Abstract
Spinal muscular atrophy (SMA) is a severe neuromuscular disorder that is caused by mutations in the survival motor neuron 1 (SMN1) gene, hindering the production of functional survival motor neuron (SMN) proteins. Antisense oligonucleotides (ASOs), a versatile DNA-like drug, are adept at binding to target RNA to prevent translation or promote alternative splicing. Nusinersen is an FDA-approved ASO for the treatment of SMA. It effectively promotes alternative splicing in pre-mRNA transcribed from the SMN2 gene, an analog of the SMN1 gene, to produce a greater amount of full-length SMN protein, to compensate for the loss of functional protein translated from SMN1. Despite its efficacy in ameliorating SMA symptoms, the cellular uptake of these ASOs is suboptimal, and their inability to penetrate the CNS necessitates invasive lumbar punctures. Cell-penetrating peptides (CPPs), which can be conjugated to ASOs, represent a promising approach to improve the efficiency of these treatments for SMA and have the potential to transverse the blood–brain barrier to circumvent the need for intrusive intrathecal injections and their associated adverse effects. This review provides a comprehensive analysis of ASO therapies, their application for the treatment of SMA, and the encouraging potential of CPPs as delivery systems to improve ASO uptake and overall efficiency.
1. Introduction
Spinal muscular atrophy (SMA) is a rare genetic condition that is characterized by progressive muscle weakness and respiratory complications that often lead to death [1]. The SMA phenotype is the result of a significant deficiency in full-length survival motor neuron (SMN) protein. Nusinersen, an ASO designed to address the present deficiency by improving the splicing efficiency of an SMN gene analog that typically is translated into a nonfunctional SMN protein, was FDA-approved in 2016 [2]. While demonstrating efficacy in improving the SMA phenotype in treated patients, Nusinersen must be administered intrathecally for CNS delivery, accompanied by notable side effects [3]. Moreover, the inherent poor cellular uptake of ASOs limits their efficacy at lower doses [4].
To overcome these challenges, several delivery systems have been explored to enhance ASO cellular uptake for improved efficiency. Among these, cell-penetrating peptides (CPPs) emerged as a promising small-molecule carrier. When directly conjugated to neutrally charged ASOs, CPPs were seen to not only improve ASO cellular uptake but also facilitate delivery into the CNS following system injection in SMA mice [5,6,7,8,9]. Despite the evident potential of CPPs to enhance ASO delivery, they have yet to be FDA-approved as a carrier due to concerns regarding their toxicity and immunogenicity [10]. This comprehensive review provides an overview of ASOs for the treatment of SMA and the observed potential of CPPs to improve their efficiency and avoid the need for invasive procedures.
2. Background on Spinal Muscular Atrophy
SMA is an autosomal recessive neuromuscular disorder that is caused by the degeneration of spinal anterior horn cells [11]. The associated denervation of the limb, trunk, bulbar, and respiratory muscles lead to progressive weakness, difficulty swallowing, and respiratory problems [12]. The estimated prevalence of SMA is between 1 and 10 in 100,000 live births, with observed intercountry variability, and it is currently one of the leading inherited causes of infant death [13,14,15]. The SMA phenotype was discovered to be due to biallelic loss or biallelic pathogenic mutations in the SMN1 gene [13,16]. The SMN protein encoded by the survival motor neuron 1 (SMN1) gene is evolutionarily conserved and ubiquitously expressed [17]. This 38 kDa protein is composed of a basic/lysine-rich domain, a tudor domain, a proline-rich domain, and a YG box. All four domains and the protein’s overall structure appear to be critical for the function of the SMN protein as pathogenic mutations causing SMA have been identified in each domain [18,19]. SMN is a multifunctional protein that plays a role in various cellular processes, including RNA metabolism [20,21,22], DNA recombination and repair [23,24], signal transduction [25,26], and intracellular trafficking [19,27,28].
The age of onset and severity of symptoms can vary greatly between patients diagnosed with SMA, primarily due to the compensatory capacity of alternative genes in mitigating the loss of functional proteins translated from SMN1. SMN is not only essential for motor neuron survival, as the absence of any SMN protein is embryonic lethal [29]. Fortunately, SMN1 is not the only gene that encodes the SMN protein on the human genome. The SMN2 gene is nearly identical to the SMN1 gene. However, SMN2 contains a single nucleotide alteration in exon 7 that disrupts the exon’s splice enhancer [30]. Due to this variation, the primary mRNA produced by SMN2 lacks exon 7 and is translated into a truncated and nonfunctional protein [31]. Although exon skipping is favoured, small amounts of full-length functional SMN proteins translated from SMN2 can compensate for the loss of functional protein from SMN1 in SMA. Individuals can have varying copy numbers (between 0 and 8) of SMN2 that impact its ability to compensate for loss of SMN1, with larger copy numbers of SMN2 typically resulting in reduced disease severity [32,33]. Mutations in exon 7 of SMN2, which result in the creation of a novel splicing enhancer, can also influence the amount of full-length SMN proteins translated from SMN2 transcripts [34]. Although dysfunctional SMN proteins transcribed from SMN1 cause SMA, the severity of the disease is strongly associated with the individual’s SMN2 copy number and sequence.
SMA has been classified into five types based on the age of symptom onset, disease severity, milestones achieved, and lifespan. Patients diagnosed with SMA type 1 begin to present with symptoms in the first 6 months of their lives and never achieve the ability to sit up on their own [35]. SMA type 1 is the most frequently diagnosed type and is also the leading cause of infantile death from a genetic disease [36]. Patients diagnosed with type 2 SMA begin to present with symptoms after 6 to 18 months of age. These patients experience progressive weakness in the arms and legs, progressive scoliosis, and restrictive lung disease due to weakening intercostal muscles. However, they have the ability to sit up on their own [35]. SMA types 3 and 4 are not associated with a reduced life expectancy, and although these patients experience progressive muscle weakness and atrophy, they achieve the ability to walk unaided [37]. Type 0 SMA, also known as prenatal SMA, is the most severe form of the disease. Prenatal SMA is associated with reduced fetal movements during pregnancy and severe muscle weakness and respiratory distress at birth [38,39]. In all prenatal SMA cases, patients die soon after birth.
3. Antisense Oligonucleotides
Short fragments of nucleic acid polymers, known as oligonucleotides, have become an incredibly useful tool for understanding gene function and represent a promising avenue for genetic disease therapeutics. ASOs are single-stranded DNA-like sequences, normally ranging from 12 to 25 nucleotides in length, that are designed to be complementary to the intended target sequence. With the proper design, these ASOs can specifically bind to their target sequence through Watson–Crick pairing, with minimal off-target pairing. The chemistry of the ASOs and their target binding site on RNA regulates their mechanism of action.
Once bound to their target RNA, ASOs can alter gene expression through two primary mechanisms, RNase H-mediated degradation of the target mRNA or steric blockage of specific sites on pre-mRNA or mRNA to modulate splicing or prevent translation (Figure 1). RNase H is a ubiquitous enzyme in all eukaryotic cells that is responsible for hydrolyzing RNA when it is bound to DNA during DNA replication [40]. ASOs can take advantage of RNase’s ability to degrade DNA by possessing a complementary sequence to that of the RNA intended to be degraded. Once the ASO-RNA heteroduplex is formed, RNase H can bind and hydrolyze the RNA so that it is unable to be translated into a disease-causing protein [41]. However, when all DNA bases in an ASO are chemically modified, RNase H is no longer able to recognize the DNA-RNA heteroduplex and elicit its enzymatic activity. In the absence of RNase H activity, ASOs bound to target RNA can block machinery involved in the translation or splicing of the RNA transcript [42,43]. Although steric blockage of translation machinery with ASOs has been observed to effectively reduce target protein translation [44], ASOs utilizing RNase H-mediated degradation are currently the preferred approach when a decrease in protein production is desired. ASOs have even been capable of increasing target protein expression by binding to 3′ and 5′ untranslated regions of pre-mRNA, resulting in improved mRNA stability [45].
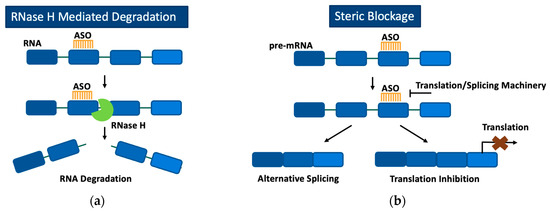
Figure 1.
