Abstract
In this study, molecular dynamics (MD) simulations were employed to elucidate the processes and underlying mechanisms that govern the adsorption and accumulation of gas (represented by N2) at the hydrophobic solid–liquid interface, using the GROMACS program with an AMBER force field. Our findings indicate that, regardless of surface roughness, the presence of water molecules is a prerequisite for the adsorption and aggregation of N2 molecules on solid surfaces. N2 molecules dissolved in water can cluster even without a solid substrate. In the gas–solid–liquid system, the exclusion of water molecules at the hydrophobic solid–liquid interface and the adsorption of N2 molecules do not occur simultaneously. A loosely arranged layer of water molecules is initially formed on the hydrophobic solid surface. The two-stage process of N2 molecule adsorption and accumulation at the hydrophobic solid/liquid interface involves initial adsorption to the solid surface, displacing water molecules, followed by N2 accumulation via self-interaction after saturating the substrate’s surface. The process and underlying mechanisms of gas adsorption and accumulation at hydrophobic solid/liquid interfaces elucidated in this study offer a molecular-level understanding of nano-gas layer formation.
1. Introduction
Nano-gas layers, which are quasi-two-dimensional gaseous aggregates at solid–liquid interfaces, have attracted extensive attention for more than ten years. Utilizing experimental techniques such as the tapping-mode atomic force microscope (TM-AFM), the stability, structural ordering, and preparation methods of nano-gas layers have been extensively studied, confirming that they hold significant applications in interface science [1,2,3,4,5]. Various studies have indicated that their presence could enhance interfacial slip lengths [1], induce protein folding [2], facilitate the nucleation of gas hydrates [3], and impact electrochemical reactions at interfaces [4,5].
In 2007, Hu et al. utilized the tapping mode of atomic force microscopy to observe gaseous layers on the surface of highly oriented pyrolytic graphite (HOPG), and these layers were not easy to form or gradually disappeared in degassed water [6]. Following the initial discovery, research on nano-gas layers progressively emerged, primarily focusing on the fundamental properties and behaviors of these layers and providing theoretical explanations. Nguyen et al. demonstrated through AFM force curves that nanobubbles on HOPG surfaces do not exist in isolation but are typically surrounded by gas layers [7]. Schlesinger et al. [8] investigated the state of gas layers near hydrophobic surfaces in water with varying degrees of gas saturation, mapping out three-dimensional structures of gaseous substances near gas–liquid interfaces. The results indicated that hydrophobic surfaces commonly exhibit gas enrichment layers with heights of 2–5 nm. Notably, these layers showed a weak dependence on the solubility of gases in water. These results confirm the widespread presence of these layers on hydrophobic interfaces, characterized by a non-uniform distribution of gas density that decreases with increasing distance. Additionally, the thickness of these layers is influenced by the gas content in the liquid.
The alcohol water replacement method involves using low-gas-solubility pure water to replace high-gas-solubility alcohols (typically ethanol) near the gas–liquid interface, creating a transient state of gas supersaturation and consequently generating nano-gas layers [9]. While this alcohol water replacement method allows for the visualization of nano-gas layers on hydrophobic surfaces, it is not an efficient method for their production. Understanding the conditions for the formation of nano-gas layers is a prerequisite for developing efficient preparation methods. Zhang et al. [10] investigated the formation of nano-gas layers on various substrates such as MoS2, talc, HOPG, and silicon, revealing that the conditions for nano-gas layer formation are stringent and influenced by the hydrophobicity of the surface, the smoothness of the material, and the presence of an ordered crystalline structure. Nevertheless, the scarcity of experimental techniques has concurrently limited further understanding of the formation of gas layers.
Molecular dynamics (MD) simulations, characterized by their femtosecond (fs) time resolution and the ability to resolve the motion of all atoms, are extensively utilized in studying dynamic and structural behaviors in various processes. Weijs et al. [11], through MD simulations, observed that nanobubbles spontaneously nucleate in heavily gas-supersaturated liquids and migrate toward the surface. Sendner et al. [12] simulated several specific gases (Ar, Ne, O2, and an “ideal” gas type “X”) dissolved in a water model on a diamond-like structure. Their findings highlighted gas enrichment within approximately 1 nm of a hydrophobic surface for all investigated gas types. The above studies demonstrate that the inherent spatial resolution of MD simulations is sufficiently high to elucidate the mechanisms of gas adsorption and accumulation.
