CrossRef]
- Acharya, B.; Behera, A.; Behera, S. Optimizing drug discovery: Surface plasmon resonance techniques and their multifaceted applications. Chem. Phys. Impact 2024, 8, 100414. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Z.; Du, H.; Ma, L.; Zhou, J.; Yuan, Z.; Sun, R.; Liu, G.; Zhang, F.; Zeng, Y. Wavelength Division Multiplexing-Based High-Sensitivity Surface Plasmon Resonance Imaging Biosensor for High-Throughput Real-Time Molecular Interaction Analysis. Molecules 2024, 29, 2811. https://doi.org/10.3390/molecules29122811
Niu Z, Du H, Ma L, Zhou J, Yuan Z, Sun R, Liu G, Zhang F, Zeng Y. Wavelength Division Multiplexing-Based High-Sensitivity Surface Plasmon Resonance Imaging Biosensor for High-Throughput Real-Time Molecular Interaction Analysis. Molecules. 2024; 29(12):2811. https://doi.org/10.3390/molecules29122811
Chicago/Turabian StyleNiu, Zhenxiao, Hao Du, Lin Ma, Jie Zhou, Zhengqiang Yuan, Ronghui Sun, Guanyu Liu, Fangteng Zhang, and Youjun Zeng. 2024. "Wavelength Division Multiplexing-Based High-Sensitivity Surface Plasmon Resonance Imaging Biosensor for High-Throughput Real-Time Molecular Interaction Analysis" Molecules 29, no. 12: 2811. https://doi.org/10.3390/molecules29122811
APA StyleNiu, Z., Du, H., Ma, L., Zhou, J., Yuan, Z., Sun, R., Liu, G., Zhang, F., & Zeng, Y. (2024). Wavelength Division Multiplexing-Based High-Sensitivity Surface Plasmon Resonance Imaging Biosensor for High-Throughput Real-Time Molecular Interaction Analysis. Molecules, 29(12), 2811. https://doi.org/10.3390/molecules29122811