Mechanism of action of ASOs through either RNase H-mediated degradation (a) or steric blockage (b). (a) ASOs bind to target RNA, and the RNA-ASO complex is recognized by RNase H, which subsequently degrades the target RNA. (b) ASOs bind to target RNA and, based on the region targeted, block translation or splicing machinery. Blocking splicing machinery results in alternative splicing of the pre-mRNA transcript. Blocking translation machinery prevents translation of the target RNA.
Alternative splicing regulates which exons will be included in the mature mRNA product after processing. It is an essential biological process that allows for the translation of related but different proteins, with differing properties and functions, from the same DNA sequence [46]. Antisense oligonucleotides can promote specific splicing events by targeting splice sites and splicing enhancers and silencers [47]. ASOs can correct reading frames that are disrupted by insertions or deletions by promoting the exclusion of the disrupted exon, resulting in the translation of a truncated, yet functional, protein. Multiple ASOs that restore the reading frame of dystrophin by promoting exon skipping are approved by the FDA for the treatment of Duchenne muscular dystrophy and are effective in minimizing symptoms of the disease [48].
ASOs also can promote the inclusion of exons by binding and blocking regions of DNA that contain intronic splicing silencers (ISSs) or exonic splicing silencers (ESSs). Once blocked by ASOs, these regions can no longer recruit splicing repressors, resulting in improved recognition of the target exon by the spliceosome. ASO-mediated exon inclusion is a promising approach for the treatment of numerous genetic disorders. For example, the most frequent variant (c. -32-13T>GP) in the GAA gene, which causes Pompe disease, results in exon 2 skipping [49]. ASOs have been effective in promoting exon 2 inclusion in patient-derived fibroblasts possessing the splice-site variant, leading to a significant improvement in GAA activity [50].
The numerous mechanisms that ASOs can utilize to modulate RNA expression or splicing events give them the potential to be effective in the treatment of a vast number of rare genetic diseases. There are currently 15 ASO therapies that have been approved by the FDA for the treatment of disease, with additional clinical trials currently underway [51]. These ASOs have a range of modifications to their chemical structure and/or utilize carriers to improve their delivery to target cells.
3.1. Chemical Modifications to Improve the Stability, Safety, and Delivery of Antisense Oligonucleotides
Oligonucleotides in their natural form lack the ability to permeate cell membranes, are highly susceptible to degradation by nucleases, and only possess suboptimal binding to target sequences [52,53]. To overcome these challenges, modifications to the chemical structure of ASOs have been effective in improving ASO stability and uptake into cells. These chemical modifications also influence the ASOs’ mechanism of action once they are bound to their target. The progress in these ASO modifications over the last two decades has allowed these oligonucleotides to be translated into a clinical setting to treat patients. FDA-approved ASOs feature a variety of chemical modifications chosen to align with the drug’s intended mode of action [54].
Replacing the nonbridging oxygens on the ASOs’ phosphate group with a sulfur group, the first chemical modification of ASOs studied, results in a phosphorothioate-modified (PS) ASO [55]. The chemical structure and negative backbone charge of PS ASOs allow them to have improved protection against nuclease degradation and a strong affinity for serum proteins that significantly improves their half-lives when compared to unmodified ASOs [56,57].
More recently, modifications to the sugar moiety of oligonucleotides have been a promising approach to improve ASO efficacy. 2′-O-methyl (2′-OME) and 2′-O-methoxyethyl (2′-MOE) ASOs are generated by using natural ribonucleotides possessing ME or MOE groups, respectively, at the 2′ position [58]. Locked nucleic acid (LNA) is formed by creating a bridge between the 2′ oxygen and 4′ carbon on the ribose moiety, resulting in reduced conformational flexibility, which increases binding affinity [59]. These ASOs with modified sugar moieties have an improved binding affinity for the target sequence when compared to PS ASOs, and 2′-OME and 2′-MOE modifications are associated with improved safety due to reduced immune stimulation [60]. However, unlike PS ASOs, RNase H-mediated degradation is not facilitated when these modifications are used for the entirety of the ASO, so they are only suitable when steric blockage is the desired effect. However, ASOs can be designed to have a chemically unmodified centre with chemically modified nucleotides flanking to induce RNase-mediated degradation, with improved safety and specificity attributed to these modifications [61].
Phosphorodiamidate morpholino oligomers (PMOs), sometimes referred to as morpholino oligos, possess a backbone consisting of morpholine rings attached by phosphorodiamidate linkages. PMOs are neutrally charged, resulting in a reduced capacity to interact with proteins when bound to their target sequence [62]. Therefore, PMO-RNA binding does not promote RNase H-mediated degradation, and PMOs are used to sterically block or modulate splicing of target sequences. PMOs appear to be highly resistant to biological nucleases and enzymes and have improved aqueous solubility [60]. Due to their neutral charge, they are also well suited for conjugation with peptides, which is discussed further in this paper.
3.2. Antisense Oligonucleotides for the Treatment of SMA
ASOs have emerged as a powerful approach for the treatment of SMA. The severity of SMA is largely associated with SMN2 copy number and its ability to compensate for the loss of functional SMN proteins translated from SMN1. ASOs have demonstrated remarkable efficiency in facilitating the inclusion of exon 7 in SMN2 transcripts and increasing the levels of full-length SMN protein [63,64]. An ISS within intron 7, known as ISS-N1, is currently the most potent target for ASOs promoting exon 7 inclusion in SMN2 transcripts [65]. 2′-OME ASOs, 2′-MOE ASOs, and PMOs designed to be complementary to ISS-N1 have been observed to significantly ameliorate SMA symptoms and improve the survival of SMA mice [66,67,68,69,70,71]. The delayed onset and progression of SMA symptoms through ASO treatment appears to be dose-dependent, exhibiting further improvement when administered at an earlier stage of the mouse’s life.
Nusinersen, a 2′-OME ASO targeting ISS-N1 for the treatment of SMA, was introduced to clinical trials led by Ionis Pharmaceuticals (Figure 2). Open-label phase 1 clinical trials involved intrathecal administration of Nusinersen to patients diagnosed with type 2 or type 3 SMA [72]. No safety or tolerability concerns from Nusinersen treatment were reported in phase 1 trials, and the observed increase in full-length SMN2 expression was associated with improved Hammersmith Functional Motor Score Expanded (HFMSE) scores, indicating improved motor function, 9–14 months after administration [72]. The phase 2 open-label, dose-escalation clinical trials included patients with type 1 SMA and evaluated the tolerability of multiple doses [73]. Uptake of the ASO into motor neurons throughout the spinal cord, resulting in increased full-length SMN protein, was confirmed in treated patients and was associated with promising clinical efficiency including improved motor function and survival [73]. The observation of statistically significant improvements in motor function of patients receiving Nusinersen in placebo-controlled double-blinded phase 3 clinical trials led to the trial’s premature termination [3]. Within months of the phase 3 clinical trial ending, Nusinersen became the first FDA-approved ASO for the treatment of SMA in December 2016 [2]. ASOs alone do not possess the capability to effectively cross the blood–brain barrier (BBB), so Nusinersen is administered intrathecally, typically via lumbar puncture, to improve the delivery of these ASOs to their primary target, motor neurons [74].
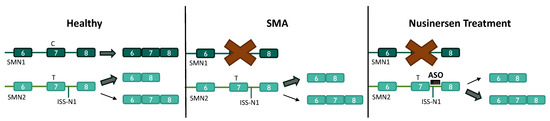
Figure 2.
Nusinersen mechanism of action for the treatment of SMA. In healthy cells, SMN1 is processed primarily into full-length SMN mRNA, containing exon 7. Exon 7 is predominantly skipped in SMN2 pre-mRNA processing, resulting in a dysfunctional SMN protein. In SMA, no functional SMN protein is translated from SMN1. Nusinersen, an ASO targeting the ISS-N1 element, promotes the inclusion of exon 7 in SMN2 pre-mRNA processing to increase full-length SMN proteins.
3.3. Limitations of Antisense Oligonucleotides for the Treatment of SMA
Nusinersen treatment, although effective at improving symptoms of SMA in patients, is also associated with several adverse events. The most reported adverse events of Nusinersen administration in patients include lower and upper respiratory tract infections and constipation [75]. Patients receiving Nusinersen have also been observed to have a higher risk for paradoxical breathing, pneumonia, and other symptoms of respiratory dysfunction [75]. However, these symptoms are largely associated with SMA progression and are unable to be attributed specifically to Nusinersen treatment. In addition, coagulation abnormalities, thrombocytopenia, and renal toxicities have been associated with ASO treatments for different diseases [76,77]. The associated risks of bleeding complications and renal toxicity require that all patients must routinely have platelet counts and urine protein levels measured prior to treatment.