In a previous study [13], we focused on investigating the impact of grooved surfaces with varying heights and widths on the adsorption and accumulation of N2 at the hydrophobic solid–liquid interface through MD simulations. The results demonstrated that an increase in the surface roughness of the solid substrate facilitated the adsorption and accumulation of N2. However, to date, there has been a lack of comprehensive research in the processes that govern the adsorption and accumulation of gas at the hydrophobic solid/liquid interface. Understanding the behavior of gas molecules at interfaces can lay the groundwork for the further exploration of the properties of nano-gas layers and guide the development of preparation methods, thereby driving their applications across various fields such as materials science, surface wetting, and biomedicine.
In this study, two gas–solid models (smooth solid and rough solid surfaces) and a gas–liquid model were developed to evaluate the significance of water in gas adsorption and accumulation. Subsequently, a gas–liquid–solid three-phase model was constructed to provide a comprehensive explanation of the gas adsorption and accumulation at the solid–liquid interface, including aspects such as the adsorption configuration, the formation of a loosely arranged water molecular layer, the density distribution, and the interaction energy. This study can provide fundamental insights into the gas adsorption and accumulation processes involved in the formation of nano-gas layers, as well as the changes in the interactions among the gas–liquid–solid phases during these processes.
2. Results and Discussion
2.1. Gas–Solid and Gas–Liquid Systems
It is evident from Figure 1a,b that when the system reached equilibrium, there was only little adsorption or aggregation of N2 molecules on either smooth or rough substrates in the gas–solid model, with most of the N2 molecule remaining uniformly distributed in the simulated box. This indicates that in the absence of water, it is difficult for N2 molecules to adsorb or aggregate on solid substrates, regardless of their roughness, signifying that the presence of water molecules is a prerequisite for the extensive adsorption and aggregation of N2 molecules on solid surfaces.
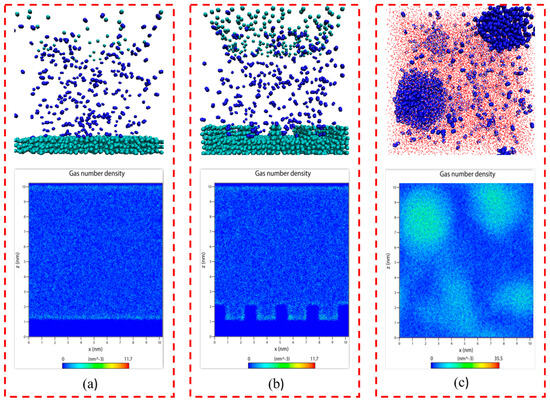
Figure 1.
Snapshots of the equilibrium model: (a) gas–solid model (smooth substrate); (b) gas–solid model (rough substrate); (c) gas–liquid model. The colors of the atoms are as follows: C, green; H, red; O, white; N, blue.
As shown in Figure 1c, N2 molecules that were initially uniformly dispersed in water showed aggregation at various locations. This indicates that N2 molecules that were dissolved in water can cluster even without a solid substrate, consistent with the formation of bulk phase micro-nanobubbles in solutions under ultrasound or intense shearing conditions.
2.2. Gas–Solid–Liquid System
2.2.1. N2 Molecular Adsorption Process and Adsorption Configuration
In the initial model, N2 molecules were randomly placed within the simulation cell. To demonstrate the adsorption and accumulation of N2 molecules, H2O molecules were hidden in the system, and snapshots at various simulation times were extracted, as shown in Figure 2. It is observed that from 0 to 6 ns, the N2 molecules in water increasingly migrated and adhered to the substrate surface, with the remaining N2 molecules dispersing into the vacuum layer above the aqueous phase.
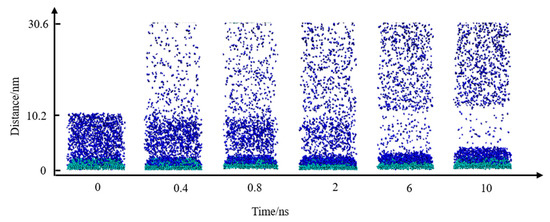
Figure 2.
Snapshots of N2 molecules’ adsorption process. The colors of the atoms are as follows: C, green; N, blue.
Figure 3a illustrates the adsorption equilibrium configuration of the solid–liquid–gas three-phase simulation system. Following the establishment of adsorption equilibrium, N2 molecules were adsorbed and accumulated within the range of 0 < Z < 3.37 nm at the solid–liquid interface. Figure 3b shows the quantity of N2 molecules within this range as a function of the simulation time. There is a minimum in the number of N2 molecules within the range of 0–0.5 ns. This phenomenon results from the specific number of N2 molecules (approximately 400–450) accommodated at the solid–liquid interface during the model construction process, along with their uniform distribution. At the beginning of the simulation, the hydrogen bond interactions among H2O molecules are more significant than interactions between N2 and H2O molecules, resulting in the separation of N2 and H2O molecules [13]. As the simulation time increases, there is a rapid rise in the number of N2 molecules adsorbed at the solid–liquid interface.
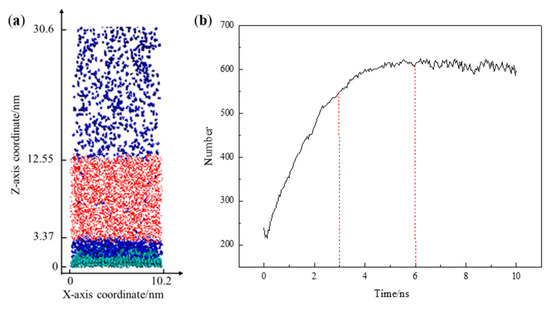
Figure 3.
(a) Snapshot of the equilibrium configuration; (b) the number of N2 molecules as a function of the simulation time. The colors of the atoms are as follows: C, green; H, red; O, white; N, blue.
During the simulation period of 3–6 ns, the growth rate of the curve representing the quantity of adsorbed N2 molecules experiences a slowdown. This suggests a gradual decrease in the rate of increase in the number of N2 molecules adsorbed at the solid–liquid interface, likely due to the adsorption sites on the substrate surface becoming occupied with increasing simulation time, with the accumulation process dominated by N2 molecule interactions becoming slower. When the simulation time exceeds 6 ns, the number of N2 molecules at the solid–liquid interface no longer changes significantly, indicating that a dynamic equilibrium between adsorption and desorption has been reached. At this juncture, the N2 molecules that were initially uniformly distributed in water were found in three locations: some aggregated at the solid–liquid interface, a minimal number were still present in the aqueous phase, and there was an excess in the vacuum layer of the simulation. It is worth noting that the minimal N2 molecules remaining in the water phase could be due to hydrogen bond (O–H···N) formations between H2O and N2 molecules [14]. N2 molecules in the vacuum are attributed to the over-saturation of nitrogen molecules during model construction. As the simulation progresses, the escape of excess N2 molecules from the aqueous phase under atmospheric pressure conditions occurs.
2.2.2. Formation of a Loosely Arranged Water Molecular Layer at the Solid–Liquid Interface
To investigate how N2 molecules displace pre-adsorbed water molecules at the solid–liquid interface, resulting in adsorption and accumulation, the density curves of H2O and N2 molecules along the Z-axis were extracted at 0 and 30 ps, as shown in Figure 4. It is observed that at a simulation time of 30 ps, the density of water molecules at the solid–liquid interface (at Z = 1.02 nm) is markedly lower than in the initial model (at 0 ps), with a difference of approximately 143.31 kg/m3. In contrast, the density of N2 molecules shows no significant change between 0 and 30 ps. This indicates that the exclusion of water molecules at the solid–liquid interface and the adsorption of N2 molecules do not occur simultaneously.
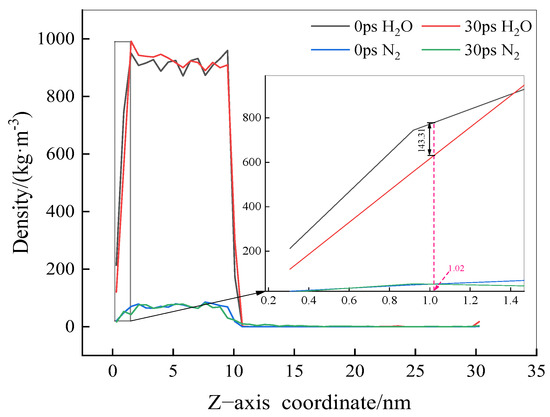
Figure 4.
Density distribution of H2O and N2 molecules at 0 ps and 30 ps.