Although chemical modifications can improve plasma stability and protect against nuclease degradation, one of the primary obstacles to improving ASO’s efficiency at eliciting their intended effect at target cells is their poor intracellular delivery [4]. ASOs, when delivered on their own, typically enter cells through endocytosis, which involves the ASO being engulfed by the cell membrane, which subsequently buds off within the cell as an ASO-containing endosome [78]. To influence their target RNA, ASOs must escape from the endosome for subsequent localization into the nucleus. If ASOs are not released from their endosome, the endosome can be fused to lysosomes, resulting in ASO degradation [79]. Poor endosomal escape is a major limiting factor of ASO efficiency [80]. Delivery systems to improve the cellular internalization of ASOs for the treatment of SMA have the potential to further improve full-length SMN levels in target cells for a superior rescue of SMA symptoms in patients at lower doses, associated with reduced side effects [81].
Side effects of current Nusinersen treatment are attributable not only to the ASO molecule itself but also to the invasive injection required for the drug’s delivery to the CNS. Intrathecal administration of Nusinersen is associated with post lumbar puncture side effects, including headaches and back pain [72]. The invasive nature of the procedure requires it to be conducted at a hospital or specialized facility. The frequency of required doses and the cost and burden associated with traveling to these facilities have led to many patients stopping treatment or failing to adhere to the recommended dosing schedule [82]. Developing a method to deliver ASOs that promote SMN2 exon 7 inclusion across the BBB would facilitate the use of substantially less invasive routes of administering these therapies to patients.
4. Cell-Penetrating Peptides
Some proteins have the distinct capacity to transverse cell membranes, a process known as protein transduction, without altering the membrane structure. The trans-activator of transcription (TAT) protein, belonging to the human immunodeficiency virus (HIV), was the first protein reported, in 1988, to possess this function [83,84]. The TAT protein contains a basic peptide domain that was determined to be responsible for facilitating the entry of the full-length TAT protein into cells [85]. This sequence was the first amino acid sequence to be reported as a CPP. CPPs, also known as protein transduction domains, are short (<40 amino acids) peptides that have a potent ability to penetrate biological membranes [86].
Since their initial discovery over three decades ago, a vast range of naturally occurring and artificially made CPPs have been reported. Due to their strong membrane-penetrating abilities, these peptides have emerged as an encouraging delivery system for drugs that would otherwise face challenges crossing these membranes. The utilization of CPPs as carriers has allowed for a significant improvement in the cellular uptake of various types of cargo, including small-molecule drugs [87], proteins [88], liposomes [89], and oligonucleotides [90]. Compared to alternative small-drug carriers currently being studied, CPPs appear to be superior due to their improved selectivity and specificity and their ability to be easily designed and synthesized [91,92]. Although recent studies have shown CPPs to be a powerful tool for small-molecule delivery, no CPPs for the delivery of therapeutics have been FDA-approved due to concerns regarding their toxicity and immunogenicity [93]. Advancing our understanding of the processes involved in their uptake and persistently assessing modifications to enhance their delivery and safety profile will facilitate the identification of superior CPP candidates appropriate for clinical translation in disease treatment.
4.1. Types of Cell-Penetrating Peptides
Over 1850 unique CPPs have been found, ranging in size, charge, solubility, and hydrophobicity [94]. Although all these features influence each CPP’s abilities, the physicochemical properties appear to be significantly regulated by the CPP’s charge [95]. As such, CPPs can be classified into three types: cationic CPPs, amphipathic CPPs, and hydrophobic CPPs.
The majority of naturally occurring CPPs fall under the classification of cationic CPPs, including the well-researched pTAT and penetratin peptides. These CPPs are typically enriched with basic amino acid residues throughout their sequence, namely, lysine and arginine, that result in a highly positively charged peptide under normal physiological conditions [96]. The positive charge of these CPPs allows them to strongly bind to negatively charged cell membrane glycoproteins for subsequent internalization into cells [93]. Stretches of polyarginine within the peptide sequence are associated with improved cellular uptake. The optimal length for repeating arginine amino acids to improve delivery efficiency appears to be between 8 and 10 arginine residues [97]. Nuclear localization sequences (NLSs) also represent a distinct group of short-cationic CPPs that can promote nuclear import of its cargo [98].
Hydrophobic CPPs are composed primarily of nonpolar amino acid residues, sometimes also containing a minimal number of charged residues. Very few hydrophobic CPPs have been reported in comparison to the other types of CPPs. Those reported include C105Y [99], Pep-7 [100], and SG3 [101]. Hydrophobic CPPs have received the least attention, and their mechanism of translocation and potential applications are not well understood [102].
Over 40% of the CPPs currently reported are amphipathic CPPs, possessing both hydrophobic and hydrophilic regions [93]. Amphipathic CPPs have been derived from both naturally occurring proteins, including pVEC [103], ARF [104], and BPrPp [105], and synthetically made proteins. Synthetic amphipathic CPPs are chimeric peptides composed of a hydrophilic domain attached through a linker to an NLS [106]. Pep-1 and MPG utilize the SV40 NLS linked to HIV glycoprotein 41’s fusion protein or a peptide cluster enriched with tryptophan, respectively [106]. Many amphipathic CPPs can possess either an α-helix or β-sheet motif, associated with improved cellular uptake [93,107].
4.2. Internalization Mechanisms of Cell-Penetrating Peptides
Despite the recent rigorous study of CPPs as delivery systems for small cargo/drugs, the mechanisms that allow CPPs to transverse across biological membranes are not clearly understood. CPPs and their cargo are believed to be internalized into cells through either energy-dependent endocytosis pathways or energy-independent direct-translocation pathways (Figure 3). The physicochemical properties appear to have a major effect on which pathway mediates CPP–cargo internalization; however, the experimental design, including drug concentration, cell type, and length of incubation, can also have an influence [108]. The internalization of low doses of cationic CPPs are observed to be primarily mediated by energy-independent pathways, whereas the cellular uptake of amphipathic CPPs at low doses is mediated by energy-independent pathways [86]. However, delivery of cationic CPPs through cell membranes can become mediated by direct translocation when high doses of these CPPs are used.
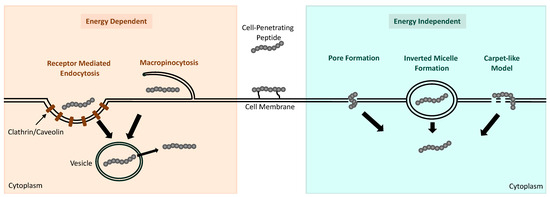
Figure 3.
Schematic representation of the mechanisms reported to facilitate the uptake of CPPs into cells. Energy-dependent pathways include receptor-mediated endocytosis and micropinocytosis, which result in the CPP being internalized in an endocytic vesicle for subsequent release into the cytoplasm. Energy-independent mechanisms include pore formation, inverted micelle formation, and the carpetlike model, which allow the CPP to enter the cell directly.
The cellular internalization of CPPs, particularly those delivered at low doses with large molecular weights or attached to cargo, is generally mediated by endocytosis. There are four endocytic mechanisms that have been reported to be involved in the uptake of CPPs, including macropinocytosis, clathrin-mediated endocytosis (CME), caveolae-mediated endocytosis (CvME), and clathrin- and caveolae-independent endocytosis. Macropinocytosis is the most reported endocytic pathway for CPP uptake and involves the plasma membrane deforming and protruding to encapsulate the CPP and its cargo in an endocytic vesicle that subsequently buds off the membrane and can release its contents within the cell [109]. Although micropinocytosis occurs independent of receptors, the protrusion of the cell membrane is regulated by a group of kinases and ATPases [86]. Receptor-mediated endocytosis involves the inward folding of the cellular membrane upon the CPP with a cell membrane receptor. In CME and CvME, the CPP-containing pit being formed is coated in clathrin or caveolin proteins for vesicle formation [110,111]. Receptor-mediated endocytosis can also occur independently of clathrin or caveolin; however, this process seems to occur only in specialized cells [112].