Due to the hydrophobic interaction of the solid surface, the density of H2O molecules at the interface decreases, forming a loosely arranged layer of water molecules. As the simulation time increases, N2 molecules gradually adsorb onto the solid surface, continuously displacing water molecules and eventually accumulating at the solid–liquid interface, forming a gaseous adsorption layer. Previous studies have shown that when hydrophobic solids are immersed in water, the density of water molecules at their surface is lower than that of bulk water molecules, extending from a few Å to several nm [15,16], and the characteristics of this low-density water layer are closely related to the hydrophobicity of the solid surface. These observations agree with the findings of the current study.
2.2.3. Density Distribution
To clearly depict the distribution of H2O and N2 in the system before and after the simulation, density distribution curves of N2 and H2O were plotted, as shown in Figure 5, with the origin at the solid surface and the variable being the height perpendicular to this surface. It is obvious that the average density of the water phase before and after simulation equilibrium is close to 950 kg/m3, which is close to the actual density of water (970 kg/m3), indicating that the model construction, force field selection, and parameter settings of the simulation system are reasonable.
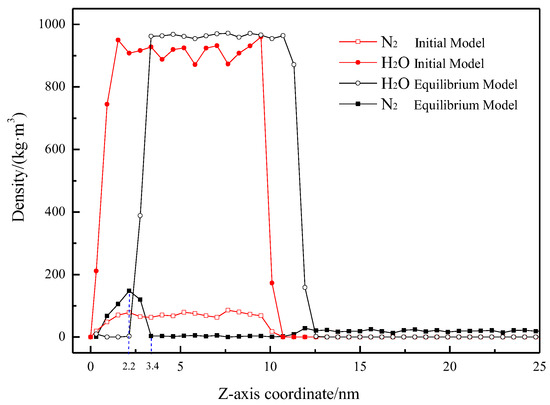
Figure 5.
Density profiles for H2O and N2 in initial and equilibrium models as a function of the distance Z.
In the initial model, due to the uniform distribution of N2 in water, the distribution of H2O and N2 perpendicular to the solid surface is consistent (distribution range is approximately 0–10.5 nm). The density of N2 in the initial stage is about 73.07 ± 6.21 kg/m3. Correspondingly, after simulation equilibrium, the density of H2O molecules is zero within the 0–2.2 nm range, and the maximum density of N2 molecules is 147.97 kg/m3. Following adsorption equilibrium, the distribution range of N2 molecules at the solid–liquid interface is approximately 0–3.4 nm, consistent with the height of the gas-rich layer depicted in Figure 2 and Figure 3. This indicates that the H2O molecules at the solid–liquid interface have been displaced, and N2 molecules have undergone adsorption and accumulation. Additionally, the density of the N2 molecular layer at the hydrophobic solid–liquid interface is significantly higher than the density of N2 molecules dissolved in water, aligning with the high-density feature of nanobubbles [17].
2.2.4. Interaction Energies
The adsorption and accumulation of N2 molecules at the solid–liquid interface involve interactions between N2, H2O, and the substrate surface; N2-N2; and N2-H2O. Figure 6 illustrates these interaction energies as a function of the simulation time. A negative interaction energy indicates a stable interaction between object phases, and the more negative the interaction energy, the more favorable the interaction.
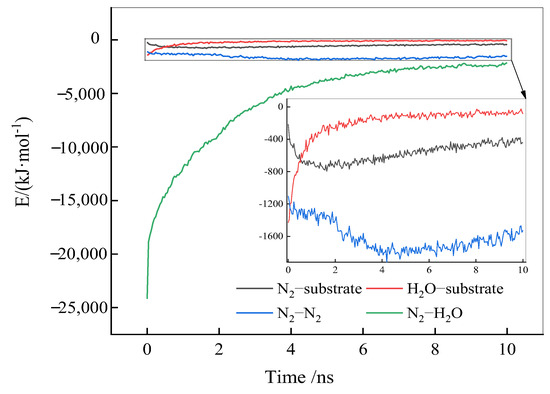
Figure 6.
Interaction energies as a function of the simulation time.
From Figure 6, as the simulation progresses, the interaction energy between N2 and H2O molecules decreases sharply, indicating a gradual separation of N2 and H2O molecules from a uniform mixed distribution to individual aggregation. At the same time, the interaction energy between N2 molecules and the substrate surface significantly increases, the interaction energy between H2O molecules and the substrate surface decreases, and the interaction energy among N2 molecules remains largely unchanged, suggesting that in this stage, N2 molecules primarily adsorb to the solid surface through interactions with the substrate, without significant clustering among N2 molecules. As the simulation progresses, the substrate surface becomes fully occupied by N2 molecules, increasing the interaction energy among N2 molecules and leading to their aggregation. In the later stages of the simulation, the reduction in interaction energy between N2 and the substrate surface, and among N2 molecules themselves, is likely due to an excess of N2 molecules escaping from the solid–liquid interface into the vacuum layer.
To summarize, the adsorption and accumulation of N2 molecules at the hydrophobic solid–liquid interface could be bifurcated into two stages. In the first stage, N2 molecules adsorb to the solid surface through their interaction with the substrate, continually displacing water molecules at the solid–liquid interface. Once all the adsorption sites on the substrate surface are occupied, the second stage involves the accumulation of N2 molecules through interactions among themselves.
3. Models and Simulations
3.1. Models
In this study, N2 molecules were selected as a representative gas, and H2O molecules were modeled using a three-point charge SPC water model. Carbon atoms were selected to construct the solid model. Approximately 3000 carbon atoms were arranged in three layers within an area of 10.20 × 10.20 nm2, with the equilibrium distance between carbon atoms set to 0.34 nm, representing the hydrophobic solid surface. To assess the conditions for gas adsorption and accumulation, two gas–solid models and a gas–liquid model were constructed. For the former, a smooth substrate representation was achieved using three layers of carbon atoms. To simulate the variation between smooth and rough substrates, grooves were added above these carbon atom layers, effectively defenestrating the groove substrate surface characteristics. These substrates were placed at the bottom of a 10.20 × 10.20 × 10.20 nm3 simulation box, with 300 N2 molecules added, forming the initial models as shown in Figure 7a,b. For the gas–liquid model, 2000 N2 molecules and 35,020 H2O molecules were randomly distributed in a 10.20 × 10.20 × 10.20 nm3 simulation box, with a water molecule density of 33 molecules per cubic nanometer, as depicted in Figure 7c.
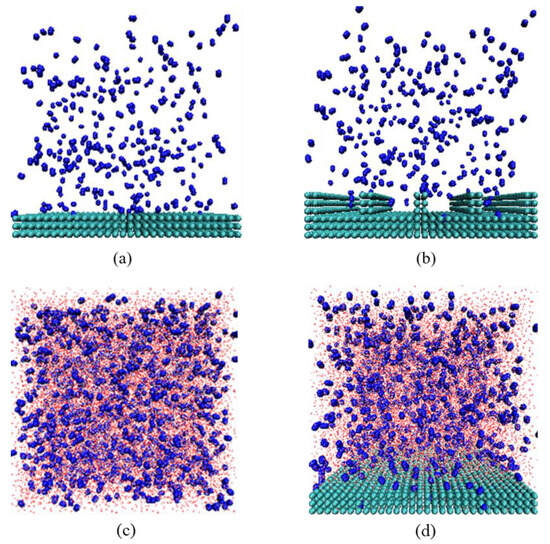
Figure 7.
Snapshot of gas–solid model: (a) smooth substrate, (b) rough substrate, (c) gas–liquid model, (d) gas–solid–liquid model. The colors of the atoms are as follows: C, green; H, red; O, white; N, blue.
To investigate the adsorption and accumulation processes of N2 molecules at the solid–liquid interface, the aforementioned carbon layers were positioned inside a 10.20 × 10.20 × 10.20 nm3 simulation box. A total of 1500 N2 molecules were added, and the remaining space was filled with SPC water molecules, totaling 29,715 H2O molecules, as shown in Figure 7d. To eliminate the impact of periodicity, a 20.40 nm vacuum layer was implemented at the top of the simulation box [18,19,20].