CPPs have also been observed to be internalized by cells through single-step, energy-independent processes. Different mechanisms for this process, including inverted micelle creation, pore formation, the carpetlike model, and membrane thinning, have been postulated as the mechanisms allowing for CPP direct translocation. In general, all mechanisms involve the CPP interacting with elements of the cell membrane that result in membrane instability [113]. In the presence of CPPs possessing hydrophobic regions that interact with the cell membrane, the formation of inverted micelles can occur, encapsulating the CPP and facilitating its intracellular transport [114]. The internalization of amphipathic CPPs with α-helical motifs appears to occur primarily due to pore formation. The bundles formed by the α-helical structure of the peptide interacting with the cell membrane create a gap in the membrane for their delivery inside [115]. The carpetlike model and membrane-thinning model are currently believed to be the mechanisms that allow cationic CPPs to translocate directly into cells at high doses. The carpet model involves the positively charged regions of the CPP lying parallel to the cell membrane, in a carpetlike manner, and the hydrophilic regions of the CPP orienting towards the hydrophilic regions of the membrane [109]. This interaction is thought to induce structural reorganization of the membrane, allowing the CPP to be internalized. The alternative membrane-thinning model involves the carpetlike formation of the CPP along the membrane. However, this model suggests that the interaction between the positively charged CPP and negatively charged cell membrane causes a rearrangement in the negative charges along the membrane and subsequent membrane thinning for the CPP to translocate through [116].
4.3. Cell-Penetrating Peptide Conjugation with Antisense Oligonucleotides
As discussed earlier, ASOs face difficulties penetrating cell membranes. CPPs, with the potential to improve the cellular uptake of their cargo, can be directly conjugated to neutrally charged ASOs through maleimide, disulfide, or amide linkages. Charged molecules are suboptimal for conjugation with CPPs that also possess a charged structure due to the potential for electrostatic interactions resulting in aggregation [112]. Therefore, neutrally charged PMOs represent a commonly used backbone chemistry that is well suited for CPP conjugation. Comparing the thermal stability of PMO/target RNA heteroduplexes in both CPP-conjugated and unconjugated forms has demonstrated that CPP conjugation does not disrupt the PMO’s affinity for its target RNA and can even lead to an increase in the heteroduplex’s melting temperature [117,118].
CPPs have been assessed for their potential to enhance PMO uptake for the treatment of a spectrum of conditions, including Duchenne muscular dystrophy (DMD) [119], myotonic dystrophy type I [120], Huntington’s disease, amyotrophic lateral sclerosis [121], and SMA [5]. For example, Eteplirsen, the first FDA-approved PMO, promotes exon skipping in the dystrophin gene to restore disrupted reading frames [122]. However, high renal clearance of the drug in patients is apparent, and high doses of the drug are required for clinical efficiency [123]. Multiple CPPs, including Pip5e, Pip6a, M12, and DG9, conjugated to PMOs mediating exon skipping for dystrophin reading frame restoration have resulted in improved cellular uptake of the PMO as well as increased levels of dystrophin protein [112,124,125,126].
5. Cell-Penetrating Peptide Conjugated Antisense Oligonucleotides for the Treatment of Spinal Muscular Atrophy
Given the increasing evidence exhibiting CPPs’ notable enhancement of ASO uptake in target cells, several studies have been undertaken employing CPPs to deliver exon inclusion-mediated ASOs for the treatment of SMA (Table 1). Nusinersen consists of 2′-OME modifications that result in an anionic molecule [127]. Since the chemical structure of Nusinersen is not ideal for CPP conjugation, studies exploring CPP-ASO conjugates for SMA treatment have opted to use PMOs targeting the same or a similar region of SMN2 pre-mRNA as Nusinersen.

Table 1.
Notable cell-penetrating peptides that are found to improve PMO efficiency and uptake through the CNS in SMA mice.
In 2016, Hammond et al. developed and evaluated a PMO-internalizing peptide (Pip), known as Pip6a, conjugated to a PMO targeting SMN2’s exon 7 ISS-N1 element [5]. Either Pip6a-PMO conjugates or PMO alone were administered to a severe mouse model of SMA through facial vein injection at PD-0 [128]. The mice treated with unconjugated PMOs displayed no survival improvements compared to saline-treated mice, which survived for a median of 12 days [5]. In contrast, Pip6a-PMO treatment extended median survival to 167 days. The Pip6a-PMOs not only significantly prolonged survival but demonstrated observable improvements in weight, muscle strength, coordination, and functional neuromuscular junctions [5]. Furthermore, the analysis of CNS tissues from SMN2 transgenic adult mice treated intravenously with Pip6a-PMO conjugates confirmed the capability of CPPs to deliver PMOs to the CNS, thereby elevating full-length SMN levels in target cells [5].
Shabanpoor et al. synthesized and assessed various CPP sequences sourced from naturally occurring proteins, such as RVG, Angiopep-2, ApoE, THR, and TET-1, conjugated to a 20-mer PMO known for promoting exon 7 inclusion in SMN2 [6,129]. These CPPs, including RVG, Angiopep2, THR, Pepc7, SynB3PFV, and a branched derivative of ApoE termed Br-ApoE (K->A), were administered to SMA type 1 patient fibroblasts to determine their efficacy in improving full-length SMN expression levels. All CPP-PMO conjugates demonstrated a dose-dependent increase in SMN expression, with Br-ApoE (K->A) exhibiting the highest efficiency [6]. Neonatal SMA mice received treatment with CPP-PMO conjugates, with unconjugated PMOs serving as a control, via facial vein injection at PD-4. Although increased levels of full-length SMN were observed in the brain, kidney, and quad of CPP-PMO-treated mice, statistically significant differences were evident only with Br-ApoE (K->A) [6].
A challenge associated with ASO-CPP delivery to the CNS is that they appear to become entrapped in, and subsequently cleared from, the endo-lysosomal barrier [130]. Therefore, Dastpeyman et al. evaluated the efficiency of a variety of CPPs with the novel capacity for endosomal escape [7]. Initial studies compared ApoE (141–150) and ApoE (133–150), which utilize receptor-mediated endocytosis for uptake, with CLIP6 and pVEC, which both appear to use-endosomal pathways for cellular uptake, as conjugates for PMOs that promote SMN2 exon 7 inclusion. All four peptides improved the delivery of PMOs in SMA patient fibroblasts, but ApoE (133–150) conjugated to PMOs showed a far superior improvement in full-length SMN levels in comparison to the other CPPs [7]. Chimeric CPPs composed of ApoE (141–150) linked at its N-terminal domain with either HA2, GALA, or NLS peptides, which possess endosomal-disrupting capabilities, or four histidine residues (H4) were observed to further improve PMO uptake [7]. The strongest CPP, ApoE (133–150), linked to the strongest endosomal escape peptide, HA2, was conjugated to PMOs and administered to SMN2+/− transgenic mice through tail vein injection, resulting in a significant improvement in SMN expression in the brain and spinal cord [7].
In 2022, Bersani et al. evaluated the delivery of a previously validated PMO targeting the ISS-N1 region of SMN2, known as HSMN2Ex7D (10–34) [131], conjugated to four CPPs: TAT, R6, r6, and (RXRRBR)2XB, also known as RXR [8]. Administered intracerebroventricularly (ICV) or intravenously (IV) to heterozygous SMAΔ7 mice, the PMO-CPP conjugates exhibited markedly elevated levels of full-length SMN protein in the brain and spinal cord compared to HSMN2Ex7D delivered alone. r6 and RXR, both arginine-rich CPPs, showed superiority over the other two CPPs in initial experiments. Therefore, RXR-PMO and r6-PMO conjugates intraperitoneally (IP) injected into symptomatic SMAΔ7 mice at PD-5 were observed to be taken up well in the CNS. The increased presence of full-length SMN in the CNS resulted in a significant improvement in neuromuscular connections and improved survival, extending to 23 and 42 days for RXR-PMO and r6-PMO, respectively [8].
In 2023, Aslesh et al. investigated the potential of the DG9 peptide, derived from the human polyhomeotic 1 homolog transcription factor, as a PMO conjugate in SMA mice [9]. Conjugated to an 18-mer PMO with the same sequence as Nusinersen, the DG9 CPP was subcutaneously administered to SMA Taiwanese mice at PD-0. Compared to R6G-PMOs, used as a benchmark control peptide PMO conjugate, as well as unconjugated PMOs and 2′-MOEs, DG9-PMO treatment yielded the most improved muscle strength and improved median lifespan, increasing it from 12 days for unconjugated PMOs to 58 days for DG9-PMO-treated mice [9]. To assess DG9-PMO uptake into the CNS at a more advanced age, a milder SMA mouse model received subcutaneous injections of DG9-PMO at PD-5. The improved CNS uptake of DG9-PMOs in comparison to unconjugated ASOs further underscores DG9 as a promising delivery system for enhancing ASO efficacy in SMA treatment.
6. Conclusions
The compelling outcomes demonstrated by the studies discussed above underscore the potential of CPPs as a highly promising strategy to enhance ASO delivery for SMA treatment. However, their translation to the clinic is impeded by the limited understanding of their in vivo stability, immunogenicity, and cellular toxicity. Additional investigations aimed at comprehensively characterizing and addressing these limitations as well as further elucidating the mechanisms facilitating CPPs’ penetration through cell membranes and the BBB are anticipated. Such efforts are crucial for the identification of superior CPP candidates capable of safely and efficiently delivering these PMOs, thereby facilitating their clinical translation.
Author Contributions
J.L. and T.Y. prepared the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. T.Y. is supported by the Muscular Dystrophy Canada, the Friends of Garrett Cumming Research Fund, the HM Toupin Neurological Science Research Fund, Canadian Institute of Health Research (CIHR), Alberta Innovates: Health Solutions (AIHS), Jesse’s Journey, the Women and Children’s Health Research Institute (WCHRI), the Heart and Stroke Foundation Canada, and the US Department of Defense. J.L. is supported by scholarships from the University of Alberta Faculty of Medicine and Dentistry and the CIHR Strategic Master’s Award.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank the University of Alberta Faculty of Medicine and Dentistry, the Canadian Institute of Health Research, the Friends of Garrett Cumming Research Funds, HM Toupin Neurological Science Research Funds, Muscular Dystrophy Canada, and the Women and Children’s Health Research Institute for their support.
Conflicts of Interest
T.Y. is a cofounder and shareholder of OligomicsTx Inc., which aims to commercialize antisense technology. Both authors declare that the research was conducted in absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Prior, T.W.; Leach, M.E.; Finanger, E. Spinal Muscular Atrophy. In GeneReviews(®); Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Neil, E.E.; Bisaccia, E.K. Nusinersen: A Novel Antisense Oligonucleotide for the Treatment of Spinal Muscular Atrophy. J. Pediatr. Pharmacol. Ther. 2019, 24, 194–203. [Google Scholar] [CrossRef]
- Garber, K. Big win possible for Ionis/Biogen antisense drug in muscular atrophy. Nat. Biotechnol. 2016, 34, 1002–1003. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, L.S.; Li, P.; Lau, C.H.; Leung, H.M.; Chin, Y.R.; Tin, C.; Lo, P.K. Cellular uptake, tissue penetration, biodistribution, and biosafety of threose nucleic acids: Assessing in vitro and in vivo delivery. Mater. Today Bio 2022, 15, 100299. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M.; Hazell, G.; Shabanpoor, F.; Saleh, A.F.; Bowerman, M.; Sleigh, J.N.; Meijboom, K.E.; Zhou, H.; Muntoni, F.; Talbot, K.; et al. Systemic peptide-mediated oligonucleotide therapy improves long-term survival in spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 2016, 113, 10962–10967. [Google Scholar] [CrossRef] [PubMed]
- Shabanpoor, F.; Hammond, S.M.; Abendroth, F.; Hazell, G.; Wood, M.J.A.; Gait, M.J. Identification of a Peptide for Systemic Brain Delivery of a Morpholino Oligonucleotide in Mouse Models of Spinal Muscular Atrophy. Nucleic Acid Ther. 2017, 27, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Dastpeyman, M.; Sharifi, R.; Amin, A.; Karas, J.A.; Cuic, B.; Pan, Y.; Nicolazzo, J.A.; Turner, B.J.; Shabanpoor, F. Endosomal escape cell-penetrating peptides significantly enhance pharmacological effectiveness and CNS activity of systemically administered antisense oligonucleotides. Int. J. Pharm. 2021, 599, 120398. [Google Scholar] [CrossRef] [PubMed]
- Bersani, M.; Rizzuti, M.; Pagliari, E.; Garbellini, M.; Saccomanno, D.; Moulton, H.M.; Bresolin, N.; Comi, G.P.; Corti, S.; Nizzardo, M. Cell-penetrating peptide-conjugated Morpholino rescues SMA in a symptomatic preclinical model. Mol. Ther. 2022, 30, 1288–1299. [Google Scholar] [CrossRef]
- Aslesh, T.; Erkut, E.; Ren, J.; Lim, K.R.Q.; Woo, S.; Hatlevig, S.; Moulton, H.M.; Gosgnach, S.; Greer, J.; Maruyama, R.; et al. DG9-conjugated morpholino rescues phenotype in SMA mice by reaching the CNS via a subcutaneous administration. JCI Insight 2023, 8, e160516. [Google Scholar] [CrossRef] [PubMed]
- Haque, U.S.; Yokota, T. Enhancing Antisense Oligonucleotide-Based Therapeutic Delivery with DG9, a Versatile Cell-Penetrating Peptide. Cells 2023, 12, 2395. [Google Scholar] [CrossRef]
- Oates, E.C.; Reddel, S.; Rodriguez, M.L.; Gandolfo, L.C.; Bahlo, M.; Hawke, S.H.; Lamandé, S.R.; Clarke, N.F.; North, K.N. Autosomal dominant congenital spinal muscular atrophy: A true form of spinal muscular atrophy caused by early loss of anterior horn cells. Brain 2012, 135, 1714–1723. [Google Scholar] [CrossRef]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Wilson, R.B. Genetic testing and risk assessment for spinal muscular atrophy (SMA). Hum. Genet. 2002, 111, 477–500. [Google Scholar] [CrossRef] [PubMed]
- Sugarman, E.A.; Nagan, N.; Zhu, H.; Akmaev, V.R.; Zhou, Z.; Rohlfs, E.M.; Flynn, K.; Hendrickson, B.C.; Scholl, T.; Sirko-Osadsa, D.A.; et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: Clinical laboratory analysis of >72,400 specimens. Eur. J. Hum. Genet. 2012, 20, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Mailman, M.D.; Heinz, J.W.; Papp, A.C.; Snyder, P.J.; Sedra, M.S.; Wirth, B.; Burghes, A.H.; Prior, T.W. Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet. Med. 2002, 4, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Osman, E.Y.; Bolding, M.R.; Villalón, E.; Kaifer, K.A.; Lorson, Z.C.; Tisdale, S.; Hao, Y.; Conant, G.C.; Pires, J.C.; Pellizzoni, L.; et al. Functional characterization of SMN evolution in mouse models of SMA. Sci. Rep. 2019, 9, 9472. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.D.; Singh, N.N.; Singh, R.N. Advances in therapeutic development for spinal muscular atrophy. Future Med. Chem. 2014, 6, 1081–1099. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.N.; Howell, M.D.; Ottesen, E.W.; Singh, N.N. Diverse role of survival motor neuron protein. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 299–315. [Google Scholar] [CrossRef]
- Pellizzoni, L.; Kataoka, N.; Charroux, B.; Dreyfuss, G. A novel function for SMN, the spinal muscular atrophy disease gene product, in pre-mRNA splicing. Cell 1998, 95, 615–624. [Google Scholar] [CrossRef]
- Zhang, R.; So, B.R.; Li, P.; Yong, J.; Glisovic, T.; Wan, L.; Dreyfuss, G. Structure of a key intermediate of the SMN complex reveals Gemin2’s crucial function in snRNP assembly. Cell 2011, 146, 384–395. [Google Scholar] [CrossRef]
- Tisdale, S.; Lotti, F.; Saieva, L.; Van Meerbeke, J.P.; Crawford, T.O.; Sumner, C.J.; Mentis, G.Z.; Pellizzoni, L. SMN is essential for the biogenesis of U7 small nuclear ribonucleoprotein and 3′-end formation of histone mRNAs. Cell Rep. 2013, 5, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Fayzullina, S.; Martin, L.J. Skeletal muscle DNA damage precedes spinal motor neuron DNA damage in a mouse model of Spinal Muscular Atrophy (SMA). PLoS ONE 2014, 9, e93329. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, Y.; Qing, Y.; Takaku, M.; Ishida, T.; Morozumi, Y.; Tsujita, T.; Kogame, T.; Hirota, K.; Takahashi, M.; Shibata, T.; et al. GEMIN2 promotes accumulation of RAD51 at double-strand breaks in homologous recombination. Nucleic Acids Res. 2010, 38, 5059–5074. [Google Scholar] [CrossRef] [PubMed]