3.2. Simulation Details
MD simulations were conducted using GROMACS2018.8 software, employing an AMBER force field to characterize the bonding and non-bonding interactions between the analyzed molecules [21,22]. The computation of interatomic non-bonding interactions was based on the Lennard–Jones potential functions [23]. The particle mesh Ewald (PME) method was applied to compute long-range electrostatic interactions with a cut-off of 1.0 nm [24]. The system initialization involved energy minimization down to 1000 kJ mol−1 nm−1 using the steepest descent method. After energy minimization, MD simulations proceeded in the NVT ensemble with a 2.0 fs time step, and temperature control at 298 K was maintained using a Nosé–Hoover thermostat [25,26,27]. The Parrinello–Rahman pressure coupling method was implemented to ensure that the pressure of the entire system was maintained at 1.01 × 105 Pa. Periodic boundary conditions were set in all directions across all simulation systems. A trajectory file was obtained every 4 × 104 fs for subsequent kinetic analysis, and VMD1.9.3 software was utilized to visualize the system’s microstructure [28].
4. Conclusions
The study provides in-depth insights into the processes of N2 adsorption and accumulation at the hydrophobic solid–liquid interface. The main conclusions obtained are as follows:
(1) Regardless of the surface’s roughness, the presence of water molecules is a prerequisite for the adsorption and aggregation of N2 molecules on solid surfaces. N2 molecules dissolved in water can cluster even without a solid substrate.
(2) In the gas–solid–liquid system, the exclusion of water molecules at the solid–liquid interface and the adsorption of N2 molecules do not occur simultaneously. A loosely arranged layer of water molecules is initially formed on the hydrophobic solid surface.
(3) The two-stage process of N2 molecule adsorption and accumulation at the hydrophobic solid-liquid interface involves initial adsorption to the solid surface, displacing water molecules, followed by N2 accumulation via self-interaction after saturating the substrate’s adsorption sites.
It is important to note that this study utilizes MD simulations to elucidate the process and underlying mechanisms of gas adsorption and accumulation at hydrophobic solid/liquid interfaces at the molecular level. However, the models constructed in this study are only based on hydrophobic surface models and only employ nitrogen as a representative gas. This study lacks an investigation into the hydrophilic model, types, and concentrations of gases. Further exploration is warranted in subsequent studies.
Author Contributions
B.L.: investigation, supervision, writing—original draft, writing—review and editing. D.S.: investigation, writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the financial support from the National Natural Science Foundation of China (22208235), the Fundamental Research Program of Shanxi Province (20210302124351), and the Research Project Supported by the Shanxi Scholarship Council of China (2022-061).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Busse, A.; Sandham, N.; McHale, G.; Newton, M. Change in drag, apparent slip and optimum air layer thickness for laminar flow over an idealised superhydrophobic surface. J. Fluid. Mech. 2013, 727, 488–508. [Google Scholar] [CrossRef]
- Baldwin, R.; Rose, G. How the hydrophobic factor drives protein folding. Proc. Natl. Acad. Sci. USA 2016, 113, 12462–12466. [Google Scholar] [CrossRef] [PubMed]
- Farhang, F.; Nguyen, A.V.; Sewell, K.B. Fundamental Investigation of the Effects of Hydrophobic Fumed Silica on the Formation of Carbon Dioxide Gas Hydrates. Energy Fuels 2014, 28, 7025–7037. [Google Scholar] [CrossRef]
- Xu, W.; Lu, Z.; Sun, X.; Jiang, L.; Duan, X. Superwetting Electrodes for Gas-Involving Electrocatalysis. Acc. Chem. Res. 2018, 51, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, X.; Liu, S.; Hao, J.; Shao, Z.; Yi, B. Study on hydrophobicity loss of the gas diffusion layer in PEMFCs by electrochemical oxidation. RSC Adv. 2014, 4, 3852–3856. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Sun, J.; Zhang, Z.; Li, G.; Fang, H.; Xiao, X.; Zeng, X.; Hu, J. Detection of novel gaseous states at the highly oriented pyrolytic graphite-water interface. Langmuir 2007, 23, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Hampton, M.A.; Nguyen, A.V. Nanobubbles do not sit alone at the solid-liquid interface. Langmuir 2013, 29, 6123–6130. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, I.; Sivan, U. Three-Dimensional Characterization of Layers of Condensed Gas Molecules Forming Universally on Hydrophobic Surfaces. J. Am. Chem. Soc. 2018, 33, 10473–10481. [Google Scholar] [CrossRef]