- Genabai, N.K.; Ahmad, S.; Zhang, Z.; Jiang, X.; Gabaldon, C.A.; Gangwani, L. Genetic inhibition of JNK3 ameliorates spinal muscular atrophy. Hum. Mol. Genet. 2015, 24, 6986–7004. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Yokokura, T.; Kankel, M.W.; Dimlich, D.N.; Manent, J.; Sanyal, S.; Artavanis-Tsakonas, S. Modeling spinal muscular atrophy in Drosophila links Smn to FGF signaling. J. Cell Biol. 2011, 192, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Rossoll, W.; Jablonka, S.; Andreassi, C.; Kröning, A.K.; Karle, K.; Monani, U.R.; Sendtner, M. Smn, the spinal muscular atrophy-determining gene product, modulates axon growth and localization of beta-actin mRNA in growth cones of motoneurons. J. Cell Biol. 2003, 163, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Gabanella, F.; Pisani, C.; Borreca, A.; Farioli-Vecchioli, S.; Ciotti, M.T.; Ingegnere, T.; Onori, A.; Ammassari-Teule, M.; Corbi, N.; Canu, N.; et al. SMN affects membrane remodelling and anchoring of the protein synthesis machinery. J. Cell Sci. 2016, 129, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Monani, U.R.; Coovert, D.D.; Burghes, A.H. Animal models of spinal muscular atrophy. Hum. Mol. Genet. 2000, 9, 2451–2457. [Google Scholar] [CrossRef] [PubMed]
- Lorson, C.L.; Hahnen, E.; Androphy, E.J.; Wirth, B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 1999, 96, 6307–6311. [Google Scholar] [CrossRef]
- Burnett, B.G.; Muñoz, E.; Tandon, A.; Kwon, D.Y.; Sumner, C.J.; Fischbeck, K.H. Regulation of SMN protein stability. Mol. Cell. Biol. 2009, 29, 1107–1115. [Google Scholar] [CrossRef]
- Calucho, M.; Bernal, S.; Alías, L.; March, F.; Venceslá, A.; Rodríguez-Álvarez, F.J.; Aller, E.; Fernández, R.M.; Borrego, S.; Millán, J.M.; et al. Correlation between SMA type and SMN2 copy number revisited: An analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul. Disord. 2018, 28, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Butchbach, M.E. Copy Number Variations in the Survival Motor Neuron Genes: Implications for Spinal Muscular Atrophy and Other Neurodegenerative Diseases. Front. Mol. Biosci. 2016, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.W.; Krainer, A.R.; Hua, Y.; Swoboda, K.J.; Snyder, P.C.; Bridgeman, S.J.; Burghes, A.H.; Kissel, J.T. A positive modifier of spinal muscular atrophy in the SMN2 gene. Am. J. Hum. Genet. 2009, 85, 408–413. [Google Scholar] [CrossRef]
- Darras, B.T. Spinal muscular atrophies. Pediatr. Clin. N. Am. 2015, 62, 743–766. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.; Mercuri, E.; Tiziano, F.D.; Bertini, E. Spinal muscular atrophy. Orphanet J. Rare Dis. 2011, 6, 71. [Google Scholar] [CrossRef]
- Kolb, S.J.; Kissel, J.T. Spinal Muscular Atrophy. Neurol. Clin. 2015, 33, 831–846. [Google Scholar] [CrossRef]
- Dubowitz, V. Very severe spinal muscular atrophy (SMA type 0): An expanding clinical phenotype. Eur. J. Paediatr. Neurol. 1999, 3, 49–51. [Google Scholar] [CrossRef]
- Grotto, S.; Cuisset, J.M.; Marret, S.; Drunat, S.; Faure, P.; Audebert-Bellanger, S.; Desguerre, I.; Flurin, V.; Grebille, A.G.; Guerrot, A.M.; et al. Type 0 Spinal Muscular Atrophy: Further Delineation of Prenatal and Postnatal Features in 16 Patients. J. Neuromuscul. Dis. 2016, 3, 487–495. [Google Scholar] [CrossRef]
- Stein, H.; Hausen, P. Enzyme from calf thymus degrading the RNA moiety of DNA-RNA Hybrids: Effect on DNA-dependent RNA polymerase. Science 1969, 166, 393–395. [Google Scholar] [CrossRef]
- Wu, H.; Lima, W.F.; Zhang, H.; Fan, A.; Sun, H.; Crooke, S.T. Determination of the role of the human RNase H1 in the pharmacology of DNA-like antisense drugs. J. Biol. Chem. 2004, 279, 17181–17189. [Google Scholar] [CrossRef]
- Stephenson, M.L.; Zamecnik, P.C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc. Natl. Acad. Sci. USA 1978, 75, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F.; Hua, Y.; Chun, S.J.; Prakash, T.P.; Krainer, A.R.; Bennett, C.F. Synthetic oligonucleotides recruit ILF2/3 to RNA transcripts to modulate splicing. Nat. Chem. Biol. 2012, 8, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Nichols, J.G.; Hsu, C.W.; Vickers, T.A.; Crooke, S.T. mRNA levels can be reduced by antisense oligonucleotides via no-go decay pathway. Nucleic Acids Res. 2019, 47, 6900–6916. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Wang, J.; Lynch, D.R.; Shen, X.; Corey, D.R.; Parekh, D.; Bhat, B.; Woo, C.; Cherry, J.J.; et al. Targeting 3′ and 5′ untranslated regions with antisense oligonucleotides to stabilize frataxin mRNA and increase protein expression. Nucleic Acids Res. 2021, 49, 11560–11574. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, O.; Convertini, P.; Zhang, Z.; Wen, Y.; Shen, M.; Falaleeva, M.; Stamm, S. Function of alternative splicing. Gene 2013, 514, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Kole, R.; Krainer, A.R.; Altman, S. RNA therapeutics: Beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discov. 2012, 11, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M. Antisense Oligonucleotide-Mediated Exon-skipping Therapies: Precision Medicine Spreading from Duchenne Muscular Dystrophy. JMA J. 2021, 4, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, O.; Thieme, A.; Claeys, K.G.; Wenninger, S.; Kley, R.A.; Kuhn, M.; Lukacs, Z.; Deschauer, M.; Gaeta, M.; Toscano, A.; et al. Homozygosity for the common GAA gene splice site mutation c.-32-13T>G in Pompe disease is associated with the classical adult phenotypical spectrum. Neuromuscul. Disord. 2015, 25, 719–724. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, E.; Bergsma, A.J.; Pijnenburg, J.M.; van der Ploeg, A.T.; Pijnappel, W. Antisense Oligonucleotides Promote Exon Inclusion and Correct the Common c.-32-13T>G GAA Splicing Variant in Pompe Disease. Mol. Ther. Nucleic Acids 2017, 7, 90–100. [Google Scholar] [CrossRef]
- Lauffer, M.C.; van Roon-Mom, W.; Aartsma-Rus, A. Possibilities and limitations of antisense oligonucleotide therapies for the treatment of monogenic disorders. Commun. Med. 2024, 4, 6. [Google Scholar] [CrossRef]
- Juliano, R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016, 44, 6518–6548. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Wang, S.; Vickers, T.A.; Shen, W.; Liang, X.H. Cellular uptake and trafficking of antisense oligonucleotides. Nat. Biotechnol. 2017, 35, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, F. Phosphorothioate oligodeoxynucleotides: What is their origin and what is unique about them? Antisense Nucleic Acid Drug Dev. 2000, 10, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, F. Phosphorothioates, essential components of therapeutic oligonucleotides. Nucleic Acid Ther. 2014, 24, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Bennett, C.F. Progress in antisense oligonucleotide therapeutics. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Prakash, T.P. An overview of sugar-modified oligonucleotides for antisense therapeutics. Chem. Biodivers. 2011, 8, 1616–1641. [Google Scholar] [CrossRef]
- Owczarzy, R.; You, Y.; Groth, C.L.; Tataurov, A.V. Stability and mismatch discrimination of locked nucleic acid-DNA duplexes. Biochemistry 2011, 50, 9352–9367. [Google Scholar] [CrossRef] [PubMed]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef]
- Hebb, M.O.; Robertson, H.A. End-capped antisense oligodeoxynucleotides effectively inhibit gene expression in vivo and offer a low-toxicity alternative to fully modified phosphorothioate oligodeoxynucleotides. Brain Res. Mol. Brain Res. 1997, 47, 223–228. [Google Scholar] [CrossRef]
- Moulton, J.D.; Jiang, S. Gene knockdowns in adult animals: PPMOs and vivo-morpholinos. Molecules 2009, 14, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Vickers, T.A.; Okunola, H.L.; Bennett, C.F.; Krainer, A.R. Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am. J. Hum. Genet. 2008, 82, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Vickers, T.A.; Baker, B.F.; Bennett, C.F.; Krainer, A.R. Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol. 2007, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.N.; Howell, M.D.; Androphy, E.J.; Singh, R.N. How the discovery of ISS-N1 led to the first medical therapy for spinal muscular atrophy. Gene Ther. 2017, 24, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Sintusek, P.; Catapano, F.; Angkathunkayul, N.; Marrosu, E.; Parson, S.H.; Morgan, J.E.; Muntoni, F.; Zhou, H. Histopathological Defects in Intestine in Severe Spinal Muscular Atrophy Mice Are Improved by Systemic Antisense Oligonucleotide Treatment. PLoS ONE 2016, 11, e0155032. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Sahashi, K.; Hung, G.; Rigo, F.; Passini, M.A.; Bennett, C.F.; Krainer, A.R. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev. 2010, 24, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Passini, M.A.; Bu, J.; Richards, A.M.; Kinnecom, C.; Sardi, S.P.; Stanek, L.M.; Hua, Y.; Rigo, F.; Matson, J.; Hung, G.; et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl. Med. 2011, 3, 72ra18. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Sahashi, K.; Rigo, F.; Hung, G.; Horev, G.; Bennett, C.F.; Krainer, A.R. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 2011, 478, 123–126. [Google Scholar] [CrossRef]
- Porensky, P.N.; Mitrpant, C.; McGovern, V.L.; Bevan, A.K.; Foust, K.D.; Kaspar, B.K.; Wilton, S.D.; Burghes, A.H. A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum. Mol. Genet. 2012, 21, 1625–1638. [Google Scholar] [CrossRef]
- Zhou, H.; Janghra, N.; Mitrpant, C.; Dickinson, R.L.; Anthony, K.; Price, L.; Eperon, I.C.; Wilton, S.D.; Morgan, J.; Muntoni, F. A novel morpholino oligomer targeting ISS-N1 improves rescue of severe spinal muscular atrophy transgenic mice. Hum. Gene Ther. 2013, 24, 331–342. [Google Scholar] [CrossRef]
- Chiriboga, C.A.; Swoboda, K.J.; Darras, B.T.; Iannaccone, S.T.; Montes, J.; De Vivo, D.C.; Norris, D.A.; Bennett, C.F.; Bishop, K.M. Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology 2016, 86, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Chiriboga, C.A.; Vajsar, J.; Day, J.W.; Montes, J.; De Vivo, D.C.; Yamashita, M.; Rigo, F.; Hung, G.; Schneider, E.; et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: A phase 2, open-label, dose-escalation study. Lancet 2016, 388, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Luu, K.T.; Norris, D.A.; Gunawan, R.; Henry, S.; Geary, R.; Wang, Y. Population Pharmacokinetics of Nusinersen in the Cerebral Spinal Fluid and Plasma of Pediatric Patients With Spinal Muscular Atrophy Following Intrathecal Administrations. J. Clin. Pharmacol. 2017, 57, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Nusinersen: First Global Approval. Drugs 2017, 77, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Narayanaswami, P. Making sense of antisense oligonucleotides: A narrative review. Muscle Nerve 2018, 57, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wahane, A.; Alhamadani, F.; Zhang, K.; Parikh, R.; Lee, S.; McCabe, E.M.; Rasmussen, T.P.; Bahal, R.; Zhong, X.B.; et al. Nephrotoxicity of marketed antisense oligonucleotide drugs. Curr. Opin. Toxicol. 2022, 32, 100373. [Google Scholar] [CrossRef] [PubMed]
- Geary, R.S.; Norris, D.; Yu, R.; Bennett, C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Carver, K.; Fisher, M.; Noel, R.; Cintrat, J.C.; Gillet, D.; Barbier, J.; Cao, C.; Bauman, J.; Juliano, R.L. The small molecule Retro-1 enhances the pharmacological actions of antisense and splice switching oligonucleotides. Nucleic Acids Res. 2013, 41, 3673–3687. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, C.; Wood, M.J.A. Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [Google Scholar] [CrossRef]
- Goyenvalle, A.; Jimenez-Mallebrera, C.; van Roon, W.; Sewing, S.; Krieg, A.M.; Arechavala-Gomeza, V.; Andersson, P. Considerations in the Preclinical Assessment of the Safety of Antisense Oligonucleotides. Nucleic Acid Ther. 2023, 33, 1–16. [Google Scholar] [CrossRef]
- Fox, D.; To, T.M.; Seetasith, A.; Patel, A.M.; Iannaccone, S.T. Adherence and Persistence to Nusinersen for Spinal Muscular Atrophy: A US Claims-Based Analysis. Adv. Ther. 2023, 40, 903–919. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Green, M.; Ishino, M.; Loewenstein, P.M. Mutational analysis of HIV-1 Tat minimal domain peptides: Identification of trans-dominant mutants that suppress HIV-LTR-driven gene expression. Cell 1989, 58, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.; Lodigiani, G.; Colombarolli, S.G.; Bergamaschi, G.; Vitali, A. Cell Penetrating Peptides: Classification, Mechanisms, Methods of Study, and Applications. ChemMedChem 2023, 18, e202300236. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, M.; Rosenthal-Aizman, K.; Saar, K.; Eiríksdóttir, E.; Jiang, Y.; Sassian, M.; Ostlund, P.; Hällbrink, M.; Langel, U. Overcoming methotrexate resistance in breast cancer tumour cells by the use of a new cell-penetrating peptide. Biochem. Pharmacol. 2006, 71, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Depollier, J.; Mery, J.; Heitz, F.; Divita, G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 2001, 19, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tang, J.; Fu, L.; Ran, R.; Liu, Y.; Yuan, M.; He, Q. A pH-responsive α-helical cell penetrating peptide-mediated liposomal delivery system. Biomaterials 2013, 34, 7980–7993. [Google Scholar] [CrossRef] [PubMed]
- Margus, H.; Padari, K.; Pooga, M. Cell-penetrating peptides as versatile vehicles for oligonucleotide delivery. Mol. Ther. 2012, 20, 525–533. [Google Scholar] [CrossRef]
- Chavda, V.P.; Solanki, H.K.; Davidson, M.; Apostolopoulos, V.; Bojarska, J. Peptide-Drug Conjugates: A New Hope for Cancer Management. Molecules 2022, 27, 7232. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, W.B.; Fuselier, T.; He, J.; Wimley, W.C. Mechanism Matters: A Taxonomy of Cell Penetrating Peptides. Trends Biochem. Sci. 2015, 40, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Bhalla, S.; Usmani, S.S.; Singh, S.; Chaudhary, K.; Raghava, G.P.; Gautam, A. CPPsite 2.0: A repository of experimentally validated cell-penetrating peptides. Nucleic Acids Res. 2016, 44, D1098–D1103. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, A.; Tornesello, A.L.; Tornesello, M.L.; Buonaguro, F.M. Cell Penetrating Peptides as Molecular Carriers for Anti-Cancer Agents. Molecules 2018, 23, 295. [Google Scholar] [CrossRef] [PubMed]
- Verdurmen, W.P.; Brock, R. Biological responses towards cationic peptides and drug carriers. Trends Pharmacol. Sci. 2011, 32, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Tammam, S.N.; Azzazy, H.M.E.; Lamprecht, A. The effect of nanoparticle size and NLS density on nuclear targeting in cancer and normal cells; impaired nuclear import and aberrant nanoparticle intracellular trafficking in glioma. J. Control. Release 2017, 253, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.; Davis, P. Mechanism of uptake of C105Y, a novel cell-penetrating peptide. J. Biol. Chem. 2006, 281, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Mao, S.; Ditzel, H.J.; Farnaes, L.; Wirsching, P.; Lerner, R.A.; Janda, K.D. A cell-penetrating peptide from a novel pVII-pIX phage-displayed random peptide library. Bioorganic Med. Chem. 2002, 10, 4057–4065. [Google Scholar] [CrossRef]