- Theodorakis, P.; Che, Z. Surface Nanobubbles: Theory, Simulation, and Experiment. A Review. Adv. Colloid. Interface Sci. 2019, 272, 101995. [Google Scholar] [CrossRef]
- Zhang, X.; Maeda, N. Interfacial gaseous states on crystalline surfaces. J. Phys. Chem. C 2011, 115, 736–743. [Google Scholar] [CrossRef]
- Weijs, J.; Snoeijer, J.; Lohse, D. Formation of surface nanobubbles and the universality of their contact angles: A molecular dynamics approach. Phys. Rev. Lett. 2012, 108, 104501. [Google Scholar] [CrossRef] [PubMed]
- Sendner, C.; Horinek, D.; Bocquet, L.; Netz, R. Interfacial water at hydrophobic and hydrophilic surfaces: Slip, viscosity, and diffusion. Langmuir 2009, 25, 10768. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhang, L.; Guo, J.; Liu, S.; Li, B. Adsorption and accumulation mechanism of N2 on groove-type rough surfaces: A molecular simulation study. J. Mol. Liq. 2022, 366, 120260. [Google Scholar] [CrossRef]
- Grein, F. CH4–N2, NH3–N2, H2O–N2 and HF–N2 complexes: Ab initio studies and comparisons—Transition to hydrogen bonding. Theor. Chem. Acc. 2020, 139, 166. [Google Scholar] [CrossRef]
- Mezger, M.; Schöder, S.; Reichert, H.; Schröder, H.; Okasinski, J.; Honkimäki, V.; Ralston, J.; Bilgram, J.; Roth, R.; Dosch, H. Water and ice in contact with octadecyl-trichlorosilane functionalized surfaces: A high resolution X-ray reflectivity study. J. Chem. Phys. 2008, 128, 244705. [Google Scholar] [CrossRef]
- Mezger, M.; Sedlmeier, F.; Horinek, D.; Reichert, H.; Pontoni, D.; Dosch, H. On the origin of the hydrophobic water gap: An X-ray reflectivity and MD simulation study. J. Am. Chem. Soc. 2010, 132, 6735–6741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, X.; Shin, H.; Wang, J.; Tai, R.; Zhang, X.; Fang, H.; Xiao, W.; Wang, L.; Wang, C.; et al. Ultrahigh density of gas molecules confined in surface nanobubbles in ambient water. J. Am. Chem. Soc. 2020, 142, 5583–5593. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, C.; Zhang, J.; Xiao, S.; Zhang, Z.; Shen, Y.; Sun, B.; He, J. Displacement mechanism of oil in shale inorganic nanopores by supercritical carbon dioxide from molecular dynamics simulations. Energy Fuels 2017, 31, 738–746. [Google Scholar] [CrossRef]
- Fang, T.; Zhang, Y.; Yan, Y.; Wang, Z.; Zhang, J. Molecular insight into the oil extraction and transport in CO2 flooding with reservoir depressurization. Int. J. Heat Mass Transf. 2020, 148, 119051. [Google Scholar] [CrossRef]
- Fang, T.; Wang, M.; Gao, Y.; Zhang, Y.; Yan, Y.; Zhang, J. Enhanced oil recovery with CO2/N2 slug in low permeability reservoir: Molecular dynamics simulation. Chem. Eng. Sci. 2019, 197, 204–211. [Google Scholar] [CrossRef]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.; Smith, J.; Kasson, P.; Van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- He, X.; Man, V.H.; Yang, W.; Lee, T.S.; Wang, J. A fast and high-quality charge model for the next generation general AMBER force field. J. Chem. Phys. 2020, 153, 114502. [Google Scholar] [CrossRef] [PubMed]
- Grubmüller, H.; Heller, H.; Windemuth, A.; Schulten, K. Generalized Verlet algorithm for efficient molecular dynamics simulations with long-range interactions. Mol. Simul. 1991, 6, 121–142. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Yu, H.; Chen, Z.; Xin, Y.; Chai, J.; Fu, L.Y.; Zhang, J.; Zhang, H. Simulation on oily contamination removal by ozone using molecular dynamics. Chemosphere 2022, 308, 136473. [Google Scholar] [CrossRef]
- Li, J.; Han, Y.; Qu, G.; Cheng, J.; Xue, C.; Gao, X.; Sun, T.; Ding, W. Molecular dynamics simulation of the aggregation behavior of N-Dodecyl-N, N-Dimethyl-3-Ammonio-1-Propanesulfonate/sodium dodecyl benzene sulfonate surfactant mixed system at oil/water interface. Colloids Surf. A Physicochem. Eng. Asp. 2017, 531, 73–80. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graphics 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).