- Gao, S.; Simon, M.J.; Hue, C.D.; Morrison, B., 3rd; Banta, S. An unusual cell penetrating peptide identified using a plasmid display-based functional selection platform. ACS Chem. Biol. 2011, 6, 484–491. [Google Scholar] [CrossRef]
- Guo, Z.; Peng, H.; Kang, J.; Sun, D. Cell-penetrating peptides: Possible transduction mechanisms and therapeutic applications. Biomed. Rep. 2016, 4, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.H.; Park, I.S.; Hahm, K.S.; Shin, S.Y. Antimicrobial activity, bactericidal mechanism and LPS-neutralizing activity of the cell-penetrating peptide pVEC and its analogs. J. Pept. Sci. 2011, 17, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.J.; El-Andaloussi, S.; Holm, T.; Mäe, M.; Jänes, J.; Maimets, T.; Langel, U. Characterization of a novel cytotoxic cell-penetrating peptide derived from p14ARF protein. Mol. Ther. 2008, 16, 115–123. [Google Scholar] [CrossRef]
- Magzoub, M.; Sandgren, S.; Lundberg, P.; Oglecka, K.; Lilja, J.; Wittrup, A.; Göran Eriksson, L.E.; Langel, U.; Belting, M.; Gräslund, A. N-terminal peptides from unprocessed prion proteins enter cells by macropinocytosis. Biochem. Biophys. Res. Commun. 2006, 348, 379–385. [Google Scholar] [CrossRef]
- Milletti, F. Cell-penetrating peptides: Classes, origin, and current landscape. Drug Discov. Today 2012, 17, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Oehlke, J.; Krause, E.; Wiesner, B.; Beyermann, M.; Bienert, M. Extensive cellular uptake into endothelial cells of an amphipathic beta-sheet forming peptide. FEBS Lett. 1997, 415, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.; Zhang, X.; Zhang, W.; Guo, S.; Jin, F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control. Release 2014, 174, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Ruseska, I.; Zimmer, A. Internalization mechanisms of cell-penetrating peptides. Beilstein J. Nanotechnol. 2020, 11, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.P.; Melikov, K.; Brooks, H.; Prevot, P.; Lebleu, B.; Chernomordik, L.V. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J. Biol. Chem. 2005, 280, 15300–15306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Chen, B.C.; Han, J.C.; Wei, L.; Pan, X.B. Delivery of cell-penetrating peptide-peptide nucleic acid conjugates by assembly on an oligonucleotide scaffold. Sci. Rep. 2015, 5, 17640. [Google Scholar] [CrossRef]
- McClorey, G.; Banerjee, S. Cell-Penetrating Peptides to Enhance Delivery of Oligonucleotide-Based Therapeutics. Biomedicines 2018, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Yesylevskyy, S.; Marrink, S.J.; Mark, A.E. Alternative mechanisms for the interaction of the cell-penetrating peptides penetratin and the TAT peptide with lipid bilayers. Biophys. J. 2009, 97, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Sharmin, S.; Moniruzzaman, M.; Yamazaki, M. Elementary processes for the entry of cell-penetrating peptides into lipid bilayer vesicles and bacterial cells. Appl. Microbiol. Biotechnol. 2018, 102, 3879–3892. [Google Scholar] [CrossRef] [PubMed]
- Deshayes, S.; Morris, M.C.; Divita, G.; Heitz, F. Interactions of amphipathic CPPs with model membranes. Biochim. Biophys. Acta 2006, 1758, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.C.; Zhang, J.; Min, K.A.; Lee, K.; Byun, Y.; David, A.E.; He, H.; Yang, V.C. Cell-penetrating peptides: Achievements and challenges in application for cancer treatment. J. Biomed. Mater. Res. A 2014, 102, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Fadzen, C.M.; Holden, R.L.; Wolfe, J.M.; Choo, A.N.; Schissel, C.K.; Yao, M.; Hanson, G.J.; Pentelute, B.L. Chimeras of Cell-Penetrating Peptides Demonstrate Synergistic Improvement in Antisense Efficacy. Biochemistry 2019, 58, 3980–3989. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.H.; Stein, D.A.; Kroeker, A.D.; Hatlevig, S.A.; Iversen, P.L.; Moulton, H.M. Arginine-rich peptide conjugation to morpholino oligomers: Effects on antisense activity and specificity. Bioconjugate Chem. 2005, 16, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, G.D.; Arzumanov, A.; Abes, R.; Yin, H.; Wood, M.J.; Lebleu, B.; Gait, M.J. Improved cell-penetrating peptide-PNA conjugates for splicing redirection in HeLa cells and exon skipping in mdx mouse muscle. Nucleic Acids Res. 2008, 36, 6418–6428. [Google Scholar] [CrossRef] [PubMed]
- Leger, A.J.; Mosquea, L.M.; Clayton, N.P.; Wu, I.H.; Weeden, T.; Nelson, C.A.; Phillips, L.; Roberts, E.; Piepenhagen, P.A.; Cheng, S.H.; et al. Systemic delivery of a Peptide-linked morpholino oligonucleotide neutralizes mutant RNA toxicity in a mouse model of myotonic dystrophy. Nucleic Acid Ther. 2013, 23, 109–117. [Google Scholar] [CrossRef]
- Tsoumpra, M.K.; Fukumoto, S.; Matsumoto, T.; Takeda, S.; Wood, M.J.A.; Aoki, Y. Peptide-conjugate antisense based splice-correction for Duchenne muscular dystrophy and other neuromuscular diseases. EBioMedicine 2019, 45, 630–645. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; Krieg, A.M. FDA Approves Eteplirsen for Duchenne Muscular Dystrophy: The Next Chapter in the Eteplirsen Saga. Nucleic Acid Ther. 2017, 27, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.; Maruyama, R.; Yokota, T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des. Dev. Ther. 2017, 11, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Saleh, A.F.; Betts, C.; Camelliti, P.; Seow, Y.; Ashraf, S.; Arzumanov, A.; Hammond, S.; Merritt, T.; Gait, M.J.; et al. Pip5 transduction peptides direct high efficiency oligonucleotide-mediated dystrophin exon skipping in heart and phenotypic correction in mdx mice. Mol. Ther. 2011, 19, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Betts, C.; Saleh, A.F.; Arzumanov, A.A.; Hammond, S.M.; Godfrey, C.; Coursindel, T.; Gait, M.J.; Wood, M.J. Pip6-PMO, A New Generation of Peptide-oligonucleotide Conjugates With Improved Cardiac Exon Skipping Activity for DMD Treatment. Mol. Ther. Nucleic Acids 2012, 1, e38. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.Q.; Woo, S.; Melo, D.; Huang, Y.; Dzierlega, K.; Shah, M.N.A.; Aslesh, T.; Roshmi, R.R.; Echigoya, Y.; Maruyama, R.; et al. Development of DG9 peptide-conjugated single- and multi-exon skipping therapies for the treatment of Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA 2022, 119, e2112546119. [Google Scholar] [CrossRef] [PubMed]
- Sazani, P.; Kang, S.H.; Maier, M.A.; Wei, C.; Dillman, J.; Summerton, J.; Manoharan, M.; Kole, R. Nuclear antisense effects of neutral, anionic and cationic oligonucleotide analogs. Nucleic Acids Res. 2001, 29, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Hsieh-Li, H.M.; Chang, J.G.; Jong, Y.J.; Wu, M.H.; Wang, N.M.; Tsai, C.H.; Li, H. A mouse model for spinal muscular atrophy. Nat. Genet. 2000, 24, 66–70. [Google Scholar] [CrossRef]
- Mitrpant, C.; Porensky, P.; Zhou, H.; Price, L.; Muntoni, F.; Fletcher, S.; Wilton, S.D.; Burghes, A.H. Improved antisense oligonucleotide design to suppress aberrant SMN2 gene transcript processing: Towards a treatment for spinal muscular atrophy. PLoS ONE 2013, 8, e62114. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor, R.; Ozmen, L.; Messaddeq, N.; Grüninger, F.; Loetscher, H.; Keller, A.; Betsholtz, C.; Freskgård, P.O.; Collin, L. Trafficking of Endogenous Immunoglobulins by Endothelial Cells at the Blood-Brain Barrier. Sci. Rep. 2016, 6, 25658. [Google Scholar] [CrossRef] [PubMed]
- Nizzardo, M.; Simone, C.; Salani, S.; Ruepp, M.D.; Rizzo, F.; Ruggieri, M.; Zanetta, C.; Brajkovic, S.; Moulton, H.M.; Müehlemann, O.; et al. Effect of combined systemic and local morpholino treatment on the spinal muscular atrophy Δ7 mouse model phenotype. Clin. Ther. 2014, 36, 340–356.e5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).